Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) is capable of causing acute respiratory illness. Laboratory-confirmed MERS-CoV cases may be asymptomatic, have mild disease, or have a life-threatening infection with a high case fatality rate. There are three patterns of transmission: sporadic community cases from presumed non-human exposure, family clusters arising from contact with an infected family index case, and healthcare-acquired infections among patients and from patients to healthcare workers. Healthcare-acquired MERS infection has become a well-known characteristic of the disease and a leading means of spread. The main factors contributing to healthcare-associated outbreaks include delayed recognition, inadequate infection control measures, inadequate triaging and isolation of suspected MERS or other respiratory illness patients, crowding, and patients remaining in the emergency department for many days. A review of the literature suggests that effective control of hospital outbreaks was accomplished in most instances by the application of proper infection control procedures. Prompt recognition, isolation and management of suspected cases are key factors for prevention of the spread of MERS. Repeated assessments of infection control and monitoring of corrective measures contribute to changing the course of an outbreak. Limiting the number of contacts and hospital visits are also important factors to decrease the spread of infection.
Keywords: Middle East respiratory syndrome coronavirus, MERS, Healthcare-associated outbreaks
Introduction
Middle East respiratory syndrome coronavirus (MERS-CoV) is capable of causing acute respiratory infection, although its spectrum ranges from asymptomatic laboratory-confirmed cases to mild infection to life-threatening disease with a high case fatality rate [1], [2]. MERS-CoV was first described in 2012 in a 60-year-old man hospitalized with suspected community-acquired pneumonia, who developed renal and respiratory failure, ultimately succumbing to progressive disease [3]. The first reported healthcare-associated MERS-CoV infection was described among multiple facilities in Al-Hasa, Saudi Arabia [4]. However, a later retrospective analysis of a respiratory outbreak in a public hospital in Jordan determined that this cluster dated back earlier, becoming the first recognized healthcare-associated MERS-CoV infection in April 2012 [5]. The disease has also been recognized in many countries with secondary transmissions. The first case of MERS-CoV infection in France caused one secondary transmission among 123 contacts [6]. In a study of 51 outbreaks, nosocomial transmissions were observed in 80.4% of clusters [7]. Another study found that 37.5% of 1797 cases were ascribed to healthcare-associated infections [8]. The percentage of healthcare workers (HCWs) involved in different outbreaks is variable, and ranges between 14% and 64% [9]. The risk of severe disease seems to be higher in people with comorbid diseases and older patients [8].
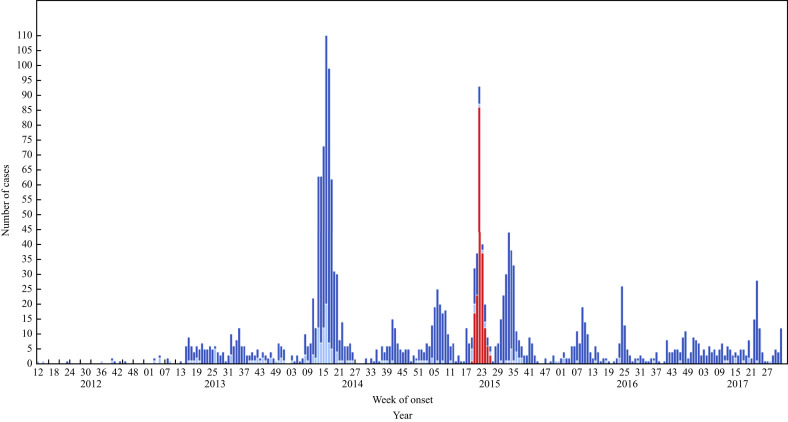
As of March 2018, the World Health Organization reported 2189 laboratory-confirmed cases of MERS-CoV from 27 countries, including 782 (35.7%) deaths [10]. Among the reported cases, peaks occurred in 2014 (due to the Jeddah outbreak) and 2015 (South Korea outbreak) (Figure 1 ).
Figure 1.
Most recent updated figure of timeline of confirmed global cases of Middle East respiratory syndrome coronavirus (MERS-CoV) reported to the World Health Organization (as of 1st September 2017), N = 2067. Red bars, Republic of Korea; dark blue bars, Saudi Arabia; light blue bars, other countries.Other countries: Algeria, Austria, Bahrain, China, Egypt, France, Germany, Greece, Iran, Italy, Jordan, Kuwait, Lebanon, Malaysia, Netherlands, Oman, Philippines, Qatar, Thailand, Tunisia, Turkey, United Arab Emirates, United Kingdom, United States of America, Yemen. Please note that the underlying data is subject to change as the investigation around cases is ongoing. Onset date estimated if not available.
There are three patterns of MERS-CoV transmission [1]: sporadic community cases from presumed non-human exposure [1], family clusters resulting from contact with an infected family index case [11], [12], [13], and healthcare-acquired infections between patients and from patients to HCWs [1], [4], [5], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31].
Although MERS-CoV has a documented ability to transmit between humans, especially in healthcare settings, there does not appear to be sustained human-to-human transmission. This is likely due to the relatively low reproduction number of MERS-CoV of 0.8–1.3 [29], [30]. The South Korean MERS outbreak was thought to have a reproduction number of 1% [31]. However, the reproduction number was estimated to be as high as 2–5% in some MERS outbreaks in Saudi Arabia and South Korea [32]. The upper reproduction estimates were probably derived from lack of sufficient infection control measures, and the estimates were lowered with improved detection and prevention practices over time. This review focuses on the available literature on healthcare-associated infections and transmission of MERS-CoV to elucidate the contributing risk factors.
Search strategy
The search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prismastatement.org). The search included MEDLINE and Scopus databases for articles published in English, as follows:
#1: ‘MERS’ OR ‘MERS-CoV’ OR ‘Middle East respiratory syndrome coronavirus’
#2: ‘Transmission’ OR ‘Outbreak’ OR ‘Healthcare associated infection’ OR ‘Nosocomial’ or ‘cluster’;
#3: #1 AND #2.
