Highlights
-
•
Here we reviewed Bacteriophage MS2 virus-like particles, including introduction to their structure, their potential as a delivery platform, and their expected use in medicine and other fields.
-
•
Bacteriophage MS2 virus-like particles represent the novel delivery platform.
-
•
Bacteriophage MS2 virus-like particles possess promising application prospect.
Abbreviations: CCPD, covalent coat protein dimer; CP, coat protein; DC, dendritic cell; FMDV, foot-and-mouth disease virus; GM-CSF, granulocyte-macrophage colony-stimulating factor; HPV, human papilloma virus; miR, microRNA; PAP, prostatic acid phosphatase; VLP, virus-like particle
Keywords: Bacteriophage MS2, Virus-like particles, Delivery platform, Vaccine, Therapy, In vitro diagnostic
Abstract
Our objective here is to review the novel delivery platform based on Bacteriophage MS2 virus-like particles (VLPs), including introduction to their structure, their potential as a delivery platform, and their expected use in medicine and other fields. Bacteriophage MS2 VLPs are nanoparticles devoid of viral genetic material and can self-assemble from the coat protein into an icosahedral capsid. As a novel delivery platform, they possess numerous features that make them suitable and attractive for targeted delivery of RNAs or DNAs, epitope peptides, and drugs within the protein capsid. In short, as a novel delivery platform, MS2 VLPs are suitable for delivery of targeted agents and hold promise for use in diagnostics, vaccines, and therapeutic modalities.
1. Introduction
Virus-like particles (VLPs)—nanoparticles devoid of viral genetic material that are usually formed by one or several structural proteins—have external structure and antigenicity similar to those of native viruses. Recently, considerable efforts have been devoted to construction of VLPs, making them attractive as possible nanocarriers (Carrico et al., 2008, Kovacs et al., 2007, Wu et al., 2005). Combining a good safety profile with strong immunogenicity, VLPs are expected to gain widespread use in numerous fields, such as in vitro diagnostics, vaccines, and therapeutic modalities.
Before 2013, more than 110 VLPs had been constructed from viruses belonging to 35 families (Zeltins, 2013), among which MS2 VLPs—an icosahedral capsid self-assembled from 180 copies of a single coat protein (CP) and measuring 22–29 nm in diameter—represent a novel delivery platform and became a hot research area within several years because of their attractive features. First, MS2 VLPs can offer effective, convenient ways to package and deliver RNAs or DNAs, epitope peptides, and drugs within bacteriophage capsids (Sun et al., 2011, Wei et al., 2009, Wu et al., 2005, Zhang et al., 2015a, Zhang et al., 2015b). Second, they can have excellent adjuvant properties and thus induce innate and cognate immune responses. Additionally, they are safer and more effective than traditional vaccines derived from attenuated or inactivated infectious viral strains. Furthermore, they are capable of tissue-specific targeting after modification with a ligand, and this feature can ensure that the targeted agents carried by MS2 VLPs are more effective.
Clinical applications of MS2 VLPs are perhaps their most exciting feature. Their main applications belong to the field of vaccine development. A series of research findings showed that an MS2 VLP-based vaccine can effectively induce innate and cognate immune responses and can be used as a specific preventive intervention in some diseases, such as foot-and-mouth disease (Bittle et al., 1982, Dong et al., 2015, Van Lierop et al., 1992, Wong et al., 2000), prostate cancer (Li et al., 2014), and illnesses caused by human papilloma virus (HPV) (Tumban et al., 2012). Another important application of MS2 VLPs is therapeutic modalities because these particles can deliver drugs or biologics to a specific tissue (Ashley et al., 2011, Pan et al., 2012a, Yao et al., 2015). Moreover, by packaging specific RNA molecules into the recombinant MS2VLPs, researchers can construct so-called armored RNAs. Because their structure is similar to that of the native virus and their concentration is already calibrated, the armored RNAs can be used as “standards” or “calibrators” for detection of the corresponding native viruses and for validation of an experiment with the help of a “control” (Das et al., 2006, Zhan et al., 2009). Our objective here is to review the novel delivery platform based on Bacteriophage MS2 VLPs, including introduction to their structure, their potential as a delivery platform, and their expected use in medicine and other fields.
2. Characteristics of MS2VLPs
2.1. Structure of MS2VLPs
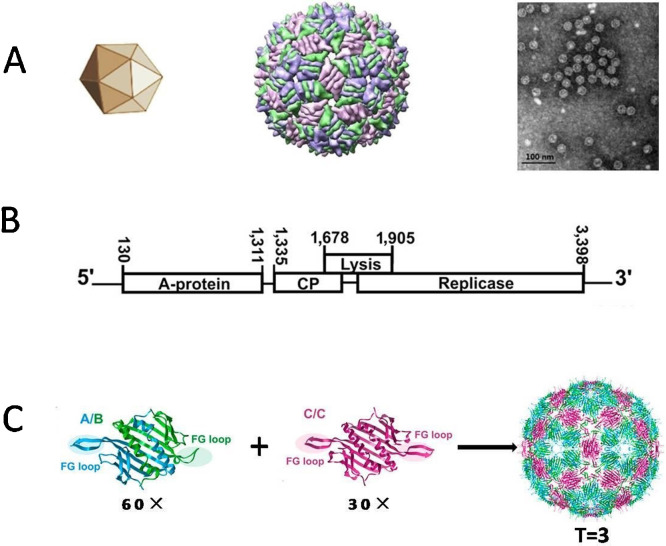
MS2 is an icosahedral RNA bacteriophage with the triangulation number T = 3, and its crystallographic structure was solved at 2.8 Å resolution (Fig. 1 A) (Borodavka et al., 2012, Golmohammadi et al., 1993, Valegård et al., 1990, Valegård et al., 1991). The genome of Bacteriophage MS2 is a positive-sense single-stranded RNA molecule of 3569 nucleotides and encodes four proteins: the major coat protein (CP), the maturation protein (A-protein), the replicase (an RNA polymerase necessary for genome multiplication), and the lysis protein (Fig. 1B).
Fig. 1.
