Abstract
Background
Zinc-based biomaterials, including biodegradable metal, nanoparticles, and coatings used in medical implants release zinc ions that may increase the whole-body and serum zinc concentrations. The impact of serum zinc concentrations on major health outcomes can provide insights for device design and clinical transformation of zinc-based biomaterials.
Methods
This nationally representative cross-sectional study enrolled participants from the National Health and Nutrition Examination Survey (NHANES, 2011-2014) including 3607 participants. Using unadjusted and multivariate-adjusted logistic regression analyses, two-piecewise linear regression model with a smoothing function and threshold level analysis, we evaluated the associations between elevated serum zinc levels and major health outcomes.
Results
Elevated serum zinc levels were significantly associated with an increase in total spine and total femur bone mineral density (BMD). Every 10 μg/dL increase was associated with a 1.12-fold increase in diabetes mellitus (DM) and 1.23-fold and 1.29-fold increase in cardiovascular diseases (CVD) and coronary heart disease (CHD), in participants with serum zinc levels ≥ 100 μg/dL. It had no significant linear or nonlinear associations with risk of fractures, congestive heart failure, heart attack, thyroid disease, arthritis, osteoarthritis, rheumatoid arthritis, dyslipidemia and cancer.
Conclusion
Serum zinc levels are significantly associated with increased BMD in the total spine and total femur, and risk of DM, and CVD/CHD among participants with serum zinc levels ≥100 μg/dL.
Keywords: Serum zinc levels, Multiple health outcomes, Zinc-based biomaterials, Odds ratio
Graphical abstract
Highlights
-
•
The impact of serum zinc levels on major health outcomes can provide insights for zinc-based biomaterials.
-
•
Serum zinc levels are significantly associated with increased BMD in the total spine and total femur.
-
•
Serum zinc levels are associated risk of DM, and CHD among participants with serum zinc levels ≥100 μg/dL.
1. Background
Zinc-based biomaterials including biodegradable zinc-based metal, nanoparticles, and coatings have been researched and explored in the field of medical implants in recent years [[1], [2], [3], [4], [5]], especially for tissue engineering, cardiovascular, orthopedic, anti-tumor and anti-infective applications [[6], [7], [8], [9]]. Most of the zinc-based biomaterials were designed to be biodegradable [[10], [11], [12], [13]], and therefore, release zinc ions upon degradation [14,15]. While they have several biological functions, including anti-bacterial, osteogenic, and angiogenic, high doses of zinc-based biomaterials can also have negative effects, such as damage to the liver, spleen, and pancreas in mice, disruption of energy metabolism, and impairment of the mitochondria and cell membrane in rat kidney [[16], [17], [18]]. Application of these biomaterials in clinical practice, therefore, leads to the concern whether elevated local or whole-body zinc concentrations will have a significant impact on human health and risk for certain diseases.
How to accurately assess the whole-body zinc content? A number of methods are used to assess zinc concentrations in the body. These include measuring its levels in the serum, plasma, hair, nails, and assessing a variety of zinc-binding proteins, such as metallothionein and other zinc metalloenzymes as possible indicators of body zinc status [2,19]. Serum zinc levels generally reflect changes in the whole-body zinc status [2]. Lowe et al. measured changes in total body zinc content using metabolic balance techniques in their depletion study, and found a strong correlation between the changes in total body zinc content and serum zinc concentration (r2 = 0.826, p < 0.001) [20]. They concluded that acute perturbations in its short-term intake and in the absence of confounding factors, changes in serum zinc concentrations might accurately reflect changes in the whole-body zinc status.
Will implantation of zinc-based biomaterials increase serum zinc levels? Wang et al. evaluated the long-term toxicity of oral zinc oxide nanoparticles (Nano-ZnO) and zinc sulfate (ZnSO4) in mice and found that both increased the serum zinc concentrations after 7 weeks (24.82 μmol/L in control group vs. 31.99 ± 1.32 μmol/L in Nano-ZnO and 37.04 ± 2.43 μmol/L in ZnSO4 groups) of oral consumption [21].
Although, there are no published studies to date demonstrating the effects of implantation of biodegradable zinc-based metal on zinc levels in the body. However, some researchers believe that more attention should be paid to the zinc toxicity after degradation of zinc-based metal compared to magnesium-based biomaterials, since the recommended daily intake of magnesium for adults (240–420 mg/day) is up to 52.5 times higher than that of zinc (8–11 mg/day) [12,22]. Pure zinc implants may be a concern because a daily intake of 100–300 mg of zinc can cause health problems and higher doses can be more harmful.
Therefore, investigating the impact of serum zinc concentrations on major health outcomes can provide additional insights for device design and clinical transformation of zinc-based biomaterials. Based on the National Health and Nutrition Examination Survey (NHANES, 2011-2014), our study mainly evaluated the associations between serum zinc levels and major human diseases such as fractures, cardiovascular diseases, stroke, arthritis, diabetes mellitus, thyroid diseases, and cancer and indicators of major outcomes such as bone mineral density, blood glucose, and blood lipids. We also aimed to ascertain the presence of non-linear correlations between serum zinc concentrations and the above-mentioned diseases and outcomes indicators and to explore possible threshold effects.
2. Methods
2.1. Study population
We analyzed the data from NHANES 2011-2014, which is an independent cross-sectional study conducted by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) designed to assess health and nutritional status in a nationally representative sample of non-institutionalized, civilian general population in the United States. The survey details regarding its plan, operation, and design have been described previously [[23], [24], [25], [26]]. Questionnaire surveys, physical examinations, household interviews including demographic, dietary, health-related questions and examinations, and laboratory tests were performed. From a total of 4848 participants who were tested for serum zinc levels, we finally selected 3607 adults after excluding those who were under 18 years of age. Among the 3607 participants, few of them have multiple conditions. The NHANES 2011-2014 was approved by the NCHS Ethics Review Board, and informed consent was obtained from all participants.
2.2. Measurement of serum zinc concentrations
Serum zinc concentrations were measured by inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS), which is a multi-element analytical technique capable of trace level elemental analysis. Detailed instructions on specimen collection and processing can be found on the NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CUSEZN_G.htm and https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CUSEZN_H.htm).
2.3. Assessments
The demographics data (age, sex, and race), physical examination data (standing height [cm], weight [kg], body mass index [kg/m2], waist circumference [cm], systolic blood pressure [SBP, mm Hg], diastolic blood pressure [DBP, mm Hg], bone mineral density [BMD, g/cm2], bone mineral content [BMC, g], and bone mineral area [BMA, cm2]), laboratory data (serum lipids [mg/dL], fasting glucose [mmol/L], glycohemoglobin [%], and insulin [pmol/L]), and questionnaire data (smoking status, alcohol drinking, activity status, fractures and major diseases, including cardiovascular diseases [congestive heart failure, coronary heart disease and heart attack], stroke, diabetes mellitus, thyroid disease, arthritis, osteoarthritis, rheumatoid arthritis, gout, and cancer) are all described on the NHANES website at https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes/default.aspx?BeginYear=2011 and https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes/default.aspx?BeginYear=2013.
For all participants, race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, other race), smoking status (yes or no), alcohol drinking (yes or no), and moderate physical activity (yes or no) were defined. BMD, BMC, and BMA in the total spine, total femur, and femoral neck were examined by dual-energy x-ray absorptiometry (Hologic QDR-4500A fan-beam densitometers, software version Hologic APEX 3.2). Serum lipids, including total cholesterol (TC), triglycerides (TG), and low-density lipoprotein-cholesterol (LDL-C) were analyzed using the Roche Modular P chemistry analyzer (enzymatic method). Whole blood glycohemoglobin was analyzed using the Tosoh G8 glycohemoglobin analyzer. Serum insulin was measured by an immunoenzymometric assay using the TOSOH AIA-900 chemistry analyzer.
Self-reported fractures were assessed by the questionnaire. Prior low trauma fractures were defined as self-reported fractures that occurred at an age ≥40 years due to a fall from a standing height or less, a trip/slip, or a fall out of bed (hip, wrist, spine) or at an age ≥ 20 years and were not due to severe trauma such as a car accident, hard fall down steps, or from a ladder (fractures other than hip, wrist, and spine). Cardiovascular diseases (CVD) were defined as congestive heart failure (CHF), coronary heart disease (CHD), and heart attack (HA). Arthritis included osteoarthritis/degenerative, rheumatoid arthritis (RA), psoriatic, and other forms of arthritis. Diabetes mellitus (DM) was defined as a self-reported diagnosis of DM and assessed by measuring blood glycohemoglobin, fasting plasma glucose levels, 2-h glucose (Oral Glucose Tolerance Test) levels, and serum insulin in participants. Dyslipidemia was defined as having high TG (≥200 mg/dL), TC (≥240 mg/dL), and LDL-C (≥160 mg/dL), or low high-density lipoprotein-cholesterol (HDL-C) (≤40 mg/dL).
