Highlights
-
•
We review events that led to the re-emergence of PEDV in Europe and its dissemination there.
-
•
Greater heterogeneity of PEDV genotypes identified.
-
•
Updated PEDV phylogeny scheme.
-
•
We discuss features in the biology of PEDV to support its control.
Keywords: PEDV, Control, Diagnosis, Outbreak, Sequence
Abstract
Porcine Epidemic Diarrhea Virus (PEDV) is a member of the genus Alphacoronavirus, in the family Coronaviridae, of the Nidovirales order and outbreaks of porcine epidemic diarrhoea (PED) were first recorded in England in the 1970s. Intriguingly the virus has since successfully made its way around the globe, while seemingly becoming extinct in parts of Europe before its recent return from Northern America. In this review we are re-evaluating the spread of PEDV, its biology and are looking at lessons learnt from both failure and success. While a new analysis of PEDV genomes demonstrates a wider heterogeneity of PEDV than previously anticipated with at least five rather than two genotypes, biological features of the virus and its replication also point towards credible control strategies to limit the impact of this re-emerging virus.
1. Introduction
Porcine Epidemic Diarrhea Virus (PEDV) is an enveloped, positive-sense, single strand RNA virus in the genus Alphacoronavirus, subfamily Coronavirinae, family Coronaviridae, of the Nidovirales order (King et al., 2011). Other recognised species in this genus are the porcine Transmissible Gastroenteritis Virus (TGEV), the human coronaviruses 229E, as well as various bat coronaviruses.
The PEDV genome is approximately 28 kb in length and inside the 5′ and 3′ untranslated regions (UTRs) encodes two overlapping non-structural polyproteins (nsp) ORFs 1a and b; the structural proteins spike (S), envelope (E), membrane (M) and nucleocapsid (N) and an accessory gene ORF3 (Kocherhans et al., 2001). ORF1a produces the replicase polyprotein 1a; ORF 1b extends polyprotein 1a to polyprotein 1ab. Both polyproteins are cleaved to produce the nsp 1-16 (Kocherhans et al., 2001).
The spike protein provides the corona-like appearance in electron micrographs that underlies the name of the viral family and mediates the entrance of virions into the cell. It comprises of five domains: a signal peptide; a S1 region, which facilitates the attachment of virions to cellular receptors; a S2 region, which mediates viral fusion to host cells; a transmembrane domain and a cytoplasmic tail. The S1 domain is further divided into two sub-domains: an N terminal domain and a C terminal domain, both of which may function as receptor binding domains (Li, 2012). Porcine aminopeptidase N (pAPN) is the receptor for PEDV’s entry to host cells (Li et al., 2007). As the major envelope glycoprotein, the spike protein stimulates the induction of neutralising antibodies. Chang et al. (2002) and Sun et al. (2007) reported the CO-26K fragment (aa7-146) and the S1D region (aa636-789), respectively to contain one or more neutralising epitopes of PEDV. Subsequently, motifs within the S1 domain (YSNIGVCK, LQDGQVKI and MQYVYTPTYYML) and at the cytoplasmic tail (GPRLQPY) were suggested as neutralising or receptor binding epitopes (Sun et al., 2008, Cruz et al., 2008, Cao et al., 2015).
PED was first described in England in the 1970s (Wood, 1977) and subsequently emerged across most of Europe, the historic events of which are summarised by Maurice Pensaert in this volume of Virus Research. PED is clinically similar to TGE of pigs mainly in young piglets characterised by watery diarrhoea sometimes preceded by vomiting (Saif, 2011, Saif et al., 2012). While PEDV spread almost globally and reached South East Asia in the 1980s, clinical reports of PED declined in Europe and no cases have been reported from the field in GB since 2002 (Williamson et al., 2013), whereas the virus still persisted in pockets of central Southern and Central Europe such as Italy until recently (Boniotti et al., 2016). Prototypic PED viruses isolated and used for further studies during the initial European epidemic included what was thought at the time to be separate strains CV777 and Br1/87 (Pensaert and de Bouck, 1978, Hofmann and Wyler, 1988, Have et al., 1992, Bridgen et al., 1993). In general, PED outbreaks caused watery diarrhoea without significant mortality in growing pigs and sows and tended to spread rapidly through the herd. While it has been speculated that the appearance of Porcine Respiratory Coronavirus (PRCV), a strain antigentically closely related to TGEV, led to the disappearance of the latter, it has remained mysterious why PEDV has disappeared from countries such as GB, whereby the absence of surveillance programs does not exclude the circulation of milder forms in the past.
With the emergence of variant PEDV strains in China in 2010, its spread into the United States in 2013 (Sun et al., 2012, Stevenson et al., 2013) and in the expectation of a re-emergence of PEDV in Europe we had reasons to revisit our historic knowledge and place it into current perspective.
2. Global emergence & spread
PEDV has been known to circulate in South East Asia since at least 1982; for example in Japan (Takahashi et al., 1983) with further records of the disease in China and Thailand since 2005 and 2007 respectively (Jinghui and Yijing, 2005, Puranaveja et al., 2009). By late summer 2010, severe PED epizootic outbreaks had been detected in China that affected pigs of all ages but more severely the neonates (Li et al., 2012, Sun et al., 2012). These viruses seemed to be geographically restricted in China at first raising questions to which extent a highly virulent form was associated with local conditions, including the widespread circulation of other endemic pig viruses that are known to exacerbate PED, such as PCV-2 (Jung et al., 2006).
In spring 2013 highly virulent Chinese PEDV strains causing watery diarrhoea, vomiting and high morbidity with little loss in weaned pigs but high mortality in neonatal piglets was first observed in Iowa and Indiana, USA (Huang et al., 2013, Stevenson et al., 2013). A full genome sequence was first published from a strain detected in Colorado in June 2013 (Marthaler et al., 2013) and for the purpose of this review, such strains will be referred to as Colorado-like PEDV viruses. In early 2014, a second variant of PEDV strains was discovered in Ohio, USA, showing minimal to no clinical signs at the time and no piglet mortality (Wang et al., 2014a). Compared to other strains circulating in the USA this strain (OH851) showed 99% nucleotide (nt) identity at the full genome level and a 96% nt identity in the complete S gene, but only around 92% identity in the first 1170 nt of the S1 region (Table 1 ). Further to several point mutations, Ohio851 had one insertion and 3 deletions in this area of the genome, which is why such strains are commonly referred to as S-INDEL (insertion/deletion) variant strains. The closer relationship with other Chinese PEDV strains such as strain CH/HBQX/10, pointed towards the fact that these mutations did not occur in the USA, but rather that a second incursion into the USA had occurred (Vlasova et al., 2014, Wang et al., 2014a).
Table 1.
Spike (S) amino acid homology of selected PEDV strains.
 |
The Colorado and Ohio851 strains resemble prototypic variants of US/Chinese PED viruses, and are most different to previous European strains. The Minnesota strain discussed in the text does not discriminate itself significantly from the Colorado strain. Also apparent is the homology between the Ohio851 variant and a strain detected previously in China (CH_HBXQ_10), which is evidence for two incursions into the USA. A) is the full S-protein sequence, B) the S1 region only.
Many further strains of PEDV have since been deposited in GenBank and described in the literature, particularly from North America. Among them another spike gene deletion (PEDV strain Minnesota188), which had a 2 amino acid (aa) deletion and further individual non-synonymous changes in the S2 region, demonstrating the emergence of new strains including such containing deletions from the pool of circulating field viruses (Marthaler et al., 2014). Since this isolate was closely related to the original USA/Colorado/2013 and not suggested to have a significantly altered pathogenicity it should not be considered separately from the clinical or impact perspective. For the purpose of this review we recognize two biological variants of the US/China PEDV viruses (Colorado-like; Ohio851-like) and reserve the term variant to make this distinction, whereby a variant requires a significant degree of genetic change and/or the demonstration of a significant biological change in behaviour. We do not distinguish here between isolates and strains, e.g. when discussing genetic data — although strictly not many isolates have been obtained and lesser strains were maintained and characterized so far.
