Abstract
Toroviruses are emergent viruses, belonging to the Nidovirales order, that remain mostly ignored, despite they are able to infect different species of domestic animals and humans, causing enteric diseases and diarrhea. Thus far, only five variants of porcine torovirus (PToV) have been identified. In this report we describe the identification and partial characterization of a new strain of porcine torovirus (PToV-BRES) that was detected by RT-PCR in a swine faecal specimen from a farm in Brescia (Italy). The complete genes coding for the nucleocapsid (N), hemagglutinin-esterase (HE) and membrane (M) proteins were amplified, and sequence analysis showed that PToV-BRES is a new PToV strain that, based on the HE gene sequence, is phylogenetically related to P4 strain, that was up to now the only member of a distinct PToV lineage. The nucleocapsid protein from PToV-BRES was expressed in insect cells as a his-tagged protein, purified by affinity chromatography and used to develop an ELISA method to detect antibodies against PToV. This assay was evaluated using a serum collection including 45 samples from three commercial farms from Spain. High antibody prevalence against PToV was observed in the three farms, both in adult animals and in piglets, which could suggest that PToV might be endemic in Spanish porcine population. The ELISA method developed in this work could be useful in future epidemiological surveys about toroviruses.
Keywords: Porcine torovirus, Phylogenetic analysis, Diagnosis, RT-PCR, ELISA
1. Introduction
Toroviruses have been described as potential gastroenteritis causing agents in horses, cows, pigs and humans. They were classified as a new genus within the family Coronaviridae, from the order Nidovirales (Cavanagh et al., 1994), although the possibility of establishing two subfamilies, Coronavirinae and Torovirinae within the family Coronaviridae has been proposed and is currently under consideration (Gonzalez et al., 2003, Coronaviridae Study Group, 2008). Torovirus genome consists of a single RNA molecule of about 25–30 kb. Genome organization is similar to that of coronavirus: the 5′ two-thirds contain two large and overlapping open reading frames, ORF1a and ORF1b, that code for the replication machinery. The last third of the genome contains four open reading frames, ORFs 2–5, coding respectively for the structural proteins: spike (S), membrane (M), hemagglutinin-esterase (HE) and nucleocapsid (N). Torovirus particles have a characteristic torus-shape nucleocapsid formed by the N protein interacting with the viral RNA. The nucleocapsid is surrounded by an envelope that contains the triple spanning M protein, and the S and HE proteins that conform the large and short spikes, respectively.
The first torovirus was identified in 1972 after examining a diarrheic faecal sample from a horse in Berne (Switzerland), and thus it was named Berne Virus or BEV (Weiss et al., 1983). A morphologically related virus was later found in a cattle farm from Breda (Iowa), and this bovine torovirus (BToV) was designated BRV (Woode et al., 1982). Over the years, there have been a few reports describing the presence of toroviral particles in faecal samples from humans (HToV) (Beards et al., 1986, Duckmanton et al., 1997, Koopmans et al., 1997, Krishnan and Naik, 1997, Jamieson et al., 1998, Uziel et al., 1999, Lodha et al., 2005) and pigs (PToV) (Scott et al., 1987, Woode, 1987, Durham et al., 1989, Penrith and Gerdes, 1992, Lavazza et al., 1996). PToV has later been detected by RT-PCR in swine faecal specimens from farms in The Netherlands, Belgium, Hungary and Italy (Kroneman et al., 1998, Matiz et al., 2002). Moreover, a partial genomic characterization of five European PToV isolates has been reported (Smits et al., 2003).
Epidemiological surveys about toroviruses have been largely concentrated on BToV, and they showed that this virus is distributed worldwide, having been detected in United States, Japan, South Korea, India, and in different European countries like United Kingdom, Germany, Belgium, France, Switzerland, and Italy (Liebler et al., 1992, Koopmans et al., 1991, Ito et al., 2007, Park et al., 2007, Van Kruiningen et al., 1992, Krishnan and Naik, 1997, Koopmans et al., 1989, Weiss et al., 1984, Lavazza, 1989). Moreover, high seroprevalences against BToV have been reported in cattle from United Kingdom (Brown et al., 1987), The Netherlands and Germany (Koopmans et al., 1989). Although there are few reports about PToV epidemiology, high seroprevalences, similar to those of BToV, have also been reported in swine populations from Switzerland (Weiss et al., 1984) and The Netherlands (Kroneman et al., 1998). Despite this extensive geographical distribution and the broad host range, these viruses have attracted little attention. This is likely due in part to the fact that torovirus infection has not been associated with disease causing important losses in livestock, but also to the lack of an “in vitro” system to work with most of these viruses, that has precluded the development of specific tools for their diagnosis. For a long time only the equine isolate BEV could be grown in cell cultures (Weiss et al., 1983), and, it has only been very recently reported the ability of a BToV variant isolated in Japan to grow in cells derived from a human rectal adenocarcinoma (Kuwabara et al., 2007). In addition, BToV can be propagated in experimentally infected gnobiotic calves (Woode et al., 1982). Thus, there have been a few reports where indirect ELISA using partially purified BToV or BEV particles were used for torovirus serodiagnosis, but purification procedures are not affordable by all laboratories and, in addition, this assay would also provide low sensitivity for detection of antibodies to human and porcine toroviruses (Brown et al., 1987). Torovirus serodiagnosis has also been performed by analyzing the ability of clinical serum samples to neutralize BEV infectivity (Weiss et al., 1984). The virus neutralization test is “a priori” a very sensitive assay that provides also information about the existence of a protective humoral immune response in the animal under study, however, since BEV, a non-homologous virus, would be used to search for antibodies to torovirus in cattle, human or swine sera, the sensitivity of the assay would be reduced. In addition seroneutralization test is a time-consuming and difficult assay that cannot be used to perform extensive studies.
Thus, alternative strategies have to be undertaken to generate torovirus specific antigens for immune detection. A frequently used alternative is the expression of immunologicaly relevant viral proteins in recombinant expression systems (Chirnside et al., 1995, Denac et al., 1997, Pelosi et al., 1999, Farkas et al., 2005, Bogdanova et al., 2007, Hou et al., 2007). For this purpose the nucleocapsid proteins from many viruses have proven to be very useful. Specifically, the N proteins of different coronaviruses including SARS have been expressed either in bacteria or through the baculovirus expression system and have been used for serodiagnosis (Timani et al., 2004, Woo et al., 2004, Wang et al., 2005). Moreover, the N protein is the most abundant protein in torovirus particles (Horzinek et al., 1985), and strong responses against it have been reported from experimental infections of gnobiotic calves with BToV (Duckmanton et al., 1998). Therefore, N protein from PToV appears as an interesting viral antigen to develop diagnostic methods. In addition, since the nucleocapsid proteins are usually highly conserved, serodiagnosis tests based on the use of PToV N protein may prove useful for detection of antibodies against other toroviruses.
The aim of this work was to characterize a new PToV strain that was detected in a faecal sample from Italy, which was previously considered as torovirus positive by electron microscopy diagnosis, and to develop an ELISA diagnostic system to detect antibodies to torovirus. The ELISA was evaluated by analyzing pig serum samples from three different Spanish farms. Our results, although obtained with a reduced number of animals, indicate a high PToV seroprevalence suggesting that PToV might be endemic in Spanish farms.
2. Materials and methods
2.1. Cells and viruses
Equine dermal (E. Derm) (ATCC® CCL-57™) cells were kindly provided by R.J. de Groot (Utrecht University. Utrecht. The Netherlands). They were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% foetal calf serum (FCS), 100 units/ml of penicillin, and 100 mg/ml of streptomycin. High Five™ (HighFive) insect cells were cultured at 27 °C in TC100 medium supplemented with antibiotics and 10% FCS. The equine torovirus strain Berne (P138/72) (BEV) was propagated in E. Derm cells as described previously (Weiss and Horzinek, 1986), and BEV particles were purified by sucrose gradient centrifugation. Porcine respiratory and reproductive virus (PRRSV) and transmissible gastroenteritis virus (TGEV), both provided to us by L. Enjuanes (CNB-CSIC, Spain), were propagated in monkey-derived MA104 (ATCC® CRL-2378.1™) cells and in swine testis fibroblasts (ST), respectively. Both viruses were partially purified by centrifugation over a 50% sucrose cushion.
