Abstract
Bacterial pneumonia is a common clinical diagnosis in dogs but seems to occur less often in cats. Underlying causes include viral infection, aspiration injury, foreign body inhalation, and defects in clearance of respiratory secretions. Identification of the specific organisms involved in disease, appropriate use of antibiotics and adjunct therapy, and control of risk factors for pneumonia improve management.
Keywords: Bacterial pneumonia, Lower respiratory tract infection, Canine, Feline, Lower airway disease
Key points
-
•
Clinically, bacterial pneumonia is diagnosed much more commonly in dogs than in cats, although it is likely under-recognized in cats.
-
•
Viral infection followed by bacterial invasion is common in young dogs, whereas aspiration pneumonia and foreign body pneumonia seem to be more common in older dogs.
-
•
Clinical signs can be acute or chronic and do not always reflect the underlying respiratory condition.
-
•
Definitive diagnosis requires detection of intracellular bacteria in airway cytology or clinically significant bacterial growth from an airway sample, although relevant clinical findings can also support a clinical diagnosis.
-
•
Treatment requires identification and management of underlying diseases associated with pneumonia, appropriate antimicrobial therapy, and control of airway secretions.
Introduction
Bacterial pneumonia remains one of the most common clinical diagnoses in dogs with acute or chronic respiratory disease. Research suggests a complex relationship between viral respiratory diseases, environmental factors, and development of bacterial and mycoplasmal respiratory infection in dogs. In cats, bacterial pneumonia is less commonly identified than is inflammatory feline bronchial disease, although it might be overlooked because of similarities in clinical presentation and diagnostic findings.
Classification of bacterial pneumonia
Aspiration
Aspiration pneumonia results from the inadvertent inhalation of gastric acid, oropharyngeal secretions, and/or ingesta and remains a common cause of bacterial pneumonia, accounting for roughly 23% of clinical diagnoses in a study of human patients admitted to the intensive care unit.1 Although the true incidence of aspiration pneumonia related to all causes is not well described in veterinary medicine, the incidence of postanesthesia or sedation aspiration pneumonia was reported to be 0.17% in 1 large multi-institution study.2 Other than anesthesia, various conditions predispose to this disease. Risk factors that have been identified for the development of aspiration pneumonia include esophageal disease, refractory vomiting, seizures, and laryngeal dysfunction3 (Table 1 ).
Table 1.
Factors associated with aspiration pneumonia
|
|
|
|
|
|
In healthy animals, physiologic and anatomic features reduce the chance of aspiration. During a normal swallow, fluid and food are propelled caudally into the oropharynx and through the upper esophageal sphincter by contraction of the oral cavity, pharynx, and tongue. Concurrently, the epiglottis retracts to cover the laryngeal aditus and protect the trachea from particulate inhalation. In addition, adduction of the arytenoid cartilages contributes to further occlusion of the upper airways. Any process impeding these primary defenses or inhibiting normal swallowing reflexes greatly enhances the likelihood of aspiration.
Aspiration injury results from inhalation of either sterile, acidic gastric contents (resulting from vomiting or gastric regurgitation) or of septic material from either gastric or oral secretions. Irritation induced by acid inhalation promotes a local environment in which bacterial colonization can develop and lead to bacterial pneumonia.4 The severity of disease varies depending on the quantity and nature of the material aspirated as well as the length of time between the event and diagnosis. Conscious animals with intact airway reflexes tend to cough and prevent massive aspiration injury. Animals under anesthesia or with reduced airway reflexes caused by neurologic disorders are less likely to cough in response to the aspiration event and are, therefore, more likely to develop diffuse pulmonary infiltrates and serious lung injury. In many instances, aspiration injuries occur under general anesthesia and it should be noted that the presence of a cuffed endotracheal tube does not prevent inadvertent aspiration. Studies have shown that concurrent use of cisapride with a proton-pump inhibitor reduces the incidence of gastroesophageal reflux under anesthesia5 , 6 and therefore might reduce the likelihood of aspiration pneumonia.
Canine Infectious Pneumonia
Infectious, or community-acquired, pneumonias in dogs often begin with viral colonization and infection of the upper respiratory tract with canine respiratory coronavirus, adenovirus, herpesvirus, pneumovirus, parainfluenza virus, or others.7 Often, such diseases are acute and self-limiting, but, in a subset of dogs, inflammation associated with these organisms immobilizes the host’s immune defenses and predisposes to infection with other (often bacterial) respiratory pathogens.8 Many bacteria have been implicated in canine infectious respiratory disease, although special focus has been directed toward Streptococcus (specifically Streptococcus equi subsp zooepidemicus and Streptococcus canis), Mycoplasma cynos, and Bordetella bronchiseptica.9
Canine infectious respiratory disease (CIRD) is especially prevalent in dogs naïve to the pathogens and exposed in overcrowded, stressful environments such as animal shelters, boarding kennels, and treatment facilities, although it is important to remember that all dogs remain susceptible to these pathogens in any environment. The pathophysiology associated with infectious respiratory disease in dogs and cats is discussed later in this article (Boxes 1 and 2 ).
Box 1. Canine infectious respiratory disease complex: changing the face of kennel cough.
CIRD complex (formerly known as kennel cough) is a syndrome in which multiple pathogens, both viral and bacterial, coinfect either naïve, immunocompromised dogs or previously vaccinated dogs. This complex is multifactorial and it seems likely that both host and environmental factors play a role in the development of illness.40 Organisms associated with this disease are ubiquitous, especially in overcrowded housing facilities such as animal shelters and training facilities. It is likely that stress induced by the new environment and exposure to novel pathogens both play a role in development of disease.
In most cases, respiratory signs are present for days to weeks and most animals show mild to moderate clinical signs. Typically, viral infections cause either a bronchopneumonia or bronchointerstitial pneumonia because of their propensity to infect and damage type I pneumocytes.41 As the condition progresses, desquamation of the respiratory epithelium and aggregation of inflammatory cells further reduce the lungs’ natural defenses, increasing the potential for secondary bacterial colonization and infection.
Previous studies have implicated viral organisms such as canine adenovirus or canine parainfluenza42 as major participants in CIRD, although recent studies have proposed novel respiratory pathogens such as canine respiratory coronavirus,7 , 43, 44, 45 canine influenza virus,43 and canine herpesvirus46 as additional important pathogens associated with CIRD. B bronchiseptica,9 , 47 S canis, S equi subsp zooepidemicus,42 and M cynos 7 , 48 have been implicated as secondary bacterial infections associated with CIRD. S equi subsp zooepidemicus infections, in particular, have been associated with a rapidly progressive and often fatal hemorrhagic pneumonia.40 , 49 Of note, some strains identified in outbreaks of this pathogen have been identified as resistant to tetracycline antibiotics, often the drug of choice prescribed for other bacterial pathogens associated with this complex.
