Research highlights
▶ PRRSV triggered the activation of IRF-3 and the activation of IFN-βpromoter in early infection. ▶ PRRSV inhibited the activation of IRF-3 and the activation of IFN-βpromoter in the following infection. ▶ PRRSV nsp1 inhibit the activation of IFN-βpromoter by antagonizing the activation of IRF-3.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), Interferon beta, IFN-regulatory factor
Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) causes an economically important disease in swine-producing area, and interferon beta (IFN-β) is the first responder against the animal virus infection. However, whether PRRSV could induce the production of IFN-β is controversial. In this paper, we first time found that PRRSV could phosphorylate IFN-regulatory factor 3 (IRF-3) and weakly activate the IFN-β promoter in MARC-145 cells in early infection, but the activations of IRF-3 and IFN-β promoter were rapidly inhibited in the following infection. Furthermore, which components or products of the invading PRRSV cause PRRSV to inhibit IFN-β promoter activity attracted our attentions. The obtained results showed that PRRSV nsp1 could inhibit Poly(I:C)-induced IFN-β promoter activity in MARC-145 cells by down-regulating the protein level of IRF-3 and inhibiting the phosphorylation of IRF-3. In conclusion, our results suggested that PRRSV could be sensed by professional IFN-β-producing system and had mechanisms to inhibit this action in MARC-145 cells.
1. Introduction
PRRSV, a positive-stranded RNA virus, is a member of family Arteriviridae (Meulenberg, 2000). Since it was firstly identified in the United States in 1987 and in Europe in 1990, PRRSV has caused one of the most economically important diseases of swine which is characterized by severe reproductive failure in sows and respiratory distress in piglets and growing pigs (Rossow, 1998). Infection with PRRSV also predisposes pigs to secondary infection by bacterial and viral pathogens, which may be due to the immunosuppression induced by the virus (Feng et al., 2001, Mateu and Diaz, 2008).
IFN-β is the first responder against animal virus infection (Muller et al., 1994, Weber et al., 2004). When virus infects, the virus could be recognized by the pathogen-associated molecular patterns (PAMPs) such as membrane bound Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I). These PAMPs recruit different adaptor proteins, for example, TLRs recruits the adaptor molecule myeloid differentiation primary-response gene 88(MyD88) and Toll/IL-1 receptor domain-containing adaptor inducing IFN(TRIF) while RIG-I recruits virus-induced signaling adapter (VISA), to make TANK-binding kinase 1 (TBK1) or IκB kinase-ɛ (IKK-ɛ) phosphorylate IRF-3 and finally to induce IFN-β transcription (Bowie and Unterholzner, 2008). Then, IFN-β induces the IFN-regulated genes responsible for the antiviral response (Sadler and Williams, 2008). However, during the co-evolution with the host cells, many viruses have developed defensive mechanisms to inhibit IFN-β production, making it difficult for host cells to defeat viral infection (Bowie and Unterholzner, 2008, Weber et al., 2004). Luo et al. (2008) and Miller et al. (2004) concluded that PRRSV does not induce IFN-β in MARC-145 cells infected with PRRSV, but Luo et al. did not detect the level of IFN-β mRNA by RT-PCR, and in Miller's paper, the level of IFN-β appears a little higher in MARC-145 cells infected by PRRSV than that in control group, which may lead to a suspicion that whether PRRSV could induce IFN-β production or not may be valued for verifying. Furthermore, Genini et al. (2008) and Loving et al. (2007) reported that PRRSV could induce the production of IFN-β in primary swine cells, which supply a clue that maybe PRRSV could also induce the production of IFN-β in MARC-145 cells. Previous studies have documented that SARS-CoV nsp3 could inhibit the IFN-β production by its papain-like protease domain (Devaraj et al., 2007) and SARS-CoV N was capable of inhibiting IFN-β production (Kopecky-Bromberg et al., 2007). It is a coincidence that PRRSV nsp1 also contained papain-like protease domain (den Boon et al., 1995) and the crystal structure of PRRSV N protein was similar to that of SARS-CoV N protein (Yu et al., 2006). So, the purpose of the present experiments is to analyze the patterns of IFN-β promoter activity in MARC-145 cells during infection with PRRSV and to analyze whether PRRSV nsp1 and N protein could inhibit IFN-β production.