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were presented as numbers and their proportion. We used the Chi-squared tests for categorical variables, one-way ANOVA for normally continuous variables, and the Kruskal-Wallis test for the skewed continuous variables. Regression coefficient and corresponding 95% confidence intervals (CI) were calculated using unadjusted and multivariate-adjusted logistic regression analyses to determine associations between 10 μg/dL increases in serum zinc levels and major diseases. The crude model was adjusted for no variables. We selected these confounders on the basis of their associations with the outcomes of interest or a change in effect estimate of more than 10%. The multivariate model was adjusted for sex, age, race, smoking, alcohol drinking, moderate activity, body mass index (BMI, kg/m2), waist circumference (cm), SBP (mm Hg), and DBP (mm Hg), CVD, DM, thyroid disease; arthritis; cancer; dyslipidemia and BMD. A two-sided p value < 0.05 was considered to be statistically significant. To examine the nonlinear association between serum zinc levels and major diseases (logOR), we further applied a two-piecewise linear regression model using a smoothing function. The threshold level was determined using trial and error, including selection of turning points along a pre-defined interval and then choosing the turning point that gave the maximum model likelihood. In addition, we conducted a log-likelihood ratio test comparing the one-line linear regression model with the two-piecewise linear model. Statistical analyses were performed using R packages (http://www.r-project.org) and Empower (R) (www.empowerstats.com, X&Y solutions Inc., Boston, MA.
3. Results
3.1. Descriptive analysis
The participant characteristics are presented in Table 1. This study included 3607 participants with fractures (n = 335), CVD (n = 229), stroke (n = 109), DM (n = 425), thyroid diseases (n = 330), arthritis (n = 864; 397 cases of osteoarthritis and 152 of RA), cancer (n = 297) and dyslipidemia (n = 1064). The average standing height, serum TC, and serum LDL-C increased across the serum zinc quartiles. BMI decreased across the serum zinc quartiles.
Table 1.
Participants characteristics. Values are mean ± SD or n (%).
| Serum Zinc (ug/dL) | <71.50 | 71.50 to 80.3 | 80.3 to 90.3 | ≥90.3 | P-value |
|---|---|---|---|---|---|
| N | 892 | 891 | 915 | 909 | |
| Age (y) | 47.23 ± 18.68 | 47.40 ± 18.74 | 47.18 ± 18.16 | 45.86 ± 18.07 | 0.258 |
| Standing Height (cm) | 166.17 ± 9.93 | 166.66 ± 9.88 | 167.44 ± 10.04 | 168.75 ± 10.17 | <0.001 |
| Weight (kg) | 81.78 ± 24.07 | 81.46 ± 23.35 | 79.73 ± 20.61 | 80.41 ± 20.49 | 0.180 |
| Body Mass Index (kg/m2) | 29.55 ± 8.00 | 29.20 ± 7.54 | 28.36 ± 6.52 | 28.12 ± 6.23 | <0.001 |
| Waist Circumference (cm) | 99.31 ± 17.82 | 98.59 ± 17.17 | 97.48 ± 16.40 | 97.46 ± 15.84 | 0.062 |
| Systolic blood pressure (mm Hg) | 123.26 ± 19.03 | 122.21 ± 17.98 | 121.33 ± 17.35 | 122.88 ± 17.89 | 0.134 |
| Diastolic blood pressure (mm Hg) | 70.00 ± 12.74 | 69.19 ± 13.51 | 69.24 ± 13.81 | 69.48 ± 13.45 | 0.588 |
| Bone mineral density (g/cm2) | |||||
| Total spine | 0.98 ± 0.15 | 1.00 ± 0.16 | 1.04 ± 0.17 | 1.01 ± 0.15 | 0.021 |
| Total femur | 0.94 ± 0.15 | 0.95 ± 0.16 | 0.95 ± 0.16 | 0.96 ± 0.15 | 0.431 |
| Femoral neck | 0.77 ± 0.14 | 0.79 ± 0.15 | 0.79 ± 0.15 | 0.79 ± 0.14 | 0.492 |
| Intertrochanter | 1.12 ± 0.18 | 1.14 ± 0.19 | 1.14 ± 0.19 | 1.15 ± 0.18 | 0.405 |
| Trochanter | 0.70 ± 0.13 | 0.72 ± 0.13 | 0.72 ± 0.13 | 0.72 ± 0.12 | 0.373 |
| Wards triangle | 0.60 ± 0.18 | 0.62 ± 0.19 | 0.61 ± 0.19 | 0.62 ± 0.17 | 0.323 |
| Bone mineral content (g) | |||||
| Total spine | 59.71 ± 14.04 | 60.46 ± 13.90 | 63.96 ± 15.68 | 63.18 ± 14.36 | 0.018 |
| Total femur | 35.37 ± 9.30 | 35.96 ± 9.85 | 35.92 ± 10.10 | 36.70 ± 9.40 | 0.503 |
| Femoral neck | 4.02 ± 0.84 | 4.14 ± 0.97 | 4.17 ± 1.01 | 4.19 ± 0.87 | 0.169 |
| Intertrochanter | 22.98 ± 6.79 | 23.09 ± 6.66 | 23.03 ± 6.92 | 23.61 ± 6.49 | 0.704 |
| Trochanter | 8.37 ± 2.27 | 8.74 ± 2.61 | 8.73 ± 2.53 | 8.89 ± 2.37 | 0.107 |
| Wards triangle | 0.70 ± 0.23 | 0.74 ± 0.25 | 0.73 ± 0.23 | 0.73 ± 0.23 | 0.355 |
| Bone mineral area (cm2) | |||||
| Total spine | 60.35 ± 8.16 | 59.92 ± 6.95 | 61.38 ± 8.03 | 61.94 ± 8.16 | 0.074 |
| Total femur | 37.40 ± 6.48 | 37.32 ± 6.24 | 37.39 ± 6.09 | 37.87 ± 5.96 | 0.756 |
| Femoral neck | 5.20 ± 0.57 | 5.23 ± 0.60 | 5.27 ± 0.56 | 5.28 ± 0.57 | 0.338 |
| Intertrochanter | 20.33 ± 4.56 | 19.99 ± 3.74 | 20.06 ± 3.83 | 20.34 ± 3.69 | 0.663 |
| Trochanter | 11.87 ± 2.23 | 12.10 ± 2.43 | 12.06 ± 2.20 | 12.24 ± 2.13 | 0.318 |
| Wards triangle | 1.17 ± 0.09 | 1.18 ± 0.09 | 1.18 ± 0.08 | 1.17 ± 0.09 | 0.896 |
| Serum lipids (mg/dL) | |||||
| Total Cholesterol | 185.10 ± 40.44 | 188.47 ± 40.14 | 189.69 ± 41.97 | 193.80 ± 41.50 | <0.001 |
| Triglyceride | 104.92 ± 74.38 | 102.65 ± 57.01 | 121.39 ± 100.02 | 132.62 ± 109.81 | <0.001 |
| HDL-Cholesterol | 52.20 ± 15.36 | 53.17 ± 15.59 | 52.43 ± 14.70 | 53.02 ± 15.77 | 0.480 |
| LDL-Cholesterol | 107.11 ± 33.93 | 110.46 ± 33.28 | 110.88 ± 37.37 | 114.50 ± 35.95 | 0.046 |
| Fasting Glucose (mmol/L) | 5.68 ± 1.41 | 5.90 ± 1.79 | 5.99 ± 2.01 | 5.91 ± 1.96 | 0.280 |
| Glycohemoglobin (%) | 5.69 ± 0.99 | 5.72 ± 0.98 | 5.76 ± 1.15 | 5.73 ± 1.12 | 0.566 |
| Insulin (pmol/L) | 72.92 ± 80.07 | 85.12 ± 92.37 | 69.07 ± 65.70 | 75.97 ± 72.14 | 0.174 |
