Highlights
-
•
This review summarizes current knowledge on the norovirus RdRp.
-
•
Multiple X-ray structures of norovirus RdRp show important conformational changes.
-
•
Norovirus RdRp recognizes specific promotor sequences to initiate RNA synthesis.
-
•
Anti-HCV nucleoside analogs such as 2CM-C also inhibit Norovirus RdRp.
-
•
Suramin and its analogs act as allosteric non-nucleoside polymerase inhibitors.
Abbreviations: 2CM-C, 2′-C-methyl-cytidine; 2′-F-2′-C-MeC, 2′-fluroro-2′-C-methyl-cytidine (PSI-6130); ACT, 2′-amino-cytidine triphosphate; EC50, half-maximal effective concentration; GI–GVII, norovirus genogroup designations; GII.4, genogroup II genotype 4; HCV, hepatitis C virus; HuNoV, human norovirus; MNV, murine norovirus; IC50, half-maximal inhibitory concentration; NCT, 5-nitrocytidine triphosphate; NIC02, a phenylthiazole carboxamide; NIC04, a pyrazole acetamide; NIC10, a triazole; NIC12, a pyrazolidinedione; NS, nonstructural; NTP, nucleoside triphosphate; ORF, open reading frame; PPNDS, pyridoxal-5′-phosphate-6-(2′-naphthylazo-6′-nitro-4′,8′-disulfonate; RdRp, RNA-dependent RNA polymerase; RTP, ribofuranosyl 5′-triphosphate; VP1, major capsid protein; VP2, minor capsid protein
Keywords: Norovirus, Caliciviridae, RNA-dependent RNA polymerase (RdRp), Virus inhibitors, Nucleoside analogs, Non-nucleoside inhibitor
Abstract
Noroviruses belong to the Caliciviridae family of single-stranded positive-sense RNA viruses. The genus Norovirus includes seven genogroups (designated GI-GVII), of which GI, GII and GIV infect humans. Human noroviruses are responsible for widespread outbreaks of acute gastroenteritis and represent one of the most common causes of foodborne illness. No vaccine or antiviral treatment options are available for norovirus infection. The RNA-dependent RNA polymerase (RdRp) of noroviruses is a key enzyme responsible for transcription and replication of the viral genome. Here, we review the progress made in understanding the structures and functions of norovirus RdRp and its use as a target for small molecule inhibitors. Crystal structures of the RdRp at different stages of substrate interaction have been determined, which shed light on its multi-step catalytic cycle. The in vitro assays and in vivo animal models that have been developed to identify and characterize inhibitors of norovirus RdRp are also summarized, followed by an update on the current antiviral research targeting different regions of norovirus RdRp. In the future, structure-based drug design and rational optimization of known nucleoside and non-nucleoside inhibitors of norovirus RdRp may pave the way towards the next generation of direct-acting antivirals.
1. Introduction to noroviruses
Noroviruses are the primary cause of foodborne illness globally, resulting in sometimes massive outbreaks of gastroenteritis. In this section, we describe in more details the medical burden associated with norovirus, the current status of biomedical efforts to address norovirus infection, and the norovirus genome organization.
1.1. Medical burden of human norovirus infection
Human noroviruses (HuNoVs) are Category B biodefense agents and the major cause of viral gastroenteritis. Less than twenty viral particles can establish a norovirus infection and prior exposure does not lead to protection from a repeat infection. The clinical symptoms of norovirus infection include vomiting, diarrhea, abdominal cramps, and nausea, usually lasting 2–4 days. The Centers for Disease Control and Prevention estimates that 900,000 clinic visits among children result from norovirus infection in developed countries and up to 200,000 deaths annually in children less than 5 years of age in developing countries (Patel et al., 2009, Scallan et al., 2011). In the United States alone, norovirus-associated foodborne illness is responsible for about 20 million cases yearly, with more than 70,000 hospitalizations and about 800 deaths (Hall et al., 2012, Lopman et al., 2012). In healthy adults, norovirus causes acute self-limiting disease lasting 1–3 days, but it causes long-lasting infection in elderly, juvenile, and immunocompromised patients. Noroviruses are a significant cause of morbidity and mortality in immunocompromised patients such as transplant recipients (Boillat Blanco et al., 2011, Capizzi et al., 2011, Roos-Weil et al., 2011, Saif et al., 2011, Schorn et al., 2010, Schwartz et al., 2011). Norovirus infections in infants can lead to seizures (Chen et al., 2009, Medici et al., 2010) and have been linked to necrotizing enterocolitis (Turcios-Ruiz et al., 2008). In adults, norovirus infection can exacerbate inflammatory bowel disease (Khan et al., 2009). Increased disease severity is linked to the use of statins to regulate cholesterol levels (Rondy et al., 2011). Cultured cells treated with statins increases norovirus replication, which is especially a concern given the number of patients using statins (Mann et al., 2008). Despite the clear need for medical intervention to prevent and treat norovirus infection, no approved vaccines or small molecule treatment options are currently available.
1.2. Strategies for preventing and controlling norovirus infection
Both vaccines and small molecules are currently being evaluated as potential strategies for medical intervention in cases of norovirus infection.
1.2.1. Vaccines
Although vaccines have been successfully developed to treat diarrheal diseases caused by other RNA viruses such as rotaviruses, efforts to develop anti-norovirus vaccines based on live-attenuated virus have been hampered by the inability to grow HuNoV efficiently in cell culture. Therefore, current strategies for developing anti-norovirus vaccines rely on the production of virus-like particles from recombinant and self-assembling protein capsids (for recent review: (Tan and Jiang, 2014), (Richardson et al., 2013)). Norovirus-like particles can be produced from a variety of expression systems such as baculovirus infection of insect cells, which is among the most advanced and is currently used in the manufacture of clinical trial materials. Both orally and nasally administered virus-like particles have been well tolerated and immunogenic in Phase 1 clinical trials and even conferred some protection against norovirus gastroenteritis in healthy adults in a human experimental challenge model (Atmar et al., 2011, El-Kamary et al., 2010, Tacket et al., 2003). A bivalent norovirus-like particle candidate that is safe and immunogenic in adults is currently under evaluation for safety in children and in the elderly and for efficacy in adults (Treanor et al., 2014) (Clinical Trial Identifiers NCT02153112, NCT02661490, and NCT02669121).
1.2.2. Small molecules
To date, the only small molecule tested in the clinic as a potential anti-norovirus drug is nitazoxanide, which was originally developed and commercialized as an antiprotozoal agent. Nitazoxanide has demonstrated some benefit in treating adults suffering from norovirus gastroenteritis (Rossignol and El-Gohary, 2006, Siddiq et al., 2011). While nitazoxanide’s mechanism of action is not well understood, it probably involves multiple host factors rather than directly targeting a norovirus protein. Nitazoxanide also inhibits a broad range of other RNA and DNA viruses including the respiratory syncytial virus, parainfluenza virus, coronavirus, rotavirus, hepatitis B virus, hepatitis C virus, dengue virus, yellow fever virus, Japanese encephalitis virus, and the human immunodeficiency virus (Rossignol, 2014).
Antiviral approaches involving direct-acting antiviral agents remain at early stages of preclinical research and mainly target two viral proteins: the protease and the RNA polymerase (Kaufman et al., 2014, Weerasekara et al., 2016). The following sections of this review focus on the structure, function, and small-molecule inhibition of norovirus polymerase.
1.3. Genomic organization and function of viral-encoded proteins
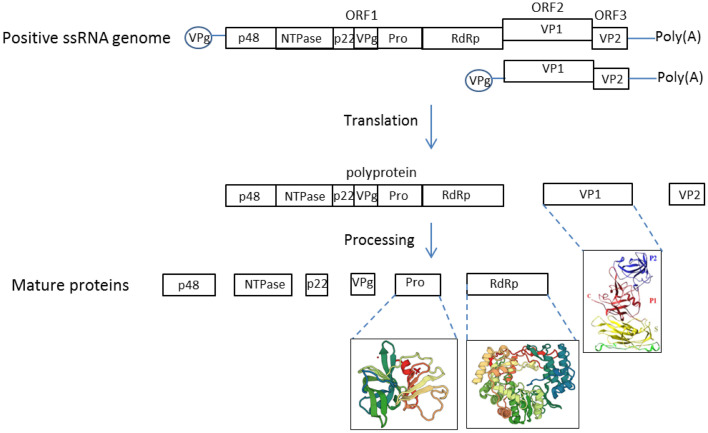
Noroviruses are a genetically diverse group within the Caliciviridae family, which includes six genera: Vesivirus, Lagovirus, Nebovirus, Sapovirus, Recovirus, and Norovirus. The genus Norovirus is subdivided into seven genogroups (designated GI–GVII), based on phylogenetic analysis of the capsid gene (Vinje, 2015). Genogroups GI, GII and GIV infect humans and cause acute gastroenteritis. Norovirus strains commonly isolated in cases of acute gastroenteritis belong to GI and GII. Within these two groups, cases of GII genotype 4 (GII.4) infection account for the majority of outbreaks of gastroenteritis. Noroviruses are non-enveloped viruses with positive-stranded genomes of 7400–7700 nucleotides. The genome typically contains three open reading frames (ORFs), named ORF1-3 (Clarke and Lambden, 2000, McFadden et al., 2011) (Fig. 1 ). ORF2 and ORF3 encode the major and minor structural capsid proteins VP1 and VP2, respectively. Ninety dimers of VP1 form the icosahedral capsid of norovirus (Donaldson et al., 2010). VP1 contains two major domains: the shell domain and the protruding domain that binds to receptors on host cell surfaces (Prasad et al., 1999, Strong et al., 2012). Only a few copies of VP2 reside on the interior surface of the capsid (Vongpunsawad et al., 2013). ORF1, located in the first two-thirds of the genome encodes a ∼200 kDa polyprotein that is proteolytically processed by the virus-encoded protease to yield 6 non-structural proteins named P48 (NS1/2), NTPase (NS3), P22 (NS4), VPg (NS5), Pro (NS6), and Pol (NS7) (Belliot et al., 2003) (Fig. 1). The function of these non-structural proteins is listed in Table 1 . P48 and P22 are involved in viral replication complex formation by recruiting host membrane vesicles. P48 promotes disassembly of the Golgi complex and prevents cell surface proteins expression and trafficking to facilitate the recruitment of cellular membranes to its replication complex (Ettayebi and Hardy, 2003, Fernandez-Vega et al., 2004). P22 contains an endoplasmic reticulum export signal that was proposed to promote P22 uptake into the coat protein complex II vesicles, leading to vesical mislocalization and Golgi body disassembly (Sharp et al., 2012, Sharp et al., 2010). Together, P22 and P48 interrupt the host protein secretion pathway and are likely responsible for the rearrangement of the cellular membrane and recruitment of membrane for the replication factory. VPg is covalently linked to the 5′ end of the genome and the subgenomic RNA. The linkage of VPg to viral RNA is thought to occur during viral genome replication whereby VPg is attached as a protein primer to the 5′ terminus of the genomic RNAs (Rohayem et al., 2006b). Protein-primed initiation of RNA synthesis is also found in Picornaviruses and Potyviruses (Goodfellow, 2011).
