Abstract
Indoor ventilation with good air quality control minimises the spread of airborne respiratory and other infections in hospitals. This article considers the role of ventilation in preventing and controlling infection in hospital general wards and identifies a simple and cost-effective ventilation design capable of reducing the chances of cross-infection. Computational fluid dynamic (CFD) analysis is used to simulate and compare the removal of microbes using a number of different ventilation systems. Instead of the conventional corridor air return arrangement used in most general wards, air return is rearranged so that ventilation is controlled from inside the ward cubicle. In addition to boosting the air ventilation rate, the CFD results reveal that ventilation performance and the removal of microbes can be significantly improved. These improvements are capable of matching the standards maintained in a properly constructed isolation room, though at much lower cost. It is recommended that the newly identified ventilation parameters be widely adopted in the design of new hospital general wards to minimise cross-infection. The proposed ventilation system can also be retrofitted in existing hospital general wards with far less disruption and cost than a full-scale refurbishment.
Keywords: Airborne respiratory diseases, Hospital general ward, Infection control, Severe acute respiratory syndrome, Ventilation design
Introduction
Hospitals frequently have to accommodate patients who may be suffering from undiagnosed infectious diseases, such as measles, chickenpox and tuberculosis, which are spread primarily through airborne routes. The risk of cross-infection from these airborne respiratory diseases can be high among vulnerable hospital patients and the healthcare staff who care for them. This is particularly relevant in general ward settings where the indoor air environment is not equipped to handle these forms of infection. The situation is further aggravated by the various patient treatment procedures that can generate large amounts of aerosols, e.g. physiotherapy. Furthermore, air ventilation systems are a recognised source of meticillin-resistant Staphylococcus aureus (MRSA) outbreaks in hospitals.1 Outbreaks of Acinetobacter infection have also been observed to be airborne spread.2 Similarly, widespread environmental contamination with Clostridium difficile has been reported.3
Hospitals face even bigger threats with the emergence of new infectious diseases such as avian influenza and severe acute respiratory syndrome (SARS). Following the large-scale SARS outbreak in 2003, an expert review reported evidence of the likelihood of airborne disease transmission, even though the exact transmission mechanism has yet to be firmly established.4 There is further evidence to demonstrate that the transmission/spread of infectious diseases, including influenza and SARS, is associated with ventilation and air movement in hospital buildings.5
When the SARS outbreak occurred, the disease was often not diagnosed before patients were admitted to a hospital general ward, hence the virus spread silently among other patients and healthcare staff, and was even redirected back into the community. In hindsight, it is now believed that this could have been minimised if the infected patients had been placed in facilities equipped with suitable isolation facilities.
It is generally agreed that proper airborne isolation facilities will reduce the spread of airborne infections. Nonetheless, although there are comprehensive engineering ventilation design guidelines for controlling the indoor air environment in operating theatres and isolation wards, there are few specifics for general wards. Accordingly, there is a genuine need to rethink the ventilation design of hospital general wards to improve infection prevention and control capabilities.
Methods
The US Centers for Disease and Control Prevention (CDC) specifies the following two key ventilation design requirements for dedicated isolation facilities:
– increased dilution efficiency through higher air ventilation rates;
– the avoidance of air flow from less clean to clean areas, by creating a pressure gradient and providing an air-locking ante room.6
No such requirements are stipulated for general wards. Most hospital general wards have corridor return airflow without air return duct design, which has the effect of potentially driving air from less clean to clean areas.
Hospital general wards accommodating more than 30 patients normally comprise a number of bed cubicles linked together through the ward corridor to a common nurse station. This rectangular, six-bed ward cubicle design is common in hospitals across the USA and the UK. In fact, the UK pioneered this ward cubicle design in 1969, by breaking the long, open Nightingale ward into smaller ward cubicles to reduce cross-infection.7
The main sources of air supply for most hospitals are natural ventilation and installed air-handling units (AHU) that draw air from outside the hospital building. The current study focuses on hospitals with an AHU, the typical air supply for hospitals with air-conditioning systems. The AHU supplies fresh air into the general ward through a high standard air filtering system to maintain reasonably good inflow air quality. As patients mostly stay inside the ward cubicle, the supply air is cleaner than that inside the rest of the ward. This inward air-flow system is also the standard requirement of the CDC for isolation room design. The isolation facility in the general ward should be able to remove and dilute microbes from within the cubicle and prevent the outflow of microbes into the corridor, nurse station and other hospital areas. The design of the ventilation system proposed in this study adapts the CDC guidelines for isolation facilities to the general ward to increase dilution efficiency and to minimise air flow from less clean to clean areas.