2. Materials and methods
2.1. Cell, virus and primary antibodies
MARC-145 cell, a fetal green monkey fibroblast cell line derived from MA-104 (Kim et al., 1993), was maintained in Dulbecco's modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum (Hyclone). PRRSV strain BJ-4, a kind gift from Dr. Hanchun Yang (China Agricultural University), was propagated in MARC-145 cells, which after 96 h post-infection (p.i.), the cells were frozen and thawed and clarified by low-speed centrifugation, and then the supernatants were stocked at −80 °C. By the same methods, the supernatants of cells which were not infected with PRRSV were also prepared as the sham virus infection in the experiment. Primary antibodies used for this study were anti-IRF-3, anti-serine 396-phosphorylated species of IRF-3 (pIRF-3) (Cell Signaling Technology), anti-actin and anti-His tag (Beijing Zhongshan Goldenbridge Biotechnology Company, China).
2.2. Plasmids
The PRRSV nsp1 and N, which contained 6× His-tag in C-terminus in their reverse primers, were cloned from PRRSV RNA, and the PCR products were cloned into pMD19-T vector (Takara) and then ligated into pcDNA3.1 (Invitrogen). TBK1, VISA, and TRIF genes were cloned from MARC-145 cells by RT-PCR and were ligated into pcDNA3.1. RIG-N gene, the constitutive active caspase recruitment domain in RIG-I, was cloned from pEF-BOS-flag RIG-N which was kindly provided by Dr. Takashi Fujita (Institute for Virus Research Kyoto University, Tokyo, Japan) (Yoneyama et al., 2004) and was ligated into pcDNA3.1. pcDNA3.1-IRF-3(5D) was constructed from the IRF-3(5D)-FLAG expression plasmid which was obtained from Dr. Rongtuan Lin (Lady Davis Institute for Medical Research, McGill University, Canada) (Lin et al., 1998). The firefly-luciferase plasmid for monitoring IFN-β promoter activation (p-284 Luc) was cloned from genetic DNA of MARC-145 cells and was ligated into pGL-417 (Prpmega). p55C1B Luc (Devaraj et al., 2007, Yoneyama et al., 1996, Yoneyama et al., 2004), a firefly-luciferase reporter gene plasmid containing repetitive pIRF-3 binding sites, was kindly provided by Dr. Takashi Fujita. phRL-TK (Promega), contained a Renilla-luciferase reporter gene, was used as an internal control in dual luciferase reporter assay system. pcDNA3.1-His was constructed by using His primers. All primers for constructing plasmids above were listed in Table 1 .
Table 1.
The primers used in plasmid construction and RT-PCR.
| Primer | Sequence (5′ → 3′) |
|---|---|
| PRRSV nsp1 For | CCCAAGCTTCGCCACCATGGGCATGTCTGG |
| PRRSV nsp1 Rev | TCAATGATGATGATGATGATGGTACCACTTGTGACTGCCAAAC |
| VISA For | CCCAAGCTTGCCACCATGCCGTTTGCTGAAGACA |
| VISA Rev | CAGACGGAATTCCTAGTGCAGGCGCCGC |
| TBK1 For | GGGGTACCGGCCACCATGCAGAGCACTTCTAATC |
| TBK1 Rev | GCAGCGTCACGCTCTAGACTAAAGACAGTCAACGTTGC |
| TRIF For | CAAGCTTCGCCACCATGGCCTGCACGGG |
| TRIF Rev | GACGTCCGACGGAATTCTCATTCTGCCTCCTGTGTT |
| RIG-N For | CAAGCTTCGCCACCATGACCACCGAGCAGC |
| RIG-N Rev | GCCTCACGGGGTACCTCATTTTTTAAGATGATGTTCACATATAAGC |
| IRF-3 For | CCCAAGCTTCGCCACCATGGGAACCCCAAAGCCA |
| IRF-3 Rev | AGCAGGTCACCGGAATTCTCAGGTCTCCCCAGGGCC |
| p-284 For | GAGATCTCTGAATTCTCAGGTCATTTG |
| p-284 Rev | GCAAGCTTAAGTTTGCAGTTAGAATGTCC |
| IRF-3 RT For | GGAGCAAGGACCCTCACGAC |
| IRF-3 RT Rev | GGGGCCAACACCATGTTACC |
| PRRSV N For | CCCAAGCTTCGCCACCATGGGCATGCCA |
| PRRSV N Rev | TCAATGATGATGATGATGATGTGCTGAGGGTGATGCTGTGAC |
| His For | CCCAAGCTTgGCCACCATGGGCCATCATCATC |
| His Rev | CGGAATTCTCAATGATGATGATGATGATGGCCCATG |
| GAPDH For | TGACAACAGCCTCAAGATCG |
| GAPDH Rev | GTCTTCTGGGTGGCAGTGAT |
2.3. Transfection and reporter gene assay
All newly prepared plasmids were verified by sequencing. Transient transfection was performed by using Lipofectamine2000 (Invitrogen). Cells grown in 24-well plates were transfected in triplicate with the indicated reporter plasmid (400 ng), phRL-TK (200 ng), and expression vector (1000 ng) or supplied with an equivalent control vector. At the appointed time, cells were harvested and the luciferase activity was measured using the dual luciferase reporter assay system (Promega) in MicroBeta® TriLux liquid scintillation and luminescence conters (Microbeta-1450) (Wallac).
