Summary
Background
The management of patients with highly infectious diseases (HIDs) is a challenge for healthcare provision requiring a high level of care without compromising the safety of other patients and healthcare workers.
Aim
To study the infection control practice in isolation facilities participating in the European Network for Highly Infectious Diseases (EuroNHID) project.
Methods
A survey was conducted during 2009 of 48 isolation facilities caring for patients with HIDs in 16 European countries. Checklists and standard evaluation forms were used to collect and interpret data on hand hygiene, routine hygiene and disinfection, and waste management.
Findings
Forty percent of HIDs had no non-hand-operated sinks or alcohol-based antiseptic distributors, while 27% did not have procedures for routine hygiene, final disinfection, or safe discarding of non-disposable objects or equipment. There was considerable variation in the management of waste and in the training of housekeeping personnel. EuroNHID has developed recommendations for hand hygiene, disinfection, routine hygiene, and waste management.
Conclusions
Most aspects of hand hygiene, routine hygiene and disinfection, and waste management were considered at least partially adequate in the majority of European isolation facilities dedicated for the care of patients with HIDs. But considerable variability was observed, with management of waste and training of housekeeping personnel being generally less satisfactory.
Keywords: Disinfection, Hand hygiene, Highly infectious diseases, Infection control, Isolation facilities, Waste management
Introduction
Healthcare facilities represent a particular challenge for infection control, since susceptible patients and healthcare workers (HCWs) may be exposed to patients with unsuspected infectious diseases. During the past decade efforts have been made to develop infection control capacity within healthcare facilities and promote safety for both patients and HCWs. However, healthcare facilities play a critical role for the spread of several emerging or re-emerging highly infectious diseases (HIDs), including viral haemorrhagic fevers, severe acute respiratory syndrome (SARS), influenza A H1N1, and extensively drug-resistant tuberculosis.1, 2, 3, 4, 5, 6, 7 The European Network for Infectious Diseases defines HID as infectious disease easily transmitted from person to person, causing life-threatening disease, presenting a serious hazard in healthcare settings and in the community, and requiring specific control measures.8 Many HIDs spread rapidly within closed settings if they are not suspected promptly or infection control measures are poorly implemented.9
The European Network for Highly Infectious Diseases (EuroNHID) is a European Union-funded project (July 2007 to December 2010), whose aim is to support isolation facilities and provide appropriate infection control advice for isolation centres responsible for managing cases of emerging, re-emerging or deliberately released HID agents. EuroNHID is coordinated by the National Institute for Infectious Diseases ‘Lazzaro Spallanzani’ (Rome, Italy). During 2009 field surveys were conducted in 48 isolation facilities in 16 European countries to assess resources and capabilities for the safe and effective management of HID patients, including infrastructure, human resources, equipment, and infection control procedures. Data are now presented from these surveys on hand hygiene, routine hygiene and disinfection, management of waste, and recommended optimum and minimum requirements for these issues.
Methods
Identification of surveyed isolation facilities
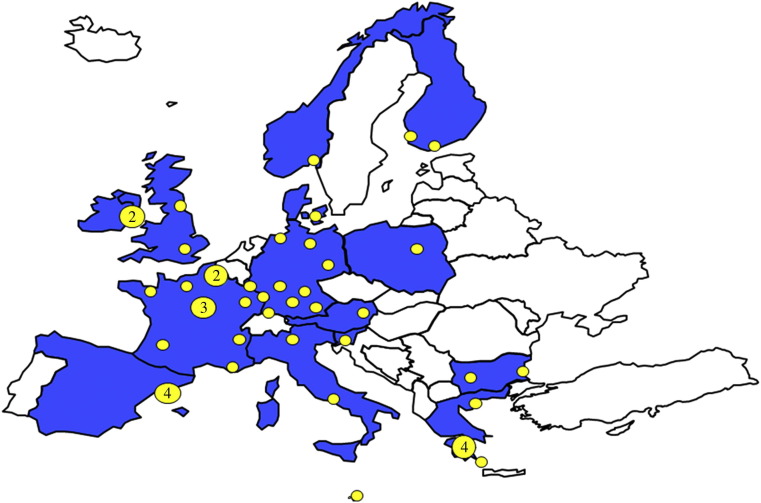
National health authorities in all European countries were contacted and asked to suggest as a project partner a physician with expertise in HID management. By this process 16 countries were included (Austria, Bulgaria, Denmark, Finland, France, Germany, Greece, Italy, Ireland, Luxembourg, Malta, Norway, Poland, Slovenia, Spain (Catalonia region only) and the UK). Most participants were clinicians working in high level isolation units or other isolation facilities designated for referral of patients with HIDs, having backgrounds in infectious diseases, intensive care, infection control, pulmonary medicine, occupational health, or public health. In order to survey only isolation facilities identified by national health authorities for the referral and management of HIDs, we asked partners to provide official documents in which these hospitals are designated as such. This process led to the identification of 48 facilities (Figure 1 ), which represent the centres identified in all participating countries except Spain, where only Catalonian centres were identified.