Fig. 1.
Genome organization of the human norovirus. HuNoV genome organization, translation and processing. The VPg linked positive single-stranded RNA genome of HuNoV contains three open reading frames, ORF1, ORF2 and ORF3. The subgenomic RNA only contains ORF2 and ORF3. The ORF1 is translated by host translation machinery into a single polypeptide which then is proteolytically cleaved by the viral protease into six nonstructural proteins, named p48, NTPase, p22, VPg, Protease (Pro), and the RNA dependent RNA polymerase (RdRp). ORF2 and ORF3 are translated into the major and the minor capsid proteins, named VP1 and VP2, respectively. Currently available structures of norovirus proteins are illustrated under each named protein. VP1 PDB: 1IHM, RdRp PDB: 1SH0, protease PDB: 2FYQ.
Table 1.
Name and function of norovirus proteins.
| Protein Name | Function(s) |
|---|---|
| P48 (NS1/2) | Viral replication complex formation, recruit host membrane vesicles |
| NTPase (NS3) | NTPase and RNA helicase |
| P22 (NS4) | Viral replication complex formation, recruit host membrane vesicles |
| VPg (NS5) | Protein primer for genomic and subgenomic RNA replication; interact with protein synthesis machinery and facilitate protein synthesis |
| Pro (NS6) | Cleave the polyprotein translated from ORF1 |
| Pol (NS7) | RdRp responsible for viral RNA synthesis |
| VP1 | Major capsid protein, part of the viral capsid, SD domain interact with RdRp and stimulate viral replication |
| VP2 | Minor capsid protein, part of the viral capsid |
NS: Nonstructural; NTP: Nucleoside triphosphate; ORF: Open reading frame; RdRp: RNA‐dependent RNA polymerase; VP: Viral protein.
2. Structure and functions of norovirus polymerase
The norovirus RdRp (NS7) is the central enzyme in norovirus RNA replication. Viral RdRps are important targets for antivirals, as evidenced by the efficacy of drugs that inhibit the hepatitis C virus (HCV) RdRp and contribute to cures of HCV infection. Therefore, structural and functional studies of norovirus RdRp are important not only to understand norovirus replication, but also for guiding rational antiviral drug candidate selection and development.
2.1. Structural features of norovirus polymerase
Multiple high-resolution structures of HuNoV RdRp have been determined (Ng et al., 2004, Zamyatkin et al., 2008, Zamyatkin et al., 2009, Zamyatkin et al., 2014, Croci et al., 2014a, Croci et al., 2014b, Mastrangelo et al., 2012). These include structures from the GI Norwalk virus and the GII.4 HuNoV that are responsible for the majority of human infections. Additional structures for related viruses in the Caliciviridae family have also been determined (Alam et al., 2012). The structures are for the apo-enzyme, RdRp with divalent metals bound at the active site, RdRp bound to nucleoside triphosphates (NTPs), the ternary complex with the template RNA and a primer, and several ternary complexes with nucleotide analogs. Structures of the HuNoV RdRp bound to allosteric compounds that inhibit RNA synthesis are also known (Croci et al., 2014a). Thus, there is extensive knowledge about the structures of the HuNoV RdRp as they relate to RdRp function.
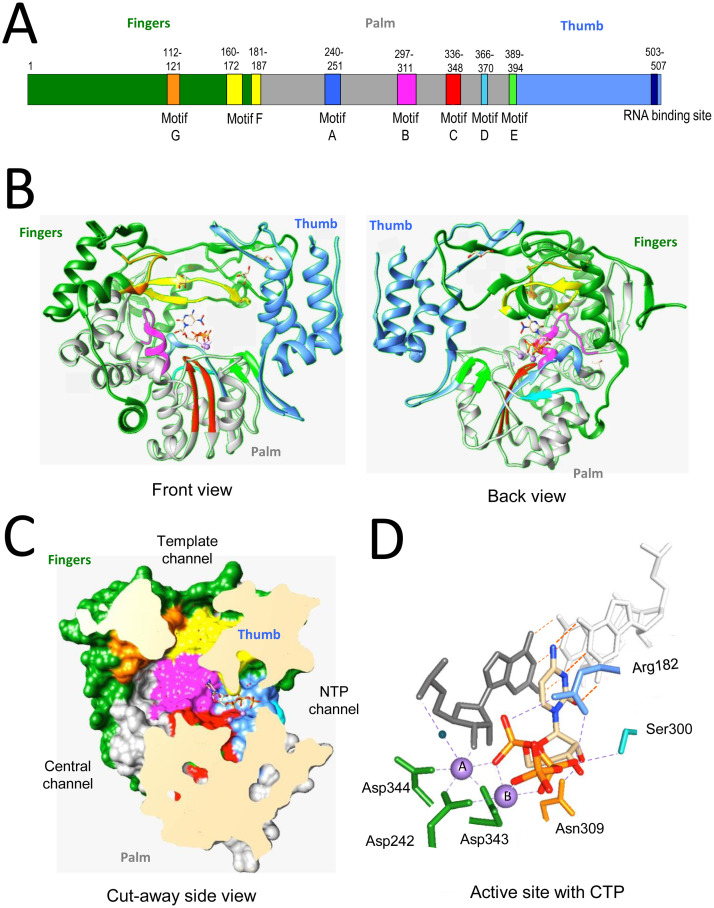
The structure of the norovirus RdRp is highly similar to those of other positive-strand RNA viruses (Alam et al., 2012, Hogbom et al., 2009, Ng et al., 2004, Zamyatkin et al., 2008). The RdRp can be described as a partially closed right hand, with fingers, thumb, and palm subdomains (Fig. 2 A and B). The fingers and the thumb subdomains interact to close the hand structure (Fig. 2B), forming a channel where the single-stranded template RNA can thread into the polymerase. The template channel merges with the central channel where the 3′ end of template RNA and the nascent RNA exit (Fig. 2C). At the confluence of the template and central channel is the NTP channel where NTPs enter. Lining the channels are highly conserved residues organized into motifs A through G, which interact with the template, the nascent RNA, and the NTPs for RNA synthesis. The active site lies at the convergence of the template channel and the NTP channel and contains aspartate residues that coordinate divalent metals to promote nucleotide polymerization (Fig. 2) (Alam et al., 2012, Zamyatkin et al., 2009).
Fig. 2.
Structure of norovirus polymerase. (A) Schematic of the HuNoV NS7 protein. The fingers, palm and thumb subdomains are colored green, grey and cornflower blue. The motifs are highlighted in distinct colors and the numbers above the motifs denote the amino acid residues that encode the motifs. The structures are based on the structure of the HuNoV complex complexed to the ternary complex and associated with CTP (pdb: 3BSN). (B) Ribbon structures of the NS7 protein. The subdomains are colored green, grey and Motifs A through F are in, respectively, light blue, magenta, grey, and cornflower blue. The motifs and their colors are: A: blue, B: magenta, C: red, D: sky blue, E: green. The front and back views are rotated by 180 °C. (C) A cut-away view of the HuNoV RdRp that illustrates the locations of the template channel, the central channel, and the NTP channel. The locations of motifs are colored as in panel C. (D) Recognition of CTP in the HuNoV ternary complex. Divalent metals, manganese that are coordinated by the active site aspartates are in purple. A water molecule that is used to H-bond to the template RNA is shown as a blue sphere. Only the side chains of amino acids that recognize the CTP are shown.
2.2. The active site of norovirus RdRp
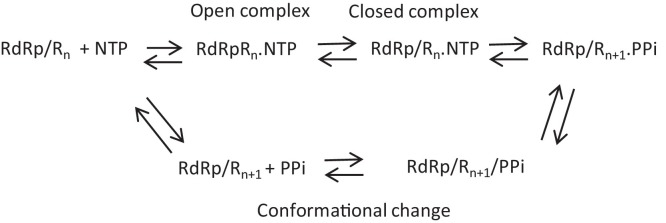
RNA synthesis by the norovirus RdRp active site occurs by a 5-step reaction that is well conserved in RdRps of different viral species (Fig. 3 ). The five steps are: (1) RdRp bound to the template binds an NTP complementary to the template base to form an open ternary complex; (2) A change in the conformation to form a closed complex; (3) Nucleotidyl transfer and translocation of the template; (4) Conformational change in the complex; (5) Release of the pyrophosphate that allows another NTP to bind to the active site. The steps in RNA synthesis involve significant conformational changes in the RdRp and the high quality structures have captured several distinct conformations. The apo-enzyme has the RdRp C-terminal tail bound to its active site (Ng et al., 2004). The ternary complex of the Norwalk virus RdRp during elongative RNA synthesis that was determined by Zamyatkin et al. (2008) shows that the binding of the template and primer RNA and the substrate NTP displaced the C-terminal tail from the active site and induced a rotation of the central helix in the thumb subdomain of the RdRp to form a binding pocket for the primer RNA (Zamyatkin et al., 2008). This effectively represents the closed conformation of the RdRp trapped just prior to nucleotidyl transfer.
Fig. 3.
The steps in catalysis of RNA extension by the norovirus RdRp.
The active site of the palm subdomain coordinates two divalent metals that mediate catalysis by the RdRp (Fig. 2D). Metal A forms an octahedral coordination with Asp242 (Motif A), Asp343, and Asp344 (Motif C), the 3′ OH of the primer, the α-phosphate of the NTP, and a water molecule. The water is activated to be a general base to extract a proton from the primer 3′OH to enable it to become a better nucleophile. Metal B also forms an octahedral coordination between the carboxylate groups of Asp242, Asp343, and Tyr243 and oxygens of all three phosphates in the NTP. The side chains of the highly conserved Arg182 in Motif F of the RdRp forms electrostatic interaction with the α-, β-phosphates of the incoming NTP, likely to stabilize the pyrophosphate as a leaving group. The ribose of the NTP is stabilized by van der Waals forces. In addition, the 2′ OH forms an H-bond with Asn309 and donates an H-bond to Ser300 that donates an H-bond to Asp247 (Fig. 2D). This network of interactions allows the RdRp to distinguish ribonucleotides from deoxyribonucleotides. Following nucleotidyl transfer and the release of the pyrophosphate, the RdRp active site remains in a closed conformation, but helices in the thumb subdomain return to the state similar to that seen in the apo-protein (Zamyatkin et al., 2014). This leads to a loosening of the contacts with the RNA duplex, likely to facilitate the translocation of the RNA as well as to open the pocket for binding of the next nucleotide.