2.1. Further circulation and initial impact
Subsequent to the initial incursion into the USA, the Colorado-like PED-viruses crossed into Canada and Mexico as neighbouring countries (Vlasova et al., 2014) and further reports described the emergence of either or both PEDV variants in several countries in Asia: for example in October 2013 Japan reported the first outbreak of PED after a period of 7 outbreak-free years, likely imported from the USA (Song et al., 2015) and further outbreaks of the disease were reported in South Korea, Taiwan, Thailand, Philippines, and Vietnam (Chung et al., 2015, Kim et al., 2015, Lin et al., 2014, Temeeyasen et al., 2014). The European Food Safety Authority (EFSA) in their first report on PEDV (EFSA, 2014) has compiled an extensive summary of PEDV detection up until the middle of 2014.
The first impression from a European perspective was that of a highly pathogenic virus and its very efficacious and efficient spread, causing devastating disease in many cases (EFSA, 2014). However, at this point in time it was difficult to assess how the nature of the virus, farming practices including biosecurity and trade practices from farming goods to pork products contributed to the spread.
Multiple investigations and studies were carried out since to investigate the route by which the virus arrived in the USA. Feed was initially suspected, but subsequent investigations failed to show a common ingredient among the farms. In late spring 2014, a Root Cause Investigation Group (RCG) was tasked to identify the most likely mechanisms by which PED reached the US. The investigation concluded that flexible intermediate bulk containers (also known as “feed totes”) provide a means to explain contamination of feed, entry into the USA and dissemination of the infection, compatible with herd investigations and assumptions regarding feed gathered (Scott et al., 2016). This does not, however, explain the local spread such as seen later across Europe, which can include various further mechanisms.
Estimates about the impact on pig industry in the US suggest that at least 7 million pigs died as a consequence of the infection in the first year of the epidemic alone (Waters, 2014) and recent figures show the infection of 2359 premises between June 2014 and March 2016 (USDA, 2016). In mid-2014, the net annual loss for U.S. economy from PED was estimated to range between $900 million and $1.8 billion (Paarlberg, 2014) and could only partially be mitigated by finishing the remaining pigs for longer, since the outbreak impacted not only through high mortality in piglets but also through decreased performance of growing pigs weaned after the outbreak (Alvarez et al., 2015) as well as the impact on sow performance (Olanratmanee et al., 2010, Dastjerdi et al., 2015, Goede and Morrison, 2016, Lin et al., 2016). The full economic impact in the USA has even been further reaching, with effects on meat packers, processors, distributors and retailers due to reduced availability of pork, and on consumers due to the increase in pork prices (Schultz and Tonsor, 2015).
2.2. European incursions, spread, circulating and extinct PEDV strains
Since spring 2014, outbreaks of diarrhoea affecting pigs of all age groups have also been reported in farms in Europe, firstly in Germany (Henniger and Schwarz, 2014, Hanke et al., 2015, Stadler et al., 2015), followed by its neighbouring countries, the Netherlands, Belgium, and Austria (Theuns et al., 2015, Steinrigl et al., 2015), as well as from the more distant Ukraine in summer 2014 (Dastjerdi et al., 2015). However, only the outbreak in the Ukraine was caused by a Colorado-like PEDV strain, whereas all the central European outbreaks were caused by the Ohio851-like variant. This in itself was interesting: whilst the Ukraine virus was at least spreading within the country at the time (Dastjerdi et al., 2015), it seemingly did not spread into neighbouring or central European countries. In contrast the Ohio851 variant spread further into Italy and Portugal (Mesquita et al., 2015, Boniotti et al., 2016). However, as of June 2016 other central and Northern European countries remained completely free (such as Scandinavia) or like France reported only one single outbreak (Grasland et al., 2015).
Overall in Central Europe, as in North America, morbidity and mortality were higher in piglets than in adult animals, sometimes reaching close to 100%. Clinical signs of PED were reported to last from 2 days in individual adult animals to up to 15 weeks in some piglet groups (EFSA, 2016). Where details of the impact of the disease were reported, they included losses directly from piglet mortalities or euthanasia, decreases piglet per sow production and abortions, and reductions in average daily weight gains (EFSA, 2016). However, it is an obvious observation that the overall impact in Europe, as a consequence of pathogenicity and spread, is so far significantly lower than in the USA, which raised questions, which factors contribute to the impact.
Given the range of clinical signs and disease patterns reported for apparently similar strains of PEDV, it is difficult to compare the pathogenicity or virulence of individual PEDV strains on the basis of literature reports. It was therefore desirable to carry out controlled in vivo studies to compare the viruses and evaluate their relative pathogenicity. Experimental infections have recently been carried out using defined virus strains. Further to establishing the proof of principle, most recent studies were able to use a molecular clone derived from strain PC22A to generate highly pathogenic viruses (Beall et al., 2016) and demonstrate the reduced pathogenicity of Ohio851-like variant strains compared to Colorado-like prototype viruses (Chen et al., 2016a). Interestingly though, viraemia levels – observed as a sign of viral spread across the animals – was similar in both variants (Chen et al., 2016a).
Intriguingly, however, not all attempts to infect pigs with PEDV have been successful recently. We aimed to infect 8 week old Large White X Landrace pigs orally in groups of six with either PEDV strain CV777 (group 1) or the Ukraine PEDV strain Poltova (group 2). CV777 virus was available in the form of culture supernatant already stored and the Ukrainian material was in the form of faeces obtained from the diagnostic case reported before (Dastjerdi et al., 2015). The amount of inoculum was adjusted by dilution with PBS on the basis of the Ct value in the real-time PCR (ranging from 13.38 to 16.15), so that each animal was to receive an equivalent amount of virus in a volume of 3 ml. Work was carried out under a Home Office (UK) licence along with approval from the Animal Welfare and Ethical Review Body at APHA and monitoring of clinical signs and sampling was carried out over 21 days. Unfortunately, no specific clinical signs were observed in any of the animals and none of the faecal samples tested were found to be positive for PEDV by qRT-PCR, nor did any of the animals seroconvert.
To account for the fact that the material had been diluted, possibly not representing the viral load available in the field, a second set of animals (4 each at 11 weeks of age) was infected with undiluted material containing 35–50× more virus than at the first attempt, this time absorbed into bread. The higher amount of virus was also to account for the fact that some of the material was possibly degraded through storage. The outcome, however, remained the same with no evidence of infection.
We finally tried to compensate for the fact that both the viruses (problems with shipment and storage) and/or the animals (age) might not have been ideal. For this purpose, two pigs of 6 weeks of age (just weaned and still on a special diet) were infected with the US isolate CO 136-025, kindly provided by Prof D. Werling and Dr R. Noad (RVC, UK). The virus had been grown over serial passage and we obtained cell culture supernatant from two passages (p2 and p3), which were used in the experiment. Tests confirmed the presence of a substantial, albeit lower amount of virus (ct values of 26 and 30). The material had been kept frozen at −80 °C since harvest and was only thawed at the time of use. Unfortunately, this attempt was not successful either.
Through personal communication, we are aware that these were not the only attempts on both sides of the Atlantic failing to establish infection in weaned pigs. We had considered post-weaned pigs advantageous for use in animal studies, as they were unlikely to succumb, consistent with 3Rs policies. Given that finishers and sows (thus older animals) were reported as affected in the field this age group could be considered susceptible.
However, all these incidental results together provide the basis for an alternative hypothesis: in that there was not enough infectious virus left to rescue. This hypothesis would also explain repeated reports from the field that back feeding of faecal material to sows failed to demonstrate a significant effect.
In contrast to other enteric viruses (e.g. Norovirus, Rotavirus, Hepatitis E virus), Coronaviruses and thus PEDV are enveloped viruses, which have a low resistance to environmental or chemical challenge. It may thus well be that PEDV stability is indeed limited and the virus cannot resist challenges such as those from the proteases present in faeces. Thus if the diarrhoeic material from clinical cases is not treated appropriately (e.g. immediate freezing at ultra-low temperatures or drying) the virus will lose its infectivity beyond recovery in due course. On the other hand, a recent observation indicated that weaned pigs require an around 1000× higher dose than non-weaned pigs for successful infection (Thomas et al., 2015) and recent publications also described the failure to infect sows, while managing to infect piglets (Goede et al., 2015, X. Liu et al., 2015). Together these observations further highlight that the spread of PEDV in the field is proportional to, if not dependent on, younger animals being involved.