The ducted return strategy
This study proposes a ‘ducted return strategy’ to induce air flow from clean to less clean areas. The new design replaces the normal corridor return without air return duct with an exhaust air duct that extracts exhaust air from inside the cubicle into the open area outside the hospital. This exhaust air duct can be installed in the cubicle at either a high ceiling or low bed level. It would be very difficult to conduct a real life experiment in a hospital to assess this ducted return strategy for a variety of reasons. Furthermore, such a study would require sophisticated instruments to measure and collect the necessary three-dimensional data and might very well impact on routine patient care. Accordingly, this study used computational fluid dynamics (CFD) to analyse the ‘ducted return strategy’ under the three air change rates of 4, 6 and 12 air changes per hour (ACH) adopted in various international ventilation design standards.6, 8
Computational fluid dynamics
CFD is a branch of fluid mechanics that uses numerical methods and algorithms to analyse and solve problems relating to fluid flows. Millions of computer calculations are executed to simulate the interaction of liquids and gases with surfaces defined by specific boundary conditions. CFD modelling is an established technique for numerically resolving a wide range of three-dimensional airflow problems, including microbial dispersion, and the effects different ventilation systems have on contaminant behaviours.9, 10 For example, CFD has been used to investigate the mechanisms that influence indoor environments under different occupancy conditions.11 In a field analysis of the ventilation performance of different building designs, the results gained from CFD simulation were in close agreement with the measured results.12 Similarly, the rate of contaminant decay in a CFD-simulated indoor air current showed excellent agreement with actual measurements.13
CFD analysis has also been widely adopted in research on air ventilation and the removal of microbes on hospital isolation wards and has produced validated results.14, 15, 16 CFD is especially appropriate for studying the movement of highly infectious pathogen particulates, as airborne field samples from an isolation room may be difficult to access and dangerous to obtain and analyse.17 In such cases, CFD can provide highly accurate estimates of air and species movements, especially in parametric studies of room airflow and contaminant dispersion.18 The CFD simulation can represent a variety of details regarding airflow field distributions and the variations in different ventilation parameters. The commercially available CFD software package ‘FloVent’ was employed in this study. This software has been used in many ventilation studies to analyse the transport of airborne contaminants, especially in hospital isolation rooms.19, 20, 21
Numerical simulation using CFD is a powerful tool for the parametric analysis of the airflow fields of different ventilation regimens and to ascertain the distribution of microbes within isolation spaces.18 To identify optimal design settings, CFD can be used to determine the effectiveness of different ventilation strategies in removing microbes.
This study first compared the ventilation performances of a typical existing hospital general ward without any built-in isolation capability and a proper hospital isolation room system designed to the highest perceived infectious disease isolation standards. The purpose of this study was to identify a relatively cost-effective way of constructing (or renovating) an existing hospital general ward resulting in a ventilation performance that matches a properly designed isolation room.
Evaluation criteria
The following performance criteria for evaluating ventilation efficiency have been consistently used to compare the relative merits of different ventilation systems: air change efficiency; contaminant (microbial) removal effectiveness (CRE).22, 23, 24
Air change efficiency measures how effectively ventilation systems replace the air present in a room with fresh air. By characterising the mix of incoming and existing air, air change efficiency expresses the spatial distribution of the ‘age’ of the air independent of the distribution or emission characteristics of the pollutants.25 Ventilation efficiency is commonly assessed in terms of the local mean age of the air (LMAA), which is defined as the average time in seconds for air to travel from the inlet to any particular point of concern in the room.26 This study focused on the movement between the inlet and the areas occupied by healthcare staff. The lower the local mean age, the less likely the air is to feel stale or stuffy.
CRE measures how quickly a contaminant is removed from a room by quantifying the efficiency with which the internal pollutant is diluted or removed. In this case, the movement and dilution of contaminants in a given space is a function of the characteristics of the air flow and the contaminants. CRE is defined as the ratio between the concentration of contaminants at the exhaust point and the concentration within the occupied zone of the ward cubicle near where healthcare staff and patients are. When using CRE as a ventilation design guideline the goal is to maximise its value.27 The aim of this study was to find a simple and relatively cost-effective ventilation regimen that matched the performance of a properly designed isolation room in terms of the data for LMAA and CRE. Therefore, the focus was not on providing absolute data for LMAA and CRE, but on the comparative performance of the respective isolation facilities under different ventilation options.