Structure of Bacteriophage MS2. (A) External morphological characteristics of Bacteriophage MS2 with the triangulation number T = 3. The left panel depicts a geometric model of MS2. The middle panel depicts surface representation of Bacteriophage MS2: a crystal structure of in vitro assembled MS2 coat protein (CP) with synthetic RNA hairpins. A/B dimers are blue and green. C/C dimers are purple. The right panel depicts an electron-microscopy image of wild-type MS2. The scale bar is 100 nm. (B) Structures of the A/B and C/C dimers. The FG loop (highlighted) exists in an extended conformation in the A and C subunits but is bent back toward the core of the protein in B subunits. Sixty A/B dimers and 30C/C dimers assemble into the icosahedron of MS2. (C) Genetic map of the MS2 genome. The genome of Bacteriophage MS2encodes four proteins: CP (the major protein), the maturation protein (A-protein), the replicase (an RNA polymerase necessary for genome multiplication), and the lysis protein. Adapted from Bleckley and Schroeder (2012), Borodavka et al. (2012), Li et al. (2014), and Pan et al., 2012a, Pan et al., 2012b. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
CP makes up the bulk of the bacteriophage, assembling into an icosahedral structure ∼26 nm in diameter. CP molecules first form a dimer. The dimer can bind to an RNA hairpin and then undergoes an allosteric conformational change in the FG loop region (Bleckley and Schroeder, 2012), forming an asymmetric A/B dimer rather than a symmetric C/C dimer (Borodavka et al., 2012, Dykeman et al., 2010, Dykeman and Twarock, 2010, Rolfsson et al., 2010, Stockley et al., 2007). The FG loops of the B conformation of CP surround the fivefold vertices of the icosahedron, and the A and C conformations interdigitate at the threefold vertices of the icosahedron, thus forming an icosahedron of 60 A/B dimers and 30 C/C dimers (Golmohammadi et al., 1993, Valegård et al., 1990). Eventually, Bacteriophage MS2 can form a simple ribonucleoprotein structure composed of 180 CP molecules.
The three quasiequivalent conformers of CP differ primarily in the conformation of the FG loop. The loops of the A subunit are similar to those of subunit C because they have extended conformations and interact with each other at the capsid’s threefold axes, but in the B subunit, the loop is bent back toward the globular core of the subunit, interacting with the other B loops at the particle’s fivefold axes (Fig. 1C) (Valegård et al., 1990).
Both in vitro (Mastico et al., 1993, Stockley et al., 1993) and in vivo (Knolle and Hohn, 1975), CP molecules are capable of self-assembling into capsids at T = 3. In very rare cases, aberrant forms, such as capsids at T = 1, have also been documented (Knolle and Hohn, 1975).
2.2. Self-assembly of Bacteriophage MS2
As discussed above, we know that Bacteriophage MS2 can assemble from 180 copies of CP into a monodisperse, icosahedral capsid with a diameter of 22–29 nm (Mastico et al., 1993, Stockley et al., 1993). Generally, the final fully formed capsids represent the state of lowest free energy of the component proteins and nucleic acids (Stonehouse and Stockley, 1993). Therefore, after its assembly, the final fully formed MS2 VLP has a stable structure, and this feature forms the basis for delivery of all kinds of targeted agents by MS2VLPs.
Regarding the self-assembly of MS2VLPs, Stockley et al. (1994) reported that the assembly of this bacteriophage can be achieved via interaction between the bacteriophage CP and a 19-nucleotide sequence-specific MS2cistron, the so-called pac site. That is to say, the MS2 RNA genome has a special functional structure when encapsidated inside the virus particle. Borodavka et al. (2012) and Rolfsson et al. (2010) showed that CPs and RNA act as mutual chaperones by altering each other’s conformation because of formation of the complex in a context-specific way, as the capsid shell grows from an initiation complex. Toropova et al., 2008, Toropova et al., 2011 reported that 90% of the encapsidated RNA is well ordered and forms two layers inside the icosahedral viral particle. Dykeman et al. (2011) stated that the symmetry of the icosahedron and the locations of the RNA hairpins in the A/B dimers point to possible assembly mechanisms for aseries of hairpins in the MS2 genome. Thus, according to the above findings, the CP-binding RNA hairpins may play an important role in the assembly of the virus particle with the correct size and symmetry (Basnak et al., 2010, Koning et al., 2003, Morton et al., 2010, Stockley et al., 2007).
Nonetheless, many viral CPs can assemble in the absence of RNA, i.e., around nonviral RNA or polyanions (Cadena-Nava et al., 2011, Hu et al., 2008) and even nanoparticles (Chen et al., 2006). The accepted mechanisms underlying the assembly of MS2 VLPs mostly represent protein-centric models. In other words, viral proteins are the key component of the process of assembly of MS2 VLPs (Zlotnick and Mukhopadhyay, 2011).
2.3. Factors influencing stability of MS2 VLPs
B acteriophage MS2 is an excellent model for studies on the factors that can affect capsid stability (Stonehouse and Stockley, 1993). Several factors, including physicochemical ones, such as temperature, pH, and ionic strength or structural ones, such as mutationsin CP, can affect MS2 VLP stability to some extent.
2.3.1. Physicochemical factors
In one experiment with temperature stability, Plevka et al. (2009) found that the melting temperature of the MS2 phage and wild-type VLPs is 66 °C. Stonehouse and Stockley (1993) reported that these particles can withstand temperatures up to 68 °C. No capsids are detectable, however, if the temperature is increased by just l °C. In order to determine the role of MS2 RNA in capsid stability, the experiment was repeated with wild-type phage particles, prepared for electron microscopy in the same way as the empty capsids. A similar result was obtained for the denaturation temperature, showing that RNA cannot exerta substantial influence on thermal stability of the capsid.
The influence of salts and natural organic matter on the stability of Bacteriophage MS2 was studied by Mylon et al. (2010). They found that aggregation of MS2 cannot be induced within a reasonable kinetic time frame in the presence of monovalent electrolytes, such as LiCl, NaCl, and KCl, and this situation is observed even at salt concentrations greater than 1.0 M. On the other hand, MS2 can aggregate in a solution containing divalent electrolytes (e.g., Ca2+); this finding points to transition from repulsive to attractive interactions among MS2 virus particles as monovalentions are replaced by divalent ones.
In addition to thermal and salt-related characteristics of MS2VLPs, pH may have a strong physicochemical influence on stability of MS2VLPs. Some studies showed that Bacteriophage MS2 can stay stable at pH 7.0 but becomes less stable as pH decreases (Da Poian et al., 1993, Lago et al., 2001, Sugiyama et al., 1967). Additionally, early in 1970, Dubovi and Akers (1970) found a decrease in the recovery of phage MS2 after prehumidification of anaerosol sample before collection, if no peptone was added to the spraymedium.
2.3.2. Structural factors
Stonehouse and Stockley (1993) indicated that a wide range of amino acid substitutions can be tolerated by MS2VLPs, even at intermolecular interfaces, with only modest effects on overall stability. Some other substitutions, however, could result in problems with CP folding or stability. Thus, a change in the amino acid sequence of this protein may lead to a functional change.
As to the effects of site-directed mutagenesis of CP on the assembly of MS2 VLPs, a study conducted by Stonehouse and Stockley (1993) showed that three mutants, L86S, G74A, and C46R, have thermal stability identical to that of wild-type CP. Another group of mutants, T59S, Y129C, and C46W, showed a small but significant 2–4 °C increase in the denaturation temperature. The third group, P78N and E76D, showed a 2 °C decrease in the denaturation temperature. In another experiment, Peabody (2003) found that only the T15C mutant among five selected amino acid substitutions (G13C, G14C, T15C, D114C, and G115C) can produce significant quantities of soluble CP that assembles into particles with the same electrophoretic mobility as those of the wild-type virus. Additionally, in comparison with the wildtype, the capsid stability decreases when mutations of CP are located in the FG loop (Axblom et al., 1998, Peabody and Ely, 1992, Stonehouse et al., 1996).