In addition, the Saudi Ministry of Health website was searched for updates, and the World Health Organization website and ProMed website were searched for any listed outbreaks.
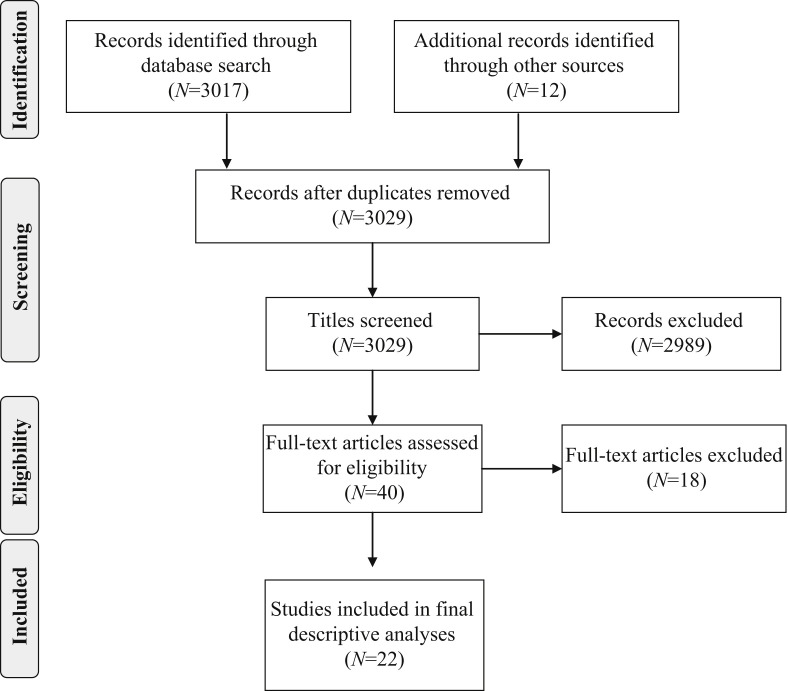
Inclusion criteria were: published papers written in English if the studies described any healthcare-associated outbreak, and included factors contributing to the outbreak or factors that were used to limit and control the spread of the infection. Case reports and reports of clinical presentations were excluded. In addition, outbreaks with no specific descriptions of contributing factors were excluded [10], [18], [26]. Of the 40 full-text articles assessed for eligibility, 18 articles were excluded as these did not describe factors related to outbreaks. In total, 22 articles were included in the final analyses for contributing factors for MERS-CoV outbreaks in healthcare settings (Figure 2 ).
Figure 2.
A flow diagram of the search strategy according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [88].
Timeline of healthcare-associated infections
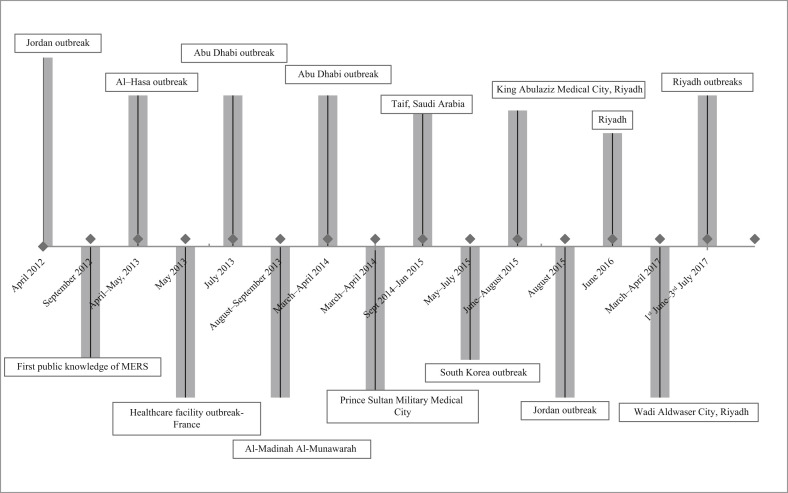
Many healthcare-associated outbreaks have occurred in Saudi Arabia, and a large outbreak arose in South Korea in 2015 [1]. A timeline of these outbreaks is shown in Figure 3 , the most recent updated figure from the World Health Organization. The first outbreak in Saudi Arabia occurred in Al-Hasa [4], followed by a significant epidemic in Jeddah in 2014 [14], [16]. Other outbreaks in 2014 were in King Faisal Specialist Hospital [17], King Fahad Medical City in Riyadh [18] and Al-Madinah Al-Mounawarh [21]. No specific factors contributing to an outbreak at Prince Sultan Military Medical City, Riyadh were listed [18], [19], [20]. In 2015, there were three outbreaks in Saudi Arabia in three public hospitals in the Al-Hasa region [22], King Abdulaziz Medical City in Riyadh [23], [24], [25] and King Fahad Cardiac Centre [26]. A multi-facility outbreak of MERS-CoV infection occurred in September 2014–January 2015 in Taif, Saudi Arabia in four healthcare facilities [27].
Figure 3.
Timeline of major healthcare associated outbreaks.
Observed infection control practices among various outbreaks
Jordan hospital outbreak, April 2012
The outbreak in Zarqa, Jordan involved 13 HCWs with pneumonia [5]. Following the identification of MERS-CoV, specimens from two fatal cases were retrospectively confirmed to be MERS-CoV by real-time reverse transcription polymerase chain reaction (RT-PCR) [5]. Based on serology and PCR testing, the attack rate was 10% among potentially exposed HCWs [15]. In this outbreak, multiple infection control issues were observed and included: absence of physical barriers between different beds in intensive care units apart from cloth drapes, lack of isolation and negative pressure rooms, and non-adherence to infection control measures [15]. Patients were transferred to two other hospitals with no further evidence of intrahospital transmission, believed to be due to adequate infection control measures in the accepting institutions [15].
