Abstract
The traffic control bundle consists of procedures designed to help prevent epidemic nosocomial infection. We retrospectively studied the serial infection control measures to determine factors most effective in preventing nosocomial infections of healthcare workers (HCWs) during the 2003 Taiwanese severe acute respiratory syndrome (SARS) epidemic. Fever screening stations, triage of fever patients, separating SARS patients from other patients, separation of entrances and passageways between patients and HCWs, and increasing hand-washing facilities all demonstrated a protective effect for HCWs (univariate analysis; P < 0.05). By multiple logistic regression: (i) checkpoint alcohol dispensers for glove-on hand rubbing between zones of risk, and (ii) fever screening at the fever screen station outside the emergency department, were the significant methods effectively minimising nosocomial SARS infection of HCWs (P < 0.05). The traffic control bundle should be implemented in future epidemics as a tool to achieve strict infection control measures.
Keywords: Nosocomial infection, Personal protective equipment, SARS (severe acute respiratory syndrome), SARS coronavirus (SARS-CoV), Taiwan, Traffic control bundle
Introduction
The severe acute respiratory syndrome (SARS) epidemic began in mainland China when the SARS coronavirus (SARS-CoV) may well have been transmitted from its animal host into humans.1 It probably spreads person to person by direct contact with body fluids and infectious droplets from coughing.1 SARS-CoV may survive in the environment for one to four days, and it may spread through ventilation systems.1 SARS outbreaks appeared in clusters, especially in hospitals where infected patients were treated, and these local epidemics spread internationally by air travel.1, 2, 3, 4, 5, 6
The 2003 SARS epidemic showed a typical pattern: a patient with SARS entering a hospital infects nearby patients, visitors, and healthcare workers (HCWs). Then SARS spread to the community.1, 2, 3, 4, 5, 6 Medical facilities responded with infection control measures to mitigate nosocomial infections.
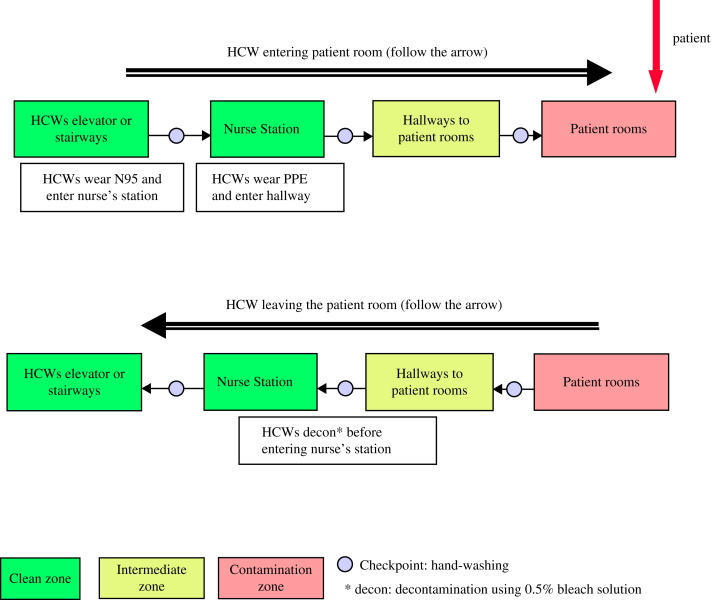
There were three main stages in Taiwan. Stage 1 began on 8 March 2003, when the first index case, who had recently returned from Guangdong, entered National Taiwan University Hospital. The patient was intubated and was admitted to the intensive care unit without a nosocomial transmission.7 In April 2003, a woman who had no history of traveling abroad entered Taipei Municipal Hoping Hospital (TMHH), leading to a nosocomial outbreak of SARS that eventually affected 113 patients and 37 HCWs.3, 5, 6 Suspecting nosocomial transmission, in Stage 2 officials quarantined TMHH on 24 April and on 26 April transferred most patients to a special isolation hospital converted from a military hospital.3, 5, 8 The special isolation hospital did not have negative pressure isolation rooms (NPIRs), but was equipped with modifications that provided a ‘negative-pressure like’ environment.5 In addition, the traffic control bundle (Figure 1 ) was implemented, comprising triage of patients before entering the hospital, division of the hospital into zones of risk, and requiring HCWs to use alcohol from dispensers for glove-on hand rubbing as they went between zones of risk.5 Meanwhile, the pattern of nosocomial superspread of SARS was repeating at other newly infected hospitals, and spread throughout the Taiwanese community.3 To test our hypothesis that the traffic control bundle significantly minimised nosocomial spread of SARS infection, we compared the rate of nosocomial SARS infection in HCWs at the special isolation hospital with that in 86 other Taiwanese hospitals that were not using the traffic control bundle.5 During three weeks (27 April to 21 May 2003) in the middle of the SARS epidemic, the special isolation hospital had only two SARS infections in HCWs (0.03 cases per bed), one of which was due to failure to follow precautions, whereas the comparison group had 43 suspected and 50 probable HCW SARS cases (0.13 cases per bed).5, 8
Figure 1.