Results
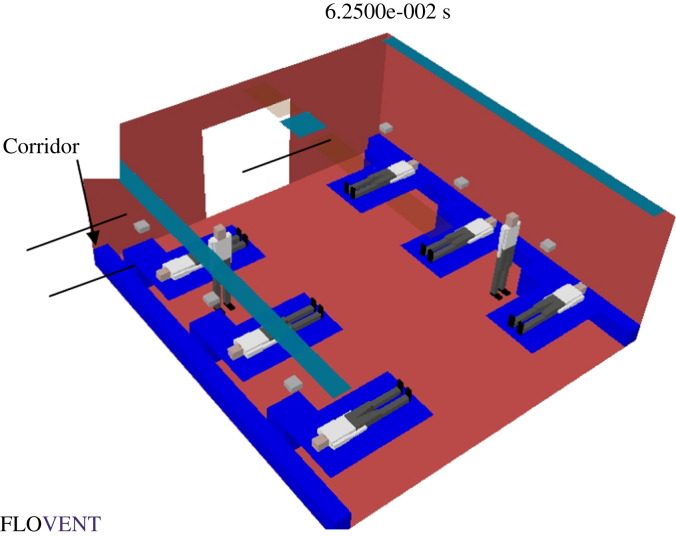
The layout of a typical hospital ward cubicle containing six patients and two standing medical staff is shown in Figure 1 . The CFD results for the various ventilation options, including the low performance limit of the existing general ward cubicle and the high performance limit of the infectious disease isolation room, are shown in Table I . The scenarios tested included air changes at the rate of 4, 6 and 12 ACH for the corridor, high and low level exhaust locations, as shown in Table I. The associated air flows in selected studies are depicted in the CFD-simulated velocity profiles shown in Figure 2 (study no. 1a), Figure 3 (study no. 2c) and Figure 4 (study no. 3c).
Figure 1.
Typical hospital general ward cubicle with six patients (the corridor is immediately outside the ward entrance).
Table I.
Summary of computational fluid dynamic simulation results
| Ventilation system in: | Study no. | Air changes | Exhaust location | Remark | CRE |
LMAA |
||
|---|---|---|---|---|---|---|---|---|
| Exhaust | Door | Patient | Healthcare staff | |||||
| Existing general ward | 1a | 4 | Corridor | No door | 0.8686 | 0.7848 | 758.1889 | 909.98 |
| 1b | 6 | Corridor | No door | 1.0040 | 0.8405 | 479.0828 | 586.94 | |
| Airborne isolation room | 2a | 6 | H | Door closed with pressure regulation | 0.9658 | NA | 197.5200 | 201.78 |
| 2b | 6 | L | Door closed with pressure regulation | 0.8626 | NA | 220.3350 | 219.36 | |
| 2c | 12 | H | Door closed with pressure regulation | 0.9568 | NA | 99.8930 | 100.41 | |
| 2d | 12 | L | Door closed with pressure regulation | 0.8865 | NA | 108.0360 | 107.18 | |
| Proposed general ward with ducted return | 3a | 4 | H | No door | 0.7432 | 0.9707 | 233.3984 | 304.65 |
| 3b | 6 | H | No door | 0.9792 | 0.1182 | 170.6100 | 195.76 | |
| 3c | 12 | H | No door | 0.9226 | 0.0090 | 101.0900 | 101.09 | |
CRE, contaminant (microbial) removal effectiveness; LMAA, local mean age of the air (average time in seconds for air to travel from the inlet to any particular point of concern in the room); H, exhaust grille installed at ceiling level; L, exhaust grille installed at low level near patient bed head; NA, not applicable.
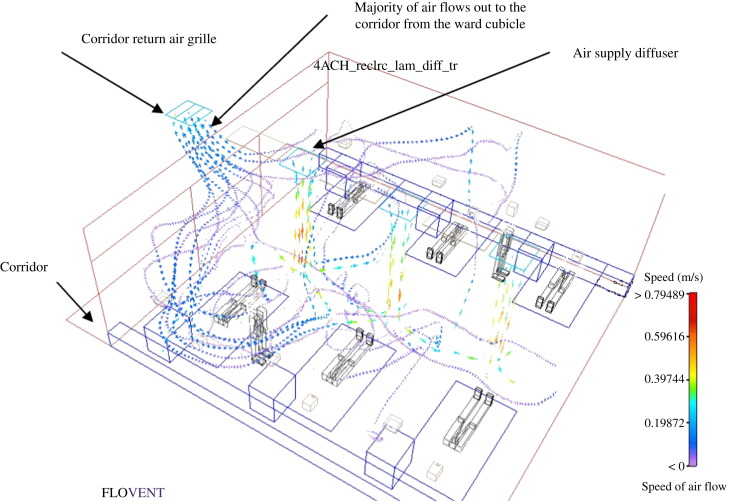
Figure 2.