Al-Hasa 2013 outbreak, April–May 2013
The outbreak involved four hospitals with 21 of the 23 cases acquired by person-to-person transmission within haemodialysis units, intensive care units or other in-patient areas [4]. Factors contributing to this outbreak included the use of aerosol-generating procedures and the performance of resuscitations [4]. A super-spreading event may also have occurred in the Al-Hasa outbreak where one patient infected seven secondary cases [4]. The outbreak abated by emphasizing basic infection control measures: hand hygiene, droplet and contact precautions for febrile patients, testing all febrile patients for MERS-CoV, use of surgical masks for all patients undergoing haemodialysis, and N95 respirators for HCWs during aerosol-generating procedures. Additional measures included enhanced environmental cleaning, and excluding non-essential staff and visitors [4].
France cases, May 2013
A French patient contracted MERS-CoV while travelling, requiring hospitalization. Of the 123 contacts of the index patient, only one (0.8%) hospitalized patient tested positive and 39 contacts of the second patient tested negative [6].
Abu Dhabi outbreak, July 2013
A patient developed community-acquired infection through camel contact, and received care in two different hospitals. Of the 277 healthcare contacts, four (1.4%) had healthcare-associated infections. These four patients were exposed to the index case before the diagnosis of MERS-CoV and institution of any respiratory protection measures [28].
Al-Madinah Al-Munawarrah outbreak, 24th August to 3rd September 2013
From 24th August to 3rd September 2014, 18 cases were linked to one cluster involving 11 healthcare-associated infections [21]. The outbreak was thought to be secondary to under-recognition and poor infection control measures [21].
Abu Dhabi outbreak, March–April 2014
In this cluster, the index case arose from camel exposure. Only two (2.2%) of 90 hospital contacts were positive for MERS-CoV [28]. Another cluster was traced to a community member who visited an emergency room three times, and was subsequently admitted to a regular unit. Evaluation of 224 contacts identified 15 (6.7%) positive cases [28].
Prince Sultan Military Medical City outbreak, March and April 2014
Among multiple outbreaks in this hospital, the largest outbreak came from 15 patients acquiring infection within the emergency room [20]. The outbreak abated after application of infection control measures.
Jeddah 2014 outbreak, 2nd March–10th May 2014
Involving 14 hospitals and more than 200 cases, 60% of infected cases resulted from healthcare-associated transmission [14], [16], [33]. Factors contributing to intrahospital transmission included inadequate separation of suspected MERS patients, crowding, and inconsistent use of infection control precautions [33]. There was no triaging or isolation of patients with respiratory illness, and patients remained in the emergency department for many days [33]. In addition, uncontrolled patient movements and high visitor traffic contributed to the outbreak [23].
Taif, Saudi Arabia outbreak, September 2014–January 2015
The outbreak in Taif, Saudi Arabia included four healthcare settings, with the largest number traced to a haemodialysis unit involving 15 patients [27]. The implicated cause was close spacing between patients of less than 2 m [27].
South Korea outbreak, May–July 2015
In the most prominent outbreak outside the Arabian Peninsula, approximately 17,000 contacts were quarantined by the summer of 2015 [34]. The index patient had been in contact with 742 people between 11th and 20th May 2015 in one hospital, subsequently infecting 28 patients [35]. A total of 186 MERS-CoV cases were identified in more than 17 healthcare settings [31], [36], [37], [38], [39]. Among the contributory reasons for MERS-CoV advancing within South Korea were HCWs unfamiliarity with MERS, suboptimal infection prevention and control measures, overcrowded emergency departments, multi-bed hospital rooms, ‘medical shopping’ by patients, visitors to infected patients, extensive MERS patient movements, and the use of aerosol-generating procedures [40], [41], [42]. Particularly problematic factors in the South Korea outbreak were contributions of overcrowding, medical shopping and super-spreaders [1], [34], [35], [40], [43], [44]. The first case in the Republic of Korea infected 27 secondary cases; one secondary patient infected 24 tertiary cases, and another secondary patient infected 73 tertiary cases [34]. Another report from South Korea found 85, 28, 23, 11 and six secondary cases arising from individual patients with MERS-CoV [40]. A secondary patient was described causing 91 tertiary MERS cases, of which 39% occurred within the emergency department and 13% were HCWs [44]. Delayed isolation of suspected patients was an important factor that contributed to the spread of MERS-CoV. This was higher in super-spreaders compared with other patients (mean 6.6 vs 2.9 days; P = 0.061) [45]. Multi-bedded rooms and nebulization treatments may also have contributed to the spread of MERS-CoV in South Korea [46].
King Abdulaziz Medical City, Riyadh outbreak, June–August 2015
One of the largest MERS outbreaks occurred in King Abdulaziz Medical City, Riyadh. Transmission appeared to be related to care in the emergency department before suspicion or diagnosis of MERS, causing 130 cases [23], [24], [25]. A major contributing factor was overcrowding in the emergency department [47].
Jordan outbreak, August 2015
Sixteen laboratory-confirmed cases from nine hospitals were identified in Amman, Jordan between August and October 2015 [36]. There were human-to-human transmissions in both cardiac care and intensive care units of two hospitals [36]. Analysed viral isolates were similar to those recovered in Riyadh, except for deletions in open reading frames 4a, although the impact of this on transmissibility or virulence remains unknown [48].
Riyadh outbreak, June 2016
A healthcare associated MERS outbreak was reported in Riyadh, Saudi Arabia between 19th and 22nd June 2016. This outbreak appeared to start with a woman admitted to the vascular surgery ward through the emergency department. Her initial symptoms were not characteristic of MERS-CoV infection. After confirmation of the diagnosis, active screening revealed 24 positive contacts (including 20 HCWs), and of all contacts, 20 (83.3%) were asymptomatic [49].
Riyadh outbreak, 1st June–3rd July 2017
The index case was a 47-year-old male who underwent emergency intubation in the emergency department. From 220 contacts, 33 additional cases were identified as positive including 16 HCWs [10]. This cluster was linked to a smaller cluster of five cases in another hospital with the involvement of three household contacts, one patient contact and one HCW [10]. A third unrelated outbreak also occurred in Riyadh in June 2017 and involved nine cases, with eight HCWs (four asymptomatic and four with mild disease) [10].