| Sex | <0.001 | ||||
| Man | 376 (42.15%) | 418 (46.91%) | 441 (48.20%) | 539 (59.30%) | |
| Woman | 516 (57.85%) | 473 (53.09%) | 474 (51.80%) | 370 (40.70%) | |
| Race | 0.293 | ||||
| Mexican American | 120 (13.45%) | 106 (11.90%) | 109 (11.91%) | 112 (12.32%) | |
| Other Hispanic | 83 (9.30%) | 92 (10.33%) | 82 (8.96%) | 89 (9.79%) | |
| Non-Hispanic White | 331 (37.11%) | 339 (38.05%) | 398 (43.50%) | 379 (41.69%) | |
| Non-Hispanic Black | 216 (24.22%) | 210 (23.57%) | 186 (20.33%) | 183 (20.13%) | |
| Other Race | 142 (15.92%) | 144 (16.16%) | 140 (15.30%) | 146 (16.06%) | |
| Smoking | 0.023 | ||||
| Yes | 328 (37.74%) | 378 (43.45%) | 378 (42.38%) | 393 (44.51%) | |
| No | 541 (62.26%) | 492 (56.55%) | 514 (57.62%) | 490 (55.49%) | |
| Alcohol drinking | 0.020 | ||||
| Yes | 546 (68.25%) | 608 (73.25%) | 585 (71.25%) | 617 (74.88%) | |
| No | 254 (31.75%) | 222 (26.75%) | 236 (28.75%) | 207 (25.12%) | |
| Moderate activity | 0.165 | ||||
| Yes | 284 (31.87%) | 269 (30.22%) | 305 (33.37%) | 318 (34.98%) | |
| No | 607 (68.13%) | 621 (69.78%) | 609 (66.63%) | 591 (65.02%) | |
| Total Fracture | 0.464 | ||||
| No | 211 (23.65%) | 199 (22.33%) | 195 (21.31%) | 201 (22.11%) | |
| Yes | 87 (9.75%) | 84 (9.43%) | 94 (10.27%) | 70 (7.70%) | |
| Spine Fracture | 0.639 | ||||
| No | 290 (32.51%) | 278 (31.20%) | 280 (30.60%) | 266 (29.26%) | |
| Yes | 8 (0.90%) | 5 (0.56%) | 9 (0.98%) | 5 (0.55%) | |
| Hip Fracture | 0.587 | ||||
| No | 293 (32.85%) | 277 (31.09%) | 280 (30.60%) | 267 (29.37%) | |
| Yes | 6 (0.67%) | 7 (0.79%) | 9 (0.98%) | 4 (0.44%) | |
| Wrist Fracture | 0.378 | ||||
| No | 277 (31.05%) | 268 (30.08%) | 261 (28.52%) | 252 (27.72%) | |
| Yes | 22 (2.47%) | 16 (1.80%) | 28 (3.06%) | 19 (2.09%) | |
| Cardiovascular diseases | 0.797 | ||||
| No | 830 (93.05%) | 836 (93.83%) | 856 (93.55%) | 856 (94.17%) | |
| Yes | 62 (6.95%) | 55 (6.17%) | 59 (6.45%) | 53 (5.83%) | |
| Stroke | 0.757 | ||||
| No | 819 (91.82%) | 816 (91.58%) | 837 (91.48%) | 826 (90.87%) | |
| Yes | 25 (2.80%) | 32 (3.59%) | 27 (2.95%) | 25 (2.75%) | |
| Congestive heart failure | 0.281 | ||||
| No | 808 (90.58%) | 823 (92.37%) | 839 (91.69%) | 833 (91.64%) | |
| Yes | 32 (3.59%) | 25 (2.81%) | 23 (2.51%) | 17 (1.87%) | |
| Coronary heart disease | 0.647 | ||||
| No | 810 (90.81%) | 814 (91.36%) | 834 (91.15%) | 820 (90.21%) | |
| Yes | 34 (3.81%) | 32 (3.59%) | 25 (2.73%) | 29 (3.19%) | |
| Heart attack | 0.886 | ||||
| No | 813 (91.14%) | 819 (91.92%) | 830 (90.71%) | 823 (90.54%) | |
| Yes | 32 (3.59%) | 29 (3.25%) | 33 (3.61%) | 29 (3.19%) | |
| Diabetes | 0.103 | ||||
| No | 760 (85.20%) | 766 (85.97%) | 772 (84.37%) | 780 (85.81%) | |
| Yes | 95 (10.65%) | 102 (11.45%) | 116 (12.68%) | 112 (12.32%) | |
| Thyroid disease | 0.432 | ||||
| No | 766 (85.87%) | 753 (84.51%) | 775 (84.70%) | 781 (85.92%) | |
| Yes | 78 (8.74%) | 93 (10.44%) | 89 (9.73%) | 70 (7.70%) | |
| Arthritis | 0.559 | ||||
| No | 621 (69.62%) | 618 (69.36%) | 646 (70.60%) | 649 (71.40%) | |
| Yes | 219 (24.55%) | 229 (25.70%) | 215 (23.50%) | 201 (22.11%) | |
| Gout | 0.303 | ||||
| No | 814 (91.26%) | 811 (91.02%) | 833 (91.04%) | 804 (88.45%) | |
| Yes | 31 (3.48%) | 37 (4.15%) | 31 (3.39%) | 47 (5.17%) | |
| Osteoarthritis | 0.559 | ||||
| No | 621 (69.62%) | 618 (69.36%) | 646 (70.60%) | 649 (71.40%) | |
| Yes | 98 (10.99%) | 114 (12.79%) | 95 (10.38%) | 90 (9.90%) | |
| Rheumatoid arthritis | 0.930 | ||||
| No | 621 (69.62%) | 618 (69.36%) | 646 (70.60%) | 649 (71.40%) | |
| Yes | 38 (4.26%) | 37 (4.15%) | 36 (3.93%) | 41 (4.51%) | |
| Cancer | 0.719 | ||||
| No | 767 (85.99%) | 768 (86.20%) | 792 (86.56%) | 785 (86.36%) | |
| Yes | 78 (8.74%) | 80 (8.98%) | 72 (7.87%) | 67 (7.37%) | |
| Dyslipidemia | 0.049 | ||||
| No | 648 (72.65%) | 637 (71.49%) | 649 (70.93%) | 609 (67.00%) | |
| Yes | 244 (27.35%) | 254 (28.51%) | 266 (29.07%) | 300 (33.00%) | |
| High TC level (mg/dL) | 0.043 | ||||
| <240 | 795 (90.14%) | 788 (89.14%) | 810 (89.50%) | 775 (86.21%) | |
| ≥240 | 87 (9.86%) | 96 (10.86%) | 95 (10.50%) | 124 (13.79%) | |
| High TG level (mg/dL) | <0.001 | ||||
| <200 | 170 (92.39%) | 334 (93.82%) | 467 (88.28%) | 555 (85.78%) | |
| ≥200 | 14 (7.61%) | 22 (6.18%) | 62 (11.72%) | 92 (14.22%) | |
| Low HDL-C level (mg/dL) | 0.815 | ||||
| ≥40 | 718 (81.41%) | 721 (81.56%) | 751 (82.98%) | 739 (82.20%) | |
| <40 | 164 (18.59%) | 163 (18.44%) | 154 (17.02%) | 160 (17.80%) | |
| High LDL-C level (mg/dL) | 0.294 | ||||
| <160 | 165 (91.16%) | 328 (92.13%) | 475 (91.00%) | 556 (88.68%) | |
| ≥160 | 16 (8.84%) | 28 (7.87%) | 47 (9.00%) | 71 (11.32%) | |
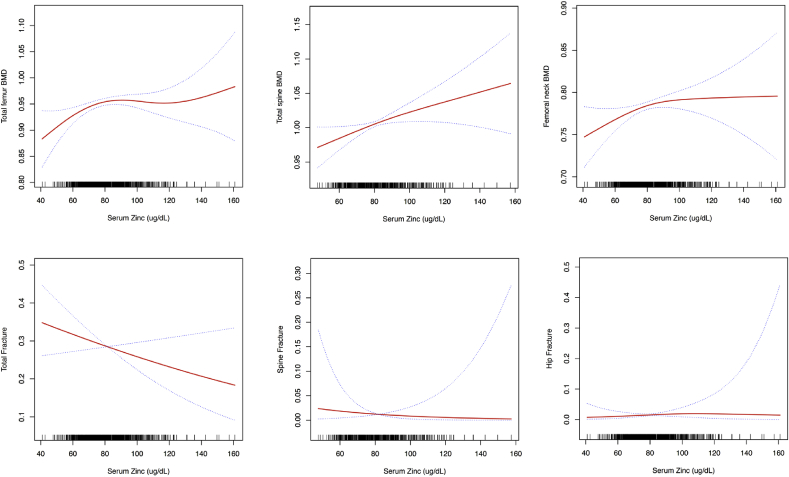
3.2. Serum zinc levels and bone mineral density
Table 2 and S2 [see Supplementary data] and Fig. 1 present the association between serum zinc levels and BMD using the logistic regression and two-piecewise linear regression models. In the multivariate logistic regression analysis, serum zinc levels were significantly associated with the total spine and total femur BMD after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, cancer and dyslipidemia. Every 10 μg/dL increase in serum zinc levels was associated with 0.009 g/cm2 (p = 0.020, 95% CI = 0.001–0.016) and 0.005 g/cm2 (p = 0.049, 95% CI = 0.000–0.010) increase in BMD of the total spine and total femur. There were significant nonlinear associations between serum zinc levels and BMD of the femoral neck.
