Highlights
-
•
Viruses are known to exploit cellular signaling pathways.
-
•
MAPK is a major cell signaling pathway activated by diverse group of viruses.
-
•
MNK1 regulates both cap-dependent and IRES-mediated mRNA translation.
-
•
This review discuss the role of MAPK, particularly the role of MNK1 in virus replication.
Keywords: Signaling pathway, MAPK, MNK1, Virus replication, Antiviral drugs
Abstract
Viruses are obligate intracellular parasites; they heavily depend on the host cell machinery to effectively replicate and produce new progeny virus particles. Following viral infection, diverse cell signaling pathways are initiated by the cells, with the major goal of establishing an antiviral state. However, viruses have been shown to exploit cellular signaling pathways for their own effective replication. Genome-wide siRNA screens have also identified numerous host factors that either support (proviral) or inhibit (antiviral) virus replication. Some of the host factors might be dispensable for the host but may be critical for virus replication; therefore such cellular factors may serve as targets for development of antiviral therapeutics. Mitogen activated protein kinase (MAPK) is a major cell signaling pathway that is known to be activated by diverse group of viruses. MAPK interacting kinase 1 (MNK1) has been shown to regulate both cap-dependent and internal ribosomal entry sites (IRES)-mediated mRNA translation. In this review we have discuss the role of MAPK in virus replication, particularly the role of MNK1 in replication and translation of viral genome.
1. Introduction
Cell signaling is a part of a complex system of communication through which living cells interact with the neighbouring cells and extracellular environment (Denef, 2008). Cells are equipped with glycoproteins or glycolipid receptors on the plasma membrane through which they respond to changes in their immediate environment. When a complementary ligand (signaling molecule) binds to the receptor, it initiates a chain of events within the cell called signal transduction, ultimately resulting into a response. This ability of the cells to respond to their microenvironment solely determines growth, development, tissue injury/repair, cell homeostasis and immunity. A cell can communicate signals to neighbouring cells in multiple ways, which include: direct transfer of ions/small molecules through pores in the membrane, endocrine signaling that utilizes hormones, paracrine signaling by secreting chemicals into the common intercellular space, autocrine signaling to alter its own extracellular environment, juxtacrine signaling by making physical contact with adjacent cells and synaptic signaling (nervous system) (Brucher and Jamall, 2014).
Cell signaling starts with an external stimuli (signals); most cell signals are chemical in nature but mechanical stimuli are also possible (Cargnello and Roux, 2011). Cells employ numerous intracellular signaling pathways for transmitting information within the cell. However, a cross talk among these cell signaling pathways is quite possible (Vert and Chory, 2011). The signaling pathways may be activated in response to external stimuli or via the messengers (ligand/information/metabolic) that are generated within the cell following insults. The information in signaling pathways is conveyed either through protein-protein interactions or via diffusible elements usually referred to as second messengers (Cargnello and Roux, 2011).
2. MAPK signaling
Mitogen activated protein kinase (MAPK) is an important cell singling pathway that converts extracellular stimuli into a wide range of cellular responses (Cargnello and Roux, 2011). MAPK signaling is activated by a variety of chemical and physical stimuli such as cytokine, hormone, growth factors, pathogens (including viruses), osmotic stress, heat, oxidative stress and microtubule disorganization (Boulton et al., 1990; Raman et al., 2007; Rose et al., 2010; Widmann et al., 1999). MAPK pathway regulates gene expression, mitosis, cell survival, apoptosis, metabolism and cell differentiation (Roux and Blenis, 2004; Shaul and Seger, 2007). The information in MAPK pathway is transmitted via protein-protein interactions. By phosphorylating serine and threonine residues in diverse groups of proteins, MAPKs convert a variety of extracellular signals into a multitude of cellular responses (Ceballos-Olvera et al., 2010; Shi et al., 2013).
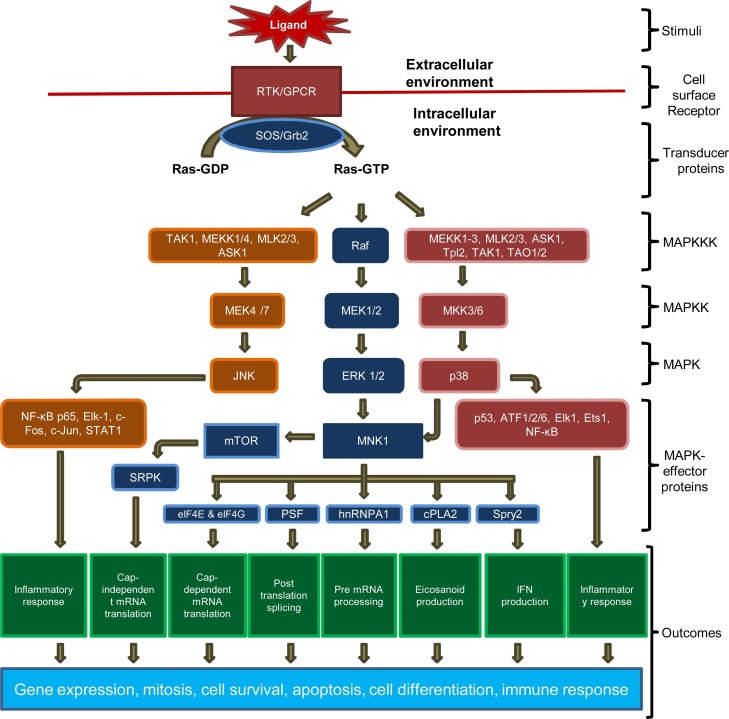
MAPK signaling toolkit is composed of a cell surface receptor that receives extracellular signals in the form of chemical or mechanical ligand, a transducer that converts extracellular stimuli into intracellular signals. MAPK module has three evolutionary conserved, sequentially acting proteins namely, MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK) and MAPK (Fig. 1 ). Their downstream targets include MAPK scaffolding protein and MAPK target protein (effectors protein) (Cargnello and Roux, 2011). To be enzymatically active, these proteins must undergo phosphorylation (Robbins et al., 1993).
Fig. 1.
MAPK/MNK signaling. MAPK/MNK signaling pathway is activated by binding of the ligands to their cognate receptors (RTKs/GPCRs). In RTKs, ligand binding induces dimerization and autophosphorylation at its Ser/Thr residues (intracytoplasmic domain). With the help of adaptor proteins- SOS/Grb2, activated RTKs convert Ras-GDP to Ras-GTP. In GPCRs, ligand binding induces structural changes in heterotrimeric subunits. These structural changes trigger generation of secondary messenger, which eventually converts Ras-GDP to Ras-GTP. Upon formation of Ras-GTP, further information in the pathway is conveyed by three sequentially acting evolutionary conserved proteins of MAPK-ERK family namely Raf (MAPKKKs), MEK1/2 (MAPKKs) and ERK1/2 (MAPK). Besides ERK signaling, Ras-GTP also activates those MAPKKKs, which are required for phosphorylation of MEK4/7 and MEK3/6 to trigger JNK and p38 pathway respectively. The ERK1/2 and p38 activation ultimately results in phosphorylation of MNK1. Activated MNK1 regulates functions of several potential downstream substrates. eIF4E and eIF4 G, which help in formation of 5′ cap-dependent mRNA translation initiation complex (eIF4 F). hnRNPA1 helps in pre-mRNA processing. PSF involves in post translational processing as well as translocation of mRNA. cPLA2, which regulates production of eicosanoids, a second messenger, plays an important role in immunity and inflammation. Spry2 is associated with interferon production and negative regulation of ERK/MAPK pathway. Besides, MNK1 also participates in regulation of cap-independent mRNA translation, which involves activation of SRPK, mediated via mTOR. By activating transcriptional factors such as c-Jun, c-Fos STAT1, ATF1/2/6, Elk1, Ets1, p53 and NF-κB, JNK and p38 induce inflammatory response.
Extracellualr signal-regulated kinase (ERK, also known as p42/44 MAPK), Janus kinase (JNK, also known as stress activated protein kinase-1, SAPK1) and p38 MAP kinase (also known as SAPK2/RK) are three major MAPK pathways in mammals (Gaur et al., 2011; Ludwig et al., 2006). ERK1 and ERK2 are key transducers of proliferation signals and are often activated by mitogens. JNKs and p38 poorly respond to mitogens but are strongly activated under cellular stress (Cargnello and Roux, 2011). Following activation, these cytosolic proteins (MAPK downstream molecules) either remain in cytoplasm or may translocate into the nucleus to activate numerous proteins and/or transcription factors (Cuadrado and Nebreda, 2010; Li et al., 2016) (Fig. 1). These regulatory molecules not only transmit information to the target effectors but also cross talk with other parallel signaling pathways. These mechanisms allow amplification of the signals and generate a threshold for activation of diverse pathways (Cargnello and Roux, 2011; Maroni et al., 2004).
In order to manipulate cellular functions in its favor, viruses interact with MAPK family members- ERK, p38 and JNK. MAPK pathway is activated by a wide variety of viruses (Table 1 ). Depending on the nature of virus, MAPK signaling may either support or down regulate virus replication (Rodriguez et al., 2014). Moreover, MAPK signaling activated by virus independent sources can also be misused by the viruses to facilitate their own replication. For instance, phorbol-12-myristate-13-acetate (PMA) and new born calf serum activate MAPK; arenaviruses use this enhanced activity to promote its own replication (Rodriguez et al., 2014). Such widespread exploitation of MAPK pathway by viruses suggests that it may be used as a target to develop broad-spectrum antiviral drugs.
Table 1.
Role of MAPK signaling in virus replication.
| Signaling pathway | Cellular mediator | Impact on virus replication | References |
|---|---|---|---|
| RTK family | Abl | Positively regulates transport and release of poxviruses | (Reeves et al., 2005) |
| EGFR | Facilitates vaccinia virus spread | (Langhammer et al., 2011) | |
| VEGF | Facilitates ORFV replication and formation of pock-like lesions | (Wise et al., 1999) | |
| Facilitates HSV-1 induced stromal keratitis | (Sharma et al., 2011) | ||
| Src kinase | Positively regulates assembly, maturation and egress of WNV | (Hirsch et al., 2005) | |
| Positively regulates assembly and maturation of DENV | (Chu and Yang, 2007) | ||
| NGFR | Positively regulates RNA synthesis, vRNP export and release (budding) of IAV | (Kumar et al., 2011a) | |
| PDGFR | Facilitates KSHV tumor progression | (Cao et al., 2015; Sturzl et al., 1992) | |
| GPCRs | GPCRs | Promotes membrane fusion and cell to cell spread in hHSV6 (mediated via viral U51 protein) | (Zhen et al., 2005) |
| Positively regulates replication and reactivation of gamma herpesvirus latency (mediated via viral CXCR2 protein) | (Lee et al., 2003) | ||
| Positively regulates flavivirus entry and RNA synthesis | (Le Sommer et al., 2012) | ||
| Ras/Akt | N-Ras | Promotes HCV replication by facilitating cell survival and establishment of persistent viral infection | (Mannova and Beretta, 2005) |
| Akt | Promotes clathrin-independent endocytosis and internalization of IAV | (Fujioka et al., 2011) | |
| Positively regulates PCV2 replication by inhibiting premature apoptosis | (Wei et al., 2012) | ||
| By expressing anti-apoptotic genes, positively regulates cowpox virus and vaccinia virus replication | (Soares et al., 2009) | ||
| Inhibits endocytic uptake of the influenza viruses | (Denisova et al., 2014) | ||
| mTORC2 | Positively regulates adenovirus replication (mediated via viral proteins that mimic nutrient/growth signals regulating mTOR pathway) | (O’Shea et al., 2005) | |
| Ras/Raf/MEK/ERK | Ras | Positively regulates reovirus replication and spread | (Shmulevitz et al., 2010) |
| Ras-GTP | Positively regulates HBV replication and transcription (mediated via HBV transcriptional transactivating protein HBx) | (Benn and Schneider, 1994; Tang et al., 2005) | |
| Cleavage of RasGAP | Positively regulates enterovirus replication | (Huber et al., 1999) | |
| Raf | Positively regulates parvovirus transport across nuclear membrane as well as capsid assembly | (Riolobos et al., 2010) | |
| Positively regulates synthesis and release of HIV-1 | (Flory et al., 1998) | ||
| RKIP | Modulation of immune response to NDV | (Yin et al., 2015) | |
| MEK | Facilitates IAV nuclear export | (Ludwig et al., 2004; Pleschka et al., 2001) | |
| Positively regulates coronavirus genome synthesis |
(Cai et al., 2007) | ||
| Positively regulates BDV spread to neighboring cells | (Planz et al., 2001) | ||
| Positively regulates replication of human neurotropic polyomavirus, JC | (Ravichandran et al., 2007) | ||
| ERK1/2 | Positively regulates RNA/protein synthesis in astroviruses | (Moser and Schultz-Cherry, 2008) | |
| Positively regulates viral protein synthesis in alphaviruses | (Voss et al., 2014) | ||
| Positively regulates CVB3 replication | (Lim et al., 2005) | ||
| Positively regulates HCV genome synthesis | (Gretton et al., 2010) | ||
| Facilitates JUNV replication | (Rodriguez et al., 2014) | ||
| Positively regulates avian leukosis virus replication and virus-induced tumorogenesis | (Dai et al., 2016) | ||
| Ras/p38 | p38 | Regulates RNA translation of encephalomyocarditis virus | (Hirasawa et al., 2003) |
| Ras/JNK | JNK | viral protein and RNA synthesis of avian and human pandemic influenza A viruses | (Nacken et al., 2012) |
| Facilitates HSV-1 replication | (McLean and Bachenheimer, 1999) | ||
| MNK1/eIF4E (cap-dependent translation) | Phosphorylation of eIF4E | Positively regulates HSV-1 genome and protein synthesis | (Walsh and Mohr, 2004) |
| Facilitates IAV protein synthesis | (Kleijn et al., 1996) | ||
| mTORC1-mediated hypophosphorylation of 4E-BP1 | Facilitates CHIKV protein synthesis | (Joubert et al., 2015) | |
| Facilitates HSV-1 replication and translation | (Chuluunbaatar et al., 2010) | ||
| MNK1/SRPK (cap-independent translation) | Inhibition of mTOR/AKT signaling | Positively regulates poliovirus replication | (Brown et al., 2014b) |
| PCB2-mediated recruitment of PIC | Positively regulates poliovirus genome synthesis | (Chase et al., 2014) | |
| Facilitates HCV replication through binding with 5’UTR | (Wang et al., 2011) | ||
| PCB2/SRp20 interaction | PCB2/SRp20 interaction is essential to effectively translate pirconavirus mRNA | (Bedard et al., 2007). | |
| SRPK | Facilitates poliovirus cap-independent translation | (Brown et al., 2014a) | |
| eIF4 G-MNK1 interaction | Negatively regulates adenovirus replication | (Cuesta et al., 2004) | |
| Cleavage of eIF4 G | Facilitates IRES type I mediated enterovirus protein synthesis | (Thompson and Sarnow, 2003) | |
| PKR | eIF2α | Regulation of immune response against viral infections | (Bergmann et al., 2000; Weber et al., 2006) |
| PP2 A | Negatively regulates DNA synthesis in simian virus 40 | (Cegielska et al., 1994) |
Depending on the nature of the virus involved, MAPK signaling may regulate single or multiple steps of virus replication (Andrade et al., 2004). For example, Ebola virus entry (Han and Harty, 2007), assembly and budding/release of HIV-1 (Hemonnot et al., 2004), replication/transcription of HCV (Pei et al., 2012), IAV protein synthesis (Gaur et al., 2011) and reactivation of KSHV latency (Jham and Montaner, 2010; Xie et al., 2008). Besides, it also regulates immune response (Gaur et al., 2011) and apoptosis (Bian et al., 2011) in virus infected cells.