Hospital general ward cubicle with six patients and the corridor return (study no. 1a).
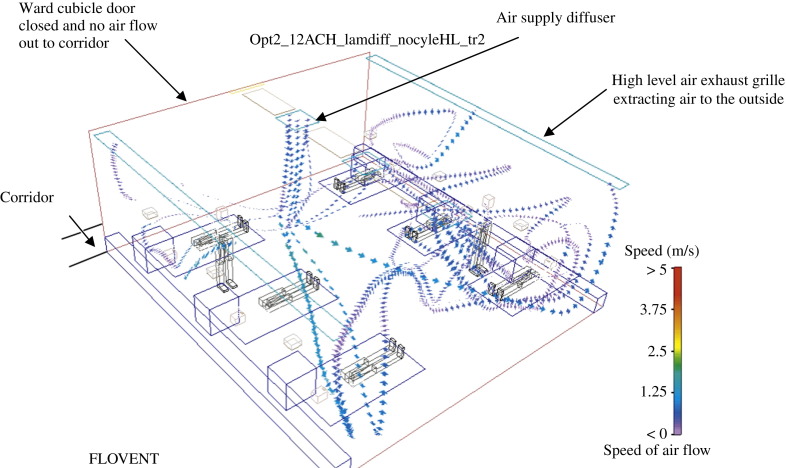
Figure 3.
Airborne isolation room with negative pressure control (study no. 2c).
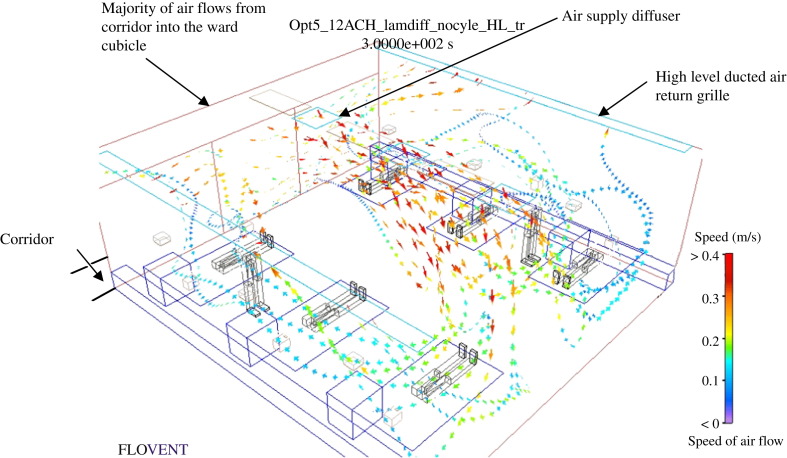
Figure 4.
Hospital general ward cubicle with ducted air return (study no. 3c).
Existing hospital general ward
The LMAA in the hospital general ward was far higher than the values of all other ventilation systems tested, i.e. the ventilation was poorest in the hospital general ward and the air was much stuffier (Figure 2). As a result of the corridor return arrangement, the CRE of the general ward (referenced at the cubicle entrance) approaches unity, which means microbes are flowing from the less clean cubicle room towards the cleaner corridor area, the opposite of what is required. Figure 2 shows the air flowing out of the ward toward the corridor area. The results demonstrate a lack of infection prevention and control capability in the existing hospital general ward.