Table 2.
Multivariate regression analysis for effect of serum zinc on multiple health outcomes.
| Per 10 μg/dL increased in serum zinc | Un-adjusted Model |
Adjusted Model I |
Adjusted Model II |
|||
|---|---|---|---|---|---|---|
| β/OR (95%CI) | P value | β/OR (95%CI) | P value | β/OR (95%CI) | P value | |
| Bone related diseases | ||||||
| Bone mineral density (g/cm2) | ||||||
| Total Spine | 0.006 (−0.002, 0.014) | 0.119 | 0.009 (0.002, 0.016) | 0.011 | 0.009 (0.001, 0.016) | 0.020a |
| Total femur | 0.004 (−0.002, 0.010) | 0.242 | 0.005 (0.000, 0.011) | 0.034 | 0.005 (0.000, 0.010) | 0.049a |
| Femoral neck | 0.003 (−0.003, 0.009) | 0.321 | 0.005 (0.000, 0.010) | 0.063 | 0.005 (−0.001, 0.010) | 0.080a |
| Fracture | ||||||
| Total Fracture | 0.938 (0.863, 1.019) | 0.131 | 0.915 (0.832, 1.006) | 0.065 | 0.930 (0.841, 1.030) | 0.164b |
| Spine Fracture | 0.855 (0.655, 1.117) | 0.251 | 0.872 (0.643, 1.182) | 0.378 | 0.818 (0.424, 1.576) | 0.548c |
| Hip Fracture | 0.899 (0.690, 1.172) | 0.433 | 0.956 (0.698, 1.310) | 0.781 | 1.042 (0.742, 1.462) | 0.814d |
| Cardiovascular diseases and stroke | ||||||
| Cardiovascular diseases | 1.035 (0.951, 1.127) | 0.421 | 1.071 (0.973, 1.180) | 0.161 | 1.056 (0.956, 1.167) | 0.282e |
| Stroke | 1.031 (0.914, 1.163) | 0.617 | 1.088 (0.949, 1.247) | 0.226 | 1.095 (0.951, 1.261) | 0.206e |
| Coronary heart disease | 1.046 (0.934, 1.172) | 0.437 | 1.085 (0.962, 1.224) | 0.184 | 1.064 (0.936, 1.209) | 0.342e |
| Congestive heart failure | 0.933 (0.814, 1.069) | 0.316 | 0.949 (0.816, 1.102) | 0.491 | 0.949 (0.814, 1.105) | 0.500e |
| Heart attack | 1.027 (0.916, 1.150) | 0.650 | 1.046 (0.919, 1.190) | 0.498 | 1.046 (0.915, 1.196) | 0.511e |
| Diabetes and Thyroid disease | ||||||
| Diabetes | 1.045 (0.980, 1.114) | 0.178 | 1.120 (1.039, 1.208) | 0.003 | 1.119 (1.035, 1.209) | 0.005f |
| Thyroid disease | 0.976 (0.906, 1.052) | 0.531 | 0.999 (0.914, 1.092) | 0.984 | 0.996 (0.911, 1.089) | 0.932g |
| Fasting Glucose (mmol/L) | 0.020 (−0.039, 0.079) | 0.498 | 0.021 (−0.042, 0.085) | 0.508 | 0.015 (−0.048, 0.079) | 0.636f |
| Glycohemoglobin (%) | 0.015 (−0.008, 0.037) | 0.196 | 0.035 (0.013, 0.058) | 0.002 | 0.034 (0.012, 0.056) | 0.003f |
| Insulin (pmol/L) | −2.579 (−5.89, 0.727) | 0.127 | −0.693 (−3.84, 2.456) | 0.666 | −0.809 (−3.985, 2.367) | 0.618f |
| Arthritis and gout | ||||||
| Arthritis | 0.970 (0.923, 1.021) | 0.242 | 0.994 (0.933, 1.060) | 0.857 | 0.992 (0.930, 1.058) | 0.807a |
| Gout | 1.075 (0.972, 1.189) | 0.158 | 1.089 (0.973, 1.218) | 0.137 | 1.092 (0.987, 1.208) | 0.089a |
| Osteoarthritis | 0.984 (0.918, 1.054) | 0.640 | 0.996 (0.913, 1.086) | 0.926 | 0.993 (0.922, 1.070) | 0.854a |
| Rheumatoid arthritis | 1.003 (0.902, 1.114) | 0.963 | 1.039 (0.921, 1.174) | 0.532 | 1.004 (0.902, 1.118) | 0.936a |
| Cancer | ||||||
| All kind of cancer | 0.982 (0.909, 1.061) | 0.650 | 1.013 (0.928, 1.106) | 0.776 | 1.001 (0.924, 1.085) | 0.980h |
| Dyslipidemia | ||||||
| Dyslipidemia | 1.052 (1.005, 1.102) | 0.029 | 1.025 (0.973, 1.081) | 0.351 | 1.022 (0.969, 1.078) | 0.420i |
| High TC level | 1.086 (1.018, 1.158) | 0.012 | 1.091 (1.015, 1.172) | 0.018 | 1.099 (1.022, 1.182) | 0.011i |
| High TG levle | 1.173 (1.071, 1.285) | 0.001 | 1.137 (1.025, 1.262) | 0.015 | 1.122 (1.009, 1.248) | 0.033i |
| Low HDL-C level | 0.981 (0.928, 1.038) | 0.504 | 0.928 (0.869, 0.992) | 0.028 | 0.920 (0.860, 0.983) | 0.014i |
| High LDL-C level | 1.074 (0.970, 1.188) | 0.169 | 1.072 (0.954, 1.203) | 0.241 | 1.078 (0.958, 1.213) | 0.212i |
| Total Cholesterol (mg/dL) | 1.891 (1.018, 2.764) | 0.001 | 2.035 (1.106, 2.964) | 0.001 | 2.219 (1.305, 3.133) | <0.001i |
| Triglyceride (mg/dL) | 6.536 (3.573, 9.499) | 0.001 | 5.483 (2.232, 8.733) | 0.001 | 4.962 (1.699, 8.224) | 0.003i |
| HDL-Cholesterol (mg/dL) | 0.227 (−0.099, 0.554) | 0.173 | 0.301 (−0.013, 0.616) | 0.060 | 0.355 (0.042, 0.667) | 0.026i |
| LDL-cholesterol (mg/dL) | 1.118 (−0.010, 2.246) | 0.052 | 1.477 (0.229, 2.726) | 0.021 | 1.602 (0.383, 2.821) | 0.010i |
Model I adjust for: Sex; Age (y); Race; Smoking; Alcohol drinking; Moderate activity; Body Mass Index (kg/m2); Waist Circumference (cm); Systolic blood pressure (mm Hg); Diastolic blood pressure (mm Hg).
Model II adjust for.
Model I plus CVD; DM; Thyroid disease; Arthritis; Cancer; Dyslipidemia.
Model I plus CVD; DM; Thyroid disease; Arthritis; Cancer; Dyslipidemia; Total femur BMD.
Model I plus CVD; DM; Thyroid disease; Arthritis; Cancer; Dyslipidemia; Spine BMD.
Model I plus CVD; DM; Thyroid disease; Arthritis; Cancer; Dyslipidemia; Femoral neck BMD.
Model I plus DM; Thyroid disease; Arthritis; Cancer; Dyslipidemia.
Model I plus CVD; Thyroid disease; Arthritis; Cancer; Dyslipidemia.
Model I plus CVD; DM; Arthritis; Cancer; Dyslipidemia.
Model I plus CVD; DM; Thyroid disease; Arthritis; Dyslipidemia.
Model I plus CVD; DM; Thyroid disease; Arthritis; Cancer.
Fig. 1.
