Abstract
In this study, we addressed the question whether cholesterol is important for transmissible gastroenteritis virus (TGEV), a porcine coronavirus, in the initiation of an infection. We found that cholesterol depletion from the cellular membrane by methyl-β-cyclodextrin (MβCD) significantly impaired the efficiency of TGEV infection. Infectivity was also reduced after depleting cholesterol from the viral envelope. This finding is surprising because coronaviruses bud from a pre-Golgi compartment which is expected to be low in cholesterol compared to the plasma membrane. Addition of exogenous cholesterol resulted in a restoration of the infectivity confirming our conclusion that efficient TGEV infection requires cholesterol in both the viral and the cellular membranes. Our data raise the possibility that the viral and cellular proteins involved in the entry process may be associated with cholesterol-rich membrane microdomains.
Keywords: TGEV, Enveloped virus, Cholesterol, Lipid rafts
1. Introduction
Transmissible gastroenteritis virus (TGEV) causes severe gastroenteritis, a highly contagious intestinal infection with high mortality rates (up to 100%) in seronegative suckling piglets (Laude et al., 1993, Saif and Wesley, 1999, Schwegmann-Wessels et al., 2003). TGEV, a member of the family Coronaviridae, is a pleomorphic enveloped virus with a positive-stranded RNA genome (Spaan et al., 1988) and four structural proteins: the spike (S) protein, the integral membrane (M) glycoprotein, the minor envelope (E) protein, and the nucleocapsid (N) protein (Laude et al., 1993, Spaan et al., 1988). The coronavirus surface protein S is a major viral antigen and its binding to the cellular receptor porcine aminopeptidase N (pAPN) is required for the initial stage of a TGEV infection including the attachment step and the subsequent membrane fusion event (Delmas et al., 1992, Winter et al., 2006). Membrane microdomains enriched in glycosphingolipids, cholesterol and certain proteins (for a review, see Schuck and Simons, 2004), also designated lipid rafts, have been described for cells from mammals, Drosophila, Dictyostelium and yeast (Drevot et al., 2002). Accumulating evidence indicates that membrane microdomains are important in the process of virus infection. Particularly, cholesterol, a critical structural component of lipid rafts, plays an important role in different aspects of the life cycle of several viruses (Imhoff et al., 2007).
The importance of cholesterol in the entry of nonenveloped viruses has been demonstrated for simian virus 40 (SV40), rotavirus, enterovirus and rhinovirus (reviewed in Suzuki and Suzuki, 2006). Entry of enveloped viruses into cells involves binding to specific receptors and fusion of the viral membrane with a cellular membrane. Successful virus entry may require cholesterol in either of the two membranes involved or in both. Previous data show that the infectivity of influenza virus is sensitive to cholesterol depletion from the viral membrane (reviewed by Smith and Helenius, 2004). More recently we have shown that canine distemper virus infection also requires cholesterol in the viral envelope (Imhoff et al., 2007). In contrast, murine leukemia virus, Ebola virus and Marburg virus are sensitive to cholesterol depletion from the cellular membrane (Bavari et al., 2002, Lu et al., 2002). In the case of human immunodeficiency virus (HIV) and herpes simplex virus, cholesterol is required in both membranes (reviewed by Smith and Helenius, 2004, Nayak and Hui, 2004), while vesicular stomatitis virus (VSV) replication is not affected by cholesterol depletion.
Current data indicates that depletion of cellular cholesterol by the drug methyl-β-cyclodextrin (MβCD), a cholesterol depletion reagent inhibits virus entry of the coronaviruses mouse hepatitis virus (Thorp and Gallagher, 2004, Choi et al., 2005), severe acute respiratory syndrome (SARS)-coronavirus (Li et al., 2007), human coronavirus 229E (Nomura et al., 2004) and avian infectious bronchitis virus (Imhoff et al., 2007). As coronaviruses differ widely in their host and tissue tropism and use quite different cellular receptors for virus entry, we investigated, for the first time, the requirement of cholesterol for a porcine coronavirus, transmissible gastroenteritis virus. We analyzed the requirement of cholesterol not only in the cellular membrane but also in the viral envelope. Our data show that depletion of cholesterol in either membrane – the viral or the cellular membrane – results in a reduction of TGEV infectivity.
