Summary
The National Taiwan University Hospital (NTUH) adopted international guidelines for surveillance and control of healthcare-associated infection (HCAI) in 1981. This report describes the secular trends in HCAI at the NTUH over the past 27 years according to site of infection, aetiological agents and control measures. Clinical and microbiological data were collected by infection prevention and control nurses using a standardised case-record form. Specific control programmes were implemented and/or intensified as needed. Poisson or negative binomial regression analysis was used to quantify time trends of the incidence of HCAI. The annual number of discharges increased from 25 074 to 91 234 with a parallel increase in the Charlson comorbidity index. Active HCAI surveillance and periodic feedback were associated with a marked decrease in surgical site infections from 1981 to 2007 (2.5 vs 0.5 episodes per 100 procedures, P < 0.0001). On the other hand, there was a 4.8-fold increase in bloodstream infections (BSIs) (0.39 vs 1.88 episodes per 100 discharges, P < 0.0001). The average annual increase of pathogen-specific HCAI incidence during 1981–2007 was 11.4% for meticillin-resistant Staphylococcus aureus (MRSA), 75.4% for extensively drug-resistant A. baumannii (XDRAB), and 7.5% for Candida albicans (P < 0.0001, respectively). The infection prevention and control programme was upgraded in 2004 by implementing annual, intensive, project-based control programmes, and decreases in rates of HCAI, BSI, MRSA and XDRAB were observed. This long term study demonstrates the need to couple surveillance of HCAI with focused control programmes. Hospitals must invest in adequate manpower to accomplish these goals.
Keywords: Healthcare-associated infection, Infection control, Surveillance
Introduction
Ongoing surveillance of healthcare-associated infection (HCAI) is an essential component of hospital infection control programmes. The goals are to assess the burden of infectious diseases, identify important problems, monitor the efficacy of specific interventions and support rational hospital policies.1 The National Nosocomial Infections Surveillance System was established in the 1980s. HCAIs were reported to be significantly decreased in hospitals that adopted surveillance programmes.2, 3 Hospital-wide surveillance programmes are highly labour intensive and tend to divert resources needed to implement control measures and prevention activities. Consequently, they are often of minimal interest to hospital policymakers except during major outbreaks such as severe acute respiratory syndrome (SARS). It has become increasingly apparent that hospital support for surveillance programmes needs to be justified by improved outcomes. To accomplish these goals they must be closely linked to effective interventional strategies.
The first infection prevention and control hospital-wide HCAI surveillance programme in Taiwan, based on international guidelines, was established at the National Taiwan University Hospital (NTUH) in 1981. A vigorous hospital-wide hand hygiene programme was initiated in 2004. This report describes the secular trends in HCAI at the NTUH during the past 27 years (1981–2007) according to site of infection, aetiological agents and control measures.
Methods
Hospital setting
The NTUH is a 2200-bedded teaching hospital located in Taipei, Taiwan. It provides both primary and tertiary medical care, and served approximately 2000 inpatients, 7000 outpatients, and 300 emergency department visits daily in 2007. There are 220 intensive care unit (ICU) beds, 1866 beds in other acute care units, and 160 beds in a psychiatric day care unit. Among the acute care units are the following beds: 700 on medical wards, 800 on surgical wards, 200 on paediatric wards, 130 on gynaecology wards, and 100 on oncology wards. There are 68 negative pressure isolation rooms.
Infection prevention and control programme
The NTUH Infection Control Team currently consists of 10 full-time infection control nurses (ICNs), two full-time technicians and is supervised by two infection disease physicians. Prospective, hospital-wide on-site surveillance of HCAI was conducted by weekly visits of ICNs to all patient units. Hospital charts were reviewed to identify patients with HCAIs according to definitions of the Centers for Disease Control and Prevention with the help of infectious disease physicians at weekly meetings.4, 5, 6 The data were collected on standardised data collection forms (Appendix 1) and incorporated into the computer database by manual entry. The unit-specific incidences of HCAI (episodes per 100 discharges) including overall and site-specific infection rates were analysed monthly and compared with historical data. Feedback was provided to each service to stimulate intervention measures.