2.2. Faecal samples
Three porcine faecal specimens (513/02, 970/02, 1318/02), taken in Brescia (Italy) in 2002, and one bovine faecal sample (1812/03) taken at the same location in 2003, were described to contain torovirus-like particles when examined by electron microscopy, but, with the exception of 1318/02, all samples were also shown to contain other viral particles that could correspond to coronavirus, enterovirus and circovirus.
2.3. Serum samples
Swine blood samples were collected from three commercial farms located in different regions in the Northern part of Spain. Ten blood samples were collected from sows in a farm located in Navarra, where diarrhea and gastroenteritis symptoms in piglets had been reported previously, but they were not observed at the time of serum collection. Twenty sow blood samples were collected from a farm located in Aragon where most newborn piglets were suffering from diarrhea at the moment of collection. Fifteen blood samples were collected from 6 to 8 week-old healthy piglets from a farm with high sanitary conditions located in Galicia. Blood samples were centrifuged at 1500 × g during 10 min at 4 °C, and the obtained sera were stored at −80 °C until their use.
A commercial porcine serum obtained from healthy pigs was purchased from AbD Serotec and has been named as CPS throughout the paper. A serum specimen from a piglet infected under field conditions with PToV, previously described (antiserum 233/90; Lavazza et al., 1996) was used as PToV positive serum control, and was referred to as αBRES. An anti-BEV hyperimmune polyclonal serum (α-BEV) was generated in our laboratory (Garzon et al., unpublished results). S. Van Gucht (Gent University, Belgium) provided us three serum samples from caesarean-derived, colostrum-deprived (CD/CD) pigs kept under germ free conditions (spf) that were used as negative control, and sera from two other pigs that were reared under these same conditions, but that had subsequently been inoculated with either porcine respiratory coronavirus (PRCV) (αPRCV) or PRRSV (αPRRSV), and that were used as cross-reactivity controls. In addition, 69 serum samples from CD/CD piglets taken at 0–36 days of age, were provided by J. Segales (CReSA. Barcelona, Spain) and were also used as negative controls.
A rabbit hyperimmune serum against the N protein from PToV-BRES was raised by immunizing a White New Zeeland rabbit with chromatography purified N chimerical protein fused to a histidine tag, and expressed in insect cells by a baculovirus recombinant (see below).
2.4. Immunoelectromicroscopy
The faecal samples containing toroviral particles were diluted 1:5 in sterile phosphate buffered saline (PBS), homogenized by vortexing and centrifuged at 3000 × g for 10 min at 4 °C. For toroviral particle aggregation the supernatants were mixed (v:v) with a convalescent serum sample from a torovirus infected pig (αBRES), previously diluted 1:40 in PBS. After a 30 min incubation period at 37 °C, viral particles were adsorbed to collodion-carbon coated copper grids by ultacentrifugation in a Beckman Airfuge, at 21 psi for 15 min as previously described (Lavazza et al., 1996). For detection of bound antibodies, particles adsorbed to the surface of the grids were incubated with protein-A conjugated with 10 nm colloidal gold particles as previously described (Rodriguez et al., 1996). After washing in distilled water, samples were negatively stained with 2% sodium phosphotungstate (PTA) in water for 1 min, allowed to dry, and observed in a JEM1010 electron microscope (Jeol, Japan). Pictures were taken with a Bioscan digital camera (Gatan).
2.5. RNA extraction
The faecal samples containing toroviral particles were diluted 1:1 in PBS, homogenized by vortexing and centrifuged at 3000 × g for 10 min at 4 °C. The supernatant was aliquoted and kept at −80 °C. 200 μl of each supernatant were used for viral RNA extraction using a commercial kit (High Pure RNA isolation kit, Roche Applied Science) following the manufacturer's recommendations. The RNA was recovered in 50 μl of DNase, RNase free water, aliquoted and maintained at −80 °C. All specimens were extracted in a dedicated class 2 laminar flow hood using dedicated pipettes and aerosol resistant tips.
2.6. Reverse transcription
Reverse transcription (RT) reaction was performed using Superscript II reverse transcriptase (Invitrogen, Corp.) following the manufacturer's instructions with minor modifications. Briefly, 8 μl of the RNA sample were mixed with a 1 mM dNTPs mix (Roche Applied Science) and 1 mM random hexamer primer (Roche Applied Science) and incubated for 10 min at 65 °C and 1 min on ice. Reverse transcription reaction was completed by adding 9 μl of reaction mix containing 1x RT buffer, 10 mM DTT, 8 mM MgCl2, 40 U ribonuclease inhibitor (Fermentas) and 200 U of Superscript II reverse transcriptase and incubating for 10 min at 25 °C, followed by 45 min at 45 °C and finally 15 min at 65 °C. To minimize cross-contamination reverse transcription reaction was carried out in an independent room using dedicated pipettes with aerosol resistant pipette tips. The cDNA preparation was aliquoted and conserved at −80 °C until use.
2.7. Primer design
Sequences from EToV, Berne strain (BEV), BToV, strains BRV-1, BRV-2, B145, B150, B155, B6 and PToV, strains Markelo, P4, P10, P9 and P78 were retrieved from Genbank database (http://www.ncbi.nlm.nih.gov) and aligned using MegAlign program (DNAstar, Inc.). Primers ToVM5′ and ToVM3′ were designed based on a conserved region from the M gene (Table 1 ). Besides, primers 593 and 620 previously described (Kroneman et al., 1998) were used to amplify a PToV specific fragment from the N gene.
Table 1.
Oligonucleotide primers: location within torovirus genome and sequences.  .
.
| Primers | Primer sequencea | Polarity | Location |
|---|---|---|---|
| ToVM 5′ | AGTATGACCT TTACTGGCTA | Forward | ORF-M |
| ToVM 3′ | TAATCTGCAA CACCTTG | Reverse | ORF-M |
| 593 | GTCAGAATAGATCACGCATT | Forward | ORF-N |
| 620 | AACTCTGCAACTCAGGTGGA | Reverse | ORF-N |
| PToVN 5′ | GGATCCATGAATTCTATGCTTA | Forward | ORF-N |
| PToVN 3′ | TCTAGATTAATTCAAAGCCACTT | Reverse | ORF-N |
| PToVM 5′ | GGATCCTGTTTGATACA AA | Forward | ORF-M |
| PToVM 3′ | TCTAGA CTACTCAAACTTTACACTTG | Reverse | ORF-M |
| PToV-BRES2-HE 5′ | GGATCCATGTTGAGGATGAT | Forward | ORF-HE |
| PToVHE 3′ | CGTCTAGA GCCTAATAACTACTTAAACA | Reverse | ORF-HE |
Recognition sequences for restriction enzymes are underlined.
To amplify the full coding sequences for the structural proteins M, N and HE from PToV, sets of primers were designed using PToV conserved sequences at both 5′ and 3′ regions from each gene (Table 1). Tails containing recognition sites for BamHI and XbaI restriction enzymes (underlined in the table) were added to each forward and reverse primer, respectively. As described below, to amplify the entire HE gene a new primer, PToV-BRES-HE5′, had to be designed.
2.8. PCR
Diagnostic PCR reactions were carried out using 2 μl of cDNA and a reaction mix containing 1× PCR reaction buffer, 0.2 mM of each dNTP, 2.5 mM MgCl2, 0.2 μM of each primer, ToV-M5′ and ToV-M3′ or 593 and 620, and 1.25 U of Taq Platinum (Invitrogen, Corp.) in a final volume of 25 μl. The mixture was subjected to an initial denaturation step of 2 min at 92 °C, followed by 30 cycles of 45 s at 92 °C, 40 s at 45 °C and 1 min at 62 °C. A final extension step of 5 min at 62 °C was added at the end.
To amplify sequences of M and N genes using the primer pairs PToV-M5′-M3′ and PToV-N5′-N3′, respectively, we used the same thermal cycling as described above.
In the case of the HE gene we could not obtain amplification from the cDNA using the designed primer pair, PToV-HE5′-HE3′. However, using primers ToV-M5′ and PToV-HE3′ a 1700 bp fragment of PToV genome comprising the 3′-end of M gene and the complete HE gene was obtained. This fragment was amplified using a high fidelity polymerase (TripleMaster polymerase mix from Eppendorf), and was sequenced to determine the 5′ end region of HE gene from PToV-BRES, and thus, the primer PToV-BRES-HE5′ was designed accordingly. To amplify just the complete HE gene, a second amplification reaction was performed using PToV-BRES-HE5′ and PToV-HE3′ primers and cDNA as template. Briefly, 2 μl of PToV-BRES cDNA were added to a reaction mix containing 1× high fidelity buffer, 200 μM of each dNTP, 200 nM of each primer and 0.71 U/μl of TripleMaster polymerase mix. Thermal cycling conditions for both reactions were as follows: 1 cycle of 94 °C; 30 cycles of denaturation at 94 °C for 20 s, annealing at 50 °C for 20 s and elongation at 72 °C for 2 min.