Traffic control bundle procedures. Following triage outside the hospital entrance, patients who are possibly infected are directed (red arrow) into the contamination zone. Healthcare workers (HCWs) and patients are separated by zones of risk with decontamination and glove-on alcohol or hand-washing, or both, between zones of risk.5 PPE, personal protective equipment.
Following the success of the traffic control bundle at the special isolation hospital, in Stage 3 the Taiwan Center for Disease Control mandated nationwide use of the traffic control bundle at all Taiwanese hospitals after 21 May 2003. Within two weeks, the SARS epidemic was under control in Taiwan.3, 5, 8
Here we report a more comprehensive retrospective study that evaluates the impact of using the traffic control bundle throughout Taiwan during the 2003 Taiwanese SARS epidemic.
Methods
Participant hospitals
The study, conducted retrospectively in 2004, covered the period from 25 February 2003, when the first hospital in Taiwan was documented as having exposure to the first proven case of SARS, until 5 July 2003, after the end of the epidemic. Taiwanese hospitals from northern and southern epicentres of the SARS epidemic were recruited.3 The 50 participating hospitals were divided into a case group (N = 19) of hospitals with one or more HCWs with a nosocomial SARS infection, and a control group (N = 31) of hospitals with no such infections.
SARS definition
SARS infection was diagnosed when clinical signs and symptoms of SARS had either two positive results (either two consecutive tests or tests of clinical specimens from two different body sites) for SARS-CoV by polymerase chain reaction, or a fourfold increase in anti-SARS antibody in an enzyme-linked immunosorbent assay or indirect fluorescent antibody test. The infection was defined as nosocomial when contact tracing and epidemiological evidence linked it to acquisition in the hospital.
Control measures
Alcohol hand rubbing
Alcohol dispensers were placed between zones of risk, so that as HCWs moved between zones they could apply alcohol to their gloved hands, which they rubbed together for disinfection. The alcohol was 75% ethanol in water, with no detergent or other additives. SARS-CoV has been shown to be susceptible to this treatment.9
Triage
The first criterion of the triage process in the fever screening station was body temperature. Using 37.5 °C as the threshold, patients without an elevated temperature were admitted to the hospital through the regular procedure. Patients showing symptoms of SARS, such as fever, respiratory symptoms, or diarrhoea, went by a controlled route to an independent controlled area (NPIRs, isolation wards, or isolation hospitals). Febrile patients with no SARS symptoms were admitted into fever wards for treatment and observation.
Zones of risk
After triage, patients with symptoms of SARS went to an independent controlled area via the independent controlled route. Therefore, a patient would be inside an encapsulated area, also known as a zone of risk, from the fever screening station outside the ED until being hospitalised in an isolation ward. There were separate entrances, passageways, and elevators for patients and HCWs. General administration and general wards (the ‘clean zone’) were outside the zones of risk. When crossing over into a zone of risk from a clean zone, an individual had to change clothing and put on personal protective equipment (PPE) first in the clean zone. When an individual crossed over from a zone of risk into a clean zone, he/she had to undergo a decontamination process in the inter-zone. Some hospitals used wooden or acrylic boards to separate zones of risk. Other than barriers, some other hospitals designed zones of risk based on layout of the building. Hand-washing checkpoints were set between clean, intermediate, and contaminated zones (Figure 1). Design of the zones in each hospital was examined and approved by experts.
Some staff members were assigned to work in zones of risk, and worked in shifts. With zoning designs and decontamination measures in place, certain staff members could enter and exit the isolation areas (during consultations, etc.). All other staff members worked in the clean zones.