Infection prevention and control events and interventions
Multiple special infection prevention and control events and interventions occurred during the study period. Prevention and control measures for surgical site infection (SSI) included intensified surveillance of SSI with feedback to the surgeons during 1988–1990, monitoring the timing and choice of prophylactic antibiotics, reinforcement of aseptic procedures, amelioration and monitoring ventilation in the operation rooms since 1991, and enhanced environmental cleaning in 1992. Organism-specific isolation precautions for meticilllin-resistant Staphylococcus aureus (MRSA) were initiated in 1990 and this was augmented in 1993 for extensively drug-resistant Acinetobacter baumannii (XDRAB) in 2001 and SARS-specific precautions during March to June 2003.7, 8, 9 The infection control guideline for XDRAB requiring contact isolation (single room cohort) was introduced in 2001. This was reinforced the following year by requiring ICNs to inform staff to isolate patients with XDRAB. Hospital-wide surveillance of HCAIs was interrupted temporarily during the SARS epidemic because the ICNs were fully occupied with containing SARS. The infection prevention and control programme was upgraded in 2004 by implementing annual, intensive, project-based control programmes. The hospital-wide hand hygiene programme was instituted in April 2004 and has continued as an annual campaign. Targeted surveillance and control for device-associated infections in ICUs (e.g. urinary catheters, central venous catheters, and ventilators) and SSI for selected surgical procedures were initiated in 2000 and intensified since 2005. Data were analysed and compared with national and international data and feedback every quarter.
Microbiological studies
Culture data were obtained from computer-generated reports issued by the hospital clinical microbiology laboratory.10 The in vitro susceptibility of Acinetobacter isolates was determined by disc diffusion method.8, 10 The following antibacterial agents were tested: cephalosporins (ceftazidime and cefepime), extended-spectrum penicillins (piperacillin-tazobactam), carbapenems (imipenem), aminoglycosides (gentamicin and amikacin), fluoroquinolones (ciprofloxacin), sulbactam and colistin. In vitro susceptibility testing of colistin was begun in July 2006. XDRAB was defined as A. baumannii isolates that were resistant to five or more classes of antibacterial agents.
Statistical analysis
Poisson regression was used to model the secular trends of the annual incidence (episodes per 100 discharges) of HCAIs during 1981–2007.11 The Poisson regression model form is written as
where Y is the number of HCAI cases at time t, PY is the number of patient-years discharged at time t, β0 represents the baseline level of HCAI rate (HCAI rate in 1981), β1 represents the annual change of HCAI, and ‘time’ indicates the linear trend of HCAI, denoted as time in year from 1981. Estimated average annual changes during 1981–2007 were calculated by . The negative binomial regression was applied while the number of HCAI cases was overdispersed, i.e. the variance was greater than the mean.12 P < 0.05 was considered to be significant. Statistical analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC, USA).
Results
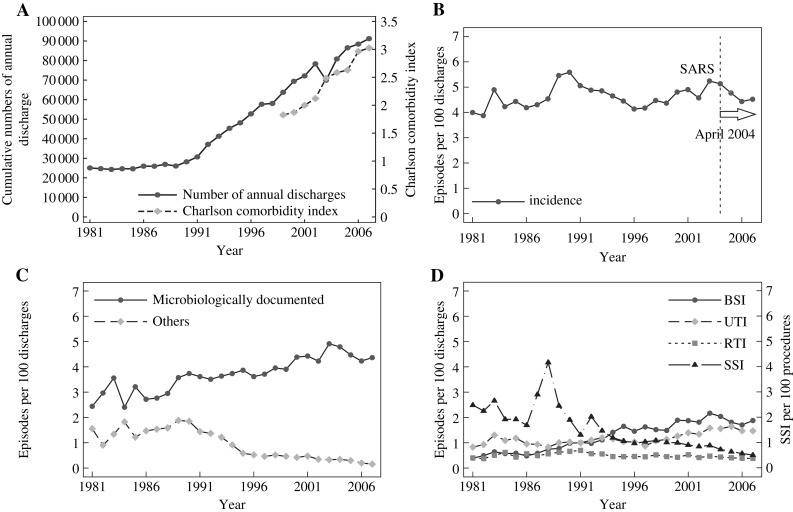
The annual number of discharges and the severity of underlying diseases on admission, as defined by Charlson comorbidity index, are shown in Figure 1 A.13 The number of acute care beds was 1846 in 1999 and 2212 in 2007. The number of discharges was 25 074 in 1981 and 91 234 in 2007. The average length of patient stay decreased from 11.3 in 1993 to 7.3 days in 2007. This was accompanied by an increasing proportion of patients admitted with multiple underlying conditions.
Figure 1.