By means of genetic engineering, two or more different polypeptides can be recombined easily. Early in 1996, Peabody and Lim (1996) fused two copies of the CP gene by removing the stop codon from the first gene and the second residue (serine) from the second copy. The final covalent CP dimer (CCPD) gene encoded 257 amino acid residues. The CCPD molecules were then found to assemble into VLPs. These particles are indistinguishable from the VLPs assembled from the wild-type CP at the resolution of an electron microscope. Another study showed that both wild-type and the CCPD particles are more stable than the dimers of the wild-type subunits (Peabody, 1997, Peabody and Lim, 1996), even though they appear to have a similar diameter: 280 ± 20 Å (Plevka et al., 2008).
3. Roles of MS2 VLPs as a nonviral delivery system
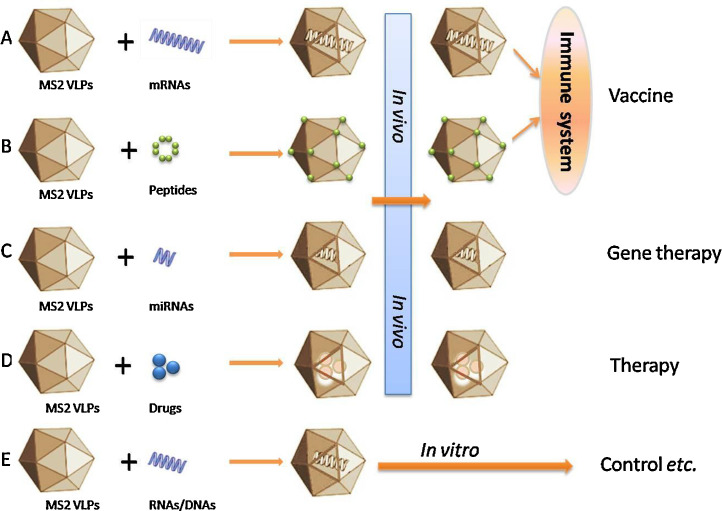
After the initial reports about MS2VLPs as a form of a nonviral delivery system (Sun et al., 2011, Wei et al., 2009, Wu et al., 2005) that can be targeted to specific cell types via chemical or covalent conjugation with ligands (Ashley et al., 2011, Brown et al., 2002), Bacteriophage MS2 VLPs have been shown to have many features as a delivery platform. On the one hand, these particles can be prepared easily by means of the recombinant-protein technology (Zhan et al., 2009). On the other hand, the MS2 capsid, which interacts with the pac site of RNA or DNA, can package the target RNA or DNA located at the 5′ terminus of the pac site and thus protect the target RNA or DNA from degradation by nucleases (Caldeira and Peabody, 2011, Zhan et al., 2009, Zhang et al., 2015a, Zhang et al., 2015b). Capable of offering effective, convenient ways of packaging all kinds of agents and ways of delivery to target cells, MS2 VLPs hold great promise as a novel delivery platform for diagnostic, vaccine-related, and therapeutic purposes (Fig. 2 ).
Fig. 2.
Roles of MS2 virus-like particles (VLPs). (A) Delivery of mRNAs. After being packaged into MS2 VLPs, an mRNA-based vaccine can easily stimulate the immune system and serve as an effective vaccine. (B) Delivery of peptides. Exogenous peptides can be easily recombined with MS2VLPs via genetic engineering, and the chimeric VLPs are strongly immunogenic when carrying either B- or T-cell epitopes. (C) Delivery of miRNAs. miRNAs perform powerful gene-regulatory functions in many diseases. As a novel attractive delivery system, MS2 VLPs bonded with miRNAs can be applied to gene therapy. (D) Delivery of drugs. MS2VLPs possess several features that make them attractive as potential nanocarriers for passive and targeted drug delivery and can be effective against certain diseases (in animal models). Adapted from Pan et al., 2012a, Pan et al., 2012b. (E) MS2 VLP-based RNAs and DNAs (armored RNA or DNA). Owing to a structure similar to that of the native RNA virus and good biosafety, recombinant MS2 VLPs are widely used as “control”, “standard”, and “calibrator” in assays for viruses.
3.1. Delivery of mRNA
As early as 1990, studies on mRNA vaccines became a hot area of research when direct injection of naked synthetic mRNA had proven to be feasible and could induce an antigen-specific immune response in clinical settings. Therefore, vaccine-based mRNA opens up a new avenue for the development of next-generation vaccines (Rittig et al., 2011). In addition, mRNA vaccines have also been considered as an efficient alternative to protein and DNA vaccines not only because of the good antigenic potential and immunostimulatory properties (Scheel et al., 2004) but also owing to safety, low cost, and high purity (Van Lint et al., 2011).
It is known that naked mRNAs are easily degraded by RNases, and this instability limits their practical applications (Ulmer et al., 2012). Several approaches, such as liposome-entrapped mRNA (Martinon et al., 1993), self-replicative mRNA (Ying et al., 1999), mRNA-coated gold particles (Qiu et al., 1996), and in vitro mRNA-transfected dendritic cells (DCs) (Gardner et al., 2012, Perche et al., 2011) have been developed to improve intracellular stability and translational efficacy of mRNA. Nevertheless, these approaches do not offer a perfect carrier for delivery. First, the greatest challenge is still the relative instability of mRNA owing to degradation by prevalent RNases. Besides, the high costs of gold particles and the gene gun with inconvenient settings for administration to experimental animals hinder the development of ballistic delivery of mRNA for vaccination. Furthermore, in vitro mRNA-transfected DCs have been used for cancer treatment in the clinic and turned out to be expensive (Van Lint et al., 2013), let alone the scarcity of the source of autologous DCs. All of these disadvantages hamper practical applications and development of mRNA vaccines. Thus, there is a high demand for a simple, safe, efficient, stable, and inexpensive method for the delivery of mRNA.
An MS2 VLP-based RNA approach can be utilized for development of an mRNA vaccine resistant to rapid extracellular degradation (Fig. 2A). Because of the highly specific interaction between CP and the pac site, the target mRNA can be packaged into CP of Bacteriophage MS2 and kept stable within 18 h (Uhlenbeck, 1998). Therefore, mRNA delivery in vivo, which does not require complicated procedures and can directly target antigen-presenting cells, may be preferable.