Box 2. Feline lower respiratory tract infections.
Organisms that have been reported as lower respiratory pathogens of cats include Pasteurella spp, Escherichia coli, Staphylococcus spp, Streptococcus spp, Pseudomonas spp, B bronchiseptica, and Mycoplasma spp,50 and specific attention has been paid to Mycoplasma spp because of a possible association with the induction and exacerbation of asthma in adult and pediatric human patients.51 However, the association between lower respiratory infection and chronic inflammatory lower airway disease in cats is unclear and a topic of ongoing interest.
Mycoplasma spp are considered normal flora in the upper respiratory tract and their role is controversial in lower respiratory tract infection. Because they are rarely identified cytologically and specific culture or polymerase chain reaction is needed to document the presence of these organisms, the role of Mycoplasma in cats (as well as in dogs) remains difficult to define.
Foreign Body
Inhaled foreign bodies carry mixed bacterial and fungal organisms into the lung and are associated with focal or lobar pneumonias that are often initially responsive to antimicrobial medications but relapse shortly after discontinuation of therapy.10 , 11 Foreign bodies reported in the veterinary literature include grass awns and plant or plastic materials.11 Organisms associated with grass awn inhalation include Pasteurella, Streptococcus, Nocardia, Actinomyces, and anaerobic bacteria.10, 11, 12 Most often, foreign material remains at the carina or enters caudodorsal principal bronchi (accessory, right and left caudal lobar bronchi).
Features associated with pulmonary foreign bodies include:
-
•
Young, sporting breeds
-
•
Environmental exposure to grass awns
-
•
Focal, recurrent radiographic alveolar pattern
-
•
History of other cutaneous or visceral foreign bodies
-
•
Spontaneous pneumothorax or pyothorax
Importantly, normal thoracic radiographs do not rule out the possibility of an airway foreign body and even computed tomography (CT) can fail to identify an affected bronchus.12 Chronic pulmonary foreign bodies are associated with marked inflammation that can lead to massive airway remodeling and bronchiectasis, which, when seen on radiographs, should raise the degree of suspicion for foreign body.10
Nosocomial
Ventilator-associated pneumonia (VAP) is a common cause of hospital-acquired pneumonia in people, although there are few reports in the veterinary literature. Colonization of the oropharynx by pathogenic and multidrug-resistant bacteria occurs and the endotracheal tube acts as a conduit to transmit pathogens into the airways, which leads to tracheobronchitis and potentially pneumonia. In addition, any animal with a compromised respiratory tract or serious systemic disease is particularly prone to development of infectious airway disease while hospitalized.
The use of mechanical ventilation in human patients increases the risk of nosocomial infection by 6-fold to 20-fold.13 No published studies assess the risk in ventilated veterinary patients, although a study investigating the difference in bacterial sensitivity between ventilated and nonventilated animals suggested that dogs requiring mechanical ventilation were more likely to be infected with bacteria resistant to the antimicrobials most commonly used empirically to treat pneumonia in veterinary practice.14 This suggestion parallels the increase in incidence of multidrug-resistant VAP in human medicine.13 In a recent outbreak of Acinetobacter calcoaceticus–Acinetobacter baumannii complex infections in a teaching hospital, 9 of 11 animals were suspected of developing pneumonia caused by use of contaminated equipment during general anesthesia.15
Immune Dysfunction
Both the innate and adaptive immune systems protect against the development of infectious airway disease, and a breakdown in either increases the likelihood of opportunistic infection (Table 2 ). Congenital immunodeficiencies have been recognized that make animals particularly sensitive to infectious organisms. Young animals are especially prone to the development of bacterial pneumonia because of their naive immune systems, and when coupled with alterations to the innate immune system, such as primary ciliary dyskinesia (PCD), complement deficiency, or bronchiectasis (congenital or acquired), the risk of life-threatening infection increases tremendously (see Veterinary Clinics of North America September 2007, Vol 37 (5): pp 845–860 for a comprehensive review of respiratory defenses in health and disease).
Table 2.
Conditions leading to impaired immune function and resulting in increased risk of pneumonia
| Congenital | Acquired | |
|---|---|---|
| Innate | Primary ciliary dyskinesia Complement deficiency Leukocyte adhesion deficiency |
Bronchiectasis Secondary ciliary dyskinesia |
| Adaptive | Immunoglobulin deficiency Severe combined immunodeficiency |
Retrovirus infection (eg, feline immunodeficiency virus, feline leukemia virus) Endocrine or metabolic disease (eg, diabetes mellitus or hyperadrenocorticism) Chemotherapy and other immunosuppressive therapy Splenectomy |
Any cause of systemic immunocompromise increases the risk for bacterial pneumonia, and any additional alterations to the body’s natural defense mechanisms dramatically increase the risk. Specifically, medications such as chemotherapy, immunosuppressive therapy, or antitussive therapy increase the likelihood of bacterial pneumonia. Underlying respiratory viruses or systemic viruses such as feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) have the potential to enhance the severity of respiratory illness.
Clinical signs
Clinical signs of bacterial pneumonia vary depending on its cause, severity, and chronicity. They can be acute or peracute in onset or can show an insidious onset, resulting in chronic illness, particularly in animals with preexisting chronic airway disease. Early in disease, mild signs such as an intermittent, soft cough might be the only evidence of disease. As infection spreads, clinical signs worsen and often include a refractory, productive cough; exercise intolerance; anorexia; and severe lethargy. Owners might note a change in the respiratory pattern, with increased panting or rapid breathing and, in cases of severe infection, cyanosis and orthopnea can be observed. In general, these systemic signs are more often recognized in dogs than in cats.
Cats with pneumonia can show similar clinical signs to dogs, although the cough can be misinterpreted as a retch or vomit by owners. Clinical signs and radiographic findings (eg, right middle lobar consolidation or collapse) can also be considered suggestive of inflammatory airway disease rather than pneumonia.16 As disease worsens, cats can become tachypneic with short, shallow breaths and nasal flaring.17 Rarely do cat owners notice exercise intolerance associated with bacterial pneumonia.
Physical examination
As with the history and clinical signs of bacterial pneumonia, physical examination findings vary greatly with the state and severity of disease. Dogs or cats with mild disease might have no abnormalities detected on physical examination. A change in the respiratory pattern, with an increase in rate and effort, can be an early clue to the diagnosis. Clinicians must pay close attention to thoracic auscultation because adventitious lung sounds (crackles and wheezes) can be subtle, focal, or intermittent. In many cases, only harsh or increased lung sounds are detected rather than crackles.18 Physical examination should assess for evidence of upper airway signs (eg, nasal congestion or discharge) that can result from lower airway infection, either as an extension of epithelial infection or from nasopharyngeal regurgitation of lower airway secretions. Thorough auscultation of the trachea and upper airway is important for detecting upper airway obstructive disease that could predispose to pneumonia.
