Abstract
Initiating the immune response to invading pathogens, the innate immune system is constituted of immune receptors (pattern recognition receptors, PRR) that sense microbe-associated molecular patterns (MAMPs). Detection of pathogens triggers intracellular defense mechanisms, such as the secretion of cytokines or chemokines to alarm neighboring cells and attract or activate immune cells. The innate immune response to viruses is mostly based on PRRs that detect the unusual structure, modification or location of viral nucleic acids. Most of the highly pathogenic and emerging viruses are RNA genome-based viruses, which can give rise to zoonotic and epidemic diseases or cause viral hemorrhagic fever. As viral RNA is located in the same compartment as host RNA, PRRs in the cytosol have to discriminate between viral and endogenous RNA by virtue of their structure or modification. This challenging task is taken on by the homologous cytosolic DExD/H-box family helicases RIG-I and MDA5, which control the innate immune response to most RNA viruses. This review focuses on the molecular basis for RIG-I like receptor (RLR) activation by synthetic and natural ligands and will discuss controversial ligand definitions.
Keywords: 5′triphosphate RNA, Immunorecognition of RNA, RNA virus, RIG-I, MDA5, Lgp2, Intracellular bacteria
Introduction
Receptors of the innate immune system sense foreign molecules and structures such as the highly conserved microbe-associated molecular patterns (MAMPs) like sugars, lipids, proteins, or nucleic acids of bacteria, fungi or viruses (Takeuchi and Akira 2010). Innate immune receptor stimulation by MAMPs triggers intracellular defense mechanisms and the induction of innate immune responses, including secretion of cytokines and chemokines, which lead to alarming of neighboring cells and attracting immune cells. Most of the known highly pathogenic and emerging viruses are RNA genome-based; they give rise to epidemic and zoonotic diseases (Flu, foot-and-mouth disease) or cause viral hemorrhagic fever including yellow fever, dengue, lassa fever and Ebola (Bray 2008). The recognition of foreign pathogenic RNA, resulting in induction of type I interferon (IFN), the most important antiviral cytokine, is therefore highly critical.
Innate immune cells express the endosomal Toll-like receptors (TLR) 7, 8 and 9, which sense GU-rich RNA and CpG-containing DNA. TLR stimulation leads to secretion of type-I IFN, IL-12 and assorted chemokines (Diebold et al., 2003, Heil et al., 2004, Hemmi et al., 2000, Hornung et al., 2005, Hornung et al., 2002, Judge et al., 2005, Krieg et al., 1995), reviewed in (Barchet et al., 2008, Schlee et al., 2007, Schlee et al., 2006). In contrast to TLR7, 8 and 9, TLR3 is expressed in more cell types (e.g. endothelial cells, fibroblasts, astrocytes) (Barchet et al., 2008, Schlee et al., 2007) and was found to detect long double-stranded RNA (Alexopoulou et al. 2001). Unlike TLRs, the RIG-I-like receptors (RLR) RIG-I, MDA5 and Lgp2 are present in the cytosol of all cell types. Similar to TLRs, RIG-I and MDA5 induce type I IFN and chemokines (but no IL12) upon activation by viral but also bacterial RNA. While the endosomal RNA detecting TLRs do contribute to antiviral immunity, RLRs are essential for the immune recognition of and response to most RNA viruses (Fig. 1 ) (Gitlin et al., 2006, Hornung et al., 2006, Kato et al., 2006, Rothenfusser et al., 2005, Venkataraman et al., 2007, Yoneyama et al., 2004). This review summarizes the biological role of and ligand recognition by RLR with special focus on RIG-I, which represents the most broadly studied and understood receptor to date.
Fig. 1.
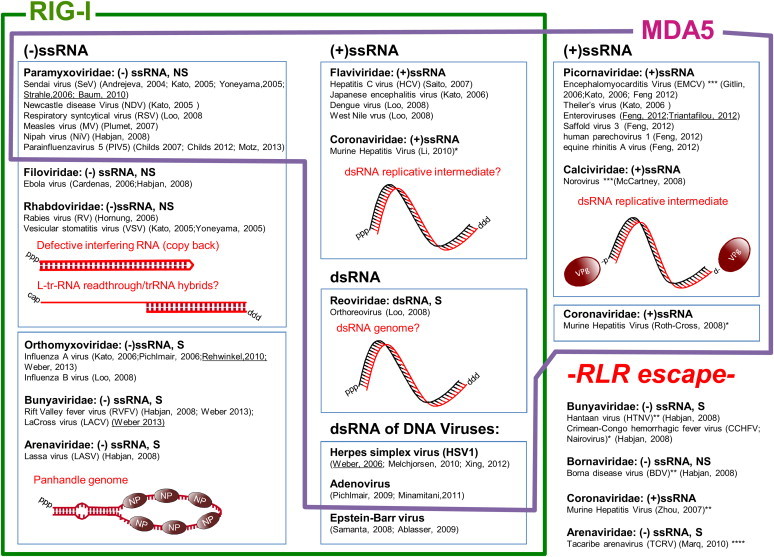
Viruses recognized by RIG-I and MDA5 and evading RLR recognition. S: segmented, NS: non-segmented, ssRNA: single-stranded RNA, dsRNA: double-stranded RNA, (+): positive strand genome, (−): negative strand genome. *Recognition by RIG-I and MDA5 in oligodendrocytes (Li et al. 2010) but no recognition by RIG-I or MDA5 in BM-DC or fibroblasts (Zhou and Perlman 2007), recognition by MDA5, not RIG-I in macrophages and microglia (Roth-Cross et al. 2008). **Evasion of RIG-I recognition by nuclease 5′ end cleavage leaving monophosphate at the 5′end of the viral genome (Garcin et al., 1995, Habjan et al., 2008). ***Evasion of RIG-I recognition via substitution of 5′triphosphate by Vpg protein at the 5′end of the viral genome (Hruby and Roberts, 1978, Lee et al., 1977, Rohayem et al., 2006). ****Evasion of RIG-I recognition by overhang at the 5′end of the viral genome (Marq et al. 2010b). Weber, 2013: papers contributing to the characterization of the real ligand structure in vivo are underlined.
RIG-I like receptors (RLRs)
RIG-I (retinoic acid-inducible gene I) and MDA5 (melanoma differentiation-associated gene-5) are closely related DExD/H-box helicase family proteins. They consist of an N-terminal tandem caspase activation and recruitment domain (CARD) fused to a DExD/H-box helicase domain (composed of Hel1, Hel2 and Hel2i) and the C-terminal domain (CTD; previously called RD = repressor domain) (Luo et al., 2013, Saito et al., 2007, Yoneyama et al., 2004) (Fig. 2 ). Stimulation of RIG-I or MDA5 by viral RNA release the associated CARDs, which aggregate with K63 polyubiquitin chains to CARD tetramers and then bind and activate the adaptor molecule MAVS (Jiang et al., 2012, Zeng et al., 2010, Zeng et al., 2009). MAVS (also known as IPS-1, Cardif or VISA (Kawai et al., 2005, Meylan et al., 2005, Seth et al., 2005, Xu et al., 2005)) recruits TBK-1, which phosphorylates IRF3 to induce transcription of type-I IFN genes (Doyle et al., 2002, Fitzgerald et al., 2003, Sharma et al., 2003). At present, the interaction of RIG-I with its corresponding ligand RNA is far better understood and analyzed than ligand–receptor interactions of MDA5 or Lgp2. Both the CTD and helicase domain (which is no active RNA helicase) possess RNA binding sites, whereas solely the CTD harbors the critical binding pocket for the RNA ligand, the features of which will be discussed below. As of now, high-resolution structure studies have been performed of the CTD alone (Cui et al., 2008, Takahasi et al., 2008), the CTD with ligand (Lu et al., 2010b, Wang et al., 2010), mouse RIG-I SF2 domain + non-hydrolysable ATP (Civril et al. 2011), human RIG-I(ΔCARDs) + ligand (Jiang et al., 2011, Luo et al., 2011), whole duck RIG-I(ligand free) and helicase + ligand (Kowalinski et al. 2011). The current model of RIG-I ligand interaction resulting from the above-mentioned studies was extensively discussed (Kolakofsky et al. 2012). Briefly, the CARD of non-stimulated RIG-I binds to the so-called Hel-2i domain within the helicase domain, mediating an auto-inhibited state. Upon stimulation the CTD-bound RNA interacts with Hel-2i, leading to dislocation of the CARDs, which now become accessible for downstream interactions (CARD multimerization, MAVS interaction, type I IFN induction) as described above. A similar activation mechanism for MDA5 is thinkable. But as long as a MDA5 recognition motif has not been clearly defined (discussed below) it remains unclear if the MDA5 CTD mediates ligand specificity.
Fig. 2.
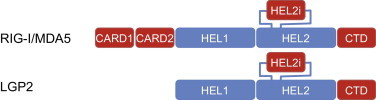
Domain structure of RIG-I, MDA5 and Lgp2.
A recently described crystal structure of MDA5(ΔCARDs) with a synthetic 11mer dsRNA (which is not a MDA5 activating ligand) revealed that the MDA5 CTD does not cap the terminus of the blunt dsRNA but rather binds the internal RNA duplex structure (Wu et al. 2013). This would provide a prerequisite for a putative MDA5 head-to-tail arrangement in a filament structure with exposed CARDs, which was suggested to be the MAVS activating structure (Wu et al. 2013).
The third RLR family member Lgp2 lacks CARDs and does not induce type I IFN. Its putative function will be discussed in the next section.
Lgp2–biological role
The role of Lgp2 in the immune response against viruses is not entirely understood. The Lgp2 CTD resembles the RIG-I CTD, albeit with different requirements for ligand binding, which will be discussed below (Li et al., 2009b, Murali et al., 2008, Pippig et al., 2009). While Lgp2 structurally shares a helicase and CTD, it lacks CARDs, suggesting a putative ligand sequestering role. Indeed, initial reports suggested a pure immune suppressive function for Lgp2 (Komuro and Horvath, 2006, Rothenfusser et al., 2005, Saito et al., 2007, Yoneyama et al., 2004). Confusingly, a RIG-I-suppressing activity was found to be independent of dsRNA binding (Li et al. 2009b).
The parainfluenzavirus type 5 V protein was reported to interact with Lgp2, to stabilize a Lgp2/RIG-I complex and in this way to cooperatively inhibit induction by RIG-I ligands (Childs et al. 2012).
Further studies on Lgp2-deficient mice revealed that Lgp2 absence impairs the immune response to viruses that are mainly detected by MDA5, and can both impair or enhance RIG-I mediated antiviral responses (Pippig et al., 2009, Satoh et al., 2010, Venkataraman et al., 2007). Suthar et al. (2012) confirmed that Lgp2 contributed to sustained RLR signaling of IFN-β expression in myeloid cells during West Nile virus (WNV) or dengue virus infection. Additionally, they discovered a role for Lgp2 in CD8(+) T cell survival: Lgp2 modulated the sensitivity of CD8(+) T cells to CD95 ligand-mediated cell death through the control of CD95 expression during WNV or lymphocytic choriomeningitis virus infection. Although the authors excluded a MDA5/Lgp2 interaction to be responsible for the observed CD95 modulation, it remained unclear if the effect in CD8(+) T cells occurs independently of RIG-I. In conclusion, Lgp2 appears to have a modulatory role in fine-tuning the innate immune response to viruses.