2.4. Western blot
MARC-145 cells grown in 24-well plates were transfected in triplicate with the pcDNA3.1-His (control vector) or pcDNA3.1-nsp1 (1000 ng), and 24 h later, transfected cells were mock-treated or treated with Poly(I:C) for 6 h and lysed in lysing buffer (1% Nonidet P-40, 0.1% sodium deoxycholate, 0.1% SDS, 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM Na3VO4, 2 mM NaF and a protease inhibitor cocktail). The detailed procedure for immunoblots has been described (Shi et al., 2008). Briefly, the total protein concentration was quantified with the Bradford protein assay (Biocolor Bioscience & Technology Company, Shanghai). Equal proteins were separated on SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Millipore Company), and then probed with appropriate antibodies. Proteins were detected by using an ECL detection system (Cell Signaling Technology).
2.5. RT-PCR
MARC-145 cells were transfected with pcDNA3.1-His (control vector) and pcDNA3.1-nsp1. Thirty hours later, the total cellular RNA was extracted by using TRIzol reagent (Invitrogen), and treated with DNase I (Takara) to remove genomic DNA contamination. Then, the RNA was reverse-transcribed to cDNA by reverse transcriptase M-MLV (Takara). The IRF-3 RT primers used in semi-quantitative analysis of the IRF-3 mRNA were listed in Table 1.
2.6. Statistical analysis
Statistical analyses was performed by Student's t-test, and the comparisons were considered as statistical significance when p < 0.05.
3. Results
3.1. PRRSV sensed by professional beta interferon-producing system
It has been extensively documented that cytopathic effect (CPE), induced by PRRSV infection in MARC-145 cells, only appeared after 72 h p.i. (Cafruny et al., 2006, Kim et al., 2002), which was also confirmed in our experiment (data not shown). Cell viability was assessed by trypan blue staining, and approximately 93% of PRRSV-infected cells were viable at 48 h p.i. (data not shown).
To investigate whether PRRSV could induce the IFN-β response in cell culture, MARC-145 cells were infected with PRRSV at an MOI of 0.05. At a certain time, cells were co-transfected with p-284 Luc and phRL-TK, and 18 h later, the cells were harvested and subjected to a dual luciferase reporter assay system. As shown in Fig. 1A, PRRSV could activate IFN-β promoter in MARC-145 Cells at 24 h p.i., but the activity of IFN-β promoter was much lower than that triggered by Poly(I:C). And the activity of IFN-β promoter was rapidly inhibited in the following infection. The expression of IFN-β mRNA was also analyzed by semi-quantitative RT-PCR at the different time points after PRRSV infection and gotten the results similar to that of Fig. 1A (data not shown). To confirm this result, the level of pIRF-3 was also detected because pIRF-3 was a necessary transcription factor to the activation of IFN-β promoter (Peters et al., 2002, Yoneyama et al., 1998). In consistent with the results of Fig. 1A, PRRSV could phosphorylate IRF-3 in early stage of infection (24 h p.i.), but after that, no detectable levels of pIRF-3 showed at certain time interval (Fig. 1B).
Fig. 1.
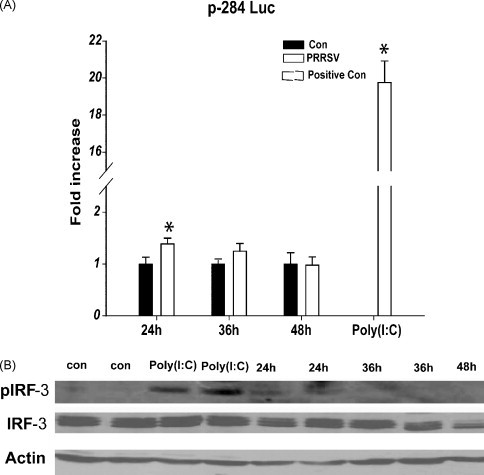
PRRSV phosphorylated IRF-3 and weakly activated the IFN-β promoter in MARC-145 at 24 h p.i., but their activities were rapidly inhibited in the following infection. (A) MARC-145 cells were infected with PRRSV at an MOI of 0.05. After 6 h, 18 h, 30 h respectively, the cells were co-transfected with p-284 Luc and phRL-TK, and 18 h later, cells were harvested for dual luciferase reporter assay system in MicroBeta-1450. Cells were transfected with Poly(I:C) for 9 h as positive controls. (B) MARC-145 cells were infected with PRRSV at an MOI of 0.05, and harvested at 24, 36, 48 h p.i. for the immunoblots of IRF-3 and pIRF-3. The results shown were from one of three independent experiments with similar observations, and each including three or two replicates. Control (Con): Sham virus mentioned in part of Section 2 were added into MARC-145 cells. *: p < 0.05 compared to the control.