(A) Trends in annual discharges and Charlson comorbidity index of patients hospitalised at the National Taiwan University Hospital from 1981 to 2007 (data of Charlson comorbidity index are available from 1999 onwards). (B) The hospital-wide annual incidence (per 100 discharges) of healthcare-associated infections during 1981–2007. Dashed line/arrow: Reorganisation of infection control team, increase in manpower and hospital-wide hand hygiene programme. SARS, severe acute respiratory syndrome. (C) Time trends of the incidences of microbiologically documented and other infections. (D) Hospital-wide annual incidence (per 100 discharges) of healthcare-associated infections by site during 1981–2007. BSI, bloodstream infection; RTI, respiratory tract infection; SSI, surgical site infection; UTI, urinary tract infection.
Secular trends in annual rates of overall infections
The annual infection rates increased slowly (average 0.3% per year, range −0.1% to 0.7%, P = 0.16) during 1981–2007 and fluctuated around 4.5 episodes per 100 discharges (Figure 1B). The major exception was an abrupt increase during 1989–1990 in HCAI with microbiological documentation and those cases without (Figure 1C). This was attributed to more intensive surveillance following adoption of the 1988 Centers for Disease Control (CDC, Atlanta, GA, USA) criteria.6
Secular trends in the distribution of infections according to site
The major sites of HCAIs over the entire study period are shown in Figure 1D. The average annual change (95% confidence interval) for bloodstream infection (BSI) was 6.4% (5.5% to 7.4%, P < 0.0001); urinary tract infection (UTI), 1.9% (1.2% to 2.5%, P < 0.0001); respiratory tract infection (RTI), −1.0% (−1.7% to −0.3%, P = 0.0083); and SSI, −6.1% (−7.2% to −5.0%, P < 0.0001). The differences in the incidence and proportion of the major sites contributing to HCAIs in 1981 and 2007 are shown in Table I . SSI was the leading cause of HCAI during 1981–1993. A peak in SSI was noted during 1988 to 1990. This was attributed to more intensive surveillance but without post-discharge surveillance (PDS). The incidence of SSI decreased sharply after intensified infection control programmes. BSI gradually increased over the study period. There was a 4.8-fold rise in BSI from 1981 to 2007 (P < 0.0001). BSI was the leading HCAI site during 1994–2007, accounting for 41.6% of HCAI in 2007. The incidence of UTI increased 1.8-fold (P < 0.0001).
Table I.
Incidence of healthcare-associated infections and the proportion by site and pathogen at the National Taiwan University Hospital, 1981–2007
| Parameters | 1981 | 2007 | Average annual change, % (95% CI) | P-valuea |
|---|---|---|---|---|
| Total no. of discharges | 25 074 | 91 234 | ||
| Total no. of infections | 1002 | 4125 | ||
| Total no. of pathogens | 1181 | 5081 | ||
| Overall incidence, per 100 discharges | 4.00 | 4.52 | 0.3 (−0.1 to 0.7) | 0.1621 |
| Site-specific infection | ||||
| Surgical site | ||||
| No. of episodes | 374 | 279 | ||
| Incidence, per 100 procedures | 2.5 | 0.5 | −6.1 (−7.2 to −5.0) | <0.0001 |
| Proportion (%) | 37.3 | 6.8 | ||
| Urinary tract | ||||
| No. of episodes | 208 | 1340 | ||
| Incidence, per 100 discharges | 0.83 | 1.47 | 1.9 (1.2 to 2.5) | <0.0001 |
| Proportion (%) | 20.8 | 32.5 | ||
| Respiratory | ||||
| No. of episodes | 100 | 347 | ||
| Incidence, per 100 discharges | 0.40 | 0.38 | −1.0 (−1.7 to −0.3) | 0.0083 |
| Proportion (%) | 10.0 | 8.4 | ||
| Bloodstream | ||||
| No. of episodes | 98 | 1714 | ||
| Incidence, per 100 discharges | 0.39 | 1.88 | 6.4 (5.5 to 7.4) | <0.0001 |
| Proportion (%) | 9.8 | 41.6 | ||
| Others | ||||
| No. of episodes | 222 | 445 | ||
| Incidence, per 100 discharges | 0.89 | 0.49 | −3.6 (−5.1 to −2.1) | <0.0001 |
| Proportion (%) | 22.2 | 10.8 | ||
| Pathogens | ||||
| Gram-positive aerobic bacteria | ||||
| Incidence, per 100 discharges | 0.91 | 1.25 | 1.1 (0.5 to 1.7) | 0.0006 |
| Proportion (%) | 21.8 | 24.6 | ||
| Gram-negative aerobic bacteria | ||||
| Incidence, per 100 discharges | 2.01 | 2.63 | 0.8 (0.3 to 1.2) | 0.0018 |
| Proportion (%) | 65.5 | 57.5 | ||
| Anaerobic bacteria | ||||
| Incidence, per 100 discharges | 0.34 | 0.16 | −4.9 (−6.3 to −3.6) | <0.0001 |
| Proportion (%) | 10.5 | 3.1 | ||
| Fungi | ||||
| Incidence, per 100 discharges | 0.09 | 0.79 | 7.0 (5.5 to 8.5) | <0.0001 |
| Proportion (%) | 1.9 | 14.6 | ||
CI, confidence interval.