MDA5 – biological role, target pathogens and ligand structure
MDA5 recognizes long double stranded RNA and contributes to or even dominates the immune response to double strand (dsRNA) and positive strand RNA [(+)ssRNA] viruses (Fig. 1) (Fredericksen et al., 2008, Gitlin et al., 2006, Kato et al., 2006, Loo et al., 2008, McCartney et al., 2008, Melchjorsen et al., 2010, Roth-Cross et al., 2008, Saito et al., 2008). It is crucial for raising innate immune responses against picornaviruses, like Theiler's virus or encephalo-myocarditis virus (EMCV), enteroviruses, Saffold virus 3, human parechovirus 1, equine rhinitis A virus or the Caliciviridae family member Norovirus, which escape RIG-I recognition (Feng et al., 2012, Gitlin et al., 2006, Kato et al., 2006, McCartney et al., 2008, Triantafilou et al., 2012) (Fig. 1). At first view, MDA5 appears to target virus types which are known to produce considerable amounts of dsRNA during their replication cycle, including (+)ssRNA, dsRNA or DNA viruses (McCartney et al., 2008, Melchjorsen et al., 2010, Pichlmair et al., 2009, Roth-Cross et al., 2008, Targett-Adams et al., 2008, Weber et al., 2006). Correspondingly, two independent groups identified the double stranded replicative intermediates of (+)ssRNA enteroviruses as MDA5-stimulating RNA species (Feng et al., 2012, Triantafilou et al., 2012). A crystal with MDA5(ΔCARD) binding to dsRNA could be obtained (Wu et al. 2013).
However, the concept of dsRNA recognition by MDA5 seems incomplete. (−)ssRNA paramyxoviruses express the immune suppressive V protein which binds to and inhibits MDA5 directly, suggesting that also (−)ssRNA viruses (which were shown not to generate long double stranded RNA (Weber et al. 2006)) produce MDA5 ligands (Andrejeva et al., 2004, Childs et al., 2007, Luthra et al., 2011, Motz et al., 2013). In light of the above-mentioned studies, it appears unexpected that many double stranded RNA species do not activate MDA5. Thus far only one artificial, albeit enzymatically generated, MDA5-stimulating ligand (polyinosine-polycytidylic acid = poly I:C) has been described. It is composed of annealed strands of long (>7000 nt) RNA polymers of inosins (polyI) and cytidines (polyC) (Gitlin et al., 2006, Kato et al., 2008, Kato et al., 2006). Most studies investigating the recognition of “long double stranded RNA” (dsRNA) in fact have used poly I:C. Of note, poly I:C is a very particular “dsRNA“, as it was reported as the only co-polymer among many other artificial dsRNAs which was capable of inducing high amounts of type I IFN in mammalian cells (Field et al. 1967). The absence of well-defined MDA5 ligands impairs systematic investigations of the MDA5–ligand interaction. Although the CTD of MDA5 binds blunt-ended dsRNA (Li et al., 2009a, Wu et al., 2013), MDA5 is not activated by short dsRNA and no contribution of the CTD in discriminating MDA5 stimulating RNA has been demonstrated (Saito et al. 2007).
Even though poly I:C also binds and can stimulate RIG-I in certain cell lines and experimental settings in vitro (Kato et al., 2008, Yoneyama et al., 2004), it is important to note that poly I:C indeed fails to induce IFN-alpha when injected intravenously into MDA5-deficient mice or transfected in vitro into MDA5-deficient peritoneal macrophages, dendritic cells or MEFs (Gitlin et al., 2006, Kato et al., 2006). By using poly I:C fragments of different sizes from RNase-III digestion, Kato and colleagues observed that MDA5 was only stimulated by long poly I:C fragments. An alternative interpretation of these results would be that RNase-III destroys certain secondary structures, which are required for recognition by MDA5. Pichlmair and colleagues concluded from testing of gel-fractionated RNAs of vaccina virus-infected cells that it was not the double-strandedness of RNA that accounted for MDA5 stimulating activity, but rather other higher order RNA structures in large RNA containing complexes (Pichlmair et al. 2009). Luthra et al. discovered a mRNA fragment from the ss(−)RNA parainfluenza virus 5 (PIV5) that activated type-I IFN expression in a MDA5-dependent manner (Luthra et al. 2011). Since type I IFN induction by this RNA required RNase L, the authors concluded that RNase L recognizes and processes viral mRNA into a MDA5 activating structure. Although a 432-nt-long region critical for MDA5 stimulation was identified, no specific features of a minimal recognition motif were found. The observation by Züst and colleagues that deficiency of the viral cap N1-2′O-methyltransferase in a type of (+)ssRNA corona virus (murine hepatitis virus; MHV) provoked recognition of this MHV by MDA5 and TLR7 (Zust et al. 2011) suggested a 5′end-dependent RNA recognition by MDA5. This finding would contrast the findings by Luthra et al., who expressed the MDA5 stimulatory mRNA from a promoter, which supports normal capping (including N1-2′O-methylation). However, binding assays documenting the interaction/non-interaction of the 5′end of viral transcripts with MDA5 were not performed. Indirect effects were therefore not excluded. Later studies on a N1-2′O-methyltransferase-lacking West Nile virus did not reveal a role of MDA5 in enhanced immune recognition of non-methylated cap structures (Szretter et al. 2012), suggesting that N1-2′O-methylation does not generally impair MDA5 engagement.
RIG-I, ligand definition
Despite increasing amounts of high resolution crystal data on the RIG-I/ligand interaction, the ligand requirements for RIG-I stimulation are still controversial in the literature. By giving an overview of the history of the RIG-I ligand definition, the following section aims to help the reader to understand how different read-out systems and ligand preparation methods could lead to conflicting interpretation of data.
RNA modification
When RIG-I was discovered as antiviral sensor by the Fujita group (Yoneyama et al. 2004), the requirements of its RNA ligand were not explored. RIG-I was shown to bind to and to be stimulated by poly I:C when overexpressed in cell lines. At the same time, the group around John Rossi, while developing siRNAs against HIV, observed that all RNAs generated by phage-polymerase in vitro transcription (Kim et al. 2004) strongly induced type-I IFN in several human cell lines (HeLa, K562, HEK293, Jurkat, CEM). By contrast, synthetic siRNAs did not show any immune stimulatory effect in the same cell lines. DNA template-dependent RNA transcription occurs primer-independently from the 5′- to the 3′-end of RNA. For this reason, RNA transcripts of all known RNA polymerases, including phage polymerase, possess a triphosphate at the 5′end (Banerjee 1980). By using phosphatase or RNase T1 (removes the 5′end pppG), Kim et al. could show that the 5′triphosphate was the crucial type-I IFN-inducing structural element of in vitro transcribed RNAs which was absent in synthetic siRNAs (Kim et al. 2004). This finding prompted us to analyze the IFN-alpha inducing capacity of in vitro transcribed 5′triphosphorylated RNA (pppRNA) in human blood cells (Hornung et al. 2006). At this time, plasmacytoid dendritic cells (PDC) were presumed to be the principal type I IFN producing cells (Cella et al., 1999, Siegal et al., 1999). They express TLR7 and 9, and secrete large amounts of IFN-alpha upon TLR7 stimulation with single or double stranded RNA (Hornung et al. 2005). Human monocytes express the RNA-sensing endosomal TLR8. However, TLR8 stimulation does not induce IFN-alpha secretion (Barchet et al. 2008). Unexpectedly, pppRNA induced high levels of IFN-alpha not only in PDC but also in human monocytes. Therefore, pppRNA represented the first agent that induced IFN-alpha in human monocytes at comparable quantities to human PDC (Hornung et al. 2006). Removal of the 5′triphosphate abrogated IFN-alpha induction by in vitro transcribed RNA in monocytes but not in PDC (Hornung et al. 2006). Integration of nucleotides with modified bases (pseudouridine, 2-thio-uridine) or backbone modifications (2′O-methyl at uridines) abolished IFN-alpha induction by pppRNA both in PDC and monocytes. Using murine RIG-I/TLR7 deficient primary cells, RIG-I was identified to be crucial for pppRNA mediated IFN-alpha induction in myeloid immune cells, while TLR7 was essential for IFN-alpha induction in PDC (Hornung et al. 2006). At the same time, Pichlmair and colleagues reported 5′phosphate-dependent type I IFN induction by Influenza virus vRNA, which contained no dsRNA detectable by a dsRNA specific antibody (Pichlmair et al. 2006).
Sequence dependence of RIG-I recognition?
Saito and colleagues suggested that RIG-I detects (+)RNA viruses (HCV) in a sequence-dependent manner (Saito et al. 2008). They screened the HCV genome for RIG-I activating motifs by in vitro transcription of small domains of the HCV genome and analyzed the results for RIG-I binding and -activation. A transcript from an 100 nt U- or A-rich region 8000 nt downstream of the 5′end showed an exceptional RIG-I inducing activity. The presence of triphosphate at the 5′end was essential for RIG-I stimulation. Of note, a polyU sequence elicited a similar IFN response as the polyA sequence, which could be explained by a phenomenon to be discussed below. While developing phage-polymerase transcription-generated shRNA without RIG-I stimulating activity, Gondai and colleagues found that 5′end extension by more than one G abolished type I IFN induction (Gondai et al. 2008). The results by Saito and Gondai suggested a sequence-dependent RNA recognition by RIG-I. However, later experiments with defined synthetic RIG-I ligands indicated that the work of both groups needs to be re-interpreted (Schlee and Hartmann, 2010, Schlee et al., 2009, Schmidt et al., 2009).
Synthetic dsRNA RIG-I ligands
Before 5′triphosphate was identified as the crucial RNA modification to induce RIG-I activation, Marques and colleagues observed that synthetic blunt ended dsRNA oligonucleotides can stimulate RIG-I (Fig. 3 ) (Marques et al. 2006). The read-out system used consisted of the glioblastoma cell line T98G, which was transfected with blunt ended or 3′overhang-possessing siRNAs. Type I IFN activity in these cells was monitored 48 or 72 h after transfection by western blot analysis of the type I IFN induced protein IFIT1 (p56), a very sensitive assay. In contrast to 3′overhangs possessing siRNA, blunt-ended siRNA induced substantial IFIT1 upregulation. Similar results were obtained with MRC-5 cells. SiRNA-mediated knockdown of RIG-I in T98G indicated involvement of RIG-I in the blunt-ended siRNA-induced type I IFN response. By contrast, HT1080 cells and HeLa cells did not respond to blunt-ended dsRNA, but exhibited IFIT1 induction after transfection of in vitro transcribed RNA. The response to blunt dsRNA could be restored in HT1080 cells by priming with type I IFN. According to the described results, the RIG-I stimulation motif was defined as double blunt-ended dsRNA longer than 23 bp. Single blunt-ended siRNAs were less active. 5′overhangs were reported to permit detectable activity after 72 h of stimulation while 3′overhangs abolished activity (Fig. 3) (Marques et al. 2006).
Fig. 3.