Multivariate adjusted smoothing spline plots of serum zinc levels and bone mineral density and fractures. The model for BMD was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, cancer, and dyslipidemia. The model for fracture was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, cancer, dyslipidemia, and total femur/total spine/femoral neck BMD. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Tables S1 and S2, and Fig. S1 [see Supplementary data] present the association between serum zinc levels and BMC and BMA using the logistic regression models. In the multivariate logistic regression analysis, serum zinc levels were significantly associated with BMC of the total spine and total femur after multivariate adjustment.
3.3. Serum zinc levels and fractures
Table 2, S2 [see Supplementary data] and Fig. 1 present the association between serum zinc levels and fractures. In the multivariate logistic regression analysis after being adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, cancer and BMD (total spine/total femur/femoral neck), serum zinc levels were not associated with risk of total, spine, or hip fractures (all p > 0.05).
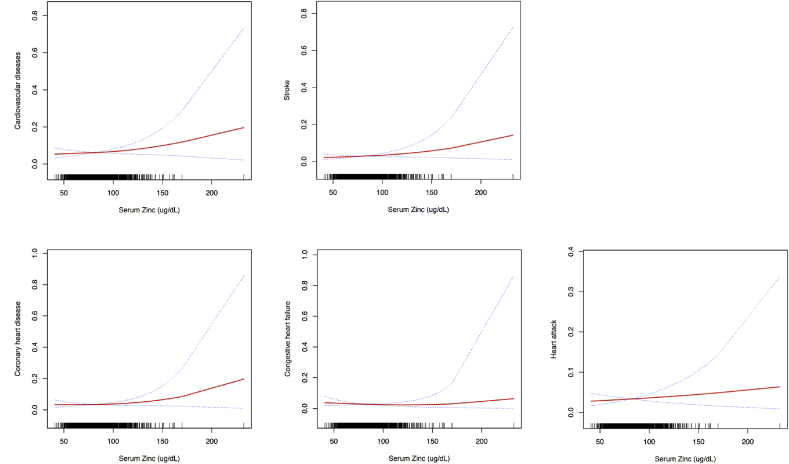
3.4. Serum zinc levels, cardiovascular diseases, and stroke
Table 2 and S3 [see Supplementary data] and Fig. 2 present the association between serum zinc levels and risk of CVD and stroke. There were no significant linear or nonlinear associations between serum zinc levels, CHF and HA in multivariate logistic regression analysis after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, DM, thyroid disease, arthritis, cancer and dyslipidemia. However, every 10 μg/dL increase in the serum zinc level was associated with a 1.23-fold (p = 0.041, 95% CI = 1.01–1.50) and 1.29-fold (p = 0.021, 95% CI = 1.04–1.61) increase in risk of CVD and CHD risk, respectively in participants with serum zinc levels ≥ 100 μg/dL. Every 10 μg/dL increase was associated with a 1.40-fold (p = 0.045, 95% CI = 1.01–1.95) increase in stroke, in participants with serum zinc levels ≥ 120 μg/dL.
Fig. 2.
Multivariate adjusted smoothing spline plots of serum zinc levels and cardiovascular diseases and stroke. This model was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, DM, thyroid disease, arthritis, cancer, and dyslipidemia. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Serum zinc levels, diabetes mellitus, and thyroid disease
Table 2 and S4 [see Supplementary data] and Fig. 3 present the association of serum zinc levels with DM and thyroid disease. There were no significant linear or nonlinear associations between serum zinc levels and thyroid disease in multivariate logistic regression analysis after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, arthritis, cancer and dyslipidemia. However, every 10 μg/dL increase in serum zinc level was associated with a 1.12-fold (p = 0.005, 95% CI = 1.04–1.21) increase in the risk of DM after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, thyroid disease, arthritis, cancer and dyslipidemia. Though multivariate logistic regression analysis revealed no significant linear or nonlinear associations between serum zinc levels and fasting glucose and insulin. And it showed that every 10 μg/dL increase in serum zinc levels, resulted in a glycohemoglobin increase by 0.034% (p = 0.003, 95% CI = 0.012–0.056).
Fig. 3.
Multivariate adjusted smoothing spline plots of serum zinc levels and diabetes mellitus, thyroid disease, and levels of fasting glucose, glycohemoglobin, and insulin. This model was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM/thyroid disease, arthritis, cancer, dyslipidemia. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
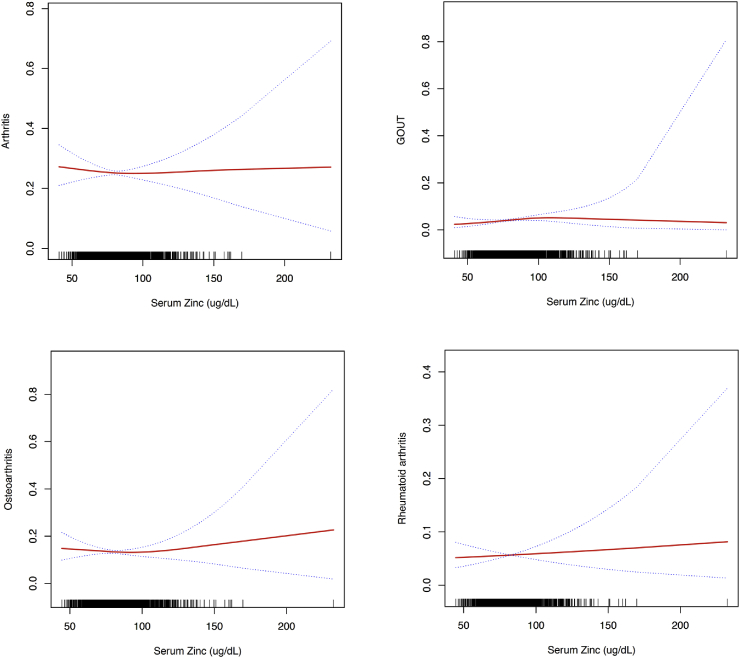
3.6. Serum zinc levels, arthritis, and gout
There were no significant linear or nonlinear associations between serum zinc levels and risk of arthritis, osteoarthritis, and RA in multivariate logistic regression analysis after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, cancer and dyslipidemia. (Table 2 and S5 [see Supplementary data] and Fig. 4). However, multivariate logistic regression analysis and threshold level analysis suggested that in participants with serum zinc levels <99 μg/dL, for every 10 μg/dL increase in serum zinc levels, the risk of gout increased by 1.24-fold (p for nonlinearly = 0.043).
Fig. 4.
Multivariate adjusted smoothing spline plots of serum zinc levels and arthritis and gout. This model was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, cancer, and dyslipidemia. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
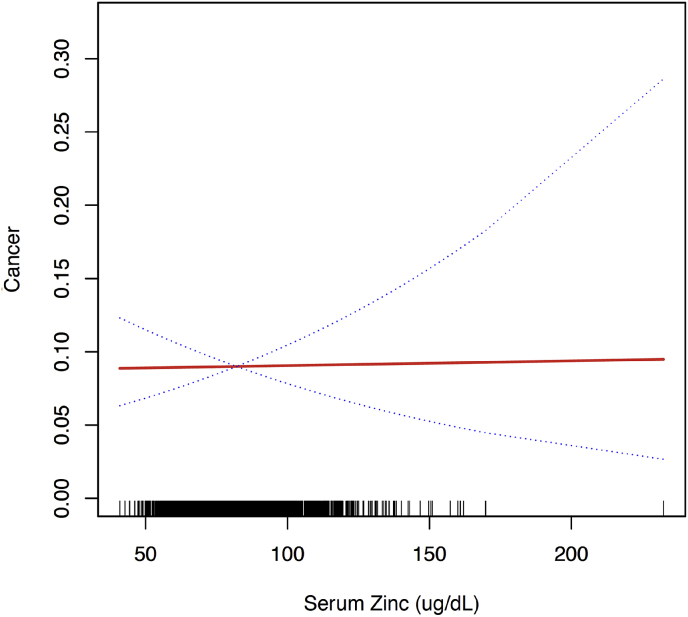
3.7. Serum zinc levels and cancer
There were no significant linear or nonlinear associations between serum zinc levels and incidence of cancer in multivariate logistic regression analysis after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, and dyslipidemia. (OR 1.001, 95% CI: 0.924, 1.085, p = 0.980) (Table 2 and S6 [see Supplementary data] and Fig. 5).
Fig. 5.