Both live as well as inactivated virus can activate MAPK signaling pathway at a similar level (Rodriguez et al., 2014). Moreover, some viral secretary proteins can also lead to sustained activation of MAPK. For example, vaccinia virus growth factor (VGF) [secretary polypeptide from vaccinia virus homologous to epidermal growth factor (EGF) and transforming growth factor (TGF)] can effectively stimulate ERK1/2 (Andrade et al., 2004).
2.1. Cell surface receptor for MAPK signaling
Cell-surface receptors are specialized integral membrane proteins that constitute a major route by which signals are conveyed from extracellular to the intracellular environment of the cells. Cell surface receptors sense the extracellular signals by binding to their cognate ligands and are of three types, ligand-gated ion channel receptor (Leite and Cascio, 2001), enzyme-linked receptor and G protein-coupled receptor (GPCR) (Jordan and Devi, 1999). In MAPK signaling, receptor tyrosine kinase (RTKs, enzyme linked receptors) and GPCRs are the major classes of cell surface receptors (Widmann et al., 1999), though integrins may also activate MAPK signaling (Widmann et al., 1999). Besides MAPK signaling, RTKs and GPCRs may also be implicated in regulation of phosphoinositide 3-kinase (PI3K)-Akt signaling pathway (Rajagopalan, 2010).
2.1.1. RTKs
RTKs are the high-affinity cell surface receptors for many growth factors, cytokines and hormones (Lemmon and Schlessinger, 2010). Besides being key regulator of normal cellular processes, RTKs also play a critical role in the development and progression of several types of cancer (Bennasroune et al., 2004). Mutations in RTKs lead to the dysregulation of a series of signaling cascades that may eventually develop disease syndromes. (Lemmon and Schlessinger, 2010). RTKs are member of the large family of proteins that contain a trans-membrane domain, an extracellular N terminal region, and an intracellular C terminal region. The cytoplasmic C terminal region of RTKs is highly conserved, encompassing kinase activity and catalyses receptor autophosphorylation including tyrosine phosphorylation of RTK substrates (Segaliny et al., 2015). Tyrosine kinase that does not possess any trans-membrane domains also exists and is called as non-receptor tyrosine kinase. Currently, over 20 different RTK classes have been identified viz; epidermal growth factor receptor (EGFR), insulin receptor family, platelet derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), cholecystokinin receptors (CCKR), nerve growth factor receptor (NGFR), hepatocyte growth factor receptor (HGFR), ephrin receptor, AXL receptors, tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE1), related to receptor tyrosine kinase receptor (RYKR), discoidin domain receptor, 1 (DDR1), rearranged during transfection (RET), ROS receptor family, leukocyte receptor tyrosine kinase (LTK), receptor tyrosine kinase-like orphan receptor (ROR), muscle-specific kinase receptors (MuSK), lemur tyrosine kinase (LMR), and an undetermined class of RTKs (Segaliny et al., 2015).
Ligand binding induces receptor dimerization and autophosphorylation (Fig. 1). Each RTK has several auto-phosphorylation sites, which recruit different Src homology 2 domains (SH2) and phospho-tyrosine binding domain (PTB) containing proteins that have intrinsic enzymatic activity. Binding of SH2 and PTB proteins with auto-phosphorylated intra-cytoplasmic domain of RTK leads to initiation of signal transduction pathways (Lemmon and Schlessinger, 2010). Besides SH2 and PTB, occasionally, adaptor proteins such as src homology 3 domain containing (SH3) and growth factor receptor-bound protein 2 (Grb2), which have no intrinsic enzymatic activity, can also initiate MAPK signaling by recruiting son of sevenless (SOS) protein (Gobert Gosse et al., 2005) (Fig. 1). Taken together, ligand binding to RTK induces RTK-Grb2-SOS-Ras/MAPK signaling (Lemmon and Schlessinger, 2010).
Besides MAPK, RTKs may also activate PI3K/Akt signaling (Cargnello and Roux, 2011; Cattaneo et al., 2014; Schlessinger, 2000) (Fig. 1). Src family of RTKs have been shown to support assembly and maturation of dengue virus (DENV) and West Nile virus (WNV) (Chu and Yang, 2007; Hirsch et al., 2005) and, entry of HIV-1 (Tokunaga et al., 1998) and HSV-1 (Qie et al., 1999). RTKs have also been shown to regulate multiple steps of influenza A virus (IAV) replication, which include RNA synthesis, Crm1-dependent nuclear export and, release of viral particles from infected cells through a pathway that is modulated by the lipid biosynthesis enzyme farnesyl diphosphate synthase (FPPS) (Kumar et al., 2011a).
2.1.2. GPCRs
GPCRs lack intrinsic enzymatic (kinases) activity and are coupled to heterotrimeric G proteins, which consist of Gα, Gβ and Gγ subunits. Ligand binding induces conformational changes in GPCRs, during which the heterotrimeric G-proteins dissociate in GTP-bound Gα and Gβγ subunits. These dissociated subunits of GPCRs regulate enzymatic activity of several key enzymes including second messengers such as adenylatecyclase, phospholipase C (PLC) which in turn regulate cellular functions by triggering different signaling pathways (Cattaneo et al., 2014; Neves et al., 2002). GPCRs have been shown to promote membrane fusion and cell-to-cell spread of human herpesvirus 6 (Zhen et al., 2005) as well as entry and RNA synthesis in flaviviruses (Le Sommer et al., 2012). Kaposi's sarcoma-associated herpesvirus (KSHV) oncogenes are assocauted with dysregulation of angeigenesis, which is mediated via GPCRs (Jham and Montaner, 2010).
2.2. Ras (transducer)
Upon activation, RTKs and GPCRs induce activation of its downstream signaling component-Rat sarcoma (Ras) which functions as GDP/GTP-regulated molecular switch (Biou and Cherfils, 2004). Ras is a 21-kDa protein belongs to the superfamily of small guanosine triphosphatases (GTPases) and is structurally related to Gα subunit of heterotrimeric G protein. In the inactive state, it binds to the nucleotide guanosine diphosphate (GDP) while in the activated state, binds to guanosine triphosphate (GTP). Ras interacts with and transmits signals to the downstream effecter molecules (MAPKKKs/MAPKKs) associated with ERK/JNK/p38 pathway (Rajalingam et al., 2007). The process of exchanging the bound nucleotide is facilitated by guanine nucleotide exchange factors (GEFs) (Li et al., 2016) and GTPase activating proteins (GAPs) (Wennerberg et al., 2005).
Ras has an intrinsic GTPase activity thereby allowing spontaneous conversion of Ras-GTP to Ras-GDP to become inactive (Campbell et al., 1998; Wennerberg et al., 2005) (Fig. 1). Ras-GTP activates rapidly accelerated fibrosarcoma (Raf) which serves as first part of ERK/MAPK module. Ras GTPase-activating proteins (Ras-GAP) are the family of regulatory proteins that bind to activated G proteins and stimulate their GTPase activity, eventually resulting in termination of the signaling event. By stimulating intrinsic Ras GTPase and consequent hydrolysis of GTP to GDP, Ras-GAP negatively regulates Ras-GTP (Bollag and McCormick, 1991; Trahey et al., 1988). Viral infections induce Ras-GAP cleavage which eventually triggers activation of Ras pathway. For example, Coxsackie virus B 3 (CVB3) infection leads to cleavage of Ras-GAP to produce Ras-GTP (activate state) which is required for efficient virus replication (Huber et al., 1999; Luo et al., 2002). Inhibition of MAPK/ERK kinase (MEK), which regulates cleavage of Ras-GAP, results in reduced CVB3 replication (Huber et al., 1999; Luo et al., 2002) (Table 1).
Ras activation depends on GEFs such as SOS and cell division cycle 25 (cdc25) phosphatase. Autophosphorylation of different intracytosolic domains of RTKs (Margolis and Skolnik, 1994) or conformational changes in GPCRs (Della Rocca et al., 1997) activate Ras by recruiting GEFs. Mutations in Ras may lead to persistent activation of Ras (Chappell et al., 2011; Li et al., 2016). Besides GEFs, some adaptor proteins also support MAPK activation. For example, growth factor receptor-bound protein 2 (Grb2), an adaptor protein, recruits SOS and triggers activation of MAPK and PI3K/Akt pathways (Fig. 1). MAPK activation results in transcription of genes involved in cell growth and differentiation (Li et al., 2016; Luo et al., 2002) whereas PI3K/Akt pathway regulates cytoskeleton integrity and apoptosis. Ras has four subtypes viz; Ha-Ras, N-Ras, KiRas 4 A and KiRas 4B of which KiRas strongly activates MAPK pathway (Li et al., 2016; Yan et al., 1998) whereas HaRas more strongly activates PI3K/Akt pathway (Li et al., 2016).
2.3. Raf/MEK/ERK pathway
ERK pathway plays multiple pivotal roles in regulating cell fate (Cagnol and Chambard, 2010; Roux and Blenis, 2004; Shaul and Seger, 2007) and cell cycle progression, thereby serving as a reasonable target for the modulation of viral multiplication. To initiate ERK signaling, Raf interacts with MEK1/2 which in turn activates ERK.
2.3.1. Raf (MAPKKK)
Raf (MAPKKK) serves as first part of ERK/MAPK module and holds a pivotal position in the MAPK signaling pathway (Hamden et al., 2005). Three kinases in Raf family viz; Raf-1, B-Raf, and A-Raf, are related with retroviral oncogenes (Roskoski, 2010). Among these, A-Raf serves as a main upstream activator of the ERK pathway (Fig. 1). Activation of Raf kinase involves interaction with Ras-GTPases (Bondeva et al., 2002; Roskoski, 2012). Besides MEK, Raf has a large number of other substrates such as JAK, PKA, ribosomal acidic P proteins (Rap), Src family kinase, Rac (also known as Ras-related C3 botulinum toxin substrate 1), PI3K, and phosphoinositide-dependent protein kinase-1 (PDK1) (Maroni et al., 2004; Zebisch and Troppmair, 2006).
Raf phosphorylates its downstream targets via MEK/ERK pathway (Cseh et al., 2014; Li et al., 2016). Among the Raf family members, only B-Raf (mutated) is able to persistently activate Raf/MEK/ERK pathway (Garnett and Marais, 2004). Mutated B-Raf can also activate MEK/ERK pathway as well as activating other Raf family members (Li et al., 2016; Rushworth et al., 2006). Furthermore, Ras, but not Raf has an intrinsic GTPase activity, thereby mutations at critical catalytic sites do not result in persistent Raf activation. Three Raf family members may induce MEK activation with varying biological effects in the order of B-Raf > Raf-1 > >A-Raf (Alessi et al., 1994; Li et al., 2016). The Raf signaling was shown to regulate calcium/calmodulin-mediated budding of Ebola virus-like particle (VLP) (Han and Harty, 2007). Drugs targeting the Raf-MAPK signaling pathways represent a promising new class of antiviral agents to treat severe acute respiratory syndrome coronavirus (SARS-CoV) and poxvirus infections (Fauci and Challberg, 2005; Mizutani et al., 2004).
2.3.2. MEK (MAPKK)
MAPKK (MEK1/2) are tyrosine and serine / threonine dual specificity protein kinases that are activated by Raf-mediated phosphorylation and are second component of ERK/MAPK module. MEK is the most characterized substrate for Raf (Hamden et al., 2005). The interaction between MAPKK and its downstream target MAPK is highly specific. Among the three types of MAPKs, p42/p44 (ERK) is phosphorylated solely by MEK 1 and 2, p38 MAPK is selectively activated by MKK3 and MKK6, while JNK is activated by MKK7 and MKK4. However, MKK4 may also activate p38 MAPK upon over expression (Maroni et al., 2004). Activated MEK has been shown to positively regulate borna disease virus (BDV) spread to neighbouring cells (Planz et al., 2001), RNA synthesis of the coronavirus (Cai et al., 2007) and nuclear export of influenza viruses (Ludwig et al., 2004; Pleschka et al., 2001).
2.3.3. ERK
ERK is a key molecule in ERK/MAPK module that is solely activated by dual specificity MEK 1 and 2 (Plotnikov et al., 2011). In order to initiate ERK signaling, Raf interacts with MEK1/2 which in turns activates ERK. ERK1 and ERK2 are two isoforms with 83% amino acid homology and are expressed in diverse tissue types, which can be activated by a large number of extracellular and intracellular stimuli (Shi et al., 2013). Activated ERK can phosphorylate downstream kinases in the cytoplasm, at cell membrane and in the nucleus, thus diversifying the signaling cascade. ERK1/2 has more than 160 downstream target molecules (Li et al., 2016; McCubrey et al., 2007) that may phosphorylate different transcription factors, such as Ets-1, c-Jun, cMyc, CREB, NFκB, Elk1, c-Fos, c-Jun and STAT1 (Nakano et al., 1998; Shi et al., 2013; Steelman et al., 2004). NF-κB and STAT1 are rapidly activated in response to viral infections and cytokines, thus modulating the expression of genes involved in proliferation, apoptosis and immune response (Kumar et al., 2008; Levy et al., 2011). Some of the downstream targets such as ribosomal S6 kinase (RSK) family members are solely activated by ERK (Chambard et al., 2007; Roux and Blenis, 2004). RSK is shown to regulate KSHV lytic replication by promoting viral protein translation (Kuang et al., 2011).