Negative binomial regression was used to model the secular trends of the annual incidence.
Changes in the distribution of pathogens
The proportions of infections by pathogens in 1981 and 2007 are shown in Table I. Gram-negative bacteria were about twice as common as Gram-positives during both time periods. There was an 8.7-fold increase in fungal infections (mainly Candida spp.) from 1981 to 2007 (P < 0.0001). Rate of C. difficile infection increased from 0.004 episodes per 100 discharges in 1983 to 0.037 in 2007 (P = 0.008).
Secular trends in the major pathogens
Annual percentage changes in the incidences of pathogens causing all HCAI during 1981–2007 are shown in Table II . The average annual increase of pathogen-specific HCAI incidence during 1981–2007 was 11.4% for MRSA, 7.5% for C. albicans, and 75.4% for XDRAB (P < 0.0001, respectively).
Table II.
Time trends of pathogen-specific healthcare-associated infections at the National Taiwan University Hospital, 1981–2007
| Pathogen | Incidence (per 100 discharges) |
Average annual change, % (95% CI) | P-valuea | |
|---|---|---|---|---|
| 1981 | 2007 | |||
| Staphylococcus aureus | 0.199 | 0.404 | 3.1 (1.9 to 4.3) | <0.0001 |
| Meticillin-resistant | 0.028 | 0.272 | 11.4 (8.3 to 14.6) | <0.0001 |
| Meticillin-susceptible | 0.164 | 0.132 | −2.8 (−3.9 to −1.7) | <0.0001 |
| Enterococcus spp. | 0.004 | 0.452 | 0.2 (−1.8 to 2.2) | 0.8463 |
| Escherichia coli | 0.634 | 0.650 | −0.2 (−1.3 to 0.9) | 0.719 |
| Klebsiella spp. | 0.447 | 0.552 | 0.5 (−0.6 to 1.5) | 0.3683 |
| Enterobacter spp. | 0.299 | 0.356 | 0.0 (−0.6 to 0.6) | 0.9234 |
| Pseudomonas aeruginosa | 0.538 | 0.516 | −1.7 (−2.4 to −1.0) | <0.0001 |
| Acinetobacter spp. | 0.227 | 0.393 | 2.4 (1.4 to 3.4) | <0.0001 |
| Extensively drug-resistant | 0.000 | 0.105 | 75.4 (46.9 to 109.3) | <0.0001 |
| Candida albicans | 0.016 | 0.272 | 7.5 (5.5 to 9.6) | <0.0001 |
CI, confidence interval.
Negative binomial regression was used to model the secular trends of the annual incidence.
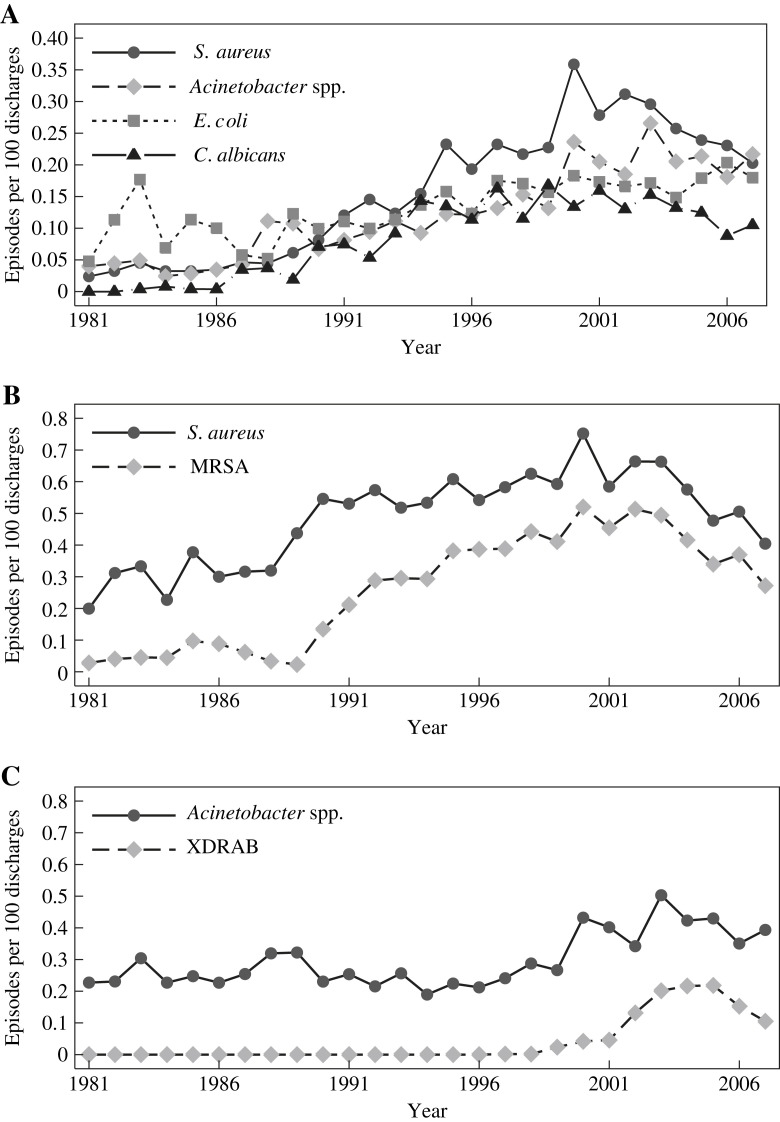