Several studies have shown that an mRNA vaccine is effective at protecting from prostate cancer when the MS2 delivery platform is used. Johnson et al. (2006) demonstrated that prostatic acid phosphatase (PAP) mRNA vaccine could induce a PAP-specific antibody and T-cell immune response, whereas a PAP DNA vaccine could not do the job without an adjuvant: granulocyte-macrophage colony-stimulating factor (GM–CSF). Mockey et al. (2007) reported that an MS2VLP-based hPAP–GM–CSF mRNA vaccine—which first showed its prophylactic potential in protection of mice from prostate cancer—proved to have a better preventive effect than a liposome-encapsulated mRNA vaccine (Mockey et al., 2007, Zhou et al., 1999) and is as effective as a naked mRNA vaccine (Kreiter et al., 2010). Nevertheless, the encapsulated mRNA is more stable than naked mRNA under the protection of CP. A study by Li et al. (2014) indicated that an MS2 VLP-based hPAP–GM–CSF mRNA vaccine could suppress prostatic-tumor growth in C57BL/6 mice, suggesting that this vaccine may evoke an efficient immune response within a short period and is a promising treatment for prostate tumors. In the above studies, the MS2VLP-based hPAP mRNA vaccine was inferior to the hPAP–GM–CSF mRNA vaccine; this observation means that GM–CSF has good adjuvanticity and is effective at activating the immune system.
3.2. Delivery of epitope peptide
In immunology, an epitope, also known as an antigenic determinant, is the part of an antigen that is recognized by the immune system, in particular, by antibodies, B cells, or T cells, and then elicits an adaptive immune response. As a nonviral delivery system, MS2 VLPs can be designed to deliver an epitope peptide for clinical purposes. First, after insertion of a DNA oligonucleotide into a unique site of the MS2CP gene, MS2 VLPs can present the epitope peptide to the immune system (Mastico et al., 1993, Peabody et al., 2008, Wei et al., 2008). It is, of course, not difficult to elicit a desired antibody response by a specific epitope peptide (Meloen et al., 2000, Partidos and Steward, 2002). Furthermore, VLPs can present viral antigens as dense repetitive arrays, which are known to be highly stimulatory to immunocytes (Bachmann et al., 1993, Milich et al., 1997). Additionally, compared to other VLP platforms MS2 VLPs are advantageous because of their good stability and proper size for effective presentation of viral antigens (Bundy et al., 2008). When MS2 VLPs are used as a platform for the presentation of epitopes, in the majority of cases, this approach is implemented through modification of the VLP gene sequence, so that fusion proteins of VLP components with the foreign epitope are assembled into VLPs during the expression of these proteins.
This feature of presenting targeted epitopes to the immune system (Fig. 2B) may lead to widespread use of MS2VLPs in clinical practice. One known clinical application of a VLP vaccine is protection from foot-and-mouth disease virus (FMDV). FMDV’s genome is a single-stranded positive sense RNA that contains a unique open reading frame encoding four structural proteins (VP1–VP4) (Acharya et al., 1989). Studies confirmed that the epitope peptide VP1141–160 (also known as EP141-160) can induce not only neutralizing antibodies but also FMDV-specific T cells (Bittle et al., 1982, Dong et al., 2015, Van Lierop et al., 1992, Wong et al., 2000). The above studies mean that EP141-160 VLPs may offer protection from FMDV.
An MS2VLP vaccine can also be used for prevention of infection with HPV. The current HPV vaccines are derived from VLPs consisting of the HPV major capsid protein L1. To develop second-generation HPV vaccines based on the HPV minor capsid protein L2, Tumban et al. (2012) inserted HPV L2 peptides into the N terminus of CP of Bacteriophage MS2 via genetic recombination and found that the VLP-based vaccine targeting HPV L2 can elicit broadly cross-reactive and cross-protective antibodies to heterologous HPVs. Thus, with the MS2 VLP platform, the recombinant L2-VLPs could serve as the basis for a broadly protective second-generation HPV vaccine.
3.3. Delivery of microRNA (miRNA)
miRNA is a type of 20- to 24-nucleotide noncoding RNA that can bind to the 3′ untranslated region of a targeted mRNA, causing its degradation or translational suppression. The powerful gene-regulatory role of miRNAs is well recognized at present, and some miRNA-based therapeutic strategies have shown effectiveness in experimental tumor models. This type of treatment may be more effective because the regulatory mechanism of a single miRNA affects hundreds of targets in multiple pathways rather than one or two transcripts (Selbach et al., 2008).
Owing to many limitations, however, such as rapid extracellular degradation and the necessity of a reliable delivery system capable of transferring miRNAs into the intracellular space without cytotoxicity (Davidson and McCray, 2011, Wang et al., 2010), the development of an efficient miRNA delivery system has been a challenge. A number of approaches to miRNA delivery involving the use of viral or nonviral vector systems have also been explored (Davidson and McCray, 2011). Nonetheless, the typically low transduction efficiency, possible cytotoxicity, and integration-induced tumorigenesis are always concerns when plasmid or viral vectors are used for miRNA expression (Davidson and McCray, 2011). Therefore, as a novel attractive delivery system, MS2VLPs recently became a focus of research attention in the field of miRNA delivery.
After delivery by MS2 VLPs, miRNAs can be used for gene therapy (Fig. 2C). Studies showed that several known miRNAs, such as miR-21, miR-125, and miR-146a, have potent capacity for gene regulation (Ceribelli et al., 2011, Dai and Ahmed, 2011). Among these miRNAs, miR-146a—an miRNA important for negative regulation of acute responses during activation of innate immunity—acts as an effective inhibitor of autoimmunity, myeloproliferation, and cancer (Boldin et al., 2011, Li et al., 2010, Tang et al., 2009). Overexpression of miR-146a can effectively suppress the expression of its target genes and proteins (Pan et al., 2012a).
Tang et al. (2009) reported that expression of miR-146a is reduced in peripheral blood mononuclear cells (PBMCs) of patients with systemic lupus erythematosus (SLE). Boldin et al. (2011) found that ablation of the miR-146a gene in mice results in several adverse immune-system-related phenotypes leading to premature death. In this study they also demonstrated that miR-146a levels in mice, after delivery via MS2VLPs, first significantly increase, and then this miRNA exerts its therapeutic action on lupus-prone mice via inhibition of production of a pathogenic autoantibody. In other words, this treatment with MS2-miR146a VLP can have profound effects on lupus-prone BXSB mice by upregulating mature miR-146a, which causes significant downregulation of autoantibodies, of total IgG, and of proinflammatory cytokines, including interferonα, IL-1β, and IL-6. Pan et al. (2012b) confirmed that the induction of dysregulated miRNAs by an MS2 VLP-based delivery system may lead to novel therapeutic interventions for SLE.
Moreover, a study by Yao et al. (2015) demonstrated that osteoclastogenesis can be inhibited in cell precursors by means of an MS2 system that transports miR-146a into human PBMCs. As a result, the number of osteoclasts decreased markedly, and a bone resorption assay demonstrated inhibition of their activity. Thus, this miR-146a delivery system can be used to induce overexpression of miR-146a and to inhibit the differentiation and function of osteoclasts.
3.4. Delivery of drug
MS2 VLPs possess several features that make them attractive as potential nanocarriers for passive and targeted drug delivery (Fig. 2D). During the self-assembly of MS2CP, MS2 VLPs can be loaded with drugs. Even for the drugs with a greater-than-average molecular weight, MS2VLPs have sufficient volume of the interior cavity and can deliver such a drug to specific target cells.