3.2. PRRSV nsp1 inhibited the activation of IFN-β promoter induced by Poly(I:C)
Previous studies have documented that SARS-CoV nsp3 could inhibit the IFN-β production by its papain-like protease domain (Devaraj et al., 2007) and SARS-CoV N was capable of inhibiting IFN-β production (Kopecky-Bromberg et al., 2007). Since PRRSV nsp1 also contains papain-like protease domain (den Boon et al., 1995) and the crystal structure of PRRSV N protein was similar to that of SARS-CoV N protein (Yu et al., 2006), PRRSV nsp1 and N proteins were chosen to probe which component of PRRSV could inhibit IFN-β production.
The expressions of pcDNA3.1 nsp1 and pcDNA3.1 N in MARC-145 cells were confirmed by RT-PCR (Fig. 2A) and western blot (Fig. 2B). Since nsp1 should be cleaved into nsp1α and nsp1β subunits (den Boon et al., 1995) and the 6× His tag was ligated to the C-terminal of nsp1 in this study, here only nsp1β could be detected (Fig. 2B).
Fig. 2.
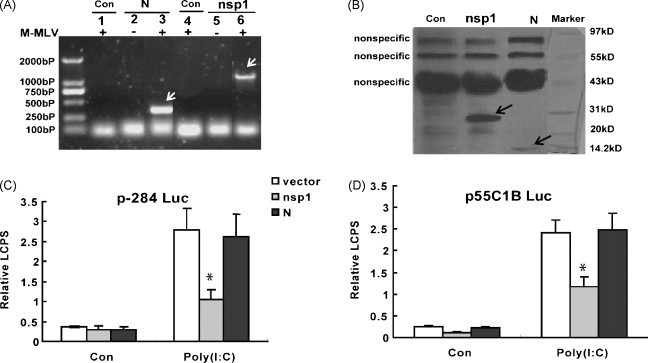
PRRSV nsp1 inhibited the activations of IFN-β promoter and pIRF-3-dependent synthetic promoter (p55C1B Luc) induced by Poly(I:C). (A) The mRNA of nsp1 and N in MARC-145 cells transfected with pcDNA3.1-nsp1 (nsp1), pcDNA3.1-N (N) or pcDNA3.1-His (Con) were analyzed by RT-PCR. Lanes 1–3 were amplified by PRRSV N primers, while lanes 4–6 were amplified by PRRSV nsp1 primers. (B) Western blot analyzed nsp1 and N protein by anti-His primary antibody in MARC-145 transfected with pcDNA3.1-nsp1 (nsp1) or pcDNA3.1-N (N). The control cells were transfected with pcDNA 3.1-His (Con). Since the nsp1 should be cleaved into nsp1α and nsp1β subunits, the arrow-pointed band was only the nsp1β subunits. (C) MARC-145 cells were co-transfected with p-284 Luc, phRL-TK and different expression plasmid, and 24 h later, cells were either mock-treated (Con) or transfected with Poly(I:C) for 6 h. Cells were analyzed by dual luciferase reporter assay. (D) MARC-145 cells were co-transfected with p55C1B Luc, phRL-TK and different expression plasmid. Twenty four hours later, cells were either mock-treated (Con) or transfected with Poly(I:C) for 6 h. Cells were analyzed by dual luciferase reporter assay. LCPS was the luminescence counts per second detected by luminescence counting on the Microbeta-1450. Bars showed relative luciferase activity (Relative LCPS) normalized to phRL-TK activity (relative LCPS = LCPS of firefly-luciferase/LCPS of Renilla-luciferase). Data represented means of three replicates, and experiments were repeated three times. Vector (pcDNA3.1-His) showed in figure was the control plasmid. *: p < 0.05 compared to the cells transfected with pcDNA3.1-His (Con).