2. Materials and methods
2.1. Cells and viruses
Swine testicle (ST) cells and Baby hamster kidney cells (BHK21) were maintained in MEM medium supplemented with 5% fetal calf serum (FCS) and passaged twice a week, respectively. TGEV strain PUR46-MAD and vesicular stomatitis virus (VSV, strain Indiana) were propagated in ST and BHK21 cells, respectively. All viruses used for the experiments were grown in serum-free medium.
2.2. Depletion and replenishment of cholesterol from cells
For removal of the cellular cholesterol, 2 × 105 cells in 1 ml medium containing 5% FCS were seeded per well of a 24-well plate and incubated in a CO2-incubator. Cell monolayers were washed two times with PBS and incubated 30 min at 37 °C with serum-free DMEM in the absence (control cells) or presence (treated cells) of MβCD (Sigma, USA) ranging form 0 to 15 mM. Then, MβCD was removed by washing the cells three times with PBS. For cholesterol replenishment, after extraction of cellular membrane cholesterol using 12 mM MβCD, cell monolayers were replenished by water-soluble cholesterol (cholesterol complexed with MβCD, Sigma) by applying final concentrations ranging from 50 to 500 μM at 37 °C for 30 min as described by Barman and Nayak (2007).
2.3. Depletion and replenishment of cholesterol from virus
For extraction of viral envelope cholesterol, the aliquots of virus suspensions with a titer of 2 × 105 pfu/ml were treated at 37 °C for 30 min with MβCD at concentrations ranging from 0 to 10 mM, respectively. For cholesterol replenishment, after extraction of viral membrane cholesterol using 4 mM MβCD, the virus suspensions were replenished with cholesterol as described above.
2.4. Cell infection analysis
To analyze the effect of cellular cholesterol depletion on virus infection, MβCD-treated or non-treated cell monolayers, respectively, were infected with 100 μl virus dilutions in DMEM at an MOI of 0.001 followed by incubation at 37 °C for 1 h on a 24-well plate. The cells were then covered with 1 ml methylcellulose (1% (w/v) in DMEM) for 36–48 h. For cell infection analysis after cholesterol replenishment, the cholesterol-replenished and non-replenished cell monolayers were washed three times with PBS, and then 100 μl of the virus suspensions with an MOI of 0.001 were applied to the cell monolayers and incubated at 37 °C for 1 h. Subsequently, the cells were washed three times, covered with methylcellulose at 37 °C for 36–48 h and the infection efficiency was determined by microscopic plaque counting.
To analyze infection efficiency after cholesterol depletion from viral membranes, cell monolayers were washed three times with PBS and then incubated with MβCD-treated or non-treated (control) virus suspensions at 37 °C for 1 h, respectively. The inoculums including the control were diluted 103-fold to avoid adverse effects of MβCD on cells. Subsequently, the cells were washed three times and overlaid with methylcellulose at 37 °C for 36–48 h.
To analyze infection efficiency after cholesterol replenishment of the viral envelopes, cell monolayers were washed three times with PBS. Then, 100 μl of the cholesterol-replenished or non-replenished (control) virus dilutions were applied to the cell monolayers and the infection efficiency was determined as described above.