In the study by Ashley et al. (2011), macromolecular drug candidates, such as doxorubicin, cisplatin, and 5-fluorouracil were encapsidated specifically and efficiently inside a protective CP shell by first covalently linking them to the RNA operator that triggers in vitro capsid assembly from disassembled CP dimers. These drug packages can then be directed toward hepatocellular carcinoma by covalent modificationof the outside surface with ligands for receptor-mediated endocytosis and eventually can selectively kill the hepatocellular carcinoma cells.
In another experiment, Brown et al. (2002) tested cytotoxicity of 5fdU/anti-DF3—anucleotide analog combined with the anti-DF3 monoclonal antibody packaged in synthetic virions—against ZR-75-1 breast carcinoma cells, which over express the DF3 antigen. The results showed that 5fdU/anti-DF3 is slightly more toxic than free 5fdU at a lower concentration, suggesting that the synthetic virions enhance the toxicity of the oligonucleotide by protecting it from degradation by nucleases in the serum or by enhancing its uptake by ZR-75-1 cells.
3.5. Other roles of MS2 VLPs
In some diagnostic methods, an absolute concentration of a specific RNA has to be detected with high sensitivity. A specific type of RNA, calibrated to known concentrations, plays a core role in the reliable detection of RNA. This kind of RNA, on the one hand, may serve as a “control” for validation of the experiment; on the other hand, it can serve as a quantitative “standard” or “calibrator” against which the samples are measured. On the other hand, this kind of RNA is easily degraded by RNases in vitro, but it is possible to place RNA in long-term storage to prevent the degradation.
In recent years, recombinant MS2 VLPs that are packaged with RNA, the so-called armored RNAs, were developed for use as “controls”, “standards”, and “calibrators” (Stevenson et al., 2008) (Fig. 2E) in diagnostic procedures for the following reasons. First, possessing structure similar to that of the native RNA virus, MS2VLPs can serve as a substitute for the native virus during the extraction procedureand thus dramatically reduce the error rate of the detection of the virus. Additionally, the RNAs in the particles are RNase resistant by virtue of their encapsulation within MS2CP. Moreover, they are not pathogenic during the experiment because these particles cannot replicate by themselves. Armored RNAs based on MS2 VLPs are now used for the detection of various viruses, such as human immunodeficiency virus (Pasloske et al., 1998), severe acute respiratory syndrome coronavirus (Drosten et al., 2001), enteroviruses (Beld et al., 2004, Donia et al., 2005), Avian influenza virus (Das et al., 2006), measles virus (Zhang et al., 2015a, Zhang et al., 2015b), and ebola virus (Wang et al., 2015).
Although armored RNA is a good candidate for an RNase-resistant positive “control” or “standard”, its application is limited by the maximal length of the exogenous RNA that can be packaged into VLPs by means of the existing armored-RNA technology. Nevertheless, Zhan et al. (2009) constructed a long armored RNA containing a 3034-base exogenous RNA, which can be used for detection of HIV-1 in a one-plasmid double-expression system. It is completely resistant to RNases, remains stable in normal human EDTA-preserved plasma at 4 °C for at least 6 months, and yields reproducible linear results in the Versant HIV-1 RNA 3.0 assay.
Additionally, in a recent study, Zhang et al., 2015a, Zhang et al., 2015b produced armored hepatitis B virus and HPV DNA for use in nucleic-acid assays; these constructs were confirmed to be stable, homogeneous, noninfectious, nuclease resistant, and safe for shipping. In that study, double-stranded DNA 1.3, 3.0, 3.5, and 6.5 kb long were successfully packaged into MS2viral capsids with high reassembly efficiency. This is the first successful use of Bacteriophage MS2 as a platform for encapsulation of double-stranded DNA. This armored DNA may become an ideal “control” and “standard” for use in clinical laboratory tests and diagnostics, holding promise for varied applications, especially as a new platform for production of assay standards for DNA viruses.
According to the above properties of MS2 VLPs, these particles seem to be ideal carriers for delivery of a variety of agents. It should be noted that when a large protein domain serves as an antigen, it may pose difficulty for display on MS2VLPs because such an insertion may disrupt the VLP assembly. In that case, chemical conjugation may be a solution. Another problem with the delivery of RNA within MS2 VLPs is that these VLPs are immunogenic, and antibody responses to the particles may interfere with the delivery to the intended destination.
4. Conclusions
Bacteriophage MS2 VLPs can self-assemble from its CP into an icosahedral capsid. These particles offer an effective and convenient way to package RNAs, DNAs, epitope peptides, and drugs into bacteriophage capsids, forming different kinds of VLPs. MS2 VLPs can not only deliver various kinds of agents with a good safety profile and strong immunogenicity but also ensure tissue-specific targeting, which is determined by the species of the virus. Thus, MS2 VLPs possess extensive prospects for practical applications. The most promising applications of MS2VLPs are vaccine development and therapeutic modalities. Besides, MS2 VLPs can be used as “control” or “calibrator” in assays for native viruses.
Acknowledgment
This work was supported by the grant from the National Natural Science Foundation of China (No. 81171981).