Figure 1.
Countries participating in the European Network for Highly Infectious Diseases project and location of surveyed isolation facilities. Numbers in the yellow circles indicate the number of isolation facilities in the same location.
Collection of data
Three checklists were developed by the EuroNHID Steering Committee (consisting of the coordination team and partners with more experience in the field of HID management) and approved by all partners on the basis of available evidence, personal experience, preparedness plans and relevant guidelines. These checklists included 44 items and 148 questions. Specific questions about hand hygiene, routine hygiene and disinfection, and waste management were included in the checklist focusing on hospital procedures (checklist 2). The checklists were tested in a pilot survey in five isolation facilities.10 All surveys were conducted during site visits in March–November 2009 by the project coordinator (F.M.F.), except in four facilities from which completed questionnaires were sent by e-mail to the coordination team.
Data interpretation and dissemination
Data were analysed and interpreted by the EuroNHID Steering Committee and returned to the respective isolation facilities for validation. We used evaluation forms developed by the steering committee in consultation with, and approved by, all partners. Groups of related questions were ranked both by strength score and by evaluation score. The strength score indicates the level of importance of the issue and was defined as: A, indispensable; B, very important; C, important; D, advisable. The evaluation score indicates the level of achievement of the observed condition in each facility against the ‘optimal condition’ defined by EuroNHID, and was defined as: A, adequate; B, partially adequate; C, not adequate; NA, not applicable.
Evaluation forms with the results and comments about identified strengths and weaknesses of surveyed facilities were sent to the Ministry of Health of the respective country, and a document summarizing the results in each country was sent to the European Commission.
Development of recommendations
On the basis of the available literature, partners' expert opinion, and data collected during the surveys, EuroNHID developed recommendations for the optimum and minimum requirements on hand hygiene, hygiene and disinfection, and waste management in isolation facilities managing HID patients. These recommendations were discussed with all partners, and a consensus agreement was reached at a meeting in Rome in May 2010.
Results
Hand hygiene
Strength and evaluation scores for hand hygiene are shown in Table I . Both non-hand-operated sinks and alcohol-based distributors were available in 60% of explored facilities. Of the remainder, 36% were partially adequate and 4% not adequate. Procedures for promoting and monitoring hand hygiene were present 59% of facilities, the remaining 41% (20) having either procedures that were not regularly monitored or no such procedures. Materials employed for hand hygiene included liquid soap (in 96%), alcohol-based solution (77%), alcohol-based gel (50%), other alcohol-based product (6%), and soap foam or solid soap (2% each).
Table I.
Implementation of hand hygiene within surveyed isolation facilities
| Issue | Evaluation score | No. of isolation facilities |
|---|---|---|
|
|
47 (98%) |
|
– | |
|
1 (2%) | |
|
|
29 (60%) |
|
17 (36%) | |
|
2 (4%) | |
|
|
28 (59%) |
|
16 (33%) | |
|
4 (8%) |
Routine hygiene and disinfection
Strength and evaluation scores for routine hygiene and disinfection are shown in Table II . Overall, written or established procedures for both routine hygiene and final disinfection of isolation rooms were not available in 17% of isolation facilities, and not available in other areas (e.g. emergency and diagnostic departments) associated with 27% of isolation facilities. For final disinfection, most isolation facilities (90%) used surface cleaning followed by disinfection, and 35% also had formalin fumigation available. Written or established procedures for routine hygiene, final disinfection, or safe discarding of non-disposable objects or equipment (e.g. bronchoscopes or specific personal protective equipment before re-use) were not in place in 27% of surveyed isolation facilities. Among the remaining 73%, 16% had established procedures only for endoscopes. Housekeeping personnel performing routine hygiene and decontamination were trained in the use of personal protective equipment, or housekeeping procedures performed by trained nurses and/or physicians in 48% of facilities.