All PCR reactions were performed in physically separated rooms and dedicated pipettes and filter tips were used. Several negative controls of distilled water for each step of the procedure, RNA isolation, reverse transcription and PCR were included in each assay.
PCR reaction products were visualized in 0.8–1% agarose gels and extracted from agarose using the commercial Gel extraction kit (Qiagen) following instructions provided by the manufacturer.
2.9. Sequence analysis
For sequencing analysis, all RT-PCR products corresponding to the N, M and HE genes were cloned in the commercial vector pGem-T®-Easy (Promega) and the resulting plasmids, pGT-PToV-M, pGT-PToV-N and pGT-PToV-HE, respectively, were used to transform E. coli DH5α competent bacteria. At least 5 independent clones were used for sequencing in the Sequencing Facility of the National Centre of Biotechnology (Madrid, Spain). Obtained sequences were analyzed using SeqMan program (DNAstar, Inc.) and aligned using ClustalX method with torovirus sequences available from NCBI database using MegAlign software (DNAstar, Inc.).
The RT-PCR amplified products and the corresponding sequences from GenBank were compared using the program MegAling (DNAstar, Inc.) employing ClustalW method. This program was used to carry out phylogenetic analysis from gene sequences. Phylogenetic analyses were performed using the neighbour-joining method, and statistical parameters of phylogenetic trees were determined by bootstrap analysis using 1000 replicates. The nucleotide sequences described in this paper have been submitted to the DDBJ nucleotide sequences database and are retrievable from GenBank. The accession numbers for the complete N, M, and HE genes of PToV-BRES are FJ232068, FJ232069, and FJ232070, respectively.
2.10. Generation of a baculovirus recombinant expressing PToV-N protein
A baculovirus recombinant that contains the nucleotide coding sequence for PToV N protein fused at its 5′-end to a sequence encoding a 6-histidine tag was generated by using the Bac-to-Bac system (Invitrogen, Corp.). For this, the pGT-PToV-N vector clone containing the consensus sequence for the N gene from PToV-BRES isolate was used to subclone this gene into the baculovirus transfer vector pFastBac™b (Invitrogen, Corp.). The N gene was extracted from pGT-PToV-N by enzymatic restriction with BamHI and XbaI and cloned into pFastBac™b, previously digested with the same restriction enzymes, giving rise to pFB-PToV-N vector. Competent E. coli DH10BAC cells, containing a bacmid (baculovirus shuttle vector plasmid) and a helper plasmid, were used to generate recombinant bacmids according to the manufacturer's instructions. The obtained recombinant bacmid, BAC-PToV-N, was transfected into monolayers of HighFive insect cells using Lipofectin (Invitrogen, Corp.). Culture medium containing recombinant baculovirus rBac-PToV-N was collected at 72 h post-transfection, clarified by centrifugation and stored at 4 °C as virus stock. Transfected cells were collected and recombinant PToV-N protein expression was analyzed by Western blot using a commercial polyclonal antibody against the histidine tag (αHis) and the αBRES serum.
2.11. Expression and purification of PToV-N protein
The recombinant N protein fused to a histidine tag at its N terminus was purified using BD TalonTM IMAC resin (BD Biosciences Clontech). HighFive cells were infected with rBac-PToV-N at a multiplicity of infection (MOI) of 5 plaque forming units per cell (pfu/cell) and maintained for 48 h at 28 °C. Infected cells were harvested, pelleted by centrifugation at 1000 × g 10 min and resuspended in lysis buffer (50 mM H2NaPO4, pH 8.0, 300 mM NaCl, 0.1% NP-40) containing protease inhibitors (Complete Mini tablets from Roche Applied Science). Cell suspensions were then incubated on ice for 30 min and sonicated. The insoluble fraction, which contains the N protein, was recovered by centrifugation at 3000 × g 5 min and solubilized with denaturing buffer containing 50 mM H2NaPO4, pH 8.0, 300 mM NaCl, and 6 M guanidine. After a second centrifugation at 3000 × g for 5 min, the supernatant was taken and mixed with the TalonTM resin equilibrated with denaturing buffer, and incubated for 1 h at 4 °C in a rotating shaker. Unbound proteins were removed by centrifugation at 500 × g for 5 min and resin was washed with 10 volumes of denaturing buffer three times, and two times with a buffer containing 50 mM H2NaPO4, pH 8.0, 300 mM NaCl, 8 M urea. The N protein was eluted from the resin with successive elution steps with equal resin volume of elution buffer (50 mM H2NaPO4, pH 8.0, 300 mM NaCl, 8 M urea and 1 M imidazol). Concentration and purity of PToV-N protein was analyzed by SDS-PAGE and Western blot. Protein concentration was determined according to BCA assay (Pierce) using a 1:5 dilution of the eluted protein in distilled water and bovine serum albumin (Sigma) as standard prepared similarly.
2.12. Serological methods
2.12.1. Western blot assay
Aliquots of the purified recombinant N protein were electrophoresed on SDS-PAGE gels (400 ng/lane). The protein was transferred to a nitrocellulose membrane that was cut into strips corresponding to each gel track. Western blots were carried out with porcine serum samples diluted 1:100. First, non-specific binding of antibodies to the nitrocellulose membrane was blocked by overnight incubation at 4 °C in PBS containing 0.05% Tween 20 (PBST) and 5% skim milk. The membranes were then incubated with porcine serum samples diluted in PBST with 5% skim milk. After extensive washings, the membranes were incubated with secondary goat anti-porcine IgG antibodies coupled to horseradish peroxidase. Western blots were developed by incubating the nitrocellulose membranes with a solution containing 0.5 mg/ml 4-chloro-1-naphtol (Sigma) in PBS and 0.015% H2O2, during 20 min at room temperature, and then overnight at 4 °C, in distilled water.
2.12.2. Serum neutralization assay
The presence of neutralizing antibodies against torovirus in swine sera was analyzed by plaque reduction test using BEV virus. This method, that is a conventional procedure to determine neutralizing antibody titers for many viruses, has not been previously used for BEV. The reason being that BEV virus titers have always been determined by establishing the 50% tissue culture infectious dose (TCID50) and neutralization assays were performed accordingly. Instead, we titrated BEV stocks by determining the number of pfu/ml (Garzon et al, unpublished results). Thus, BEV neutralization assay was performed as follows: 25 μl of heat inactivated diluted serum (two-fold serial dilutions in DMEM, from 1:4 to 1:4096) were incubated with an equal volume of a viral suspension containing 100 pfu of BEV for 1 h at 37 °C. Virus-serum mixtures were added to confluent monolayers of E. Derm cells grown in 12-well plates. After 1 h of virus adsorption at 37 °C, the infection medium was removed and 2 ml per well of 2× DMEM medium mixed (V/V) with 1.9% agar and containing 0.05 mg/ml of DEAE-dextrane and 2% FCS was added, and the cells were maintained at 37 °C for 3 days. After removing the agar overlay, cells were stained with crystal violet and lysis plaques were counted. The virus-neutralizing titer (ND50) of each serum sample corresponds to the reciprocal of the highest serum dilution giving 50% reduction in the number of plaques with respect to that obtained with the virus without serum. Serum samples were tested by duplicate and negative (spf) and positive (CPS) control sera were included in each test. Neutralization titers were calculated using Spearman-Kärber formula. Serum samples with neutralizing antibody titers equal to or higher than 4 (or 0.6 that is the equivalent value in logarithmic scale) were considered as positive.