Animals with bacterial pneumonia generally present with mixed inspiratory and expiratory signs, similar to those seen with other diseases of the pulmonary parenchyma. Fever is detected in 16% to 50% of cases, so it is not a reliable indicator of disease.8 , 16 , 18, 19, 20
Diagnosis
Bacterial pneumonia implies sepsis of the lower airway and lungs; consequently, the diagnosis is confirmed by the presence of septic suppurative inflammation on airway cytology obtained through bronchoalveolar lavage (BAL) or tracheal wash, along with a positive microbiology culture. In some cases, this is completed easily and yields results consistent with clinical suspicion. However, financial limitations or anesthetic concerns sometimes inhibit the ability to collect the samples needed to confirm a bacterial infection, and in those cases a clinical diagnosis of bacterial pneumonia might be presumed based on available information.
A clinical diagnosis of bacterial pneumonia should be reached after obtaining compelling evidence to suggest a bacterial cause for the animal’s clinical signs (after excluding other causes), and is confirmed by resolution of signs following appropriate antimicrobial therapy. Acute bacterial pneumonia is a common diagnosis in the small animal clinic and can often be easily identified; however, early and chronic pneumonias are more challenging to recognize because clinical signs can be subtle.
Hematology
The complete blood count is a useful diagnostic test in animals with respiratory signs. Bacterial pneumonias are often associated with an inflammatory leukogram, characterized primarily by a neutrophilia, with or without a left shift and variable evidence of toxic changes,12 , 21 although the absence of inflammatory change does not exclude the possibility of pneumonia.8 , 18 Furthermore, the leukogram and differential can provide clues that suggest bacterial pneumonia is less likely. For example, eosinophilia in an animal with respiratory signs would be more suggestive of eosinophilic bronchopneumopathy, granulomas, or parasitic lung diseases as an underlying cause than a bacterial cause. The erythrogram and platelet evaluation are generally not helpful in determining a bacterial cause of respiratory disease.
A biochemistry panel and urinalysis do not always contribute to the diagnosis of bacterial pneumonia but can provide clues to the presence of metabolic or endocrine diseases that could make the development of bacterial pneumonia more likely. Similarly, fecal flotation, sedimentation, and Baermann or heartworm test do not provide evidence for bacterial pneumonia but can be helpful in excluding parasitic pneumonia in areas where these organisms are endemic. Cats with respiratory conditions should be screened for FeLV and FIV to detect systemic causes of immunosuppression.
Pulmonary Function Testing
Arterial blood gas analysis is a useful test to measure the lung’s ability to oxygenate. Ideally, for animals with significant respiratory compromise, arterial blood samples should be collected and analyzed to determine the severity of pulmonary disease. Furthermore, trends in arterial oxygen partial pressure can be used to track progression or resolution of disease. In many cases, blood gas analysis is not available or patient factors preclude the acquisition of samples. Pulse oximetry is a quick, noninvasive evaluation of oxygen delivery to body tissues that measures percentage of hemoglobin saturation with oxygen. It provides only a crude assessment of oxygenation and is subject to variability; however, trends in hemoglobin saturation can provide additional clinical support to progression or resolution of disease. In addition, pulse oximetry provides a practical measure of oxygen desaturation during anesthesia for airway lavage and should be monitored closely during this procedure.
Thoracic Radiography
Thoracic radiographs are crucial diagnostic tests in the evaluation of lower airway and pulmonary parenchymal disease. Radiographic evidence of bacterial pneumonia can appear as a focal, multifocal, or diffuse alveolar pattern, although early in the disease process infiltrates can be primarily interstitial (Figs. 1 and 2 ).16 , 22 Ventral lung lobes are most commonly affected in aspiration pneumonia, and a caudodorsal pattern would be expected with inhaled foreign bodies or hematogenous bacterial spread. A lobar sign is often seen in cases of aspiration pneumonia in which the right middle lung lobe is affected (Table 3 ).
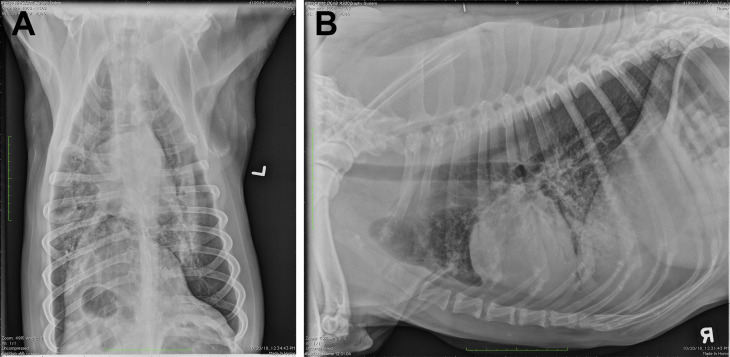
Fig. 1.
Dorsoventral (A) and right lateral (B) thoracic radiographs from a dog with an alveolar pattern in the cranioventral lung lobes, suggestive of aspiration. Note that in many cases the right middle lung lobe is most affected, which is best seen on a left lateral orthogonal view.
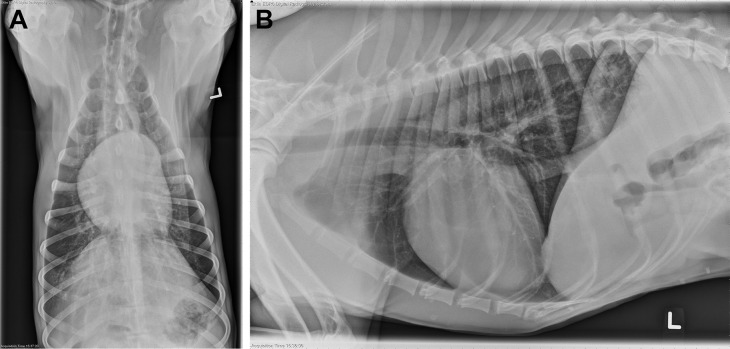
Fig. 2.
Dorsoventral (A) and right lateral (B) thoracic radiographs of a dog with a focal, patchy interstitial to alveolar pattern in the left cranial lung lobe. This dog was diagnosed with a foxtail foreign body, which was removed thoracoscopically via lung lobectomy.
(From Dear JD. Bacterial pneumonia in dogs and cats. Vet Clin North Am Small Anim Pract. 2014; 44(1): 143-159; with permission.)
Table 3.
Differential diagnoses for specific radiographic patterns
|
|
|
|
Modified from Dear JD. Bacterial pneumonia in dogs and cats. Vet Clin North Am Small Anim Pract. 2014; 44(1): 143-159; with permission.