2.5. Determination of cholesterol
To determine cellular cholesterol, confluent monolayers of ST cells grown on six-well plate were treated with various concentrations of MβCD as above. In parallel, confluent ST monolayers were replenished by adding various concentrations of exogenous cholesterol after the treatment with 12 mM MβCD as described above. Following the different treatments, the cell monolayers were washed three times with PBS, trypsinised and centrifuged at 1400 rpm at 4 °C for 10 min. The cells were resuspended in PBS and subjected to cell counting. Equal cell samples (2.6 × 104 cells/sample) were pelleted at 3000 rpm at 4 °C for 5 min. Subsequently, the cellular cholesterol concentrations were determined with Amplex® Red Cholesterol Assay Kit (Molecular Probes, USA), according to the manufacturer's instructions.
To determine viral cholesterol content, 1 ml TGEV particles (2.5 × 107 pfu/ml) were treated with various concentrations of MβCD or with various concentrations of cholesterol after MβCD depletion. Non-treated viruses were used as controls. After centrifugation at 1500 rpm, 4 °C, for 10 min, the viruses were sedimented by ultracentrifugation at 140,000 × g at 4 °C for 1 h. The virus pellets were suspended in 100 μl PBS. Virus suspensions of 75 μl were mixed with an equal volume of lysis buffer, incubated with 150 μl work solution, and subjected to cholesterol concentration determination in triplicates as described above. It should be noted that TGEV was grown in serum-free medium; therefore, cholesterol measurements are not affected by serum cholesterol.
3. Results
3.1. Effect of cellular cholesterol on TGEV infection
To determine the importance of cholesterol-rich microdomains for TGEV infection, we applied MβCD that is commonly used to deplete cholesterol from cellular membranes. As shown in Fig. 1A, MβCD treatment of ST cells resulted in a dose-dependent reduction of the cholesterol content. At 12 mM of the drug, the amount of cholesterol was reduced by about 60%. Cells pretreated with 12 mM MβCD were used to analyze the replenishment of cellular cholesterol by addition of exogenous cholesterol. As shown in Fig. 1B, adding cholesterol in increasing amounts resulted in an increase of the cholesterol content of the cellular membranes. At a concentration of 500 μM, the cholesterol values of the cellular membranes were similar to the values determined prior to MβCD treatment.
Fig. 1.

Depletion and replenishment of cholesterol from the cellular membrane. (A) Effect of MβCD treatment on the cholesterol content of ST cells. After treatment of ST cells with various concentrations of MβCD, the cellular cholesterol content was determined. (B) Effect of addition of exogenous cholesterol to cholesterol-depleted cells on the cholesterol content to ST cells. The recovery of cellular cholesterol was determined after addition of exogenous cholesterol to cells that have been depleted by 12 mM MβCD.
To analyze the effect of cholesterol depletion from the host cells on TGEV infection, MβCD-treated or mock-treated cell monolayers were infected with virus dilutions at an MOI of 0.001. The effect of cholesterol depletion was determined 36–48 h.p.i. by a plaque-reduction assay. As shown in Fig. 2A, the virus infection efficiency was reduced in a dose-dependent fashion. At a concentration of 12 mM, the infection rate was reduced by about 85%. In contrast, the infection of cholesterol-depleted cells by VSV was not affected. To confirm the importance of cholesterol for virus infection, we analyzed the recovery of virus infection when exogenous cholesterol was added to cholesterol-depleted cells. Cells pretreated with 12 mM MβCD were incubated with various cholesterol concentrations and then infected by TGEV. As shown in Fig. 2B, infectivity increased with increasing concentration of cholesterol. At a concentration of 500 μM, infectivity was restored to values that were close to the infectivity determined prior to MβCD treatment. These results confirm the importance of cellular cholesterol for TGEV infection.
Fig. 2.

Effect of cellular cholesterol depletion and replenishment on TGEV infection. (A) Effect of MβCD treatment of cells on the infection by TGEV and VSV, respectively. ST and BHK21 cell monolayers were treated with various concentrations of MβCD; subsequently, cells were infected with TGEV or VSV, respectively. The 100% infectivity values of TGEV and VSV represent average plaque numbers of 160 and 100, respectively. (B) Effect of addition of exogenous cholesterol to cholesterol-depleted cells on the infection by TGEV. ST cell monolayers were treated with 12 mM MβCD and subsequently exogenous cholesterol was added. TGEV was used to infect the monolayers; the 100% infectivity value corresponds to an average plaque number of 100.