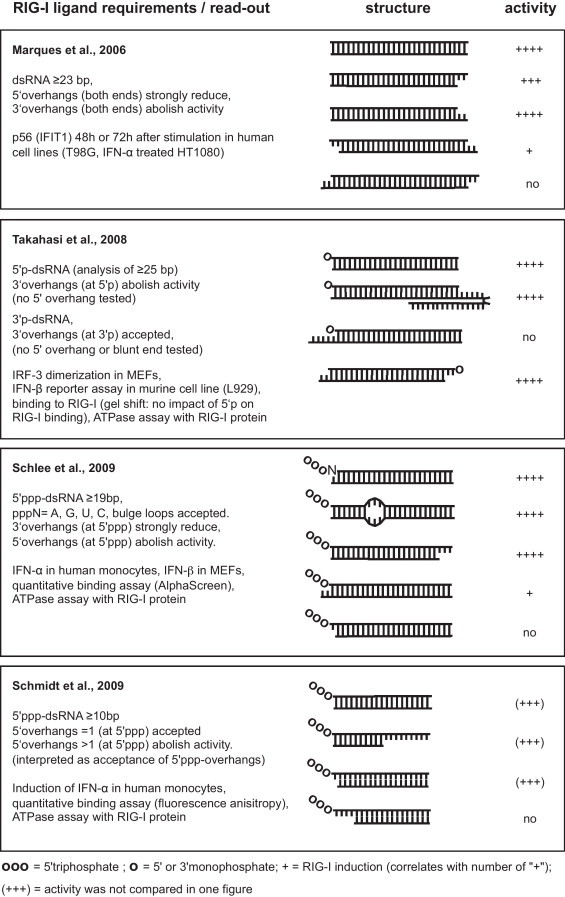
Synthetic RNA ligands tested for RIG-I activation. Upper strands are in 5′–3′ direction, lower strands in 3′–5′ direction.
Further studies analyzed the physical interaction of recombinant full-length RIG-I or CARD- or CTD deficient mutants with synthetic blunt-ended dsRNA in comparison to in vitro transcribed single stranded pppRNA (ivtppp-ssRNA) (Cui et al. 2008). While full length RIG-I was highly activated by ivtppp-ssRNA, synthetic non-phosphorylated dsRNA induced RIG-I to a much weaker degree. Unexpectedly, for RIG-I lacking the CARD domain, dsRNA and ivtpppRNA showed comparable ATPase activity. By contrast, interaction studies using fluorescence anisotropy with recombinant RIG-I protein or the recombinant RIG-I CTD domain confirmed the requirement of the 5′triphosphate for substantial interaction with full-length RIG-I (Cui et al. 2008). Takahasi and colleagues reported a RIG-I-dependent type I IFN response to synthetic 5′-monophosphorylated and 3′-monophosphorylated dsRNA oligonucleotides in an IFN-beta-primed murine cell line and in IFN-beta-treated mouse embryonic fibroblasts (MEF). The type I IFN response was monitored by IFNbeta promoter reporter assays and IRF-3 dimerization (Fig. 3) (Takahasi et al. 2008). In this setting non-modified synthetic dsRNA did not induce type I IFN (Takahasi et al. 2008). In accordance with earlier studies (Marques et al. 2006), 3′-overhangs at the 5′-monophosphorylated end abrogated the type I IFN response, while 5′overhangs were not tested (Fig. 3) (Takahasi et al. 2008). By contrast, 2 nt 3′overhangs in 3′monophosphorylated dsRNA induced a type-I IFN response (no other end structures, e.g. blunt, 5′overhang, were analyzed). Unexpectedly, the authors found that monophosphorylation did not enforce RIG-I binding of dsRNA but increased RNA stability in the cells, suggesting that increased RNA stability is responsible for the particular RIG-I stimulating activity (Takahasi et al. 2008).
Synthetic triphosphorylated dsRNA ligands
Since the 5′triphosphorylated end sequence of RNAs generated by phage polymerase is restricted to a conserved consensus starting nucleotide G (or A followed by G), in vitro transcription is not applicable for screening of sequence variations at the 5′end of triphosphorylated oligonucleotides. Therefore, our group established a method to generate synthetic triphosphorylated RNAs (Schlee et al. 2009) which is based on the standard cyclotriphosphate protocol of triphosphate synthesis (Ludwig and Eckstein 1989). Unexpectedly, synthetic single stranded triphosphorylated RNA (ppp-ssRNA) did not induce type-I IFN in human monocytes, while the “same” RNA sequence generated by in vitro transcription (ivtppp-ssRNA) was a strong type-I IFN inducer. Sequencing of products from the ivtppp-ssRNA transcription mix revealed the presence of complementary sequences and double stranded hairpin species, which were obviously generated by template-dependent RNA transcription, a side activity of phage polymerase that had been reported earlier (Cazenave and Uhlenbeck, 1994, Triana-Alonso et al., 1995). Transcription reaction conditions that did not allow synthesis of complementary RNA abrogated RIG-I activation by ivtppp-ssRNA completely, suggesting that RIG-I was not stimulated by the intended ssRNA transcript but rather by side products (Schlee et al. 2009). Hence, hybridization of a complementary ssRNA strand reconstituted RIG-I stimulation by synthetic ppp-ssRNA. Optimal RIG-I agonists appeared to be blunt ended, while 2 nt 3′overhangs at the 5′triphosphate end impaired RIG-I activation by more than 70% (Fig. 3). 5′overhangs of the triphosphorylated end were not tolerated, thus demonstrating that base pairing of the nucleotide carrying the 5′triphosphate is essential for RIG-I activation. The non-phosphorylated end structure had no substantial impact on RIG-I stimulation, as long as the dsRNA encompassed at least 19 base pairs. Small (3 nt) bulge loops in the center of the sequence were tolerated. All four nucleotides constituted active triphosphorylated 5′ends of the RIG-I ligand. Activity of pppA, pppG and pppU differed only slightly (A = G > U), whereas pppC induced around 50% less type I IFN. Of note, this sequence-dependency that was observed is based on only one dsRNA sequence (NACACACACACACACACACACUUU), and remains to be verified in another sequence context. According to the public databases, no genomic viral RNAs (vRNA) start with pppC but most start vRNA with pppA.
At the same time, by testing synthetic ppp-ssRNA, Schmidt and colleagues (Fig. 3) confirmed the importance of dsRNA (Schmidt et al. 2009). In disparity with our results, they observed that RIG-I tolerates a 1 nt 5′overhang at the ppp bearing end for one tested sequences. By using phage polymerase-generated hairpin pppRNA structures with intended 5′ppp overhangs, they concluded that longer (>1 nt) 5′overhangs in hairpin pppRNAs are tolerated. However, this interpretation may be misleading since in vitro transcribed pppRNA hairpins with overhangs (accurate transcription/identity was not analyzed by mass spectrometry) are likely to be contaminated with completely double stranded material (Cazenave and Uhlenbeck, 1994, Triana-Alonso et al., 1995). In our experience, one-time size fractionation of hairpin RNA is not sufficient to exclude contamination of transcripts with small size differences completely. On the other hand, it has to be considered that hairpins are in equilibrium with their self-complementary duplex (Nakano et al. 2007), which is supposed to be a more active ligand than the monomeric hairpin (Binder et al. 2011). Thus, small contaminations can cause substantial effects. Schmidt et al. reported that a double stranded region of minimum 10 bp length is sufficient for RIG-I activation. In their test they included three different sizes of ppp-dsRNA (15, 10 and 5 bp, blunt at the ppp-end). Curiously, a 10mer ppp-dsRNA induced a stronger type-I IFN response than a 15mer. As a direct comparison of the full 19mer duplex pppRNA to the 15mer and 10mer is missing, the interpretation of the result remains difficult (Schmidt et al. 2009). Altogether, it remains unclear whether just any 10mer duplex ppp-dsRNA sequence can activate RIG-I. It has to be considered that the possibility of non-canonical base pairings of RNA (e.g. G-U wobbles) provides manifold alternatives to form double stranded structures, all of which have to be kept in mind when claiming that RNA structures are single stranded.
To date only a small number of synthetic ppp-dsRNA sequences have been analyzed (seven in our work (Schlee et al. 2009), one in the work of Schmidt et al. (2009)). It is possible that a stabilizing nucleotide sequence next to the 5′ppp end enables a tolerance of 1 nt 5′-ppp-overhangs. Using highly purified in vitro transcribed pppRNA from arenavirus sequences Marq et al. (2010b) confirmed the requirement of a base paired 5′-ppp end of dsRNA for RIG-I activation and suggested that some arenaviruses and bunyaviruses use a prime and realign mechanism for genome synthesis, leading to 5′overhangs in order to evade RIG-I recognition (Marq et al. 2010b).
The need of a base-paired 5′-ppp end of dsRNA was also validated by the assembly of the RIG-I ligand within the RIG-I CTD ppp-dsRNA binding cleft (Lu et al., 2010b, Wang et al., 2010). 5′ppp-terminal base pairing supports an essential stacking interaction with a conserved phenylalanine residue in the RNA binding cleft of the CTD. In addition, in contrast to a single base pair, which allows free rotation of the following sequence, the double strand assembly stabilizes the helix in a fixed optimum position for interaction of the adjoining phosphodiester backbone with the CTD and the helicase domain (Fig. 4 ) (Kolakofsky et al. 2012). In disparity to previous studies using type-I IFN-primed murine cells as a read-out (Takahasi et al. 2008), no considerable type I IFN induction was observed after 24 h when human monocytes were transfected with 5′monophosphorylated dsRNAs (Schlee et al., 2009, Schmidt et al., 2009). Of note, Schmidt and colleagues tested the same sequences, which were previously reported to induce type I IFN in type I IFN-primed MEFs (Schmidt et al., 2009, Takahasi et al., 2008). By contrast, in both studies monophosphorylated and non-phosphorylated dsRNA induced a substantial ATPase activity of RIG-I protein at higher RNA doses (Schlee et al., 2009, Schmidt et al., 2009). This indicates that RIG-I activation observed by Marques et al. (2006) and Takahasi et al. (2008) could occur because of relatively high local RNA concentrations in the cytosol. Further factors, sensitizing the readout and leading to contradictory results are most likely due to the use of highly RIG-I responsive cell lines (T98G) or murine cells combined with long incubation times (48–72 h (Marques et al. 2006)), pre-activation by incubation with type I IFN (Marques et al., 2006, Takahasi et al., 2008) and sensitive detection methods (IFIT1 western blot, IFN-beta reporter assay, IRF-3 dimerization (Marques et al., 2006, Takahasi et al., 2008)). Using gel shift experiments with radioactive labeled ligand, Vela et al. could calculate that the triphosphate moiety of dsRNA enhanced binding to the CTD 127 fold (Vela et al. 2012). The crystallization of RIG-I CTD with 5′OH-dsRNA by Lu and colleagues revealed that binding of 5′OH-dsRNA in the CTD RNA binding cleft is possible (Lu et al. 2010a). Although the assembly for 5′OH-dsRNA resembles that of 5′ppp-dsRNA, the crystal data reveal that 5′OH-dsRNA binds in a different angle, and with other amino acid positions. Habjan and colleagues observed that Crimean-Congo hemorrhagic fever virus (CCHTV), Hantaan virus (HTNV), and Borna disease virus (BDV) can prevent RIG-I mediated detection of their genomes by a prime and realign mechanism and cleavage of the 5′ terminal base of their genomic RNA leaving monophosphorylated 5′ends (Habjan et al. 2008) (Fig. 1). In contrast to 5′ppp ended genomic RNA, the genomic RNAs of CCHTV, HTNV or BDV with 5′monophosphorylated ends (pRNA) failed to bind or to activate RIG-I when transfected into HEK293 cells. This laborious procedure of (−)ssRNA viruses to generate 5′monophosphorylated genomes to prevent RIG-I recognition does not support the concept that pRNA is a preferred target structure for RIG-I during viral infection.
Fig. 4.
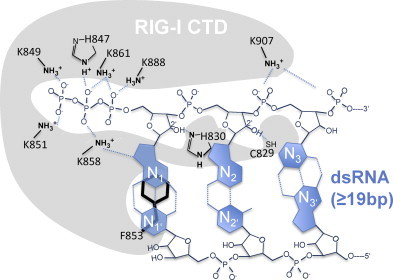
RIG-I CTD interaction with blunt ppp-dsRNA. K858, K851, K849, H847, K861, K888 constitute a basic binding cleft which binds 5′triphosphate. K907 interacts with backbone phosphates. H830 and C829 bind to the 2′OH groups of the first two nucleotides of the ppp-dsRNA.