Furthermore, we noted in the literature that those groups that succeeded in infecting pigs with PEDV have often used cell bound material from in vivo or in vitro propagation of the virus. This is mirrored by information from the field that minced intestines are far superior as material for back feeding in attempts to create herd immunity. While our results could be dismissed as a lack of opportunity to establish the viral load, we think that they rather reflect particular requirements of PEDV before reaching the target cells, i.e. the virus needs to be protected. In vivo, and for example during diarrhoea, parts of the mucosal cell layer are contained in the faeces, providing protection for PEDV. Those cells are however, subject to damage by many procedures that preceded our infection (transport, storage, harvesting virus from supernatant) that was mimicking the natural route of infection, which in weaned pigs includes a passage through the stomach. It has to be noted that most of the above-mentioned, successful infections use orogastric inoculation as a means to ensure reproducible infection, which is, however, not reflective of the field situation.
Another way of assessing the threat of emerging variants from the global PEDV circulation was to assess strain differences between known strains such as CV777, review our historical archive of strains and analyse the heterogeneity of PEDV in field material.
A 15 year analysis of Korean PEDV strains observed little genetic variation (Kim et al., 2015), which demonstrates a rather slow evolution and maintenance of fitness by accruing recombination and/or mutations. This supports the idea of high fidelity replication in coronaviruses due to the presence of 3′ to 5′ proofreading endonuclease activity, which maintains the stability of the viral genome (Smith and Denison, 2013). Similarly, CV777 and material thereof that was for almost a decade (until 1987) passaged in pigs in GB (at APHA) before being sent to DTU, Denmark, where it was further passage twice in pigs before being isolated as Br1/87 (Have et al., 1992, Bridgen et al., 1993), demonstrate only few changes over the decades of maintenance in vivo and in vitro (Table 2 ).
Table 2.
Spike (S) amino acid homology of selected isolates of PEDV CV777.
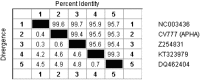 |
Strains of CV777 were maintained in various labs around the globe, passaged partially in vivo and in vitro as discussed in the text. The original CV777 (GenBank AccNo NC_003436.1) and its derivatives maintained through sequential passage in pigs or Vero cells (CV777 APHA and Br1/87/GenBank AccNo Z254831) are almost identical, demonstrating their common origin. Only the presumed Chinese CV777 vaccine strain (GenBank AccNo KT323979, for which the authors could not find further history), whose S-protein is in fact almost identical to the Korean strain DR13 (GenBank AccNo DQ462404) is different, raising questions about the provenance and relation to this strain.
While the genetic drift of Coronaviruses is relatively slow, two events need to be accounted for. Firstly recombination and deletion events across the genome (not only in the S gene) contribute significantly to the overall evolution of PEDV (S. Park et al., 2014, Masuda et al., 2015, Jarvis et al., 2016). While most of these recombination were with other PEDV strains (Tian et al., 2014, Li et al., 2016), recent reports from Italy and Germany highlighted the ability of PEDV to exchange sequences with other Coronaviruses, here TGEV (Boniotti et al., 2016, Akimkin et al., 2016), leading to the creation of novel alpha-Coronavirus, tentatively named swine enteric Coronavirus (SeCoV), not further considered here since its backbone is mainly TGEV rather than PEDV.
Secondly, the scale and the size of the outbreaks in modern pig production units cannot be regarded as one in vivo passage per outbreak. Rather, upon the first incursion the virus undertakes some (few) passages while spreading across the unit, but subsequently leads to a mass production and secretion of virus from one generation of piglets to the next as long as the virus is not controlled, which in some cases was for months, creating a cloud of viruses. When further analysing a single NGS sample from the Ukraine outbreak, that delivered the consensus sequence published (Dastjerdi et al., 2015) we found several areas of mutational hot-spots in, but not exclusively limited to the S gene (e.g. positions 1312–1382, 1782–1822, 1922–1972, 2022–2072, 2222–2272, 2822–2852, 3022–3072, 3532–3572, 3672–3722) with evidence for 29 SNPs therein, each underpinned by several reads, clearly indicating a slow but steady evolution during such outbreaks.
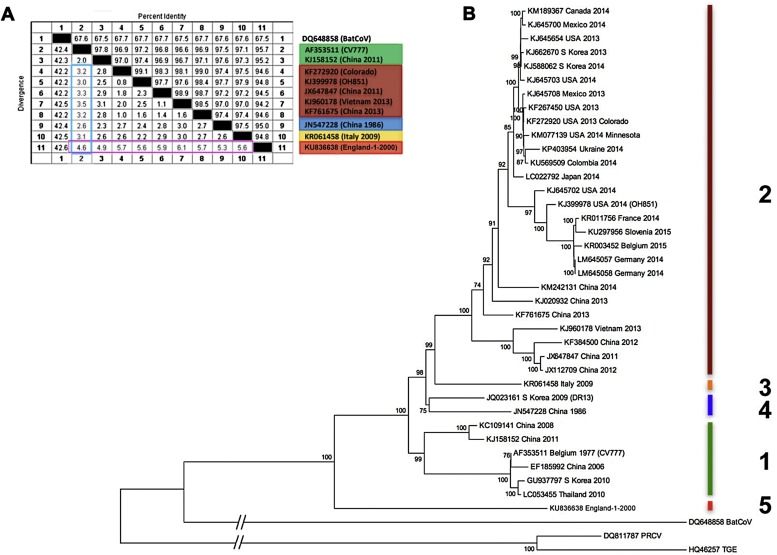
While most of the historic material held in our storage is from earlier passages of CV777, we also had material from one of the last GB PEDV cases in the year 2000 available for analysis (Steinbach et al., 2016). This PEDV strain, termed England-1-2000, is indeed significantly different not only to CV777, but also to all other previously known strains and can be seen as a prototype of a further genotype of PEDV (Fig. 1 ). This and other recently identified PEDV strains, such as the PEDV strain circulating in Italy in 2009 (Boniotti et al., 2016) also provide the basis for an update of the previously proposed nomenclature of PEDV genotypes (Huang et al., 2013), whereby Genotype 1 is formed through a distinct clade comprising the prototype strain CV777 and related strains circulating in Asia until recently at least, but also a second group of viruses from Asia, which points towards an older ancestor of this genotype. Genotype 2 has various clades arising, which are statistically fully supported by high bootstrap values, amongst them individual strains from China that are not closely related to the highly pathogenic strains. To which extend these subgroups in both Genotype 1 and 2 will prevail is not certain, as they are likely many PEDV strains not fully sequenced yet. We are thus not favouring any further division — also since these are currently not underpinned by antigenetic or biological differences. However, the strain from Italy mentioned above is succinctly different to both genotypes 1 and 2 so that it warrants to be kept separate and by genetic distance would be the prototype of genotype 3. We see not merit in calling a genotype R (recombinant) since recombination between strains is a common event among Coronaviruses. It thus seems more meritorious to accept this clade as genotype 4, being similarly distant from the others. Finally the strain England-1-2000 is significantly different to all other PEDV strains known so far and can be seen as the prototype of Genotype 5 (Fig. 1).
Fig. 1.
Phylogenetic analysis of 40 representative PEDV full genome RNA sequences. The evolutionary history was inferred by using the Neighbor-Joining method, as described by Huang et al., 2013. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013). Accession numbers, country and year of isolation are indicated, as provided by GenBank and the references therein. The resulting genotypes are numbered and colour-coded. A) Percentage identity/differences across the different genotypes. The genetic differences between genotypes are at least 2.5% as highlighted in the light blue box using the prototype PEDV strain CV777 as reference. This table also demonstrates that PEDV England-1-2000 (Genotype 5) is more diverse to any other genotype than these are to each other (>4.5%, pink box). B) The tree is drawn to scale, except for the out-groups bat coronavirus (BatCoV), porcine respiratory coronavirus (PRCV) and transmissible gastroenteritis virus (TGEV), which are shown for reference. Branch lengths other than the out-groups reflect the number of substitutions per site. The percentage of trees in which the associated taxa clustered together is shown next to the branches for values greater than 70%. Genotypes 1 and 2 are as inferred by Huang et al. (2013); Genotype 3 is represented by the recently sequenced Italian strain from 2009, while Genotype 4 is represented by two strains derived from recombination thus previously termed R (Huang et al., 2013). Genotype 5 is represented by the strain England-1-2000, the most distinct PEDV strain discovered so far.