The incidences for all BSI pathogens increased significantly (Table III ). The secular trends of pathogen-specific BSI rates closely mirrored the overall rates of HCAIs, except for anaerobes. The secular trends of the major pathogens causing BSI are shown in Figure 2 A. Fungi (mainly Candida spp.) were rare in the 1980s and increased rapidly in later years. Note the decrease in BSI caused by S. aureus, Acinetobacter spp., and C. albicans, but not Escherichia coli after the implementation of a hand hygiene programme.
Table III.
Time trends of pathogen-specific healthcare-associated bloodstream infections at the National Taiwan University Hospital during 1981 and 2007
| Pathogen | Incidence (per 100 discharges) |
Average annual change, % (95% CI) | P-valuea | |
|---|---|---|---|---|
| 1981 | 2007 | |||
| Gram-positive aerobic bacteria | 0.092 | 0.617 | 8.4 (7.0 to 9.8) | <0.0001 |
| Staphylococcus aureus | 0.024 | 0.203 | 10.3 (8.1 to 12.5) | <0.0001 |
| Meticillin-resistant | 0.000 | 0.138 | 23.4 (16.9 to 30.2) | <0.0001 |
| Meticillin-susceptible | 0.020 | 0.066 | 3.0 (1.2 to 4.8) | 0.0009 |
| Enterococcus spp. | 0.000 | 0.180 | 7.85 (6.3 to 9.3) | <0.0001 |
| Gram-negative aerobic bacteria | 0.291 | 1.137 | 5.4 (4.7 to 6.1) | <0.0001 |
| Escherichia coli | 0.048 | 0.180 | 3.3 (2.3 to 4.3) | <0.0001 |
| Klebsiella spp. | 0.076 | 0.246 | 5.8 (4.6 to 7.0) | <0.0001 |
| Enterobacter spp. | 0.048 | 0.166 | 5.2 (3.7 to 6.8) | <0.0001 |
| Pseudomonas aeruginosa | 0.016 | 0.153 | 4.0 (2.9 to 5.0) | <0.0001 |
| Acinetobacter spp. | 0.040 | 0.217 | 7.7 (6.3 to 9.1) | <0.0001 |
| Extensively drug-resistant | 0.000 | 0.045 | 62.1 (37.7 to 90.8) | <0.0001 |
| Anaerobic bacteria | 0.004 | 0.052 | 2.3 (1.0 to 3.6) | 0.0005b |
| Fungi | 0.008 | 0.213 | 15.0 (9.9 to 20.4) | <0.0001 |
| Candida albicans | 0.000 | 0.105 | 13.5 (8.7 to 18.4) | <0.0001 |
Negative binomial regression.
Poisson regression.
Figure 2.
(A) Annual incidence (per 100 discharges) of the major pathogens causing healthcare-associated bloodstream infections during 1981–2007. (B) Annual incidence (per 100 discharges) of healthcare-associated infections due to Staphylococcus aureus and meticillin-resistant S. aureus (MRSA) during 1981–2007. (C) Annual incidence (per 100 discharges) of healthcare-associated infections due to Acinetobacter spp. and extensively resistant Acinetobacter baumannii during 1981–2007. XDRAB, extensively drug-resistant A. baumannii.