Overexpression of the dominant negative mutants of Raf/ERK2 and MEK was shown to block influenza virus (Pleschka et al., 2001) and HCV replication respectively (Gretton et al., 2010). Likewise, overexpression of an active form of the Raf catalytic domain enhances parvovirus (Riolobos et al., 2010) and HIV-1 (Flory et al., 1998) replication. These evidences suggest that enhanced signaling activity may be misused by the viruses for their effective replication. For example, IAV interacts with ERK1/2 that allows effective trafficking of viral ribonucleoprotein (vRNP) (Pleschka et al., 2001), viral genome synthesis (Gaur et al., 2011) and induction of immune response, mediated via RANTES (regulated on activation, normal T cell expressed and secreted) (Kujime et al., 2000). Adenovirus type 7 activates Raf/MEK/ERK pathway that results in induction of IL-8 which plays an important role in viral pathogenesis (Alcorn et al., 2001). ERK2 is involved in regulating HIV-1 virion assembly and release by phosphorylating p6Gag (Hemonnot et al., 2004; Snyder et al., 2010). In HCV infection, MAPK/ERK pathway regulates viral RNA translation by directing the viral genome towards IRES (Pei et al., 2012). By regulating expression of lytic gene (switching viral latency from latent to lytic phase), MAPK pathway also facilitates Kaposi's sarcoma-associated herpesvirus (KSHV) replication (Xie et al., 2008).
Most common viral infections induce biphasic activation of ERK1/2 in the target cells. First peak of ERK1/2 activation occurs immediately following infection, suggesting that signaling is initiated by binding of the virus to its cognate receptors/co-receptor complex. For instance, engagement of HIV-1 p56lck and/or p59fyn with RTKs leads to ERK activation (Shenoy-Scaria et al., 1992). Only replication-competent (but not UV-irradiated/inactivated) viruses are capable of triggering late ERK activation thereby suggesting that high level of late-phase ERK activation is dependent on accumulation of viral gene/gene products; the late phase ERK activation is associated with expression of HCV core protein (Shenoy-Scaria et al., 1992) and the HIV-1 Tat protein (Rusnati et al., 2001).
2.4. JNK pathway
In mammals, JNKs are encoded by three distinct genes (JNK1, JNK2 and JNK3). JNK1 and JNK2 are ubiquitously expressed, while JNK3 is limited to brain, heart and testis (Yan et al., 2010). During JNK activation, initial signaling pathway starts with the activation of several MAPKKKs including TAK1, MEKK1/4, MLK2/3, and ASK1, which phosphorylate MEK4 or MEK7 and thus lead to activation of JNK (Krishna and Narang, 2008) (Fig. 1). JNK induces activation of transcription factors such as NF-κB p65, ELK-1, c-Fos, c-Jun and STAT1 which play a pivotal role in regulating the immune response to viral infections. In addition, activated JNK induces secretion of proinflammatory cytokines (Das and Muniyappa, 2010) such as TNFα, IFNs, IL1, IL2 and IL6 (Ting et al., 2010). JNK is also associated with regulation of apoptosis via modulation of c-Jun/AP1 and transforming growth factor-β (TGF-β) (Xing et al., 2010). Although JNK is important for apoptosis, it also contributes to cell survival. These opposite effects rely on duration and magnitude of the pathway activation as well as concurrent activation of other signaling pathways. Prolonged activation of JNK mediates apoptosis whereas transient activation allows cell survival (Shi et al., 2013). Transient JNK activation is therefore essential for effective virus replication such as HSV-1 (McLean and Bachenheimer, 1999), DENV (Ceballos-Olvera et al., 2010), IAV (Ludwig et al., 2001) and varicella-zoster virus (VZV) (Rahaus et al., 2004).
2.5. p38 MAPK pathway
p38 MAPK pathway modulates macrophage and neutrophil functions including chemotaxis, respiratory burst activity, granular exocytosis, adherence and apoptosis, as well as mediating T cell differentiation and apoptosis, by regulating IFN-γ production (O’Sullivan et al., 2009). MKK3 and MKK6 serve as the major MAPKKs responsible for p38 activation (Derijard et al., 1995). MKK3/6 itself are activated by a number of MAPKKKs, including MEKK1-3, MLK2/3, ASK1, Tpl2, TAK1, and TAO1/2 (Cuadrado and Nebreda, 2010) (Fig. 1, Fig. 2 ). After activation, p38 phosphorylates a large number of substrates in both cytoplasm (cPLA2, MNK1/2, MK2/3, HuR, Bax, and Tau) and the nucleus (ATF1/2/6, MEF2, Elk-1, GADD153, Ets1, p53, and MSK1/2) (Cuadrado and Nebreda, 2010) (Fig. 1). These transcription factors regulate expression of RANTES, IL-8 and TNF-α, which are believed to regulate proinflammatory responses following viral infections (Medders and Kaul, 2011).
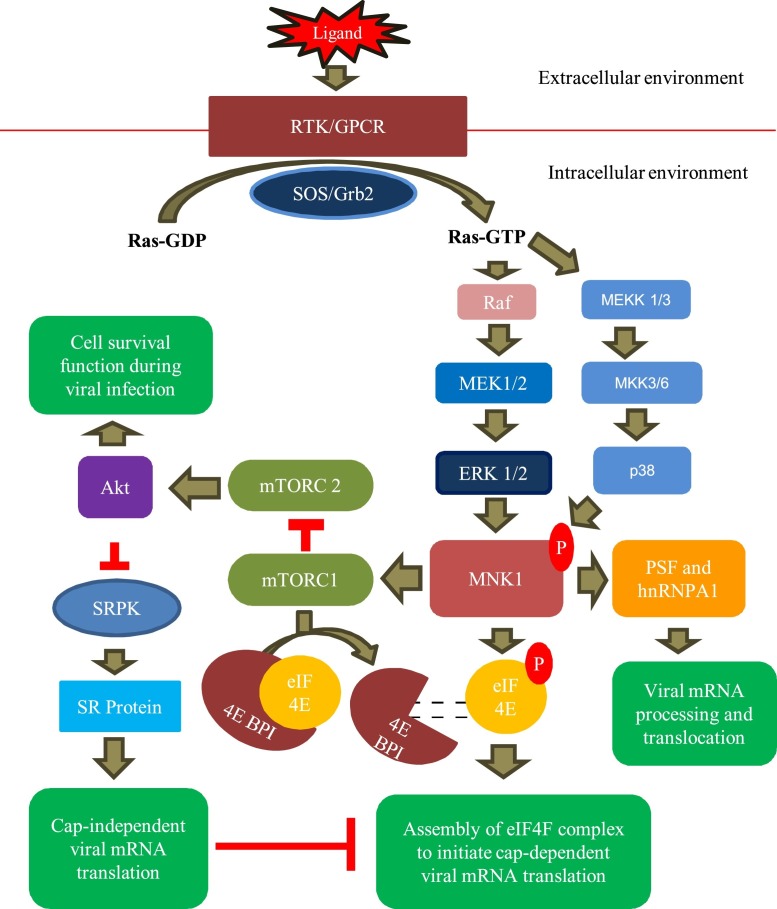
Fig. 2.
Role of MNK1 in viral mRNA translation. MAPK pathway is activated by binding of the ligands to their cognate receptors (RTKs/GPCRs). With the help of adaptor proteins (SOS/Grb2), activated receptors convert Ras-GDP to Ras-GTP. Further information in the pathway is conveyed by phosphorylation (activation) of a series of enzymes, namely Raf, MEK1/2, and ERK1/2 which leads to activation of MNK1. Alternatively, MNK1 may be acitivated by p38. Besides regulating cell proliferation, cell survival and immune response, activated MNK1 also regulates viral mRNA translation. Activated MNK1 leads to dissociation of 4EBP1-eIF4E complex, which results in release (mediated via mTORC1) and subsequently phosphorylation of eIF4E. Activated eIF4E is required for assembly of eIF4 F to initiate cap-dependent viral mRNA translation. Alternatively, MNK1 may modulate SRPK function by directly inhibiting mTORC2-Akt signaling which is prerequisite for cell survival during viral infection. Activated SRPK phosphorylates SRPs that bind with IRES in the viral genome to initiate cap-independent viral mRNA translation. Activated MNK1 also facilitates pre-mRNA and post-translational processing, mediated via hnRNPA1 and PSF respectively.
In conclusion, MAPK pathway positively regulates virus replication in diverse group of viruses (Table 1) except herpesvirus, NDV and polyomavirus where it has negative impact on virus replication cycle. In herpesviruses and polyomaviruses, MAPK/ERK function is performed by MAPK/p38/JNK pathway (Table 1).
3. MNK1
MAPK interacting kinase 1 (MNK1) is one of the MAPKs activated kinase encoded by Mnk1 gene (O’Loghlen et al., 2004). It is expressed in all adult tissues except the brain (Waskiewicz et al., 1997) and is mainly activated by ERK1/2, although p38 can also activate it (Hargett et al., 2005; Roth et al., 2017). The downstream enzymatic activity of MNK1 depends on the upstream regulators (ERK1/2 and p38) which phosphorylate it at specific residues; these phophorylation sites have been mapped using phospho peptide analysis of MNK1 by mass spectrometry (Cargnello and Roux, 2011). MNK1 has several potential downstream substrates, which include (i) eukaryotic initiation factor 4E (eIF4E) and eukaryotic initiation factor 4 G (eIF4 G) that helps in 5′ m7G-dependent mRNA translation and regulate cell growth and proliferation (Richter and Sonenberg, 2005). (ii) heterogenous nuclear ribonucleoprotein A 1 (hnRNPA1) that facilitates processing, metabolism and transport of pre-mRNA. Besides, it also helps in production of TNFα and plays an important role in regulating inflammation (Locksley et al., 2001). (iii) p54 together with PSF [polypyrimidine tract binding (PTB)-associated splicing factor] form transcription splicing factor that is implicated in diverse biological functions such as alternative processing and translation of RNAs, as well as regulation of tissue-specific gene expression (Buxade et al., 2008; Valcarcel and Gebauer, 1997). (iv) Cytosolic phospholipase A2 (cPLA2) leads to production of eicosanoids (a second messenger) and plays an important role in immunity and inflammation (Hefner et al., 2000). (v) Sprouty 2 (Spry2) membrane associated protein negatively regulates ERK/MAPK signaling pathway (Cabrita and Christofori, 2008).
4. Role of MNK in virus replication
Viruses completely depend on the host translational machinery for synthesis of the viral proteins (Walsh and Mohr, 2004), therefore they have developed strategies to modulate host cell translation for optimizing their replication and spread. Viral mRNAs may have a range of structures at its 5′ end. It may be capped with m7G or linked with viral genome-linked proteins (VPg). Uncapped viral mRNAs may carry cis-acting regulatory elements (CREs) such as internal ribosomal entry site (IRES) and protein binding sites. These 5′ end structures have been shown to regulate viral mRNA translation (Brown et al., 2014a). MNK1 is involved in regulating both IRES- and cap-dependent viral mRNA translation.
4.1. Role of MNK1 in initiation of cap-dependent viral mRNA translation
Most DNA viruses such as HSV-1, African swine fever virus (ASFV) (Castello et al., 2009), vaccinia virus (Walsh et al., 2008) as well as some RNA viruses such as chikungunya virus (CHIKV) (Walsh et al., 2013), SARS-CoV (Cencic et al., 2011), orthomyxoviruses (Aragon et al., 2000; Yanguez et al., 2012), rhabdoviruses (VSV, rabies virus) (Komarova et al., 2007), reoviruses (Chulu et al., 2010), hantavirus (Mir and Panganiban, 2008), and alphaviruses (Voss et al., 2014) contain a 5′ cap in their mRNA. Some of the viruses such as influenza virus steal the 5′ cap from the cellular mRNAs (Gu et al., 2015; Plotch et al., 1981) whereas several other viruses synthesize it by using host-encoded capping apparatus, for instance, vaccinia virus and reoviruses (Harwig et al., 2017). Viruses such as flaviviruses (Saeedi and Geiss, 2013) and nidoviruses synthesise the 5′ cap by their own capping apparatus (Harwig et al., 2017). Caliciviruses and picornaviruses contain VPg at its 5′end (Chaudhry et al., 2006; Goodfellow et al., 2005). These 5′ end structures in viral genome mimic 5′ m7G cap of the host mRNA, therefore viral RNA is translated like cellular mRNA. These viruses do not completely shutoff host protein synthesis. For initiation of 5′ m7G cap-dependent viral mRNA translation, eIF4 F plays a central role in recruiting protein synthesis machinery to fully process capped mRNAs (Fig. 2). eIF4 F consists of a large adaptor protein eIF4 G that binds with eIF4E, a cap-binding protein eIF4 A, an RNA helicase (Svitkin et al., 2005), poly(A)-binding protein (PABA) that links 5′ and 3′ ends of properly spliced mRNA (Imataka et al., 1998) and eIF3 that assembles mRNA to the 40S ribosomal subunit.
Activated MNK1 may directly bind to elF4 G in the initiation complex and phosphorylate elF4E which subsequently binds to 5′ cap of mRNA. In resting stage, eIF4E binding protein 1 (4E-BP1) remains associated with eIF4E. This dimer gets dissociated by phophorylation of 4E-BP1 by metabolic sensor-mammalian target of rapamycin (mTORC1), which may be activated by either MNK1 or by ATP (representing high metabolic energy in the cell) (Waskiewicz et al., 1999). MNK1/eIF4E-mediated viral mRNA translation is known to take place in poxviruses, herpesvirus, alpahviruses, orthomyxoviruses, reovirses and coronavirus (Table 1).
4.2. MNK1 in initiation of cap-independent viral mRNA translation
Cap-independent translation is usually mediated by IRES which regulates interaction with eIFs and/or ribosomal proteins, and are able to initiate translation in the middle of a mRNA (Jackson, 2013). The mechanism of association of MNK1 with viral IRES-mediated translation is not completely understood. IRES-containing viruses can misuse activated MNK1 only when the classical function of MNK1 is being usurped, i. e. phosphorylation of eIF4E and its binding with eIF4 G to form eIF4 F complex, to initiation translation (Cuesta et al., 2000) (Fig. 2). Activated MNK1 is misused by the viruses by three distinct mechanisms, which include (i) Dephosphorylation of eIF4E as seen in poliovirus and EMCV (Kleijn et al., 1996). (ii) Cleavage of eIF4 G as seen in poliovirus and pestivirus (Kempf and Barton, 2008; Willcocks et al., 2011) and (iii) Inhibiting 4E-BP1 phosphorylation as seen among adenoviruses (Gingras and Sonenberg, 1997). These events, which are associated with suppressing classical MNK1 function, trigger a switch from cap-dependent to cap-independent translation (Gingras and Sonenberg, 1997), eventually preventing translation of celllular but not viral mRNA.