Data collection regarding prevention
Questionnaires, interviews, and on-site inspections were used to gather data from hospitals, HCWs, and infection control procedure documents. We recorded the countermeasures in place against SARS, the risk factors of exposure to SARS virus, the date of SARS patient admission, the date of first SARS signs and symptoms in HCWs, and the date when protective countermeasures against SARS were initiated. From the raw data, 27 ‘control measures’ against nosocomial infection were recognised (Table I ). We grouped these measures into the following categories: triage, division of the hospital into zones of risk, hand-washing facilities, NPIR design and PPE usage, and hospital administrative management.
Table I.
Univariate analysis of control measures for prevention of severe acute respiratory syndrome
| Control group (non-infected) | Case group (infected) | P | |
|---|---|---|---|
| (N = 31) | (N = 19) | ||
| A. Triage used in hospital | |||
| Triage for patients with fever of unknown origin in EDa | 28 (90.3%) | 9 (47.4%) | 0.001 |
| Set up fever ED stations outside of EDb | 23 (74.2%) | 2 (10.5%) | <0.001 |
| Body temperature screening in main entrancea | 31 (100%) | 11 (57.9%) | <0.001 |
| Body temperature screening for patientsa | 30 (96.8%) | 11 (57.9%) | 0.001 |
| Body temperature screening for HCWsa | 30 (96.8%) | 11 (57.9%) | 0.001 |
| B. Zones of risk | |||
| Separation of fever patients within physical barrier isolated region in EDb | 13 (41.9%) | 3 (15.8%) | NS |
| Moving patient into a special designated centralised isolation ward or evacuate patients within a general wardb | 27 (87.1%) | 4 (21.1%) | <0.001 |
| Separate elevators and routes for patients and HCWsb | 25 (80.6%) | 5 (26.3%) | <0.001 |
| Installation of physical barriers between zones of risk of isolation wardb | 19 (61.3%) | 2 (10.5%) | <0.001 |
| C. Hand-washing/disinfection | |||
| Installation of hand-washing station in EDb | 28 (90.3%) | 6 (31.6%) | <0.001 |
| Disinfectant solution available at main entrance (of hospital)a | 30 (96.8%) | 10 (52.6%) | <0.001 |
| Set up hand-washing facilities around whole hospitalb | 20 (64.5%) | 5 (26.3%) | 0.009 |
| Set up alcohol dispensers at checkpoints for glove-on hand rubbing between zones of riska | 30 (96.8%) | 5 (26.3%) | <0.001 |
| D. NPIR/PPE | |||
| Set up of standardised NPIR in hospitala | 19 (61.3%) | 4 (21.1%) | 0.006 |
| Set up of simplified NPIR within hospitalb | 21 (67.7%) | 8 (42.1%) | NS |
| Wearing N95 mask in EDa | 30 (96.8%) | 14 (73.7%) | NS |
| Wearing N95 mask within zones of riska | 31 (100%) | 12 (63.2%) | <0.001 |
| Mask worn when entering hospitala | 31 (100%) | 11 (57.9%) | <0.001 |
| Wearing surgical mask in OPDa | 30 (96.8%) | 14 (73.7%) | 0.015 |
| Wearing surgical mask in warda | 30 (96.8%) | 14 (73.7%) | 0.015 |
| Number (layer) of gowning in EDcd | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | NS |
| Number (layer) of gloves in EDcd | 2.0 (2.0–2.5) | 2.0 (1.0–2.0) | NS |
| E. Administration | |||
| Establishing crisis response teama | 31 (100%) | 11 (57.9%) | <0.001 |
| Exclude visitors from hospitalb | 25 (80.6%) | 6 (31.6%) | 0.001 |
| Support from administration for ICPb | 25 (83.3%) | 6 (31.6%) | <0.001 |
| Support from administration for IDa | 29 (96.7%) | 11 (57.9%) | 0.001 |
| Support from superintendent/directors for infection controla | 29 (96.7%) | 10 (52.6%) | <0.001 |
ED, emergency department; HCW, healthcare worker; NPIR, negative-pressure isolation room; PPE, personal protective equipment; OPD, outpatient department; ICP, infection control practitioner; ID, infectious diseases specialist or physician; NS, non-significant. Number (percentage) is shown for categorical variables and median (interquartile range) is shown for continuous variables.
Fisher’s exact test.
χ2-Test.
Mann–Whitney U-test.
Incomplete data.
The duration of risk for each HCW of exposure to SARS-CoV within the hospital was defined as being from the date the first SARS patient was admitted until two weeks after the last SARS patient was admitted. The date of contracting nosocomial SARS infection was defined as the date the HCW first presented with SARS signs and symptoms, minus seven days of incubation period.