2.3. Initiation of RNA synthesis by norovirus RdRp
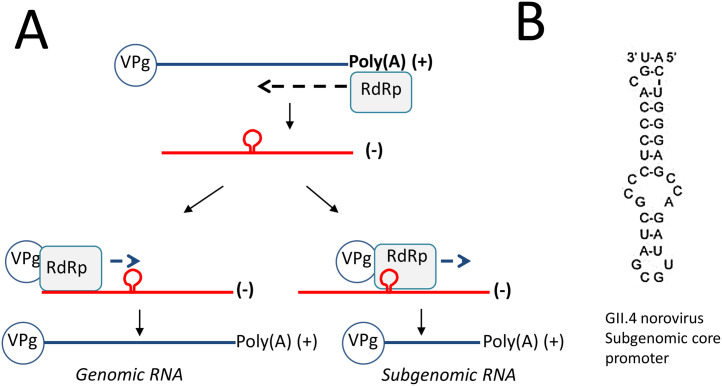
Accurate initiation of RNA synthesis is critical to prevent the loss of viral genetic information (Kao et al., 2001). The norovirus RdRp specifically recognizes the viral genome for RNA synthesis. In murine norovirus (MNV) the positive- or minus-sense RNA from the viral sgRNA stimulates VPg nucleotidylation, possibly by stabilizing the structure of the viral RdRp (Goodfellow, 2011). The MNV and HuNoV variants can also preferentially recognize RNA segments to direct initiation of the RNA towards subgenomic RNA (Lin et al., 2015). Subgenomic RNA synthesis by caliciviruses occurs by internal initiation on the minus-strand RNA (Morales et al., 2004) (Fig. 4 A). Within the minus-strand RNA, a highly conserved stem-loop structure upstream of the start site of the sgRNA initiation nucleotide was identified in all caliciviruses (Fig. 4B). In MNV, mutations in this RNA structure debilitated MNV sgRNA synthesis and suppressor mutations that restored the RNA structure were found to restore MNV infectivity (Simmonds et al., 2008, Yunus et al., 2015). NS7 from MNV and HuNoV preferentially recognize their cognate subgenomic promoters and a short stretch of template sequence that contributes to accurate initiation of the subgenomic RNAs and efficient RNA synthesis. Crosslinking of the subgenomic RNA hairpin to the MNV and HuNoV RdRp showed that helix 1 in the thumb subdomain of the MNV RdRp contacts the MNV subgenomic promoter hairpin. Notably, this mode of recognition of the promoter for RNA synthesis is similar to that of phage RNA polymerases, which recognize promoter sequences for accurate initiation of RNA synthesis (Cheetham et al., 1999, Imburgio et al., 2000).
Fig. 4.
Mechanism of RNA replication by norovirus RdRp. (A) Norovirus RdRp recognizes the 3′ end of the viral RNA(+) genome (blue), and produces the negative-strand intermediate by de novo initiation of RNA synthesis (red). This RNA serves as template to recruit VPg as primer for synthesis of new RNA(+) copies of viral genomic RNA. Subgenomic RNA is also produced when norovirus RdRp recognizes a promoter sequence present on the downstream of the VP-1 gene on the negative-strand intermediate RNA. (B) Promoter sequence for human norovirus genome.
2.4. Other viral proteins involved in RNA synthesis by the norovirus RdRp
Norovirus can initiate RNA synthesis through two distinct modes (Rohayem et al., 2006b) (Fig. 4A). The replication intermediate minus-strand RNA is thought to be initiated by a de novo mechanism (i.e., in the absence of a pre-existing primer). In this case, the first nucleotide serves as a primer to provide the ribose 3′ OH for the attachment of subsequent nucleotides. De novo initiation allows RNA synthesis to take place opposite to the 3′-terminal nucleotide in the template RNA, thus preventing the loss of genetic information. The positive-strand genomic RNA and the subgenomic RNAs contain the protein VPg covalently attached to their 5′ terminus. The NS7 protein or the unprocessed NS6-7 proteins are involved in both de novo-initiated and VPg-primed RNA synthesis (Belliot et al., 2005).
VPg is essential for translation initiation from the norovirus genomic and subgenomic RNAs and appears to also act in the recruitment of ribosomes (Daughenbaugh et al., 2003, Thorne and Goodfellow, 2014). For RNA synthesis, the norovirus VPg contains a tyrosine, likely Tyr27 for HuNoV, whose hydroxyl side chain is involved in RNA synthesis, which has recently been confirmed by mass spectrometry (Olspert et al., 2016). In vitro, purified NS7 can add uridylates to purified VPg and then use the VPg-polyU to prime the synthesis of the full-length subgenomic RNA (Rohayem et al., 2006b). VPg-primed RNA synthesis was not observed with minimal promoter-RNA templates, perhaps due to the lack of a sequence such as the cis-replication element that directs the uridylation of the VPg (Lin et al., 2015). The cis-acting replication element of noroviruses remain to be identified. De novo nucleotide-initiated norovirus subgenomic RNA synthesis suggests that the VPg could be recognized in a way functionally equivalent to an initiation NTP. VPg also increases the activity of the RdRp in a cell-based reporter assay without the production of VPg-primed RNA, suggesting that VPg can promote a state of the RdRp that is more competent for RNA synthesis (Subba-Reddy et al., 2011, Chaudhry et al., 2006). Mutation in the nucleotidylation residue reduced the stimulatory effect. The structure of the central region of the MNV VPg determined by nuclear magnetic resonance spectroscopy supports the hypothesis that a large structural rearrangement in the RdRp is required for it to recognize the cognate tyrosine needed for uridylation (Leen et al., 2013). However, details of the molecular interaction between NS7 and VPg are unknown, mainly because a crystal structure of the protein complex is not yet available.
Activities of the norovirus RdRp are also modulated by the norovirus capsid proteins. VP1 can enhance RNA synthesis by the RdRp in a concentration-dependent manner when the proteins are co-expressed in cells. The shell domain of VP1 is responsible for the stimulatory activity and can bind to the RdRp (Subba-Reddy et al., 2012). Overexpression of the MNV VP1 also stimulated the kinetics of MNV replicon replication in cells. The VP1 enhancement of RNA synthesis is species specific since the MNV VP1 could not enhance the HuNoV GII.4 RdRp activity. A regulatory role of the activity of viral structural proteins on viral RNA synthesis is becoming an increasingly important theme in RNA virology (Ni and Kao, 2013).
2.5. Role of norovirus RdRp in pathogenesis and epidemiology
A significant increase in the global incidence of norovirus outbreaks since 2002 has been associated with the emergence of the highly transmittable HuNoV GII.4 lineage (Lopman et al., 2004, Siebenga et al., 2009). In this pandemic strain, the RdRp displays reduced replication fidelity, which results in higher mutation rate and rate of viral evolution compared to the nonpandemic and less frequently detected strains (Bull et al., 2010). During the winter season of 2014–2015, a newly emerging GII.17 isolate outcompeted the GII.4 in parts of Asia, with risk of spreading globally (Lu et al., 2016). The GII.17 isolate displays evolutionary rates at least 1 order of magnitude higher than those seen with GII.4, further establishing the role of the RdRp in the emergence of new epidemic strains (Chan et al., 2015). The hypothesis that RdRp-driven genetic diversity is vital for viral fitness and pathogenesis is not unique to norovirus, and has been well documented for other virus families such as Picornaviruses (Vignuzzi et al., 2006, Peersen, 2017). Consistent with reports on the poliovirus polymerase, Arias et al. reported a high-fidelity MNV polymerase variant associated with the I391L mutation (Arias et al., 2016). When mutated at this position, the corresponding MNV clone displayed delayed replication kinetics in vivo and lower transmission rate compared to the wild-type virus. The impact of a single point mutation in the RdRp on viral replication and transmission further highlights the dynamic interplay between replication fidelity, viral fitness, and the emergence of dominant strains of norovirus.
3. Inhibitors of norovirus polymerase
Although a number of small molecule anti-norovirus agents have been described, the majority of them remain at early stages of preclinical development. This section reviews these efforts, as well as the assays that have been developed to study such molecules.
3.1. In vitro assays and in vivo models available to characterize norovirus polymerase inhibitors
3.1.1. Biochemical and biophysical tools
The production of recombinant norovirus NS7 protein is amenable to high yield and purity, which has facilitated the development of norovirus polymerase assays used for inhibitor testing. The most common RdRp assays are based on the incorporation of a radiolabeled nucleotide onto the RNA primer strand (Fukushi et al., 2004, Rohayem et al., 2006a). RNA synthesis is initiated de novo on nonpolyadenylated negative-strand RNAs containing a specific promoter sequence. Although gel-based assays provide qualitative information about the types of RNAs formed enzymatically, their application to inhibitor testing was optimized by adapting the radiometric readout to a 96-well format for higher throughput purposes (Jin et al., 2015). The use of fluorescence readout based on the binding of the PicoGreen dye to double-stranded RNA products even further enhanced the compatibility of norovirus RdRp assay with high-throughput screening (Mastrangelo et al., 2012). As described earlier in this review, X-ray crystallography is also a valuable tool to understand the binding interactions at the atomic level between norovirus RdRp and small molecule inhibitors. This has been done both with the apo-protein and with protein in complex with primer/template (Ng et al., 2004, Zamyatkin et al., 2008). Finally, other biophysical methods have been employed to characterize the binding interaction between norovirus polymerase and small molecules (e.g., the thermal-shift assay measuring changes in melting temperature associated with compound binding (Mastrangelo et al., 2012) and the fluorescent polarization assay to measure protein-RNA binding (Lin et al., 2015)).
3.1.2. Cell-based assays
Until recently, all attempts to develop a robust HuNoV in vitro replication assay have failed because of the lack of permissive cells, including human macrophages and dendritic cells from susceptible humans (Duizer et al., 2004, Herbst-Kralovetz et al., 2013, Lay et al., 2010, Papafragkou et al., 2014, Takanashi et al., 2014). Despite this limitation, the in vitro effect of small molecules on HuNoV replication has been studied using a stable human hepatoma cell line expressing the part of the Norwalk virus RNA genome encoding the non-structural proteins and carrying the neomycin resistance gene (Chang et al., 2006). In this norovirus minigenome, or replicon, production of the viral RNA leads to the reconstitution of a functional norovirus RNA replication complex. The challenge associated with developing an authentic in vitro infection assay for HuNoV has also led to the exploration of calicivirus surrogates more amenable to cell-based assays. Multiple non-human enteric calicivirus systems have been described (for complete review, see Rocha-Pereira et al., 2014). Among them, MNV is the closest to HuNoV, and therefore the most broadly used for inhibition studies. MNV replicates well in mouse monocytes and macrophages (Wobus et al., 2004, Wobus et al., 2006). In 2014, Jones et al., demonstrated successful infection of human B cells with HuNoV from GII.4, using commensal bacteria as cofactor (Jones et al., 2015, Jones et al., 2014). The infection of B cells resulted in measurable translation of viral proteins and production of infectious progeny, and the increase in viral genome copies was significant, but overall low. B cells are not the only site of HuNoV infection. Ettayebi et al. recently reported the successful cultivation of human GII.4 norovirus in stem cell-derived human intestinal enteroids isolated from intestinal crypts in human intestinal tissues (Ettayebi et al., 2016). The addition of bile to enteroids was not required for GII.4 virus but enhanced its replication, while other strains only replicated in the presence of bile. Taken together, it is likely that the establishment of B cell and enteroid in vitro infection systems will find broad applications in the development of diagnostics, vaccines, and therapeutics.