Table II.
Management of disinfection issues within the surveyed isolation facilities
| Issue | Evaluation score | No. of isolation facilities |
|---|---|---|
|
|
40 (83%) |
|
7 (15%) | |
|
1 (2%) | |
|
|
35 (73%) |
|
4 (8%) | |
|
9 (19%) | |
|
|
35 (73%) |
|
13 (27%) | |
|
||
|
|
23 (48%) |
|
– | |
|
25 (52%) |
Waste management
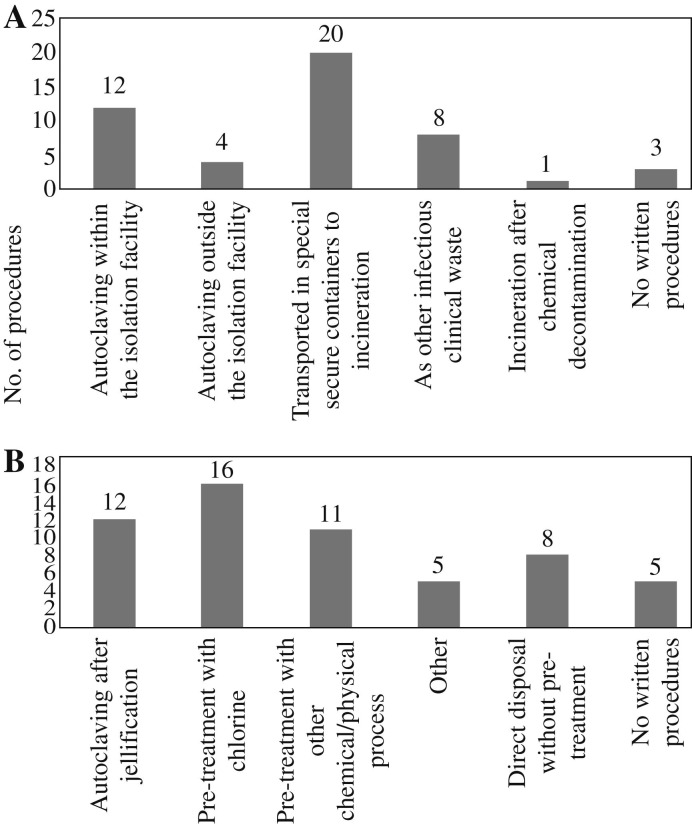
Strength and evaluation score distributions for waste management are presented in Table III . Ninety-four percent of surveyed isolation facilities had established procedures for the management of solid waste, either by autoclaving nearby or by the use of secure transportation in specific containers for incineration at an external facility. Three isolation facilities (6%) had no protocols for solid waste management. As for liquid waste, 33 (69%) of facilities decontaminated it before disposal, 10 (21%) had established procedures but did not decontaminate it before disposal, while 5 (10%) isolation facilities had no procedures for liquid waste at all. Equipment for the safe management of both solid and liquid waste (autoclaves, secure containers, chlorination basins or other collectors) was present in 11 facilities. Twenty-five facilities had equipment only for solid waste management, four had equipment only for liquid waste management, and 8 (17%) had no such equipment. Figure 2 details the available procedures for the disposal of the clinical waste.
Table III.
Management of waste within the surveyed isolation facilities
| Issue | Evaluation score | No. of isolation facilities |
|---|---|---|
|
|
14 (29%) |
|
31 (65%) | |
|
3 (6%) | |
|
|
33 (69%) |
|
10 (21%) | |
|
5 (10%) | |
|
|
11 (23%) |
|
29 (60%) | |
|
8 (17%) |
Figure 2.
Procedures for waste disposal in the surveyed isolation facilities: (A) clinical solid waste; (B) liquid waste. Isolation facilities were able to supply more than one answer.