Discussion
The World Health Organization continues to tally laboratory-confirmed MERS-CoV infections. In the recent update from September 2012 to July 2017, there were 2040 cases with healthcare-associated infections (40 HCWs, patients and visitors), representing 31% of cases [10]. The initial symptoms of MERS-CoV are non-specific, often thought to be pedestrian respiratory infections, and thus may go unnoticed. Adherence to standard precautions at all times, given the unpredictability of MERS-CoV infection, appears to be the critical factor for the prevention of transmission in healthcare facilities [10]. Additionally, environmental contamination may play a role in some transmission of MERS, as viral RNA has been detected for up to five days on surfaces [36].
Multiple factors contribute to the development of hospital outbreaks of MERS infections as summarized in Table I . A well-characterized outbreak in Abu Dhabi, United Arab Emirates offers insights into important infection control issues. The overwhelming reason for MERS-CoV spread centred on the delayed diagnosis of MERS-CoV, as 93% of infected contacts were exposed before the patient's diagnosis. Also, use of personal protective equipment (PPE) during care was inconsistent among these HCWs, especially during aerosol-generating procedures. Although improved PPE use would be of benefit, a larger preventative impact would be to place patients presenting with respiratory complaints and potential MERS-CoV infection in proper isolation with infection control practices until MERS-CoV infection is ruled out using the standard PCR diagnostic method.
Table I.
Factors contributing to hospital outbreaks
| Infection control issues | Examples | Number of instances where this was an issue | Involved hospitals | Reference |
|---|---|---|---|---|
| Hospital design | Absence of physical barriers between different beds, inadequate separation of suspected MERS patients, lack of isolation and negative pressure rooms | 3 | Jordan, Jeddah, Taif | [5], [14], [16], [27], [33], [87] |
| Healthcare workers' adherence | Suboptimal adherence to infection control measures | 4 | Jordan, Al-Madinah Al-Muwnawarah, Jeddah, Riyadh | [5], [14], [16], [21], [23], [24], [25], [33], [87] |
| Contacts prior to MERS diagnosis and under-recognition | 1 | Abu Dhabi | [28] | |
| Contact without respiratory protection | 1 | Abu Dhabi | [28] | |
| Overcrowding | 2 | South Korea, | [1], [14], [16], [33], [34], [35], [36], [40], [41], [42], [43], [44], [87] | |
| Patient flow | No triaging and isolation of patients with respiratory illness, patients remained in the emergency room for many days, use of multi-bed rooms, extensive patients movements, | 2 | South Korea, | [1], [14], [16], [33], [34], [35], [36], [40], [41], [42], [43], [44], [87] |
| Unfamiliarity with MERS infection | 1 | South Korea | [1], [34], [35], [36], [40], [41], [42], [43], [44] | |
| Under-recognition | 2 | Al-Madinah Al-Muwnawarah | [10], [21], [49] | |
| Aerosol-generating procedures | Use of CPAP and nebulized medications and the performance of resuscitations | 3 | South Korea, Al-Hasa | [1], [4], [10], [34], [35], [36], [40], [41], [42], [43], [44] |
| Patients' characteristics | Contribution of super-spreaders | 1 | South Korea | [1], [34], [35], [36], [40], [41], [42], [43], [44] |
| Social norms | ‘Medical shopping’, presence of multiple friends and family members with patients | 1 | South Korea | [1], [34], [35], [36], [40], [41], [42], [43], [44] |
MERS, Middle East respiratory syndrome; CPAP, continuous positive airways pressure.
Protocols to address such patients in emergency departments or patients developing an apparent viral illness while hospitalized for other conditions should include the ability to rapidly isolate patients and run laboratory testing, which would likely mitigate spread within healthcare systems. Proper triaging of patients with acute respiratory illness is a fundamental step towards the application of a unified process to deal with such patients, as suggested by the World Health Organization and the United States Centers for Disease Control and Prevention [50], [51]. Despite such advice, not all healthcare systems in regions endemic for MERS-CoV or in non-endemic regions caring for a potentially infected traveller follow these steps. In a simulation of a ‘mystery’ infectious patient, 95 drills including 42 drills specifically for patients with possible MERS were conducted in 49 emergency departments in New York, USA. Hospitals were variable in the identification of potentially infectious patients and implementation of appropriate infection control measures, such as suboptimal adherence to hand hygiene, PPE use and isolation signage posting [52].
The Saudi Ministry of Health has recently introduced visual triage using a scoring system to aid in the assessment of patients presenting with respiratory symptoms in emergency departments, dialysis units and other clinical settings [53]. Also, the Saudi Ministry of Health has developed a rapid response team that visits hospitals upon the identification of any positive MERS cases to help streamline contact tracing and implementation of infection control measures [54]. A similar rapid response team was also formed following the MERS outbreak in South Korea [55]. An interesting observation of the outbreaks of MERS-CoV infection is the variability in intrahospital transmission of the virus. The South Korea and Jeddah outbreaks witnessed patients causing significant spread of secondary cases, while in other outbreaks, the index patient resulted in few secondary cases despite lack of infection control practices [1]. One reason may lie with prolonged viral shedding, observed more commonly in patients with multiple comorbidities [1]. One study found that 30% of contacts and 76% of cases were still positive for MERS-CoV by PCR 12 days after initial positive samples [56]. A case report showed that a HCW shed MERS-COV for approximately 42 days after initial sampling for diagnosis [57]. Whether viral detection by molecular methods always equates to an infectious risk remains unclear. An additional factor likely rests in patient movements. Intra- and interhospital transmission were documented arising from patient movements within and between hospitals [4] [42], [44]. Control of patient flow within and between healthcare settings with proper infection prevention measures is necessary to decrease the chance of transmission of MERS-CoV.
It is not clear how much asymptomatic and mildly symptomatic patients contribute to viral dissemination in the healthcare setting. Studies have found variable percentages of asymptomatic individuals from 7% to 66% [10], [16], [23], [24], [33], [58]. Data from South Korea showed that none of 82 contacts of an asymptomatic carrier were positive [59], and transmission occurred from asymptomatic individuals [11], [60], [61]. These differences do not yet provide defined answers for the variability in intrahospital transmission; however, they do highlight the need to review and refine infection control practices to help prevent the spread of infection within healthcare settings, and prevent unwitting spread, especially by asymptomatic individuals, by screening HCW contacts by PCR.