3.1.3. In vivo models
Replication of HuNoV has been described using large animals such as chimpanzees, pigs, and calves (for review, see (Rocha-Pereira et al., 2014), but mouse is the most common species for in vivo inhibition studies with small molecules. Immunocompetent mice infected with MNV are typically asymptomatic and represent a natural host for MNV infection (Karst et al., 2003). For MNV-1 alone, reactive antibodies have been found in 22% of mouse serum samples from research colonies across the United States and Canada (Hsu et al., 2005). Other strains of norovirus such as MNV-3, MNV CR6, and MNV O7 have been shown to cause a persistent yet asymptomatic infection in immunocompetent mice (Arias et al., 2012, Hsu et al., 2006, Kahan et al., 2011, Shortland et al., 2014). MNV infection in immunocompromised mice lacking innate immunity factors such as STAT1 or interferon receptors can result in persistent or acute virus replication associated with severe diarrhea, weight loss, and mortality (Karst et al., 2003, Wobus et al., 2004). In particular, the knockout mouse strain AG129 defective of interferon alpha/beta and gamma receptors has been a widely utilized model to test the in vivo efficacy of norovirus polymerase inhibitors (Rocha-Pereira et al., 2013). This particular strain of mice is also used as model for dengue and Zika virus infection (Aliota et al., 2016, Johnson and Roehrig, 1999, Zmurko et al., 2016). Small-animal models have also been developed to study HuNoV infection. BALB/c mice deficient in recombination activation gene (Rag) 1 or 2 and common gamma chain (γc) (Rag-γc) are permissive to HuNoV infection (Taube et al., 2013).
3.2. Nucleoside analogs as inhibitors of norovirus polymerase
3.2.1. Ribavirin
Replicon cell lines expressing subgenomic components of Norwalk virus have enabled fast and simple testing of norovirus replication inhibitors. Ribavirin was one of the first small molecules identified to inhibit norovirus in in vitro replicon assays. Ribavirin reduced the viral genome and proteins with a weak half-maximal effective concentration (EC50) value of about 40 μM (Chang and George, 2007, Jin et al., 2015) (Fig. 5 A). Combining interferon-alpha with ribavirin showed additive effects on the inhibition of norovirus replication. The mechanism of action was evaluated by adding guanosine to the ribavirin treatment, resulting in moderately reversed antiviral effect. The reversal in the presence of guanosine indicates that ribavirin’s effect may be at least partially associated with the depletion of guanosine triphosphate in the cells, suggesting a mechanism other than direct targeting of the polymerase. Recent in vitro work from Julian et al. using MNV suggested that ribavirin treatment also increases viral quasispecies diversity, which could be contributing to its antiviral effects (Julian et al., 2016).
Fig. 5.
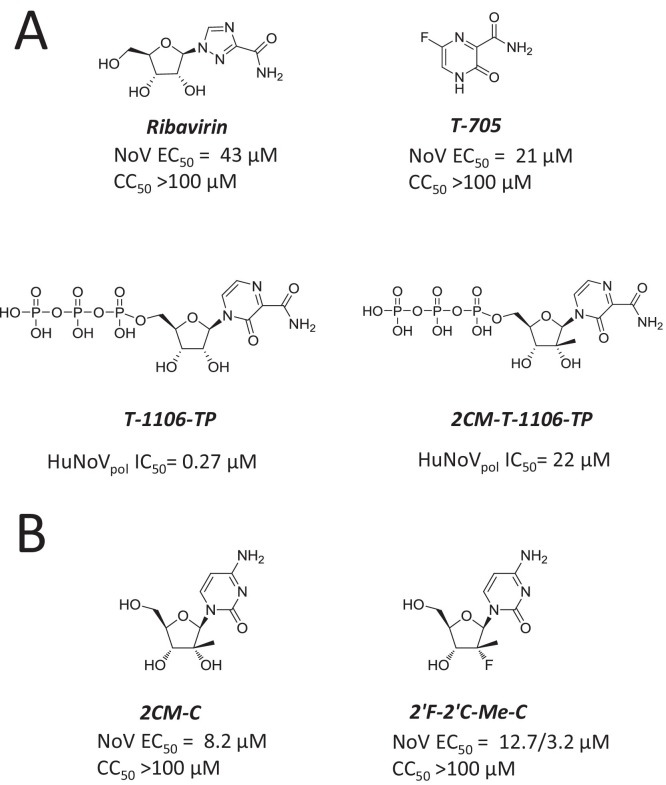
Nucleoside analogs inhibiting norovirus RdRp. (A) Structure and inhibition potency of ribavirin, T-705/Favipiravir, and T-705 ribonucleoside triphosphate analogs T-1106-TP and 2CM-T-1106-TP. (B) Structure and inhibition potency of 2′-C-methyl-cytidine (2-CMC) and 2′-C-methyl-2′-fluroro-cytidine (2′F-2′C-Me-C).
3.2.2. T-705 (Favipiravir) and its analogs
The nucleoside precursor 6-fluoro-3-hydroxy-2-pyrazinecarboxamide (T-705, favipiravir) is structurally related to ribavirin (Fig. 5A). T-705 was originally developed against influenza virus (for review: (Furuta et al., 2013)). The molecule has also been shown to inhibit other RNA viruses including members of Orthomyxo-, Noro-, Bunya-, Arena-, and Flaviviridae (Furuta et al., 2013). In vitro, the antiviral potency of T-705 against MNV replication is fairly modest, but more potent than ribavirin (Rocha-Pereira et al., 2012b) (Arias et al., 2014, Jin et al., 2015). Time-of-addition assays revealed that T-705 exerts its activity at a timepoint that coincides with the onset of viral RNA synthesis, suggesting that T-705 might target the polymerase of norovirus. In cells, T-705 is efficiently converted to a ribofuranosyl 5′-triphosphate form (T-705 RTP) by cellular enzymes (Furuta et al., 2009). Similar to previous work with influenza, T-705 RTP is recognized as substrate during norovirus RNA synthesis, which results in enzyme inhibition by delayed and incomplete chain termination (Furuta et al., 2005, Jin et al., 2013, Jin et al., 2015). Treatment of norovirus-infected cells with T-705 induces viral mutagenesis in vivo through a mechanism similar to that of ribavirin that may be associated with incorporation of the nucleotide analog into viral RNA (Arias et al., 2014). The intrinsically low antiviral potency of T-705 prevents experiments aimed to select drug-associated resistance mutations, which would otherwise represent an important orthogonal validation of its proposed mechanism of action. Attempts to identify analogs of T-705 with increased selectivity against human polymerases by adding a 2′C-methyl substitution had limited success, with a loss of recognition both by human mitochondrial RNA polymerase and norovirus RdRp (Jin et al., 2015) (Fig. 5A). Other promising analogs of T-705 have been tested against influenza virus and its polymerase, but antiviral effect of those analogs against the norovirus counterpart has not been reported (Wang et al., 2016).
3.2.3. 2CM-C and 2′-F-2′-C-MeC
The nucleoside analog 2′-C-methyl-cytidine (2CM-C) was originally developed against the polymerase of HCV and advanced as valopicitabine/NM283, an orally bioavailable 3′-valine ester prodrug (Pierra et al., 2006) (Fig. 5B). Although valopicitabine was efficacious in chronically HCV-infected patients, its clinical development was halted because of adverse events. 2CM-C was later found to inhibit the replication of MNV (Rocha-Pereira et al., 2012a). In this assay, viral RNA and virus-induced plaque formation were inhibited with an EC50 value of about 2 μM. Similar to prior work with T-705, time-of-addition assays demonstrated that 2CM-C acts during viral RNA synthesis, which is consistent with the viral RNA polymerase being the antiviral target. Target identification was confirmed in biochemical assays showing that 2CM-C triphosphate directly inhibits norovirus polymerase by terminating RNA synthesis (Jin et al., 2015). In vitro combination of 2CM-C with ribavirin induced a marked antagonistic antiviral effect by a mechanism that was not clearly understood (Rocha-Pereira et al., 2012a). In a separate study, however, no antagonism was observed between 2CM-C and ribavirin (Costantini et al., 2012). 2CM-C also inhibits HuNoV replication in a Norwalk virus replicon assay, with reduced inhibition potency compared with the MNV assay (Rocha-Pereira et al., 2013) (Jin et al., 2015). Importantly, the antiviral activity of 2CM-C against HuNoV was confirmed in the B‐cell line BJAB infection assay with an EC50 value of 0.3 μM, which is significantly more potent than the value obtained with the HuNoV replicon (Kolawole et al., 2016). Treatment of MNV-infected AG129 mice with 2CM-C prevented virus-induced onset of diarrhea and mortality by reducing viral load in organs (Rocha-Pereira et al., 2013), even when treatment started between 12 h and 2 days post-infection (Rocha-Pereira et al., 2016). In contrast, 2CM-C did not reduce mortality when treatment was initiated after the onset of symptoms 3 days post-infection. In a norovirus transmission model, prophylactic treatment of sentinel mice with 2CM-C also protected against transmission of MNV infection (Rocha-Pereira et al., 2015). 2CM-C also had an antiviral effect against HuNoV in BALB/c Rag-γc-deficient mice (Kolawole et al., 2016).
The nucleoside analog 2′-fluroro-2′-C-methyl-cytidine (2′-F-2′-C-MeC, or PSI-6130) is structurally related to 2CM-C (Fig. 5B). It is the parent nucleoside of the prodrug mericitabine, an HCV inhibitor that acts as a chain terminator and has reached Phase 2 clinical development during which it was well tolerated. In cell-based assays, 2′-F-2′-C-MeC and 2CM-C have comparable antiviral activity against both MNV and HuNoV (Costantini et al., 2012). Although no biochemical data directly compare the inhibition effect on the target of each NTP, this result suggests that 2′-F-2′-C-MeC and 2CM-C are both recognized as a cytidine analog for the norovirus polymerase with similar substrate efficiency. For this reason, it would be interesting to further evaluate the antiviral efficacy of 2′-F-2′-C-MeC in a mouse model of norovirus infection.