References
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989;337:709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Ashley C.E., Carnes E.C., Phillips G.K., Durfee P.N., Buley M.D., Lino C.A., Padilla D.P., Phillips B., Carter M.B., Willman C.L., Brinker C.J., Caldeira J., do C., Chackerian B., Wharton W., Peabody D.S. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano. 2011;5:5729–5745. doi: 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axblom C., Tars K., Fridborg K., Oma L., Bundule M., Liljas L. Structure of phage fr capsids with a deletion in the FG loop: implications for viral assembly. Virology. 1998;249:80–88. doi: 10.1006/viro.1998.9279. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Rohrer U.H., Kündig T.M., Bürki K., Hengartner H., Zinkernagel R.M. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Basnak G., Morton V.L., Rolfsson O., Stonehouse N.J., Ashcroft A.E., Stockley P.G. Viral genomic single-stranded RNA directs the pathway toward a T = 3 capsid. J. Mol. Biol. 2010;395:924–936. doi: 10.1016/j.jmb.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beld M., Minnaar R., Weel J., Sol C., Damen M., van der Avoort H., Wertheim-van Dillen P., van Breda A., Boom R. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J. Clin. Microbiol. 2004;42:3059–3064. doi: 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittle J.L., Houghten R.A., Alexander H., Shinnick T.M., Sutcliffe J.G., Lerner R.A., Rowlands D.J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982;298:30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Bleckley S., Schroeder S.J. Incorporating global features of RNA motifs in predictions for an ensemble of secondary structures for encapsidated MS2 bacteriophage. RNA. 2012;18:1309–1318. doi: 10.1261/rna.032326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M.P., Taganov K.D., Rao D.S., Yang L., Zhao J.L., Kalwani M., Garcia-Flores Y., Luong M., Devrekanli A., Xu J., Sun G., Tay J., Linsley P.S., Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodavka A., Tuma R., Stockley P.G. Evidence that viral RNAs have evolved for efficient, two-stage packaging. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15769–15774. doi: 10.1073/pnas.1204357109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.L., Mastico R.A., Wu M., Heal K.G., Adams C.J., Murray J.B., Simpson J.C., Lord J.M., Taylor-Robinson A.W., Stockley P.G. RNA bacteriophage capsid-mediated drug delivery and epitope presentation. Intervirology. 2002;45:371–380. doi: 10.1159/000067930. [DOI] [PubMed] [Google Scholar]
- Bundy B.C., Franciszkowicz M.J., Swartz J.R. Escherichia coli-based cell-free synthesis of virus-like particles. Biotechnol. Bioeng. 2008;100:28–37. doi: 10.1002/bit.21716. [DOI] [PubMed] [Google Scholar]
- Cadena-Nava R.D., Hu Y., Garmann R.F., Nq B., Zelikin A.N., Knobler C.M., Gelbart W.M. Exploiting fluorescent polymers to probe the self-assembly of virus-like particles. J. Phys. Chem. B. 2011;115:2386–2391. doi: 10.1021/jp1094118. [DOI] [PubMed] [Google Scholar]
- Caldeira J.C., Peabody D.S. Thermal stability of RNA phage virus-like particles displaying foreign peptides. J. Nanobiotechnol. 2011;9:22. doi: 10.1186/1477-3155-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico Z.M., Romanini D.W., Mehl R.A., Francis M.B. Oxidative coupling of peptides to a virus capsid containing unnatural amino acids. Chem. Commun. (Cambridge) 2008;14:1205–1207. doi: 10.1039/b717826c. [DOI] [PubMed] [Google Scholar]
- Ceribelli A., Yao B., Dominguez-Gutierrez P.R., Nahid M.A., Satoh M., Chan E.K. MicroRNAs in systemic rheumatic diseases. Arthritis Res. Ther. 2011;13:229. doi: 10.1186/ar3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Daniel M.C., Quinkert Z.T., De M., Stein B., Bowman V.D., Chipman P.R., Rotello V.M., Kao C.C., Dragnea B. Nanoparticle-templated assembly of viral protein cages. Nano Lett. 2006;6:611–615. doi: 10.1021/nl0600878. [DOI] [PubMed] [Google Scholar]
- Dai R., Ahmed S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Poian A.T., Oliveira A.C., Gaspar L.P., Silva J.L., Weber G. Reversible pressure dissociation of R17 bacteriophage: the physical individuality of virus particles. J. Mol. Biol. 1993;231:999–1008. doi: 10.1006/jmbi.1993.1347. [DOI] [PubMed] [Google Scholar]
- Das A., Spackman E., Senne D., Pedersen J., Suarez D.L. Development of an internal positive control for rapid diagnosis of avian influenza virus infections by real-time reverse transcription-PCR with lyophilized reagents. J. Clin. Microbiol. 2006;44:3065–3073. doi: 10.1128/JCM.00639-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B.L., McCray P.B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.M., Zhang G.G., Huang X.J., Chen L., Chen H.T. Promising MS2 mediated virus-like particle vaccine against foot-and-mouth disease. Antivir. Res. 2015;117:39–43. doi: 10.1016/j.antiviral.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Donia D., Divizia M., Pana A. Use of armored RNA as a standard to construct a calibration curve for real-time RT-PCR. J. Virol. Methods. 2005;126:157–163. doi: 10.1016/j.jviromet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Drosten C., Seifried E., Roth W.K. TaqMan 5′-nuclease human immunodeficiency virus type 1 PCR assay with phage-packaged competitive internal control for high-throughput blood donor screening. J. Clin. Microbiol. 2001;39:4302–4308. doi: 10.1128/JCM.39.12.4302-4308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E.J., Akers T.G. Airborne stability of tailless bacterial viruses S-13 and MS-2. Appl. Microbiol. 1970;19:624–628. doi: 10.1128/am.19.4.624-628.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykeman E.C., Grayson N.E., Toropova K., Ranson N.A., Stockley P.G., Twarock R. Simple rules for efficient assembly predict the layout of a packaged viral RNA. J. Mol. Biol. 2011;408:399–407. doi: 10.1016/j.jmb.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Dykeman E.C., Stockley P.G., Twarock R. Dynamic allostery controls coat protein conformer switching during MS2 phage assembly. J. Mol. Biol. 2010;395:916–923. doi: 10.1016/j.jmb.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Dykeman E.C., Twarock R. All-atom normal-mode analysis reveals an RNA-induced allostery in a bacteriophage coat protein. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2010;81:31,908. doi: 10.1103/PhysRevE.81.031908. [DOI] [PubMed] [Google Scholar]
- Gardner T.A., Elzey B.D., Hahn N.M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum. Vaccin Immunother. 2012;8:534–539. doi: 10.4161/hv.19795. [DOI] [PubMed] [Google Scholar]
- Golmohammadi R., Valegård K., Fridborg K., Liljas L. The refined structure of bacteriophage MS2 at 28 A resolution. J. Mol. Biol. 1993;234:620–639. doi: 10.1006/jmbi.1993.1616. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zandi R., Anavitarte A., Knobler C.M., Gelbart W.M. Packaging of a polymer by a viral capsid: the interplay between polymer length and capsid size. Biophys. J. 2008;94:1428–1436. doi: 10.1529/biophysj.107.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.E., Frye T.P., Arnot A.R., Marquette C., Couture L.A., Gendron-Fitzpatrick A., McNeel D.G. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- Knolle P., Hohn T. RNA Phages. In: Zinder N.D., editor. Cold Spring Harbor; Chapter 6: 1975. pp. 147–201. [Google Scholar]