What is the contribution of the persistence of the MERS-CoV virus in the environment to its spread? As an RNA virus, MERS-CoV survives surprisingly well on surfaces and in the air, especially at low temperature and low humidity [62]. Sixty minutes after aerosolization, the virus remains infectious at 63.5% at 25 °C and 79% humidity, compared with only 4.7% at conditions 38 °C and 24% humidity [63]. MERS-CoV was detected by RT-PCR and viral culture up to 18–27 days after symptom onset from most of the touchable environments in patients' rooms [64]. Bed controller devices and thermometers were PCR positive until five days after the last positive PCR result from the patient's respiratory specimen [64]. MERS-CoV was cultured in four of seven air samples from patients' rooms, a restroom and a common corridor, and from 15 (22%) of 68 surface swabs [37]. MERS-CoV RNA was detected from anterooms, medical devices, air-ventilating equipment, bed sheets, bed rails, intravenous fluid hangers and X-ray devices [65]. These data suggest that environmental cleaning needs to be meticulous, and that laboratory data may be required to prove adequate disinfection to allow discontinuation of isolation procedures. Further studies are required to assess infectious risks of the environment in the presence of molecularly detected MERS-CoV RNA.
The prospect for control of MERS-CoV relies on multi-faceted programmes to educate HCWs on the disease, to identify potential patients early in their course of illness, to isolate suspected and confirmed cases appropriately, and to implement proper infection control (Table II ). The best prospect for MERS prevention and control rests on basic principles of infection prevention and control in healthcare settings. Balkhy argues that three pillars are necessary: policies and procedures, facility and human resources, and accountability and leadership [66], [67]. How these goals could be achieved is demonstrated, in part, in one study using a core group of nurses for an education programme consisting of an online skill module, donning and doffing of PPE, and hands-on practice to obtain nasopharyngeal swabs [68]. Another study called for the use of the ‘3Is’ (identify–isolate–inform) tool in the emergency department to rapidly identify and isolate suspected cases with any emerging infectious disease [69].
Table II.
A summary of highlights of areas for concern and possible interventions to control Middle East respiratory syndrome (MERS) infection
| Infection control domain | Infection control issues | Suggested interventions |
|---|---|---|
| Environmental | Absent physical barriers between beds, inadequate isolation of suspected MERS patients | Establish respiratory triage areas, adequate isolation of suspected patients: contact and airborne |
| Lack of isolation and negative pressure rooms | Build capacity to accommodate increasing number of patients with respiratory illness | |
| System | Unfamiliarity and under-recognition of MERS infection | Education and periodic review of MERS case definitions |
| Insufficient compliance with infection control measures | Strengthening education, compliance monitoring and feedback | |
| Aerosol-generating procedures | Include training of HCWs on how to protect themselves during these procedures and to carry them in an airborne infection isolation room | |
| Personal | Presence of multiple friends and family members with patients | Limiting visitors to suspected and confirmed cases until patients are no longer infectious |
| ‘Medical shopping’ | Education of the public on the risk of exposure to multiple medical venues |
HCWs, healthcare workers.
Table II provides a summary of potential concern and possible interventions in infection prevention and control to halt MERS-CoV transmission with appropriate interventions. Some listed items are, by nature, parochial, including facility structure, knowledge and skills of HCWs, and patient-related issues. These can be individualized incorporating strategies that include education, feedback and tackling facility-related issues [70]. In a survey of 607 HCWs, hospital infrastructure and design, staffing shortfalls, and absence of infection control training were cited as the primary contributing factors for the spread of MERS-CoV infection in healthcare facilities [71]. In a study from Saudi Arabia of 228 trainees, half of the respondents indicated that their hospital had a clear plan while 28% did not feel that they were well prepared for MERS [72]. A targeted educational strategy increased HCWs' knowledge, skills and attitudes in private hospitals in Saudi Arabia [73]. Raising public awareness and recognition of the disease will also assist in preventing transmission [74].
MERS-CoV presents with a variety of clinical and laboratory findings [4], [13], [75]. There are notable challenges in diagnosis through laboratory and radiological methods, including the possibility of negative swab results by PCR methodology. Currently, the diagnosis of MERS-CoV infection best relies on viral detection by RT-PCR [75], [76], [77]. Lower respiratory samples have been shown to yield better diagnostic results than nasal or oral swabs. In two studies, lower respiratory samples showed higher Ct values than upper respiratory samples, and MERS-CoV virus was detected later in the course of the disease [76], [78]. The World Health Organization recommends that both upper and lower respiratory tract specimens should be collected whenever possible [79]. Improved diagnostic tools are needed for early diagnosis of the disease.
In conclusion, MERS-CoV, like severe acute respiratory syndrome (SARS), requires leadership insisting on formal and continued training of HCWs to be sufficiently prepared for emerging respiratory infectious diseases. Hospitals have varied in their ability to correctly identify potentially infectious patients and quickly implement appropriate infection control measures [52]. Prompt recognition, isolation and management of the suspected cases are vital factors for the prevention of MERS transmission. It has been shown that MERS-CoV nucleic acid was detected by PCR in serum, faeces and urine [80], [81], [82], [83], [84]. Thus, it is important to maintain proper infection control practices when caring for these patients.