3.2.4. 5-Nitro and 2′-amino cytidine
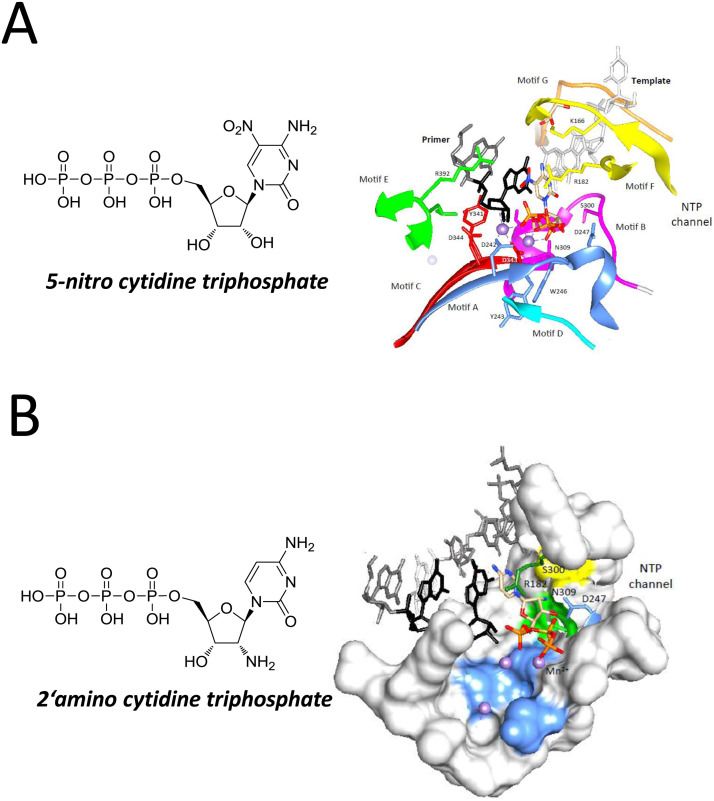
The antiviral activity of 5-nitrocytidine was first described against poliovirus and coxsackievirus B3, demonstrating greater inhibition potency than the control drug ribavirin (Harki et al., 2007). In the same study, 5-nitrocytidine triphosphate (NCT) inhibited poliovirus RdRp activity. A high-resolution structure of norovirus polymerase bound to NCT was determined by X-ray crystallography (Zamyatkin et al., 2008). In this structure, the RdRp is trapped in a ternary complex that includes a self-complementary primer-template RNA and two metal ions, mimicking the conformation of the protein during RNA synthesis. The NCT molecule occupies the nucleotide binding site and its interactions with the active site residues in the HuNoV are different from those of natural cytidine triphosphate (i.e., the negatively-charged nitro group of the NCT is positioned close to the primer 3′OH to destabilize the transition state of the nucleotidyl transfer reaction) (Fig. 6 A). The nitro group also displaces a water molecule from interacting with the α-phosphate of the NCT and results in increased distance in the coordination between Asp344 and metal A. Finally, the side chain of Arg182 is spatially affected to decrease its interaction with the β-phosphate of the NCT, likely decreasing the effectiveness of Arg182 from stabilizing the pyrophosphate leaving group. Although 2′-amino-cytidine triphosphate (ACT) makes overall similar interactions with the polymerase of norovirus, it also features some significant differences (Zamyatkin et al., 2009). The nucleobase of ACT forms Watson-Crick hydrogen bonds with the template nucleotide and the ribose exhibits primarily a C3′-endo pucker typically found in RNA (Fig. 6B). The ribose and triphosphates of the ACT are shifted in position to accommodate the 2′-amino group. Asp247, which normally forms a hydrogen bond with Ser300 to recognize the ribose 2′OH, is out of place. Asp242 that binds both divalent metal ions is displaced by 2 Å and could impact divalent metal occupancy in the active site. As a result of these relatively subtle changes, the primer 3′OH and the α-phosphate in ACT are not positioned to allow catalysis of nucleotidyl transfer. These interactions could have important implications in the inhibition of norovirus RNA replication by nucleotide analogs. Although prior studies have shown that other 2′-amino nucleosides can have an antiviral effect on viruses unrelated to Caliciviridae (De Clercq et al., 1980), more work is needed to demonstrate that 5-nitro and 2′-amino cytidine have the potential to inhibit norovirus replication.
Fig. 6.
Structure of 5-nitro and 2′-amino cytidine triphosphate and their molecular interaction with the HuNoV RdRp. (A) Structure of 5-nitro cytidine triphosphate and interaction with HuNoV RdRp. (B) Structure of 2′-amino cytidine triphosphate and interaction with HuNoV RdRp.
3.3. Non-nucleoside inhibitors of norovirus polymerase
3.3.1. Suramin and its analogs
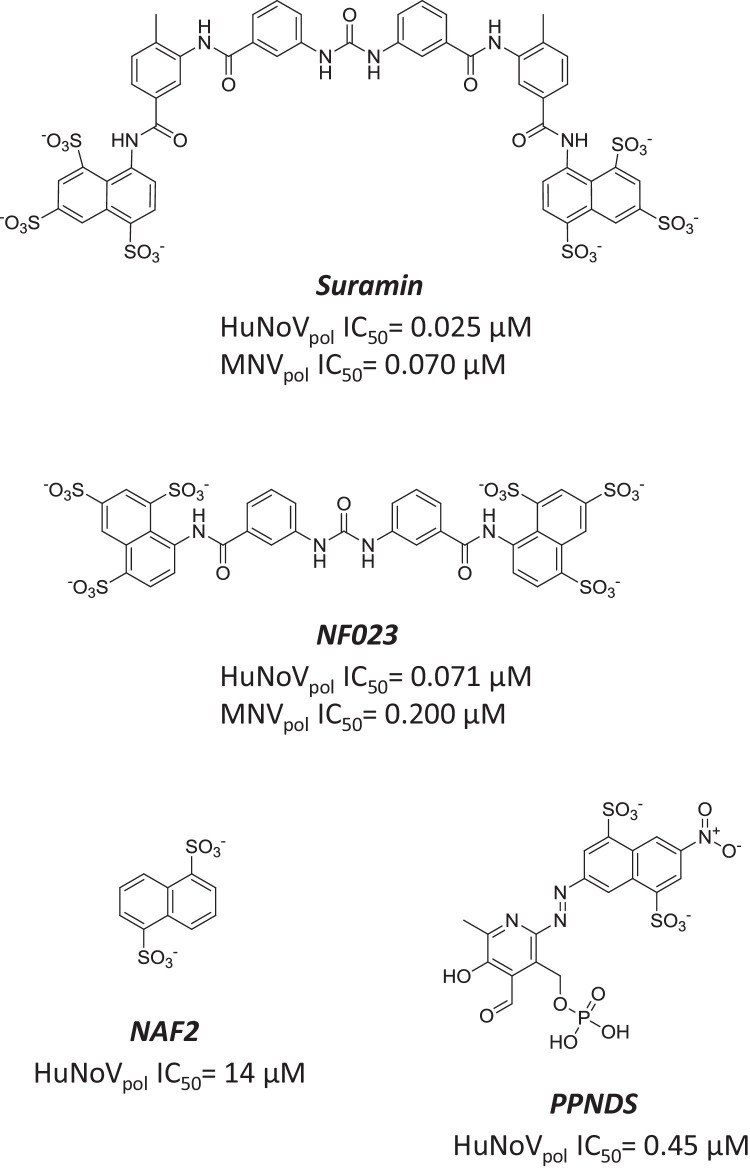
Suramin is an antiparasitic molecule originally developed by Bayer in 1916. It has a long symmetric structure containing two charged naphthalene-trisulfonic acid heads (Fig. 7 ). Suramin has also been identified in vitro as an antiviral agent potent against human immunodeficiency virus reverse transcriptase and hepatitis B virus (Jentsch et al., 1987, Offensperger et al., 1993). More recently, suramin was identified in an in silico docking search for compounds predicted to bind to the active site of norovirus polymerase (Mastrangelo et al., 2012). This modeling exercise was confirmed by measuring the inhibition of the RdRp activity of HuNoV and MNV polymerase by suramin, resulting in IC50 values 25 and 70 nM, respectively. NF023, an analog of suramin harboring the same two groups connected by a shorter linker, displayed similar inhibition potency (Fig. 7). Interestingly, NF023 is also a known competitive and reversible P2 × 1 receptor antagonist (Sneddon et al., 2000, Soto et al., 1999). The analysis of the detailed binding mode of suramin and NF023 to the MNV polymerase domain was made possible by X-ray crystallography. It showed that both molecules occupy the same binding site, between the fingers and the thumb subdomains. The importance of the interaction of the two molecules with tryptophan 42 was demonstrated by site-directed mutagenesis, which resulted in significant loss in inhibition potency. Two smaller analogs of suramin, naphthalene di-sulfonate and pyridoxal-5′-phosphate-6-(2′-naphthylazo-6′-nitro-4′,8′-disulfonate (PPNDS), retained some level of biochemical effect and were also able to bind to a second region of the protein located in the thumb subdomain (Croci et al., 2014a, Croci et al., 2014b, Tarantino et al., 2014) (Fig. 7). One would predict that the high inhibition potency of suramin and its analogs in biochemical assays would translate to a significant antiviral effect in cells infected with MNV. However, suramin has only a modest antiviral effect on norovirus replication, presumably due to low cell permeability (Mastrangelo et al., 2014). In order to further advance these compounds as potential anti-norovirus drugs, attempts have been made to improve cellular permeability by delivering suramin through liposomes (Mastrangelo et al., 2014). A separate study showed the in vitro and in vivo antiviral activity of suramin against enterovirus 71, a member of the Picornaviridae family (Ren et al., 2014). The same study reported that suramin blocked enterovirus 71 replication by interfering with the viral capsid assembly process. Taken together, these results indicate that suramin and its structural analogs are promiscuous binders that can interact with multiple classes of proteins, including but not limited to the norovirus polymerase (Morgan et al., 2011, Torrente et al., 2014). To minimize the off-target liability caused by promiscuous binding, it may be possible to increase the selectivity of this class of compounds by further building binding interactions between naphthalene di-sulfonate or PPNDS and the second thumb binding pocket in norovirus polymerase.
Fig. 7.
Suramin and its analogs as inhibitors of norovirus RdRp. Structure and inhibition potency of suramin, NF023, naphthalene di-sulfonate, and PPNDS.
3.3.2. Other non-nucleoside inhibitors
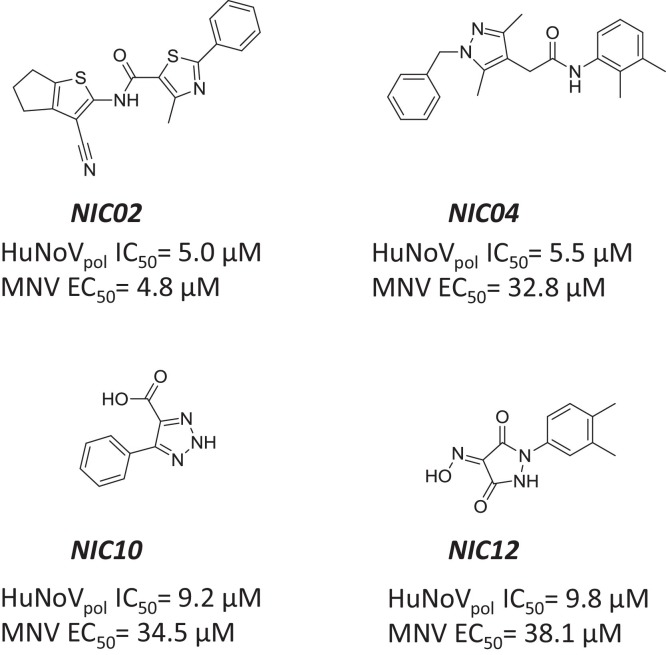
the fluorescence-based PicoGreen biochemical assay has been used in a high throughput screening campaign to identify small molecule inhibitors of GII.4 HuNoV RdRp activity (Eltahla et al., 2014), using a model previously established for HCV polymerase (Eltahla et al., 2013). The four most interesting hits were a phenylthiazole carboxamide (NIC02) and a pyrazole acetamide (NIC04) with IC50 values around 5 μM, and a triazole (NIC10) and a pyrazolidinedione (NIC12) that were about 2-fold less potent in the polymerase assay (Fig. 8 ). Interestingly, NIC12 had already been reported to weakly inhibit poliovirus RdRp (Campagnola et al., 2011), while the three other compounds were not previously known to inhibit viral polymerases. NIC02 was the only compound to also show consistent cell-based inhibition in the Norwalk replicon and MNV infection assays. In comparison, NIC04 was significantly less potent. NIC10 and NIC12 were inactive in the replicon, and weak (EC50 ∼35 μM) in the MNV assay. Mode of action inhibition studies suggested that NIC02 and NIC04 occupy a different binding pocket of HuNoV RdRp than NIC10 and NIC12. However, these findings will need to be confirmed by other methods such as x-ray crystallography and/or identification of resistance-associated mutations.