Four distinct classes of IRES are known: (i) Class I IRES directly cleave eIF4 G (Bordeleau et al., 2006; de Breyne et al., 2009) into two fragments. The larger fragment that contains binding sites for eIF4 A and eIF3 recruits 40 s preinitiation complex (PIC) (required for translation). For example, poliovirus 2 A protease cleaves eIF4 G in order to selectively translate viral mRNA (Kempf and Barton, 2008; Willcocks et al., 2011). (ii) class II IRES induce conformational change in eIF4 G but do not result in its cleavage. The host protein shutoff is achieved by inhibiting 4E-BP1 phosphorylation concomitant with recruitment of 40 s PIC, for example adenovirus (Gingras and Sonenberg, 1997) and HIV-1 (Borman et al., 2001). (iii) class III IRES utilize prokaryotic-like mode of translation initiation. By interacting with eIF3 and ribosomes, it facilitates positioning of ribosomes at mRNA (Babaylova et al., 2009; Berry et al., 2010; Locker et al., 2007), for example pestivirus, HCV, HIV-1 (Locker et al., 2011) and simian picornavirus (SPV9) (de Breyne et al., 2008). (iv) class IV IRES directly recruit 40S subunits without utilizing any eIFs, for example members of the family Dicistroviridae (Jan and Sarnow, 2002; Kamoshita et al., 2009; Wilson et al., 2000). Trans-acting host factors such as SRPKs, hNRNPA1 and PSF (MNK1 downstream substrates) interact with the CREs (positive-stranded RNA viruses and ds RNA viruses) to regulate viral RNA replication, subgenomic mRNA synthesis, translation and encapsidation. (Sullivan and Ahlquist, 1997; Pathak et al., 2012; Wang and Zhang, 1999).
MNK1 regulates IRES-containing mRNA translation by modulating SRPK1/2. However, SRPK1/2 is not a direct substrate for MNK1, its being regulated by MNK1-induced mTORC1-mediated inhibition of mTORC2-AKT signaling (Brown et al., 2014b) (Fig. 2). Ser/Arg (SR)-rich proteins (SRPs) that are prime substrates of SRPK1/2 (Brown et al., 2014b) constitute a large family of nuclear phosphoproteins that found in both nucleus and cytoplasm (Sanford et al., 2004) and are associated with host-RNA-processing and alternative splicing. Upon direct interaction with viral mRNA, SRPs facilitates splicing of viral pre-mRNA in SINV, HIV-1, CMV (Fukuhara et al., 2006) and HCV (Karakama et al., 2010).
To recruit PIC in class I and class II IRES containing viruses, the SRPs are also believed to interact with the poly (rC) binding protein 2 (PCBP2) (Bedard et al., 2007). PCBP2 may alter IRES conformation to enhance eIF4 G binding, thereby facilitating ribosome recruitment (Blyn et al., 1996; Sweeney et al., 2014). PCBP2/SRp20 interaction is essential for picornavirus mRNA translation (Bedard et al., 2007). However, in IRES class III and class IV, SRPs do not seem to play any role because PIC assembly does not require eIF4 G.
MNK1 substrate hnRNPA1 has been shown to colocalize with viral nucleoprotein in the cytoplasm, particularly in the perinuclear region of virus-infected cells (Shi et al., 2000; Wang and Zhang, 1999) and is believed to regulates not only IRES-dependent translation initiation in enteroviruses (Tolbert et al., 2017), but also non-IRES-dependent translation initiation in SINV (Lin et al., 2009) and HCV (Kim et al., 2007; Li et al., 1997).
PSF, an another MNK1 substrate has been shown to directly interact with viral mRNA in order to support posttranscriptional processing as well as translation of viral mRNA (Fig. 2). For example, PSF directly interacts with the cloverleaf CVB3 RNA as well as the IRES elements to regulate viral mRNA translation (Dave et al., 2017). In Hepatitis delta virus (HDV), PSF directly binds to the terminal stem loop domain of the viral RNA to regulate viral mRNA translation (Greco-Stewart et al., 2006). MNK1/SRPK mediated viral mRNA translation occurs in flaviviruses, picornaviruses, retroviruses, polyomaviruses and adenoviruses (Table 1).
4.3. Role of MNK1 in viral genome synthesis
CREs in positive-sense RNA viruses participate in RNA/RNA and RNA/prtotein interactions, thereby modulating viral RNA replication/transcription/translation as well as encapsidation (Sullivan and Ahlquist, 1997; Liu et al., 2009; Pathak et al., 2012; Wang and Zhang, 1999). MNK1 substrate hnRNPA1 colocalize with viral RNA in the cytoplasm and regulate genome synthesis of coronavirus mouse hepatitis virus (Shi et al., 2000, 2003). Besides MNK1, mTORC1 (which may be activated by both MNK1 and ATP) has also been shown to support synthesis of the viral genome (Waskiewicz et al., 1999). For example, in a study with HCV, activated mTORC1 was shown to support viral RNA synthesis (Stohr et al., 2016) without any direct involvement of MNK1.
4.4. Role of MNK1 in cell survival during viral infection
mTOR is a metabolic sensor of cells with highly conserved serine/threonine residues (Hall, 2008) and is found in two structurally and functionally distinct multiprotein complexes, mTORC1 and mTORC2. mTORC1 is rapamycin sensitive and contains mTOR, regulatory associated protein of mTOR (RAPTOR) and mammalian lethal with SEC13 protein 8 (mLST8). (Hall, 2008). The high ATP/AMP ratio promotes and activates mTORC1, which leads to the dissociation of eIF4E from 4E-BP, ultimately resulting in enhanced protein synthesis (Brett et al., 2014), to support virus replication (Kuss-Duerkop et al., 2017; McNulty et al., 2013; Stohr et al., 2016). Conversely, antiviral role of mTORC1 has been demonstrated in CHIKV where virus has evolved a mechanism to bypass mTORC1 inhibition via MNK1/eIF4E mediated translation (Joubert et al., 2015) (Fig. 2). Antiviral function of mTORC1 in VSV is believed to be due to mTORC1-dependent type I IFN production, and initiation of autophagy (Alain et al., 2010).
mTORC2 is resistant to rapamycin and contains mTOR, rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR), mammalian stress-activated protein kinase-interaction protein 1 (mSIN1), proline-rich protein 5 (PRR5) and mLST8 (Hall, 2008). Energy depletion (low ATP levels) leads to adenosine monophosphate-activated protein kinase (AMPK)-mediated accumulation of AMP, which inhibits mTORC1 and activates mTORC2 (Brett et al., 2014). Upregulation of various apoptotic factors and inflammatory mediators during viral infection leads to cellular stress (reduced ATP level), which results in inactivation of mTORC1 and AMPK-mediated activation of mTORC2. Activated mTORC2 then promotes Akt phosphorylation (Vadlakonda et al., 2013) and MNK1 dephosphorylation. The Akt-mTORC2 signaling plays a critical role in survivalbility of cells under stressful conditions such as: (i) activation of PKCα that maintains microtubule integrity (cytoskeletal dynamics) (ii) controlling ion transport and cellular growth via serine/threonine-protein kinase (SGK1) phosphorylation (iii) inhibiting PKR-mediated inflammatory response and upregulation of interferons (iv) interfering caspase protein and p53 mediated apoptosis and (iv) inhibition of MNK1 that shutoff both cap-dependent and cap-independent translation. This global inhibition of cellular protein synthesis aids in immune evasion (Ersahin et al., 2015; Porta et al., 2014). For example, influenza virus (Kuss-Duerkop et al., 2017), vaccinia virus and cowpox virus (Soares et al., 2009) activate mTORC2-Akt signaling which play a critical role in survivalbility at late stage of viral infection. However, in a study, DENV polyprotein was found to suppress only host cell translation at the level of translation initiation whereas viral protein synthesis remained unaffected. DENV infection triggered activation of p38/MNK1signaling that eventually resulted in phosphorylation of eIF4E, which was sufficient to translate viral genome (Roth et al., 2017).
4.5. MNK1 signaling in immune response to viral infections
Virus has evolved strategies to avoid a complete arrest in synthesis of cellular and viral proteins. Many of them directly modulate PKR activity while others inhibit phosphorylation of eIF2α. PKR, a member of eIF2α family of kinases contributes in cellular homeostasis by inhibiting translation of nonessential proteins. It acts as a pattern recognition receptor to sense dsRNA, a replicative intermediate formed in host cells during RNA virus replication. It has three sensor units viz; (i) general control nonrepressed 2 (GCN2) that is activated in response to amino-acid deprivation (ii) PKR-like endoplasmic reticulum kinase (PERK) that is activated in response to overloaded or misfolded proteins in the endoplasmic reticulum and (iii) heme-regulated eIF2α kinase (HRI) that is activated in response to reduced heme levels as well as heat shock (Dang Do et al., 2009). Activated PKR phosphorylates α subunit of eIF2-α as well as activating stress pathways- JNK, p38, NFκB (Fernandes, 2016) and protein phosphatase 2 A (PP2 A) (Ahn et al., 2007).
PKR is known to antagonize MNK1 (Castello et al., 2009) and AKT signaling (Pataer et al., 2009). Phosphorylated eIF2α antagonize MNK1 functions by arresting both cellular and viral mRNA translation (Srivastava et al., 1998). For example, PKR mediated phosphorylation of eIF2α is shown to inhibit translation of VSV (Connor and Lyles, 2005), rotavirus (Rojas et al., 2010) and alphavirus (Ventoso et al., 2006), besides inducing shutoff of the host cell translational machinery. PKR-mediated activation of NFκB (Dabo and Meurs, 2012), JNK and p38 leads to upregulation of various transcription factors including interferons and cytokines, thereby inducing an antiviral state (Taghavi and Samuel, 2012). PKR-mediated activation of tumor suppressor PP2 A leads to negative regulation of ERK1/2 (Yu et al., 2004) and AKT signaling (Ugi et al., 2004). Inhibition of ERK1/2 impedes MNK1 activation (Waskiewicz et al., 1997) whereas AKT inhibition may negatively affect cell survival (lack of maintenance of microtubule integrity and interference with p53 mediated apoptosis) (Ersahin et al., 2015; Porta et al., 2014), thereby preventing further viral spread and augmenting abortive viral replication. Besides PKR, SPRY2 (MNK1 substrate) also triggers interferon production. In addition, SPRY2 negatively regulates ERK-MNK1 signaling (Cabrita and Christofori, 2008). By upregulating TNFα (Locksley et al., 2001) and eicosanoids (Hefner et al., 2000), MNK1 substrates- hnRNPA1 and cPLA2 also play an important role in immunity and inflammation.
5. Genome-wide siRNA screens to identify cellular factors required for virus replication
Gene silencing through RNAi includes sequence-specific targeting of mRNAs by delivering 20–25 nucleotide long dsRNA homologous to gene concerned. RNAi has enabled systematic exploration of host genes involved in virus replication and virulence, thereby allowing identification of novel drug targets (Radoshitzky et al., 2016). Since the last decade, a large number of genome-scale RNAi screens have been conducted, which identified cellular factors required for WNV (Krishnan et al., 2008), DENV (Morchang et al., 2017; Sessions et al., 2009), HIV-1 (Zhou et al., 2008), HCV (Li et al., 2009; Lupberger et al., 2015; Tai et al., 2009), IAV (Han et al., 2018; Karlas et al., 2010), JUNV (Lavanya et al., 2013), coronavirus (Staff, 2015), poxvirus (Kilcher et al., 2014), herpesvirus (Griffiths et al., 2013) and polyomavirus (Zhao and Imperiale, 2017) replication. siRNA screens reveal both proviral and antiviral cellular factors and therefore help in understanding the precise nature of virus-host interactions (Watanabe et al., 2010).
Using reductionist approach, only few of the host factors were known to be implicated in virus replication. However, genome-wide siRNA screens enabled to identify almost every potential host candidate gene that may regulate virus replication. Each virus requires over a thousand host proteins to effectively replicate inside the host. Each siRNA screen identified multiple MAPK components that influence virus replication. Nevertheless, genomewide siRNA screens conducted on Zika Virus (ZIKV), DENV (Savidis et al., 2016), HCV (Lupberger et al., 2015; Tai et al., 2009), Ebola Virus (Kolokoltsov et al., 2009) and SARS-CoV (de Wilde et al., 2015) have identified MNK1 as one of the cellular factor that regulates virus replication. MNK1 has been identified as a proviral factor in all the siRNA screens conducted so far. However, MNK2 has both proviral and antiviral (de Wilde et al., 2015) functions, depending on the virus prototypes involved. More detailed functional analyses of these host genes (identified in genome-wide screens) will provide insights for development of novel antiviral therapeutics.
6. Kinase inhibitors as antiviral agents
In 2002, kinome-the protein kinase complement of the human genome, identified 518 protein kinase genes. Protein kinases play a pivotal role in cellular transduction signaling. Their malfunction, hyperactivity or overexpression is usually associated with disease. Kinase is one of the most intensively studied classes of drug targets (Zhang et al., 2009). Understanding the structural basis of kinase is essential for developing selective inhibitors to target most possible member of the kinome (Cao et al., 2010). Over 80 kinase inhibitors have been evaluated to some stage of clinical trial and over 30 distinct kinase inhibitors have been developed to the level of a Phase I clinical trial (Zhang et al., 2009). The vast majority of these inhibitors were investigated for the treatment of cancer (Player, 2009). However, dysregulation of kinase function has been implicated in several other disorders, including metabolic, immunological, neurological and infectious disease. Most of the known kinase inhibitors target at the kinase activation loop in the ATP binding site (Zhang et al., 2009).
Besides genome-wide siRNA screens, screening kinase inhibitor libraries has also enabled identification of kinases required for efficient virus replication (Kumar et al., 2011a). These inhibitor libraries identified both virus-specific and broad-spectrum antiviral agents (Kumar et al., 2011b). While some of the inhibitors were shown to impair single step of virus life cycle (Kumar et al., 2008), others block multiple steps of virus replication cycle (Kumar et al., 2011a). Kinase inhibitors that target MAPK pathway and may have potential to act as antiviral agents are summarized in Table 2 . More comprehensive information about the kinase inhibitors could be accessed elsewhere in several excellent reviews (Bogoyevitch et al., 2004; Burkhard and Shapiro, 2010; Gangwal et al., 2013; Genovese, 2009; Janne et al., 2013; Kim and Sim, 2012; Levitzki and Gazit, 1995; Matsuda and Fukumoto, 2011; Michelangeli and East, 2011; Sweeney and Firestein, 2006; Uehling and Harris, 2015).
Table 2.