- Koning R., Van den Worm S., Plaiser J.R., Van Duin J., Abrahams J.P., Koerten H. Visualization by cryo-electron microscopy of genomic RNA that binds to the protein capsid inside bacteriophage MS2. J. Mol. Biol. 2003;332:415–422. doi: 10.1016/s0022-2836(03)00846-5. [DOI] [PubMed] [Google Scholar]
- Kovacs E.W., Hooker J.M., Romanini D.W., Holder P.G., Berry K.E., Francis M.B. Dual-surface-modified bacteriophage MS2 as an ideal scaffold for a viral capsid-based drug delivery system. Bioconjugate Chem. 2007;18:1140–1147. doi: 10.1021/bc070006e. [DOI] [PubMed] [Google Scholar]
- Kreiter S., Selmi A., Diken M., Koslowski M., Britten C.M., Huber C., Türeci O., Sahin U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70:9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- Lago H., Parrott A.M., Moss T., Stonehouse N.J., Stockley P.G. Probing the kinetics of formation of the bacteriophage MS2 translational operator complex: identification of a protein conformer unable to bind RNA. J. Mol. Biol. 2001;305:1131–1144. doi: 10.1006/jmbi.2000.4355. [DOI] [PubMed] [Google Scholar]
- Li J., Sun Y., Jia T., Zhang R., Wang Zhang. K. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int. J. Cancer. 2014;134:1683–1694. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- Li L., Chen X.P., Li Y.J. MicroRNA-146a and human disease. Scand. J. Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., Lévy J.P., Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- Mastico R.A., Talbot S.J., Stockley P.G. Multiple presentation of foreign peptides on the surface of an RNA-free spherical bacteriophage capsid. J. Gen. Virol. 1993;74:541–548. doi: 10.1099/0022-1317-74-4-541. [DOI] [PubMed] [Google Scholar]
- Meloen R.H., Puijk W.C., Slootstra J.W. Mimotopes: realization of an unlikely concept. J. Mol. Recognit. 2000;13:352–359. doi: 10.1002/1099-1352(200011/12)13:6<352::AID-JMR509>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Milich D.R., Chen M., Schödel F., Peterson D.L., Jones J.E., Hughes J.L. Role of B cells in antigen presentation of the hepatitis B core. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylon S.E., Rinciog C.I., Schmidt N., Gutierrez L., Wong G.C., Nguyen T.H. Influence of salts and natural organic matter on the stability of bacteriophage MS2. Langmuir. 2010;26:1035–1042. doi: 10.1021/la902290t. [DOI] [PubMed] [Google Scholar]
- Mockey M., Bourseau E., Chandrashekhar V., Chaudhuri A., Lafosse S., Le Cam E., Quesniaux V.F., Ryffel B., Pichon C., Midoux P. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14:802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- Morton V.L., Dykemna E.C., Stonehouse N.J., Ashcroft A.E., Twarock R., Stockley P.G. The impact of viral RNA on assembly pathway selection. J. Mol. Biol. 2010;401:298–308. doi: 10.1016/j.jmb.2010.05.059. [DOI] [PubMed] [Google Scholar]
- Pan Y., Jia T., Zhang Y., Zhang K., Zhang R., Li J., Wang L. MS2 VLP-based delivery of microRNA-146a inhibits autoantibody production in lupus-prone mice. Int. J. Nanomed. 2012;7:5957–5967. doi: 10.2147/IJN.S37990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang Y., Jia T., Zhang K., Li J., Wang L. Development of a microRNA delivery system based on bacteriophage MS2 virus-like particles. FEBS J. 2012;279:1198–1208. doi: 10.1111/j.1742-4658.2012.08512.x. [DOI] [PubMed] [Google Scholar]
- Partidos C.D., Steward M.W. Mimotopes of viral antigens and biologically important molecules as candidate vaccines and potential immunotherapeutics. Comb. Chem. High Throughput Screen. 2002;5:15–27. doi: 10.2174/1386207023330589. [DOI] [PubMed] [Google Scholar]
- Pasloske B.L., Walkerpeach C.R., Obermoeller R.D., Winkler M., DuBois D.B. Armored RNA technology for production of ribonuclease-resistant viral RNA controls and standards. J. Clin. Microbiol. 1998;36:3590–3594. doi: 10.1128/jcm.36.12.3590-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S., Ely K.R. Control of translational repression by protein–protein interactions. Nucleic Acids Res. 1992;20:1649–1655. doi: 10.1093/nar/20.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S., Lim F. Complementation of RNA binding site mutations in MS2 coat protein heterodimers. Nucleic Acids Res. 1996;24:2352–2359. doi: 10.1093/nar/24.12.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S., Manifold-Wheeler B., Medford A., Jordan S.K., do C., armo C., aldeira J., Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J. Mol. Biol. 2008;380:252–263. doi: 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S. A viral platform for chemical modification and multivalent display. J. Nanobiotechnol. 2003;1:5. doi: 10.1186/1477-3155-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S. Subunit fusion confers tolerance to peptide insertions in a virus coat protein. Arch. Biochem. Biophys. 1997;347:85–92. doi: 10.1006/abbi.1997.0312. [DOI] [PubMed] [Google Scholar]
- Perche F., Benvegnu T., Berchel M., Lebegue L., Pichon C., Jaffrès P.A., Midoux P. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7:445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Plevka P., Tars K., Liljas L. Crystal packing of a bacteriophage MS2 coat protein mutant corresponds to octahedral particles. Protein Sci. 2008;17:1731–1739. doi: 10.1110/ps.036905.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevka P., Tars K., Liljas L. Structure and stability of icosahedral particles of a covalent coat protein dimer of bacteriophage MS2. Protein Sci. 2009;18:1653–1661. doi: 10.1002/pro.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Ziegelhoffer P., Sun J., Yang N.S. Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunization. Gene Ther. 1996;3:262–268. [PubMed] [Google Scholar]
- Rittig S.M., Haentschel M., Weimer K.J., Heine A., Muller M.R., Brugger W., Horger M.S., Maksimovic O., Stenzl A., Hoerr I., Rammensee H.G., Holderried T.A., Kanz L., Pascolo S., Brossart P. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfsson O., Toropova K., Ranson N.A., Stockley P.G. Mutually-induced conformational switching of RNA and coat protein underpins efficient assembly of a viral capsid. J. Mol. Biol. 2010;401:309–322. doi: 10.1016/j.jmb.2010.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel B., Braedel S., Probst J., Carralot J.P., Wagner H., Schild H., Jung G., Rammensee H.G., Pascolo S. Immunostimulating capacities of stabilized RNA molecules. Eur. J. Immunol. 2004;34:537–547. doi: 10.1002/eji.200324198. [DOI] [PubMed] [Google Scholar]
- Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Stevenson J., Hymas W., Hillyard D. The use of armored RNA as a multi-purpose internal control for RT-PCR. J. Virol. Methods. 2008;150:73–76. doi: 10.1016/j.jviromet.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P.G., Rolfsson O., Thompson G.S., Basnak G., Francese S., Stonehouse N.J., Homans S.W., Ashcroft A.E. A simple, RNA-mediated allosteric switch controls the pathway to formation of a T = 3 viral capsid. J. Mol. Biol. 2007;369:541–552. doi: 10.1016/j.jmb.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P.G., Stonehouse N.J., Valegård K. Molecular mechanism of RNA phage morphogenesis. Int. J. Biochem. 1994;26:1249–1260. doi: 10.1016/0020-711x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stockley P.G., Stonehouse N.J., Walton C., Walters D.A., Medina G., Macedo J.M., Hill H.R., Goodman S.T., Talbot S.J., Tewary H.K., Golmohammadi R., Liljas L., Valegtird K. Molecular mechanism of RNA-phage morphogenesis. Biochem. Soc. Trans. 1993;21:627–634. doi: 10.1042/bst0210627. [DOI] [PubMed] [Google Scholar]
- Stonehouse N.J., Stockley P.G. Effects of amino acid substitution on the thermal stability of MS2 capsids lacking genomic RNA. FEBS Lett. 1993;334:355–359. doi: 10.1016/0014-5793(93)80711-3. [DOI] [PubMed] [Google Scholar]
- Stonehouse N.J., Valegård K., Golmohammadi R., van den Worm S., Walton C., Stockey P.G., Liljas L. Crystal structures of MS2 capsids with mutations in the subunit FG loop. J. Mol. Biol. 1996;256:330–339. doi: 10.1006/jmbi.1996.0089. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Hebert R.R., Hartman K.A. Ribonucleoprotein complexes formed between bacteriophages MS2 RNA and MS2 protein in vitro. J. Mol. Biol. 1967;25:455–463. doi: 10.1016/0022-2836(67)90198-2. [DOI] [PubMed] [Google Scholar]
- Sun S., Li W., Sun Y., Pan Y., Li J. A new RNA vaccine platform based on MS2 virus-like particles produced in saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2011;407:124–128. doi: 10.1016/j.bbrc.2011.02.122. [DOI] [PubMed] [Google Scholar]
- Tang Y., Luo X., Cui H., Ni X., Yuan M., Guo Y., Huang X., Zhou H., de Vries N., Tak P.P., Chen S., Shen N. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- Toropova K., Basnak G., Twarock R., Stockley P.G., Ranson N.A. The three-dimensional structure of genomic RNA in bacteriophage MS2: implications for assembly. J. Mol. Biol. 2008;375:824–836. doi: 10.1016/j.jmb.2007.08.067. [DOI] [PubMed] [Google Scholar]
- Toropova K., Stockley P.G., Ranson N.A. Visualising a viral RNA genome poised for release from its receptor complex. J. Mol. Biol. 2011;408:408–419. doi: 10.1016/j.jmb.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Tumban E., Peabody J., Tyler M., Peabody D.S., Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papilloma virus. PLoS One. 2012;7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O.C. A coat for all sequences. Nat. Struct. Biol. 1998;5:174–176. doi: 10.1038/nsb0398-174. [DOI] [PubMed] [Google Scholar]
- Ulmer J.B., Mason P.W., Geall A., Mandl C.W. RNA-based vaccines. Vaccine. 2012;30:4414–4418. doi: 10.1016/j.vaccine.2012.04.060. [DOI] [PubMed] [Google Scholar]
- Valegård K., Liljas L., Fridborg K., Unge T. Structure determination of the bacteriophage MS2. Acta Crystallogr. B. 1991;47:949–960. doi: 10.1107/s0108768191006821. [DOI] [PubMed] [Google Scholar]
- Valegård K., Liljas L., Fridborg K., Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Van Lierop M.J., van Maanen K., Meloen R.H., Rutten V.P., de Jong M.A., Hensen E.J. Proliferative lymphocyte responses to foot-and-mouth disease virus and three FMDV peptides after vaccination or immunization with these peptides in cattle. Immunology. 1992;75:406–413. [PMC free article] [PubMed] [Google Scholar]
- Van Lint S., Heirman C., Thielemans K., Breckpot K. mRNA: from a chemical blueprint for protein production to an off-the-shelf therapeutic. Hum. Vaccin Immunother. 2013;9:265–274. doi: 10.4161/hv.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint S., Thielemans K., Breckpot K. mRNA: delivering an antitumor message? Immunotherapy. 2011;3:605–607. doi: 10.2217/imt.11.28. [DOI] [PubMed] [Google Scholar]
- Wang G., Sun Y., Zhang K., Jia T., Hao M., Zhang D., Chang L., Zhang L., Zhang R., Lin G., Peng R., Li J. External quality assessment of molecular detection of ebola virus in China. PLoS One. 2015;10:e0132659. doi: 10.1371/journal.pone.0132659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lu Z., Wientjes M.G., Au J.L. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, B., Wei, Y., Zhang, K., Wang, J., Xu, R., Zhan, S., Lin, G., Wang, W., Liu, M., Wang, L., Zhang, R., Li, j., 2009. Development of an antisense RNA delivery system using conjugates of the MS2 bacteriophage capsids and HIV-1 TAT cell-penetrating peptide. Biomed. Pharmacother. 63, 313–318. [DOI] [PubMed]
- Wei Y., Yang C., Wei B., Huang J., Wang L., Meng S., Zhang R., Li J. RNase-resistant virus-like particles containing long chimeric RNA sequences produced by two-plasmid coexpression system. J. Clin. Microbiol. 2008;46:1734–1740. doi: 10.1128/JCM.02248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.T., Cheng S.C., Chan E.W., Sheng Z.T., Yan W.Y., Zheng Z.X., Xie Y. Plasmids encoding foot-and-mouth disease virus VP1 epitopes elicited immune responses in mice and swine and protected swine against viral infection. Virology. 2000;278:27–35. doi: 10.1006/viro.2000.0607. [DOI] [PubMed] [Google Scholar]
- Wu M., Sherwin T., Brown W.L., Stockley P.G. Delivery of antisense oligonucleotides to leukemia cells by RNA bacteriophage capsids. Nanomedicine. 2005;1:67–76. doi: 10.1016/j.nano.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Yao Y., Jia T., Pan Y., Gou H., Li Y., Sun Y., Zhang R., Zhang K., Lin G., Xie J., Li J., Wang L. Using a novel MicroRNA delivery system to inhibit osteoclastogenesis. Int. J. Mol. Sci. 2015;16:8337–8350. doi: 10.3390/ijms16048337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H., Zaks T.Z., Wang R.F., Irvine K.R., Kammula U.S., Marincola F.M., Leitner W.W., Restifo N.P. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999;5:823–837. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltins A. Construction and characterization of virus-like particles: a review. Mol. Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan S., Li J., Xu R., Wang L., Zhang K., Zhang R. Armored long RNA controls or standards for branched DNA assay for detection of human immunodeficiency virus type 1. J. Clin. Microbiol. 2009;47:2571–2576. doi: 10.1128/JCM.00232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Sun Y., Jia T., Zhang L., Wang G., Zhang R., Zhang K., Lin G., Xie J., Wang L., Li J. External quality assessment for the detection of measles virus by reverse transcription-PCR using armored RNA. PLoS One. 2015;10:e0134681. doi: 10.1371/journal.pone.0134681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sun Y., Chang L., Jia T., Wang G., Zhang R., Zhang K., Li J. A novel method to produce armored double-stranded DNA by encapsulation of MS2 viral capsids. Appl. Microbiol. Biotechnol. 2015;99:7047–7057. doi: 10.1007/s00253-015-6664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.Z., Hoon D.S., Huang S.K., Fujii S., Hashimoto K., Morishita R., Kaneda Y. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum. Gene Ther. 1999;10:2719–2724. doi: 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- Zlotnick A., Mukhopadhyay S. Virus assembly, allostery and antivirals. Trends Microbiol. 2011;19:14–23. doi: 10.1016/j.tim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]