Fig. 8.
Other non-nucleoside inhibitors of norovirus RdRp. Structure and inhibition potency of NIC02, NIC04, NIC10, and NIC12.
4. Conclusions
The key role that the norovirus RdRp plays in viral genome replication and the fact that host cells lack an equivalent function make norovirus RdRp an attractive target for development of norovirus-specific antiviral therapies. Although many in vitro and in vivo systems are now available to identify and develop inhibitors of norovirus RdRp, current research in this field remains at an early stage of drug discovery, with nitazoxanide being the only small molecule to be evaluated in human clinical trials. The fact that norovirus typically causes a short and self-limiting infection, with a narrow time window for therapeutic intervention has limited the effort and interest in the development of antivirals. However, the consequences of norovirus infection can be severe and prolonged in vulnerable populations such as children, the elderly, and immunocompromised patients. Therefore, increased commitment to the development of small molecules treatments alongside the current anti-norovirus vaccine approach is needed. In fact, much is already known about the specific recognition of nucleotides and nucleotide analogs that should guide structure-based inhibitor design. Additional locations in the HuNoV RdRp that bind nonnucleoside inhibitors have also been identified and characterized. Finally, since norovirus RdRp shares functional and structural features with proteins from other RNA viruses such as HCV, it may be possible to identify nucleo(s/t)ide analogs, like 2CM-C, which inhibits a range of viral polymerases. Identifying novel broad-spectrum nucleoside or non-nucleoside molecules that block RdRp and inhibit a panel of positive-strand RNA viruses, including norovirus, provides an exciting prospect for future antiviral therapies.
Acknowledgements
We thank X. Lin for helpful discussions of the Norovirus structure during this work. We also thank Peggy Korn and Anh Truong for their editorial comments.
References
- Alam I., Lee J.H., Cho K.J., Han K.R., Yang J.M., Chung M.S., Kim K.H. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012;426(2):143–151. doi: 10.1016/j.virol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Aliota M.T., Caine E.A., Walker E.C., Larkin K.E., Camacho E., Osorio J.E. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 2016;10(4):e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A., Bailey D., Chaudhry Y., Goodfellow I. Development of a reverse-genetics system for murine norovirus 3: long-term persistence occurs in the caecum and colon. J. Gen. Virol. 2012;93(Pt. 7):1432–1441. doi: 10.1099/vir.0.042176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A., Thorne L., Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. eLife. 2014;3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A., Thorne L., Ghurburrun E., Bailey D., Goodfellow I. Norovirus polymerase fidelity contributes to viral transmission in vivo. mSphere. 2016;1(5) doi: 10.1128/mSphere.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar R.L., Bernstein D.I., Harro C.D., Al-Ibrahim M.S., Chen W.H., Ferreira J., Estes M.K., Graham D.Y., Opekun A.R., Richardson C., Mendelman P.M. Norovirus vaccine against experimental human Norwalk virus illness. N. Engl. J. Med. 2011;365(23):2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliot G., Sosnovtsev S.V., Mitra T., Hammer C., Garfield M., Green K.Y. In vitro proteolytic processing of the MD145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J. Virol. 2003;77(20):10957–10974. doi: 10.1128/JVI.77.20.10957-10974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliot G., Sosnovtsev S.V., Chang K.O., Babu V., Uche U., Arnold J.J., Cameron C.E., Green K.Y. Norovirus proteinase-polymerase and polymerase are both active forms of RNA-dependent RNA polymerase. J. Virol. 2005;79(4):2393–2403. doi: 10.1128/JVI.79.4.2393-2403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillat Blanco N., Kuonen R., Bellini C., Manuel O., Estrade C., Mazza-Stalder J., Aubert J.D., Sahli R., Meylan P. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl. Infect. Dis. 2011;13(2):213–215. doi: 10.1111/j.1399-3062.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- Bull R.A., Eden J.S., Rawlinson W.D., White P.A. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 2010;6(3):e1000831. doi: 10.1371/journal.ppat.1000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry Y., Nayak A., Bordeleau M.E., Tanaka J., Pelletier J., Belsham G.J., Roberts L.O., Goodfellow I.G. Caliciviruses differ in their functional requirements for eIF4F components. J. Biol. Chem. 2006;281(September (35)):25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- Campagnola G., Gong P., Peersen O.B. High-throughput screening identification of poliovirus RNA-dependent RNA polymerase inhibitors. Antivir. Res. 2011;91(3):241–251. doi: 10.1016/j.antiviral.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzi T., Makari-Judson G., Steingart R., Mertens W.C. Chronic diarrhea associated with persistent norovirus excretion in patients with chronic lymphocytic leukemia: report of two cases. BMC Infect. Dis. 2011;11:131. doi: 10.1186/1471-2334-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.C., Lee N., Hung T.N., Kwok K., Cheung K., Tin E.K., Lai R.W., Nelson E.A., Leung T.F., Chan P.K. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat. Commun. 2015;6:10061. doi: 10.1038/ncomms10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., George D.W. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J. Virol. 2007;81(22):12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., Sosnovtsev S.V., Belliot G., King A.D., Green K.Y. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353(2):463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Cheetham G.M., Jeruzalmi D., Steitz T.A. Structural basis for initiation of transcription from an RNA polymerase-promoter complex. Nature. 1999;399(6731):80–83. doi: 10.1038/19999. [DOI] [PubMed] [Google Scholar]
- Chen S.Y., Tsai C.N., Lai M.W., Chen C.Y., Lin K.L., Lin T.Y., Chiu C.H. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin. Infect. Dis. 2009;48(7):849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- Clarke I.N., Lambden P.R. Organization and expression of calicivirus genes. J. Infect. Dis. 2000;181(Suppl. 2):S309–316. doi: 10.1086/315575. [DOI] [PubMed] [Google Scholar]
- Costantini V.P., Whitaker T., Barclay L., Lee D., McBrayer T.R., Schinazi R.F., Vinje J. Antiviral activity of nucleoside analogues against norovirus. Antivir. Ther. 2012;17(6):981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci R., Pezzullo M., Tarantino D., Milani M., Tsay S.C., Sureshbabu R., Tsai Y.J., Mastrangelo E., Rohayem J., Bolognesi M., Hwu J.R. Structural bases of norovirus RNA dependent RNA polymerase inhibition by novel suramin-related compounds. PLoS One. 2014;9(3):e91765. doi: 10.1371/journal.pone.0091765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci R., Tarantino D., Milani M., Pezzullo M., Rohayem J., Bolognesi M., Mastrangelo E. PPNDS inhibits murine Norovirus RNA-dependent RNA-polymerase mimicking two RNA stacking bases. FEBS Lett. 2014;588(9):1720–1725. doi: 10.1016/j.febslet.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Daughenbaugh K.F., Fraser C.S., Hershey J.W., Hardy M.E. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22(11):2852–2859. doi: 10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Balzarini J., Descamps J., Eckstein F. Antiviral, antimetabolic and antineoplastic activities of 2′- or 3′-amino or -azido-substituted deoxyribonucleosides. Biochem. Pharmacol. 1980;29(12):1849–1851. doi: 10.1016/0006-2952(80)90149-5. [DOI] [PubMed] [Google Scholar]
- Donaldson E.F., Lindesmith L.C., LoBue A.D., Baric R.S. Viral shape-shifting: norovirus evasion of the human immune system. Nat. Rev. Microbiol. 2010;8(3):231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duizer, E., van Duynhoven, Y., Vennema, H., Koopmans, M., 2004. Failure to detect norovirus in a large group of asymptomatic individuals by Marshall et al. (Public Health Vol 118 (3) 230–233). Public health 118 (6), 455–456; author reply 456–457. [DOI] [PubMed]
- El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J., Ferreira J., Chen W.H., Sublett R., Richardson C., Bargatze R.F., Sztein M.B., Tacket C.O. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 2010;202(11):1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltahla A.A., Lackovic K., Marquis C., Eden J.S., White P.A. A fluorescence-based high-throughput screen to identify small compound inhibitors of the genotype 3a hepatitis C virus RNA polymerase. J. Biomol. Screen. 2013;18(9):1027–1034. doi: 10.1177/1087057113489883. [DOI] [PubMed] [Google Scholar]
- Eltahla A.A., Lim K.L., Eden J.S., Kelly A.G., Mackenzie J.M., White P.A. Nonnucleoside inhibitors of norovirus RNA polymerase: scaffolds for rational drug design. Antimicrob. Agents Chemother. 2014;58(6):3115–3123. doi: 10.1128/AAC.02799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K., Hardy M.E. Norwalk virus nonstructural protein p48 forms a complex with the SNARE regulator VAP-A and prevents cell surface expression of vesicular stomatitis virus G protein. J. Virol. 2003;77(21):11790–11797. doi: 10.1128/JVI.77.21.11790-11797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K., Crawford S.E., Murakami K., Broughman J.R., Karandikar U., Tenge V.R., Neill F.H., Blutt S.E., Zeng X.L., Qu L., Kou B., Opekun A.R., Burrin D., Graham D.Y., Ramani S., Atmar R.L., Estes M.K. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vega V., Sosnovtsev S.V., Belliot G., King A.D., Mitra T., Gorbalenya A., Green K.Y. Norwalk virus N-terminal nonstructural protein is associated with disassembly of the golgi complex in transfected cells. J. Virol. 2004;78(9):4827–4837. doi: 10.1128/JVI.78.9.4827-4837.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S., Kojima S., Takai R., Hoshino F.B., Oka T., Takeda N., Katayama K., Kageyama T. Poly(A)- and primer-independent RNA polymerase of Norovirus. J. Virol. 2004;78(8):3889–3896. doi: 10.1128/JVI.78.8.3889-3896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009;82(3):95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow I. The genome-linked protein VPg of vertebrate viruses—a multifaceted protein. Curr. Opin. Virol. 2011;1(5):355–362. doi: 10.1016/j.coviro.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.J., Eisenbart V.G., Etingue A.L., Gould L.H., Lopman B.A., Parashar U.D. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg. Infect. Dis. 2012;18(10):1566–1573. doi: 10.3201/eid1810.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harki D.A., Graci J.D., Edathil J.P., Castro C., Cameron C.E., Peterson B.R. Synthesis of a universal 5-nitroindole ribonucleotide and incorporation into RNA by a viral RNA-dependent RNA polymerase. ChemBioChem. 2007;8(12):1359–1362. doi: 10.1002/cbic.200700160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz M.M., Radtke A.L., Lay M.K., Hjelm B.E., Bolick A.N., Sarker S.S., Atmar R.L., Kingsley D.H., Arntzen C.J., Estes M.K., Nickerson C.A. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg. Infect. Dis. 2013;19(3):431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogbom M., Jager K., Robel I., Unge T., Rohayem J. The active form of the norovirus RNA-dependent RNA polymerase is a homodimer with cooperative activity. J. Gen. Virol. 2009;90(Pt. 2):281–291. doi: 10.1099/vir.0.005629-0. [DOI] [PubMed] [Google Scholar]