Three-view thoracic radiographs (left lateral, right lateral, and either dorsoventral or ventrodorsal views) should be obtained when screening for pneumonia because differential aeration associated with positional atelectasis can either mask or highlight pulmonary changes. For example, a radiograph taken in left lateral recumbency is preferred when aspiration is suspected because it increases aeration of the right middle lung lobe, the most commonly affected lobe.
Diffuse radiographic involvement would be expected to suggest more severe disease, although radiographic changes lag behind clinical disease. Consequently, bacterial pneumonia cannot be ruled out in animals with acute onset of clinical signs and unremarkable radiographs.12
Advanced Imaging
Advanced imaging is rarely necessary in the diagnosis of uncomplicated bacterial pneumonia, although it can be helpful in more complicated cases. Thoracic ultrasonography can be used to characterize peripheral areas of consolidation and to obtain fine-needle aspirates for cytology. Cytology is often helpful in distinguishing inflammation from neoplastic or fungal disease. In addition, sonographic evaluation can be useful in the detection of superficial foxtail foreign bodies when they remain in the periphery of the lobe (Figs. 3 and 4 ).12
Fig. 3.
A foxtail foreign body retrieved bronchoscopically from the left principle bronchus of a dog with chronic respiratory signs. Foxtails are endemic to the Western and Midwestern United States as well as some parts of Europe and are associated with mixed aerobic and anaerobic infections. Fungal infections seem to occur rarely as a consequence of bronchopulmonary foreign bodies.
(From Dear JD. Bacterial pneumonia in dogs and cats. Vet Clin North Am Small Anim Pract. 2014; 44(1): 143-159; with permission.)
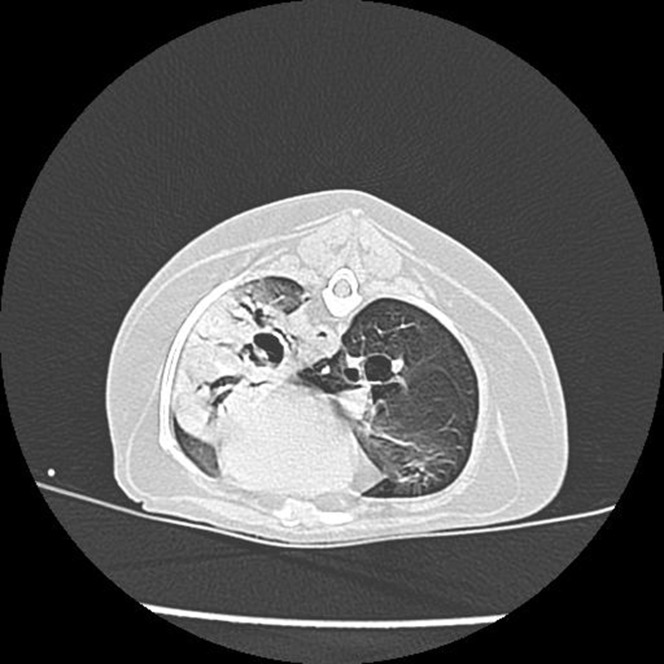
Fig. 4.
CT image of a dog with severe, diffuse pneumonia resulting from a chronic foxtail foreign body (see Fig. 3). The foreign body was not visible on thoracic radiographs, but is clearly evident in the left principal bronchus on this image.
(From Dear JD. Bacterial pneumonia in dogs and cats. Vet Clin North Am Small Anim Pract. 2014; 44(1): 143-159; with permission.)
CT provides greater detail and resolution of lesions within the pulmonary parenchyma and gives clinicians better spatial information regarding the severity and extent of pulmonary involvement. In particular, CT is much better at identifying the presence and extent of bronchiectasis compared with thoracic radiography. In some cases, CT can be useful to identify migration tracts associated with inhaled foreign bodies.12 However, in most cases general anesthesia is required for CT acquisition, and prolonged recumbency can lead to atelectasis, which is difficult to differentiate radiographically from infiltrates. Repeating the CT in a different position after providing several maximal inspirations can alleviate atelectasis. Nuclear scintigraphy can be useful for the evaluation of ciliary dyskinesia, although secondary causes of mucociliary stasis (eg, infection with Mycoplasma or Bordetella, exposure to smoke) must be excluded before assuming the diagnosis of PCD. Because of the time necessary for image acquisition, MRI is not commonly used for the diagnosis of most respiratory diseases. PET has not been evaluated for use in bacterial pneumonia, although it might be useful in evaluating patients with atypical infiltrates or mass lesions when a definitive diagnosis is not forthcoming.
Bronchoscopic Evaluation
Examination of the trachea and bronchial tree should be performed systematically. Endoscopists should note the color and character of the mucosa and any airway sections, making sure to evaluate all branches of the lower airways for evidence of foreign bodies, bronchiectasis, or collapse (diffuse or focal changes). Airway mucosa in normal animals should be pale pink with visible mucosal and pulmonary vessels. Airway bifurcations should appear as narrow, crisp mucosal margins.
Animals with pneumonia can have hyperemia of the epithelium, prominent mucosal vessels, and evidence of airway inflammation, appearing as rounded, thickened airway bifurcations and airway nodules. Airway secretions are usually opaque, therefore viscous and discolored (brown, yellow-green, or red-tinged) secretions can indicate inflammation or pneumonia.
Airway Sampling
When available, BAL is preferred for collection of lower airway samples rather than tracheal wash because the trachea and carina are not sterile, even in healthy dogs.23 In addition, the sensitivity for detecting cytologic features of sepsis is greater with BAL than with tracheal wash.19 However, when only a tracheal wash specimen can be obtained, because of the lack of equipment for BAL or because of patient instability, collection of a lower airway sample is desirable to identify infecting bacteria and to determine appropriate antimicrobial therapy through susceptibility testing. Oropharyngeal swabs are not suitable substitutes for making a diagnosis of pneumonia.
BAL cell counts in animals with bacterial pneumonia are markedly higher than in dogs with chronic bronchitis or other respiratory disease.21 Septic, suppurative inflammation is a reliable indicator of bacterial pneumonia in dogs21 and is likely to indicate bacterial pneumonia in cats. In cases that lack evidence of airway sepsis (intracellular bacteria), BAL cytology generally reveals suppurative or mixed inflammation.20 Note that animals with mycoplasma pneumonia can have positive culture in the absence of cytologic evidence of sepsis.