For each control measure, the date the control measure was put into place in a hospital was compared with when the last HCW nosocomial SARS infection took place at that hospital (date of first SARS symptoms minus seven days). If the date of implementation of the control measure was after the last nosocomial SARS infection, the control measure was considered ‘effective’. Likewise, if any HCWs contracted a nosocomial SARS infection after the date of introduction of a given control measure at a given hospital, the control measure was an ‘ineffective control measure’.
Statistical analysis
Continuous variables are expressed as the median (interquartile range, IQR) due to their skewed distribution, and were tested by the Mann–Whitney U-test. Categorical variables are expressed as a number (percentage) and examined by the χ2-test. When more than 20% of cells had an expected value <5, the χ2-test was replaced with Fisher's exact test. Stepwise logistic regression analysis was performed to identify factors related to SARS prevention. All factors with P < 0.2 in the univariate analysis were left in the analysis to establish the stepwise logistic regression model. When a variable was highly correlated with others (correlation coefficient ≥0.6), the variable was dropped to construct the stepwise logistic regression model. Two-sided P < 0.05 was defined as statistically significant. We used statistical software SPSS 15.0 (SPSS Inc., Chicago, IL, USA) for the analysis.
Results
The control group consisted of 31 hospitals that had no nosocomial infections with SARS-CoV among HCWs; there were 149 SARS patients admitted to these hospitals during the study period. The case group consisted of 19 hospitals that had one or more nosocomial infections with SARS-CoV among HCWs; during the study period, 191 SARS patients were admitted to these hospitals, and HCWs had 115 nosocomial cases of SARS infections.
Table I summarises the results of univariate analysis of control measures for SARS prevention. The following factors were significantly associated (all P < 0.05) with effective prevention of nosocomial SARS infection: measures relating to triage used in hospital (e.g. body temperature screening; set up of an isolation room in the ED); most of the measures to identify the zones of risk (e.g. installation of physical barriers between zones of risk of isolation ward); installation of hand-washing station (e.g. set up alcohol dispensers at checkpoints for glove-on hand rubbing between zones of risk); use of NPIRs and wearing personal protective equipment (PPE) (e.g. set up standardised NPIR, wearing of an N95 or surgical mask); and support from administration (e.g. established crisis response team, support from superintendent/directors for infection control).
We then performed a multivariate analysis of control measures that were significantly associated in the univariate analysis with preventing nosocomial SARS infections among HCWs. Table II shows the two control measures that were significantly associated with protection against nosocomial infection: set up of fever stations outside of the ED; and alcohol dispensers for glove-on hand rubbing at checkpoints between zones of risk.
Table II.
Stepwise logistic regression model of severe acute respiratory syndrome prevention in 32 hospitalsa
| OR | 95% CI | P | |
|---|---|---|---|
| Set up fever screen station outside of ED | |||
| Ineffective | Reference | – | – |
| Effective | 0.051 | (0.004–0.692) | 0.025 |
| Set up alcohol dispensers at checkpoint for glove-on hand rubbing between zones of risk | |||
| Ineffective | Reference | – | – |
| Effective | 0.043 | (0.003–0.627) | 0.021 |
OR, odds ratio; CI, confidence interval; ED, emergency department.
Fifty hospitals were used to establish a stepwise multiple logistic regression model, and 32 were left after eliminations.
Discussion
The highly contagious SARS-CoV can spread through aerosol or contact transmission, giving HCWs a very high risk of acquiring SARS while caring for SARS patients. HCWs became the amplifier in the chain of transmission in the 2003 outbreak. Although PPE and NPIRs were implemented as recommended by health authorities, nosocomial SARS infections still occurred among HCWs. Given that PPE and NPIRs were available throughout Taiwan in the early phase of the Taiwanese SARS epidemic, we postulated that PPE and NPIRs do not effectively protect HCWs from becoming infected with SARS-CoV. The present study demonstrated that neither the frequency of change and double layers of gowns and gloves, nor use of NPIRs alone, were effective protection. Similar situations had been observed in Canada and Hong Kong during the second wave of SARS outbreaks.1, 10 We hypothesised that the traffic control bundle was responsible for almost completely eliminating nosocomial SARS infections among HCWs in Taiwan. The results of our multivariate analysis of control measures used during the Taiwanese SARS epidemic showed that outdoor fever triage and checkpoint glove-on alcohol disinfection were effective protection against nosocomial SARS transmission (Table II). Because checkpoints for glove-on alcohol disinfection were set between zones of risk, glove-on alcohol disinfection could be effective only after the zone of risk was delineated. Therefore, the three integrated components of the traffic control bundle were all effective. None of the other standard control measures, including NPIRs and PPE usage alone, was effective without implementing the traffic control bundle.