2.12.3. Enzyme-linked immunosorbent assay (ELISA)
An indirect ELISA was established in our laboratory to test for the presence of antibodies against porcine torovirus in pig serum samples using PToV-N protein as antigen. Optimal concentration of reagents was established by checkerboard titration. Paired rows of a 96-well microtiter plate (Maxisorp, Nunc) were coated with 400 ng/well of purified N protein diluted in 50 mM carbonate-bicarbonate buffer (pH 9.6) by overnight incubation at 4 °C. After coating and at each washing step, plates were rinsed 3 times with 150 μl/well of PBST. Subsequently, plates were blocked for 2 h at 37 °C with 150 μl/well of PBST-3% BSA. Then 50 μl of each serum sample diluted 1:100 in PBST-1% BSA was added in paired wells and the plates were incubated 1 h at 37 °C. Next, commercial goat anti-pig IgG antibodies conjugated to horseradish peroxidase (Sigma) diluted in PBST-1% BSA was added and the plates were incubated 1 h at 37 °C. The enzymatic reaction was developed using o-phenylenediamine dihydrochloride (OPD FAST™, Sigma). After 10 min at room temperature the reaction was stopped by adding 50 μl/well of 2N sulphuric acid, and absorbance at 492 nm was measured with a multichannel spectrophotometer (Titertek Multiscan MCC/340).
3. Results
3.1. Porcine torovirus identification
Torovirus-like particles were observed by electron microscopy analysis in three porcine (513/02, 970/02, 1318/02) and one bovine (1812/03) faecal samples, in the course of a diagnostic examination of animals suffering diarrhea in Brescia, Italy. In some of these samples (513/02, 970/02 and 1812/03), other viral particles were also observed that could correspond to coronavirus, circovirus and enterovirus (data not shown). Moreover, in two of the porcine samples (513/02 and 970/02), the torovirus-like particles were not well preserved and were difficult to detect. To enhance the efficacy and specificity of the electron microscopy method for toroviral particle detection, faecal suspensions were incubated with convaslescent serum from a torovirus infected pig (α-BRES) facilitating particle aggregation, as previously described (Lavazza et al., 1996). Samples were then ultracentrifuged onto a grid, and adsorbed viral particles were incubated with protein A-gold for antibody detection, and negatively stained with 2% PTA. Fig. 1 shows immunogold-labeled toroviral particles in one of the porcine faecal samples (1318/02), which will later be identified as PToV-BRES. The results obtained with the other three samples were not conclusive.
Fig. 1.
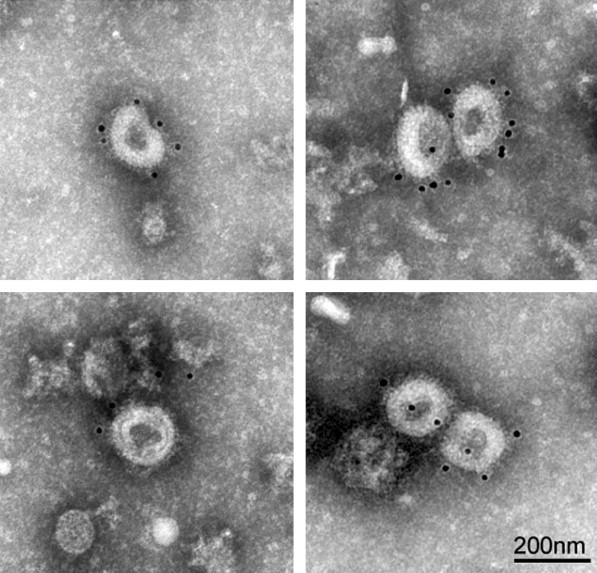
Immunolabeled PToV particles visualized by negative staining. Toroviral particles present in swine faecal specimens were agglutinated by incubating the clarified faecal suspensions with convalescent serum from an infected pig (αBRES). The aggregated particles were loaded on a grid by ultracentrifugation in a Beckman Airfuge, and treated with 10 nm gold-conjugated protein A, and negative stained with 2% PTA. The electron micrographs correspond the 1318/02 porcine faecal sample.
To detect toroviral genomic RNA in these samples we designed a new primer set based on a region from the M gene that is conserved in all available torovirus sequences (ToVM5′ and ToVM3′ described in Table 1). Optimal RT-PCR conditions were established using serial dilutions of a titrated BEV stock and using primers ToVM5′ and ToVM3′ we were able to detect up to 103 pfu/ml (data not shown). These primers were then used for RT-PCR detection of PToV and BToV in the faecal samples from three pigs and one calf described above. The expected 410 bp fragment was amplified from one of the porcine specimens (1318/02) (Fig. 2A, lane 1) and also from the bovine stool sample (Fig. 2B, lane 1). Sequence of the PToV fragment showed a 95% similarity with respect to other available PToV sequences and 80% with respect to BToV sequences. Conversely, the fragment amplified from BToV showed a 98% similarity with respect to other BToV isolates, but only an 80% identity with respect to PToV isolates. Both fragments showed a 79–80% similarity with respect to BEV. A second diagnostic PCR was carried out to amplify specifically a fragment from PToV N gene, using PToV specific primers 593 and 620 (Table 1). A 180 bp fragment was obtained only from the 1318/02 sample (Fig. 2A, lane 2), but not from the other two porcine samples (not shown), nor from the bovine one. The amplified fragment was of the expected size and sequence of this second fragment showed a homology of 87–89% with respect to PToV strains, 85% with respect to BToV strains and only 57% with BEV. Negative water controls were analyzed in parallel with each primer set to discard sample cross-contamination (Fig. 2A and B, lanes 3 and 4).
Fig. 2.
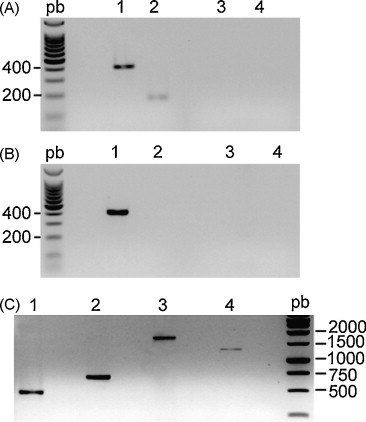
Detection of PToV and BToV in field faecal samples by RT-PCR. Porcine (A) and bovine (B) torovirus genomes were detected by RT-PCR using ToV-M5′-ToV-M3 primers (lanes 1 in panels A and B) from faecal samples 1318/02 and 1812/03 respectively, in which torovirus-like particles had been observed by electron microscopy. Primers 593–620 were used as positive control for specific amplification of PToV (lanes 2 in panels A and B). Distilled water negative controls were processed in parallel in each step (RT, lanes 3, and PCR, lanes 4, in panels A and B). (C) PCR products amplified form PToV-BRES cDNA corresponding to ORF-N (lane 1), ORF-M (lane 2), ORF-M 3′ end plus the full ORF-HE (lane 3), and ORF-HE (lane 4). Ladder DNA (pb) was run in parallel and sizes in bp are indicated.
Thus, a new PToV strain has been identified in this work and it has been named as PToV-BRES throughout this report.
3.1.1. Phylogenetic characterization of PToV-BRES isolate
To establish the phylogenetic relationship of PToV-BRES with other PToV strains, we amplified the sequences corresponding to the genes coding for M, HE and N proteins from PToV-BRES cDNA. The amplicons were analyzed by agarose gel electrophoresis, purified from the gel, and cloned into pGemT®-Easy vector and at least 5 independent clones were sequenced in both directions for each gene.
The N gene of PToV-BRES was amplified as 505 bp fragment (Fig. 2C, lane 1) that showed 91.7–93.1% homology with respect to other PToV strains, 91.3–92.1% with respect to BToV and 70% with BEV (Fig. 3A). The 700 bp DNA fragment corresponding to M gene (Fig. 2C, lane 2) showed 94.4–95.6% homology with other PToV strains, 80.6–81.1% with respect to BToV strains, and 81.2% with respect to the BEV isolate (Fig. 3C).
Fig. 3.