The occurrence of aberrant dsRNA during phage polymerase in vitro transcription questions the data interpretation from earlier studies, which intended to identify RIG-I recognition sequences based on experiments with in vitro transcribed RNA. The observations of Gondai and colleagues can easily be explained by the non-acceptance of 5′ppp-overhang structures (Gondai et al. 2008): shRNAs consisting of a RNA hairpin with base paired 5′ppp-ends and a UU 3′-overhang induced RIG-I. Extra Gs at the 5′-ppp end generated single stranded or mismatched 5′ppp ends (5′ppp-overhang), which fail to stimulate RIG-I.
The finding that pppRNA composed of polyA or poly U stretches are equally potent RIG-I inducers can be explained by the possibility that both complementary RNA species are generated in the phage-polymerase transcription reaction that was intended to produce only one ssRNA species and form a duplex (Saito et al. 2008). By using the same template as used by Saito and colleagues for phage polymerase-mediated generation of the RIG-I stimulating “poly A” rich sequence (which in fact is composed of starting Gs and A), Schmidt and colleagues did not receive any RIG-I stimulating activity when UTP or CTP were omitted in the transcription mix (Saito et al., 2008, Schmidt et al., 2009). By contrast, addition of UTP and CTP yielded RIG-I-activating RNA as reported (Saito et al., 2008, Schmidt et al., 2009), suggesting that a double stranded polyA/polyU rich sequence constitutes the RIG-I activating agent. Potent RIG-I stimulation by this structure can be explained by the fact that poly A and poly U represent sequences that are not able to form stable secondary structures. Absence of secondary structures facilitates the hybridization of complementary RNAs at low temperature to uniform dsRNA structures in comparison to mixed high melting G/C containing sequences. It is important to note that subgenomic (single stranded) RNAs of HCV were reported to have 5′monophosphorylated ends (Takahashi et al. 2005). Since RIG-I activation by polyU strictly depended on the presence of 5′triphosphate (Saito et al. 2008), triphosphate-dependent RIG-I stimulation in vivo can happen by recognition of replicative pppRNA intermediates, which are generated during replication and are, in fact, double stranded (Targett-Adams et al. 2008). In summary, for RIG-I recognition structural features appear to be more important than the sequences of candidate ppp-dsRNA.
Mechanisms of RNA recognition by RIG-I like receptors – insights from structural data
RIG-I possesses two RNA binding domains (DECH domain and CTD). Pioneering studies involving crystal structure analysis or NMR from the Hopfner and the Fuijita lab identified a basic binding cleft within the CTD of RIG-I (amino acids 802–925) as the crucial pppRNA binding structure that determines ligand specificity (Cui et al., 2008, Kolakofsky et al., 2012, Takahasi et al., 2008). As described above, ppp-dsRNA bound to the CTD displaces autoinactivated CARDs from binding to the helicase domain, leading to liberation and activation of CARDs and downstream events that culminate in the induction of type I IFN [reviewed in Kolakofsky et al. (2012)]. Using synthetic or highly purified in vitro transcribed triphosphorylated RNA led to the resolution of the crystal structure of the RIG-I CTD bound to 12mer ppp-dsRNA palindromic sequences (Lu et al., 2010b, Wang et al., 2010). The crystal structures revealed an RNA binding basic binding cleft with highly conserved amino acids involved in binding of the 5′terminal base pair, the triphosphate structure itself, and backbone phosphate (Fig. 4). K849 and K851 (amino acid numbers in the text always refer to human RIG-I sequence) are in proximity to the gamma phosphate of the triphosphate. However, conservative K849 and K851 mutations to alanine (A) impaired RIG-I activation only at very low ligand concentrations (Wang et al. 2010), suggesting a minor role of this interaction in RIG-I mediated recognition of viral RNA. K858, H847 and K861 were identified to interact with the beta phosphate of the triphosphate. Substitution of K861 to A, which is also in contact with alpha phosphate, or double substitution of H847 and K858 to A, abrogated RIG-I activation by ppp-dsRNA. Likewise substitution of K888, being in contact with the alpha phosphate group, inhibited recognition of ppp-dsRNA by RIG-I. The side chain of K907 is in contact with either the backbone phosphate between N2 and N3 (Wang et al. 2010) or N3 and N4 (Lu et al. 2010b). Since substitution of K907 to A abolishes RIG-I activation completely (Wang et al. 2010) this backbone phosphate interaction appears to be crucial for the detection of the ribose backbone of a dsRNA structure. The contact of K907 to the phosphate between N2 and N3 (Wang et al. 2010) or N3 and N4, as reported in another study (Lu et al. 2010b), could either depend on the incorporated oligonucleotide sequence (pppGACGCUAGCGUC (Wang et al. 2010) or pppGGCGCGCGCGCGCC (Lu et al. 2010b)) or the crystal packaging. In summary, both studies exhibited very similar pppRNA/CTD structures.
Of note, the amino acids mediating RNA ligand binding (H847, K858, K861, K888 and K907) are 100% conserved in the RIG-I sequences of vertebrates, highlighting the importance of these positions for RIG-I-mediated RNA-virus recognition. F853 is also essential for RIG-I activation (Wang et al. 2010). It conducts the crucial stacking interaction with the 5′terminal base pair (Fig. 4), mediating the RIG-I selectivity for base paired triphosphorylated RNA. The stacking interaction is stabilized by base pairing of N1. In addition, base pairing of the interacting base pair prevents free rotation of the following RNA strand and in this way can assure appropriate assembly in the CTD structure (Kolakofsky et al. 2012), another argument for the strict necessity of a base pairing at the triphosphate-bearing end of dsRNA to stimulate RIG-I. As suggested by the comparison of RIG-I CTD of different vertebrate species, F853 can only be substituted by the functionally related tyrosine (Y). H830 and C829 mediate possibly important backbone interactions with the 2′OH groups of the ribose of N1 and N2, suggesting a strict discrimination of RNA versus DNA at those positions.
The conclusion by Lu and colleagues that RIG-I can bind and simultaneously be stimulated by single-stranded pppRNA has to be questioned, since in vitro transcribed RNA (which usually contains ppp-dsRNA species) was used for stimulation of cells (Lu et al. 2010b). Although the RIG-I CTD appears to interact only with the ppp-bearing RNA strand, helix formation should be still crucial for appropriate interaction of the RNA with the contacting amino acid residues of H853, K907, H830 and C829. Marq and colleagues found that non-stimulatory ppp-dsRNA (ppp-dsRNA with 5′ppp-overhang) can bind RIG-I with comparable affinity as active ligands (complete ppp-dsRNA) indicating the possibility of stimulatory (“productive”) and non-stimulatory (“non-productive”) ligand binding modes to RIG-I (Marq et al. 2010a). These data suggest that binding to RIG-I is essential but not sufficient for activation of RIG-I. Two independent studies found that the presence of CARDs strongly reduce the tolerance for binding of OH-dsRNA, thus represent a considerable contribution to the selective recognition 5′triphosphorylated RNA (Cui et al., 2008, Vela et al., 2012). Additionally, based on energetic parameters of the RIG-I dsRNA interaction, Vela et al. suggested that a relatively low affinity of full-length RIG-I for dsRNA and, therefore, enhanced target specificity is mediated through antagonistic domain binding between helicase and CTD (Vela et al. 2012).
The CTD of the RIG-I inhibiting helicase Lgp2 is closely related to the RIG-I CTD (Li et al., 2009b, Pippig et al., 2009). Similar to RIG-I, Lgp2 was reported to preferentially bind to blunt ended dsRNA (Li et al., 2009b, Murali et al., 2008, Pippig et al., 2009), albeit in a 5′triphosphate independent manner (Pippig et al. 2009). Amino acids mediating the interaction with the 5′terminal base pair and the ribose backbone (H830RIG-I, F853RIG-I, K888 RIG-I, K907RIG-I = H576Lgp2, W604Lgp2, K634Lgp2, K651Lgp2) are conserved or at least functionally related between the RIG-I and the Lgp2 CTD, while triphosphate-interacting amino acids (H847, K849, K858 and K861) are missing in the Lgp2 CTD. H576Lgp2, W604Lgp2 K634Lgp2 and K651Lgp2were found to be involved in dsRNA binding of the Lgp2 CTD (Li et al., 2009b, Pippig et al., 2009). Conversely, the binding mode of OH-dsRNA to Lgp2 differed considerably from the binding mode of ppp-dsRNA. Confusingly, mutation of the amino acids in the Lgp2 CTD corresponding to K888RIG-I (K634Lgp2 → E) and K907RIG-I (K651Lgp2 → E) led to loss of RNA binding but did not impair Lgp2-mediated inhibition of RIG-I activation, suggesting a ligand-independent RIG-I inhibiting mechanism by Lgp2 (Li et al. 2009b).
Triphosphate independent recognition of long double stranded RNA by RIG-I
The RIG-I ligand requirements for short dsRNA described above is in contrast to the initial finding that RIG-I can be activated by poly I:C (Yoneyama et al. 2004), a dsRNA polymer with monophosphates at the 5′ end (Grunberg-Manago 1967). In order to characterize ligand structure motifs differentiating RIG-I and MDA5 recognition, Kato and colleagues fractionated RNase III-digested poly I:C (resulting in 5′monophosphates and 2 nt 3′overhangs). While 7 kb poly I:C fragments (high molecular weight) were preferentially detected by MDA5, fractions equal to 300 bp or smaller were exclusively recognized by RIG-I (Kato et al. 2008). Binder et al. (2011) found an inverse correlation: in their experiments, dsRNAs of different length (40–1600 bp) were assessed for RIG-I stimulating activity in RIG-I transgenic Huh7.5 cells. They observed that molecular weight of ppp-dsRNA positively correlated with RIG-I activation. Of note, Binder et al. compared RIG-I activation at relatively low concentration with constant molarity while Kato et al. analyzed RIG-I activity transfecting high concentrations (1 μg/ml) at constant mass concentration (Binder et al., 2011, Kato et al., 2008). Binder et al. could reproduce the results of Kato et al. when transfecting high amounts (0.1 pmol/well) of RNA. Nevertheless, both studies observed 5′triphosphate independent activation of RIG-I by very long (≥100 bp) dsRNA. Length-dependent, 5′end-independent RIG-I activation cannot be explained with the current model of CTD-mediated recognition. Binder et al. proposed an alternative CTD-independent recognition mechanism (cooperative multimerization of RIG-I) in which binding of one RIG-I molecule facilitates the binding of a second, etc. (Binder et al. 2011). In this case, binding can only occur in a helicase-dependent manner and would enable displacement of the CARDs, leading to initiation of the signaling cascade. Based on multi-angle light scattering and size-exclusion chromatography-coupled small-angle X-ray scattering of RIG-I/29mer dsRNA complexes, Beckham e al. suggested that binding of a first RIG-I molecule changes the RNA structure, thus constructively influencing the binding of a second RIG-I molecule (Beckham et al. 2013).
However, if RIG-I can be activated by long dsRNA in a 5′end independent manner, the question still remains why long double-stranded replicative RNA intermediates purified from (+)ssRNA picornaviruses, are not recognized by RIG-I (Feng et al. 2012).
RNase cleavage products–ligands for RIG-I and MDA5?