- Hsu C.C., Wobus C.E., Steffen E.K., Riley L.K., Livingston R.S. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 2005;12(10):1145–1151. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.C., Riley L.K., Wills H.M., Livingston R.S. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 2006;56(4):247–251. [PubMed] [Google Scholar]
- Imburgio D., Rong M., Ma K., McAllister W.T. Studies of promoter recognition and start site selection by T7 RNA polymerase using a comprehensive collection of promoter variants. Biochemistry. 2000;39(34):10419–10430. doi: 10.1021/bi000365w. [DOI] [PubMed] [Google Scholar]
- Jentsch K.D., Hunsmann G., Hartmann H., Nickel P. Inhibition of human immunodeficiency virus type I reverse transcriptase by suramin-related compounds. J. Gen. Virol. 1987;68(Pt. 8):2183–2192. doi: 10.1099/0022-1317-68-8-2183. [DOI] [PubMed] [Google Scholar]
- Jin Z., Smith L.K., Rajwanshi V.K., Kim B., Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS One. 2013;8(7):e68347. doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Tucker K., Lin X., Kao C.C., Shaw K., Tan H., Symons J., Behera I., Rajwanshi V.K., Dyatkina N., Wang G., Beigelman L., Deval J. Biochemical evaluation of the inhibition properties of favipiravir and 2′-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob. Agents Chemother. 2015;59(12):7504–7516. doi: 10.1128/AAC.01391-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.J., Roehrig J.T. New mouse model for dengue virus vaccine testing. J. Virol. 1999;73(1):783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.K., Watanabe M., Zhu S., Graves C.L., Keyes L.R., Grau K.R., Gonzalez-Hernandez M.B., Iovine N.M., Wobus C.E., Vinje J., Tibbetts S.A., Wallet S.M., Karst S.M. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.K., Grau K.R., Costantini V., Kolawole A.O., de Graaf M., Freiden P., Graves C.L., Koopmans M., Wallet S.M., Tibbetts S.A., Schultz-Cherry S., Wobus C.E., Vinje J., Karst S.M. Human norovirus culture in B cells. Nat. Protoc. 2015;10(12):1939–1947. doi: 10.1038/nprot.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian T.R., Baugher J.D., Rippinger C.M., Pinekenstein R., Kolawole A.O., Mehoke T.S., Wobus C.E., Feldman A.B., Pineda F.J., Schwab K.J. Murine norovirus (MNV-1) exposure in vitro to the purine nucleoside analog Ribavirin increases quasispecies diversity. Virus Res. 2016;211:165–173. doi: 10.1016/j.virusres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Kahan S.M., Liu G., Reinhard M.K., Hsu C.C., Livingston R.S., Karst S.M. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011;421(2):202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst S.M., Wobus C.E., Lay M., Davidson J., Virgin H.W.t. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299(5612):1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Kaufman S.S., Green K.Y., Korba B.E. Treatment of norovirus infections: moving antivirals from the bench to the bedside. Antivir. Res. 2014;105:80–91. doi: 10.1016/j.antiviral.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C., Sighn P., Ecker D. De novo initiation of viral RNA-dependent RNA synthesis. Minirev. Virol. 2001;287:251–260. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- Khan R.R., Lawson A.D., Minnich L.L., Martin K., Nasir A., Emmett M.K., Welch C.A., Udall J.N., Jr. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2009;48(3):328–333. doi: 10.1097/mpg.0b013e31818255cc. [DOI] [PubMed] [Google Scholar]
- Kolawole A.O., Rocha-Pereira J., Elftman M.D., Neyts J., Wobus C.E. Inhibition of human norovirus by a viral polymerase inhibitor in the B cell culture system and in the mouse model. Antivir. Res. 2016;132:46–49. doi: 10.1016/j.antiviral.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay M.K., Atmar R.L., Guix S., Bharadwaj U., He H., Neill F.H., Sastry K.J., Yao Q., Estes M.K. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology. 2010;406(1):1–11. doi: 10.1016/j.virol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen E.N., Kwok K.Y., Birtley J.R., Simpson P.J., Subba-Reddy C.V., Chaudhry Y., Sosnovtsev S.V., Green K.Y., Prater S.N., Tong M., Young J.C., Chung L.M., Marchant J., Roberts L.O., Kao C.C., Matthews S., Goodfellow I.G., Curry S. Structures of the compact helical core domains of feline calicivirus and murine norovirus VPg proteins. J. Virol. 2013;87(10):5318–5330. doi: 10.1128/JVI.03151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Thorne L., Jin Z., Hammad L.A., Li S., Deval J., Goodfellow I.G., Kao C.C. Subgenomic promoter recognition by the norovirus RNA-dependent RNA polymerases. Nucleic Acids Res. 2015;43(1):446–460. doi: 10.1093/nar/gku1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman B., Vennema H., Kohli E., Pothier P., Sanchez A., Negredo A., Buesa J., Schreier E., Reacher M., Brown D., Gray J., Iturriza M., Gallimore C., Bottiger B., Hedlund K.O., Torven M., von Bonsdorff C.H., Maunula L., Poljsak-Prijatelj M., Zimsek J., Reuter G., Szucs G., Melegh B., Svennson L., van Duijnhoven Y., Koopmans M. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363(9410):682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- Lopman B., Gastanaduy P., Park G.W., Hall A.J., Parashar U.D., Vinje J. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2012;2(1):96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Lu J., Fang L., Zheng H., Lao J., Yang F., Sun L., Xiao J., Lin J., Song T., Ni T., Raghwani J., Ke C., Faria N.R., Bowden T.A., Pybus O.G., Li H. The evolution and transmission of epidemic GII.17 noroviruses. J. Infect. Dis. 2016;214(4):556–564. doi: 10.1093/infdis/jiw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D., Reynolds K., Smith D., Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann. Pharmacother. 2008;42(9):1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- Mastrangelo E., Pezzullo M., Tarantino D., Petazzi R., Germani F., Kramer D., Robel I., Rohayem J., Bolognesi M., Milani M. Structure-based inhibition of norovirus RNA-dependent RNA polymerases. J. Mol. Biol. 2012;419(3–4):198–210. doi: 10.1016/j.jmb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Mastrangelo E., Mazzitelli S., Fabbri J., Rohayem J., Ruokolainen J., Nykanen A., Milani M., Pezzullo M., Nastruzzi C., Bolognesi M. Delivery of suramin as an antiviral agent through liposomal systems. ChemMedChem. 2014;9(5):933–939. doi: 10.1002/cmdc.201300563. [DOI] [PubMed] [Google Scholar]
- McFadden N., Bailey D., Carrara G., Benson A., Chaudhry Y., Shortland A., Heeney J., Yarovinsky F., Simmonds P., Macdonald A., Goodfellow I. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011;7(12):e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M.C., Abelli L.A., Dodi I., Dettori G., Chezzi C. Norovirus RNA in the blood of a child with gastroenteritis and convulsions—a case report. J. Clin. Virol. 2010;48(2):147–149. doi: 10.1016/j.jcv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Morales M., Barcena J., Ramirez M.A., Boga J.A., Parra F., Torres J.M. Synthesis in vitro of rabbit hemorrhagic disease virus subgenomic RNA by internal initiation on (−)sense genomic RNA: mapping of a subgenomic promoter. J. Biol. Chem. 2004;279(17):17013–17018. doi: 10.1074/jbc.M313674200. [DOI] [PubMed] [Google Scholar]
- Morgan H.P., McNae I.W., Nowicki M.W., Zhong W., Michels P.A., Auld D.S., Fothergill-Gilmore L.A., Walkinshaw M.D. The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site. J. Biol. Chem. 2011;286(36):31232–31240. doi: 10.1074/jbc.M110.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.K., Pendas-Franco N., Rojo J., Boga J.A., Machin A., Alonso J.M., Parra F. Crystal structure of norwalk virus polymerase reveals the carboxyl terminus in the active site cleft. J. Biol. Chem. 2004;279(16):16638–16645. doi: 10.1074/jbc.M400584200. [DOI] [PubMed] [Google Scholar]
- Offensperger W.B., Offensperger S., Walter E., Blum H.E., Gerok W. Suramin prevents duck hepatitis B virus infection in vivo. Antimicrob. Agents Chemother. 1993;37(7):1539–1542. doi: 10.1128/aac.37.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olspert A., Hosmillo M., Chaudhry Y., Peil L., Truve E., Goodfellow I. Protein-RNA linkage and posttranslational modifications of feline calicivirus and murine norovirus VPg proteins. PeerJ. 2016;4:e2134. doi: 10.7717/peerj.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafragkou E., Hewitt J., Park G.W., Greening G., Vinje J. Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS One. 2014;8(6):e63485. doi: 10.1371/journal.pone.0063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M.M., Hall A.J., Vinjé J., Parashar U.D. Noroviruses: a comprehensive review. J. Clin. Virol. 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Peersen O.B. Picornaviral polymerase structure, function and fidelity modulation. Virus Res. 2017;234:4–20. doi: 10.1016/j.virusres.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierra C., Amador A., Benzaria S., Cretton-Scott E., D'Amours M., Mao J., Mathieu S., Moussa A., Bridges E.G., Standring D.N., Sommadossi J.P., Storer R., Gosselin G. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J. Med. Chem. 2006;49(22):6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- Prasad B.V.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286(5438):287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Ren P., Zou G., Bailly B., Xu S., Zeng M., Chen X., Shen L., Zhang Y., Guillon P., Arenzana-Seisdedos F., Buchy P., Li J., von Itzstein M., Li Q., Altmeyer R. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection-suramin inhibits EV71 infection in vitro and in vivo. Emerg. Microbes Infect. 2014;3(9):e62. doi: 10.1038/emi.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Bargatze R.F., Goodwin R., Mendelman P.M. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev. Vaccines. 2013;12(2):155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Cunha R., Costa I., Nascimento M.S., Neyts J. Inhibition of norovirus replication by the nucleoside analogue 2′-C-methylcytidine. Biochem. Biophys. Res. Commun. 2012;427(4):796–800. doi: 10.1016/j.bbrc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Nascimento M.S., Neyts J. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem. Biophys. Res. Commun. 2012;424(4):777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J., Jochmans D., Debing Y., Verbeken E., Nascimento M.S., Neyts J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J. Virol. 2013;87(21):11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Pereira J., Neyts J., Jochmans D. Norovirus: targets and tools in antiviral drug discovery. Biochem. Pharmacol. 2014;91(1):1–11. doi: 10.1016/j.bcp.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Pereira J., Jochmans D., Neyts J. Prophylactic treatment with the nucleoside analogue 2′-C-methylcytidine completely prevents transmission of norovirus. J. Antimicrob. Chemother. 2015;70(1):190–197. doi: 10.1093/jac/dku363. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J., Kolawole A.O., Verbeken E., Wobus C.E., Neyts J. Post-exposure antiviral treatment of norovirus infections effectively protects against diarrhea and reduces virus shedding in the stool in a mortality mouse model. Antivir. Res. 2016;132:76–84. doi: 10.1016/j.antiviral.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohayem J., Jager K., Robel I., Scheffler U., Temme A., Rudolph W. Characterization of norovirus 3Dpol RNA-dependent RNA polymerase activity and initiation of RNA synthesis. J. Gen. Virol. 2006;87(Pt. 9):2621–2630. doi: 10.1099/vir.0.81802-0. [DOI] [PubMed] [Google Scholar]