The traffic control bundle includes triage and diversion of the patient before entrance to the hospital, delineation of zones of risk between the contaminated zone and clean zone, and hand disinfection at checkpoints between zones of risk. The traffic control bundle is similar to a ‘traffic light system’, which separates zones of risk using wooden or acrylic boards with different colours. Another way to implement the traffic control bundle, used by some hospitals, is to design zones of risk based on layout of the building.
Since the ‘traffic control bundle’ SARS control system was a novel system, the Department of Health in Taiwan did not promote the system at first. Instead, the government conducted a trial in a special isolation hospital, which was converted from a military hospital in Taipei city. Following success of the traffic control bundle at the trial hospital, the Taiwan Center for Disease Control finally mandated nationwide use of the traffic control bundle at all Taiwanese hospitals after 21 May 2003. That was two-and-a-half months after the first case of SARS in Taiwan. Before that time, most of the hospitals used only traditional infectious disease control measures, such as setting up simplified NPIRs within a hospital or NPIR with anteroom design (Table I). Some of the hospitals did not even strictly require wearing a mask when entering the hospital or wearing a surgical mask in a ward. That could be the reason different institutions implemented different parts of the system at the early stage of the SARS epidemic.
During the Taiwanese SARS epidemic, fever screening was originally at the entrance of the hospital and ED, but later in an outdoor fever screening station due to the upsurge of fever patients coming to the hospital and crowding the ED. A report on the ED of the National Taiwan University Hospital demonstrated that when there was no outdoor fever screening and triage, all fever patients were managed and triaged within the ED of the hospital.7 Triage was also found to be a significant protective control measure in Singapore during the SARS epidemic.2 During the height of the epidemic, with crowded space, HCWs tended to spread the virus unintentionally despite being gowned and gloved, contaminating the environment.7
A minimum distance between beds of ≤1 m was found to be a risk factor for acquiring SARS.6 However, a different result was found in the study at the National Taiwan University Hospital where a distance <1 m from the SARS patient helped to protect HCWs from contracting symptoms of fever and diarrhoea.11 It is intriguing because <1 m is considered as a zone of risk in a hospital even without implementing the traffic control bundle, and is more cautious in terms of infection control. A distance >1 m from SARS beds would blur the zones of risk, and there would be more chance to contract SARS-CoV through casual contact with the contaminated environment. In that case, the traffic control bundle would be an effective tool to achieve strict infection control measures. The effect of bed spacing on spread of infectious disease in hospitals highlights the importance of the traffic control bundle.
It has been suggested that when HCWs worked in a given area without any designated zones of risk, the HCWs developed a false sense of security when they were working away from SARS patients.7 Likewise, being gloved and gowned also may have led to a false sense of security. Thus, HCWs may not follow the strict infection control procedures and accidentally spread and contract the virus through contact transmission if zones of risk have not been applied. In the present study, however, merely having the physical barrier between zones of risk showed no benefit in preventing nosocomial transmission. Instead, use of a glove-on hand disinfection checkpoint between zones of risk was the effective protective measure (Table II). Strategic installation of alcohol dispensers to enforce hand disinfection between zones of risk not only demonstrated to HCWs the significance of zones of risk, it also strengthened the adherence to and increased the frequency of hand-washing to 100%.
An infection control strategy analogous to glove-on hand-washing is double-gloving, in which the second pair of gloves is routinely exchanged. In Guangdong, double-gloving was found to be an effective control measure during the SARS epidemic.1 Our study found double-gloving in the ED to be an ineffective control measure in the univariate analysis (Table I). The discrepancy may be due to differences in the policy of when, where, and how the second pair of gloves was exchanged, because the process can be contaminated also.
Leadership has been an important factor during disaster crisis situations. The superintendent's support for infection control, as well as the presence of well-trained infection control professionals is expected to be a prerequisite to the success of infection control measures. Other studies of the SARS epidemic identified administrative support as a significant contributor to protection against nosocomial infection.6 We also found administrative support measures to be effective in the univariate analysis (Table I), but not in the multivariate analysis (Table II). Nevertheless, we recommend that administrators support implementation of the traffic control bundle.