Multivariate adjusted smoothing spline plots of serum zinc levels and cancer. This model was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis, and dyslipidemia. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
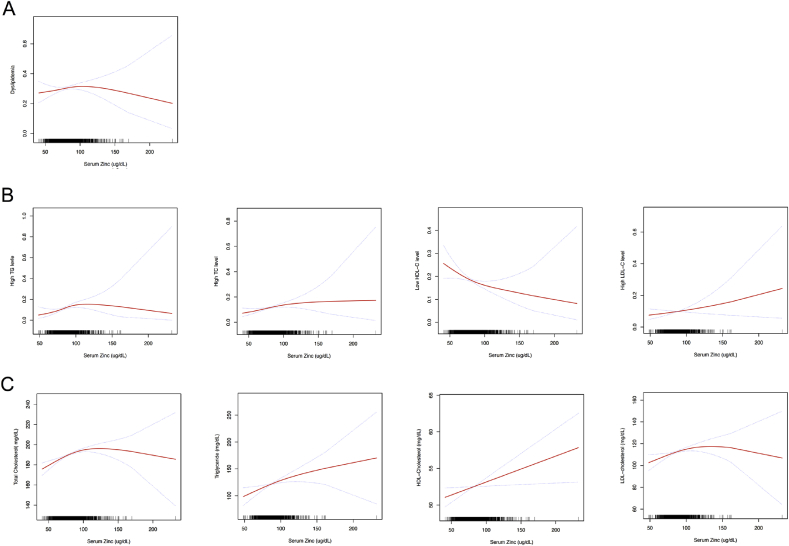
3.8. Serum zinc levels and dyslipidemia
Table 2 and S7 [see Supplementary data] and Fig. 6 present the associations of serum zinc levels with dyslipidemia and serum lipids. There were no significant linear or nonlinear associations between serum zinc levels and total dyslipidemia in multivariate logistic regression analysis after multivariate adjustment for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM, thyroid disease, arthritis and cancer. However, every 10 μg/dL increase in serum zinc level was associated with a 1.10-fold (p = 0.011, 95% CI = 1.022–1.182), and a 1.12-fold (p = 0.033, 95% CI = 1.010–1.248) increase in high TC level and high TG level, respectively. Every 10 μg/dL increase in serum zinc levels was also associated with 2.219 mg/dL, 4.962 mg/dL, 0.355 mg/dL and 1.602 mg/dL increase in serum TC, serum TG, HDL-Cholesterol and LDL-cholesterol.
Fig. 6.
Multivariate adjusted smoothing spline plots of serum zinc levels and dyslipidemia (A, B) and serum lipids (B). This model was adjusted for sex, age, race, smoking status, alcohol drinking, moderate activity, BMI, waist circumference, SBP, DBP, CVD, DM/thyroid disease, arthritis, and cancer. The red line represents the best-fit line, and the blue lines are 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
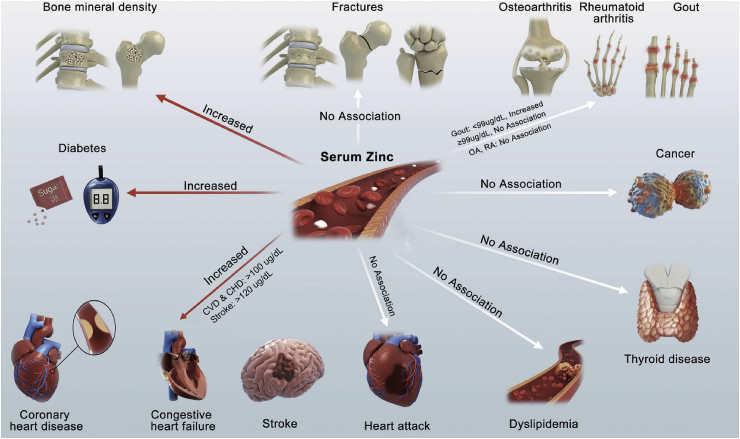
This study analyzed the data from the NHANES (2011-2014) including 3607 participants and found that the high serum zinc levels were significantly associated with an increase in the total spine and total femur BMD; risk of DM; and CVD/CHD in participants with serum zinc levels 100 μg/dL. It had no significant linear or nonlinear associations with risk of fractures, CHF, HA, thyroid disease, arthritis, osteoarthritis, RA, dyslipidemia and cancer (Fig. 7 and Fig. 8).
Fig. 7.
Forest plots of serum zinc levels and major human diseases.
Fig. 8.
Conceptual diagram of serum zinc levels and major human diseases.
Zinc-based biomaterials, especially zinc-based nanoparticles, can increase the concentration of zinc ions in the blood, raising concerns about their whole body zinc ion status and toxicity [21,[27], [28], [29]]. A study has shown that high doses of nano-ZnO can cause acute toxicity, which can damage the lung, liver, spleen, and pancreas in mice, disturb energy metabolism and impair the mitochondria and cell membrane in rat kidney. They were also shown to increase lactate dehydrogenase (LDH) leakage and cause genotoxicity to primary mouse embryo fibroblasts [[16], [17], [18]]. Studying the long-term toxicity of nano-ZnO, Wang et al. reported that 7 weeks after their gastrointestinal administration, reduction in body weight was seen from weeks 8–11, and reported increased serum glutamic-pyruvic transaminase activity, and elevated zinc concentrations in the serum, liver, and kidney [21]. Some studies provided convincing evidence that zinc ions played important roles in the toxicity of zinc-based nanoparticles [30,31]. George et al. have reported that decreasing the nano-ZnO dissolution rate could slow the release of zinc ions, thereby reducing their toxicity [30,31].
Furthermore, it is estimated that around two billion people in the developing world have zinc deficiency due to high phytate-containing cereal protein intake [32]. On the other hand, in the developed countries there are rising concerns over excessive levels of zinc in the body [33]. The use of zinc-based biomaterials may further increase the risk of excessive zinc. Therefore, investigating the relationship between serum zinc ions level and human health outcomes can provide evidence and standards for the future designs, applications, contraindications, and post-implantation strategies for zinc-containing biomaterials.
It has been suggested that zinc deficiency can increase the risk of chronic diseases, though its toxicity and possible negative effects on chronic diseases have not been studied enough [33]. In the past, some studies have explored the correlation between serum zinc levels and certain types of chronic diseases [2,[34], [35], [36]]. However, most of these studies were based on patients in hospitals, which suggests that their findings might be biased and therefore, cannot be extrapolated to the general population.
Additionally, none of the studies validated the relationship between serum zinc levels and multiple health outcomes in the same subject population, which makes it difficult to compare the results since different studies had different inclusion criteria for participants, different characteristics of the included population, different disease assessment methods, and used different methods for detecting serum levels of zinc. Our research using data from NHANES to evaluate multiple health outcomes is a comprehensive study. Moreover, we have used a variety of models to verify the associations between serum zinc levels and different diseases, which makes our findings solid and reliable.
The benefits of zinc for bone health have been demonstrated by many studies [[37], [38], [39]], and our findings indicate that serum zinc levels were significantly associated with the total spine and total femur BMD. Bone zinc content has been shown to decrease with aging, skeletal unloading, and following menopause, suggesting its role in bone physiology [[37], [38], [39]]. Zinc has been demonstrated to have a stimulatory effect on osteoblastic proliferation, differentiation, and mineralization, which might be related to zinc-induced activation of a variety of osteogenesis-related genes and proteins thereby promoting bone formation [38,40]. It has also been shown to activate the expression of ALP, osteocalcin, Runx2 genes, and proteins, which play a role in the differentiation of osteoblastic cells [41]. Moreover, zinc enhances protein synthesis in osteoblastic cells by activating aminoacyl-tRNA synthetase, a rate-limiting enzyme in the translational machinery and therefore has a potent stimulatory effect on the osteoblastic bone formation. Zinc activates MAPK kinase and has a stimulatory effect on the proliferation of osteoblastic cells in the bone tissues of newborn rats. It also increases the production of IGF-I and TGF-b1, which activate osteoblastic cells and modulate cell proliferation [[42], [43], [44]]. Zinc has also been shown to inhibit osteoclastic bone resorption by inhibiting the formation of osteoclasts from the bone marrow cells and inducing apoptosis of mature osteoclasts. It has a suppressive effect on the receptor activator of nuclear factor kappa-Β ligand (RANKL) and TNFα-induced osteoclastogenesis. Zinc also stimulates OPG gene expression in osteoblastic cells, which can inhibit the binding of RANKL to RANK receptors in pre-osteoclastic cells [41,45].