To which extent the genetic variation limits the immunity conferred by infection or vaccination remains to be determined. Antibodies (Abs) are most important to protect newborn piglets in particular, and maternally-derived, colostral-transferred Abs that neutralise PEDV (neutralising Abs, nAbs) confer protection to newborn piglets (Song et al., 2015). However, not only has the efficiency of existing live-attenuated vaccines been discussed critically (Song et al., 2015), there is also evidence that vaccine strains might escape and further evolve, not least through recombination with other field strains (Song and Park, 2012). Furthermore, it has been demonstrated that genetic variability in the spike protein not only affects the pathogenicity as discussed above, but also occurs within areas correlated to neutralisation, specifically the CO-26K fragment (Song et al., 2015). Here, further studies are required to determine if particular mutations occurring within the spike protein have a significant impact upon the binding of nAbs, limiting cross-protection between strains in particular.
3. Challenges for the control of PEDV
PEDV cannot be diagnosed on the basis of clinical findings — not least since there are far too many similar Coronavirus infections. The early identification of PEDV was carried out through electron microscopy (Pensaert and de Bouck, 1978) before the virus had even been isolated.
While virus isolation is no longer the gold standard for disease confirmation, it remains an important tool for studying strains to determine the existence of biological variants. However, PEDV is notoriously difficult to isolate (Chen et al., 2014) and its propagation causes specific challenges.
The first adaptation of PEDV to enable growth in Vero cells by serial passage was reported in 1988 and the addition of trypsin to the cell culture medium has since been found to be essential for virus isolation and passage (Hofmann and Wyler, 1988, Y. Wang et al., 2015). This trypsin-dependence, which seemingly mimics the situation in the small intestines, has been the subject of several subsequent studies that found it to be critical for the cleavage of the spike (S) protein which mediates activation of membrane fusion (Park et al., 2011, Wicht et al., 2014). In addition, trypsin is required for virus-induced haemagglutination (Park et al., 2010), and also for cell-to-cell fusion and syncytia formation, which precedes the lytic cytopathogenic effect (Hofmann and Wyler, 1988, Wicht et al., 2014). Thus, trypsin is required for multi-cycle replication, i.e. without proteases clusters of virions were retained on the cell surface (Shirato et al., 2011) hence the maintenance of cell cultures during infection becomes more challenging.
Pig intestinal epithelial cells (IEC), representing a natural target cell, could be infected with PEDV (CV777), but interestingly were less susceptible than green monkey Vero E6 cells (Cong et al., 2015). Furthermore, PEDV has been shown to infect a range of other cells, such as Vero CCL-81, human liver (Huh-7), human lung (MRC-5), porcine kidney (PK-15), swine testis (ST), as well as bat lung (Tb1-Lu) cells (C. Liu et al., 2015). These studies were also able to confirm earlier reports that aminopeptidase N (APN), CD13, from both porcine and other species, acts as the cellular receptor for PEDV that upon transfection can render non-permissive cells (such as MDCK) permissive and that N-acetylneuraminic acid (Neu5Ac) is a co-receptor, such as described for other alpha-Coronaviruses (Li et al., 2007, Cong et al., 2015, C. Liu et al., 2015). However, as PEDV ultimately enters via clathrin-mediated endocytosis, independent of caveolae-coated pit assembly (J.E. Park et al., 2014), other receptors and co-receptors are conceivable, such as heparan-sulfate (Huan et al., 2015), which is widely expressed on macrophages for example and PEDV has been shown to infect porcine alveolar macrophages in vitro and in vivo, which may be a secondary target upon the well described viraemia (Park and Shin, 2014, Chen et al., 2016a).
It is worth noting, however, that there were significant differences between the levels of PEDV protein expression between susceptible cell lines mentioned above. ST and PK-15 cells for example showed only a very limited infection at low infection dose with slight increase in MRC-5 and Tb1-Lu, while Vero CCL-81 and Huh-7 cells were seemingly comparable in their infection (C. Liu et al., 2015). This is in line with Cong et al. (2015) demonstrating the need for a much higher moi10 to be used for an efficient infection of IEC. It also has to be taken into account that some reports (such as C. Liu et al., 2015) only investigate the uptake of PEDV and protein expression, not the complete replication, i.e. by determining the resulting titres. Thus it is quite possible that some cells rather display an abortive infection cycle than promoting virus production. The impact of viral strain variation has not been much further evaluated so far, but it has been suggested that primary isolates might grow preferably in IEC (Cong et al., 2015).
In our attempt to rescue the Ukrainian PEDV strain, we reviewed several components of the original Vero cell protocol as the combination of trypsin, yeast extract and tryptose phosphate broth seemed a very unlikely combination required, with no explanation provided for the latter two components (Hofmann and Wyler, 1988). While trypsin was required to increase the resulting viral titres, it could be abandoned after infection, whereas yeast extract was required for cell viability when trypsin was added. Tryptose phosphate broth on the other hand increased the slightly detrimental effect of trypsin to the cell culture, but its absence also correlated with a decrease in cytopathogenic effect (cpe). There are, however, alternatives on the market now, such as the ultra-MDCK medium (Lonza), which did not require any other additives than trypsin and antibiotics and resulted in similar viral titres, albeit slightly smaller sized plaques compared to the standard medium (data not shown).
Intriguingly, there are at least three clones of African green monkey (Chlorocebus sabaeus) kidney (Vero) cells, which have been used for PEDV infections: the original Vero cell line isolated in 1962 (ATCC CCL-81) has been widely used (e.g. Hofmann and Wyler, 1988, Wicht et al., 2014, Y. Wang et al., 2015, Chen et al., 2014, C. Liu et al., 2015). Vero 76 cells were derived from these in 1968 and have rarely been used for PEDV propagation (Okda et al., 2015). Vero E6 (also referred to as Vero C1008) is a clone of Vero 76 cells, derived in 1979, also successfully used for PEDV culture (Cong et al., 2015). The two subclones of the original Vero cell line are slower-growing cells, which exhibit some degree of contact inhibition (according to the ATCC website). The differences between Vero CCL-81 and Vero E6 in PEDV infection remain unclear, but in our hands Vero CCL-81 were by far superior to E6 in propagating CV777 to a lytic replication.
The reason for Vero cells being uniquely superior to other cells have not been full elucidated and it has mainly been suggested that receptor density of CD13 plays a key role, since CD13 overexpression rendered ST cells more susceptible to PEDV infection (Nam and Lee, 2010). Another key feature of Vero cells, however, is their inability to produce type I interferon (IFN-I), which might limit coronavirus infection (Desmyter et al., 1968). To test the impact IFN deficiency has on PEDV replication we used a PK-15 line stably transfected with CSFn-pro (kindly provided by Dr S.A. La Rocca, APHA), a protein known to inhibit IFN production if overexpressed. There was, however, no difference between the PK15 cells and the PK15n-pro cells and we are able to confirm that PK-15 are relatively refractory to Vero cells, but not resistant, requiring a 10× higher concentration than Vero cells to propagate the virus (data not shown), which led the original isolation team to conclude that PK-15 were non-permissive (Hofmann and Wyler, 1988). However, while PK15 became infected at high concentration and developed a cpe we did not manage to rescue virus from the supernatant. Also when reviewing the Vero cell replication we had noted that a virus titres obtained from serial titration was not reproducible when trying to plate out a distinct number of plaque forming units.
Whilst problems to isolate the virus were traditionally attributed to faecal inoculums and maintenance of the cells with trypsin containing medium, our results are in line with the general observation by both our US and European colleagues that the isolation and maintenance of PEDV is extremely challenging. In one recent study 68 attempts to rescue a PEDV S-INDEL-variant strain from clinical material failed and virus isolation was only successful from small intestine homogenates or cecum contents (Chen et al., 2016b). This suggests that the key to successful PEDV isolation lies in a high concentration of the virus in the sample as well as immediate virus isolation from fresh samples, which supports our earlier hypothesis that viable virus requires protection and that upon transport and storage the amount of viable virus is often significantly reduced, thereby emphasizing the relatively low tenacity of PEDV (Hofmann and Wyler, 1989, Jung and Saif, 2015, Quist-Rybachuk et al., 2015), which does not necessarily affect the detection of viral RNA by RT-PCR (Jung and Chae, 2004), but neither does the successful application of disinfectants (Bowman et al., 2015).