In animals with suspected or confirmed foreign bodies, a BAL sample should always be obtained from the affected airway as well as an additional site, with both submitted individually for cytologic analysis. Airway bacteria are more likely to be seen in the cytologic sample from the site of the foreign body than from an alternate site.11 Furthermore, cytology of BAL samples obtained from multiple lobes can reveal different findings, even in cases of sterile inflammatory diseases such as feline bronchial disease, thus reliance on single-segment BAL cytology could lessen the chance of yielding diagnostic results.24
Microbiology
Diagnosis of bacterial pneumonia relies on identification of septic inflammation in conjunction with a positive bacterial culture. Typically, aerobic and Mycoplasma culture and sensitivity are requested, and, in cases with markedly purulent secretions or a history of known aspiration or foreign bodies, anaerobic cultures should also be requested. Samples should be refrigerated in sterile containers until submitted. If multiple alveolar segments are sampled during BAL, these are usually pooled for culture submission. When anaerobic cultures are desired, BAL fluid should be inoculated into the appropriate transport media and kept at room temperature until submission.
Cultures should be performed whenever possible in order to guide appropriate antimicrobial therapy. With overly liberal use of antibiotics, increasing populations of resistant microbes are being identified, particularly in animals with hospital-acquired pneumonia.25 , 26 However, airway samples cannot be collected in all animals, and, in those instances, recommendations regarding antimicrobial stewardship should be followed.27
Bacteria commonly isolated from lung washes of cats or dogs with bacterial pneumonia include enteric organisms (Escherichia coli, Klebsiella spp), Pasteurella spp, coagulase-positive Staphylococcus spp, beta-hemolytic Streptococcus spp, Mycoplasma spp, and B bronchiseptica (Table 4 ).20 , 22
Table 4.
Bacteria commonly isolated from airway samples of canine patients with pneumonia
| Organism | Isolates (%) |
|---|---|
| B bronchiseptica | 22–71 |
| E coli | 11–51 |
| Klebsiella pneumoniae | 2–25 |
| Pasteurella spp | 3–21 |
| Mycoplasma spp | 30–70 |
| Streptococcus spp | 6–21 |
| Staphylococcus spp | 7–20 |
| Anaerobes | 5–17 |
| Enterococcus spp | 4–11 |
Treatment
Treatment of bacterial pneumonia varies depending on the severity of disease, and appropriate antimicrobial therapy is essential. The International Society for Companion Animal Infectious Disease (ISCAID) has published guidelines for treatment of dogs and cats with respiratory infections and these should be consulted for further details about recommendations.27 For stable animals with mild disease, outpatient therapy consisting of administration of a single, oral antibiotic is often all that is necessary (Table 5 ). Ideally, antimicrobial choices should be based on culture and sensitivity results from airway lavage samples because resistance to antimicrobials selected empirically has been reported in up to 26% of cases.28 For critically ill animals in which airways samples cannot be obtained, blood cultures might be considered, although there is a lack of data on sensitivity in veterinary patients. Regardless, in cases of severe pneumonia, initial empiric therapy should be instituted while awaiting culture results. Traditionally, antimicrobials have been administered for 3 to 6 weeks, and at least 1 to 2 weeks beyond the resolution of clinical and/or radiographic signs of disease, although there is no evidence to support this practice. ISCAID recommendations suggest that shorter durations might be appropriate, but there are few data to support this suggestion. One observational study found similar radiographic and clinical cures in dogs treated with a short course of antibiotic (<14 days) compared with those that received a longer duration of treatment.29 Regardless of the intended duration of therapy, reevaluation within 10 to 14 days of starting treatment is important to determine response and to define optimal length of treatment.
Table 5.
Empiric antibiotic choice for patients with pneumonia
| Stable patient, mild clinical signs |
|
| Moderate clinical signs |
|
| Critical patient, severe clinical signs |
|
Abbreviations: IV, intravenous; PO, by mouth; SQ, subcutaneous.
Data from Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279-294.
Animals with more advanced disease require more intensive care, including hospitalization with intravenous fluids to maintain hydration. Adequate hydration is essential to facilitate clearance of respiratory exudates. Nebulization to create liquid particles that enter the lower airways (<5 μm) can also enhance clearance of secretions. Nebulizer types include ultrasonic devices, compressed air nebulizers, and mesh nebulizers. Nebulization with sterile saline can be achieved by directing the hosing from the nebulizer into a cage or animal carrier covered in plastic. Depending on how viscous secretions are, therapy can be provided for 15 to 20 minutes 2 to 4 times daily. In many cases, nebulization coupled with coupage can help the animal expectorate airway secretions, although no specific studies in veterinary medicine have evaluated this technique. Coupage is performed by cupping the hands and gently, rhythmically pounding on both lateral thoracic walls in a dorsal to ventral and caudal to cranial direction. Coupage should not be performed in animals with regurgitation because any increase in intrathoracic pressure could exacerbate regurgitation and subsequent reaspiration.
Supplemental oxygen is necessary for animals with moderate to marked hypoxemia (documented by a Pao 2 <80 mm Hg or SpO2 <94% on room air) in conjunction with increased respiratory effort. Oxygen supplementation at 40% to 60% is provided until respiratory difficulty lessens and the animal can be weaned to room air. Animals with refractory pneumonia that fail to improve on supplemental oxygen can succumb to ventilatory fatigue and need to be referred to an intensive care facility for mechanical ventilation.
Clinically, it seems that administration of an oral mucolytic agent such as N-acetylcysteine can be useful for animals with retention of thick respiratory secretions. In particular, this can be helpful in dogs with moderate to severe bronchiectasis that are prone to chronic or recurrent pneumonia. Decreasing the viscosity of airway secretions might improve expectoration of fluid and debris that accumulates in dependent airways, although no published information is available on the use of mucolytics in animals. N-acetylcysteine is typically not used via nebulization because of risks of bronchoconstriction and epithelial toxicity. Under no circumstances is it appropriate to use cough suppressants (eg, butorphanol or hydrocodone) in the management of bacterial pneumonia, particularly when it is complicated by bronchiectasis. By decreasing the cough reflex, these drugs perpetuate retention of mucus, debris, and other material in the airways and therefore hinder clearance of infection. Also, furosemide should not be used because drying of secretions traps material in the lower airway and perpetuates infection.
In cases in which aspiration pneumonia is suspected, strategies should be used to reduce the chance of reaspirating through appropriate treatment of the underlying condition. With disorders of esophageal motility, upright feedings of either slurry or meatballs can enhance esophageal transit. Furthermore, diets low in fat can increase gastric emptying. In patients with refractory vomiting, antiemetic and prokinetic agents can be used to reduce the episodes of vomiting. Drugs such as maropitant (Cerenia; 1 mg/kg intravenously or subcutaneously once daily) or ondansetron (Zofran; 0.3–1 mg/kg intravenously or subcutaneously once to twice daily) act peripherally or centrally to decrease the urge to vomit and are safe to use in both cats and dogs.