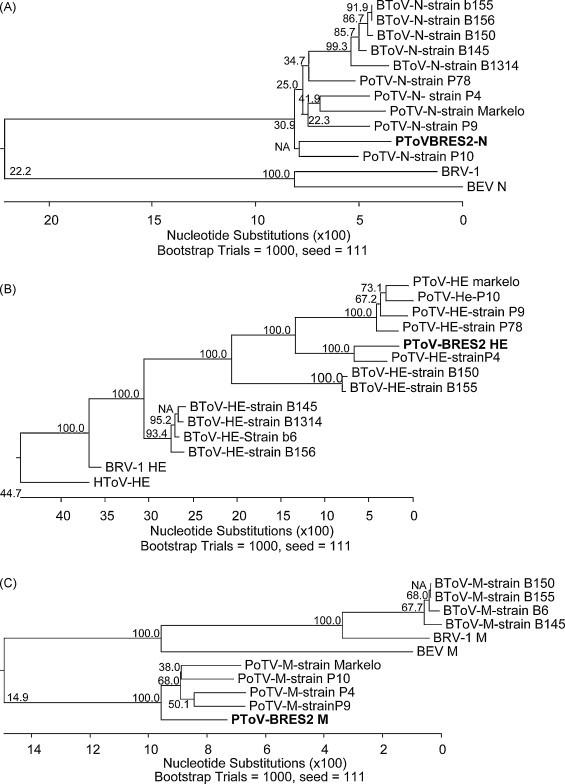
Phylogenetic analysis of ORF N (A), HE (B) and M (C) from PToV-BRES. Sequences from ORF N, HE and M were aligned with torovirus sequences available in GenBank. Comparisons were performed by neighbour-joining method using 1000 bootstrap. The numbers represent the distance to the nearest node and bar indicate the number of nucleotide substitutions (×100).
The HE gene from PToV-BRES was amplified using a two-step strategy. First, we amplified a 1700 bp (Fig. 2C, lane 3). This fragment was sequenced and it showed to contain the 3′ end from M gene, the intergenic region containing the canonical Translation Regulatory Sequence (TRS) and the complete HE gene. A new primer, PToV-BRES-HE5′, was designed to amplify only the complete HE gene without extra sequences (1.2 kb fragment) using the cDNA as template (Fig. 2C, lane 4). Consensus sequence for PToV-BRES HE gene shows 92% homology with the previously described P4 strain of PToV, and 75–78% homology with the other PToV strains. With respect to BToV strains a 67–70% identity was obtained. Only 52% homology was observed with respect to the HToV-HE gene sequence and 63% with respect to the HE gene fragment conserved in BEV (Fig. 3B). These results strongly suggest that PToV-BRES is a new strain of PToV not detected previously.
3.2. Expression and purification of the nucleocapsid protein from PToV-BRES in insect cells
We have generated a recombinant baculovirus that expressed the nucleocapsid protein from PToV-BRES fused at its N-terminus to a histidine tag (rBac-PToV-N). HighFive insect cells infected with rBac-PToV-N showed a prominent 20 kDa protein band when analyzed by SDS-PAGE and Coomassie brilliant blue staining at 48 and 72 hpi (data not shown), and this protein was specifically recognized by αHis (Fig. 4A) and αBRES (Fig. 4B) antibodies in Western blot. The estimated size of this main product was in agreement with the predicted size for the recombinant fusion protein. Other protein bands of lower molecular weight, likely corresponding to degradation products, and a slower migrating protein were also observed by Western blot.
Fig. 4.
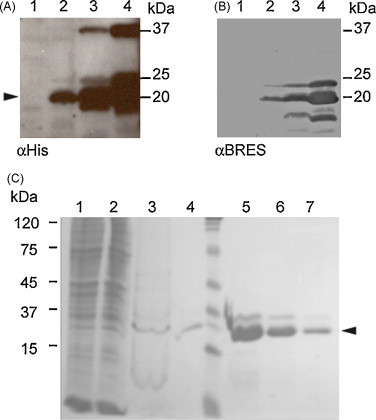
Expression and purification of recombinant N protein from PToV-BRES. A recombinant baculovirus carrying PToV-BRES N gene (rBac-PToV-N) was used to infect HighFive cells. Infected cells were harvested at 24, 48 and 72 hpi (lanes 2, 3 and 4, respectively). Uninfected cells (lane 1) served as negative control. Protein expression was analyzed by SDS-PAGE and Western blot using (A) a commercial αHis antibody and (B) serum from a pig naturally infected with torovirus (αBRES). (C) PToV-N protein was purified from HighFive cells infected with rBac-PToV-N by affinity chromatography. The purification steps were analyzed by SDS-PAGE and Coomassie blue staining. The cell extract (lane 1) was prepared by lysis with NP40 and the insoluble fraction (lane 2) was treated with 6 M guanidine. After centrifugation the soluble fraction was incubated with Talon™ resin 1 h at 4 °C. After 3 washing steps, the bound protein (lane 3) was eluted from the resin by three elution steps (lanes 5, 6 and 7) with 1 M imidazol. A small amount of protein remained bound to the resin after elution (lane 4). Arrowhead indicates the position of the recombinant protein. The sizes of the molecular weight markers in kDa are indicated at the left or right margins.
The recombinant N protein was solubilized by treatment of the infected cells with 6 M guanidine, and was purified by affinity chromatography using a cobalt-coated resin. The protein was recovered by three rounds of elution with imidazole in presence of 8 M urea. Purity of the protein was analyzed by SDS-PAGE and Coomassie brilliant blue staining. Fig. 4C shows the analysis by SDS-PAGE of the different steps of the purification procedure. After elution, only the bands corresponding to the recombinant N protein were observed (Fig. 4C, lanes 5–7), indicating that the protein was highly purified. Protein was stored in 8 M urea to keep it soluble.
To generate an anti-PToV N protein polyclonal serum (RαPToV-N) the purified protein was dialysed against distilled water, freeze-dried and resuspended in PBS before being inoculated in a rabbit. Specificity of antibodies was analyzed by Western blot using extracts from rBac-PToV-N infected and non-infected HighFive insect cells (data not shown).
3.3. Development of an ELISA to detect IgG antibodies against PToV N protein
Purified recombinant N protein was used to develop an ELISA to detect antibodies against PToV in porcine serum samples. Optimal concentrations of reagents was determined by checkerboard titration using the above described polyclonal serum, RαPToV-N, two porcine serum samples, αBRES and CPS, as positive controls, and five serum samples from spf animals that were used as negative controls. The optimal protein concentration that provided higher discrimination between negative and positive sera was 400 ng/well, and the optimal porcine serum dilution was 1:100 (data not shown).
Since there are no reference sera available for porcine torovirus, ELISA cut-off value was established using 5 spf serum samples and 69 serum samples from CD/CD animals that had been collected between 0 and 36 days after birth. All these serum samples were analyzed for IgG antibodies in five individual assays performed on different days. These control serum samples showed a mean ELISA reactivity of 0.175 ± 0.03. Therefore, ELISA cut-off values were established as the mean value obtained from control animals plus three times the standard deviation (O.D. 492 nm = 0.270). None of these control serum samples showed ELISA O.D. values above the cut off.
Lack of cross-reactivity between PToV and antibodies to other related viruses infecting pigs was confirmed by ELISA, Western blot and virus neutralization tests using αPRRSV, αPRCV, and αBRES serum samples, purified PToV N protein or BEV particles as toroviral antigens, and purified PRRSV and TGEV virions as related viruses (data not shown).
3.4. ELISA evaluation
In order to evaluate the potential use of the PToV-N protein as antigen for diagnostic ELISA to detect antibodies against porcine torovirus, 45 serum samples were collected from three Spanish farms located in Galicia, Navarra and Aragon and were analyzed by ELISA, virus neutralization assay and/or Western blot.
All 45 serum samples analyzed by ELISA were positive against torovirus and these results were confirmed by Western blot and/or serum neutralization test in 44 cases (93%). Most serum samples from the farm in Navarra showed moderate ELISA values (between 0.5 and 1.5) and low neutralizing antibodies titers (log ND50 < 1.5) (Fig. 5A). One sample showed positive ELISA value (O.D.492nm = 1.30) and a neutralizing titer close to the cut-off (log ND50 = 0.63) (Fig. 5A, arrow), however, it was positive by Western blot (Fig. 5A, strip 1). From the farm located in Galicia, more dispersed results were obtained by ELISA (O.D.492nm between 0.3 and 2.3), neutralization test (log ND50 between 0.5 and 3.2) and Western blot (Fig. 5B). All 15 serum samples were ELISA positive, and that result was confirmed by Western blot in 9 samples and by neutralization test in 14 samples. The only sample that was positive by ELISA (O.D492nm = 0.325) but negative by the other two tests (Fig. 5B, strip 13 and arrow) had a neutralizing titer that was close to the cut-off value (log ND50 = 0.54). Serum samples from Aragon showed high titers of antibodies against PToV-N (O.D.492nm between 1.0 and 2.3), and accordingly, they were highly neutralizing (log ND50 between 1.5 and >3.2), and for some of them we could not determine the neutralizing titer since the highest serum dilution used, 1:4096, still reduces the number of viral plaques to more than 50%, and they were all positive by Western blot (Fig. 5C).