Repeated assessments of infection control and monitoring implementation of corrective measures leading to constant readiness will change the course of an outbreak or halt it before it starts [85]. Limiting the number of contacts and hospital visits are also important factors to decrease the spread of infection [86]. Healthcare facilities should take all the steps to learn from past experiences and build capacity for the prevention of outbreaks such as MERS, SARS and Ebola. Healthcare leadership and workers should strive to prevent future outbreaks. Failure to do so would mean that lessons learned from SARS, MERS and Ebola would be relegated to history, leaving patients, hospitals and HCWs vulnerable. Many infection control questions remain requiring further research, including a better understanding of the disease dynamics. Leading concerns include the role of asymptomatic individuals in transmission of the disease, and precise risk factors for transmission in healthcare facilities. Although data accrued have concentrated on respiratory transmission, the role of other body fluids and the built environment in transmission remains unknown. As zoonotic reservoirs for MERS-CoV may continue to produce sporadic human infections, rigorous infection control practices should prevent the virus from spreading past the hospital door.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Al-Tawfiq J.A., Memish Z.A. Drivers of MERS-CoV transmission: what do we know? Exp Rev Respir Med. 2016;10:331–338. doi: 10.1586/17476348.2016.1150784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist. 2014;7:281–287. doi: 10.2147/IDR.S51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijawi B., Abdallat M., Sayaydeh A., Alqasrawi S., Haddadin A., Jaarour N. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(Suppl. 1):S12–S18. [PubMed] [Google Scholar]
- 6.Mailles A., Blanckaert K., Chaud P., van der Werf S., Lina B., Caro V. First cases of middle east respiratory syndrome coronavirus (MERS-COV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. EuroSurveillance. 2013;18 pii:20502. [PubMed] [Google Scholar]
- 7.Majumder M.S., Brownstein J.S., Finkelstein S.N., Larson R.C., Bourouiba L. Nosocomial amplification of MERS-coronavirus in South Korea, 2015. Trans R Soc Trop Med Hyg. 2017;111:261–269. doi: 10.1093/trstmh/trx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aly M., Elrobh M., Alzayer M., Aljuhani S., Balkhy H. Occurrence of the Middle East respiratory syndrome coronavirus (MERS-CoV) across the Gulf Corporation Council countries: four years update. PLoS One. 2017;12:e0183850. doi: 10.1371/journal.pone.0183850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tawfiq J.A., Perl T.M. Middle East respiratory syndrome coronavirus in healthcare settings. Curr Opin Infect Dis. 2015;28:392–396. doi: 10.1097/QCO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO; Geneva: 2017. Middle East respiratory syndrome coronavirus (MERS-CoV) WHO MERS-CoV global summary and assessment of risk global summary 2017.http://www.who.int/emergencies/mers-cov/risk-assessment-july-2017.pdf?ua=1 Available at: [last accessed September 2017] [Google Scholar]
- 11.Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memish Z., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 13.Memish Z.A., Cotten M., Watson S.J., Kellam P., Zumla A., Alhakeem R.F. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W. An observational, laboratory-based study of outbreaks of middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah – a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alraddadi B., Bawareth N., Omar H., Alsalmi H., Alshukairi A., Qushmaq I. Patient characteristics infected with Middle East respiratory syndrome coronavirus infection in a tertiary hospital. Ann Thorac Med. 2016;11:128–131. doi: 10.4103/1817-1737.180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagbo S.F., Skakni L., Chu D.K.W., Garbati M.A., Joseph M., Peiris M. Molecular epidemiology of hospital outbreak of Middle East respiratory syndrome, Riyadh, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:1981–1988. doi: 10.3201/eid2111.150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almekhlafi G.A., Albarrak M.M., Mandourah Y., Hassan S., Alwan A., Abudayah A. Presentation and outcome of Middle East respiratory syndrome in Saudi intensive care unit patients. Crit Care. 2016;20:123. doi: 10.1186/s13054-016-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memish Z.A., Al-Tawfiq J.A., Alhakeem R.F., Assiri A., Alharby K.D., Almahallawi M.S. Middle East respiratory syndrome coronavirus (MERS-CoV): a cluster analysis with implications for global management of suspected cases. Travel Med Infect Dis. 2015;13:311–314. doi: 10.1016/j.tmaid.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Bushra H.E., Abdalla M.N., Al Arbash H., Alshayeb Z., Al-Ali S., Latif Z.A.-A. An outbreak of Middle East Respiratory Syndrome (MERS) due to coronavirus in Al-Ahssa Region, Saudi Arabia, 2015. East Mediterr Health J. 2016;22:468–475. [PubMed] [Google Scholar]
- 23.Balkhy H.H., Alenazi T.H., Alshamrani M.M., Baffoe-Bonnie H., Al-Abdely H.M., El-Saed A. Notes from the field: nosocomial outbreak of Middle East respiratory syndrome in a large tertiary care hospital – Riyadh, Saudi Arabia, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:163–164. doi: 10.15585/mmwr.mm6506a5. [DOI] [PubMed] [Google Scholar]
- 24.Balkhy H.H., Alenazi T.H., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y., Hijazi R. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37:1147–1155. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assiri A.M., Biggs H.M., Abedi G.R., Lu X., Bin Saeed A., Abdalla O. Increase in Middle East respiratory syndrome-coronavirus cases in Saudi Arabia linked to hospital outbreak with continued circulation of recombinant virus, July 1–August 31, 2015. Open Forum Infect Dis. 2016;3:ofw165. doi: 10.1093/ofid/ofw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazer R.I. Outbreak of Middle East respiratory syndrome-coronavirus causes high fatality after cardiac operations. Ann Thorac Surg. 2017;104:e127–e129. doi: 10.1016/j.athoracsur.2017.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assiri A., Abedi G.R., Bin Saeed A.A., Abdalla M.A., al-Masry M., Choudhry A.J. Multifacility outbreak of Middle East respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22:32–40. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter J.C., Nguyen D., Aden B., Al Bandar Z., Al Dhaheri W., Abu Elkheir K. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis. 2016;22:647–656. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauchemez S., Van Kerkhove M.D., Riley S., Donnelly C.A., Fraser C., Ferguson N.M. Transmission scenarios for Middle East respiratory syndrome coronavirus (MERS-CoV) and how to tell them apart. EuroSurveillance. 2013;18 pii:20503. [PMC free article] [PubMed] [Google Scholar]