Recommendations for infection control in isolation facilities
EuroNHID has developed recommendations for optimum and minimum requirements concerning hand hygiene, routine hygiene and disinfection, and waste management in isolation facilities for HIDs, in order to contribute to the standardization of procedures and enhance the preparedness of these facilities in Europe (Box 1, Box 2, Box 3 ).11, 12, 13, 14, 15, 16, 17, 18, 19
Box 1. Recommendations for hand hygiene by the European Network for Highly Infectious Diseases.
Optimum requirements
Within an isolation facility, hand hygiene should be always and strictly practised
-
•
before wearing and after removing gloves.
HCWs should wear (non-sterilized) gloves during:
-
•
patient contact;
-
•
any contact with biological material (e.g. blood, urine);
-
•
procedures that carry a risk for contact with biological material (e.g. during blood sampling);
-
•
contact with contaminated objects or surfaces;
-
•
during cleaning–decontaminating procedures.
Hand hygiene and use of gloves concern all patients in an isolation facility, all HCW categories that may be at risk for contact with a biological material or an infectious agent, and all potentially contaminated objects and surfaces. Hand hygiene is a fundamental part of the appropriate process of PPE removal. HCWs should keep in mind that gloves do not protect from needlesticks or accidents with sharp objects, and that they are not a substitute for hand hygiene.
Regarding the practice for hand hygiene, the following apply.
-
•
When there is visible dirt, hands should be washed thoroughly under running water and soap.
-
•
In all other cases, an antiseptic agent should be preferred.
-
•
The appropriate quantity (3–5 mL, in accordance with the manufacturer's instructions) should be applied and all surfaces (between fingers, wrists, under nails) should be covered for ≥15 s.
-
•
Plain soap should not be used.
-
•
Hands should be dried using disposable paper towels. Air dryers should not be used in isolation facilities.
-
•
If non-hand-operated sinks are not available, disposable paper towels should be used in order to close the drain.
-
•
HCWs' nails should be kept short.
-
•
Artificial nails and jewellery should be avoided.
-
•
Warm water should be avoided for hand washing
Posters using pictures in order to promote appropriate techniques for hand hygiene should be posted near sinks and antiseptic solutions. Written protocols about hand hygiene should be in place. All HCWs who may be in contact with infectious agents or biological material (e.g. HCWs in direct patient care, laboratory personnel, housekeeping personnel, personnel working in the laundry) should be trained in hand hygiene regularly. Monitoring and audit procedures should be also in place. Attention should be paid in order to ensure that temporary and short-term employees (e.g. through time-limited contracts with private laundry and housekeeping companies) have been trained appropriately. Alcohol-based antiseptic distributors should be available at sinks.
Minimum requirements
In order for an isolation facility to manage a patient with HID without compromising safety of HCWs and other patients, written protocols about procedures for hand hygiene should be in place. Posters and pictures indicating the appropriate techniques should be posted above sinks and alcohol-based distributors. One sink (preferably non-hand-operated) should be available per anteroom. Alcohol-based antiseptic distributor should be available by the sink.
Isolation facilities should promote hand hygiene through campaigns and educational events in order to train HCWs and promote hand hygiene. These should be done on a regular, continuous basis (e.g. annual ‘Hand Hygiene Week’), in order that high compliance rates among HCWs are achieved and sustained. Compliance with recommendations for hand hygiene should be regularly monitored and audit should be available.
HCW, healthcare worker; PPE, personal protective equipment; HID, highly infectious disease.
Box 2. Recommendations for routine hygiene and decontamination by the European Network for Highly Infectious Diseases.
Optimum requirements
-
•
The isolation room should be designed in order to facilitate routine hygiene and decontamination procedures.
-
•
The materials used in the construction of the isolation room (walls, furniture) should be easily cleaned and disinfected, resist frequent and intensive disinfection, be non-porous, and repel dust.
-
•
Decontamination should be one of the principal factors for selection of equipment and medical devices in these facilities. Disposable items/devices or items/devices that can be safely decontaminated should be preferred.
-
•
Non-disposable medical equipment (e.g. bronchoscope) should be dedicated to the care of HID patients only and during their entire hospitalization. Where this is not feasible, medical equipment may be used for other patients, only after high-level disinfection using a disinfectant with wide-spectrum antibacterial and antiviral action has been accomplished, and only after the nature of the HID is taken under consideration.