Secular trends for S. aureus infections
The secular trends of the annual incidence of S. aureus and MRSA are shown in Figure 2B. S. aureus steadily increased over the study period, peaked in 2001 and fell thereafter (year 2003 vs 2007; P < 0.0001). The incidence of MRSA paralleled that of all S. aureus infections and accounted for 69.2% of S. aureus infections at their peak in 2000.
Secular trends for Acinetobacter infections
The secular trends of the annual incidence of Acinetobacter and XDRAB are shown in Figure 2C. XDRAB began to appear in 1999, increased rapidly and peaked in 2005. XDRAB markedly decreased in association with the hand hygiene programme and possibly with other interventions between 2003 and 2007 (P < 0.0001).
Changes in the distribution of the five most common pathogens
There were modest place changes of the leading pathogens causing any HCAI, BSI and UTI during five-year intervals from 1981 to 2007. The leading pathogen causing BSI was Klebsiella spp. (18.6%) in 1981, E. coli (20.9%) in 1986, Candida spp. and other yeasts (18.6%) in 1996, S. aureus (12.7%) in 2001, and Klebsiella spp. (11.0%) in 2007. Gram-negative bacilli accounted for 60% of healthcare-associated BSI in 2007. Among the leading five pathogens causing UTI, E. coli was the first or second most common pathogen followed by C. albicans and other yeasts, Enterococcus spp. Enterobacter spp., Pseudomonas aeruginosa and Klebsiella spp.
Discussion
This report describes the secular trends of HCAIs over the course of a 27 year prospective hospital-wide surveillance programme in a large university hospital in Taiwan. The major findings are similar to those in tertiary care hospitals in western countries.3, 14, 15, 16 There was a gradual increase in admissions of patients with severe underlying disease. The overall rate of the HCAI increased slowly and has fluctuated at approximately 4.5 episodes per 100 discharges. This is in the high range reported by other tertiary care facilities.17, 18, 19, 20 The time trend of overall infection rates tended to obscure the substantial changes in SSI rates, a 4.8-fold increase in BSI, an increase in UTI, the appearance of XDRAB and MRSA, and an 8.7-fold increase in fungal infections.
The remarkable decrease in SSI may result from improvements in surgical prophylaxis, active surveillance and periodic feedback, and changes in surgical practice including the increasing shift to ‘day surgery’ which led to decreased detection of SSIs in a system which depended on ICNs going through inpatient charts. However, there was no PDS apart from those patients readmitted for postoperative complications. Changes in HCAIs may be due to multiple factors (e.g. implementation of targeted surveillance, SARS outbreak, multidrug-resistant organism outbreaks, active surveillance, etc.), and the rates of HCAI may fluctuate over time. We instituted several focused control programmes to deal with the increasing occurrence of MRSA and XDRAB. These consisted of more intense surveillance, strict isolation procedures for patients with XDRAB and a hand hygiene programme to control multidrug-resistant organisms.
A notable decrease was observed in the overall HCAI rates since 2004 when a hospital-wide hand hygiene programme was launched. However, due to a limited postintervention period, it is not possible to attribute the decrease to the hand hygiene programme. Therefore further analysis is required by expanding the follow-up periods which might make the formal statistical inference possible.
Regarding pathogens causing healthcare-associated BSI, a multicentre study in the USA [Surveillance and Control of Pathogens of Epidemiological importance (SCOPE)] from 1995 to 2002 showed that Gram-positive organisms accounted for 65% of cases and the leading pathogens were coagulase-negative staphylococci, S. aureus, and enterococci.21 Furthermore, the proportion of healthcare-associated BSI due to Gram-positive cocci increased gradually.21, 22, 23 Most of the studies showed coagulase-negative staphylococci were the leading cause of healthcare-associated BSI.21, 24, 25, 26, 27, 28 However, in this study, Gram-negative bacilli accounted for 60% of healthcare-associated BSI in 2007, and the leading pathogen was Klebsiella spp., which is very unusual in Europe and North America. Therefore when considering HCAIs, regional factors should be considered. Despite these achievements we have not as yet been able to achieve our goal to reduce overall infection rates below 4.5%. It may be impossible to reduce the rate in a structurally and immunocompromised patient population that requires aggressive antimicrobial therapy for endogenous infections. A third of patients had one or more malignancies. This hospital also maintains very active organ and bone marrow transplantation programmes and ICUs. Rates of infection are more meaningful when adjusted for major underlying diseases and disease severity, such as the Charlson comorbidity index, the McCabe–Jackson criteria and Acute Physiological Assessment and Chronic Health Evaluation (APACHE) score.13, 29, 30 It is apparent from these observations that there needs to be a major shift in hospital infection prevention and control measures from passive surveillance to focused control programmes of proven efficacy.5 The CDC 12-step campaign to prevent antimicrobial resistance in healthcare settings provides a good start. Some of the key measures optimise conditions that prevent infections, e.g. removing an unnecessary device, minimising broad spectrum antibiotics, avoiding long term antimicrobial prophylaxis, treating infection rather than colonisation, and preventing transmission. Our major successes were achieved when we adopted some of these measures.