- 30.Cauchemez S., Fraser C., Van Kerkhove M.D., Donnelly C.A., Riley S., Rambaut A. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14:50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowell G., Abdirizak F., Lee S., Lee J., Jung E., Nishiura H. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi S., Jung E., Choi B.Y., Hur Y.J., Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2018;99:162–168. doi: 10.1016/j.jhin.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastings D.L., Tokars J.I., Aziz Izam Abdel, Alkhaldi K.Z., Bensadek A.T., Alraddadi B.M. Outbreak of Middle East respiratory syndrome at Tertiary Care Hospital, Jeddah, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:794–801. doi: 10.3201/eid2205.151797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowling B.J., Park M., Fang V.J., Wu P., Leung G.M., Wu J.T. Preliminary epidemiologic assessment of MERS-CoV outbreak in South Korea, May–June 2015. EuroSurveillance. 2015;20:7–13. doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y.-S., Lee C., Kim K.M., Kim S.W., Lee K.-J., Ahn J. The first case of the 2015 Korean Middle East respiratory syndrome outbreak. Epidemiol Health. 2015;37:e2015049. doi: 10.4178/epih/e2015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . WHO; Geneva: 2016. Middle East respiratory syndrome coronavirus (MERS-CoV) WHO MERS-CoV global summary and risk assessment global summary, December 2016.http://www.who.int/emergencies/mers-cov/mers-summary-2016.pdf Available at: [last accessed September 2017] [Google Scholar]
- 37.Kim S.-H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi I., Lee D.H., Kim Y. Effects of timely control intervention on the spread of Middle East respiratory syndrome coronavirus infection. Osong Public Heal Res Perspect. 2017;8:373–376. doi: 10.24171/j.phrp.2017.8.6.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y., Lee S., Chu C., Choe S., Hong S., Shin Y. The characteristics of Middle Eastern respiratory syndrome coronavirus transmission dynamics in South Korea. Osong Public Heal Res Perspect. 2016;7:49–55. doi: 10.1016/j.phrp.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H.Y., Lee E.J., Ryu Y.W., Kim Y., Kim H., Lee H. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. EuroSurveillance. 2015;20:1–5. doi: 10.2807/1560-7917.es2015.20.25.21169. [DOI] [PubMed] [Google Scholar]
- 42.Kim K.H., Tandi T.E., Choi J.W., Moon J.M., Kim M.S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95:207–213. doi: 10.1016/j.jhin.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishiura H., Endo A., Saitoh M., Kinoshita R., Ueno R., Nakaoka S. Identifying determinants of heterogeneous transmission dynamics of the Middle East respiratory syndrome (MERS) outbreak in the Republic of Korea, 2015: a retrospective epidemiological analysis. BMJ Open. 2016;6:e009936. doi: 10.1136/bmjopen-2015-009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh M., Choe P.G., Oh H.S., Park W.B., Lee S.-M., Park J. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci. 2015;30:1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang C.K., Song K.H., Choe P.G., Park W.B., Bang J.H., Kim E.S. Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Korean Med Sci. 2017;32:744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nam H.-S., Park J.W., Ki M., Yeon M.-Y., Kim J., Kim S.W. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alenazi T.H., Al Arbash H., El-Saed A., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y.M. Identified transmission dynamics of Middle East respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65:675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamers M.M., Raj V.S., Shafei M., Ali S.S., Abdallh S.M., Gazo M. Deletion variants of Middle East respiratory syndrome coronavirus from humans, Jordan, 2015. Emerg Infect Dis. 2016;22:716–719. doi: 10.3201/eid2204.152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization . WHO; Geneva: 2016. WHO update and clarification on recent MERS cases reported by the Kingdom of Saudi Arabia 23 June 2016.http://who.int/emergencies/mers-cov/saudi-arabia-update/en/ Available at: [last accessed September 2017] [Google Scholar]
- 50.World Health Organization . WHO; Geneva: 2015. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection.http://apps.who.int/iris/bitstream/handle/10665/174652/WHO_MERS_IPC_15.1_eng.pdf;jsessionid=E60EEABCA96F2383F0F1D82C3A44C13B?sequence=1 Available at: [last accessed April 2017] [Google Scholar]
- 51.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2015. Interim infection prevention and control recommendations for hospitalized patients with Middle East respiratory syndrome coronavirus (MERS-CoV)https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html Available at: [last accessed March 2017] [Google Scholar]
- 52.Foote M.M.K., Styles T.S., Quinn C.L. Assessment of hospital emergency department response to potentially infectious diseases using unannounced mystery patient drills — New York City, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:945–949. doi: 10.15585/mmwr.mm6636a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Command and Control Center Ministry of Health Kingdom of Saudi Arabia Scientific Advisory Board . 4th ed. 2017. Infection prevention and control guidelines for the Middle East respiratory syndrome coronavirus (MERS-CoV) infection.http://www.moh.gov.sa/endepts/Infection/Documents/Guidelines-for-MERS-CoV.PDF Available at: [last accessed May 2017] [Google Scholar]
- 54.Saudi Ministry of Health . Saudi Ministry of Health (website); Riyadh, Saudi Arabia: 2015. MOH’s command and control center forms rapid response teams including 120 health specialists.http://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2015-04-12-001.aspx Available at: [last accessed September 2017] [Google Scholar]