Malathi et al. (2007) observed that activated antiviral endoribonuclease RNase L generates small RNA cleavage products from self-RNA that induce type I IFN production (Malathi et al. 2007). In this study both, MDA5 and RIG-I were reported to contribute to recognition of small (<200 nt) RNase L cleavage products of total cellular RNA. Importantly, the type I IFN-induced RNase L digests single-stranded RNA into RNA products with 5′-OH and 3′-monophosphate groups. By reverse sequencing in a follow-up study, Malathi et al. discovered HCV genome sequence-derived RNase-L cleavage products that bind to RIG-I (Malathi et al. 2010). One of 15 RIG-I binding RNA sequences was able to significantly activate RIG-I in a 3′monophosphate-dependent but 5′triphosphate-independent manner. The putative structure of RIG-I activating sequence included long (>20 bp) dsRNA regions but also long (>5 nt) single stranded 5′ and 3′ ends. As the sequence was generated by in vitro transcription and not validated by mass spectrometry, it remains unclear whether the intended structure or co-purified side products from in vitro transcription are responsible for RIG-I stimulation. Nevertheless, the study indicates that special RNA structures exist, which can stimulate RIG-I in a 3′monophosphate-dependent manner and that these kinds of structures can be generated by RNase L cleavage of RNA virus genomes. Of note, 3′monophosphate-dependent RIG-I stimulation was also reported earlier by the Fujita group (Takahasi et al. 2008).
RNA polymerase III transcripts
At the same time, the Akira lab and the Medzhitov lab reported a TLR9-independent type I IFN induction when dsDNA was transfected into the cytosol of cells (Ishii et al., 2006, Stetson and Medzhitov, 2006). Knock-down of MAVS in 293T cells significantly reduced dsDNA-induced type I IFN, suggesting a MAVS-dependent pathway of dsDNA recognition in these cells (Ishii et al. 2006). Unexpectedly, MAVS-deficient murine cells still responded to dsDNA (Sun et al. 2006). Importantly, the Akira lab used a special type of dsDNA, the heteropolymer dAdT (a polymer of the alternating sequence AT). Cheng et al. observed that human cell lines (Huh7, HEK293) secrete type I IFN in a MAVS and RIG-I-dependent manner after transfection of dAdT but not plasmid DNA (Cheng et al. 2007). The dAdT enigma was later solved by two independent groups (Ablasser et al., 2009, Chiu et al., 2009): Ablasser et al. and Chiu et al. discovered that dAdT functions as a template for the endogenous RNA polymerase III, which generates 5′triphosphorylated AU-polymers in the cytosol (Chiu et al. 2009). AT-rich DNA is in two ways a special kind of DNA: first, RNA polymerase III prefers transcription of AT-rich sequences; second, the resulting pppAU-polymers are self-complementary and can easily anneal to ppp-dsRNA. They therefore represent excellent RIG-I target structures (Ablasser et al. 2009). Thus, it has to be kept in mind that “RIG-I stimulating DNA” needs to provide two features: first, the DNA needs to be able to serve as a template for RNA polymerase III, second, the transcript has to form an appropriate RIG-I ligand structure (base paired 5′ppp end + dsRNA > 19 bp). Most natural dsDNA structures do not provide both features. Some DNA virus (e.g. Epstein Barr virus and Adenovirus) encode for small RNAs (EBER and VAI) under the control of a polymerase III-driven promoter; these were observed to stimulate RIG-I (Ablasser et al., 2009, Minamitani et al., 2011, Samanta et al., 2008).
Unlike murine cells and human monocytic cells, most human cell lines are not stimulated by dsDNAs (e.g. plasmid DNA or PCR products) other than dAdT. This indicates the absence of a receptor in the cytosol, which can recognize DNA directly in those cell lines (Ablasser et al., 2009, Cheng et al., 2007). In those cells, the RNA Polymerase III/RIG-I pathway constitutes the only alternative to detect cytosolic dsDNA. Since some DNA viruses and facultative intracellular bacteria induced a MAVS or RIG-I-dependent type I IFN response in non-immune cells, it was suggested that in these cells the innate immune response to intracellular dsDNA-containing pathogens can occur via stimulation of RIG-I by pathogen-DNA templated polymerase III transcripts (Ablasser et al., 2009, Chiu et al., 2009). As will be summarized in the next section, intracellular bacteria were actually shown to release RNA into the cytosol of cells, which is then recognized by RIG-I (Abdullah et al., 2012, Hagmann et al., 2013).
RIG-I senses pppRNA from bacteria
In general, all RNA polymerase-dependent transcribed RNAs initially possess a 5′triphosphorylated start nucleotide. Posttranscriptional 5′processing or modification such as capping are key features of mRNA translation regulation in eucaryotes. In contrast to eucaryotes, one third of Escherichia coli (bacterial) mRNA remains 5′triphosphorylated (Bieger and Nierlich 1989). Regulation of the 5′phosphorylation status of bacterial mRNA, which determines mRNA decay (Celesnik et al. 2007), occurs via the pyrophosphatase RppH (Deana et al. 2008). The 5′triphosphate moiety prevents degradation of bacterial mRNA by RNase-E (Celesnik et al. 2007). Importantly, pyrophosphatase RppH preferentially targets single-stranded triphosphorylated 5′nucleotides as a substrate, consequently leaving 5′triphosphorylated dsRNA (Deana et al. 2008). This conclusion is supported by the finding that 5′-terminal stem-loops prolong the lifetime of bacterial RNAs (Emory et al., 1992, Mackie, 2000). Thus, base-paired 5′triphosphorylated RNAs (ppp-dsRNA), which are an excellent target structure for RIG-I, appear to represent a characteristic molecular pattern (MAMP) for bacteria.
In a siRNA-based approach, Opitz et al. observed that Legionella pneumophilae, a facultative intracellular gram-negative bacterium with type IV secretion system, raised a MAVS-dependent but RIG-I- or MDA5-independent type-I IFN response in a human endothelial cell line (A549) (Opitz et al. 2006). The involvement of MAVS in the L. pneumophilae induced immune response was later affirmed in experiments with MAVS- and MDA5-deficient, anti-RIG-I shRNA-expressing bone marrow-derived macrophage cell lines (Monroe et al. 2009). In contrast to findings by Opitz et al. (2006), contribution of MDA5 and RIG-I to the type I IFN response was reported (Monroe et al. 2009). While knock-down of RIG-I substantially reduced the response to transfected purified bacterial RNA, it remained an open question whether Legionella-derived RNA actually gains access to the cytosol of the host cell during infection. As MDA5 appeared to not directly sense bacterial RNA, the authors speculated about an indirect mechanism leading to activation of RIG-I and MDA5. Importantly, DNA from L. pneumophilae did not induce type I IFN in HEK293 cells, thus excluding RIG-I-mediated recognition of RNA polymerase-III transcripts in the host cell, as previously suggested (Chiu et al., 2009, Monroe et al., 2009). Recognition of bacterial RNA by RIG-I was also observed for the bacterium Helicobacter pylori (Rad et al. 2009). Abdullah et al. discovered that the facultative intracellular bacterium Listeria monocytogenes actively secrete small RNAs via its SecA2 secretion system resulting in strong RIG-I activating activity (Abdullah et al. 2012). Recently, we visualized translocation of bacterial RNA from L. monocytogenes into the cytosol of several human cell lines (Hagmann et al. 2013). Previously, L. monocytogenes was reported to induce type I IFN exclusively via the STING pathway, an adaptor/receptor sensing second messenger molecules or cyclic nucleic acids either directly secreted by L. monocytogenes (cyclic di-AMP) or generated downstream of cytosolic DNA recognition (GMP-AMP) in mammalian cells (Ishikawa et al., 2009, Sauer et al., 2011, Sun et al., 2012, Woodward et al., 2010, Wu et al., 2012, Zhong et al., 2008). We found that the L. monocytogenes-triggered type I IFN response is dependent on RIG-I recognition when the STING pathway is not present in cells, this being the case in tested non-immune cells (Hagmann et al. 2013). In immune cells such as the human cell line THP-1 the STING-dependent recognition pathway dominates the immune response to L. monocytogenes while RIG-I appears to not play any role in recognition (Hagmann et al. 2013). In this respect, data for murine cells are conflicting: while some studies excluded involvement of RIG-I/MAVS in L. monocytogenes recognition in macrophages (Soulat et al., 2006, Sun et al., 2006) another study observed a substantial contribution of the RIG-I pathway (Abdullah et al. 2012). Culturing conditions of bacteria and murine cells may influence the amount of transferred bacterial pppRNA and the balance between RIG-I and the STING pathway in murine macrophages, thus leading to controversial findings. Interestingly, Li et al. found that RNA of commensal bacteria is recognized in a MAVS-dependent manner and that MAVS in cells of non-hematopoietic origin plays a dominant role in preventing DSS-induced colitis (Li et al. 2011), supporting that RIG-I-inducing bacterial RNA indeed has access to non-immune cells in vivo and mediates important effects. This finding explained the observation by Wang et al. that RIG-I deficient mice easily develop colitis (Wang et al. 2007).
Considering the fact that mice with a defect in the type I IFN pathway exhibit a strong resistance to Listeria-induced pathogenesis, it remains to be determined if the observed RIG-I-dependent recognition of bacterial RNA contributes more to the pathogenicity or to the clearance of Listeria (Auerbuch et al., 2004, O’Connell et al., 2004).
Detection of viral RNA – which structure stimulates RIG-I?
(+)ssRNA/dsRNA viruses
In addition to dsRNA viruses positive single-strand RNA [(+)ssRNA] viruses also generate cytosolic dsRNA species, such as replicative dsRNA intermediates, during their replication (Feng et al., 2012, Targett-Adams et al., 2008, Triantafilou et al., 2012, Weber et al., 2006). Together with 5′triphosphate, such RNA species represent ideal RIG-I target structures.
Since picornaviruses were shown not to activate RIG-I during infection (Gitlin et al., 2006, Kato et al., 2006) it was presumed that picornaviruses (and caliciviruses) (Fig. 1) are able to escape RIG-I recognition because instead of a 5′triphosphate their RNA genomes possess a peptide (Vpg) linked via a tyrosine residue to 5′monophosphate (Hruby and Roberts, 1978, Lee et al., 1977, Rohayem et al., 2006). In line with these findings, Feng et al. observed that purified picorna virus RNA stimulated MDA5 but not RIG-I (Feng et al. 2012). Additionally and rather unexpectedly, picornaviruses were observed to degrade RIG-I during infection. The apparent need to degrade RIG-I indicates the occurrence of some RIG-I stimulating activity, the identity of which has not been clarified so far (Barral et al., 2009, Papon et al., 2009).
(−)ssRNA viruses
RIG-I dominates the immune response to many (−)ssRNA viruses (Cardenas et al., 2006, Habjan et al., 2008, Hornung et al., 2006, Kato et al., 2005, Kato et al., 2006, Loo et al., 2008, Plumet et al., 2007, Yoneyama et al., 2005). However, three out of four so far analyzed (−)ssRNA viruses were described not to generate double-stranded RNA species during infection (no dsRNA: influenza; Sendai virus, SeV; La Crosse Virus, LACV; dsRNA detectable: New Castle disease virus, NDV) (Pichlmair et al., 2006, Takeuchi et al., 2008, Weber et al., 2006). This finding may seem conflicting at first, but can be explained by the fact that the dsRNA-visualizing antibody used in these studies is only able to bind dsRNA longer than 40 bases (Bonin et al. 2000) but RIG-I can recognize ppp-dsRNA ≥ 19 bp.