3.2. Importance of viral cholesterol for TGEV infection
MβCD was also used to deplete cholesterol from the viral membrane. As shown in Fig. 3A, increasing drug concentrations resulted in a dose-dependent decrease of the cholesterol content. At a concentration of 4 mM MβCD, the amount of cholesterol in the viral membrane was reduced by 75%. When cholesterol-depleted virions were incubated with 500 μM exogenous cholesterol, the cholesterol content of the viral membrane recovered to values measured prior to depletion by MβCD treatment (Fig. 3B).
Fig. 3.
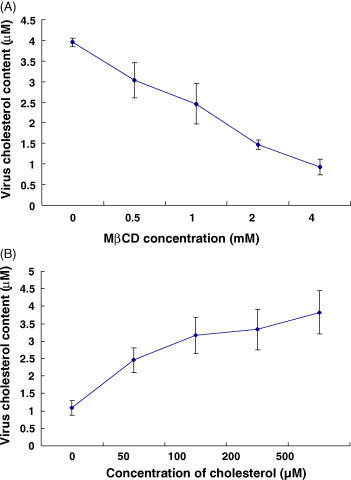
Changes of viral cholesterol content. (A) Effect of MβCD treatment of TGEV on the content of cholesterol in the viral membrane. After the treatment with MβCD at various concentrations, the viral cholesterol content was determined with cholesterol detection kit. (B) Replenishment of cholesterol in the viral membrane by addition of exogenous cholesterol. Viruses treated with 4 mM MβCD were subjected to addition of exogenous cholesterol and the virus pellets were used for cholesterol measurement.
To analyze the effect of cholesterol depletion from the viral membrane on virus infectivity, TGEV and VSV were treated with various concentrations of MβCD, respectively. As shown in Fig. 4A, TGEV was found to be sensitive to MβCD. At a concentration of 4 mM MβCD, infectivity was reduced by about 80%. For comparison, in our analysis we included VSV which buds from the plasma membrane. As reported previously (Imhoff et al., 2007) the infectivity of VSV is reduced only at higher concentrations of MβCD (Fig. 4A). To see whether the effect of cholesterol depletion was reversible, TGEV was treated with 4 mM MβCD followed by the addition of exogenous cholesterol. As shown in Fig. 4B, replenishment of cholesterol resulted in an increase of the infectivity of MβCD-treated TGEV. At a concentration of 500 μM of exogenous cholesterol, infectivity reached 80% of the value determined prior to cholesterol depletion. These results demonstrate that cholesterol in the viral envelope is an important factor for the infectivity of TGEV.
Fig. 4.
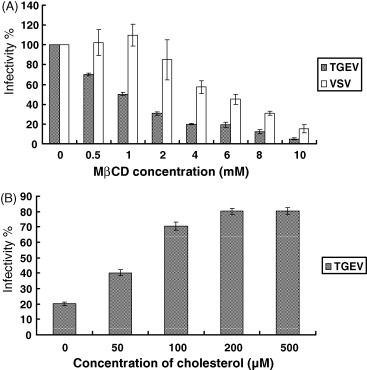
Importance of cholesterol in the viral envelope on the infectivity of TGEV. (A) Effect of MβCD treatment of virions on virus infectivity. TGEV and VSV were treated with 0–10 mM MβCD for 30 min at various concentrations, and the MβCD-treated viruses were employed to infect the cell monolayers. The 100% infectivity values of TGEV and VSV correspond to average plaque numbers of 180 and 270, respectively. (B) Effect of replenishment of cholesterol in the viral membrane on the infectivity of TGEV. Virions were treated with MβCD at a concentration of 4 mM, and replenished with the 0–500 μM exogenous cholesterol for 30 min. The 100% infectivity value corresponds to an average plaque number of 160.