Existing airborne isolation room
The CRE of the isolation room (referenced at the low level exhaust grille installed inside the cubicle) also approaches unity under different rates of air change, i.e. most contaminants move toward the exhaust (Figure 3). This indicates an effective microbe or contaminant removal regimen. Contaminants are extracted from the ward cubicle through the exhaust point situated at the low level exhaust grille inside the ward cubicle. Figure 3 shows the air flowing from the surrounding areas in the room towards the ward cubicle exhaust points, thereby forming a clean to less clean air flow that restricts contaminants spreading to the corridor area. This is in line with the isolation control measures required by the CDC.6
Whereas a sensitivity analysis of the reduced air change rate of 6 ACH revealed no significant drop in CRE, the LMAA was twice that measured for 12 ACH. Further analysis of the location of the exhaust point indicated that the high level exhaust grille layout provided even better CRE results. This can be explained by the interaction between body thermal plumes and the downward supply air stream. These findings agree with experimental results obtained for the dispersion of exhaled droplet nuclei in hospital wards with different ventilation systems.28, 29 This further suggests that the low level exhaust design should be abandoned, as it is difficult to implement in existing hospital general wards due to space constraints.
Proposed general ward with ducted return
The CRE of the various ducted return strategies are estimated at both the entrance to the cubicle and at the exhaust points located inside the cubicle (Figure 4). The 4 ACH design does not provide good results owing to air flowing from the cubicle to the corridor. Although at 6 ACH most of the air is exhausted through the high level exhaust grille, a significant portion of the air still flows toward the corridor via the cubicle entrance. When operating at the higher rate of 12 ACH, the CRE at the exhaust approaches unity and only an extremely small amount of air leaks into the ward corridor. This shows that, when the supply and exhaust of air is maintained at 12 ACH, the main air stream flows from the corridor towards the ward cubicle and a clean to less clean air flow direction is achieved.
Discussion
The findings of the present study reveal a simple and relatively cost-effective system of ventilation that can be incorporated into the design of new or retrofitted hospital general wards to enhance their capacity to minimise the risk of airborne disease transmission. Moreover, this enhanced air-handling capability will enable hospital general wards to accommodate large influxes of infectious patients in a pandemic situation. This can serve as an effective means of augmenting the limited number of isolation facilities currently available.
Based on the CFD results, the performance of the proposed ventilation system is comparable to a properly designed isolation room in restricting the flow of contaminated air and, thus, potentially airborne infections. The relatively simple design of the proposed system is based on an air-ducted exhaust situated at the ward cubicle ceiling level and a higher non-recirculating airflow supply of 12 ACH. The AHU should also be designed to run at lower rates of air change to save energy during the non-peak seasons for infectious disease. In terms of cost-effectiveness, the work and capital outlay required to install the additional exhaust return air duct and a larger capacity AHU represent a very small fraction of the construction cost of a new hospital general ward. Similarly, the cost of retrofitting an existing general ward would be far less than that of a full-scale refurbishment.
Nevertheless, the degree of safety achieved is far from absolute. This contribution of the susceptibility of patients to infection and the inhaled bioburden required needs further investigation. The issue is further complicated by the effects of different environmental conditions and the physical layouts of different hospitals. Ideally, it would be useful to compare the projected infection rates relating to airborne pathogens in a conventionally ventilated general ward and what is proposed here. This would require close collaboration between engineering and medical research professionals to conduct an extensive and large-scale prospective study involving patients.
Perhaps the simplest and most cost-effective way of reducing infection from airborne disease, especially in a pandemic situation, is to provide natural ventilation by opening hospital windows to achieve a high rate of air change. A number of recent studies have looked at natural ventilation as a means of preventing airborne disease transmission. Even so, a number of questions remain to be answered, including the need to locate the hospital where there are sufficient and regular air movements or wind, the types of hospital design layout suitable for natural ventilation, and whether specific indoor environmental conditions are required. Until such time that the natural ventilation approach has been fully explored and endorsed, the ventilation design proposed in this study remains a useful and effective alternative.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Kumari D.N.P., Haji T.C., Keer V., Hawley P.M., Duncanson V., Flower E. Ventilation grilles as a potential source of methicillin-resistant Staphylococcus aureus causing an outbreak in an orthopaedic ward at a district general hospital. J Hosp Infect. 1998;39:127–133. doi: 10.1016/s0195-6701(98)90326-7. [DOI] [PubMed] [Google Scholar]
- 2.Allen K.D., Green H.T. Hospital outbreak of multi-resistant Acinetobacter anitratus: an airborne mode of spread? J Hosp Infect. 1987;9:110–119. doi: 10.1016/0195-6701(87)90048-x. [DOI] [PubMed] [Google Scholar]
- 3.Best E.L., Fawley W.N., Parnell P., Wilcox M.H. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis. 2010;50:1450–1457. doi: 10.1086/652648. [DOI] [PubMed] [Google Scholar]
- 4.Yu I.T.S., Li Y., Wong T.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 5.Li Y., Leung G.M., Tang J.W. Role of ventilation on airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17:2–8. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . CDC; Atlanta: 2003. Guidelines for environmental infection control in healthcare facilities. [Google Scholar]
- 7.Whyte W., Howie J.G.R., Eakin J.E. Bacteriological observations in a mechanically ventilated experimental ward and in two open-plan wards. J Med Microbiol. 1969;2:335–345. doi: 10.1099/00222615-2-3-335. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Heating, Refrigerating and Air-Conditioning Engineers Inc . 2003. Handbook – HVAC applications. [Google Scholar]
- 9.Hensen JLM. Integrated building and airflow simulation: an overview. In: Ninth International Conference on computing in civil and building engineering, April 2002, p. 3–5.