-
•
Spillages of blood and other biological material should be immediately removed and decontaminated.
-
•
All surfaces and equipment within the patient's isolation room should be cleaned and decontaminated twice per day (routine hygiene).
-
•
Final decontamination should be applied after discharge of a patient with HID.
-
•
Final decontamination should cover all objects/devices/equipment/furniture of the isolation room, including filtration system.
-
•
Cleaning and decontamination of horizontal and vertical surfaces and furniture of the isolation room should be accomplished with appropriate hospital detergents.
-
•
Where hypochlorite solution (1000 ppm) is used, the working dilution should be prepared on site at the time of use.
-
•
In order to eliminate the possibility of nosocomial spread of HID agents, dry mopping and household vacuuming should be forbidden within isolation facilities; wet vacuuming should be preferred.
-
•
The disinfection procedure of items/equipment should be performed in the anteroom of the isolation room.
-
•
For decontamination of large and complex equipment, a pre-identified, dedicated area within the isolation facility should be available.
-
•
For mechanical devices that cannot be immersed within a disinfectant, appropriate procedures of disinfection of environmental surfaces should be implemented. In case this is not feasible, it is advisable to use an autoclave.
-
•
Large and complex equipment may require decontamination on site before disassembly, and a fumigation procedure may be applied.
-
•
Fumigation (preferably using 5% H2O2) may be applied following terminal decontamination.
-
•
Integral autoclave facilities or safe access to pre-identified, dedicated autoclave facilities should be in place.
-
•
Housekeeping personnel and other HCWs involved in routine hygiene and final decontamination in isolation facilities should be trained appropriately and on a regular basis, including the use of personal protective equipment. Monitoring and audit in order to investigate compliance of HCWs with written procedures should be conducted periodically.
Minimum requirements
Written protocols about procedures for routine hygiene and final disinfection should be in place, including other areas (e.g. emergency and diagnostic departments). Written protocols about procedures for routine hygiene, final disinfection or safe discarding of non-disposable items/devices/instruments should also be in place. When possible, disposable items/devices/instruments should be used. Housekeeping personnel should be specifically and routinely trained, or alternate procedures (i.e. housekeeping performed by nurses or physicians) should be in place. HCWs should be familiar with procedures about hygiene and decontamination procedures. Periodical monitoring and audit is recommended.
HID, highly infectious disease; HCW, healthcare worker.
Box 3. Recommendations for waste management by the European Network for Highly Infectious Diseases.
Optimum requirements
-
•
Solid waste should be decontaminated by autoclaving before being released from isolation facilities.
-
•
Solid waste volume should be reduced by leaving packaging and other non-clinical material, such as information leaflets, outside of the isolation area.
-
•
With the exception of fluids resulting from hand washing and showers, all other fluids should be decontaminated before being released in the hospital waste water drain.
-
•
Liquid waste may be decontaminated by chlorine and other chemical or physical treatments of proven efficacy.
-
•
Solidification of the liquid waste is an option before being decontaminated as solid waste.
-
•
A policy must be available for special circumstances, such as the emergency repair of drains and pipe work in the facility.
-
•
Appropriate supervision and personal protective equipment must be available for workers performing these tasks.
Minimum requirements
-
•
Solid waste must be securely packed and decontaminated by autoclaving in a nearby autoclave (shared autoclave in the same hospital).
-
•
Solid waste must be securely packed and decontaminated by incineration in the hospital system if this complies with national transportation rules of infectious substances.
-
•
Compacting solid waste is an option before autoclaving or transportation to incinerator but should be carried out with caution.
-
•
Liquid waste resulting from care or body fluids must be decontaminated by chlorine addition in the receptacle before being released into the hospital drain.
Discussion
The hospitalization and management of a patient with HID is a challenge for HCWs and the health system, since it almost always requires the provision of sophisticated healthcare services without compromising the safety of HCWs and other hospitalized patients. To our knowledge, this is the first study detailing the implementation of infection control measures in isolation facilities dedicated to the care for patients with HIDs in Europe. This study included a large number of isolation facilities from several European countries. The checklists for the standardized collection of data are now available to hospital administrators and health authorities for internal or external surveys of other isolation facilities. The checklists may also assist countries to assess their level of preparedness for infectious public health threats, to identify safety issues for patients and HCWs, to prioritize interventions and to monitor their implementation.