4. Discussion
Lipid rafts are membrane microdomains enriched in sphingolipids and cholesterol. They contain lipids in liquid ordered phase and may correspond to those membrane structures described as detergent-resistant membranes. Apart from various cellular processes, lipid rafts have been reported to play a critical role in different aspects of the virus life cycle such as viral entry, protein transport and targeting, and assembly and budding (Nayak and Hui, 2004). Cholesterol is a characteristic structural component of lipid rafts. Cholesterol depletion may therefore result in disorganization of these membrane microdomains (Scheiffele et al., 1997). The drug methyl-β-cyclodextrin, a cholesterol depleting reagent can reduce the cholesterol content and cause disorganization of lipid rafts efficiently. At the same time, unlike other cholesterol-binding agents that become incorporated into membranes, MβCD is a strictly surface-acting agent and can rapidly remove cholesterol from the plasma membrane.
In this study, we were especially interested in understanding whether cholesterol is required for TGEV infection and if so, whether cholesterol is important as a constituent of the virus, of the host cells or of both. To address this question, we used MβCD as cholesterol depleting agent to treat either TGEV or swine testicle (ST) cells prior to virus infection. Our results show that depletion of cholesterol from either the viral or the cellular membrane resulted in a decrease of the infectivity of TGEV on ST cells. The concentration of MβCD and cholesterol we used in this study do not produce significant adverse effect on cell viability as shown by trypan blue staining (data not shown). The drug concentration and the protocol applied are similar to those described for the analysis of other viruses (Nayak and Hui, 2004, Imhoff et al., 2007). Other members of the family Coronaviridae, MHV, SARS-CoV, HCoV-229E and IBV (Thorp and Gallagher, 2004, Nomura et al., 2004, Li et al., 2007, Imhoff et al., 2007) are sensitive to MβCD treatment of host cells. Therefore, the importance of cholesterol-rich microdomains appears to be a general feature of the entry mechanism coronaviruses have developed. In the case of TGEV, the cholesterol dependence is consistent with the presence of porcine aminopeptidase N in detergent-resistant membrane microdomains. This holds also true for the human coronavirus 229E which interacts with human aminopeptidase N. MHV and SARS-CoV use different receptors, MHVR and ACE2, respectively. Surprisingly, both of these receptors have been reported to be nonraft-proteins (Thorp and Gallagher, 2004, Warner et al., 2005). However, MHVR has been shown to redistribute to some extent into lipid rafts after interaction with MHV (Choi et al., 2005). Thus, cholesterol-rich microdomains may contribute to coronavirus entry either by providing platforms for efficient virus binding to receptors presented in these membrane domains or by recruiting virus-receptor complexes to promote the entry process.
We have shown that cholesterol is important not only in the host cell membrane but also in the viral membrane. This finding may be surprising because coronaviruses mature by a budding process at a pre-Golgi compartment at the transition from the endoplasmic reticulum to the Golgi apparatus (Tooze et al., 1984). At the early compartments of the secretory pathway, the content of cholesterol and sphingolipids is lower compared to the plasma membrane. Therefore, the cholesterol content of coronaviruses is expected to be lower than that of viruses budding from the plasma membrane. Nevertheless, even the membrane of the endoplasmic reticulum has been shown to contain lipid microdomains (Sevlever et al., 1999). At present we do not know how cholesterol in the viral membrane affects virus infectivity. However, our results raise the possibility that lipid microdomains exist in the membrane of coronaviruses. The low concentration of cholesterol may explain that the infectivity of TGEV is affected by concentrations of MβCD that are lower than those that affect infectivity of viruses like HIV and influenza virus. Cholesterol treatment of TGEV without prior MβCD treatment did not affect virus infectivity (data not shown). It will be interesting in future studies to confirm the importance of viral cholesterol for other coronaviruses and to analyze the membrane microdomains in the coronavirus envelope.