Since virus isolation as a reliable routine method for the detection of PEDV is not an option, other tests are more important. Several assays have been developed for the detection of the virus. Recently, antigen-capture ELISA’s based on the monoclonal antibody 5D7 were suggested to have a sensitivity and specificity comparable to RT-PCR at detecting clinical disease (Z. Wang et al., 2015). Lateral flow pen-side devices are also available to confirm clinical suspicion of PEDV where rapid access to a diagnostic laboratory is limited, and were used for example to confirm suspicion of PEDV in Ukraine (Dastjerdi et al., 2015).
Detection of the viral RNA through RT-PCR, however, has become the gold standard for PEDV detection. Several of such PCR assays including real-time (qPCR) assays have been described in the literature (e.g. Kim et al., 2000, Song and Park, 2012) and commercial test kits have been developed after the outbreak in the USA not least. An interesting concept thereby is the detection of several enteric porcine viruses, or different porcine Coronaviruses, as now exist with PEDV, TGEV, SeCoV and the Delta-Coronavirus, depending on geographical requirements (Kim et al., 2007, Ben Salem et al., 2010). From our perspective, there seems to be little merit, however, in trying to discriminate Colorado-like and Ohio-like strains or recent and classical PEDV variants (Wang et al., 2014b, Zhao et al., 2014), as there is no difference for the control arising. Insulated isothermal amplification systems do not offer the ability to multiplex, but might provide the opportunity to deploy tests in the field in countries where laboratories are not easily accessible (Zhang et al., 2016). Importantly, it has to be taken into consideration that some of the early PCR primers, designed on the available virus sequences at the time, may no longer be suitable for detection of the new virus variants.
Considering the high virus load excreted by acutely affected pigs a range of qPCR assays were feasible to detect disease. It needs to be considered, though, that testing in an outbreak might also include environmental sampling to trace the origin of the outbreak. Also as shedding in older animals has been demonstrated to occur for quite some time after infection (Crawford et al., 2015, Madson et al., 2014), it has to be considered that sensitive qPCRs might be required to detect carriers of infection, such as relevant for trade and tracing purposes. For this purpose, test sensitivity needs to be a focus of the analysis (Miller et al., 2016).
In order to trace infections and to allow for biosecurity and control measures to be implemented, it is also helpful to determine the genetic composition of PEDV from each outbreak. While traditionally PCRs in combination with Sanger sequencing would have been used, next generation sequencing (NGS) has recently been applied extensively (e.g. Dastjerdi et al., 2015; Hanke et al., 2015) and can be used to determine recombination events as seen in Italy alike. The widely used Illumina or IonTorrent technologies, however, are still prohibitively expensive to make this approach affordable. Latest technologies, such as the MINIon, however, could provide a breakthrough for the wider applicability of sequencing technologies into the control toolbox.
In order to assess the on-going situation in Europe it may be of value to implement serological testing, e.g. for confirming freedom from infection and to enhance trade of live pigs.
While variation in the S glycoprotein has been related to attenuation in vivo and growth adaptation in vitro (Sato et al., 2011, Chen et al., 2015), there appears to be only a single PEDV serotype to date. Thus virus neutralisation (VN) as a well-characterised method is feasible, but is comparatively demanding due to the cell culture requirements discussed above and time-consuming (Oh et al., 2005). However, the sensitivity of the VN allows for the detection of PEDV antibodies as early as 7 dpi (Thomas et al., 2015). An adaptation of the assay, as Fluorescent Focus Neutralisation (FFN) assay, is a more rapid assay measuring infection through labelling infected cells rather than waiting for cpe (Okda et al., 2015).
To screen larger herds, though, high-throughput technologies, such as ELISAs are more appropriate. The first ELISA described for PEDV used cell-culture grown PEDV as antigen and was considered more specific and sensitive test than indirect immunofluorescence at the time (Hofmann and Wyler, 1990). Immunoperoxidase monolayer assay (IPMA), however, can detect the presence of the virus as early as 6dpi (Z. Wang et al., 2015) and offers the ability to verify the result not only by quantity (titre), but also be quality (specificity of the staining). While earlier indirect ELISAs were based on whole virus preparations (Knuchel et al., 1992, Oh et al., 2005), more recently developed indirect ELISA’s are based on recombinant forms of viral membrane, N or S proteins (Fan et al., 2015, Hou et al., 2007, Li et al., 2015). Alternatively blocking ELISA’s are used and have been postulated to have greater levels of specificity and sensitivity when compared with other assays (van Nieuwstadt and Zetstra, 1991, Carvajal et al., 1995). To bridge the contrasting requirements for high specificity and high sensitivity has always been a challenge for ELISAs and unfortunately none of the ELISAs has so far been further validated, e.g. against the ISO standard 17025 and our experience so far suggests that neither blocking nor indirect ELISAs are matching requirements for single animal analysis.
Fluorescent microsphere immunoassay (FMIA) or Luminex assays promise simpler setup with the ability to detect several pathogens simultaneously and a recently developed PEDV assay showed the highest specificity and was able to detect antibodies earlier than the ELISAs (Okda et al., 2015).
4. Conclusions
Despite a considerable amount of research not least in recent years, PEDV remains an enigmatic virus with complex requirements for replication that render it difficult to draw conclusions that can be translated from the laboratory to the field. International collaboration has been a key factor for progress not least in Europe, but is limited on a global scale with too few funding opportunities for diseases such as PEDV that are not recognised priorities, which contribute to the vulnerability towards globally emerging viruses.
Fortunately, the re-emergence of PEDV in Europe has not had similar devastating effects as in the US or China, but its economic impact and potential must not be underestimated (EFSA, 2016). With rapid technologies for the detection of PEDV in place, effective control measures can be designed and various pieces of evidence discussed above, such as the limited spread in Europe, the low tenacity of the virus and the effective measures for disinfection support the Canadian experience i.e. strict biosecurity measures are able to control outbreaks (Ménard, 2015).
Consequently, the biggest challenges in the re-emergence of PEDV seem to be associated with pig producing practices, many of which are not common in central Europe. One way by which PEDV overcomes the limitation of its low tenacity is the amount of virus produced during an outbreak. Taking the qPCR values into account PEDV is more than 1000 and possible up to 100,000 fold more concentrated in pig faeces than other pig viruses such as PRRSV in other pig secretions (such as saliva for PRRSV). This, plus the scale of diarrhoea per piglet plus the number of piglets per sow (with a dissemination rate close to 100%) and the scale of some pig production units, where thousands of piglets are produced per week (Dastjerdi et al., 2015) created an almost unimaginable amount of virus under some circumstances, where even a high dilution of starting material still suffices to transmit virus effectively — including to older pigs which are more resistant. Even the possibility of airborne spread to close-by farms has to be considered as a route of virus transmission under those circumstances (Alonso et al., 2014, Alonso et al., 2015, Beam et al., 2015; Dastjerdi et al., 2015). Other routes of transmission between close by farms include contaminated trucks (Lowe et al., 2014), equipment, or people and it has been determined that the risk is associated with proximity to an infected farm, which in turn correlates with the time the virus maintains its viability (Sasaki et al., 2016).
Best practice in preparation of an incursion into countries with a naïve population, such as the UK remains to focus on horizon scanning of suspect cases and biosecurity practises to enable local control, in particular in the absence of a registered vaccine that could be of additional assistance, hence a key in this concept is the ability of highly sensitive tests to detect the low level shedding in pigs that do not succumb as it might occur for a prolonged period.
Acknowledgements
Our work on PEDV was co-funded by Defra, Scottish and Welsh government project ED1200 (Scanning surveillance for Pig Diseases in England and Wales), the Agriculture and Horticulture Development Board (AHDB) Pork and APHA. We are grateful to members of our teams particularly the Mammalian Virology Investigation Unit (MVIU) at APHA for providing support and to members of the CoVetLab group (DTU, Copenhagen; CVI, Lelystad; ANSES, France; SVA, Uppsala), associated teams from Germany (FLI, Riems) and Italy (IZLER, Brescia) and Dr Ian Brown APHA for helpful discussion.