Our study found that every 10 μg/dL increase in serum zinc levels was associated with a 1.12-fold increase in the incidence of DM. Another prospective study including 2220 Finnish men from the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) has demonstrated that higher serum zinc levels are significantly and independently associated with an increased risk of incident type 2 DM in middle-aged and older men even after adjustment for potential confounders [33]. However, the potential mechanisms underlying this relationship between higher serum zinc levels and DM are not elucidated. Previously published studies have also shown that high levels of zinc can target hormones such as leptin and impact the hormonal homeostasis which in turn could result in insulin resistance. Zinc has been shown to play an important role in the function of β-cells and secretion of insulin [46]. The activity of β-cells is suggested to be increased in order to manage glucose levels in subjects with DM [46]. Excess zinc can cause hyperactivity of β-cells and the resultant increase in insulin production can lead to insulin resistance through exhaustion of insulin receptors. Over-stimulation by long-term elevated zinc levels, therefore, can be harmful to the β-cells [33]. Previous studies have shown lower levels of serum zinc and higher levels of urinary zinc in subjects with DM compared to control subjects [47], suggesting that this could be a mechanism to release excess zinc and avoid toxicity [33].
Our study also found that every 10 μg/dL increase was associated with a 1.23-fold and 1.29-fold increase in CVD and CHD, in participants with serum zinc levels ≥ 100 μg/dL. Some studies have explored the association between serum zinc levels and CVD, and the results are controversial [48]. Most of these studies are based on patient-based case-control studies, and the conclusions are difficult to generalize to the general population. A large prospective study conducted by Milton et al. found a positive association between dietary zinc intake and incidence of CVD in women even after adjusting for potential confounders. Compared to those in the lowest quintile of zinc intake, those in the highest quintile (odds ratio [OR] = 1.67, 95% CI = 1.08–2.62) had almost twice the odds of developing CVD (p = 0.007) [49]. However, the potential mechanisms underlying this relationship are not clarified. Our study findings are preliminary and need to be investigated further. Future research is necessary to confirm this association in other populations as well.
4.1. Implications and conclusion
Our study found that serum zinc ion concentrations are associated with BMD, which strongly supports the use of zinc-based biomaterials for the treatment of bone-related diseases, bone fixation, bone repair materials, and bone loss prevention. While our study found no correlation between serum zinc levels and most of the health outcomes we evaluated, we did find associations with DM and CHD, which indicated that patients at high risk of DM or CHD should be extremely cautious to control serum zinc ions when they were using products that increase serum zinc ions,.
Future in vivo studies are recommended to focus on changes in serum zinc concentrations (total zinc, free Zn ion and protein-bound Zn) following implantation of zinc-based biomaterials. At the same time, an evaluation of the dose-effect relationship, the relationship between different zinc biomaterials, different treatment methods (such as oral and surgical implantation), and different implanted sites (cardiovascular, cortical bone marrow cavity, muscle, subcutaneous) is also required. Further studies focusing on the application of zinc biomaterials in pre-diabetic, diabetic, and CHD animal models is suggested. Glycated hemoglobin testing should be considered in pre-diabetic or diabetic animal models. Investigations into the impact of zinc from zinc-based biomaterials on human health are also needed to evaluate and modify the currently recommended guidelines for the clinical transformation of zinc-based biomaterials.
However, in our study, some potential confounding factors were possibly omitted due to inherent bias of representative cross-sectional study. Although most confounding factors were adjusted, other known and unknown risk factors cannot be excluded as a potential explanation for the observed findings.
In conclusion, our study demonstrates that serum zinc levels are significantly associated with (a) increased BMD in the total spine and total femur and (b) risk of DM, and CVD/CHD among participants with serum zinc levels ≥100 μg/dL. However, there were no significant associations between serum zinc levels and risk of fractures, CHF, HA, stroke, thyroid disease, arthritis, dyslipidemia, and cancer. This study reveals an association between serum zinc concentration and BMD, which indicate that biodegradable zinc-based materials have good prospects for orthopedic applications. In future studies, the application of zinc-based biomaterials in pre-diabetic or diabetic models should be further evaluated. In addition, further exploration of the threshold of zinc release from zinc-based biomaterials in cardiovascular disease models can aid in the application of zinc-based biomaterials for development of cardiovascular stents.
Funding
This work was supported by National Natural Science Foundation of China [grant numbers 51931001, 51631009, 51431002, 51871004], and NSFC/RGC Joint Research Scheme [grant number 51661165014]. Shanghai "Rising Stars of Medical Talent" Youth Development Program (Youth Medical Talents - Specialist Program).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request. Online Supplementary data are available.
Ethics approval
The NHANES 2011-2014 was approved by the NCHS Ethics Review Board, and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
CRediT authorship contribution statement
Xinhua Qu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - original draft. Hongtao Yang: Conceptualization, Investigation, Methodology. Zhifeng Yu: Data curation, Funding acquisition, Investigation, Methodology. Bo Jia: Formal analysis, Investigation. Han Qiao: Writing - original draft. Yufeng Zheng: Conceptualization, Project administration, Writing - original draft. Kerong Dai: Conceptualization, Project administration, Writing - original draft.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Minqi Wang, Jingtian Mei, Zihao He, Yifu Zhuang, for their technical assistance.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2020.03.006.
Contributor Information
Yufeng Zheng, Email: yfzheng@pku.edu.cn.
Kerong Dai, Email: krdai@163.com.
List of abbreviations
- BMA
Bone mineral area
- BMC
Bone mineral content
- BMD
Bone mineral density
- CI
Confidence interval
- CDC
Centers for Disease Control and Prevention
- DBP
Diastolic blood pressure
- DXA
Dual-energy x-ray absorptiometry
- HDL-C
High-density lipoprotein-cholesterol
- LDL-C
Low-density lipoprotein-cholesterol
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- SBP
Systolic blood pressure
- SD
Standard deviation
- TC
Total cholesterol
- TG
Triglycerides
- Nano-ZnO
Zinc oxide nanoparticles
- ZnSO4
Zinc sulfate
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li G., Yang H., Zheng Y., Chen X.H., Yang J.A., Zhu D., Ruan L., Takashima K. Challenges in the use of zinc and its alloys as biodegradable metals: perspective from biomechanical compatibility. Acta Biomater. 2019;97:23–45. doi: 10.1016/j.actbio.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Hess S.Y., Peerson J.M., King J.C., Brown K.H. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr. Bull. 2007;28(3 Suppl):S403–S429. doi: 10.1177/15648265070283S303. [DOI] [PubMed] [Google Scholar]
- 3.Su Y., Yang H., Gao J., Qin Y.X., Zheng Y., Zhu D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. (Weinh) 2019;6(14):1900112. doi: 10.1002/advs.201900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H., Qu X., Lin W., Wang C., Zhu D., Dai K., Zheng Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018;71:200–214. doi: 10.1016/j.actbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Su Y., Cockerill I., Wang Y., Qin Y.X., Chang L., Zheng Y., Zhu D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019;37(4):428–441. doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katarivas Levy G., Goldman J., Aghion E. The prospects of zinc as a structural material for biodegradable implants—a review paper. Metals. 2017;7(10):402. [Google Scholar]
- 7.Prakasam M., Locs J., Salma-Ancane K., Loca D., Largeteau A., Berzina-Cimdina L. Biodegradable materials and metallic implants-A review. J. Funct. Biomater. 2017;8(4) doi: 10.3390/jfb8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Gu X., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. [Google Scholar]