Hand hygiene, routine hygiene and disinfection, and waste management are crucial components of infection control, especially for HID. A significant proportion of the surveyed isolation facilities had structural or procedural deficits in their arrangements for hand hygiene. Routine hygiene and disinfection was adequate in the isolation areas of most of the surveyed facilities, but these procedures were often inadequate, particularly as regards final disinfection or safe handling of non-disposable objects, in other areas where the HID patients may stay or pass through, such as diagnostic and emergency departments. Another serious safety issue was identified, in that less than half of the surveyed facilities had established training of housekeeping personnel in procedures for infection control and personal protective equipment. This is vitally important, since the risk for acquisition of a HID is real during housekeeping procedures.20 Housekeeping personnel are often not permanent and their basic training may not include the management of HIDs.
There was considerable variation in the management of waste. In two-thirds of the facilities, procedures for the management of solid waste were in place but the decontamination was performed outside the facility. This is considered a partially adequate solution by EuroNHID, because it implies risks during the management, packaging and transportation of contaminated material. The solution suggested as optimal – decontamination by autoclaving within the facility – was not available in most facilities. The management of liquid waste was generally more satisfactory, although several facilities lacked decontamination procedures for liquid waste, with possible environmental consequences. Some isolation facilities had no established procedures for solid or liquid waste management. Such variations may be attributed partly to different legal requirements for waste management across Europe, partly to differences in experiences with HIDs, and partly to national economic differences.
A limitation of our study is the fact that the assessments were performed mainly before August 2009. Most of the data were therefore collected before the 2009 influenza A/H1N1 pandemic. The experience gained during the pandemic may have caused modifications and improvements of procedures and capacities that are not reflected in our data. Furthermore, the study's focus on isolation facilities facing HIDs limits its applicability to this setting. Facilities facing HIDs may put more emphasis on isolation and infection control issues than ordinary hospitals but the latter may also have to manage HIDs. Data were also collected on the availability of procedures, but no assessment was made of how appropriate these procedures were nor the level of compliance. The recommendations for adequate management also have limitations. Because of the infrequency of suspected and confirmed HIDs, no high-quality studies exist. Consequently, the recommendations are not evidence-based and no ranking of recommendations is possible. The inadequacy of infection control procedures was particularly evident during the 2003 SARS pandemic and the 2009 influenza H1N1 pandemic, when hospitals played a central role in the dissemination of both infections.3, 4, 5, 6 Our recommendations are therefore based on experience reported in the literature or revealed during this study, and on the partners' expert opinion. Consensus recommendations for biocontainment patient care units specifically designed to care for patients with serious communicable diseases have also been issued in the USA.21 Generally, written procedures and protocols facilitate the management of HID cases and ensure safety during medical practice. Such protocols should ideally be syndrome-specific (e.g. viral haemorrhagic fever) or pathogen-specific (e.g. smallpox) and specify the appropriate procedures and techniques in detail.
In conclusion, most aspects of hand hygiene, routine hygiene and disinfection, and waste management were considered at least partially adequate in the majority of European isolation facilities dedicated for the care of patients with HIDs. But considerable variability was observed, with management of waste and training of housekeeping personnel being generally less satisfactory.
Acknowledgements
We would like to acknowledge the healthcare personnel who participated in the survey. We also thank Ramona Iacovino for administrative assistance and additional members of EuroNHID Working Group for data contribution: N. Vetter (Austria), M. Kojouharova (Bulgaria), K. Parmakova (Bulgaria), P. Skinhoej (Denmark), H. Siikamaki (Finland), C. Perronne (France), O. Adrami (Greece), J. Lambert (Republic of Ireland), S. Lanini (Italy), R. Hemmer (Luxembourg), M. Borg (Malta), A.L. Fjellet (Norway), A.B. Brantsæter (Norway), A. Horban (Poland), F. Strle (Slovenia), A. Trilla (Spain).
Conflict of interest
None declared.