MARC-145 cells were co-transfected with pcDNA3.1-nsp1 or pcDNA3.1-N, p-284 Luc and phRL-TK plasmids. As control group, pcDNA3.1-His was in place of pcDNA3.1 nsp1. Twenty four hours later, MARC-145 cells were either mock-treated or Poly(I:C)-treated for 6 h, and then the cells were harvested for luciferase reporter assay. The experimental data showed that only nsp1 suppressed the activation of IFN-β promoter (Fig. 2C), but N protein did not.
Because the pIRF-3 was a necessary component to the activation of IFN-β promoter, after the Poly(I:C) treatment or the mock treatment, pIRF-3-dependent synthetic promoter, p55C1B-Luc (Devaraj et al., 2007, Yoneyama et al., 1998, Yoneyama et al., 2004), was detected with luciferase reporter assays,. As shown in Fig. 2D, only nsp1 could inhibit the activation of p55C1B-Luc, while N protein could not. The results in Fig. 2D confirmed the results in Fig. 2C.
3.3. PRRSV nsp1 inhibited the activity of IFN-β promoter induced by RIG-N, VISA, TRIF or TBK1, but did not inhibit the activity of IFN-β promoter induced by IRF-3(5D)
RIG-I, VISA, TRIF and TBK1 were the upstream signaling proteins of IRF-3 in the signal pathway of IFN-β production. The over-expression of RIG-I, VISA, TRIF or TBK1 could induce the activation of IRF-3 and promote the activation of IFN-β promoter (Devaraj et al., 2007, Yoneyama et al., 2004, Zhong et al., 2008). RIG-N was the constitutive active caspase recruitment domain in RIG-I (Yoneyama et al., 2004). PRRSV nsp1 strongly suppressed the activation of IFN-β promoter induced by over-expression of RIG-N or ectopic expression of VISA, TRIF, TBK1 (Fig. 3A). In contrast, IRF-3(5D), a constitutively active and phospho-mimetic IRF-3 mutant (Lin et al., 1998), activated the IFN-β promoter, and that was no difference between cells expressing nsp1 and cells transfected with a control vector (Fig. 3A). Similar results were obtained when p55C1B Luc was in place of p-284 Luc (Fig. 3B).
Fig. 3.
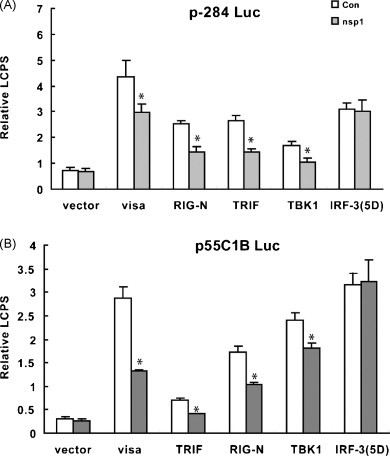
PRRSV nsp1 inhibited activities of IFN-β promoter and pIRF-3 dependent promoters (p55C1B Luc) induced by RIG-N, VISA, TRIF or TBK1, but did not inhibit the activities of IFN-β promoter and pIRF-3 dependent promoter induced by IRF-3(5D). MARC-145 cells were co-transfected with p-284 Luc (A) or p55C1B Luc (B), phRL-TK and different expression plasmids. Thirty hours later, cells were harvested for dual luciferase reporter assay. Con: cells transfected with pcDNA3.1-His. Data represented means of three replicates, and experiments were repeated three times. *: p < 0.05 compared to cells transfected with pcDNA3.1-His (Con).
3.4. PRRSV nsp1 down-regulated the protein level of IRF-3 and inhibited the phosphorylation of IRF-3 induced by Poly(I:C)
Immunoblot analyzed the activation and latent status of IRF-3. And the results showed that nsp1 not only down-regulated the protein level of IRF-3, but also inhibited the phosphorylation of IRF-3 induced by Poly(I:C) (Fig. 4A).
Fig. 4.
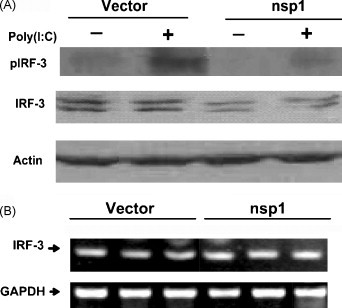
PRRSV nsp1 inhibited the phosphorylation of IRF-3 induced by Poly(I:C) but had no effect on the level of IRF-3 mRNA. (A) MARC-145 cells were transfected with pcDNA3.1-nsp1 (nsp1) or control vector (Vector). Twenty four hours later, the cells were either mock-treated or transfected with Poly(I:C) for 6 h. Then the cells were analyzed by immunoblots. (D) MARC-145 cells were transfected with pcDNA3.1-nsp1 or control vector (Vector). Thirty hours later, the total cellular RNA was extracted and used for semi-quantitative analysis of the IRF-3 mRNA (22 cycle amplification). The results shown were from one of three independent experiments with similar observations.