Kinase inhibitors as antiviral agents.
| Cellular Target | Inhibitor | Virus(es) involved* | References |
|---|---|---|---|
| Abl-family | STI-571 (Gleevec) | Poxvirus, variola virus and monkeypox virus | (Reeves et al., 2005, 2011) |
| EGFR | Gefitinib (Iressa) | Poxvirus and HCMV | (Herget et al., 2004; Langhammer et al., 2011) |
| NGFR | AG879 | IAV, Sendai virus, HSV-1, MHV, and rotavirus | (Kumar et al., 2011a, b) |
| PDGFR | Tyrphostin A9 (A9) | IAV, Sendai virus, HSV-1, MHV, and rotavirus | (Kumar et al., 2011a, b) |
| Src family kinases | TG100572 | HSV-1 | (Sharma et al., 2011) |
| Raf (MAP3K) | Vemurafenib | IAV | (Holzberg et al., 2017) |
| MEK1/2 (MAP2K) | U0126 | IAV, IAB, PEDV, Astrovirus, BDV, Coronavirus, JUNV and HSV-1 | (Cai et al., 2007; Colao et al., 2017; Kim and Lee, 2015; Ludwig et al., 2004; Moser and Schultz-Cherry, 2008; Planz et al., 2001; Rodriguez et al., 2014) |
| Cl-1040 (PD184352) | IAV | (Haasbach et al., 2017) | |
| ERK1/2 (MAPK) | FR180204 | DENV and Lentivirus | (Bukong et al., 2010; Sreekanth et al., 2014) |
| Ag-126 | VEEV | (Voss et al., 2014) | |
| p38 (MAPK) | SB203580 | EMCV and HSV-1 | (Hirasawa et al., 2003; Walsh and Mohr, 2004) |
| SB 202190 | Influenza (H5N1, H7N7) virus, EMCV and Avian reovirus | (Borgeling et al., 2014; Chulu et al., 2010; Hirasawa et al., 2003) | |
| JNK (MAPK) | AS601245 | IAV | (Nacken et al., 2012) |
| SP600125 | IAV and HCMV | (Nacken et al., 2012; Zhang et al., 2015) | |
| MNK1 | CGP57380 | HSV-1, Poxvirus and HCMV | (Walsh et al., 2008; Walsh and Mohr, 2004; Walsh et al., 2005) |
| eIF4E/eIF4 G | 4E2RCat | Coronavirus | (Cencic et al., 2011) |
| mTORC1 | Rapamycin | HCV | (Stohr et al., 2016) |
| Akt | MK2206 | IAV | (Denisova et al., 2014) |
*Abbreviations: IAV: Influenza A virus, DENV: dengue virus, EMCV: Encephalomyocarditis Virus, HCV: Hepatitis C virus, HCMV: Human cytomegalovirus, HSV: Herpes simplex virus, MHV: Mouse hepatitis virus, PEDV: Porcine epidemic dirrhoea virus, JUNV: Junin virus, VEEV: Venezuelan equine encephalitis virus.
7. Concluding remarks
Classically, antiviral agents are developed by targeting certain viral components. Viral genome is highly unstable and undergoes frequent mutations; under selection pressure of a drug, virus quickly acquires drug resistance at druggable sites. After the advent of high throughput genome sequencing and genome-wide siRNA screens, thousands of cellular factors that support virus replication have been identified. Since it’s not quite easy for the virus to replace the missing cellular functions by mutations, host-directed antiviral therapies usually do not induce generation of drug-resistant virus variants. In this review, we have described numerous cellular factors/cell signaling pathways which are exploited by the viruses for their effective replication. These cellular factors may serve as potential drug targets to develop novel antiviral therapeutics. However, their further validation, in vivo efficacy and clinical trials are essential before actually translating them from research into the clinical settings.
Acknowledgments
Science and Engineering Research Board (India) supported this work [grant number SB/SO/AS-20/2014]. The funding agency has no role in design, data collection and interpretation, or the decision to submit this work for publication.
References
- Ahn J.H., McAvoy T., Rakhilin S.V., Nishi A., Greengard P., Nairn A.C. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc. Natl. Acad. Sci. U. S. A. 2007;104(8):2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain T., Lun X., Martineau Y., Sean P., Pulendran B., Petroulakis E., Zemp F.J., Lemay C.G., Roy D., Bell J.C., Thomas G., Kozma S.C., Forsyth P.A., Costa-Mattioli M., Sonenberg N. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc. Natl. Acad. Sci. U. S. A. 2010;107(4):1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn M.J., Booth J.L., Coggeshall K.M., Metcalf J.P. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J. Virol. 2001;75(14):6450–6459. doi: 10.1128/JVI.75.14.6450-6459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., Saito Y., Campbell D.G., Cohen P., Sithanandam G., Rapp U., Ashworth A., Marshall C.J., Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13(7):1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A.A., Silva P.N., Pereira A.C., De Sousa L.P., Ferreira P.C., Gazzinelli R.T., Kroon E.G., Ropert C., Bonjardim C.A. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 2004;381(Pt 2):437–446. doi: 10.1042/BJ20031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon T., de la Luna S., Novoa I., Carrasco L., Ortin J., Nieto A. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol. 2000;20(17):6259–6268. doi: 10.1128/mcb.20.17.6259-6268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaylova E., Graifer D., Malygin A., Stahl J., Shatsky I., Karpova G. Positioning of subdomain IIId and apical loop of domain II of the hepatitis C IRES on the human 40S ribosome. Nucleic Acids Res. 2009;37(4):1141–1151. doi: 10.1093/nar/gkn1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K.M., Daijogo S., Semler B.L. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J. 2007;26(2):459–467. doi: 10.1038/sj.emboj.7601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn J., Schneider R.J. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc. Natl. Acad. Sci. U. S. A. 1994;91(22):10350–10354. doi: 10.1073/pnas.91.22.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennasroune A., Gardin A., Aunis D., Cremel G., Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit. Rev. Oncol. Hematol. 2004;50(1):23–38. doi: 10.1016/j.critrevonc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Garcia-Sastre A., Carnero E., Pehamberger H., Wolff K., Palese P., Muster T. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 2000;74(13):6203–6206. doi: 10.1128/jvi.74.13.6203-6206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K.E., Waghray S., Doudna J.A. The HCV IRES pseudoknot positions the initiation codon on the 40S ribosomal subunit. RNA. 2010;16(8):1559–1569. doi: 10.1261/rna.2197210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J., Wang K., Kong X., Liu H., Chen F., Hu M., Zhang X., Jiao X., Ge B., Wu Y., Meng S. Caspase- and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus. Arch. Virol. 2011;156(8):1335–1344. doi: 10.1007/s00705-011-0987-y. [DOI] [PubMed] [Google Scholar]
- Biou V., Cherfils J. Structural principles for the multispecificity of small GTP-binding proteins. Biochemistry. 2004;43(22):6833–6840. doi: 10.1021/bi049630u. [DOI] [PubMed] [Google Scholar]
- Blyn L.B., Swiderek K.M., Richards O., Stahl D.C., Semler B.L., Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5’ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 1996;93(20):11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M.A., Boehm I., Oakley A., Ketterman A.J., Barr R.K. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim. Biophys. Acta. 2004;1697(1-2):89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Bollag G., McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature. 1991;351(6327):576–579. doi: 10.1038/351576a0. [DOI] [PubMed] [Google Scholar]
- Bondeva T., Balla A., Varnai P., Balla T. Structural determinants of Ras-Raf interaction analyzed in live cells. Mol. Biol. Cell. 2002;13(7):2323–2333. doi: 10.1091/mbc.E02-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau M.E., Mori A., Oberer M., Lindqvist L., Chard L.S., Higa T., Belsham G.J., Wagner G., Tanaka J., Pelletier J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol. 2006;2(4):213–220. doi: 10.1038/nchembio776. [DOI] [PubMed] [Google Scholar]
- Borgeling Y., Schmolke M., Viemann D., Nordhoff C., Roth J., Ludwig S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J. Biol. Chem. 2014;289(1):13–27. doi: 10.1074/jbc.M113.469239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A.M., Michel Y.M., Kean K.M. Detailed analysis of the requirements of hepatitis A virus internal ribosome entry segment for the eukaryotic initiation factor complex eIF4F. J. Virol. 2001;75(17):7864–7871. doi: 10.1128/JVI.75.17.7864-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T.G., Yancopoulos G.D., Gregory J.S., Slaughter C., Moomaw C., Hsu J., Cobb M.H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Brett K.E., Ferraro Z.M., Yockell-Lelievre J., Gruslin A., Adamo K.B. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int. J. Mol. Sci. 2014;15(9):16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.C., Bryant J.D., Dobrikova E.Y., Shveygert M., Bradrick S.S., Chandramohan V., Bigner D.D., Gromeier M. Induction of viral, 7-methyl-guanosine cap-independent translation and oncolysis by mitogen-activated protein kinase-interacting kinase-mediated effects on the serine/arginine-rich protein kinase. J. Virol. 2014;88(22):13135–13148. doi: 10.1128/JVI.01883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.C., Dobrikov M.I., Gromeier M. Mitogen-activated protein kinase-interacting kinase regulates mTOR/AKT signaling and controls the serine/arginine-rich protein kinase-responsive type 1 internal ribosome entry site-mediated translation and viral oncolysis. J. Virol. 2014;88(22):13149–13160. doi: 10.1128/JVI.01884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucher B.L., Jamall I.S. Cell-cell communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell. Physiol. Biochem. 2014;34(2):213–243. doi: 10.1159/000362978. [DOI] [PubMed] [Google Scholar]
- Bukong T.N., Hall W.W., Jacque J.M. Lentivirus-associated MAPK/ERK2 phosphorylates EMD and regulates infectivity. J. Gen. Virol. 2010;91(Pt 9):2381–2392. doi: 10.1099/vir.0.019604-0. [DOI] [PubMed] [Google Scholar]
- Burkhard K., Shapiro P. Use of inhibitors in the study of MAP kinases. Methods Mol. Biol. 2010;661:107–122. doi: 10.1007/978-1-60761-795-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxade M., Morrice N., Krebs D.L., Proud C.G. The PSF.p54nrb complex is a novel Mnk substrate that binds the mRNA for tumor necrosis factor alpha. J. Biol. Chem. 2008;283(1):57–65. doi: 10.1074/jbc.M705286200. [DOI] [PubMed] [Google Scholar]
- Cabrita M.A., Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11(1):53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Cai Y., Liu Y., Zhang X. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J. Virol. 2007;81(2):446–456. doi: 10.1128/JVI.01705-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.L., Khosravi-Far R., Rossman K.L., Clark G.J., Der C.J. Increasing complexity of Ras signaling. Oncogene. 1998;17(11 Reviews):1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Cao R., Mi N., Zhang H. 3D-QSAR study of c-Src kinase inhibitors based on docking. J. Mol. Model. 2010;16(2):361–375. doi: 10.1007/s00894-009-0530-1. [DOI] [PubMed] [Google Scholar]
- Cao W., Vyboh K., Routy B., Chababi-Atallah M., Lemire B., Routy J.P. Imatinib for highly chemoresistant Kaposi sarcoma in a patient with long-term HIV control: a case report and literature review. Curr. Oncol. 2015;22(5):e395–399. doi: 10.3747/co.22.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello A., Quintas A., Sanchez E.G., Sabina P., Nogal M., Carrasco L., Revilla Y. Regulation of host translational machinery by African swine fever virus. PLoS Pathog. 2009;5(8):e1000562. doi: 10.1371/journal.ppat.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo F., Guerra G., Parisi M., De Marinis M., Tafuri D., Cinelli M., Ammendola R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int. J. Mol. Sci. 2014;15(11):19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Olvera I., Chavez-Salinas S., Medina F., Ludert J.E., del Angel R.M. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology. 2010;396(1):30–36. doi: 10.1016/j.virol.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Cegielska A., Shaffer S., Derua R., Goris J., Virshup D.M. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol. Cell. Biol. 1994;14(7):4616–4623. doi: 10.1128/mcb.14.7.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencic R., Desforges M., Hall D.R., Kozakov D., Du Y., Min J., Dingledine R., Fu H., Vajda S., Talbot P.J., Pelletier J. Blocking eIF4E-eIF4G interaction as a strategy to impair coronavirus replication. J. Virol. 2011;85(13):6381–6389. doi: 10.1128/JVI.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J.C., Lefloch R., Pouyssegur J., Lenormand P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta. 2007;1773(8):1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chappell W.H., Steelman L.S., Long J.M., Kempf R.C., Abrams S.L., Franklin R.A., Basecke J., Stivala F., Donia M., Fagone P., Malaponte G., Mazzarino M.C., Nicoletti F., Libra M., Maksimovic-Ivanic D., Mijatovic S., Montalto G., Cervello M., Laidler P., Milella M., Tafuri A., Bonati A., Evangelisti C., Cocco L., Martelli A.M., McCubrey J.A. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2(3):135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase A.J., Daijogo S., Semler B.L. Inhibition of poliovirus-induced cleavage of cellular protein PCBP2 reduces the levels of viral RNA replication. J. Virol. 2014;88(6):3192–3201. doi: 10.1128/JVI.02503-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry Y., Nayak A., Bordeleau M.E., Tanaka J., Pelletier J., Belsham G.J., Roberts L.O., Goodfellow I.G. Caliciviruses differ in their functional requirements for eIF4F components. J. Biol. Chem. 2006;281(35):25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- Chu J.J., Yang P.L. C-src protein kinase inhibitors block assembly and maturation of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 2007;104(9):3520–3525. doi: 10.1073/pnas.0611681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulu J.L., Huang W.R., Wang L., Shih W.L., Liu H.J. Avian reovirus nonstructural protein p17-induced G(2)/M cell cycle arrest and host cellular protein translation shutoff involve activation of p53-dependent pathways. J. Virol. 2010;84(15):7683–7694. doi: 10.1128/JVI.02604-09. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chuluunbaatar U., Roller R., Feldman M.E., Brown S., Shokat K.M., Mohr I. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24(23):2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao I., Pennisi R., Venuti A., Nygardas M., Heikkila O., Hukkanen V., Sciortino M.T. The ERK-1 function is required for HSV-1-mediated G1/S progression in HEP-2 cells and contributes to virus growth. Sci. Rep. 2017;7(1):9176. doi: 10.1038/s41598-017-09529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J.H., Lyles D.S. Inhibition of host and viral translation during vesicular stomatitis virus infection. eIF2 is responsible for the inhibition of viral but not host translation. J. Biol. Chem. 2005;280(14):13512–13519. doi: 10.1074/jbc.M501156200. [DOI] [PubMed] [Google Scholar]