Funding sources
This work is supported by the EC grant EuroNHID (2006205).
References
- 1.Vorou R., Pierroutsakos I.N., Maltezou H.C. Crimean Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–600. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- 2.Paweska J.T., Sewlall N.H., Ksiazek T.G. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg Infect Dis. 2009;15:1598–1602. doi: 10.3201/eid1510.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho P.L., Tang X.P., Seto W.H. SARS: hospital infection control and admission strategies. Respirology. 2003; Nov;(8 Suppl.):S41–S45. doi: 10.1046/j.1440-1843.2003.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng V.C., Tai J.W., Wong L.M. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect. 2010;74:271–277. doi: 10.1016/j.jhin.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltezou H.C. Novel (pandemic) influenza A H1N1 in healthcare facilities: implications for prevention and control. Scand J Infect Dis. 2010;42:412–420. doi: 10.3109/00365541003699649. [DOI] [PubMed] [Google Scholar]
- 6.Cehn L.F., Daily N.J., Rao A.K. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients – North Carolina, 2009. J Infect Dis. 2011;203:838–846. doi: 10.1093/infdis/jiq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell M.R., Jarand J., Loveday M. High incidence of hospital admissions with multidrug-resistant and extensively drug-resistant tuberculosis among South African health care workers. Ann Intern Med. 2010;153:516–522. doi: 10.1059/0003-4819-153-8-201010190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouqui P., Puro V., Fusco F.M. Infection control in the management of highly pathogenic infectious diseases: consensus statement from the European Network for Infectious Diseases. Lancet Infect Dis. 2009;9:301–311. doi: 10.1016/S1473-3099(09)70070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannister B., Puro V., Fusco F.M. Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Lancet Infect Dis. 2009;9:45–56. doi: 10.1016/S1473-3099(08)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fusco F.M., Schilling S., Puro V. EuroNHID checklists for the assessment of high-level isolation units and referral centres for highly infectious diseases: results from the pilot phase of a European survey. Clin Microbiol Infect. 2009;15:711–719. doi: 10.1111/j.1469-0691.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- 11.National Health and Medical Research Council . Commonwealth of Australia; Canberra: 2010. Australian Guidelines for the Prevention and Control of Infection in Healthcare. [Google Scholar]
- 12.Centers for Disease Control and Prevention Guidelines for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morb Mortal Wkly Rev. 2002;51:1–45. [Google Scholar]
- 13.World Health Organization . WHO; Geneva: 2009. Guidelines on hand hygiene in health care first global patient safety challenge. Clean care is safer care. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . CDC; Atlanta: 2008. Guideline for disinfection and sterilization in health-care facilities. [Google Scholar]
- 15.Blenkharn J.L. Standards of clinical waste management in UK hospitals. J Hosp Infect. 2006;62:300–303. doi: 10.1016/j.jhin.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Kanemitsu K., Inden K., Kunishima H. Does incineration turn infectious waste aseptic? J Hosp Infect. 2005;60:304–306. doi: 10.1016/j.jhin.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman P.N., Hanley M.J. Assessment of microwave-based clinical waste decontamination unit. J Appl Bacteriol. 1994;77:607–612. doi: 10.1111/j.1365-2672.1994.tb02808.x. [DOI] [PubMed] [Google Scholar]
- 18.Coronel B., Duroselle P., Behr H., Moskovtchenko J.F., Freney J. In situ decontamination of medical wastes using oxidative agents: a 16-month study in a polyvalent intensive care unit. J Hosp Infect. 2002;50:207–212. doi: 10.1053/jhin.2002.1188. [DOI] [PubMed] [Google Scholar]
- 19.Santamaria J., Toranzos G.A. Enteric pathogens and soil: a short review. Int Microbiol. 2003;6:5–9. doi: 10.1007/s10123-003-0096-1. [DOI] [PubMed] [Google Scholar]
- 20.Tarantola A., Abiteboul D., Rachline A. Infection risks following accidental exposure to blood or bloody fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006;34:367–375. doi: 10.1016/j.ajic.2004.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith P.W., Anderson A.O., Christopher G.W. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror. 2006;4:351–365. doi: 10.1089/bsp.2006.4.351. [DOI] [PubMed] [Google Scholar]