Fig. 5.
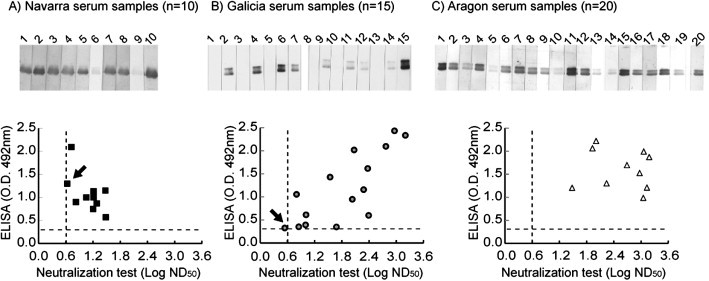
ELISA evaluation. 45 porcine serum samples collected from farms located in (A) Navarra (n = 10), (B) Galicia (n = 15) and (C) Aragon (n = 20), were analyzed by ELISA and Western blot using PToV N protein (400 ng) and sera diluted at 1:100. In addition, sera were analyzed by virus neutralization test at two-fold serial dilutions using BEV. Neutralization titers are expressed as the log ND50. Western blot strips are shown in the upper part of the figure, and ELISA and neutralization values for each serum sample are represented on Y and X axes, respectively. IgG ELISA cut-off (O.D. 492nm = 0.270) (- - -) and virus neutralization cut-off (log ND50 = 0.6) (|) are indicated. The arrows indicate serum samples with controversial ELISA and neutralization values from the farms in Navarra (A) and Galicia (B).
The results obtained by the ELISA test using PToV N protein as antigen are in good agreement with those obtained by neutralization assay, indicating that the ELISA could be used instead for detection of antibodies to torovirus, providing a simpler and more feasible diagnostic system.
4. Discussion
Toroviruses have been described as potential gastroenteritis causing agents that appear to infect many animal species, including humans. Most torovirus epidemiological information comes from studies on BToV performed in different countries, and they indicate that torovirus are broadly dispersed around the world, with high prevalences in animal populations. However, few studies have been reported about PToV, despite they indicate an important prevalence of PToV at least in European countries. The lack of a growing system for PToV has hindered its study and the development of diagnostic tools. This work reports the identification and partial characterization of a new PToV strain and the development of an ELISA assay to detect antibodies against PToV in porcine serum samples.
First, we describe a new primer pair, ToVM5′ and ToVM3′, whose sequences are highly conserved between torovirus species. Using this primer set we were able to detect a new strain, PToV-BRES. In addition, primer set ToVM5′ and ToVM3′ was able to amplify BEV and BToV genomes from cell culture medium and a stool sample, respectively. Moreover, although HToV detection using ToVM5′ and ToVM3′ primer pairs has not been tested yet, these primers might also amplify HToV since they were designed in conserved regions from all torovirus M gene available sequences. Hence, we propose that this new primer set could be used for torovirus epidemiological trials.
At present, little information about PToV genome sequence is available since only five PToV strains have been described (Kroneman et al., 1998, Matiz et al., 2002, Smits et al., 2003). Genomic information about other PToV variants is needed to understand torovirus evolution and to develop new diagnostic tools. In this work we describe the amplification and sequencing of M, HE and N genes from PToV-BRES. Interestingly, phylogenetic analysis of HE gene showed a high sequence homology (92%) with the HE gene from strain P4, but only 73–78% with other PToV strains. Two PToV lineages had previously been described based on HE gene sequences, which are represented by P4 and Markelo strains. It has been suggested that these two PToV lineages were generated by recombination events into the HE gene between a parental PToV and an unknown donor (Smits et al., 2003). PToV-P4 was detected in Italy in 1990, during an enteritis outbreak (Lavazza et al., 1996), and since then no other strains have shown a phylogenetic relationship with it. Therefore, P4 was considered as the likely PToV parental strain, and Markelo-like strains (Markelo, P78, P9 and P10) could then be considered as recombinant progeny originated from it. Our finding of a new PToV strain phylogenetically related to P4 in a sample collected in 2002 suggests that at least these two PToV lineages might be currently circulating in porcine populations. Antigenic differences between HE proteins from both PToV lineages have not been determined but this possibility should be considered. In this regard, antigenic differences between the known BToV lineages have been previously described (Smits et al., 2005).
In this work, we also describe the expression of the N protein of PToV-BRES and its use for the development of an ELISA method to detect antibodies to torovirus in swine serum samples. To our knowledge, no specific serodiagnostic assay for PToV has been developed to date. Antibodies against PToV were previously analyzed by BEV serum neutralization test (Weiss et al., 1984, Liebermann, 1990, Kroneman et al., 1998), however, this method is time consuming and its sensitivity could be compromised by the fact that it is based on cross-reactivity between torovirus species. Besides, two ELISA assays to detect antibodies against BToV and BEV have been previously reported. Both methods were developed using sucrose gradient purified virus, obtained either from faeces from gnobiotic calves infected with BToV (Brown et al., 1987, Van Kruiningen et al., 1992) or from culture medium from BEV infected cells (Weiss et al., 1984). However, these approaches cannot be applied to porcine torovirus, since PToV cannot be propagated in cell cultures yet, and no experimental infection protocol has been reported. Therefore, alternative strategies to obtain specific PToV antigens are needed.
Expression of viral proteins in insect cells to develop diagnostic assays have been reported for other viruses (Pelosi et al., 1999, Sestak et al., 1999, Guo et al., 2001, Deng et al., 2003, Timani et al., 2004, Farkas et al., 2005, Shin et al., 2007). The torovirus nucleocapsid protein is the most abundant protein in the virions, and is highly antigenic as shown by the strong antibody response developed against it in gnobiotic calves experimentally infected with BToV. The N and HE proteins from BToV strain BRV-1 were expressed previously in E. coli (Duckmanton et al., 1998) and in insect cells (Duckmanton et al., 1999), respectively, and both proteins showed immunoreactivity to specific antisera and field serum samples by Western blot. However, these proteins were not used to develop any diagnostic assay. Therefore, we sought to use N protein from PToV as antigen to develop diagnostic methods. Thus, recombinant nucleocapsid protein from PToV-BRES isolate was expressed in insect cells and purified by affinity chromatography. Using this approach, large amounts of highly purified protein were achieved. Recombinant PToV-BRES-N protein was used to develop an ELISA assay to detect antibodies against torovirus in swine serum samples. Moreover, given the high degree of N protein conservation, the developed assay may also be useful to study the seroprevalence to bovine and even human toroviruses.
ELISA optimization was carried out using a serum sample from a pig naturally infected by PToV (αBRES), a commercial serum mixture from healthy pigs (CPS) and serum samples from spf pigs inoculated or not with PRRSV or PRCV. The assay prove to be very reproducible since very little variation was observed in the O.D. values of positive and negative control sera among assays performed in different days. Cross-reactivity of PToV with other related viruses infecting pigs has been discarded in the three assays tested (ELISA, Western blot and virus neutralization) and these results confirm that these viruses are serologically unrelated, and are in agreement with previous results described by other authors (Woode et al., 1982, Woode et al., 1985, Lamouliatte et al., 1987). However, CPS antibodies recognized both PToV-N and BEV-N proteins (not shown), indicating that the animals used to obtain the serum had been infected by, or, exposed to PToV. Similar observations had previously been described with different batches of commercial newborn calf serum that were shown to react with BEV (Vanopdenbosch et al., 1992b).
Due to the lack of reference sera for PToV, ELISA cut-off value was empirically determined using serum samples from spf and CD/CD animals. Overall, ELISA results from adult animals from different Spanish farms located in Aragon (n = 20) and Navarra (n = 10) suggest a high prevalence of antibodies in Spanish pig population, since all 30 adult pigs analyzed from these farms were positive by ELISA, and these results were confirmed by neutralization test and Western blot. On the other hand, the high prevalence of antibodies in serum samples from piglets from the farm from Galicia suggests that PToV infection occurs early in piglets. This prevalence rate is higher than others previously reported for PToV in The Netherlands (81%) using neutralization assay (Kroneman et al., 1998) and for BToV (Brown et al., 1987, Koopmans et al., 1989). However, these PToV prevalence rate data can only be considered as preliminary, since both, our, and the study performed in The Netherlands, were carried out with a short number of animals. Further epidemiological studies are needed to obtain definitive information regarding PToV prevalences in Spain and in other countries such as Italy, where PToV strains seem to normally circulate.