In general, the complementary 5′ and 3′ terminal sequences of all (−)ssRNA viruses genomes bear the potential to hybridize to so-called dsRNA panhandle structures with blunt ended 5′triphosphorylated ends (Fig. 1, left bottom). A certain degree of self-complementarity cannot be avoided by (−)ssRNA viruses since the same viral polymerase which recognizes its start sequence needs to start mRNA transcription or replication from the (−)ssRNA or (+)ssRNA intermediates. For Bunyaviridae, including LACV, it was validated by psoralen-crosslinking and electron microscopy that the 5′- and 3′-ends of the viral genome indeed constituted a panhandle structure in vivo (Hewlett et al., 1977, Raju and Kolakofsky, 1989). The LACV panhandle consists of a blunt-ended 24–27 bp dsRNA stretch with only a few mismatched nucleotides (Raju and Kolakofsky 1989), thereby achieving almost all RIG-I ligand requirements (Schlee et al. 2009) without being detected by the 40pb RNA-specific antibody. Marq et al. suggested that for this reason some arenaviruses and bunyaviruses circumvent RIG-I recognition by conducting a prime and realign mechanism which enables generation of 5′overhangs in their genomes (Marq et al. 2010b). As mentioned above, Habjan et al. identified viruses (Bornaviridae, Bunyaviridae), which escape RIG-I recognition by combination of a prime and realign mechanism to 5′terminal cleavage, leaving a 5′monophosphorylated end (Fig. 1, “RLR escape”).
By using replication and translation blocking agents, applying enzymatic probing and visualization by superresolution microscopy, Weber et al. confirmed that RIG-I indeed recognizes viral capsids of LACV upon entry into the cell by binding to the panhandle structure (Weber et al. 2013).
Similar to LACV, influenza virus genomic pppRNA (comprising 8 segments with conserved 5′ends) forms triphosphorylated, panhandle structures, albeit with quite short double stranded stretches (about 15 bp), including mismatches/bulge loop structures (Desselberger et al., 1980, Hsu et al., 1987). This panhandle structure represents the site of RNA transcription-initiation for the viral RNA polymerase complex in the nucleus of the host cell (Portela and Digard 2002). Dauber et al. observed that the Influenza panhandle is accessible for the antiviral double-stranded RNA-dependent protein kinase (PKR) after export from the nucleus into the cytosol of the host cell (Dauber et al. 2009). Since PKR and RIG-I recognize comparable dsRNA structures–short triphosphorylated dsRNA (Nallagatla et al., 2007, Schlee et al., 2009), it is likely that RIG-I also has access to the Influenza panhandle structure in the cytosol. If the Influenza panhandle is indeed sufficient to activate RIG-I still remains to be verified using well-defined RIG-I ligands. Rehwinkel and colleagues generated mutated Influenza RNA polymerases which either selectively produce viral mRNA or replicative genomic RNA (Rehwinkel et al. 2010). Using this tool, they confirmed that RIG-I activation occurs exclusively by the genomic RNA and not mRNA of Influenza (Rehwinkel et al. 2010). Additionally, analysis of RIG-I-bound viral RNA from Influenza infected cells revealed that only 5′triphosphorylated viral genomic RNA co-precipitated with RIG-I.
In contrast to LACV and Influenza, viral particles of Sendai Virus (SeV) and Measles Virus (MeV) which belong to the group of Mononegavirales contain predominantly linear nucleocapsids because encapsidation with structural proteins prevents formation of double stranded or panhandle structures (Bhella et al., 2004, Gerlier and Lyles, 2011, Loney et al., 2009). But SeV and VSV were found to produce defective interfering (DI) viral genomes during replication (Kolakofsky, 1976, Lazzarini et al., 1981, Perrault and Leavitt, 1978). Three kinds of DI genomes were identified: DI genomes with internal deletions, 5′promoter duplications with completely complementary 5′–3′ ends, and hairpin DI genomes “snap back”, consisting of a dsRNA hairpin of 100–1000 bp (Fig. 1). “Panhandle” and “snap back” DI RNAs should result in excellent RIG-I ligands. Indeed, Strahle et al. could correlate Sendai virus-induced RIG-I activation with the occurrence of snap back DI genomes (DI-H4) which are generated during infection without encapsidation and thus are able to form panhandle structures in infected cells (Strahle et al., 2006, Strahle et al., 2007). In concordance with these data, by applying a deep sequencing approach after purification of RNA attached to RIG-I from Sendai virus infected cells, Baum et al. determined preferred binding of DI genomes to RIG-I (Baum et al. 2010).
It appears plausible that the genomic RNA should be targeted by RIG-I, primarily. Actually, purified genomic RNA from most examined (−)ssRNA viruses like Rabies virus (Hornung et al. 2006), Lassa virus, Nipah virus, Rift Valley fever virus (Habjan et al. 2008) activated RIG-I. Nevertheless, RNA purification by denaturating agents removes RNA-interacting viral nuclear proteins, allowing formation of secondary RIG-I-activating RNA structures which do not occur in vivo (e.g. hybridization of (−)ssRNA and (+)ssRNA). Therefore it is uncertain if these viral genomes would activate RIG-I during infection. However, the phenomenon that (−)ssRNA viruses possess mechanisms to modify their genomic 5′end, preventing RIG-I recognition (Habjan et al., 2008, Marq et al., 2010b), implies that the dsRNA structures that (can) form at the 5′end of viral genomes are critical for the viruses with RIG-I escape mechanisms (Arenaviridae, Bunyaviridae, Bornaviridae).
In fact, the principle of detecting panhandle structures represents an intelligent strategy to detect (−)ssRNA viruses because replication of (−)ssRNA viruses necessitates highly conserved promoters at both ends of the genome, consequently yielding self-complementary 5′ and 3′ ends. The intolerance of VSV for artificially introduced extra nucleotides at the 5′ and 3′ ends of its (−)ssRNA genome suggests lack of flexibility of (−)ssRNA viruses concerning promoter sequences (Pattnaik et al. 1992). Therefore, a blunt-ended double-stranded RNA structure with 5′triphosphate, the consequence of two conserved promoters, constitutes a negative strand virus-associated molecular pattern recognized by RIG-I.
Correctly processed viral mRNA is unlikely to be targeted by RIG-I, because RNA viruses possess mechanisms to cap their mRNA either by viral-encoded capping enzymes or cap-snatching mechanisms (Fechter and Brownlee 2005) leading to loss of, or masking of the 5′triphosphate. Nevertheless, other studies suggest that type I IFN induction by Mononegavirales (e.g. SeV, VSV, MeV) is associated with mRNA transcription rather than replication (reviewed in Gerlier and Lyles (2011)). E.g., in contrast to Influenza, replication-disabled Measles virus that was still capable of transcription was observed to still activate a type I IFN response (Plumet et al. 2007). Leader and trailer RNAs (leRNA and trRNA) represent the only non-capped 5′triphosphorylated transcripts occurring during the transcription of the genome. Plumet et al. and Bitko et al. observed (−)ssRNA virus LeRNA dependent stimulation of RIG-I (Bitko et al., 2008, Plumet et al., 2007). However, the viral RNA which forms together with the LeRNA the RIG-I activating dsRNA species still needs to be determined. Gerlier and Lyles proposed that L-trRNA read-through transcripts (viral L-mRNA extended by the trRNA template) hybridized to ppp-trRNA could also reconstitute a source of perfect blunt-ended RIG-I target structures during the non-replicative transcription phase (Fig. 1, left panel) (Gerlier and Lyles 2011).
DNA viruses
DNA genome-based viruses like Adenovirus, vaccinia virus and Herpesviridae (Herpes simplex virus, HSV) generate copious amounts of dsRNA during their life cycle (Weber et al. 2006); these dsRNAs are most probably generated by overlapping converging transcription (Jacobs and Langland 1996). While Melchjorsen et al. reported MDA5 but not RIG-I-dependent type I IFN induction by HSV1 (Melchjorsen et al. 2010), Xing et al. reported that the HSV1 encoded US11 protein interacts with and inhibits both RIG-I and MDA5, indicating that HSV1-derived RNA is also recognized by RIG-I (Xing et al. 2012). Finally, EBV and Adenovirus-encoded small RNA polymerase III transcripts (EBER and VAI) were described to activate RIG-I (Ablasser et al., 2009, Minamitani et al., 2011, Samanta et al., 2008).
Concluding remarks
Despite the presence of crystal data, conflicting results on ligand motif definition still obscure the understanding of MDA5 pathogen RNA detection. As demonstrated for Paramyxoviruses, conclusions from studies testing immune responses in RIG-I/MDA5 knock-out cells to whole viruses can be misleading as long as the function of viral proteins is unknown: viruses appear to be recognized by RIG-I because they express proteins efficiently inhibiting MDA5, and the opposite is also thinkable. By contrast, recent progress in the synthesis and purification of defined synthetic ligands, native preparation and visualization of natural RIG-I ligands (nucleocapsids) and crystallization of RIG-I/ligand complexes has greatly improved the understanding of the molecular basis of RIG-I-dependent virus recognition. Most of the studies agree on terminal base pair recognition by RIG-I, which is dependent on the presence of 5′triphosphate in a physiological relevant ligand concentration range. Completely end base pair-independent RIG-I recognition as proposed for long (≥100 bp) dsRNA needs further investigation since it can currently not be explained by the model derived from recent crystal data. Naturally occurring RIG-I ligands are double stranded triphosphorylated replicative intermediates of (+)ssRNA viruses (formal scientific proof is missing though) and the triphosphorylated panhandle structures of genomes of (−)ssRNA viruses with the exception of Mononegavirales, which are able to avoid forming panhandle structures in vivo. Other viruses avoid RIG-I recognition by laborious procedures leading to 5′overhangs or 5′monophosphorylation. Interestingly, in analogy to phage polymerases in vitro, RNA polymerases of Mononegavirales generate RIG-I ligands due to an error-prone transcription process, therefore counteracting their – in principle – perfect camouflage against RIG-I recognition.
On the other hand, a successful virus has to protect its host from detrimental consequences of infection. The best examples are Herpesviruses, the infection of which usually proceeds asymptomatic: >95% of adults are, for example, infected by EBV, which persists lifelong in its host without causing detrimental symptoms, making EBV a very successful virus (Thorley-Lawson 2005). EBV is known for its close interaction with the immune system, limiting its own infection in the host (Thorley-Lawson 2005). Thus immune recognition of the virus at a certain stage of its spread in the host should be evolutionarily favored and complete immune evasion not the goal of virus adaption.
Conflict of interest statement
The author is listed as inventor on a patent application covering structures described in a manuscript, which is cited in this review (Schlee et al. 2009).
Acknowledgments
I thank Janos Ludwig for illuminating discussions and Cristina Amparo Hagmann for critically reading the manuscript. Present work in the laboratory was supported by grants from the Deutsche Forschungsgemeinschaft (SFB670 and DFG Research Grants Program SCHL 1930/1-1).