Time-trend analysis performed in this study can be considered a screening tool to decide whether a more intensive investigation into underlying causes is justified. Also regression analysis allows us to state the annual changes in HCAI rates. However, there are limitations to this study from the methodological viewpoint. This study has demonstrated that changes in HCAIs were due to multiple factors which occurred in different time periods. Thus, the underlying trend might not be continuously increasing or decreasing. Also, the time trends might not be the same for overall HCAI rate, site-specific or pathogen-specific HCAI rates. The model used in our study to quantify the linear trend could not account for other variations such as possible serial correlation. However, a longer time period of one year was used in our analysis, to minimise the correlations between adjacent periods.12
In conclusion, this long term prospective hospital-wide surveillance study indicates what needs to be addressed to prevent and control HCAI. There needs to be a shift in the paradigm from surveillance with feedback to implementation of proven, control measures using focused surveillance to monitor efficacy. This will require a greater investment on the part of hospitals, if we want to reduce HCAI to the minimum and thus improve the quality and safety of patient care.
Acknowledgements
We are grateful to members of the Center for Infection Control for the contribution of the hospital-wide healthcare-associated infection surveillance programme and all the staff in the hospital for their commitment to improving patient safety and reducing healthcare-associated infection. We are also grateful to members of the Biostatistics Laboratory, College of Public Health, National Taiwan University Taipei for contributing to the statistical analysis. The authors are indebted to Prof. C. Kunin and Prof. W.-C. Hsieh for their critical reviewing and comments.
Footnotes
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.jhin.2010.05.001.
Conflict of interest statement
None declared.
Funding source
Y.-C. Chen received grants from the Center for Disease Control, Department of Health, Taiwan (DOH96-DC-1010, DOH97-DC-1005).
Appendix. Supplementary data
References
- 1.McKibben L., Horan T.C., Tokars J.I. Guidance on public reporting of healthcare-associated infections: recommendations of the Healthcare Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 2005;26:580–587. doi: 10.1086/502585. [DOI] [PubMed] [Google Scholar]
- 2.Haley R.W., Culver D.H., White J.W. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 3.Schwab F., Geffers C., Barwolff S., Ruden H., Gastmeier P. Reducing neonatal nosocomial bloodstream infections through participation in a national surveillance system. J Hosp Infect. 2007;65:319–325. doi: 10.1016/j.jhin.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control . US Department of Health, Education and Welfare, Public Health Service; Washington, DC: 1972. Outline for surveillance and control of nosocomial infections, revised. [Google Scholar]
- 5.Centers for Disease Control . Centers for Disease Control; Atlanta: 1982. National Nosocomial Infections Study: instruction manual, revised. [Google Scholar]
- 6.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections. Am J Infect Control. 1988;1988(16):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.T., Chang S.C., Ko W.J. A hospital-acquired outbreak of methicillin-resistant Staphylococcus aureus infection initiated by a surgeon carrier. J Hosp Infect. 2001;47:104–109. doi: 10.1053/jhin.2000.0878. [DOI] [PubMed] [Google Scholar]
- 8.Chan P.C., Huang L.M., Lin H.C. Control of an outbreak of pandrug-resistant Acinetobacter baumannii colonization and infection in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2007;28:423–429. doi: 10.1086/513120. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.C., Chen M.F., Liu S.Z., Romeis J.C., Lee Y.T. SARS in teaching hospital, Taiwan. Emerg Infect Dis. 2004;10:1886–1887. doi: 10.3201/eid1010.040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsueh P.R., Chen M.L., Sun C.C. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in Taiwan, 1981–1999. Emerg Infect Dis. 2002;8:63–68. doi: 10.3201/eid0801.000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnbaum D. Nosocomial infection surveillance programs. Infect Control. 1987;8:474–479. doi: 10.1017/s0195941700069800. [DOI] [PubMed] [Google Scholar]