References
- Akimkin V., Beer M., Blome S., Hanke D., Hoper D., Jenckel M., Pohlmann A. New chimeric porcine Coronavirus in swine feces, Germany, 2012. Emerg. Infect. Dis. 2016 doi: 10.3201/eid2207.160179. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C., Goede D.P., Morrison R.B., Davies P.R., Rovira A., Marthaler D.G., Torremorell M. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet. Res. 2014;45:73. doi: 10.1186/s13567-014-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C., Raynor P.C., Davies P.R., Torremorell M. Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS One. 2015;10:e0135675. doi: 10.1371/journal.pone.0135675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J., Sarradell J., Morrison R., Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS One. 2015;10(3):e0120532. doi: 10.1371/journal.pone.0120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall A., Yount B., Lin C.M., Hou Y., Wang Q., Saif L., Baric R. Characterization of a pathogenic full-length cDNA clone and transmission model for porcine epidemic diarrhea virus strain PC22A. mBio. 2016;7:e01451–01415. doi: 10.1128/mBio.01451-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam A., Goede D., Fox A., McCool M.J., Wall G., Haley C., Morrison R. A porcine epidemic diarrhea virus outbreak in one geographic region of the United States: descriptive epidemiology and investigation of the possibility of airborne virus spread. PLoS One. 2015;10:e0144818. doi: 10.1371/journal.pone.0144818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salem A.N., Chupin Sergei A., Bjadovskaya Olga P., Andreeva Olga G., Mahjoub A., Prokhvatilova Larissa B. Multiplex nested RT-PCR for the detection of porcine enteric viruses. J. Virol. Methods. 2010;165:283–293. doi: 10.1016/j.jviromet.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniotti M.B., Papetti A., Lavazza A., Alborali G., Sozzi E., Chiapponi C., Faccini S., Bonilauri P., Cordioli P., Marthaler D. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric Coronavirus, Italy. Emerg. Infect. Dis. 2016;22:83–87. doi: 10.3201/eid2201.150544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A.S., Nolting J.M., Nelson S.W., Bliss N., Stull J.W., Wang Q., Premanandan C. Effects of disinfection on the molecular detection of porcine epidemic diarrhea virus. Vet. Microbiol. 2015;179:213–218. doi: 10.1016/j.vetmic.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen A., Duarte M., Tobler K., Laude H., Ackermann M. Sequence determination of the nucleocapsid protein gene of the porcine epidemic diarrhoea virus confirms that this virus is a coronavirus related to human coronavirus 229E and porcine transmissible gastroenteritis virus. J. Gen. Virol. 1993;74(Pt. 9):1795–1804. doi: 10.1099/0022-1317-74-9-1795. [DOI] [PubMed] [Google Scholar]
- Liu C. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015;89(11):6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Ge X., Gao Y., Zarlenga D.S., Wang K., Li X., Qin Z., Yin X., Liu J., Ren X., Li G. Putative phage-display epitopes of the porcine epidemic diarrhea virus S1 protein and their anti-viral activity. Virus Genes. 2015;51:217–224. doi: 10.1007/s11262-015-1234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal A. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J. Vet. Diagn. Invest. 1995;7(1):60–64. doi: 10.1177/104063879500700109. [DOI] [PubMed] [Google Scholar]
- Chang S.H., Bae J.L., Kang T.J., Kim J., Chung G.H., Lim C.W., Laude H., Yang M.S., Jang Y.S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cells. 2002;14(October (2)):295–299. [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52(January (1)):234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhu Y., Wu M., Ku X., Ye S., Li Z., Guo X., He Q. Comparative genomic analysis of classical and variant virulent parental/attenuated strains of porcine epidemic diarrhea virus. Viruses. 2015;7:5525–5538. doi: 10.3390/v7102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Gauger P.C., Stafne M.R., Thomas J.T., Madson D.M., Huang H., Zheng Y., Li G., Zhang J. Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J. Gen. Virol. 2016;97:1107–1121. doi: 10.1099/jgv.0.000419. [DOI] [PubMed] [Google Scholar]
- Chen Q., Thomas J.T., Giménez-Lirola L.G., Hardham J.M., Gao Q., Gerber P.F., Opriessnig T., Zheng Y., Li G., Gauger P.C., Madson D.M., Magstadt D.R., Zhang J. Evaluation of serological cross-reactivity and cross-neutralization between the United States porcine epidemic diarrhea virus prototype and S-INDEL-variant strains. BMC Vet. Res. 2016;12(April (1)):70. doi: 10.1186/s12917-016-0697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.C., Nguyen V.G., Moon H.J., Lee J.H., Park S.J., Lee G.E., Kim H.K., Noh Y.S., Lee C.H., Goede D., Park B.K. Isolation of porcine epidemic diarrhea virus during outbreaks in South Korea, 2013–2014. Emerg. Infect. Dis. 2015;21(12):2238–2240. doi: 10.3201/eid2112.150437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Li X., Bai Y., Lv X., Herrler G., Enjuanes L., Zhou X., Qu B., Meng F., Cong C., Ren X., Li G. Porcine aminopeptidase N mediated polarized infection by porcine epidemic diarrhea virus in target cells. Virology. 2015;478:1–8. doi: 10.1016/j.virol.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K., Lager K., Miller L., Opriessnig T., Gerber P., Hesse R. Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Vet. Res. 2015;46:49. doi: 10.1186/s13567-015-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D.J., Kim C.J., Shin H.J. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize Porcine epidemic diarrhea virus. Virus Res. 2008;132(March (1–2)):192–196. doi: 10.1016/j.virusres.2007.10.015. Epub 2007 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi A., Carr J., Ellis R.J., Steinbach F., Williamson S. Porcine Epidemic Diarrhea Virus among Farmed Pigs, Ukraine. Emerging Infect. Dis. 2015;21:2235–2237. doi: 10.3201/eid2112.150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter J., Melnick J.L., Rawls W.E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero) J. Virol. 1968;2:955–961. doi: 10.1128/jvi.2.10.955-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Opinion on porcine epidemic diarrhoea and emerging pig deltacoronavirus. EFSA J. 2014;12(10):3877. doi: 10.2903/j.efsa.2014.3877. 68 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Scientific Report on the collection and review of updated epidemiological data on porcine epidemic diarrhoea. EFSA J. 2016;14(2):4375. doi: 10.2903/j.efsa.2016.4375. 52 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J.H. Development of an enzyme-linked immunosorbent assay for the monitoring and surveillance of antibodies to porcine epidemic diarrhea virus based on a recombinant membrane protein. J. Virol. Methods. 2015;225:90–94. doi: 10.1016/j.jviromet.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede D., Morrison R.B. Production impact & time to stability in sow herds infected with porcine epidemic diarrhea virus (PEDV) Prev. Vet. Med. 2016;123:202–207. doi: 10.1016/j.prevetmed.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Goede D., Murtaugh M.P., Nerem J., Yeske P., Rossow K., Morrison R. Previous infection of sows with a mild strain of porcine epidemic diarrhea virus confers protection against infection with a severe strain. Vet. Microbiol. 2015;176(1/2):161–164. doi: 10.1016/j.vetmic.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Grasland B., Bigault L., Bernard C., Quenault H., Toulouse O., Fablet C., Rose N., Touzain F., Blanchard Y. Complete genome sequence of a porcine epidemic diarrhea S gene indel strain isolated in France in December 2014. Genome Announc. 2015;3(June (3)) doi: 10.1128/genomeA.00535-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D., Jenckel M., Petrov A., Ritzmann M., Stadler J., Akimkin V., Blome S., Pohlmann A., Schirrmeier H., Beer M., Hoper D. Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerg. Infect. Dis. 2015;21:493–496. doi: 10.3201/eid2103.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Have P., Moving V., Svansson V., Uttenthal A., Bloch B. Coronavirus infection in mink (Mustela vison). Serological evidence of infection with a coronavirus related to transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Vet. Microbiol. 1992;31:1–10. doi: 10.1016/0378-1135(92)90135-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henniger T., Schwarz B.A. Porcine epidemic diarrhoea-neuausbrüche in deutschen Mastschweinebeständen. Tierärztliche Umschau. 2014;69:394–397. [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26(11):2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Quantitation, biological and physicochemical properties of cell culture-adapted porcine epidemic diarrhea coronavirus (PEDV) Vet. Microbiol. 