Acknowledgements
This work was performed in partial fulfillment of the requirements for the Dr. Vet. Med. degree at University of Veterinary Medicine Hannover, Germany. X.R. received a scholarship from German Academic Exchange Service (DAAD). The technical assistance of Sandra Bauer is gratefully acknowledged. Financial supports to X.R. were from National Natural Science Foundation of China (30700590), Heilongjiang Provincial Science and Technology Department (LC06C01) and Harbin Science and Technology Bureau (2006RFLXN004), and Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (NO706019). The work was also supported by funds from the Deutsche Forschungsgemeinschaft (SFB621) to G.H.
References
- Barman S., Nayak D.P. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J. Virol. 2007;81:12169–12178. doi: 10.1128/JVI.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavari S., Bosio C.M., Wiegand E., Ruthel G., Will A.B., Geisbert T.W., Hevey M., Schmaljohn C., Schmaljohn A., Aman M.J. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Aizaki H., Lai M.M. Murine coronavirus requires lipid rafts for virus entry and cell–cell fusion but not for virus release. J. Virol. 2005;79:9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L’Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevot P., Langlet C., Guo X.J., Bernard A.M., Colard O., Chauvin J.P., Lasserre R., He H.T. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002;21:1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff H., von Messling V., Herrler G., Haas L. Canine distemper virus infection requires cholesterol in the viral envelope. J. Virol. 2007;81:4158–4165. doi: 10.1128/JVI.02647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Reeth K.V., Pensaert M. Porcine respiratory coronavirus: molecular features and virus–host interactions. Vet. Res. 1993;24:125–150. [PubMed] [Google Scholar]
- Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Xiong Y., Silver J. Asymmetric requirement for cholesterol in receptor-bearing but not envelope-bearing membranes for fusion mediated by ecotropic murine leukemia virus. J. Virol. 2002;76:6701–6709. doi: 10.1128/JVI.76.13.6701-6709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D.P., Hui E.K. The role of lipid microdomains in virus biology. Subcell Biochem. 2004;37:443–491. doi: 10.1007/978-1-4757-5806-1_14. [DOI] [PubMed] [Google Scholar]
- Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Wesley R.D. Transmissible gastroenteritis and porcine respiratory coronavirus. In: Straw B.E., D’Allaire S., Mengeling W.L., Taylor D.J., editors. Diseases of Swine. 8th ed. Iowa State University Press; Ames: 1999. pp. 295–325. [Google Scholar]
- Scheiffele P., Roth M.G., Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S., Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Zimmer G., Schröder B., Breves G., Herrler G. Binding of transmissible gastroenteritis coronavirus to brush border membrane sialoglycoproteins. J. Virol. 2003;77:11846–11848. doi: 10.1128/JVI.77.21.11846-11848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever D., Pickett S., Mann K.J., Sambamurti K., Medof M.E., Rosenberry T.L. Glycosylphosphatidylinositol-anchor intermediates associate with triton-insoluble membranes in subcellular compartments that include the endoplasmic reticulum. Biochem. J. 1999;343:627–635. [PMC free article] [PubMed] [Google Scholar]
- Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek C. Coronaviruses: structure and genome expression. J. Gen. Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Suzuki Y. Virus infection and lipid rafts. Biol. Pharm. Bull. 2006;29:1538–1541. doi: 10.1248/bpb.29.1538. [DOI] [PubMed] [Google Scholar]
- Thorp E.B., Gallagher T.M. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac-cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 1984;33:281–293. [PubMed] [Google Scholar]
- Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J. Biol. Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- Winter C., Schwegmann-Wessels C., Cavanagh D., Neumann U., Herrler G. Sialic acid is a receptor determinant for infection of cells by avian Infectious bronchitis virus. J. Gen. Virol. 2006;87:1209–1216. doi: 10.1099/vir.0.81651-0. [DOI] [PubMed] [Google Scholar]