- Cseh B., Doma E., Baccarini M. RAF" neighborhood: protein-protein interaction in the Raf/Mek/Erk pathway. FEBS Lett. 2014;588(15):2398–2406. doi: 10.1016/j.febslet.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Cuesta R., Xi Q., Schneider R.J. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 2000;19(13):3465–3474. doi: 10.1093/emboj/19.13.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta R., Xi Q., Schneider R.J. Structural basis for competitive inhibition of eIF4G-Mnk1 interaction by the adenovirus 100-kilodalton protein. J. Virol. 2004;78(14):7707–7716. doi: 10.1128/JVI.78.14.7707-7716.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabo S., Meurs E.F. dsRNA-dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses. 2012;4(11):2598–2635. doi: 10.3390/v4112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Feng M., Ye Y., Wu X., Liu D., Liao M., Cao W. Exogenous avian leukosis virus-induced activation of the ERK/AP1 pathway is required for virus replication and correlates with virus-induced tumorigenesis. Sci. Rep. 2016;6:19226. doi: 10.1038/srep19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Do A.N., Kimball S.R., Cavener D.R., Jefferson L.S. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol. Genom. 2009;38(3):328–341. doi: 10.1152/physiolgenomics.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K.C., Muniyappa H. c-Jun-NH2 terminal kinase (JNK)-mediates AP-1 activation by thioredoxin: phosphorylation of cJun, JunB, and Fra-1. Mol. Cell. Biochem. 2010;337(1-2):53–63. doi: 10.1007/s11010-009-0285-0. [DOI] [PubMed] [Google Scholar]
- Dave P., George B., Sharma D.K., Das S. Polypyrimidine tract-binding protein (PTB) and PTB-associated splicing factor in CVB3 infection: an ITAF for an ITAF. Nucleic Acids Res. 2017;45(15):9068–9084. doi: 10.1093/nar/gkx519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breyne S., Yu Y., Pestova T.V., Hellen C.U. Factor requirements for translation initiation on the Simian picornavirus internal ribosomal entry site. RNA. 2008;14(2):367–380. doi: 10.1261/rna.696508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breyne S., Yu Y., Unbehaun A., Pestova T.V., Hellen C.U. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106(23):9197–9202. doi: 10.1073/pnas.0900153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Wannee K.F., Scholte F.E., Goeman J.J., Ten Dijke P., Snijder E.J., Kikkert M., van Hemert M.J. A kinome-Wide small interfering RNA screen identifies proviral and antiviral host factors in severe acute respiratory syndrome coronavirus replication, including double-stranded RNA-activated protein kinase and early secretory pathway Proteins. J. Virol. 2015;89(16):8318–8333. doi: 10.1128/JVI.01029-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca G.J., van Biesen T., Daaka Y., Luttrell D.K., Luttrell L.M., Lefkowitz R.J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J. Biol. Chem. 1997;272(31):19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 2008;20(1):1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova O.V., Soderholm S., Virtanen S., Von Schantz C., Bychkov D., Vashchinkina E., Desloovere J., Tynell J., Ikonen N., Theisen L.L., Nyman T.A., Matikainen S., Kallioniemi O., Julkunen I., Muller C.P., Saelens X., Verkhusha V.V., Kainov D.E. Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrob. Agents Chemother. 2014;58(7):3689–3696. doi: 10.1128/AAC.02798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B., Raingeaud J., Barrett T., Wu I.H., Han J., Ulevitch R.J., Davis R.J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Ersahin T., Tuncbag N., Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015;11(7):1946–1954. doi: 10.1039/c5mb00101c. [DOI] [PubMed] [Google Scholar]
- Fauci A.S., Challberg M.D. Host-based antipoxvirus therapeutic strategies: turning the tables. J. Clin. Invest. 2005;115(2):231–233. doi: 10.1172/JCI24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J. Oncogenes: the passport for viral oncolysis through PKR inhibition. Biomark. Cancer. 2016;8:101–110. doi: 10.4137/BIC.S33378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory E., Weber C.K., Chen P., Hoffmeyer A., Jassoy C., Rapp U.R. Plasma membrane-targeted Raf kinase activates NF-kappaB and human immunodeficiency virus type 1 replication in T lymphocytes. J. Virol. 1998;72(4):2788–2794. doi: 10.1128/jvi.72.4.2788-2794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Tsuda M., Hattori T., Sasaki J., Sasaki T., Miyazaki T., Ohba Y. The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses. PLoS One. 2011;6(1):e16324. doi: 10.1371/journal.pone.0016324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T., Hosoya T., Shimizu S., Sumi K., Oshiro T., Yoshinaka Y., Suzuki M., Yamamoto N., Herzenberg L.A., Herzenberg L.A., Hagiwara M. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proc. Natl. Acad. Sci. U. S. A. 2006;103(30):11329–11333. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwal R.P., Bhadauriya A., Damre M.V., Dhoke G.V., Sangamwar A.T. p38 Mitogen-activated protein kinase inhibitors: a review on pharmacophore mapping and QSAR studies. Curr. Top. Med. Chem. 2013;13(9):1015–1035. doi: 10.2174/1568026611313090005. [DOI] [PubMed] [Google Scholar]
- Garnett M.J., Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6(4):313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Gaur P., Munjhal A., Lal S.K. Influenza virus and cell signaling pathways. Med. Sci. Monit. 2011;17(6):RA148–154. doi: 10.12659/MSM.881801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M.C. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 2009;60(2):317–320. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Sonenberg N. Adenovirus infection inactivates the translational inhibitors 4E-BP1 and 4E-BP2. Virology. 1997;237(1):182–186. doi: 10.1006/viro.1997.8757. [DOI] [PubMed] [Google Scholar]
- Gobert Gosse S., Bourgin C., Liu W.Q., Garbay C., Mouchiroud G. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cell. Signal. 2005;17(11):1352–1362. doi: 10.1016/j.cellsig.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Goodfellow I., Chaudhry Y., Gioldasi I., Gerondopoulos A., Natoni A., Labrie L., Laliberte J.F., Roberts L. Calicivirus translation initiation requires an interaction between VPg and eIF 4 E. EMBO Rep. 2005;6(10):968–972. doi: 10.1038/sj.embor.7400510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco-Stewart V.S., Thibault C.S., Pelchat M. Binding of the polypyrimidine tract-binding protein-associated splicing factor (PSF) to the hepatitis delta virus RNA. Virology. 2006;356(1-2):35–44. doi: 10.1016/j.virol.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Gretton S., Hughes M., Harris M. Hepatitis C virus RNA replication is regulated by Ras-Erk signalling. J. Gen. Virol. 2010;91(Pt 3):671–680. doi: 10.1099/vir.0.016899-0. [DOI] [PubMed] [Google Scholar]
- Griffiths S.J., Koegl M., Boutell C., Zenner H.L., Crump C.M., Pica F., Gonzalez O., Friedel C.C., Barry G., Martin K., Craigon M.H., Chen R., Kaza L.N., Fossum E., Fazakerley J.K., Efstathiou S., Volpi A., Zimmer R., Ghazal P., Haas J. A systematic analysis of host factors reveals a Med23-interferon-lambda regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 2013;9(8):e1003514. doi: 10.1371/journal.ppat.1003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Gallagher G.R., Dai W., Liu P., Li R., Trombly M.I., Gammon D.B., Mello C.C., Wang J.P., Finberg R.W. Influenza A virus preferentially snatches noncoding RNA caps. RNA. 2015;21(12):2067–2075. doi: 10.1261/rna.054221.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasbach E., Muller C., Ehrhardt C., Schreiber A., Pleschka S., Ludwig S., Planz O. The MEK-inhibitor CI-1040 displays a broad anti-influenza virus activity in vitro and provides a prolonged treatment window compared to standard of care in vivo. Antivir. Res. 2017;142:178–184. doi: 10.1016/j.antiviral.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Hall M.N. mTOR-what does it do? Transpl. Proc. 2008;40(10 Suppl):S5–8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Hamden K.E., Whitman A.G., Ford P.W., Shelton J.G., McCubrey J.A., Akula S.M. Raf and VEGF: emerging therapeutic targets in Kaposi’s sarcoma-associated herpesvirus infection and angiogenesis in hematopoietic and nonhematopoietic tumors. Leukemia. 2005;19(1):18–26. doi: 10.1038/sj.leu.2403532. [DOI] [PubMed] [Google Scholar]
- Han Z., Harty R.N. Influence of calcium/calmodulin on budding of Ebola VLPs: implications for the involvement of the Ras/Raf/MEK/ERK pathway. Virus Genes. 2007;35(3):511–520. doi: 10.1007/s11262-007-0125-9. [DOI] [PubMed] [Google Scholar]
- Han J., Perez J.T., Chen C., Li Y., Benitez A., Kandasamy M., Lee Y., Andrade J., tenOever B., Manicassamy B. Genome-wide CRISPR/Cas9 screen identifies host factors essential for influenza virus replication. Cell. Rep. 2018;23(2):596–607. doi: 10.1016/j.celrep.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargett D., McLean T., Bachenheimer S.L. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 2005;79(13):8348–8360. doi: 10.1128/JVI.79.13.8348-8360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwig A., Landick R., Berkhout B. The Battle of RNA synthesis: virus versus host. Viruses. 2017;9(10) doi: 10.3390/v9100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner Y., Borsch-Haubold A.G., Murakami M., Wilde J.I., Pasquet S., Schieltz D., Ghomashchi F., Yates J.R., 3rd, Armstrong C.G., Paterson A., Cohen P., Fukunaga R., Hunter T., Kudo I., Watson S.P., Gelb M.H. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J. Biol. Chem. 2000;275(48):37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- Hemonnot B., Cartier C., Gay B., Rebuffat S., Bardy M., Devaux C., Boyer V., Briant L. The host cell MAP kinase ERK-2 regulates viral assembly and release by phosphorylating the p6gag protein of HIV-1. J. Biol. Chem. 2004;279(31):32426–32434. doi: 10.1074/jbc.M313137200. [DOI] [PubMed] [Google Scholar]
- Herget T., Freitag M., Morbitzer M., Kupfer R., Stamminger T., Marschall M. Novel chemical class of pUL97 protein kinase-specific inhibitors with strong anticytomegaloviral activity. Antimicrob. Agents Chemother. 2004;48(11):4154–4162. doi: 10.1128/AAC.48.11.4154-4162.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Kim A., Han H.S., Han J., Jun H.S., Yoon J.W. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 2003;77(10):5649–5656. doi: 10.1128/JVI.77.10.5649-5656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A.J., Medigeshi G.R., Meyers H.L., DeFilippis V., Fruh K., Briese T., Lipkin W.I., Nelson J.A. The Src family kinase c-Yes is required for maturation of West Nile virus particles. J. Virol. 2005;79(18):11943–11951. doi: 10.1128/JVI.79.18.11943-11951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg M., Boergeling Y., Schrader T., Ludwig S., Ehrhardt C. Vemurafenib limits influenza a virus propagation by targeting multiple signaling pathways. Front. Microbiol. 2017;8:2426. doi: 10.3389/fmicb.2017.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Watson K.A., Selinka H.C., Carthy C.M., Klingel K., McManus B.M., Kandolf R. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 1999;73(5):3587–3594. doi: 10.1128/jvi.73.5.3587-3594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka H., Gradi A., Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17(24):7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.J. The current status of vertebrate cellular mRNA IRESs. Cold Spring Harb. Perspect. Biol. 2013;5(2) doi: 10.1101/cshperspect.a011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan E., Sarnow P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002;324(5):889–902. doi: 10.1016/s0022-2836(02)01099-9. [DOI] [PubMed] [Google Scholar]
- Janne P.A., Shaw A.T., Pereira J.R., Jeannin G., Vansteenkiste J., Barrios C., Franke F.A., Grinsted L., Zazulina V., Smith P., Smith I., Crino L. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- Jham B.C., Montaner S. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor: lessons on dysregulated angiogenesis from a viral oncogene. J. Cell. Biochem. 2010;110(1):1–9. doi: 10.1002/jcb.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B.A., Devi L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert P.E., Stapleford K., Guivel-Benhassine F., Vignuzzi M., Schwartz O., Albert M.L. Inhibition of mTORC1 enhances the translation of chikungunya proteins via the activation of the MnK/eIF4E pathway. PLoS Pathog. 2015;11(8):e1005091. doi: 10.1371/journal.ppat.1005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshita N., Nomoto A., RajBhandary U.L. Translation initiation from the ribosomal A site or the P site, dependent on the conformation of RNA pseudoknot I in dicistrovirus RNAs. Mol. Cell. 2009;35(2):181–190. doi: 10.1016/j.molcel.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakama Y., Sakamoto N., Itsui Y., Nakagawa M., Tasaka-Fujita M., Nishimura-Sakurai Y., Kakinuma S., Oooka M., Azuma S., Tsuchiya K., Onogi H., Hagiwara M., Watanabe M. Inhibition of hepatitis C virus replication by a specific inhibitor of serine-arginine-rich protein kinase. Antimicrob. Agents Chemother. 2010;54(8):3179–3186. doi: 10.1128/AAC.00113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlas A., Machuy N., Shin Y., Pleissner K.P., Artarini A., Heuer D., Becker D., Khalil H., Ogilvie L.A., Hess S., Maurer A.P., Muller E., Wolff T., Rudel T., Meyer T.F. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463(7282):818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- Kempf B.J., Barton D.J. Poliovirus 2A(Pro) increases viral mRNA and polysome stability coordinately in time with cleavage of eIF4G. J. Virol. 2008;82(12):5847–5859. doi: 10.1128/JVI.01514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcher S., Schmidt F.I., Schneider C., Kopf M., Helenius A., Mercer J. siRNA screen of early poxvirus genes identifies the AAA++ ATPase D5 as the virus genome-uncoating factor. Cell. Host Microbe. 2014;15(1):103–112. doi: 10.1016/j.chom.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee C. Extracellular signal-regulated kinase (ERK) activation is required for porcine epidemic diarrhea virus replication. Virology. 2015;484:181–193. doi: 10.1016/j.virol.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Sim T. Novel small molecule Raf kinase inhibitors for targeted cancer therapeutics. Arch. Pharm. Res. 2012;35(4):605–615. doi: 10.1007/s12272-012-0403-5. [DOI] [PubMed] [Google Scholar]
- Kim C.S., Seol S.K., Song O.K., Park J.H., Jang S.K. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J. Virol. 2007;81(8):3852–3865. doi: 10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn M., Vrins C.L., Voorma H.O., Thomas A.A. Phosphorylation state of the cap-binding protein eIF4E during viral infection. Virology. 1996;217(2):486–494. doi: 10.1006/viro.1996.0143. [DOI] [PubMed] [Google Scholar]