1989;20:131–142. doi: 10.1016/0378-1135(89)90036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Vet. Microbiol. 1990;21(3):263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X.L. Development and evaluation of enzyme-linked immunosorbent assay based on recombinant nucleocapsid protein for detection of porcine epidemic diarrhea (PEDV) antibodies. Vet. Microbiol. 2007;123(1–3):86–92. doi: 10.1016/j.vetmic.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan C.C. Porcine epidemic diarrhea virus uses cell-surface heparan sulfate as an attachment factor. Arch. Virol. 2015;160(7):1621–1628. doi: 10.1007/s00705-015-2408-0. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Piñeyro P., Li L., Fang L., Kiehne R. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio. 2013;4(5) doi: 10.1128/mBio.00737-13. 00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014;191:21–29. doi: 10.1016/j.virusres.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M.C., Lam H.C., Zhang Y., Wang L., Hesse R.A., Hause B.M., Vlasova A., Wang Q., Zhang J., Nelson M.I., Murtaugh M.P., Marthaler D. Genomic and evolutionary inferences between American and global strains of porcine epidemic diarrhea virus. Prev. Vet. Med. 2016;123(January):175–184. doi: 10.1016/j.prevetmed.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinghui F., Yijing L. Cloning and sequence analysis of the M gene of porcine epidemic diarrhea virus LJB/03. Virus Genes. 2005;30(January (1)):69–73. doi: 10.1007/s11262-004-4583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Chae C. Effect of temperature on the detection of porcine epidemic diarrhea virus and transmissible gastroenteritis virus in fecal samples by reverse transcription-polymerase chain reaction. J. Vet. Diagn. Invest. 2004;16:237–239. doi: 10.1177/104063870401600312. [DOI] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204(2):134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. The effects of transplacental porcine circovirus type 2 infection on porcine epidemic diarrhoea virus-induced enteritis in preweaning piglets. Vet. J. 2006;171(3):445–450. doi: 10.1016/j.tvjl.2005.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet. Rec. 2000;146:637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Kim I.J., Pyo H.M., Tark D.S., Song J.Y., Hyun B.H. Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J. Virol. Methods. 2007;146:172–177. doi: 10.1016/j.jviromet.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee J.M., Jung J., Kim I.J., Hyun B.H., Kim H.I., Park C.K., Oem J.K., Kim Y.H., Lee M.H., Lee K.K. Genetic characterization of porcine epidemic diarrhea virus in Korea from 1998 to 2013. Arch. Virol. 2015;160(April (4)):1055–1064. doi: 10.1007/s00705-015-2353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. The international code of virus classification and nomenclature. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy—Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press; London, United Kingdom: 2011. pp. 782–795. [Google Scholar]
- Knuchel M. An ELISA for detection of antibodies against porcine epidemic diarrhoea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Vet. Microbiol. 1992;32(2):117–134. doi: 10.1016/0378-1135(92)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23(2):137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365(August (1)):166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li H., Liu Y., Pan Y., Deng F., Song Y., Tang X., He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012;18:1350–1353. doi: 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Development of an indirect ELISA based on a truncated S protein of the porcine epidemic diarrhea virus. Can. J. Microbiol. 2015;61(11):811–817. doi: 10.1139/cjm-2015-0213. [DOI] [PubMed] [Google Scholar]
- Li R., Qiao S., Yang Y., Guo J., Xie S., Zhou E., Zhang G. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes. 2016;52:91–98. doi: 10.1007/s11262-015-1254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J. Virol. 2012;86:2856–2858. doi: 10.1128/JVI.06882-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.N., Chung W.B., Chang S.W. US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013–2014. J. Vet. Med. Sci. 2014;76:1297–1299. doi: 10.1292/jvms.14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.D., Lin C.F., Chung W.B., Chiou M.T., Lin C.N. Impact of mated female nonproductive days in breeding herd after porcine epidemic diarrhea virus outbreak. PLoS One. 2016;11:e0147316. doi: 10.1371/journal.pone.0147316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin C.M., Annamalai T., Gao X., Lu Z., Esseili M.A., Jung K., El-Tholoth M., Saif L.J., Wang Q. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Vet. Res. 2015;46:109. doi: 10.1186/s13567-015-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Gauger P., Harmon K., Zhang J., Connor J., Yeske P., Loula T., Levis I., Dufresne L., Main R. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 2014;20:872–874. doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard J. Porcine epidemic diarrhea (PED)—a constant threat. Proceedings of the 7th European Symposium of Porcine Health Management; Nantes, France April; 2015. p. 53. [Google Scholar]
- Madson D.M., Magstadt D.R., Arruda P.H., Hoang H., Sun D., Bower L.P., Bhandari M., Burrough E.R., Gauger P.C., Pillatzki A.E., Stevenson G.W., Wilberts B.L., Brodie J., Harmon K.M., Wang C., Main R.G., Zhang J., Yoon K.J. Pathogenesis of porcine epidemic diarrhoea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014;174:60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Marthaler D., Jiang Y., Otterson T., Goyal S., Rossow K., Collins J. Complete genome sequence of porcine epidemic diarrhea virus strain USA/Colorado/2013 from the United States. Genome Announc. 2013;1(4):e00555–13. doi: 10.1128/genomeA.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Bruner L., Collins J. Rossow Third strain of porcine epidemic diarrhea virus, United States. Emerg. Infect. Dis. 2014;20(December (12)):2162–2163. doi: 10.3201/eid2014.140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Murakami S., Takahashi O., Miyazaki A., Ohashi S., Yamasato H., Suzuki T. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch. Virol. 2015;160:2565–2568. doi: 10.1007/s00705-015-2522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J.R., Hakze-van der Honing R., Almeida A., Lourenço M., van der Poel W.H., Nascimento M.S. Outbreak of porcine epidemic diarrhea virus in Portugal, 2015. Transbound. Emerg. Dis. 2015;62(December (6)):586–588. doi: 10.1111/tbed.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.C., Crawford K.K., Lager K.M., Kellner S.G., Brockmeier S.L. Evaluation of two real-time polymerase chain reaction assays for Porcine epidemic diarrhea virus (PEDV) to assess PEDV transmission in growing pigs. J. Vet. Diagn. Invest. 2016;28:20–29. doi: 10.1177/1040638715621949. [DOI] [PubMed] [Google Scholar]
- Nam E., Lee C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet. Microbiol. 2010;144(1–2):41–50. doi: 10.1016/j.vetmic.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S. Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. J. Vet. Sci. 2005;6(4):349–352. [PubMed] [Google Scholar]
- Okda F. Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Vet. Res. 2015;11:180. doi: 10.1186/s12917-015-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanratmanee E.O., Kunavongkrit A., Tummaruk P. Impact of porcine epidemic diarrhea virus infection at different periods of pregnancy on subsequent reproductive performance in gilts and sows. Anim. Reprod. Sci. 2010;122:42–51. doi: 10.1016/j.anireprosci.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Paarlberg P.L. 2014. Updated estimated economic welfare impacts of porcine epidemic diarrhea virus (PEDV) http://ageconsearch.umn.edu/bitstream/174517/2/14-4.Updated%20Estimated%20Economic%20Welfare%20Impacts%20of%20PEDV.pdf (accessed 30.03.16.) [Google Scholar]