Pathogenic potential of PToV as diarrhea causing agent remains unclear. In this regard, pigs from the farm in Aragon, where an acute gastroenteritis outbreak was reported without an identified causing agent, show high levels of antibodies against torovirus. More studies are needed to understand the potential pathogenesis of PToV.
In conclusion, we have developed an ELISA for torovirus diagnosis that could be very useful for future epidemiological studies, to determine seroprevalence to porcine torovirus, but also to bovine and even human toroviruses. Moreover, the nucleocapsid protein expressed by the baculovirus expression system could also be evaluated in immunization protocols to study its potential application for vaccination purposes against toroviruses. In this regard, protective responses elicited against the nucleocapsid protein have been reported for different coronaviruses (Wasmoen et al., 1995, Seo et al., 1997, Cavanagh, 2003). In addition, we have reported a new PToV strain and three genes coding for structural proteins have been fully sequenced providing information about torovirus evolution. This work contributes to increase our knowledge about this scarcely studied, but highly prevalent group of viruses, provides important tools for future studies, and presents, for the first time, preliminary evidences on the presence of antibodies to torovirus in Spanish livestock.
Acknowledgments
We thank Raquel Blanco and Juan Ramón Rodríguez for critical reading of the manuscript. We also want to thank R.J. de Groot (Utrecht University, Utrecht, The Netherlands) for providing the E. Derm cells and BEV virus, S. Van Gucht (Gent University, Belgium) for providing the serum samples from spf piglets, J. Segales for providing the serum samples from CD/CD animals and L. Enjuanes for supplying TGEV and PRRSV viruses.
This work was supported by grants CICYT-BIO2006-10988 and CONSOLIDER-PORCIVIR CSD2006-00007 to D.R. J.P. was recipient of FPI fellowship from the Spanish Ministry of Education and Science.
References
- Beards G.M., Brown D.W., Green J., Flewett T.H. Preliminary characterisation of torovirus-like particles of humans: comparison with Berne virus of horses and Breda virus of calves. J. Med. Virol. 1986;20(1):67–78. doi: 10.1002/jmv.1890200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova V.S., Tsibezov V.V., Grabovetskii V.V., Eliseeva O.V., Grebennikova T.V., Verkhovskii O.A., Zabereshchnyi A.D., Aliper T.I. Enzyme-linked immunosorbent assay for detection of antibodies to porcine reproductive and respiratory syndrome virus, by using recombinant nucleocapsid protein N. Vopr. Virusol. 2007;52(2):45–49. [PubMed] [Google Scholar]
- Brown D.W., Beards G.M., Flewett T.H. Detection of Breda virus antigen and antibody in humans and animals by enzyme immunoassay. J. Clin. Microbiol. 1987;25(4):637–640. doi: 10.1128/jcm.25.4.637-640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Brian D.A., Briton M., Enjuanes L., Holmes K.V., Lai M.M., Laude H., Plagemann P.G., Siddell S.G., Spaan W.J., Taguchi F., Talbot P. Revision of the taxonomy of the Coronavirus, Torovirus and Arterivirus genera. Arch. Virol. 1994;135(1–2):227–237. doi: 10.1007/BF01309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirnside E.D., Francis P.M., de Vries A.A., Sinclair R., Mumford J.A. Development and evaluation of an ELISA using recombinant fusion protein to detect the presence of host antibody to equine arteritis virus. J. Virol. Methods. 1995;54(1):1–13. doi: 10.1016/0166-0934(95)00020-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group. XIth International Symposium on Nidoviruses. Oxford. U.K. June 22-27, 2008.
- Denac H., Moser C., Tratschin J.D., Hofmann M.A. An indirect ELISA for the detection of antibodies against porcine reproductive and respiratory syndrome virus using recombinant nucleocapsid protein as antigen. J. Virol. Methods. 1997;65(2):169–181. doi: 10.1016/s0166-0934(97)02186-1. [DOI] [PubMed] [Google Scholar]
- Deng Y., Batten C.A., Liu B.L., Lambden P.R., Elschner M., Gunther H., Otto P., Schnurch P., Eichhorn W., Herbst W., Clarke I.N. Studies of epidemiology and seroprevalence of bovine noroviruses in Germany. J. Clin. Microbiol. 2003;41(6):2300–2305. doi: 10.1128/JCM.41.6.2300-2305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckmanton L., Luan B., Devenish J., Tellier R., Petric M. Characterization of torovirus from human fecal specimens. Virology. 1997;239(1):158–168. doi: 10.1006/viro.1997.8879. [DOI] [PubMed] [Google Scholar]
- Duckmanton L., Tellier R., Richardson C., Petric M. The novel hemagglutinin-esterase genes of human torovirus and Breda virus. Virus Res. 1999;64(2):137–149. doi: 10.1016/S0168-1702(99)00088-X. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duckmanton L.M., Tellier R., Liu P., Petric M. Bovine torovirus: sequencing of the structural genes and expression of the nucleocapsid protein of Breda virus. Virus Res. 1998;58(1–2):83–96. doi: 10.1016/S0168-1702(98)00104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham P.J., Hassard L.E., Armstrong K.R., Naylor J.M. Coronavirus-associated diarrhea (winter dysentery) in adult cattle. Can. Vet. J. 1989;30(10):825–827. [PMC free article] [PubMed] [Google Scholar]
- Farkas T., Nakajima S., Sugieda M., Deng X., Zhong W., Jiang X. Seroprevalence of noroviruses in swine. J. Clin. Microbiol. 2005;43(2):657–661. doi: 10.1128/JCM.43.2.657-661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.M., Gomez-Puertas P., Cavanagh D., Gorbalenya A.E., Enjuanes L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch Virol. 2003;148(11):2207–2235. doi: 10.1007/s00705-003-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Evermann J.F., Saif L.J. Detection and molecular characterization of cultivable caliciviruses from clinically normal mink and enteric caliciviruses associated with diarrhea in mink. Arch Virol. 2001;146(3):479–493. doi: 10.1007/s007050170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzinek M.C., Ederveen J., Weiss M. The nucleocapsid of Berne virus. J. Gen. Virol. 1985;66(Pt 6):1287–1296. doi: 10.1099/0022-1317-66-6-1287. [DOI] [PubMed] [Google Scholar]
- Hou X.L., Yu L.Y., Liu J. Development and evaluation of enzyme-linked immunosorbent assay based on recombinant nucleocapsid protein for detection of porcine epidemic diarrhea (PEDV) antibodies. Vet. Microbiol. 2007;123(1–3):86–92. doi: 10.1016/j.vetmic.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Okada N., Fukuyama S.I. Epidemiological analysis of bovine torovirus in Japan. Virus Res. 2007 doi: 10.1016/j.virusres.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson F.B., Wang E.E., Bain C., Good J., Duckmanton L., Petric M. Human torovirus: a new nosocomial gastrointestinal pathogen. J. Infect. Dis. 1998;178(5):1263–1269. doi: 10.1086/314434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans M., van den Boom U., Woode G., Horzinek M.C. Seroepidemiology of Breda virus in cattle using ELISA. Vet. Microbiol. 1989;19(3):233–243. doi: 10.1016/0378-1135(89)90069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans M., van Wuijckhuise-Sjouke L., Schukken Y.H., Cremers H., Horzinek M.C. Association of diarrhea in cattle with torovirus infections on farms. Am. J. Vet. Res. 1991;52(11):1769–1773. [PubMed] [Google Scholar]
- Koopmans M.P., Goosen E.S., Lima A.A., McAuliffe I.T., Nataro J.P., Barrett L.J., Glass R.I., Guerrant R.L. Association of torovirus with acute and persistent diarrhea in children. Pediatr. Infect. Dis. J. 1997;16(5):504–507. doi: 10.1097/00006454-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Krishnan T., Naik T.N. Electronmicroscopic evidence of torovirus like particles in children with diarrhoea. Indian J. Med. Res. 1997;105:108–110. [PubMed] [Google Scholar]