- Rohayem J., Robel I., Jager K., Scheffler U., Rudolph W. Protein-primed and de novo initiation of RNA synthesis by norovirus 3Dpol. J. Virol. 2006;80(14):7060–7069. doi: 10.1128/JVI.02195-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondy M., Koopmans M., Rotsaert C., Van Loon T., Beljaars B., Van Dijk G., Siebenga J., Svraka S., Rossen J.W., Teunis P., Van Pelt W., Verhoef L. Norovirus disease associated with excess mortality and use of statins: a retrospective cohort study of an outbreak following a pilgrimage to Lourdes. Epidemiol. Infect. 2011;139(3):453–463. doi: 10.1017/S0950268810000993. [DOI] [PubMed] [Google Scholar]
- Roos-Weil D., Ambert-Balay K., Lanternier F., Mamzer-Bruneel M.F., Nochy D., Pothier P., Avettand-Fenoel V., Anglicheau D., Snanoudj R., Bererhi L., Thervet E., Lecuit M., Legendre C., Lortholary O., Zuber J. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92(1):61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F., El-Gohary Y.M. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment. Pharmacol. Ther. 2006;24(10):1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif M.A., Bonney D.K., Bigger B., Forsythe L., Williams N., Page J., Babiker Z.O., Guiver M., Turner A.J., Hughes S., Wynn R.F. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr. Transplant. 2011;15(5):505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.-A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorn R., Hohne M., Meerbach A., Bossart W., Wuthrich R.P., Schreier E., Muller N.J., Fehr T. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin. Infect. Dis. 2010;51(3):307–314. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Vergoulidou M., Schreier E., Loddenkemper C., Reinwald M., Schmidt-Hieber M., Flegel W.A., Thiel E., Schneider T. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117(22):5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T.M., Guix S., Katayama K., Crawford S.E., Estes M.K. Inhibition of cellular protein secretion by Norwalk virus nonstructural protein p22 requires a mimic of an endoplasmic reticulum export signal. PLoS One. 2010;5(10):e13130. doi: 10.1371/journal.pone.0013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T.M., Crawford S.E., Ajami N.J., Neill F.H., Atmar R.L., Katayama K., Utama B., Estes M.K. Secretory pathway antagonism by calicivirus homologues of Norwalk virus nonstructural protein p22 is restricted to noroviruses. Virol. J. 2012;9:181. doi: 10.1186/1743-422X-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland A., Chettle J., Archer J., Wood K., Bailey D., Goodfellow I., Blacklaws B.A., Heeney J.L. Pathology caused by persistent murine norovirus infection. J. Gen. Virol. 2014;95(Pt. 2):413–422. doi: 10.1099/vir.0.059188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq D.M., Koo H.L., Adachi J.A., Viola G.M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63(5):394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenga J.J., Vennema H., Zheng D.P., Vinje J., Lee B.E., Pang X.L., Ho E.C., Lim W., Choudekar A., Broor S., Halperin T., Rasool N.B., Hewitt J., Greening G.E., Jin M., Duan Z.J., Lucero Y., O'Ryan M., Hoehne M., Schreier E., Ratcliff R.M., White P.A., Iritani N., Reuter G., Koopmans M. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J. Infect. Dis. 2009;200(5):802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Karakasiliotis I., Bailey D., Chaudhry Y., Evans D.J., Goodfellow I.G. Bioinformatic and functional analysis of RNA secondary structure elements among different genera of human and animal caliciviruses. Nucleic Acids Res. 2008;36(8):2530–2546. doi: 10.1093/nar/gkn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Westfall T.D., Todorov L.D., Todorova S.M., Westfall D.P., Nickel P., Kennedy C. The effect of P2 receptor antagonists and ATPase inhibition on sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens. Br. J. Pharmacol. 2000;129(6):1089–1094. doi: 10.1038/sj.bjp.0703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F., Lambrecht G., Nickel P., Stuhmer W., Busch A.E. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38(1):141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- Strong D.W., Thackray L.B., Smith T.J., Virgin H.W. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J. Virol. 2012;86(6):2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Reddy C.V., Goodfellow I., Kao C.C. VPg-primed RNA synthesis of norovirus RNA-dependent RNA polymerases by using a novel cell-based assay. J. Virol. 2011;85(24):13027–13037. doi: 10.1128/JVI.06191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Reddy C.V., Yunus M.A., Goodfellow I.G., Kao C.C. Norovirus RNA synthesis is modulated by an interaction between the viral RNA-dependent RNA polymerase and the major capsid protein, VP1. J. Virol. 2012;86(18):10138–10149. doi: 10.1128/JVI.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tacket C.O., Sztein M.B., Losonsky G.A., Wasserman S.S., Estes M.K. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. 2003;108(3):241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Takanashi S., Saif L.J., Hughes J.H., Meulia T., Jung K., Scheuer K.A., Wang Q. Failure of propagation of human norovirus in intestinal epithelial cells with microvilli grown in three-dimensional cultures. Arch. Virol. 2014;159(2):257–266. doi: 10.1007/s00705-013-1806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M., Jiang X. Vaccine against norovirus. Hum. Vaccines Immunother. 2014;10(6):1449–1456. doi: 10.4161/hv.28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino D., Pezzullo M., Mastrangelo E., Croci R., Rohayem J., Robel I., Bolognesi M., Milani M. Naphthalene-sulfonate inhibitors of human norovirus RNA-dependent RNA-polymerase. Antivir. Res. 2014;102:23–28. doi: 10.1016/j.antiviral.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Taube S., Kolawole A.O., Hohne M., Wilkinson J.E., Handley S.A., Perry J.W., Thackray L.B., Akkina R., Wobus C.E. A mouse model for human norovirus. mBio. 2013;4(4) doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne L.G., Goodfellow I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014;95(Pt. 2):278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- Torrente M.P., Castellano L.M., Shorter J. Suramin inhibits Hsp104 ATPase and disaggregase activity. PLoS One. 2014;9(10):e110115. doi: 10.1371/journal.pone.0110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor J.J., Atmar R.L., Frey S.E., Gormley R., Chen W.H., Ferreira J., Goodwin R., Borkowski A., Clemens R., Mendelman P.M. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate-reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 2014;210(11):1763–1771. doi: 10.1093/infdis/jiu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcios-Ruiz R.M., Axelrod P., St John K., Bullitt E., Donahue J., Robinson N., Friss H.E. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J. Pediatr. 2008;153(3):339–344. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignuzzi M., Stone J.K., Arnold J.J., Cameron C.E., Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439(7074):344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015;53(2):373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongpunsawad S., Prasad B.V.V., Estes M.K. Norwalk virus minor capsid protein VP2 associates within the VP1 shell domain. J. Virol. 2013;87(9):4818–4825. doi: 10.1128/JVI.03508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wan J., Hu Y., Wu X., Prhavc M., Dyatkina N., Rajwanshi V.K., Smith D.B., Jekle A., Kinkade A., Symons J.A., Jin Z., Deval J., Zhang Q., Tam Y., Chanda S., Blatt L., Beigelman L. Synthesis and anti-Influenza activity of pyridine, pyridazine, and pyrimidine C-nucleosides as favipiravir (T-705) analogues. J. Med. Chem. 2016;59(10):4611–4624. doi: 10.1021/acs.jmedchem.5b01933. [DOI] [PubMed] [Google Scholar]
- Weerasekara S., Prior A.M., Hua D.H. Current tools for norovirus drug discovery. Expert Opin. Drug Discov. 2016;11(6):529–541. doi: 10.1080/17460441.2016.1178231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus C.E., Karst S.M., Thackray L.B., Chang K.O., Sosnovtsev S.V., Belliot G., Krug A., Mackenzie J.M., Green K.Y., Virgin H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2(12):e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus C.E., Thackray L.B., Virgin H.W.t. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 2006;80(11):5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus M.A., Lin X., Bailey D., Karakasiliotis I., Chaudhry Y., Vashist S., Zhang G., Thorne L., Kao C.C., Goodfellow I. The murine norovirus core subgenomic RNA promoter consists of a stable stem-loop that can direct accurate initiation of RNA synthesis. J. Virol. 2015;89(2):1218–1229. doi: 10.1128/JVI.02432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamyatkin D.F., Parra F., Alonso J.M., Harki D.A., Peterson B.R., Grochulski P., Ng K.K. Structural insights into mechanisms of catalysis and inhibition in Norwalk virus polymerase. J. Biol. Chem. 2008;283(12):7705–7712. doi: 10.1074/jbc.M709563200. [DOI] [PubMed] [Google Scholar]
- Zamyatkin D.F., Parra F., Machin A., Grochulski P., Ng K.K. Binding of 2′-amino-2′-deoxycytidine-5′-triphosphate to norovirus polymerase induces rearrangement of the active site. J. Mol. Biol. 2009;390(1):10–16. doi: 10.1016/j.jmb.2009.04.069. [DOI] [PubMed] [Google Scholar]
- Zamyatkin D., Rao C., Hoffarth E., Jurca G., Rho H., Parra F., Grochulski P., Ng K.K. Structure of a backtracked state reveals conformational changes similar to the state following nucleotide incorporation in human norovirus polymerase. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2014;70(Pt. 12):3099–3109. doi: 10.1107/S1399004714021518. [DOI] [PubMed] [Google Scholar]
- Zmurko J., Marques R.E., Schols D., Verbeken E., Kaptein S.J., Neyts J. The viral polymerase inhibitor 7-deaza-2′-C-methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl. Trop. Dis. 2016;10(5):e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]