The expression of IRF-3 mRNA was analyzed by semi-quantitative RT-PCR. The results in Fig. 4B demonstrated that nsp1 have no effect on the transcription of IRF-3.
4. Discussion
Our present work was for the first time to analyze the dynamic activities of IFN-β promoter and IRF-3, a necessary transcription factor to the activity of IFN-β promoter (Yoneyama et al., 1998) during PRRSV infection in MARC-145 cell line which was capable of producing IFN-β (McKimm-Breschkin and Holmes, 1982) and supported the replication of PRRSV (Kim et al., 1993). The results indicated that PRRSV could trigger the activation of IRF-3 as well as induce the activation of IFN-β promoter at 24 h p.i., but the activities were much lower than that triggered by the Poly(I:C). And their activities were rapidly inhibited in following infection (Fig. 1). So it is reasonable to speculate that PRRSV could be sensed by professional IFN-β-producing system and had mechanisms to inhibit this action in MARC-145 cells.
PRRSV nsp1 contained papain-like protease domain (den Boon et al., 1995) and the crystal structure of PRRSV N protein was similar to that of SARS-CoV N (Yu et al., 2006). Previous studies have documented that SARS-CoV nsp3 could inhibit the IFN-β production by its papain-like protease domain (Devaraj et al., 2007) and SARS-CoV N was capable of inhibiting IFN-β production (Kopecky-Bromberg et al., 2007). Our present work indicated that PRRSV nsp1 inhibited the IFN-β production induced by Poly(I:C), while N protein did not (Fig. 2). It's unexpected that PRRSV N protein could not inhibit the IFN-β production, because a protein's function generally depended on its three-dimension structure or conformation. Perhaps, SARS-CoV N protein could inhibit IFN-β production by its peptide or other unclear mechanism.
Poly(I:C), a double-stranded RNA, could be recognized by TLR3 (Yamamoto et al., 2003) and RIG-I (Kato et al., 2006). Then through TBK1, TLR3 recruited TRIF and RIG-I recruited VISA to phosphorylate IRF-3 and finally activate IFN-β promoter (Bowie and Unterholzner, 2008). Over-expression of RIG-I, VISA, TBK1 or TRIF could induce the activation of IRF-3 and promote the activation of IFN-β promoter (Devaraj et al., 2007, Yoneyama et al., 2004, Zhong et al., 2008). Our data showed that nsp1 antagonized the IFN-β production induced by RIG-I, VISA, TBK1 or TRIF, which indicated that nsp1 inhibited the IFN-β production induced by both TLR3 and RIG-I pathways (Fig. 3). Nevertheless, nsp1 had no effect on the activity of IFN-β promoter induced by IRF-3(5D), which suggested that nsp1 inhibited IFN-β production by suppressing the activation of IRF-3. This speculation has been confirmed. PRRSV nsp1 down-regulated the protein level of IRF-3 and inhibited Poly(I:C)-induced phosphorylation of IRF-3, but had no effect on the level of IRF-3 mRNA (Fig. 4). Perhaps, nsp1 inhibited the translation of IRF-3 or induced the degradation of IRF-3 protein or inhibited the activation of kinase to phosphorylate IRF-3. SARS-CoV nsp3 inhibited phosphorylation of IRF-3 through its papain-like protease domain to be interacted with IRF-3 but did not down-regulated the protein level of IRF-3 (Devaraj et al., 2007), while the Npro protein of Classical Swine Fever Virus, similar to the group of papain-like proteases (Stark et al., 1993), could antagonize IRF-3 activity by inducing its proteasomal degradation (Bauhofer et al., 2007). PRRSV nsp1 also contains papain-like protease domain (den Boon et al., 1995) and PRRSV nsp1 down-regulated the protein level of IRF-3. So it is possible that PRRSV nsp1 inhibits the IRF-3 activation by inducing IRF-3 degradation or inhibits the activation of kinase to phosphorylate IRF-3. And this suppositional mechanism deserves a further study.