- Kroneman A., Cornelissen L.A., Horzinek M.C., de Groot R.J., Egberink H.F. Identification and characterization of a porcine torovirus. J. Virol. 1998;72(5):3507–3511. doi: 10.1128/jvi.72.5.3507-3511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M., Wada K., Maeda Y., Miyazaki A., Tsunemitsu H. First isolation of cytopathogenic bovine torovirus in cell culture from a calf with diarrhea. Clin. Vaccine Immunol. 2007;14(8):998–1004. doi: 10.1128/CVI.00475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouliatte F., du Pasquier P., Rossi F., Laporte J., Loze J.P. Studies on bovine Breda virus. Vet. Microbiol. 1987;15(4):261–278. doi: 10.1016/0378-1135(87)90015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazza A., Candotti P., Perini S., Vezzali L. Electron microscopic identification of torovirus-like particles in post-weaned piglets with enteritis. 14th IPVS Congress; Bologna, Italy; 1996. [Google Scholar]
- Lavazza A. Diarrea neonatale dei vitelli: identificazione al M.E. in colorazione negativa di particelle virali morfologicamente riferibili al Bredavirus. Atti Soc Ital Buiatria. 1989;21:121–127. [Google Scholar]
- Liebermann H. New types of virus infections of domestic animals in the German Democratic Republic. 1. Serologic survey studies of the distribution of equine torovirus infections in the GDR. Arch. Exp. Veterinarmed. 1990;44(2):251–253. [PubMed] [Google Scholar]
- Liebler E.M., Kluver S., Pohlenz J., Koopmans M. The significance of bredavirus as a diarrhea agent in calf herds in Lower Saxony. Dtsch. Tierarztl. Wochenschr. 1992;99(5):195–200. [PubMed] [Google Scholar]
- Lodha A., de Silva N., Petric M., Moore A.M. Human torovirus: a new virus associated with neonatal necrotizing enterocolitis. Acta Paediatr. 2005;94(8):1085–1088. doi: 10.1111/j.1651-2227.2005.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Matiz K., Kecskemeti S., Kiss I., Adam Z., Tanyi J., Nagy B. Torovirus detection in faecal specimens of calves and pigs in Hungary: short communication. Acta Vet. Hung. 2002;50(3):293–296. doi: 10.1556/AVet.50.2002.3.5. [DOI] [PubMed] [Google Scholar]
- Park S.J., Oh E.H., Park S.I., Kim H.H., Jeong Y.J., Lim G.K., Hyun B.H., Cho K.O. Molecular epidemiology of bovine toroviruses circulating in South Korea. Vet. Microbiol. 2007 doi: 10.1016/j.vetmic.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E., Lambden P.R., Caul E.O., Liu B., Dingle K., Deng Y., Clarke I.N. The seroepidemiology of genogroup 1 and genogroup 2 Norwalk-like viruses in Italy. J. Med. Virol. 1999;58(1):93–99. [PubMed] [Google Scholar]
- Penrith M.L., Gerdes G.H. Breda virus-like particles in pigs in South Africa. J. S. Afr. Vet. Assoc. 1992;63(3):102. [PubMed] [Google Scholar]
- Rodriguez D., Risco C., Rodriguez J.R., Carrascosa J.L., Esteban M. Inducible expression of the vaccinia virus A17L gene provides a synchronized system to monitor sorting of viral proteins during morphogenesis. J. Virol. 1996;70(11):7641–7653. doi: 10.1128/jvi.70.11.7641-7653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A.C., Chaplin M.J., Stack M.J., Lund L.J. Porcine torovirus? Vet. Rec. 1987;120(24):583. doi: 10.1136/vr.120.24.583-a. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71(10):7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K., Zhou Z., Shoup D.I., Saif L.J. Evaluation of the baculovirus-expressed S glycoprotein of transmissible gastroenteritis virus (TGEV) as antigen in a competition ELISA to differentiate porcine respiratory coronavirus from TGEV antibodies in pigs. J. Vet. Diagn. Invest. 1999;11(3):205–214. doi: 10.1177/104063879901100301. [DOI] [PubMed] [Google Scholar]
- Shin G.C., Chung Y.S., Kim I.S., Cho H.W., Kang C. Antigenic characterization of severe acute respiratory syndrome-coronavirus nucleocapsid protein expressed in insect cells: The effect of phosphorylation on immunoreactivity and specificity. Virus Res. 2007;127(1):71–80. doi: 10.1016/j.virusres.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., Gerwig G.J., van Vliet A.L., Lissenberg A., Briza P., Kamerling J.P., Vlasak R., de Groot R.J. Nidovirus sialate-O-acetylesterases: evolution and substrate specificity of coronaviral and toroviral receptor-destroying enzymes. J. Biol. Chem. 2005;280(8):6933–6941. doi: 10.1074/jbc.M409683200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., Lavazza A., Matiz K., Horzinek M.C., Koopmans M.P., de Groot R.J. Phylogenetic and evolutionary relationships among torovirus field variants: evidence for multiple intertypic recombination events. J. Virol. 2003;77(17):9567–9577. doi: 10.1128/JVI.77.17.9567-9577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timani K.A., Ye L., Ye L., Zhu Y., Wu Z., Gong Z. Cloning, sequencing, expression, and purification of SARS-associated coronavirus nucleocapsid protein for serodiagnosis of SARS. J. Clin. Virol. 2004;30(4):309–312. doi: 10.1016/j.jcv.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel Y., Laxer R.M., Petric M. Torovirus gastroenteritis presenting as acute abdomen. Clin. Infect. Dis. 1999;28(4):925–926. doi: 10.1086/517251. [DOI] [PubMed] [Google Scholar]
- Van Kruiningen H.J., Castellano V.P., Koopmans M., Harris L.L. A serologic investigation for coronavirus and Breda virus antibody in winter dysentery of dairy cattle in the northeastern United States. J. Vet. Diagn. Invest. 1992;4(4):450–452. doi: 10.1177/104063879200400415. [DOI] [PubMed] [Google Scholar]
- Vanopdenbosch E., Wellemans G., Charlier G., Petroff K. Bovine torovirus: cell culture propagation of a respiratory isolate and some epidemiological data. Vlaams Diergeneeskundig Tijdschrift. 1992;61:45–49. [Google Scholar]
- Wang Y., Chang Z., Ouyang J., Wei H., Yang R., Chao Y., Qu J., Wang J., Hung T. Profiles of IgG antibodies to nucleocapsid and spike proteins of the SARS-associated coronavirus in SARS patients. DNA Cell Biol. 2005;24(8):521–527. doi: 10.1089/dna.2005.24.521. [DOI] [PubMed] [Google Scholar]
- Wasmoen T.L., Kadakia N.P., Unfer R.C., Fickbohm B.L., Cook C.P., Chu H.J., Acree W.M. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus. Adv. Exp. Med. Biol. 1995;380:221–228. doi: 10.1007/978-1-4615-1899-0_36. [DOI] [PubMed] [Google Scholar]
- Weiss M., Steck F., Horzinek M.C. Purification and partial characterization of a new enveloped RNA virus (Berne virus) J. Gen. Virol. 1983;64(Pt 9):1849–1858. doi: 10.1099/0022-1317-64-9-1849. [DOI] [PubMed] [Google Scholar]
- Weiss M., Steck F., Kaderli R., Horzinek M.C. Antibodies to Berne virus in horses and other animals. Vet. Microbiol. 1984;9(6):523–531. doi: 10.1016/0378-1135(84)90014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M., Horzinek M.C. Morphogenesis of Berne virus (proposed family Toroviridae) J Gen Virol. 1986;67(7):1305–1314. doi: 10.1099/0022-1317-67-7-1305. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Chan K.H., Chu C.M., Tsoi H.W., Huang Y., Peiris J.S., Yuen K.Y. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin. Diagn. Lab. Immunol. 2004;11(4):665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G.N. Breda and Breda-like viruses: diagnosis, pathology and epidemiology. Ciba Found Symp. 1987;128:175–191. doi: 10.1002/9780470513460.ch11. [DOI] [PubMed] [Google Scholar]
- Woode G.N., Reed D.E., Runnels P.L., Herrig M.A., Hill H.T. Studies with an unclassified virus isolated from diarrheic calves. Vet. Microbiol. 1982;7(3):221–240. doi: 10.1016/0378-1135(82)90036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G.N., Saif L.J., Quesada M., Winand N.J., Pohlenz J.F., Gourley N.K. Comparative studies on three isolates of Breda virus of calves. Am. J. Vet. Res. 1985;46(5):1003–1010. [PubMed] [Google Scholar]