The role of antacids in management of aspiration pneumonia remains controversial. By neutralizing the pH of gastric secretions, animals with refractory vomiting or regurgitation are less likely to succumb to chemical injury related to aspiration. However, in cases treated with acid suppression, the aspirant could be more likely to contain a greater concentration of bacteria that can colonize the airways and lead to bacterial pneumonia. Although treatment with proton pump inhibitors has been shown to reduce the incidence of acid reflux events in both dogs and cats undergoing anesthesia,5 , 30 no controlled studies have assessed the severity of aspiration pneumonia or relative risk of using antacid therapy in dogs or cats as a preventive measure.
Because radiographic findings lag behind clinical disease, recheck radiographs are not helpful early in the disease process, although they are useful to document resolution of disease and should be obtained either before or within a week of discontinuation of antimicrobial therapy. In cases of refractory pneumonia, recheck radiographs midway through therapy can assess resolution or progression of disease and help to guide further diagnostics and therapy.
Serum biomarkers such as acute phase proteins are associated with inflammatory disease. Being nonspecific, these biomarkers are not clinically useful for diagnosis but might be helpful in determining treatment response, facilitating better antimicrobial stewardship by suggesting resolution of disease more rapidly than thoracic radiographs.31 Further studies are required to establish their utility in management of pneumonia.
In animals suspected of having contagious or multidrug-resistant pathogens, appropriate contact precautions should be used. Isolation gowns, examination gloves, and good hand washing technique along with appropriate quarantine facilities are essential to prevent transmission of disease to other animals or members of the health care team.
Prognosis
Prognosis for animals with bacterial pneumonia varies depending on the severity of disease, the animal’s immunocompetence, and the virulence of the infectious agent. In general, between 77% and 94% of patients diagnosed with pneumonia are discharged from the hospital.8 , 28 , 32 No large, long-term studies have assessed the overall prognosis of animals with multidrug-resistant bacteria or recurrent pneumonia. Presumably, the outcome associated with these cases will be worse. In a recent case series of presumed nosocomial multidrug resistant A calcoaceticus–A baumannii complex infections, 8 out of 11 animals with pneumonia died or were euthanized as a consequence of their disease.15
Case studies
Case Study 1
A 7-year-old male castrated bichon frise presented for a chronic cough.
History
Six-year history of progressive cough since adoption. The cough is described as nonproductive, worse in the morning, and exacerbated by aerosols and heavy fragrances. Previous treatment with theophylline and doxycycline have not lessened the severity of cough.
Physical Examination
Temperature (38.9°C [101.9°F]), pulse (72 beats/min), and respiratory rate (32 breaths/min) were normal. No heart murmur but soft crackles were auscultated on inspiration. A cough was elicited on tracheal palpation.
Diagnostic Evaluation
Chronic cough in a small-breed dog is often associated with airway collapse or chronic bronchitis; however, infectious and neoplastic disease must remain on the differential list. Congestive heart failure is unlikely in this case given the lack of a heart murmur and normal heart rate.
A white blood cell count was normal (6650 cells/μL) with 4722 neutrophils. Thoracic radiographs revealed dynamic lower airway narrowing between lateral projections and a diffuse prominent bronchointerstitial pattern, most prominent in the caudal thorax (Fig. 5 ). The larynx seemed to have normal function at anesthetic induction. Bronchoscopy revealed mild to moderate dynamic lower airway collapse and bronchiectasis of caudodorsal bronchi along with airway exudate. BAL samples were hypercellular on cytology (2500 cells/μL) and revealed septic suppurative inflammation (55%, normal 5%–8%) with degenerate neutrophils. Bacterial cultures were positive for Pasteurella dagmatis and Fusobacterium sp. In this case, chronic inflammatory airway disease likely contributed to the dog’s bronchiectasis, which then predisposed to bronchopneumonia.
Fig. 5.
Dorsoventral (A) and right lateral (B) thoracic radiographs revealing bronchiectasis and a diffuse prominent bronchointerstitial pattern, most prominent in the caudal thorax (case study 1).
Case Study 2
A 5-year-old MC domestic medium hair was presented for evaluation of acute respiratory distress.
History
Lethargy and anorexia had been noted 3 days before the onset of respiratory signs.
Physical Examination
Temperature (38.7°C [101.6°F]) and pulse (210 beats/min) were normal. Tachypnea was noted (respiratory rate 60 breaths/min) along with increased respiratory effort on inspiration and expiration. Diffuse expiratory wheezes were auscultated.
Diagnostic Evaluation
Acute onset of respiratory difficulty in a cat is most commonly related to inflammatory airway disease. The physical examination is consistent with this diagnosis, although it is uncommon for affected cats to show lethargy and anorexia. Infectious and neoplastic diseases were also on the differential diagnosis list, along with aspiration and foreign body pneumonia.
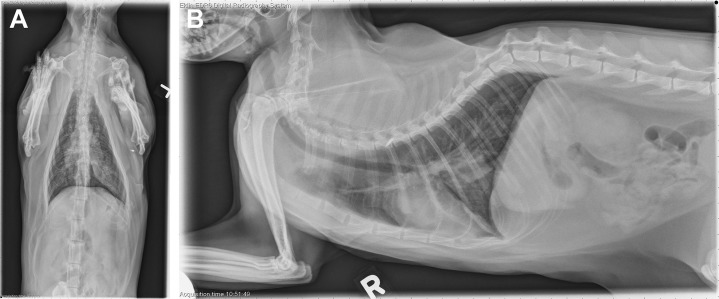
Thoracic radiographs showed a focal opacity in the left caudal lung lobe and a diffuse bronchial pattern (Fig. 6 ). Complete blood count revealed a normal white blood cell count (8500 cells/μL) with a left shift (6800 neutrophils, 1000 bands). Bronchoscopy with lavage was performed. A moderate amount of airway hyperemia and edema was noted along with purulent material obstructing several airways. BAL cytology had increased cellularity (1500 cells/μL, normal 500 cells/μL) with neutrophilic inflammation (84%, normal 5%–8%). Neutrophils contained dark blue granular debris, suspicious for sepsis. Aerobic and anaerobic cultures were negative, but a pure culture of Mycoplasma was isolated on special media. A diagnosis of mycoplasma bronchopneumonia was made.
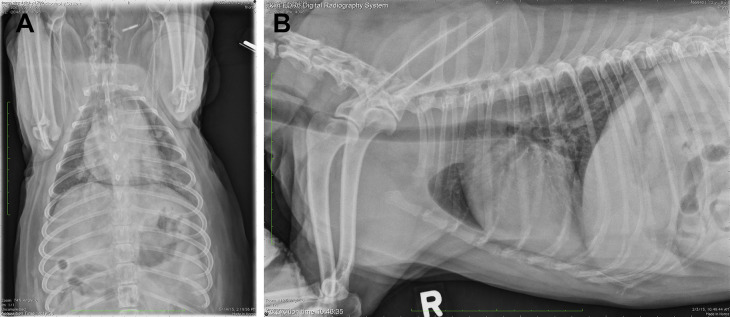
Fig. 6.