Our study had several limitations. First, the study was limited because it did not measure variables related to severity of disease in the patients. Studies of the 2003 SARS outbreak identified the severity of illness of patients, whether patients received intubation, and whether or not HCWs saw patients in the ED, as significant factors increasing the chance of HCWs contracting SARS after patient contact.1, 4, 6, 12 Second, our study was also limited by not measuring compliance with alcohol hand rubbing between zones of risk. However, we believe compliance should have approached 100%, because this disinfection step was part of the standard operating procedures that were implemented and HCWs were on extremely high alert when passing through checkpoints. Finally, susceptibility of individual HCWs to SARS infection was not taken into consideration. At TMHH during the 2003 outbreak, certain HCWs were less likely to acquire SARS due to their human leucocyte antigen (HLA) genotype.13 Although such bias is specific only to SARS, potential genetic variations in the HCW sample biased our sample to an unknown degree.
Nosocomial infections are not only a key stage in creation of an epidemic but also compromise healthcare facilities by reducing the workforce during a time of great need. The control measures from the 2003 SARS epidemic are expected to be useful in a future influenza epidemic.14 The established efficacy, ease and speed of implementation, and low cost of the traffic control bundle make it an attractive option for application to emerging infectious diseases besides SARS.5 Screening for emerging epidemic infectious diseases ideally is performed in outdoor, isolated quarantine or triage stations. SARS can be triaged by fever screening because SARS-CoV is shed only by febrile patients. Screening for other emerging epidemic infectious diseases may require using criteria other than fever depending on the disease.
In conclusion, we have shown that elements of the traffic control bundle were the significant control measures protecting HCWs from nosocomial infection by SARS-CoV during the 2003 Taiwanese SARS epidemic. In response to future emerging infectious diseases, we recommend adoption of the traffic control bundle, or modified forms of it, as additional measures of non-pharmaceutical intervention.
Conflict of interest statement
None declared.
Funding source
Supported by Taiwan CDC Grant: DOH92-DC-SA01.
References
- 1.Chen W.Q., Ling W.H., Lu C.Y. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009;9:81. doi: 10.1186/1471-2458-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh K.T., Cutter J., Heng B.H. Epidemiology and control of SARS in Singapore. Ann Acad Med Singapore. 2006;35:301–316. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Severe acute respiratory syndrome – Taiwan, 2003. Morb Mortal Wkly Rep. 2003;52:461–466. [PubMed] [Google Scholar]
- 4.Nishiyama A., Wakasugi N., Kirikae T. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis. 2008;61:388–390. [PubMed] [Google Scholar]
- 5.Yen M.Y., Lin Y.E., Su I.J. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2006;62:195–199. doi: 10.1016/j.jhin.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu I.T., Xie Z.H., Tsoi K.K. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y.C., Huang L.M., Chan C.C. SARS in hospital emergency room. Emerg Infect Dis. 2004;10:782–788. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung C.P., Hsieh T.L., Tan K.H. Rapid creation of a temporary isolation ward for patients with severe acute respiratory syndrome in Taiwan. Infect Control Hosp Epidemol. 2004;25:1026–1032. doi: 10.1086/502339. [DOI] [PubMed] [Google Scholar]
- 9.Rabenau H.F., Kampf G., Cinatl J., Doerr H.W. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigayeva A., Green K., Raboud J.M. Factors associated with critical-care healthcare workers’ adherence to recommended barrier precautions during the Toronto severe acute respiratory syndrome outbreak. Infect Control Hosp Epidemiol. 2007;28:1275–1283. doi: 10.1086/521661. [DOI] [PubMed] [Google Scholar]
- 11.Wang S.H., Chen Y.C., Jiang D.D. Risk factors for development of fever and diarrhea among healthcare workers caring for SARS patients. Infect Control J. 2005;15:205–220. [in Chinese] [Google Scholar]
- 12.Wang F.D., Chen Y.Y., Lee Y.M. Positive rate of serum SARS-CoV immunoglobulin G antibody among healthcare workers. Scand J Infect Dis. 2007;39:152–156. doi: 10.1080/00365540600951226. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.M., Liang S.Y., Shih Y.P. Epidemiological and genetic correlates of severe acute respiratory syndrome coronavirus infection in the hospital with the highest nosocomial infection rate in Taiwan in 2003. J Clin Microbiol. 2006;44:359–365. doi: 10.1128/JCM.44.2.359-365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai D.Y. SARS: how to manage future outbreaks? Ann Acad Med Singapore. 2006;35:368–373. [PubMed] [Google Scholar]