- Kolokoltsov A.A., Saeed M.F., Freiberg A.N., Holbrook M.R., Davey R.A. Identification of novel cellular targets for therapeutic intervention against Ebola virus infection by siRNA screening. Drug. Dev. Res. 2009;70(4):255–265. doi: 10.1002/ddr.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova A.V., Real E., Borman A.M., Brocard M., England P., Tordo N., Hershey J.W., Kean K.M., Jacob Y. Rabies virus matrix protein interplay with eIF3, new insights into rabies virus pathogenesis. Nucleic Acids Res. 2007;35(5):1522–1532. doi: 10.1093/nar/gkl1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna M., Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 2008;65(22):3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M.N., Ng A., Sukumaran B., Gilfoy F.D., Uchil P.D., Sultana H., Brass A.L., Adametz R., Tsui M., Qian F., Montgomery R.R., Lev S., Mason P.W., Koski R.A., Elledge S.J., Xavier R.J., Agaisse H., Fikrig E. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455(7210):242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang E., Fu B., Liang Q., Myoung J., Zhu F. Phosphorylation of eukaryotic translation initiation factor 4B (EIF4B) by open reading frame 45/p90 ribosomal S6 kinase (ORF45/RSK) signaling axis facilitates protein translation during Kaposi sarcoma-associated herpesvirus (KSHV) lytic replication. J. Biol. Chem. 2011;286(48):41171–41182. doi: 10.1074/jbc.M111.280982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujime K., Hashimoto S., Gon Y., Shimizu K., Horie T. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 2000;164(6):3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- Kumar N., Xin Z.T., Liang Y., Ly H., Liang Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 2008;82(20):9880–9889. doi: 10.1128/JVI.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Liang Y., Parslow T.G., Liang Y. Receptor tyrosine kinase inhibitors block multiple steps of influenza a virus replication. J. Virol. 2011;85(6):2818–2827. doi: 10.1128/JVI.01969-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Sharma N.R., Ly H., Parslow T.G., Liang Y. Receptor tyrosine kinase inhibitors that block replication of influenza a and other viruses. Antimicrob. Agents Chemother. 2011;55(12):5553–5559. doi: 10.1128/AAC.00725-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss-Duerkop S.K., Wang J., Mena I., White K., Metreveli G., Sakthivel R., Mata M.A., Munoz-Moreno R., Chen X., Krammer F., Diamond M.S., Chen Z.J., Garcia-Sastre A., Fontoura B.M.A. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog. 2017;13(9):e1006635. doi: 10.1371/journal.ppat.1006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhammer S., Koban R., Yue C., Ellerbrok H. Inhibition of poxvirus spreading by the anti-tumor drug Gefitinib (Iressa) Antivir. Res. 2011;89(1):64–70. doi: 10.1016/j.antiviral.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lavanya M., Cuevas C.D., Thomas M., Cherry S., Ross S.R. siRNA screen for genes that affect Junin virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci. Transl. Med. 2013;5(204) doi: 10.1126/scitranslmed.3006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Sommer C., Barrows N.J., Bradrick S.S., Pearson J.L., Garcia-Blanco M.A. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLoS Negl. Trop Dis. 2012;6(9):e1820. doi: 10.1371/journal.pntd.0001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.J., Koszinowski U.H., Sarawar S.R., Adler H. A gammaherpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J. Immunol. 2003;170(1):243–251. doi: 10.4049/jimmunol.170.1.243. [DOI] [PubMed] [Google Scholar]
- Leite J.F., Cascio M. Structure of ligand-gated ion channels: critical assessment of biochemical data supports novel topology. Mol. Cell. Neurosci. 2001;17(5):777–792. doi: 10.1006/mcne.2001.0984. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267(5205):1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Levy D.E., Marie I.J., Durbin J.E. Induction and function of type I and III interferon in response to viral infection. Curr. Opin. Virol. 2011;1(6):476–486. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.P., Zhang X., Duncan R., Comai L., Lai M.M. Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc. Natl. Acad. Sci. U. S. A. 1997;94(18):9544–9549. doi: 10.1073/pnas.94.18.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Brass A.L., Ng A., Hu Z., Xavier R.J., Liang T.J., Elledge S.J. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhao G.D., Shi Z., Qi L.L., Zhou L.Y., Fu Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016;12(5):3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B.K., Nam J.H., Gil C.O., Yun S.H., Choi J.H., Kim D.K., Jeon E.S. Coxsackievirus B3 replication is related to activation of the late extracellular signal-regulated kinase (ERK) signal. Virus Res. 2005;113(2):153–157. doi: 10.1016/j.virusres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lin J.Y., Shih S.R., Pan M., Li C., Lue C.F., Stollar V., Li M.L. hnRNP A1 interacts with the 5’ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 2009;83(12):6106–6114. doi: 10.1128/JVI.02476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wimmer E., Paul A.V. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim. Biophys. Acta. 2009;1789(9-10):495–517. doi: 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker N., Easton L.E., Lukavsky P.J. HCV and CSFV IRES domain II mediate eIF2 release during 80S ribosome assembly. EMBO J. 2007;26(3):795–805. doi: 10.1038/sj.emboj.7601549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker N., Chamond N., Sargueil B. A conserved structure within the HIV gag open reading frame that controls translation initiation directly recruits the 40S subunit and eIF3. Nucleic Acids Res. 2011;39(6):2367–2377. doi: 10.1093/nar/gkq1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Ludwig S., Ehrhardt C., Neumeier E.R., Kracht M., Rapp U.R., Pleschka S. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 2001;276(24):10990–10998. [PubMed] [Google Scholar]
- Ludwig S., Wolff T., Ehrhardt C., Wurzer W.J., Reinhardt J., Planz O., Pleschka S. MEK inhibition impairs influenza B virus propagation without emergence of resistant variants. FEBS Lett. 2004;561(1-3):37–43. doi: 10.1016/S0014-5793(04)00108-5. [DOI] [PubMed] [Google Scholar]
- Ludwig S., Pleschka S., Planz O., Wolff T. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell. Microbiol. 2006;8(3):375–386. doi: 10.1111/j.1462-5822.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Luo H., Yanagawa B., Zhang J., Luo Z., Zhang M., Esfandiarei M., Carthy C., Wilson J.E., Yang D., McManus B.M. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 2002;76(7):3365–3373. doi: 10.1128/JVI.76.7.3365-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupberger J., Casanova C., Fischer B., Weiss A., Fofana I., Fontaine N., Fujiwara T., Renaud M., Kopp A., Schuster C., Brino L., Baumert T.F., Thoma C. PI4K-beta and MKNK1 are regulators of hepatitis C virus IRES-dependent translation. Sci. Rep. 2015;5:13344. doi: 10.1038/srep13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannova P., Beretta L. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J. Virol. 2005;79(14):8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Skolnik E.Y. Activation of Ras by receptor tyrosine kinases. J. Am Soc. Nephrol. 1994;5(6):1288–1299. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- Maroni P.D., Koul S., Meacham R.B., Koul H.K. Mitogen activated protein kinase signal transduction pathways in the prostate. Cell. Commun. Signal. 2004;2(1):5. doi: 10.1186/1478-811X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Fukumoto M. Sorafenib: complexities of Raf-dependent and Raf-independent signaling are now unveiled. Med. Mol. Morphol. 2011;44(4):183–189. doi: 10.1007/s00795-011-0558-z. [DOI] [PubMed] [Google Scholar]
- McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A.M., Franklin R.A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean T.I., Bachenheimer S.L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 1999;73(10):8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S., Flint M., Nichol S.T., Spiropoulou C.F. Host mTORC1 signaling regulates andes virus replication. J. Virol. 2013;87(2):912–922. doi: 10.1128/JVI.02415-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medders K.E., Kaul M. Mitogen-activated protein kinase p38 in HIV infection and associated brain injury. J. Neuroimmune Pharmacol. 2011;6(2):202–215. doi: 10.1007/s11481-011-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelangeli F., East J.M. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc. Trans. 2011;39(3):789–797. doi: 10.1042/BST0390789. [DOI] [PubMed] [Google Scholar]
- Mir M.A., Panganiban A.T. A protein that replaces the entire cellular eIF4F complex. EMBO J. 2008;27(23):3129–3139. doi: 10.1038/emboj.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem Biophys Res. Commun. 2004;319(4):1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morchang A., Lee R.C.H., Yenchitsomanus P.T., Sreekanth G.P., Noisakran S., Chu J.J.H., Limjindaporn T. RNAi screen reveals a role of SPHK2 in dengue virus-mediated apoptosis in hepatic cell lines. PLoS One. 2017;12(11):e0188121. doi: 10.1371/journal.pone.0188121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser L.A., Schultz-Cherry S. Suppression of astrovirus replication by an ERK1/2 inhibitor. J. Virol. 2008;82(15):7475–7482. doi: 10.1128/JVI.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacken W., Ehrhardt C., Ludwig S. Small molecule inhibitors of the c-Jun N-terminal kinase (JNK) possess antiviral activity against highly pathogenic avian and human pandemic influenza A viruses. Biol. Chem. 2012;393(6):525–534. doi: 10.1515/hsz-2011-0270. [DOI] [PubMed] [Google Scholar]
- Nakano H., Shindo M., Sakon S., Nishinaka S., Mihara M., Yagita H., Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc. Natl. Acad. Sci. U. S. A. 1998;95(7):3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves S.R., Ram P.T., Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- O’Loghlen A., Gonzalez V.M., Pineiro D., Perez-Morgado M.I., Salinas M., Martin M.E. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp. Cell. Res. 2004;299(2):343–355. doi: 10.1016/j.yexcr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- O’Shea C., Klupsch K., Choi S., Bagus B., Soria C., Shen J., McCormick F., Stokoe D. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 2005;24(6):1211–1221. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan A.W., Wang J.H., Redmond H.P. The role of P38 MAPK and PKC in BLP induced TNF-alpha release, apoptosis, and NFkappaB activation in THP-1 monocyte cells. J. Surg. Res. 2009;151(1):138–144. doi: 10.1016/j.jss.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Pataer A., Swisher S.G., Roth J.A., Logothetis C.J., Corn P.G. Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biol. Ther. 2009;8(3):245–252. doi: 10.4161/cbt.8.3.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak K.B., Pogany J., Xu K., White K.A., Nagy P.D. Defining the roles of cis-acting RNA elements in tombusvirus replicase assembly in vitro. J. Virol. 2012;86(1):156–171. doi: 10.1128/JVI.00404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Zhang X., Xu S., Meng Z., Roggendorf M., Lu M., Chen X. Regulation of hepatitis C virus replication and gene expression by the MAPK-ERK pathway. Virol Sin. 2012;27(5):278–285. doi: 10.1007/s12250-012-3257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planz O., Pleschka S., Ludwig S. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 2001;75(10):4871–4877. doi: 10.1128/JVI.75.10.4871-4877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Player M.R. Protein kinase inhibitors for the treatment of inflammatory disease. Curr. Top. Med. Chem. 2009;9(7):598. doi: 10.2174/156802609789007354. [DOI] [PubMed] [Google Scholar]
- Pleschka S., Wolff T., Ehrhardt C., Hobom G., Planz O., Rapp U.R., Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell. Biol. 2001;3(3):301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- Plotch S.J., Bouloy M., Ulmanen I., Krug R.M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813(9):1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie L., Marcellino D., Herold B.C. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology. 1999;256(2):220–227. doi: 10.1006/viro.1999.9673. [DOI] [PubMed] [Google Scholar]
- Radoshitzky S.R., Pegoraro G., Chi X.O., L D.N., Chiang C.Y., Jozwick L., Clester J.C., Cooper C.L., Courier D., Langan D.P., Underwood K., Kuehl K.A., Sun M.G., Cai Y., Yu S.Q., Burk R., Zamani R., Kota K., Kuhn J.H., Bavari S. siRNA screen identifies trafficking host factors that modulate alphavirus infection. PLoS Pathog. 2016;12(3):e1005466. doi: 10.1371/journal.ppat.1005466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaus M., Desloges N., Wolff M.H. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 2004;85(Pt 12):3529–3540. doi: 10.1099/vir.0.80347-0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S. Endosomal signaling and a novel pathway defined by the natural killer receptor KIR2DL4 (CD158d) Traffic. 2010;11(11):1381–1390. doi: 10.1111/j.1600-0854.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- Rajalingam K., Schreck R., Rapp U.R., Albert S. Ras oncogenes and their downstream targets. Biochim. Biophys. Acta. 2007;1773(8):1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Ravichandran V., Jensen P.N., Major E.O. MEK1/2 inhibitors block basal and transforming growth factor 1beta1-stimulated JC virus multiplication. J. Virol. 2007;81(12):6412–6418. doi: 10.1128/JVI.02658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.M., Bommarius B., Lebeis S., McNulty S., Christensen J., Swimm A., Chahroudi A., Chavan R., Feinberg M.B., Veach D., Bornmann W., Sherman M., Kalman D. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 2005;11(7):731–739. doi: 10.1038/nm1265. [DOI] [PubMed] [Google Scholar]
- Reeves P.M., Smith S.K., Olson V.A., Thorne S.H., Bornmann W., Damon I.K., Kalman D. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J. Virol. 2011;85(1):21–31. doi: 10.1128/JVI.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J.D., Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433(7025):477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Riolobos L., Valle N., Hernando E., Maroto B., Kann M., Almendral J.M. Viral oncolysis that targets Raf-1 signaling control of nuclear transport. J. Virol. 2010;84(4):2090–2099. doi: 10.1128/JVI.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D.J., Zhen E., Owaki H., Vanderbilt C.A., Ebert D., Geppert T.D., Cobb M.H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 1993;268(7):5097–5106. [PubMed] [Google Scholar]
- Rodriguez M.E., Brunetti J.E., Wachsman M.B., Scolaro L.A., Castilla V. Raf/MEK/ERK pathway activation is required for Junin virus replication. J. Gen. Virol. 2014;95(Pt 4):799–805. doi: 10.1099/vir.0.061242-0. [DOI] [PubMed] [Google Scholar]
- Rojas M., Arias C.F., Lopez S. Protein kinase R is responsible for the phosphorylation of eIF2alpha in rotavirus infection. J. Virol. 2010;84(20):10457–10466. doi: 10.1128/JVI.00625-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B.A., Force T., Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 2010;90(4):1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. RAF protein-serine/threonine kinases: structure and regulation. Biochem. Biophys. Res. Commun. 2010;399(3):313–317. doi: 10.1016/j.bbrc.2010.07.092. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. MEK1/2 dual-specificity protein kinases: structure and regulation. Biochem. Biophys. Res. Commun. 2012;417(1):5–10. doi: 10.1016/j.bbrc.2011.11.145. [DOI] [PubMed] [Google Scholar]
- Roth H., Magg V., Uch F., Mutz P., Klein P., Haneke K., Lohmann V., Bartenschlager R., Fackler O.T., Locker N., Stoecklin G., Ruggieri A. Flavivirus infection uncouples translation suppression from cellular stress responses. MBio. 2017;8(1) doi: 10.1128/mBio.02150-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P.P., Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth L.K., Hindley A.D., O’Neill E., Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 2006;26(6):2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M., Urbinati C., Musulin B., Ribatti D., Albini A., Noonan D., Marchisone C., Waltenberger J., Presta M. Activation of endothelial cell mitogen activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium. 2001;8(1):65–74. doi: 10.3109/10623320109063158. [DOI] [PubMed] [Google Scholar]