Dorsoventral (A) and right lateral (B) thoracic radiographs revealing a focal opacity in the left caudal lung lobe and a diffuse bronchial pattern (case study 2).
Acknowledgments
Disclosure
The author has nothing to disclose.
References
- 1.Leroy O., Vandenbussche C., Coffinier C. Community-acquired aspiration pneumonia in intensive care units. Epidemiological and prognosis data. Am J Respir Crit Care Med. 1997;156(6):1922–1929. doi: 10.1164/ajrccm.156.6.9702069. [DOI] [PubMed] [Google Scholar]
- 2.Ovbey D.H., Wilson D.V., Bednarski R.M. Prevalence and risk factors for canine post-anesthetic aspiration pneumonia (1999-2009): a multicenter study. Vet Anaesth Analg. 2014;41(2):127–136. doi: 10.1111/vaa.12110. [DOI] [PubMed] [Google Scholar]
- 3.Tart K.M., Babski D.M., Lee J.A. Potential risks, prognostic indicators, and diagnostic and treatment modalities affecting survival in dogs with presumptive aspiration pneumonia: 125 cases (2005-2008) J Vet Emerg Crit Care (San Antonio) 2010;20(3):319–329. doi: 10.1111/j.1476-4431.2010.00542.x. [DOI] [PubMed] [Google Scholar]
- 4.Mitsushima H., Oishi K., Nagao T. Acid aspiration induces bacterial pneumonia by enhanced bacterial adherence in mice. Microb Pathog. 2002;33(5):203–210. doi: 10.1006/mpat.2002.0529. [DOI] [PubMed] [Google Scholar]
- 5.Zacuto A.C., Marks S.L., Osborn J. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med. 2012;26(3):518–525. doi: 10.1111/j.1939-1676.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogden J., Ovbey D., Saile K. Effects of preoperative cisapride on postoperative aspiration pneumonia in dogs with laryngeal paralysis. J Small Anim Pract. 2019;60(3):183–190. doi: 10.1111/jsap.12940. [DOI] [PubMed] [Google Scholar]
- 7.Brownlie J., Mitchell J., Walker C.A. Mycoplasmas and novel viral pathogens in canine infectious respiratory disease. J Vet Intern Med (Seattle) 2013 [Google Scholar]
- 8.Radhakrishnan A., Drobatz K.J., Culp W.T.N. Community-acquired infectious pneumonia in puppies: 65 cases (1993-2002) J Am Vet Med Assoc. 2007;230(10):1493–1497. doi: 10.2460/javma.230.10.1493. [DOI] [PubMed] [Google Scholar]
- 9.Taha-Abdelaziz K., Bassel L.L., Harness M.L. Cilia-associated bacteria in fatal Bordetella bronchiseptica pneumonia of dogs and cats. J Vet Diagn Invest. 2016;28(4):369–376. doi: 10.1177/1040638716646806. [DOI] [PubMed] [Google Scholar]
- 10.Workman H.C., Bailiff N.L., Jang S.S. Capnocytophaga cynodegmi in a rottweiler dog with severe bronchitis and foreign-body pneumonia. J Clin Microbiol. 2008;46(12):4099–4103. doi: 10.1128/JCM.00173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenwolde A.C., Johnson L.R., Hunt G.B. The role of bronchoscopy in foreign body removal in dogs and cats: 37 cases (2000-2008) J Vet Intern Med. 2010;24(5):1063–1068. doi: 10.1111/j.1939-1676.2010.0580.x. [DOI] [PubMed] [Google Scholar]
- 12.Schultz R.M., Zwingenberger A. Radiographic, computed tomographic, and ultrasonographic findings with migrating intrathoracic grass awns in dogs and cats. Vet Radiol Ultrasound. 2008;49(3):249–255. doi: 10.1111/j.1740-8261.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 13.Craven D.E., Hjalmarson K.I. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–S66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 14.Epstein S.E., Mellema M.S., Hopper K. Airway microbial culture and susceptibility patterns in dogs and cats with respiratory disease of varying severity. J Vet Emerg Crit Care (San Antonio) 2010;20(6):587–594. doi: 10.1111/j.1476-4431.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuzi S., Blum S.E., Kahane N. Multi-drug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex infection outbreak in dogs and cats in a veterinary hospital. J Small Anim Pract. 2016;57(11):617–625. doi: 10.1111/jsap.12555. [DOI] [PubMed] [Google Scholar]
- 16.Levy N., Ballegeer E., Koenigshof A. Clinical and radiographic findings in cats with aspiration pneumonia: retrospective evaluation of 28 cases. J Small Anim Pract. 2019;60(6):356–360. doi: 10.1111/jsap.12990. [DOI] [PubMed] [Google Scholar]
- 17.Egberink H., Addie D., Belak S. Bordetella bronchiseptica infection in cats. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):610–614. doi: 10.1016/j.jfms.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogan D.A., Johnson L.R., Jandrey K.E. Clinical, clinicopathologic, and radiographic findings in dogs with aspiration pneumonia: 88 cases (2004-2006) J Am Vet Med Assoc. 2008;233(11):1742–1747. doi: 10.2460/javma.233.11.1742. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins E.C., DeNicola D.B., Plier M.L. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of spontaneous respiratory tract disease in dogs: a retrospective study. J Vet Intern Med. 1995;9(6):386–392. doi: 10.1111/j.1939-1676.1995.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson L.R., Queen E.V., Vernau W. Microbiologic and cytologic assessment of bronchoalveolar lavage fluid from dogs with lower respiratory tract infection: 105 cases (2001-2011) J Vet Intern Med. 2013;27(2):259–267. doi: 10.1111/jvim.12037. [DOI] [PubMed] [Google Scholar]
- 21.Peeters D.E., McKiernan B.C., Weisiger R.M. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens in dogs. J Vet Intern Med. 2000;14(5):534–541. doi: 10.1892/0891-6640(2000)014<0534:qbcace>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Cohn L.A. Pulmonary parenchymal disease. In: Ettinger S.J., Feldman E.C., editors. 7th edition. vol. 2. Saunders, Elsevier; St Louis (MO): 2010. (Textbook of veterinary internal medicine: diseases of the dog and the cat). [Google Scholar]
- 23.McKiernan B.C., Smith A.R., Kissil M. Bacterial isolates from the lower trachea of clinically healthy dogs. J Am Anim Hosp Assoc. 1984;20:139–142. [Google Scholar]