- Park J.E., Shin H.J. Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res. 2014;191:143–152. doi: 10.1016/j.virusres.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E. Trypsin-induced hemagglutination activity of porcine epidemic diarrhea virus. Arch. Virol. 2010;155(4):595–599. doi: 10.1007/s00705-010-0620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011;156(10):1749–1756. doi: 10.1007/s00705-011-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;5824:3–7. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranaveja S., Poolperm P., Lertwatcharasarakul P., Kesdaengsakonwut S., Boonsoongnern A., Urairong K., Kitikoon P., Choojai P., Kedkovid R., Teankum K., Thanawongnuwech R. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009;15(July (7)):1112–1115. doi: 10.3201/eid1507.081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist-Rybachuk G.V., Nauwynck H.J., Kalmar I.D. Sensitivity of porcine epidemic diarrhea virus (PEDV) to pH and heat treatment in the presence or absence of porcine plasma. Vet. Microbiol. 2015;181:283–288. doi: 10.1016/j.vetmic.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Park S., Kim S., Song D., Park B. Novel porcine epidemic diarrhea virus variant with large genomic deletion, South Korea. Emerg. Infect. Dis. 2014;20:2089–2092. doi: 10.3201/eid2012.131642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Pensaert M.B., Sestak K., Yeo S.G., Jung K. Coronaviruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Diseases of Swine. 10th edition. Wiley-Blackwell; 2012. pp. 501–524. [Google Scholar]
- Saif L.J. Coronaviridae. In: MacLachlan N.J., Dubovi E.J., editors. Fenner's Veterinary Virology. fourth edition. Academic Press of Elsevier; 2011. pp. 393–413. (Chapter 24) [Google Scholar]
- Sasaki Y., Alvarez J., Sekiguchi S., Sueyoshi M., Otake S., Perez A. Epidemiological factors associated to spread of porcine epidemic diarrhea in Japan. Prev. Vet. Med. 2016;123:161–167. doi: 10.1016/j.prevetmed.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Sato T., Takeyama N., Katsumata A., Tuchiya K., Kodama T., Kusanagi K. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes. 2011;43(August (1)):72–78. doi: 10.1007/s11262-011-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L.L., Tonsor G.T. Assessment of the economic impacts of porcine epidemic diarrhea virus in the United States. J. Anim. Sci. 2015;93:5111–5118. doi: 10.2527/jas.2015-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A., McCluskey B., Brown-Reid M., Grear D., Pitcher P., Ramos G., Spencer D., Singrey A. Porcine epidemic diarrhea virus introduction into the United States: root cause investigation. Prev. Vet. Med. 2016;123:192–201. doi: 10.1016/j.prevetmed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K. Role of proteases in the release of porcine epidemic diarrhea virus from infected cells. J. Virol. 2011;85(15):7872–7880. doi: 10.1128/JVI.00464-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.C., Denison M.R. Coronaviruses as DNA wannabes: a new model for the regulation of RNA virus replication fidelity. PLoS Pathog. 2013;9(12):e1003760. doi: 10.1371/journal.ppat.1003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Moon H., Kang B. Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015;4(July (2)):166–176. doi: 10.7774/cevr.2015.4.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Zoels S., Fux R., Hanke D., Pohlmann A., Blome S., Weissenböck H., Weissenbacher-Lang C., Ritzmann M., Ladinig A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015 Jul;2(11):142. doi: 10.1186/s12917-015-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach F., Dastjerdi A., Peake J., La Rocca S.A., Tobin F., Frossard J.P., Williamson S. A retrospective study detects a novel variant of porcine epidemic diarrhea virus in England in archived material from the year 2000. PeerJ. 2016 doi: 10.7717/peerj.2564. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrigl A., Fernández S.R., Stoiber F., Pikalo J., Sattler T., Schmoll F. First detection, clinical presentation and phylogenetic characterization of Porcine epidemic diarrhea virus in Austria. BMC Vet. Res. 2015;30(December (11)):310. doi: 10.1186/s12917-015-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25(5):649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Sun D.B., Feng L., Shi H.Y., Chen J.F., Liu S.W., Chen H.Y., Wang Y.F. Spike protein region (aa 636–789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta Virol. 2007;51:149–156. [PubMed] [Google Scholar]
- Sun D., Feng L., Shi H., Chen J., Cui X., Chen H., Liu S., Tong Y., Wang Y., Tong G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet. Microbiol. 2008;131(September (1–2)):73–81. doi: 10.1016/j.vetmic.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R.Q., Cai R.J., Chen Y.Q., Liang P., Chen D., Song C. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012;18(1):161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Okada K., Ohshima K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi. 1983;45:829–832. doi: 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeeyasen G., Srijangwad A., Tripipat T. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect. Genet. Evol. 2014;21:205–213. doi: 10.1016/j.meegid.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Theuns S., Conceição-Neto N., Christiaens I., Zeller M., Desmarets L.M., Roukaerts I.D., Acar D.D., Heylen E., Matthijnssens J., Nauwynck H.J. Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in Belgium, January 2015. Genome Announc. 2015;21(May (3)) doi: 10.1128/genomeA.00506-15. pii: e00506-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.T., Chen Q., Gauger P.C., Giménez-Lirola L.G., Sinha A., Harmon K.M., Madson D.M., Burrough E.R., Magstadt D.R., Salzbrenner H.M., Welch M.W., Yoon K.J., Zimmerman J.J., Zhang J. Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naïve conventional neonatal and weaned pigs. PLoS One. 2015;10(10):e0139266. doi: 10.1371/journal.pone.0139266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P.F., Jin Y.L., Xing G., Qv L.L., Huang Y.W., Zhou J.Y. Evidence of recombinant strains of porcine epidemic diarrhea virus, United States, 2013. Emerg. Infect. Dis. 2014;20:1735–1738. doi: 10.3201/eid2010.140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture . 2016. Swine Enteric Coronavirus Disease (SECD) Situation Report. Available at http://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/secd_sit_rep_03_17_16.pdf (accessed 30.03.16.) [Google Scholar]
- van Nieuwstadt A.P., Zetstra T. Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhea virus. Am. J. Vet. Res. 1991;52(7):1044–1050. [PubMed] [Google Scholar]
- Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A., Collins J., Saif L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014;20(October (10)):1620–1628. doi: 10.3201/eid2010.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang Y., Byrum B. Development and evaluation of a duplex real-time RT-PCR for detection and differentiation of virulent and variant strains of porcine epidemic diarrhea viruses from the United States. J. Virol. Methods. 2014;207:154–157. doi: 10.1016/j.jviromet.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao X., Yao Y., Zhang Y., Lv C., Sun Z., Wang Y., Jia X., Zhuang J., Xiao Y., Li X., Tian K. The dynamics of Chinese variant porcine epidemic diarrhea virus production in Vero cells and intestines of 2-day old piglets. Virus Res. 2015;208:82–88. doi: 10.1016/j.virusres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Wang Z., Jiyuan Y., Su C., Lijie T., Yijing L. Development of an antigen capture enzyme-linked immunosorbent assay for virus detection based on porcine epidemic diarrhea virus monoclonal antibodies. Viral Immunol. 2015;28(3):184–189. doi: 10.1089/vim.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters T., 2014. U.S. hog losses from virus at about 8 million, but rate slows. Reuters June 5.
- Wicht O., Li W., Willems L., Meuleman T.J., Wubbolts R.W., van Kuppeveld F.J.M., Rottier P.J.M., Bosch B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014;88(14):7952–7961. doi: 10.1128/JVI.00297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S., Strugnell B., Thomson J., Webster G., McOrist S., Clarke H., Armstrong D. Emergence of porcine epidemic diarrhoea in pigs in the USA. Vet. Rec. 2013;173:146–148. doi: 10.1136/vr.f4947. [DOI] [PubMed] [Google Scholar]
- Wood E.N. An apparently new syndrome of porcine epidemic diarrhea. Vet. Rec. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]
- Zhang J., Tsai Y.L., Lee P.A., Chen Q., Zhang Y., Chiang C.J., Shen Y.H., Li F.C., Chang H.G., Gauger P.C., Harmon K.M., Wang H.T. Evaluation of two singleplex reverse transcription-Insulated isothermal PCR tests and a duplex real-time RT-PCR test for the detection of porcine epidemic diarrhea virus and porcine deltacoronavirus. J. Virol. Methods. 2016;234:34–42. doi: 10.1016/j.jviromet.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P.D., Bai J., Jiang P., Tang T.S., Li Y., Tan C., Shi X. Development of a multiplex TaqMan probe-based real-time PCR for discrimination of variant and classical porcine epidemic diarrhea virus. J. Virol. Methods. 2014;206:150–155. doi: 10.1016/j.jviromet.2014.06.006. [DOI] [PubMed] [Google Scholar]