References
- Abdullah Z., Schlee M., Roth S., Mraheil M.A., Barchet W., Bottcher J., Hain T., Geiger S., Hayakawa Y., Fritz J.H. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012;31:4153–4164. doi: 10.1038/emboj.2012.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablasser A., Bauernfeind F., Hartmann G., Latz E., Fitzgerald K.A., Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Andrejeva J., Childs K.S., Young D.F., Carlos T.S., Stock N., Goodbourn S., Randall R.E. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbuch V., Brockstedt D.G., Meyer-Morse N., O’Riordan M., Portnoy D.A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A.K. 5’-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol. Rev. 1980;44:175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchet W., Wimmenauer V., Schlee M., Hartmann G. Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr. Opin. Immunol. 2008;20:389–395. doi: 10.1016/j.coi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Barral P.M., Sarkar D., Fisher P.B., Racaniello V.R. RIG-I is cleaved during picornavirus infection. Virology. 2009;391:171–176. doi: 10.1016/j.virol.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Sachidanandam R., Garcia-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl. Acad. Sci. U.S.A. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham S.A., Brouwer J., Roth A., Wang D., Sadler A.J., John M., Jahn-Hofmann K., Williams B.R., Wilce J.A., Wilce M.C. Conformational rearrangements of RIG-I receptor on formation of a multiprotein:dsRNA assembly. Nucleic Acids Res. 2013;41:3436–3445. doi: 10.1093/nar/gks1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhella D., Ralph A., Yeo R.P. Conformational flexibility in recombinant measles virus nucleocapsids visualised by cryo-negative stain electron microscopy and real-space helical reconstruction. J. Mol. Biol. 2004;340:319–331. doi: 10.1016/j.jmb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Bieger C.D., Nierlich D.P. Distribution of 5′-triphosphate termini on the mRNA of Escherichia coli. J. Bacteriol. 1989;171:141–147. doi: 10.1128/jb.171.1.141-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Eberle F., Seitz S., Mucke N., Huber C.M., Kiani N., Kaderali L., Lohmann V., Dalpke A., Bartenschlager R. Molecular mechanism of signal perception and integration by the innate immune sensor retinoic acid-inducible gene-I (RIG-I) J. Biol. Chem. 2011;286:27278–27287. doi: 10.1074/jbc.M111.256974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitko V., Musiyenko A., Bayfield M.A., Maraia R.J., Barik S. Cellular La protein shields nonsegmented negative-strand RNA viral leader RNA from RIG-I and enhances virus growth by diverse mechanisms. J. Virol. 2008;82:7977–7987. doi: 10.1128/JVI.02762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin M., Oberstrass J., Lukacs N., Ewert K., Oesterschulze E., Kassing R., Nellen W. Determination of preferential binding sites for anti-dsRNA antibodies on double-stranded RNA by scanning force microscopy. RNA. 2000;6:563–570. doi: 10.1017/s1355838200992318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. Highly pathogenic RNA viral infections: challenges for antiviral research. Antiviral Res. 2008;78:1–8. doi: 10.1016/j.antiviral.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Cardenas W.B., Loo Y.M., Gale M., Jr., Hartman A.L., Kimberlin C.R., Martinez-Sobrido L., Saphire E.O., Basler C.F. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Uhlenbeck O.C. RNA template-directed RNA synthesis by T7 RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6972–6976. doi: 10.1073/pnas.91.15.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesnik H., Deana A., Belasco J.G. Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol. Cell. 2007;27:79–90. doi: 10.1016/j.molcel.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Cheng G., Zhong J., Chung J., Chisari F.V. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9035–9040. doi: 10.1073/pnas.0703285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs K., Randall R., Goodbourn S. Paramyxovirus V proteins interact with the RNA Helicase LGP2 to inhibit RIG-I-dependent interferon induction. J. Virol. 2012;86:3411–3421. doi: 10.1128/JVI.06405-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs K., Stock N., Ross C., Andrejeva J., Hilton L., Skinner M., Randall R., Goodbourn S. Mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Chiu Y.H., Macmillan J.B., Chen Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civril F., Bennett M., Moldt M., Deimling T., Witte G., Schiesser S., Carell T., Hopfner K.P. The RIG-I ATPase domain structure reveals insights into ATP-dependent antiviral signalling. EMBO Rep. 2011;12:1127–1134. doi: 10.1038/embor.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Eisenacher K., Kirchhofer A., Brzozka K., Lammens A., Lammens K., Fujita T., Conzelmann K.K., Krug A., Hopfner K.P. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Dauber B., Martinez-Sobrido L., Schneider J., Hai R., Waibler Z., Kalinke U., Garcia-Sastre A., Wolff T. Influenza B virus ribonucleoprotein is a potent activator of the antiviral kinase PKR. PLoS Pathog. 2009;5:e1000473. doi: 10.1371/journal.ppat.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana A., Celesnik H., Belasco J.G. The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature. 2008;451:355–358. doi: 10.1038/nature06475. [DOI] [PubMed] [Google Scholar]
- Desselberger U., Racaniello V.R., Zazra J.J., Palese P. The 3′ and 5′-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980;8:315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- Diebold S.S., Montoya M., Unger H., Alexopoulou L., Roy P., Haswell L.E., Al-Shamkhani A., Flavell R., Borrow P., Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- Doyle S., Vaidya S., O’Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Emory S.A., Bouvet P., Belasco J.G. A 5′-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- Fechter P., Brownlee G.G. Recognition of mRNA cap structures by viral and cellular proteins. J. Gen. Virol. 2005;86:1239–1249. doi: 10.1099/vir.0.80755-0. [DOI] [PubMed] [Google Scholar]
- Feng Q., Hato S.V., Langereis M.A., Zoll J., Virgen-Slane R., Peisley A., Hur S., Semler B.L., van Rij R.P., van Kuppeveld F.J. MDA5 detects the double-stranded RNA replicative form in picornavirus-infected cells. Cell Rep. 2012;2:1187–1196. doi: 10.1016/j.celrep.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.K., Tytell A.A., Lampson G.P., Hilleman M.R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl. Acad. Sci. U.S.A. 1967;58:1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K.A., Rowe D.C., Barnes B.J., Caffrey D.R., Visintin A., Latz E., Monks B., Pitha P.M., Golenbock D.T. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen B.L., Keller B.C., Fornek J., Katze M.G., Gale M., Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Lezzi M., Dobbs M., Elliott R.M., Schmaljohn C., Kang C.Y., Kolakofsky D. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J. Virol. 1995;69:5754–5762. doi: 10.1128/jvi.69.9.5754-5762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlier D., Lyles D.S. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol. Mol. Biol. Rev. 2011;75:468–490. doi: 10.1128/MMBR.00007-11. second page of table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R.A., Diamond M.S., Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondai T., Yamaguchi K., Miyano-Kurosaki N., Habu Y., Takaku H. Short-hairpin RNAs synthesized by T7 phage polymerase do not induce interferon. Nucleic Acids Res. 2008;36:e18. doi: 10.1093/nar/gkm1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M. Polynucleotide phosphorylase: structure and mechanism of action. Biochem. J. 1967;103:62P. [PMC free article] [PubMed] [Google Scholar]
- Habjan M., Andersson I., Klingstrom J., Schumann M., Martin A., Zimmermann P., Wagner V., Pichlmair A., Schneider U., Muhlberger E. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE. 2008;3:e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann C.A., Herzner A.M., Abdullah Z., Zillinger T., Jakobs C., Schuberth C., Coch C., Higgins P.G., Wisplinghoff H., Barchet W. RIG-I detects triphosphorylated RNA of Listeria monocytogenes during infection in non-immune cells. PLoS ONE. 2013;8:e62872. doi: 10.1371/journal.pone.0062872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hewlett M.J., Pettersson R.F., Baltimore D. Circular forms of Uukuniemi virion RNA: an electron microscopic study. J. Virol. 1977;21:1085–1093. doi: 10.1128/jvi.21.3.1085-1093.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Hornung V., Guenthner-Biller M., Bourquin C., Ablasser A., Schlee M., Uematsu S., Noronha A., Manoharan M., Akira S., de Fougerolles A. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdorfer B., Giese T., Endres S., Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Hruby D.E., Roberts W.K. Encephalomyocarditis virus RNA. III. Presence of a genome-associated protein. J. Virol. 1978;25:413–415. doi: 10.1128/jvi.25.1.413-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M.T., Parvin J.D., Gupta S., Krystal M., Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc. Natl. Acad. Sci. U.S.A. 1987;84:8140–8144. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K.J., Coban C., Kato H., Takahashi K., Torii Y., Takeshita F., Ludwig H., Sutter G., Suzuki K., Hemmi H. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B.L., Langland J.O. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- Jiang F., Ramanathan A., Miller M.T., Tang G.Q., Gale M., Jr., Patel S.S., Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Kinch L.N., Brautigam C.A., Chen X., Du F., Grishin N.V., Chen Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O., Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K.J., Takeuchi O., Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Longo M., Han Y., Lundberg P., Cantin E., Rossi J.J. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotechnol. 2004;22:321–325. doi: 10.1038/nbt940. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976;8:547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Kowalinski E., Cusack S. A structure-based model of RIG-I activation. RNA. 2012;18:2118–2127. doi: 10.1261/rna.035949.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A., Horvath C.M. RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J. Virol. 2006;80:12332–12342. doi: 10.1128/JVI.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalinski E., Lunardi T., McCarthy A.A., Louber J., Brunel J., Grigorov B., Gerlier D., Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Krieg A.M., Yi A.K., Matson S., Waldschmidt T.J., Bishop G.A., Teasdale R., Koretzky G.A., Klinman D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R.A., Keene J.D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981;26:145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lee Y.F., Nomoto A., Detjen B.M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc. Natl. Acad. Sci. U.S.A. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu Y., Zhang X. Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J. Virol. 2010;84:6472–6482. doi: 10.1128/JVI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lu C., Stewart M., Xu H., Strong R.K., Igumenova T., Li P. Structural basis of double-stranded RNA recognition by the RIG-I like receptor MDA5. Arch. Biochem. Biophys. 2009;488:23–33. doi: 10.1016/j.abb.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Li X., Ranjith-Kumar C.T., Brooks M.T., Dharmaiah S., Herr A.B., Kao C., Li P. The RIG-I-like receptor LGP2 recognizes the termini of double-stranded RNA. J. Biol. Chem. 2009;284:13881–13891. doi: 10.1074/jbc.M900818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.D., Chiu Y.H., Ismail A.S., Behrendt C.L., Wight-Carter M., Hooper L.V., Chen Z.J. Mitochondrial antiviral signaling protein (MAVS) monitors commensal bacteria and induces an immune response that prevents experimental colitis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17390–17395. doi: 10.1073/pnas.1107114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney C., Mottet-Osman G., Roux L., Bhella D. Paramyxovirus ultrastructure and genome packaging: cryo-electron tomography of sendai virus. J. Virol. 2009;83:8191–8197. doi: 10.1128/JVI.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo Y.M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M.A., Garcia-Sastre A., Katze M.G., Gale M., Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Ranjith-Kumar C.T., Hao L., Kao C.C., Li P. Crystal structure of RIG-I C-terminal domain bound to blunt-ended double-strand RNA without 5′ triphosphate. Nucleic Acids Res. 2010;39:1565–1575. doi: 10.1093/nar/gkq974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Xu H., Ranjith-Kumar C.T., Brooks M.T., Hou T.Y., Hu F., Herr A.B., Strong R.K., Kao C.C., Li P. The structural basis of 5′ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure. 2010;18:1032–1043. doi: 10.1016/j.str.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Eckstein F. Rapid and efficient synthesis of nucleoside 5′-0-(1-thiotriphosphates), 5′-triphosphates and 2′,3′-cyclophosphorothioates using 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one. J. Org. Chem. 1989;54:631–635. [Google Scholar]