- 9.Krol A., Pomastowski P., Rafinska K., Railean-Plugaru V., Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017;249:37–52. doi: 10.1016/j.cis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Nescakova Z., Zheng K., Liverani L., Nawaz Q., Galuskova D., Kankova H., Michalek M., Galusek D., Boccaccini A.R. Multifunctional zinc ion doped sol - gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019;4:312–321. doi: 10.1016/j.bioactmat.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver J.N., Su Y., Lu X., Kuo P.H., Du J., Zhu D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019;4:261–270. doi: 10.1016/j.bioactmat.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H., Jia B., Zhang Z., Qu X., Li G., Lin W., Zhu D., Dai K., Zheng Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020;11(1):401. doi: 10.1038/s41467-019-14153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S., Kent D., Doan N., Dargusch M., Wang G. Effects of deformation twinning on the mechanical properties of biodegradable Zn-Mg alloys. Bioact. Mater. 2019;4(1):8–16. doi: 10.1016/j.bioactmat.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Yan J., Zhou W., Xiong P., Wang P., Yuan W., Zheng Y., Cheng Y. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (Mg/Zn/Fe) for the application of tracheobronchial stenosis. Bioact. Mater. 2019;4:114–119. doi: 10.1016/j.bioactmat.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Y., Liu L., Zhang D., Dong C., Yan Y., Volinsky A.A., Wang L.N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019;4(1):87–96. doi: 10.1016/j.bioactmat.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J.S., Park M.K., Kim M.S., Lim J.H., Park G.J., Maeng E.H., Shin J.H., Kim Y.R., Kim M.K., Lee J.K., Park J.A., Kim J.C., Shin H.C. Effect of zinc oxide nanoparticles on dams and embryo-fetal development in rats. Int. J. Nanomed. 2014;9(Suppl 2):145–157. doi: 10.2147/IJN.S57931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Feng W., Wang M., Wang T., Gu Y., Zhu M., Ouyang H., Shi J., Zhang F., Zhao Y. Acute toxicological impact of nano-and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanoparticle Res. 2008;10(2):263–276. [Google Scholar]
- 18.Yan G., Huang Y., Bu Q., Lv L., Deng P., Zhou J., Wang Y., Yang Y., Liu Q., Cen X., Zhao Y. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J. Environ. Sci. Health - Part A Toxic Hazard. Subst. Environ. Eng. 2012;47(4):577–588. doi: 10.1080/10934529.2012.650576. [DOI] [PubMed] [Google Scholar]
- 19.International Zinc Nutrition Consultative G., Brown K.H., Rivera J.A., Bhutta Z., Gibson R.S., King J.C., Lonnerdal B., Ruel M.T., Sandtrom B., Wasantwisut E., Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004;25(1 Suppl 2):S99–S203. [PubMed] [Google Scholar]
- 20.Jackson M.J., Jones D.A., Edwards R.H. Tissue zinc levels as an index of body zinc status. Clin. Physiol. 1982;2(4):333–343. doi: 10.1111/j.1475-097x.1982.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Yang J., Wang L., Ran B., Jia Y., Zhang L., Yang G., Shao H., Jiang X. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano. 2017;11(6):5737–5745. doi: 10.1021/acsnano.7b01240. [DOI] [PubMed] [Google Scholar]
- 22.Seitz J.M., Durisin M., Goldman J., Drelich J.W. Recent advances in biodegradable metals for medical sutures: a critical review. Adv. Healthc. Mater. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Woo J.G., Zhang N. Association between urinary manganese and blood pressure: results from national health and nutrition examination survey (NHANES), 2011-2014. PloS One. 2017;12(11) doi: 10.1371/journal.pone.0188145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostchega Y., Zhang G., Kit B.K., Nwankwo T. Factors associated with home blood pressure monitoring among US adults: national health and nutrition examination survey, 2011-2014. Am. J. Hypertens. 2017;30(11):1126–1132. doi: 10.1093/ajh/hpx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu E.W., Chastain H.M., Shin S.H., Wiegand R.E., Kruszon-Moran D., Handali S., Jones J.L. Seroprevalence of antibodies to toxocara species in the United States and associated risk factors, 2011-2014. Clin. Infect. Dis. 2018;66(2):206–212. doi: 10.1093/cid/cix784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelman S.V., Polonsky W.H. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425–1432. doi: 10.2337/dc16-1974. [DOI] [PubMed] [Google Scholar]
- 27.Konduru N.V., Murdaugh K.M., Swami A., Jimenez R.J., Donaghey T.C., Demokritou P., Brain J.D., Molina R.M. Surface modification of zinc oxide nanoparticles with amorphous silica alters their fate in the circulation. Nanotoxicology. 2016;10(6):720–727. doi: 10.3109/17435390.2015.1113322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kura A.U., Saifullah B., Cheah P.S., Hussein M.Z., Azmi N., Fakurazi S. Acute oral toxicity and biodistribution study of zinc-aluminium-levodopa nanocomposite. Nanoscale Res. Lett. 2015;10:105. doi: 10.1186/s11671-015-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konduru N.V., Murdaugh K.M., Sotiriou G.A., Donaghey T.C., Demokritou P., Brain J.D., Molina R.M. Bioavailability, distribution and clearance of tracheally-instilled and gavaged uncoated or silica-coated zinc oxide nanoparticles. Part. Fibre Toxicol. 2014;11:44. doi: 10.1186/s12989-014-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George S., Pokhrel S., Xia T., Gilbert B., Ji Z., Schowalter M., Rosenauer A., Damoiseaux R., Bradley K.A., Madler L., Nel A.E. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano. 2010;4(1):15–29. doi: 10.1021/nn901503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao Y.Y., Chen Y.C., Cheng T.J., Chiung Y.M., Liu P.S. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 2012;125(2):462–472. doi: 10.1093/toxsci/kfr319. [DOI] [PubMed] [Google Scholar]
- 32.Prasad A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009;28(3):257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- 33.Yary T., Virtanen J.K., Ruusunen A., Tuomainen T.P., Voutilainen S. Serum zinc and risk of type 2 diabetes incidence in men: the Kuopio ischaemic heart disease risk factor study. J. Trace Elem. Med. Biol. : Organ Soc. Miner. Trace Elem. 2016;33:120–124. doi: 10.1016/j.jtemb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Sun H., Liu X., Ge H., Wang T., Wang Y., Li W. Association between serum zinc levels and the risk of Parkinson's disease: a meta-analysis. Biol. Trace Elem. Res. 2017;179(1):45–51. doi: 10.1007/s12011-017-0941-2. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y., Shen X., Zhang D. The relationship between serum zinc level and preeclampsia: a meta-analysis. Nutrients. 2015;7(9):7806–7820. doi: 10.3390/nu7095366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J., Mao X., Ling J., He Q., Quan J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: a meta-analysis. Biol. Trace Elem. Res. 2014;160(1):15–23. doi: 10.1007/s12011-014-0031-7. [DOI] [PubMed] [Google Scholar]
- 37.Fukada T., Hojyo S., Furuichi T. Zinc signal: a new player in osteobiology. J. Bone Miner. Metabol. 2013;31(2):129–135. doi: 10.1007/s00774-012-0409-6. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010;338(1–2):241–254. doi: 10.1007/s11010-009-0358-0. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi M. Nutritional factors and bone homeostasis: synergistic effect with zinc and genistein in osteogenesis. Mol. Cell. Biochem. 2012;366(1–2):201–221. doi: 10.1007/s11010-012-1298-7. [DOI] [PubMed] [Google Scholar]
- 40.Hashizume M., Yamaguchi M. Effect of beta-alanyl-L-histidinato zinc on differentiation of osteoblastic MC3T3-E1 cells: increases in alkaline phosphatase activity and protein concentration. Mol. Cell. Biochem. 1994;131(1):19–24. doi: 10.1007/BF01075720. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M., Goto M., Uchiyama S., Nakagawa T. Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol. Cell. Biochem. 2008;312(1–2):157–166. doi: 10.1007/s11010-008-9731-7. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M., Hashizume M. Effect of beta-alanyl-L-histidinato zinc on protein components in osteoblastic MC3T3-El cells: increase in osteocalcin, insulin-like growth factor-I and transforming growth factor-beta. Mol. Cell. Biochem. 1994;136(2):163–169. doi: 10.1007/BF00926077. [DOI] [PubMed] [Google Scholar]
- 43.Matsui T., Yamaguchi M. Zinc modulation of insulin-like growth factor's effect in osteoblastic MC3T3-E1 cells. Peptides. 1995;16(6):1063–1068. doi: 10.1016/0196-9781(95)00067-t. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi M., Fukagawa M. Role of zinc in regulation of protein tyrosine phosphatase activity in osteoblastic MC3T3-E1 cells: zinc modulation of insulin-like growth factor-I's effect. Calcif. Tissue Int. 2005;76(1):32–38. doi: 10.1007/s00223-004-0052-x. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi M., Uchiyama S. Receptor activator of NF-kappaB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int. J. Mol. Med. 2004;14(1):81–85. [PubMed] [Google Scholar]
- 46.Rink L., Gabriel P. Extracellular and immunological actions of zinc. Biometals. 2001;14(3–4):367–383. doi: 10.1023/a:1012986225203. [DOI] [PubMed] [Google Scholar]
- 47.Al-Maroof R.A., Al-Sharbatti S.S. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med. J. 2006;27(3):344–350. [PubMed] [Google Scholar]
- 48.Chu A., Foster M., Samman S. Zinc status and risk of cardiovascular diseases and type 2 diabetes mellitus-A systematic review of prospective cohort studies. Nutrients. 2016;8(11) doi: 10.3390/nu8110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milton A.H., Vashum K.P., McEvoy M., Hussain S., McElduff P., Byles J., Attia J. Prospective study of dietary zinc intake and risk of cardiovascular disease in women. Nutrients. 2018;10(1) doi: 10.3390/nu10010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request. Online Supplementary data are available.