- 10.Song F., Zhao B., Yang X. A new approach on zonal modelling of indoor environment with mechanical ventilation. Build Environ. 2008;43:278–286. [Google Scholar]
- 11.Assimakopoulos V.D., Stathopoulou O.I., Halios C., Helmis C.G. Numerical investigation of indoor environmental conditions in an office. Int J Ventilation. 2008;6:315–326. [Google Scholar]
- 12.Xing H., HaHon A., Awbi H.B. A study of the air quality in the breathing zone in a room with displacement ventilation. Build Environ. 2001;36:809–820. [Google Scholar]
- 13.Chung I.-Ping, Dunn-Rankin D. Using numerical simulation to predict ventilation efficiency in model room. Energy Build. 1998;28:43–50. [Google Scholar]
- 14.Duncan AP, Sinclair RJ, Schuyler GD. Isolation room ventilation design case studies. In: ASHRAE IAQ 2004 Conf Proc.
- 15.Kumar R., Kumar R., Gupta A. Analysis of the ventilation system of an isolation room for a hospital. Int J Ventilation. 2008;7:139–149. [Google Scholar]
- 16.Cheong K.W.D., Phua S.Y. Development of ventilation design strategy for effective removal of pollutant in the isolation room of a hospital. Build Environ. 2006;41:1161–1170. [Google Scholar]
- 17.Richmond-Bryant J. Transport of exhaled particulate matter in air borne infection isolation rooms. Build Environ. 2009;44:44–45. doi: 10.1016/j.buildenv.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami S., Kato S., Suyama Y. Numerical and experimental study on turbulent diffusion fields in conventional clean rooms. ASHRAE Trans. 1988;94:469–488. [Google Scholar]
- 19.Mazumdas S., Chen Q. A one-dimensional analytical model for airborne contaminant transport in airliner cabins. Indoor Air. 2009;19:3–13. doi: 10.1111/j.1600-0668.2008.00553.x. [DOI] [PubMed] [Google Scholar]
- 20.Memarzh F., Jiang J. Methodology for minimizing risk from airborne organisms in hospital isolation rooms. ASHRAE Trans. 2000 [Google Scholar]
- 21.Kim S.H., Augenbroe G. Ventilation operation in hospital isolation room: a multi-criterion assessment considering organisational behaviours. Eleventh Int IBPSA Conf. 2009 [Google Scholar]
- 22.Sandberg M. What is ventilation efficiency? Build Environ. 1981;19:123–135. [Google Scholar]
- 23.Sandberg M. The use of moments for assessing air quality in ventilated rooms. Build Environ. 1983;18:181–197. [Google Scholar]
- 24.Skavet E. Contaminant removal performance in terms of ventilation effectiveness. Indoor Air ’84 Stockholm. 1984 [Google Scholar]
- 25.Sutcliffe H. Technical note AIVC 28. Air Infiltration and Ventilation Centre; 1990. A guide to air changes efficiency. [Google Scholar]
- 26.Brouns C., Waters R. Air Infiltration and Ventilation Centre; 1991. A guide to contaminant removal effectiveness. Technical note AIVC 28.2. [Google Scholar]
- 27.Liddament M.W. Air Infiltration and Ventilation Centre; 1996. Guide to energy efficiency ventilation. [Google Scholar]
- 28.Li Y., Qian H., Nielsen P.V., Hyldgaard C.E., Wong T.W., Chwang A.T.W. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air. 2006;6:111–128. doi: 10.1111/j.1600-0668.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 29.Qian H., Li Y., Nielsen P.V., Hyldgaard C.E. Dispersion of exhalation pollutants in a two-bed hospital ward with a downward ventilation system. Build Environ. 2008;43:344–354. [Google Scholar]