- 55.Lee J., Rapid Response Team, Kim W.J. Collaborative intervention of Middle East respiratory syndrome: rapid response team. Infect Chemother. 2016;48:71–74. doi: 10.3947/ic.2016.48.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Memish Z.A., Assiri A.M., Al-Tawfiq J.A. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Gethamy M., Corman V.M., Hussain R., Al-Tawfiq J.A., Drosten C., Memish Z.A. A case of long-term excretion and subclinical infection with middle east respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60:973–974. doi: 10.1093/cid/ciu1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Hosani F.I., Pringle K., Al Mulla M., Kim L., Pham H., Alami N.N. Response to emergence of Middle East respiratory syndrome coronavirus, Abu Dhabi, United Arab Emirates, 2013–2014. Emerg Infect Dis. 2016;22:1162–1168. doi: 10.3201/eid2207.160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon S., Son J.S. Infectivity of an asymptomatic patient with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2017;64:1457–1458. doi: 10.1093/cid/cix170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alfaraj S.H., Al-Tawfiq J.A., Altuwaijri T.A., Memish Z.A. Middle East respiratory syndrome coronavirus and pulmonary tuberculosis coinfection: implications for infection control. Intervirology. 2017;60:53–55. doi: 10.1159/000477908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alfaraj S.H., Al-Tawfiq J.A., Altuwaijri T.A., Alanazi M., Alzahrani N., Memish Z.A. Middle East respiratory syndrome coronavirus transmission among health care workers: implication for infection control. Am J Infect Control. 2018;46:165–168. doi: 10.1016/j.ajic.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. EuroSurveillance. 2013;18 doi: 10.2807/1560-7917.es2013.18.38.20590. pii:20590. [DOI] [PubMed] [Google Scholar]
- 63.Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeong H.W., Heo J.Y., Kim H.-W., Choi Y.K., Song M.-S., Bin Seo Y. Persistent environmental contamination and prolonged viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Open Forum Infect Dis. 2015;2(Suppl 1):1978a. [Google Scholar]
- 65.Bin Seo Y., Heo J.Y., Song M.-S., Lee J., Kim E.-H., Park S.-J. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis. 2016;62:755–756. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balkhy H. MERS CoV: a trigger for healthcare transformation. J Infect Public Health. 2016;9:1–2. doi: 10.1016/j.jiph.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balkhy H. The emergence of a new corona virus – MERS-CoV: hind sight is always 20/20. J Infect Public Health. 2013;6:317–318. doi: 10.1016/j.jiph.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Tawfiq J.A., Rothwell S., Mcgregor H.A., Khouri Z.A. A multi-faceted approach of a nursing led education in response to MERS-CoV infection. J Infect Public Health. 2018;11:260–264. doi: 10.1016/j.jiph.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koenig K. Identify–isolate–inform: a modified tool for initial detection and management of Middle East respiratory syndrome patients in the emergency department. West J Emerg Med. 2015;16:619–624. doi: 10.5811/westjem.2015.7.27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J.Y., Kim G., Lim D.-G., Jee H.-G., Jang Y., Joh J.-S. A Middle East respiratory syndrome screening clinic for health care personnel during the 2015 Middle East respiratory syndrome outbreak in South Korea: a single-center experience. Am J Infect Control. 2018;46:436–440. doi: 10.1016/j.ajic.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabaan A.A., Alhani H.M., Bazzi A.M., Al-Ahmed S.H. Questionnaire-based analysis of infection prevention and control in healthcare facilities in Saudi Arabia in regards to Middle East respiratory syndrome. J Infect Public Health. 2017;10:548–563. doi: 10.1016/j.jiph.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aldrees T., Al Ghobain M., Alenezi A., Alqaryan S., Aldabeeb D., Alotaibi N. Medical residents’ attitudes and emotions related to Middle East respiratory syndrome in Saudi Arabia. A cross-sectional study. Saudi Med J. 2017;38:942–947. doi: 10.15537/smj.2017.9.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nour M.O., Babalghith A.O., Natto H.A., Alawneh S.M., Elamin F.O. Raising awareness of health care providers about MERSCoV infection in public hospitals in Mecca, Saudi Arabia. East Mediterr Health J. 2017;23:534–542. [PubMed] [Google Scholar]
- 74.Bawazir A., Al-Mazroo E., Jradi H., Ahmed A., Badri M. MERS-CoV infection: mind the public knowledge gap. J Infect Public Health. 2018;11:89–93. doi: 10.1016/j.jiph.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A. Middle East respiratory syndrome-coronavirus (MERS-CoV): a case–control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Tawfiq J.A., Hinedi K. The calm before the storm: clinical observations of Middle East respiratory syndrome (MERS) patients. J Chemother. 2018;30:179–182. doi: 10.1080/1120009X.2018.1429236. [DOI] [PubMed] [Google Scholar]
- 78.Memish Z.A.Z.A., Al-Tawfiq J.A.J.A., Makhdoom H.Q.H.Q., Assiri A., Alhakeem R.F.R.F., Albarrak A. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.World Health Organization . WHO; Geneva: 2015. Laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV)http://apps.who.int/iris/bitstream/10665/176982/1/WHO_MERS_LAB_15.1_eng.pdf?ua=1 Available at: [last accessed December 2016] [Google Scholar]
- 80.Zhou J., Li C., Zhao G., Chu H., Wang D., Yan H.H.-N. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3:eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abroug F., Slim A., Ouanes-Besbes L., Hadj Kacem M.-A., Dachraoui F., Ouanes I. Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis. 2014;20:1527–1530. doi: 10.3201/eid2009.140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kraaij-Dirkzwager M., Timen A., Dirksen K., Gelinck L., Leyten E., Groeneveld P. Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. EuroSurveillance. 2014;19 doi: 10.2807/1560-7917.es2014.19.21.20817. pii:20817. [DOI] [PubMed] [Google Scholar]
- 83.Poissy J., Goffard A., Parmentier-Decrucq E., Favory R., Kauv M., Kipnis E. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol. 2014;61:275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cha R., Yang S.H., Moon K.C., Joh J.-S., Lee J.Y., Shin H.-S. A case report of a Middle East respiratory syndrome survivor with kidney biopsy results. J Korean Med Sci. 2016;31:635. doi: 10.3346/jkms.2016.31.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Bushra H.E., Al Arbash H.A., Mohammed M., Abdalla O., Abdallah M.N., Al-Mayahi Z.K. Outcome of strict implementation of infection prevention control measures during an outbreak of Middle East respiratory syndrome. Am J Infect Control. 2017;45:502–507. doi: 10.1016/j.ajic.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim S.W., Park J.W., Jung H.-D., Yang J.-S., Park Y.-S., Lee C. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin Infect Dis. 2017;64:551–557. doi: 10.1093/cid/ciw768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization . WHO; Geneva: 2014. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update as of 9 May 2014.http://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_Update_09_May_2014.pdf?ua=1 Available at: [last accessed September 2017] [Google Scholar]
- 88.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]