- Saeedi B.J., Geiss B.J. Regulation of flavivirus RNA synthesis and capping. Wiley Interdiscip. Rev. RNA. 2013;4(6):723–735. doi: 10.1002/wrna.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J.R., Gray N.K., Beckmann K., Caceres J.F. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 2004;18(7):755–768. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G., McDougall W.M., Meraner P., Perreira J.M., Portmann J.M., Trincucci G., John S.P., Aker A.M., Renzette N., Robbins D.R., Guo Z., Green S., Kowalik T.F., Brass A.L. Identification of zika virus and dengue virus dependency factors using functional genomics. Cell. Rep. 2016;16(1):232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Segaliny A.I., Tellez-Gabriel M., Heymann M.F., Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 2015;4(1):1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions O.M., Barrows N.J., Souza-Neto J.A., Robinson T.J., Hershey C.L., Rodgers M.A., Ramirez J.L., Dimopoulos G., Yang P.L., Pearson J.L., Garcia-Blanco M.A. Discovery of insect and human dengue virus host factors. Nature. 2009;458(7241):1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Mulik S., Kumar N., Suryawanshi A., Rouse B.T. An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology. J. Virol. 2011;85(12):5995–6007. doi: 10.1128/JVI.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y.D., Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim. Biophys. Acta. 2007;1773(8):1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A.M., Kwong J., Fujita T., Olszowy M.W., Shaw A.S., Lublin D.M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 1992;149(11):3535–3541. [PubMed] [Google Scholar]
- Shi S.T., Huang P., Li H.P., Lai M.M. Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J. 2000;19(17):4701–4711. doi: 10.1093/emboj/19.17.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.T., Yu G.Y., Lai M.M. Multiple type A/B heterogeneous nuclear ribonucleoproteins (hnRNPs) can replace hnRNP A1 in mouse hepatitis virus RNA synthesis. J. Virol. 2003;77(19):10584–10593. doi: 10.1128/JVI.77.19.10584-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Hou X., Li X., Peng H., Shi M., Jiang Q., Liu X., Ji Y., Yao Y., He C., Lei X. Differential gene expressions of the MAPK signaling pathway in enterovirus 71-infected rhabdomyosarcoma cells. Braz. J. Infect. Dis. 2013;17(4):410–417. doi: 10.1016/j.bjid.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulevitz M., Marcato P., Lee P.W. Activated Ras signaling significantly enhances reovirus replication and spread. Cancer Gene Ther. 2010;17(1):69–70. doi: 10.1038/cgt.2009.46. [DOI] [PubMed] [Google Scholar]
- Snyder A., Alsauskas Z.C., Leventhal J.S., Rosenstiel P.E., Gong P., Chan J.J., Barley K., He J.C., Klotman M.E., Ross M.J., Klotman P.E. HIV-1 viral protein r induces ERK and caspase-8-dependent apoptosis in renal tubular epithelial cells. AIDS. 2010;24(8):1107–1119. doi: 10.1097/QAD.0b013e328337b0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.A., Leite F.G., Andrade L.G., Torres A.A., De Sousa L.P., Barcelos L.S., Teixeira M.M., Ferreira P.C., Kroon E.G., Souto-Padron T., Bonjardim C.A. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 2009;83(13):6883–6899. doi: 10.1128/JVI.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekanth G.P., Chuncharunee A., Sirimontaporn A., Panaampon J., Srisawat C., Morchang A., Malakar S., Thuwajit P., Kooptiwut S., Suttitheptumrong A., Songprakhon P., Noisakran S., Yenchitsomanus P.T., Limjindaporn T. Role of ERK1/2 signaling in dengue virus-induced liver injury. Virus Res. 2014;188:15–26. doi: 10.1016/j.virusres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Srivastava S.P., Kumar K.U., Kaufman R.J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 1998;273(4):2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- Staff P.P. Correction: coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2015;11(2):e1004709. doi: 10.1371/journal.ppat.1004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman L.S., Pohnert S.C., Shelton J.G., Franklin R.A., Bertrand F.E., McCubrey J.A. JAK/ STAT, Raf/ MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Stohr S., Costa R., Sandmann L., Westhaus S., Pfaender S., Anggakusuma, Dazert E., Meuleman P., Vondran F.W., Manns M.P., Steinmann E., von Hahn T., Ciesek S. Host cell mTORC1 is required for HCV RNA replication. Gut. 2016;65(12):2017–2028. doi: 10.1136/gutjnl-2014-308971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzl M., Roth W.K., Brockmeyer N.H., Zietz C., Speiser B., Hofschneider P.H. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc. Natl. Acad. Sci. U. S. A. 1992;89(15):7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M.L., Ahlquist P. 1997. cis-Acting Signals in Bromovirus RNA Replication and Gene Expression: Networking With Viral Proteins and Host Factors; pp. 221–230. 8. [Google Scholar]
- Svitkin Y.V., Herdy B., Costa-Mattioli M., Gingras A.C., Raught B., Sonenberg N. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol. Cell. Biol. 2005;25(23):10556–10565. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney S.E., Firestein G.S. Mitogen activated protein kinase inhibitors: where are we now and where are we going? Ann. Rheum. Dis. 2006;65(Suppl 3):iii83–88. doi: 10.1136/ard.2006.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T.R., Abaeva I.S., Pestova T.V., Hellen C.U. The mechanism of translation initiation on type 1 picornavirus IRESs. EMBO J. 2014;33(1):76–92. doi: 10.1002/embj.201386124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi N., Samuel C.E. Protein kinase PKR catalytic activity is required for the PKR-dependent activation of mitogen-activated protein kinases and amplification of interferon beta induction following virus infection. Virology. 2012;427(2):208–216. doi: 10.1016/j.virol.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai A.W., Benita Y., Peng L.F., Kim S.S., Sakamoto N., Xavier R.J., Chung R.T. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell. Host Microbe. 2009;5(3):298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Delgermaa L., Huang F., Oishi N., Liu L., He F., Zhao L., Murakami S. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J. Virol. 2005;79(9):5548–5556. doi: 10.1128/JVI.79.9.5548-5556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S.R., Sarnow P. Enterovirus 71 contains a type I IRES element that functions when eukaryotic initiation factor eIF4G is cleaved. Virology. 2003;315(1):259–266. doi: 10.1016/s0042-6822(03)00544-0. [DOI] [PubMed] [Google Scholar]
- Ting J.P., Duncan J.A., Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327(5963):286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Kiyokawa E., Nakaya M., Otsuka N., Kojima A., Kurata T., Matsuda M. Inhibition of human immunodeficiency virus type 1 virion entry by dominant-negative Hck. J. Virol. 1998;72(7):6257–6259. doi: 10.1128/jvi.72.7.6257-6259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert M., Morgan C.E., Pollum M., Crespo-Hernandez C.E., Li M.L., Brewer G., Tolbert B.S. HnRNP A1 alters the structure of a conserved enterovirus IRES domain to stimulate viral translation. J. Mol. Biol. 2017;429(19):2841–2858. doi: 10.1016/j.jmb.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G.A., Ladner M., Long C.M., Crosier W.J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Uehling D.E., Harris P.A. Recent progress on MAP kinase pathway inhibitors. Bioorg. Med. Chem. Lett. 2015;25(19):4047–4056. doi: 10.1016/j.bmcl.2015.07.093. [DOI] [PubMed] [Google Scholar]
- Ugi S., Imamura T., Maegawa H., Egawa K., Yoshizaki T., Shi K., Obata T., Ebina Y., Kashiwagi A., Olefsky J.M. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 2004;24(19):8778–8789. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlakonda L., Dash A., Pasupuleti M., Anil Kumar K., Reddanna P. The paradox of Akt-mTOR interactions. Front. Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel J., Gebauer F. Post-transcriptional regulation: the dawn of PTB. Curr. Biol. 1997;7(11):R705–708. doi: 10.1016/s0960-9822(06)00361-7. [DOI] [PubMed] [Google Scholar]
- Ventoso I., Sanz M.A., Molina S., Berlanga J.J., Carrasco L., Esteban M. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 2006;20(1):87–100. doi: 10.1101/gad.357006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G., Chory J. Crosstalk in cellular signaling: background noise or the real thing? Dev. Cell. 2011;21(6):985–991. doi: 10.1016/j.devcel.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K., Amaya M., Mueller C., Roberts B., Kehn-Hall K., Bailey C., Petricoin E., 3rd, Narayanan A. Inhibition of host extracellular signal-regulated kinase (ERK) activation decreases new world alphavirus multiplication in infected cells. Virology. 2014;468-470:490–503. doi: 10.1016/j.virol.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18(6):660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Perez C., Notary J., Mohr I. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 2005;79(13):8057–8064. doi: 10.1128/JVI.79.13.8057-8064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Arias C., Perez C., Halladin D., Escandon M., Ueda T., Watanabe-Fukunaga R., Fukunaga R., Mohr I. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol. Cell. Biol. 2008;28(8):2648–2658. doi: 10.1128/MCB.01631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Mathews M.B., Mohr I. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harb. Perspect. Biol. 2013;5(1):a012351. doi: 10.1101/cshperspect.a012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Jeng K.S., Lai M.M. Poly(C)-binding protein 2 interacts with sequences required for viral replication in the hepatitis C virus (HCV) 5’ untranslated region and directs HCV RNA replication through circularizing the viral genome. J. Virol. 2011;85(16):7954–7964. doi: 10.1128/JVI.00339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang X. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology. 1999;265(1):96–109. doi: 10.1006/viro.1999.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz A.J., Flynn A., Proud C.G., Cooper J.A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16(8):1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz A.J., Johnson J.C., Penn B., Mahalingam M., Kimball S.R., Cooper J.A. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 1999;19(3):1871–1880. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Watanabe S., Kawaoka Y. Cellular networks involved in the influenza virus life cycle. Cell. Host Microbe. 2010;7(6):427–439. doi: 10.1016/j.chom.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Wagner V., Rasmussen S.B., Hartmann R., Paludan S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80(10):5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Zhu S., Wang J., Liu J. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway during porcine circovirus type 2 infection facilitates cell survival and viral replication. J. Virol. 2012;86(24):13589–13597. doi: 10.1128/JVI.01697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K., Rossman K.L., Der C.J. The Ras superfamily at a glance. J. Cell. Sci. 2005;118(Pt 5):843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson S., Jarpe M.B., Johnson G.L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Willcocks M.M., Locker N., Gomwalk Z., Royall E., Bakhshesh M., Belsham G.J., Idamakanti N., Burroughs K.D., Reddy P.S., Hallenbeck P.L., Roberts L.O. Structural features of the Seneca Valley virus internal ribosome entry site (IRES) element: a picornavirus with a pestivirus-like IRES. J. Virol. 2011;85(9):4452–4461. doi: 10.1128/JVI.01107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.E., Pestova T.V., Hellen C.U., Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102(4):511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Wise L.M., Veikkola T., Mercer A.A., Savory L.J., Fleming S.B., Caesar C., Vitali A., Makinen T., Alitalo K., Stacker S.A. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc. Natl. Acad. Sci. U. S. A. 1999;96(6):3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Ajibade A.O., Ye F., Kuhne K., Gao S.J. Reactivation of Kaposi’s sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371(1):139–154. doi: 10.1016/j.virol.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Cardona C.J., Anunciacion J., Adams S., Dao N. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. J. Gen. Virol. 2010;91(Pt 2):343–351. doi: 10.1099/vir.0.015578-0. [DOI] [PubMed] [Google Scholar]
- Yan J., Roy S., Apolloni A., Lane A., Hancock J.F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 1998;273(37):24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]
- Yan F., Wang X.M., Liu Z.C., Pan C., Yuan S.B., Ma Q.M. JNK1, JNK2, and JNK3 are involved in P-glycoprotein-mediated multidrug resistance of hepatocellular carcinoma cells. Hepatobiliary Pancreat. Dis. Int. 2010;9(3):287–295. [PubMed] [Google Scholar]
- Yanguez E., Rodriguez P., Goodfellow I., Nieto A. Influenza virus polymerase confers independence of the cellular cap-binding factor eIF4E for viral mRNA translation. Virology. 2012;422(2):297–307. doi: 10.1016/j.virol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R., Liu X., Bi Y., Xie G., Zhang P., Meng X., Ai L., Xu R., Sun Y., Stoeger T., Ding Z. Expression of Raf kinase inhibitor protein is downregulated in response to Newcastle disease virus infection to promote viral replication. J. Gen. Virol. 2015;96(9):2579–2586. doi: 10.1099/jgv.0.000228. [DOI] [PubMed] [Google Scholar]
- Yu L.G., Packman L.C., Weldon M., Hamlett J., Rhodes J.M. Protein phosphatase 2A, a negative regulator of the ERK signaling pathway, is activated by tyrosine phosphorylation of putative HLA class II-associated protein I (PHAPI)/pp32 in response to the antiproliferative lectin, jacalin. J. Biol. Chem. 2004;279(40):41377–41383. doi: 10.1074/jbc.M400017200. [DOI] [PubMed] [Google Scholar]
- Zebisch A., Troppmair J. Back to the roots: the remarkable RAF oncogene story. Cell. Mol. Life Sci. 2006;63(11):1314–1330. doi: 10.1007/s00018-006-6005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang P.L., Gray N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Niu X., Qian Z., Qian J., Xuan B. The c-Jun N-terminal kinase inhibitor SP600125 inhibits human cytomegalovirus replication. J. Med. Virol. 2015;87(12):2135–2144. doi: 10.1002/jmv.24286. [DOI] [PubMed] [Google Scholar]
- Zhao L., Imperiale M.J. Identification of Rab18 as an essential host factor for BK polyomavirus infection using a whole-genome RNA interference screen. mSphere. 2017;2(4) doi: 10.1128/mSphereDirect.00291-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z., Bradel-Tretheway B., Sumagin S., Bidlack J.M., Dewhurst S. The human herpesvirus 6 G protein-coupled receptor homolog U51 positively regulates virus replication and enhances cell-cell fusion in vitro. J. Virol. 2005;79(18):11914–11924. doi: 10.1128/JVI.79.18.11914-11924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Xu M., Huang Q., Gates A.T., Zhang X.D., Castle J.C., Stec E., Ferrer M., Strulovici B., Hazuda D.J., Espeseth A.S. Genome-scale RNAi screen for host factors required for HIV replication. Cell. Host Microbe. 2008;4(5):495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]