- Luo D., Ding S.C., Vela A., Kohlway A., Lindenbach B.D., Pyle A.M. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Kohlway A., Pyle A.M. Duplex RNA activated ATPases (DRAs): platforms for RNA sensing, signaling and processing. RNA Biol. 2013;10:111–120. doi: 10.4161/rna.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra P., Sun D., Silverman R.H., He B. Activation of IFN-β expression by a viral mRNA through RNase L and MDA5. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2118–2123. doi: 10.1073/pnas.1012409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G.A. Stabilization of circular rpsT mRNA demonstrates the 5′-end dependence of RNase E action in vivo. J. Biol. Chem. 2000;275:25069–25072. doi: 10.1074/jbc.C000363200. [DOI] [PubMed] [Google Scholar]
- Malathi K., Dong B., Gale M., Jr., Silverman R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K., Saito T., Crochet N., Barton D.J., Gale M., Jr., Silverman R.H. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA. 2010;16:2108–2119. doi: 10.1261/rna.2244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marq J.B., Hausmann S., Veillard N., Kolakofsky D., Garcin D. Short double-stranded RNAs with an overhanging 5′ ppp-nucleotide, as found in arenavirus genomes, act as RIG-I decoys. J. Biol. Chem. 2010;286:6108–6116. doi: 10.1074/jbc.M110.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marq J.B., Kolakofsky D., Garcin D. Unpaired 5′ ppp-nucleotides, as found in arenavirus double-stranded RNA panhandles, are not recognized by RIG-I. J. Biol. Chem. 2010;285:18208–18216. doi: 10.1074/jbc.M109.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques J.T., Devosse T., Wang D., Zamanian-Daryoush M., Serbinowski P., Hartmann R., Fujita T., Behlke M.A., Williams B.R. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat. Biotechnol. 2006;24:559–565. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- McCartney S.A., Thackray L.B., Gitlin L., Gilfillan S., Virgin H.W., Colonna M. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J., Rintahaka J., Soby S., Horan K.A., Poltajainen A., Ostergaard L., Paludan S.R., Matikainen S. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J. Virol. 2010;84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Minamitani T., Iwakiri D., Takada K. Adenovirus virus-associated RNAs induce type I interferon expression through a RIG-I-mediated pathway. J. Virol. 2011;85:4035–4040. doi: 10.1128/JVI.02160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe K.M., McWhirter S.M., Vance R.E. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 2009;5:e1000665. doi: 10.1371/journal.ppat.1000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz C., Schuhmann K.M., Kirchhofer A., Moldt M., Witte G., Conzelmann K.K., Hopfner K.P. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- Murali A., Li X., Ranjith-Kumar C.T., Bhardwaj K., Holzenburg A., Li P., Kao C.C. Structure and function of LGP2, a DEX(D/H) helicase that regulates the innate immunity response. J. Biol. Chem. 2008;283:15825–15833. doi: 10.1074/jbc.M800542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Kirihata T., Fujii S., Sakai H., Kuwahara M., Sawai H., Sugimoto N. Influence of cationic molecules on the hairpin to duplex equilibria of self-complementary DNA and RNA oligonucleotides. Nucleic Acids Res. 2007;35:486–494. doi: 10.1093/nar/gkl1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla S.R., Hwang J., Toroney R., Zheng X., Cameron C.E., Bevilacqua P.C. 5′-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science. 2007;318:1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- O’Connell R.M., Saha S.K., Vaidya S.A., Bruhn K.W., Miranda G.A., Zarnegar B., Perry A.K., Nguyen B.O., Lane T.F., Taniguchi T. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B., Vinzing M., van Laak V., Schmeck B., Heine G., Gunther S., Preissner R., Slevogt H., N’Guessan P.D., Eitel J. Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J. Biol. Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- Papon L., Oteiza A., Imaizumi T., Kato H., Brocchi E., Lawson T.G., Akira S., Mechti N. The viral RNA recognition sensor RIG-I is degraded during encephalomyocarditis virus (EMCV) infection. Virology. 2009;393:311–318. doi: 10.1016/j.virol.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Pattnaik A.K., Ball L.A., LeGrone A.W., Wertz G.W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992;69:1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R.W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J. Gen. Virol. 1978;38:35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Naslund T.I., Liljestrom P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Rehwinkel J., Kato H., Takeuchi O., Akira S., Way M., Schiavo G., Reis E.S.C. Activation of MDA5 requires higher order RNA structures generated during virus infection. J. Virol. 2009;83:10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig D.A., Hellmuth J.C., Cui S., Kirchhofer A., Lammens K., Lammens A., Schmidt A., Rothenfusser S., Hopfner K.P. The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 2009;37:2014–2025. doi: 10.1093/nar/gkp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumet S., Herschke F., Bourhis J.M., Valentin H., Longhi S., Gerlier D. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE. 2007;2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A., Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002;83:723–734. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- Rad R., Ballhorn W., Voland P., Eisenacher K., Mages J., Rad L., Ferstl R., Lang R., Wagner H., Schmid R.M. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. The ends of La Crosse virus genome and antigenome RNAs within nucleocapsids are base paired. J. Virol. 1989;63:122–128. doi: 10.1128/jvi.63.1.122-128.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Tan C.P., Goubau D., Schulz O., Pichlmair A., Bier K., Robb N., Vreede F., Barclay W., Fodor E., Reis E.S.C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Rohayem J., Robel I., Jager K., Scheffler U., Rudolph W. Protein-primed and de novo initiation of RNA synthesis by norovirus 3Dpol. J. Virol. 2006;80:7060–7069. doi: 10.1128/JVI.02195-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Cross J.K., Bender S.J., Weiss S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B.G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K.A. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- Saito T., Hirai R., Loo Y.M., Owen D., Johnson C.L., Sinha S.C., Akira S., Fujita T., Gale M., Jr. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. U.S.A. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Owen D.M., Jiang F., Marcotrigiano J., Gale M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M., Iwakiri D., Takada K. Epstein–Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene. 2008;27:4150–4160. doi: 10.1038/onc.2008.75. [DOI] [PubMed] [Google Scholar]
- Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J.D., Sotelo-Troha K., von Moltke J., Monroe K.M., Rae C.S., Brubaker S.W., Hyodo M., Hayakawa Y., Woodward J.J., Portnoy D.A., Vance R.E. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Barchet W., Hornung V., Hartmann G. Beyond double-stranded RNA-type I IFN induction by 3pRNA and other viral nucleic acids. Curr. Top. Microbiol. Immunol. 2007;316:207–230. doi: 10.1007/978-3-540-71329-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Hartmann G. The chase for the RIG-I ligand-recent advances. Mol. Ther. 2010;18:1254–1262. doi: 10.1038/mt.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Hornung V., Hartmann G. siRNA and isRNA: two edges of one sword. Mol. Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Schwerd T., Hamm W., Hellmuth J.C., Cui S., Wenzel M., Hoffmann F.S., Michallet M.C., Besch R., Hopfner K.P. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12067–12072. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Sharma S., tenOever B.R., Grandvaux N., Zhou G.P., Lin R., Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Soulat D., Bauch A., Stockinger S., Superti-Furga G., Decker T. Cytoplasmic Listeria monocytogenes stimulates IFN-beta synthesis without requiring the adapter protein MAVS. FEBS Lett. 2006;580:2341–2346. doi: 10.1016/j.febslet.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Stetson D.B., Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Strahle L., Garcin D., Kolakofsky D. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology. 2006;351:101–111. doi: 10.1016/j.virol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Strahle L., Marq J.B., Brini A., Hausmann S., Kolakofsky D., Garcin D. Activation of the beta interferon promoter by unnatural Sendai virus infection requires RIG-I and is inhibited by viral C proteins. J. Virol. 2007;81:12227–12237. doi: 10.1128/JVI.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2012;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Sun L., Liu H.H., Chen X., Seth R.B., Forman J., Chen Z.J. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Suthar M.S., Ramos H.J., Brassil M.M., Netland J., Chappell C.P., Blahnik G., McMillan A., Diamond M.S., Clark E.A., Bevan M.J., Gale M., Jr. The RIG-I-like receptor LGP2 controls CD8(+) T cell survival and fitness. Immunity. 2012;37:235–248. doi: 10.1016/j.immuni.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter K.J., Daniels B.P., Cho H., Gainey M.D., Yokoyama W.M., Gale M., Jr., Virgin H.W., Klein R.S., Sen G.C., Diamond M.S. 2′-O methylation of the viral mRNA cap by West Nile virus evades ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 2012;8:e1002698. doi: 10.1371/journal.ppat.1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Yamaji M., Hosaka M., Kishine H., Hijikata M., Shimotohno K. Analysis of the 5′ end structure of HCV subgenomic RNA replicated in a Huh7 cell line. Intervirology. 2005;48:104–111. doi: 10.1159/000081736. [DOI] [PubMed] [Google Scholar]
- Takahasi K., Yoneyama M., Nishihori T., Hirai R., Kumeta H., Narita R., Gale M., Jr., Inagaki F., Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Komatsu T., Kitagawa Y., Sada K., Gotoh B. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J. Virol. 2008;82:10102–10110. doi: 10.1128/JVI.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Targett-Adams P., Boulant S., McLauchlan J. Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J. Virol. 2008;82:2182–2195. doi: 10.1128/JVI.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D.A. EBV the prototypical human tumor virus – just how bad is it? J. Allergy Clin. Immunol. 2005;116:251–261. doi: 10.1016/j.jaci.2005.05.038. quiz 262. [DOI] [PubMed] [Google Scholar]
- Triana-Alonso F.J., Dabrowski M., Wadzack J., Nierhaus K.H. Self-coded 3′-extension of run-off transcripts produces aberrant products during in vitro transcription with T7 RNA polymerase. J. Biol. Chem. 1995;270:6298–6307. doi: 10.1074/jbc.270.11.6298. [DOI] [PubMed] [Google Scholar]
- Triantafilou K., Vakakis E., Kar S., Richer E., Evans G.L., Triantafilou M. Visualisation of direct interaction of MDA5 and the dsRNA replicative intermediate form of positive strand RNA viruses. J. Cell Sci. 2012;125:4761–4769. doi: 10.1242/jcs.103887. [DOI] [PubMed] [Google Scholar]
- Vela A., Fedorova O., Ding S.C., Pyle A.M. The thermodynamic basis for viral RNA detection by the RIG-I innate immune sensor. J. Biol. Chem. 2012;287:42564–42573. doi: 10.1074/jbc.M112.385146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman T., Valdes M., Elsby R., Kakuta S., Caceres G., Saijo S., Iwakura Y., Barber G.N. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ludwig J., Schuberth C., Goldeck M., Schlee M., Li H., Juranek S., Sheng G., Micura R., Tuschl T. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat. Struct. Mol. Biol. 2010;17:781–787. doi: 10.1038/nsmb.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang H.X., Sun Y.P., Liu Z.X., Liu X.S., Wang L., Lu S.Y., Kong H., Liu Q.L., Li X.H. Rig-I−/− mice develop colitis associated with downregulation of G alpha i2. Cell Res. 2007;17:858–868. doi: 10.1038/cr.2007.81. [DOI] [PubMed] [Google Scholar]
- Weber F., Wagner V., Rasmussen S.B., Hartmann R., Paludan S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Gawanbacht A., Habjan M., Rang A., Borner C., Schmidt A.M., Veitinger S., Jacob R., Devignot S., Kochs G. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe. 2013;13:336–346. doi: 10.1016/j.chom.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J.J., Iavarone A.T., Portnoy D.A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Peisley A., Richards C., Yao H., Zeng X., Lin C., Chu F., Walz T., Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2012;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Wang S., Lin R., Mossman K.L., Zheng C. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5. J. Virol. 2012;86:3528–3540. doi: 10.1128/JVI.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.G., Wang Y.Y., Han K.J., Li L.Y., Zhai Z., Shu H.B. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zeng W., Sun L., Jiang X., Chen X., Hou F., Adhikari A., Xu M., Chen Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Xu M., Liu S., Sun L., Chen Z.J. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol. Cell. 2009;36:315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Yang Y., Li S., Wang Y.Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., Shu H.B. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zhou H., Perlman S. Mouse hepatitis virus does not induce Beta interferon synthesis and does not inhibit its induction by double-stranded RNA. J. Virol. 2007;81:568–574. doi: 10.1128/JVI.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