Based on the experimental data discussed above, we propose a model to illustrate how PRRSV nsp1 negatively regulates IFN-β signaling pathways (Fig. 5 ). First, PRRSV could be sensed by the professional beta interferon-producing system, although it is unclear that PRRSV could be recognized by TLRs or RIG-I-like receptors (RLRs) or by the both. Second, PRRSV nsp1 could negatively regulate IFN-β production by inhibiting the phosphorylation of IRF-3.
Fig. 5.
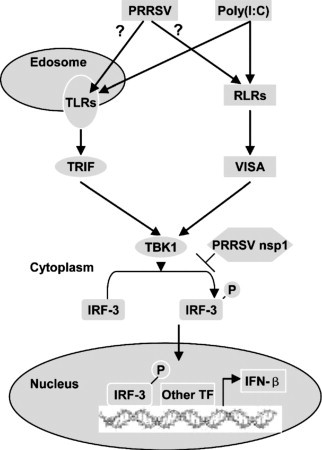
Proposed model illustrating how PRRSV nsp1 negatively regulates IFN-β signaling pathways. Other TF: other transcription factor.?: it is unclear that PRRSV could be recognized by TLRs or RIG-I-like receptors (RLRs) or by the both.
It is known that type I IFN system (IFN-α/β) provides an important first-host response to viral intruders (Weber et al., 2004), and type I IFN is essential to the initial control of virus infection and the establishment of an adaptive immune response (Muller et al., 1994). Many viruses developed defensive mechanisms to inhibit IFN-β production (Bowie and Unterholzner, 2008, Devaraj et al., 2007, Weber et al., 2004). The influenza virus NS1 protein, a multi-functional facilitator of virus replication, could antagonize the IFN-β production (Hale et al., 2008). Deletion of the IFN-β antagonist NS1 could produce a novel type of influenza vaccine (Wacheck et al., 2010). Novel antagonists against influenza virus NS1, only significantly and specifically inhibiting influenza A replication in cell capable of interferon production, could be a potential drug for influenza virus-treatment (Basu et al., 2009). Previous studies also showed intriguing observations that recombinant IFN-β not only protected swine alveolar macrophages and MARC-145 cells from infection with PRRSV (Overend et al., 2007), but also could reduce the yield of PRRSV in vivo (Buddaert et al., 1998). Perhaps, inhibition of IFN-β production leads to the persistent infection of PRRSV. Our data demonstrated that PRRSV could inhibit the IFN-β production by PRRSV nsp1. In the future, it will be interesting to research on the function or characterization of PRRSV nsp1 in virus-induced immunosuppression and virus replication, and PRRSV nsp1 may be used as a potent target for exploiting new drags for PRRSV treatment or PRRSV vaccine.
Acknowledgements
We thank Takashi Fujita, Rongtuan Lin for providing reagents. This work was supported by the Key Program National Natural Science Foundation of China (30730068).
References
- Basu D., Walkiewicz M.P., Frieman M., Baric R.S., Auble D.T., Engel D.A. Novel influenza virus NS1 antagonists block replication and restore innate immune function. J. Virol. 2009;83(4):1881–1891. doi: 10.1128/JVI.01805-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauhofer O., Summerfield A., Sakoda Y., Tratschin J.D., Hofmann M.A., Ruggli N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J. Virol. 2007;81(7):3087–3096. doi: 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A.G., Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 2008;8(12):911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddaert W., Van Reeth K., Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv. Exp. Med. Biol. 1998;440:461–467. doi: 10.1007/978-1-4615-5331-1_59. [DOI] [PubMed] [Google Scholar]
- Cafruny W.A., Duman R.G., Wong G.H., Said S., Ward-Demo P., Rowland R.R., Nelson E.A. Porcine reproductive and respiratory syndrome virus (PRRSV) infection spreads by cell-to-cell transfer in cultured MARC-145 cells, is dependent on an intact cytoskeleton, and is suppressed by drug-targeting of cell permissiveness to virus infection. Virol. J. 2006;3:90. doi: 10.1186/1743-422X-3-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon J.A., Faaberg K.S., Meulenberg J.J., Wassenaar A.L., Plagemann P.G., Gorbalenya A.E., Snijder E.J. Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papain like cysteine proteases. J. Virol. 1995;69(7):4500–4505. doi: 10.1128/jvi.69.7.4500-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S.G., Wang N., Chen Z., Chen Z., Tseng M., Barretto N., Lin R., Peters C.J., Tseng C.T., Baker S.C., Li K. Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2007;282(44):32208–32221. doi: 10.1074/jbc.M704870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Laster S.M., Tompkins M., Brown T., Xu J.S., Altier C., Gomez W., Benfield D., McCaw M.B. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis type II. J. Virol. 2001;75(10):4889–4895. doi: 10.1128/JVI.75.10.4889-4895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genini S., Delputte P.L., Malinverni R., Cecere M., Stella A., Nauwynck H.J., Giuffra E. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008;89(10):2550–2564. doi: 10.1099/vir.0.2008/003244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale B.G., Randall R.E., Ortin J., Jackson D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008;89(10):2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., Yamaguchi O., Otsu K., Tsujimura T., Koh C.S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Kwang J., Yoon I.J., Joo H.S., Frey M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993;133(3–4):477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Kim T.S., Benfield D.A., Rowland R.R. Porcine reproductive and respiratory syndrome virus-induced cell death exhibits features consistent with a nontypical form of apoptosis. Virus Res. 2002;85(2):133–140. doi: 10.1016/s0168-1702(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81(2):548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Heylbroeck C., Pitha P.M., Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 1998;18(5):2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Sacco R.E. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120(2):217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R., Xiao S., Jiang Y., Jin H., Wang D., Liu M., Chen H., Fang L. Porcine reproductive and respiratory syndrome virus (PRRSV) suppresses interferon-beta production by interfering with the RIG-I signaling pathway. Mol. Immunol. 2008;45(10):2839–2846. doi: 10.1016/j.molimm.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu E., Diaz I. The challenge of PRRS immunology. Vet. J. 2008;177(3):345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J.L., Holmes I.H. Conditions required for induction of interferon by rotaviruses and for their sensitivity to its action. Infect. Immun. 1982;36(3):857–863. doi: 10.1128/iai.36.3.857-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J. PRRSV, the virus. Vet. Res. 2000;31(1):11–21. doi: 10.1051/vetres:2000103. [DOI] [PubMed] [Google Scholar]
- Miller L.C., Laegreid W.W., Bono J.L., Chitko-McKown C.G., Fox J.M. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol. 2004;149(12):2453–2463. doi: 10.1007/s00705-004-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Overend C., Mitchell R., He D., Rompato G., Grubman M.J., Garmendia A.E. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with Porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007;88(3):925–931. doi: 10.1099/vir.0.82585-0. [DOI] [PubMed] [Google Scholar]
- Peters K.L., Smith H.L., Stark G.R., Sen G.C. IRF-3-dependent, NFkappa B- and JNK-independent activation of the 561 and IFN-beta genes in response to double-stranded RNA. Proc. Natl. Acad. Sci. U.S.A. 2002;99(9):6322–6327. doi: 10.1073/pnas.092133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998;35(1):1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Sadler A.J., Williams B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008;8(7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Qin L., Liu G., Zhao S., Peng N., Chen X. Dynamic balance of pSTAT1 and pSTAT3 in C57BL/6 mice infected with lethal or nonlethal Plasmodium yoelii. Cell. Mol. Immunol. 2008;5(5):341–348. doi: 10.1038/cmi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R., Meyers G., Rumenapf T., Thiel H.J. Processing of pestivirus polyprotein: cleavage site between autoprotease and nucleocapsid protein of classical swine fever virus. J. Virol. 1993;67(12):7088–7095. doi: 10.1128/jvi.67.12.7088-7095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacheck V., Egorov A., Groiss F., Pfeiffer A., Fuereder T., Hoeflmayer D., Kundi M., Popow-Kraupp T., Redlberger-Fritz M., Mueller C.A., Cinatl J., Michaelis M., Geiler J., Bergmann M., Romanova J., Roethl E., Morokutti A., Wolschek M., Ferko B., Seipelt J., Dick-Gudenus R., Muster T. A novel type of influenza vaccine: safety and immunogenicity of replication-deficient influenza virus created by deletion of the interferon antagonist NS1. J. Infect. Dis. 2010;201(3):354–362. doi: 10.1086/649428. [DOI] [PubMed] [Google Scholar]
- Weber F., Kochs G., Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17(4):498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Suhara W., Fukuhara Y., Fukuda M., Nishida E., Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17(4):1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Suhara W., Fukuhara Y., Sato M., Ozato K., Fujita T. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3) J. Biochem. 1996;120(1):160–169. doi: 10.1093/oxfordjournals.jbchem.a021379. [DOI] [PubMed] [Google Scholar]
- Yu I.M., Oldham M.L., Zhang J., Chen J. Crystal structure of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein dimerization domain reveals evolutionary linkage between corona- and arteriviridae. J. Biol. Chem. 2006;281(25):17134–17139. doi: 10.1074/jbc.M602107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Yang Y., Li S., Wang Y.Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., Shu H.B. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]