- 12.Ely J.W., Dawson J.D., Lemke J.H., Rosenberg J. An introduction to time-trend analysis. Infect Control Hosp Epidemiol. 1997;18:267–274. doi: 10.1086/647609. [DOI] [PubMed] [Google Scholar]
- 13.D’Hoore W., Sicotte C., Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32:382–387. [PubMed] [Google Scholar]
- 14.Altman L.K. Experts see need to control antibiotics and hospital infection. New York Times. 12 March 1998 [Google Scholar]
- 15.Haley R.W., Culver D.H., White J.W., Morgan W.M., Emori T.G. The nationwide nosocomial infection rate. A new need for vital statistics. Am J Epidemiol. 1985;121:159–167. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- 16.Ebnother C., Tanner B., Schmid F., La Rocca V., Heinzer I., Bregenzer T. Impact of an infection control program on the prevalence of nosocomial infections at a tertiary care center in Switzerland. Infect Control Hosp Epidemiol. 2008;29:38–43. doi: 10.1086/524330. [DOI] [PubMed] [Google Scholar]
- 17.Erdinc F.S., Yetkin M.A., Ataman Hatipoglu C. Five-year surveillance of nosocomial infections in Ankara Training and Research Hospital. J Hosp Infect. 2006;64:391–396. doi: 10.1016/j.jhin.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Berthelot P., Garnier M., Fascia P. Conversion of prevalence survey data on nosocomial infections to incidence estimates: a simplified tool for surveillance? Infect Control Hosp Epidemiol. 2007;28:633–636. doi: 10.1086/513536. [DOI] [PubMed] [Google Scholar]
- 19.Botterel F., Faibis F., Chevalier C. Advantages and limits of the surveillance of nosocomial infections from the microbiology laboratory: experience of Meaux hospital. Pathol Biol (Paris) 2004;52:469–473. doi: 10.1016/j.patbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Al-Helali N.S., Al-Asmary S.M., Abdel-Fattah M.M., Al-Jabban T.M., Al-Bamri A.L. Epidemiologic study of nosocomial urinary tract infections in Saudi military hospitals. Infect Control Hosp Epidemiol. 2004;25:1004–1007. doi: 10.1086/502336. [DOI] [PubMed] [Google Scholar]
- 21.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 22.Cockerill F.R., 3rd, Hughes J.G., Vetter E.A. Analysis of 281,797 consecutive blood cultures performed over an eight-year period: trends in microorganisms isolated and the value of anaerobic culture of blood. Clin Infect Dis. 1997;24:403–418. doi: 10.1093/clinids/24.3.403. [DOI] [PubMed] [Google Scholar]
- 23.Lark R.L., Chenoweth C., Saint S., Zemencuk J.K., Lipsky B.A., Plorde J.J. Four year prospective evaluation of nosocomial bacteremia: epidemiology, microbiology, and patient outcome. Diagn Microbiol Infect Dis. 2000;38:131–140. doi: 10.1016/s0732-8893(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 24.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986–April 1996, issued May 1996. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 25.Suljagic V., Cobeljic M., Jankovic S. Nosocomial bloodstream infections in ICU and non-ICU patients. Am J Infect Control. 2005;33:333–340. doi: 10.1016/j.ajic.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Lyytikainen O., Lumio J., Sarkkinen H., Kolho E., Kostiala A., Ruutu P. Nosocomial bloodstream infections in Finnish hospitals during 1999–2000. Clin Infect Dis. 2002;35:e14–e19. doi: 10.1086/340981. [DOI] [PubMed] [Google Scholar]
- 27.Douglas M.W., Lum G., Roy J., Fisher D.A., Anstey N.M., Currie B.J. Epidemiology of community-acquired and nosocomial bloodstream infections in tropical Australia: a 12-month prospective study. Trop Med Int Health. 2004;9:795–804. doi: 10.1111/j.1365-3156.2004.01269.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu C.J., Lee H.C., Lee N.Y. Predominance of Gram-negative bacilli and increasing antimicrobial resistance in nosocomial bloodstream infections at a university hospital in southern Taiwan, 1996–2003. J Microbiol Immunol Infect. 2006;39:135–143. [PubMed] [Google Scholar]
- 29.McCabe W.R., Jackson G.G. Gram-negative bacteremia. I. Etiology and ecology. Arch Intern Med. 1962;110:847–855. [Google Scholar]
- 30.Perl T.M., Dvorak L., Hwang T., Wenzel R.P. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338–345. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.