- 24.Ybarra W.L., Johnson L.R., Drazenovich T.L. Interpretation of multisegment bronchoalveolar lavage in cats (1/2001-1/2011) J Vet Intern Med. 2012;26(6):1281–1287. doi: 10.1111/j.1939-1676.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 25.Chalker V.J., Waller A., Webb K. Genetic diversity of Streptococcus equi subsp. zooepidemicus and doxycycline resistance in kennelled dogs. J Clin Microbiol. 2012;50(6):2134–2136. doi: 10.1128/JCM.00719-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley J.E., Rand C., Bannasch M.J. Molecular epidemiology of feline bordetellosis in two animal shelters in California, USA. Prev Vet Med. 2002;54(2):141–156. doi: 10.1016/s0167-5877(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Lappin M.R., Blondeau J., Boothe D. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279–294. doi: 10.1111/jvim.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proulx A., Hume D.Z., Drobatz K.J. In vitro bacterial isolate susceptibility to empirically selected antimicrobials in 111 dogs with bacterial pneumonia. J Vet Emerg Crit Care (San Antonio) 2014;24(2):194–200. doi: 10.1111/vec.12128. [DOI] [PubMed] [Google Scholar]
- 29.Wayne A., Davis M., Sinnott V.B. Outcomes in dogs with uncomplicated, presumptive bacterial pneumonia treated with short or long course antibiotics. Can Vet J. 2017;58(6):610–613. [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia R.S., Belafsky P.C., Della Maggiore A. Prevalence of gastroesophageal reflux in cats during anesthesia and effect of omeprazole on gastric pH. J Vet Intern Med. 2017;31(3):734–742. doi: 10.1111/jvim.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viitanen S.J., Lappalainen A.K., Christensen M.B. The utility of acute-phase proteins in the assessment of treatment response in dogs with bacterial pneumonia. J Vet Intern Med. 2017;31(1):124–133. doi: 10.1111/jvim.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kogan D.A., Johnson L.R., Sturges B.K. Etiology and clinical outcome in dogs with aspiration pneumonia: 88 cases (2004-2006) J Am Vet Med Assoc. 2008;233(11):1748–1755. doi: 10.2460/javma.233.11.1748. [DOI] [PubMed] [Google Scholar]
- 33.Viitanen S.J., Lappalainen A.K., Koho N.M. Recurrent bacterial pneumonia in Irish Wolfhounds: clinical findings and etiological studies. J Vet Intern Med. 2019;33(2):846–855. doi: 10.1111/jvim.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBrearty A., Ramsey I., Courcier E. Clinical factors associated with death before discharge and overall survival time in dogs with generalized megaesophagus. J Am Vet Med Assoc. 2011;238(12):1622–1628. doi: 10.2460/javma.238.12.1622. [DOI] [PubMed] [Google Scholar]
- 35.Bedu A.S., Labruyere J.J., Thibaud J.L. Age-related thoracic radiographic changes in golden and labrador retriever muscular dystrophy. Vet Radiol Ultrasound. 2012;53(5):492–500. doi: 10.1111/j.1740-8261.2012.01948.x. [DOI] [PubMed] [Google Scholar]
- 36.Watson P.J., Herrtage M.E., Peacock M.A. Primary ciliary dyskinesia in Newfoundland dogs. Vet Rec. 1999;144(26):718–725. doi: 10.1136/vr.144.26.718. [DOI] [PubMed] [Google Scholar]
- 37.Watson P.J., Wotton P., Eastwood J. Immunoglobulin deficiency in Cavalier King Charles spaniels with pneumocystis pneumonia. J Vet Intern Med. 2006;20(3):523–527. doi: 10.1892/0891-6640(2006)20[523:idickc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Jezyk P.F., Felsburg P.J., Haskins M.E. X-linked severe combined immunodeficiency in the dog. Clin Immunol Immunopathol. 1989;52(2):173–189. doi: 10.1016/0090-1229(89)90170-0. [DOI] [PubMed] [Google Scholar]
- 39.Foster S.F., Martin P., Braddock J.A. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995-2000) J Feline Med Surg. 2004;6(3):189–198. doi: 10.1016/j.jfms.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesavento P.A., Hurley K.F., Bannasch M.J. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet Pathol. 2008;45(1):51–53. doi: 10.1354/vp.45-1-51. [DOI] [PubMed] [Google Scholar]
- 41.Mellema M. Viral pneumonia. In: King L.G., editor. vol. 1. Saunders; St Louis (MO): 2004. pp. 431–445. (Textbook of respiratory disease in dogs and cats). [Google Scholar]
- 42.Chalker V.J., Brooks H.W., Brownlie J. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet Microbiol. 2003;95(1–2):149–156. doi: 10.1016/S0378-1135(03)00155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An D.J., Jeoung H.Y., Jeong W. A serological survey of canine respiratory coronavirus and canine influenza virus in Korean dogs. J Vet Med Sci. 2010;72(9):1217–1219. doi: 10.1292/jvms.10-0067. [DOI] [PubMed] [Google Scholar]
- 44.Knesl O., Allan F.J., Shields S. The seroprevalence of canine respiratory coronavirus and canine influenza virus in dogs in New Zealand. N Z Vet J. 2009;57(5):295–298. doi: 10.1080/00480169.2009.58624. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J.A., Brooks H.W., Szladovits B. Tropism and pathological findings associated with canine respiratory coronavirus (CRCoV) Vet Microbiol. 2013;162(2–4):582–594. doi: 10.1016/j.vetmic.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakami K., Ogawa H., Maeda K. Nosocomial outbreak of serious canine infectious tracheobronchitis (kennel cough) caused by canine herpesvirus infection. J Clin Microbiol. 2010;48(4):1176–1181. doi: 10.1128/JCM.02128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keil D.J., Fenwick B. Canine respiratory bordetellosis: keeping up with an evolving pathogen. In: Carmichael L.E., editor. Recent advances in canine infectious disease. International Veterinary Information Service; Ithaca (NY): 2000. http://www.ivis.org/advances/Infect_Dis_Carmichael/keil/chapter.asp?LA=1 Available at: Accessed November 23, 2019. [Google Scholar]
- 48.Chalker V.J., Owen W.M., Paterson C. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150(Pt 10):3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- 49.Priestnall S., Erles K. Streptococcus zooepidemicus: an emerging canine pathogen. Vet J. 2011;188(2):142–148. doi: 10.1016/j.tvjl.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster S.F., Martin P., Allan G.S. Lower respiratory tract infections in cats: 21 cases (1995-2000) J Feline Med Surg. 2004;6(3):167–180. doi: 10.1016/j.jfms.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood P.R., Hill V.L., Burks M.L. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2013;110(5):328–334 e321. doi: 10.1016/j.anai.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]