Abstract

Effects of preparation parameters of NiAl oxide-supported Au nanocatalysts on their performance in the chemoselective hydrogenation of nitro compounds were investigated. The deposition–precipitation method, low Au loading, and low Ni/Al molar ratio of the support contributed to the generation of small-sized Au nanoparticles. High catalytic properties were related to the small sizes of Au particles and appropriate basicity of supports. Accordingly, the 0.43% Au/NiAlO-2-500 (the Ni/Al molar ratio of the support = 2) showed high activity and excellent selectivity for nitro hydrogenation. It also showed good versatility for other nitro compounds and good recyclability. Interestingly, for the first time, this Au catalyst switched its selectivity to vinyl hydrogenation by mere regulation of the composition of the support (the Ni/Al molar ratio of the support = 4). The observed shift in selectivity was ascribed to the different adsorption behaviors of the nitro and vinyl group on Au nanocatalysts. It provides a novel and facile strategy to construct Au nanocatalysts with high activity and switchable selectivity for hydrogenation of nitro compounds by the fine tuning of preparation parameters.
1. Introduction
The chemoselective hydrogenation of nitro compounds to the corresponding aromatic amines is one of the most important reactions in both academia and industry, as the resulting compounds serve as the important intermediates for pharmaceuticals, agrochemicals, fine chemicals, dyes, and polymers.1−4 This kind of reaction has been successfully reduced by stoichiometric reducing agents such as sodium hydrosulfite, iron, and zinc, generating a large number of byproducts which are very harmful to our environment.2,5 From the viewpoints of green chemistry and sustainable development, it is our objective that the reaction mentioned above is carried out over heterogeneous catalysts with H2 as the reductant under mild conditions.
As one of the most used heterogeneous hydrogenation catalysts, the platinum-group metal catalysts such as platinum and palladium are usually employed for this kind of reaction, but they suffer from low selectivity, especially when the nitro group and other reductive functional groups co-exist on the same substrate because of the simultaneous hydrogenation of these reductive groups.6,7 The preferential activation and hydrogenation of the nitro group could be achieved by tuning Pt absorption characteristic with phosphorous additives.8 The Pt single-atom and pseudo single-atom on FeOx displayed outstanding activity and high selectivity for hydrogenation of the nitro group.9 Recently, atomically dispersed Pt over α-MoC was reported to be a highly CO-resistant and selective catalyst for hydrogenation of nitrobenzene derivatives.10 Although these catalytic processes are effective, the addition of phosphorous additives are detrimental to our environment, especially, the preparation of Pt single-atom catalysts remains critical and usually must be controlled precisely. It is still highly desirable to develop the kind of heterogeneous catalysts with high performance for chemoselective hydrogenation of nitro compounds.
Because Haruta et al. discovered the unprecedented catalytic activity of supported Au nanoparticles for low temperature CO oxidation, gold nanocatalysts have gained increasingly much attention and are widely used in a variety of important reactions, especially in selective hydrogenations.4,11−17 Corma and Serna2 first reported that Au/TiO2 afforded >95% selectivity toward 3-aminostyrene at high conversion in the chemoselective hydrogenation of 3-nitrostyrene, which was far more selective than Pd/C and Pt/C. Recently, using the colloidal deposition methods, gold nanoparticles positioned on the edge/corner sites of TiO2 showed the 99% conversion and 99% selectivity in the selective hydrogenation of 3-nitrobenzene.18 Gold catalysts with the well-controlled size (2.0 nm) were also obtained through the calcination of cysteine-capped Au25 nanoclusters on ZnAl-hydrotalcite, giving >98% selectivity to 3-vinylaniline at complete conversion.19 The modification of the support with a single-site Sn promoter, or the change in the types of divalent metal ions of the hydrotalcite support also affected catalytic performances and even hydrogenation reaction pathways.20,21 Therefore, it is a general and facile approach to construct gold nanocatalysts with high property by the fine control of preparation parameters.
Following the aforementioned rule, gold nanocatalysts were obtained with the use of the deposition–precipitation method and NiAl-hydrotalcite as a precursor of the support. The as-prepared Au nanocatalysts exhibited high activity, excellent selectivity to nitro hydrogenation for chemoselective hydrogenation of 4-nitrostyrene. It also showed good performance for the selective reduction of other substrates containing nitro groups. Moreover, the selectivity toward nitro hydrogenation could be drastically switched to vinyl hydrogenation by changing the composition of the support. To the best of our knowledge, it is the first report to shift the selectivity from hydrogenation of the nitro group to the vinyl group via the modification of the composition of the support. The observed properties probably resulted from the small sizes of gold nanoparticles and nature of NiAl oxides.
2. Results & Discussion
The NiAl-layered double hydroxide (LDH) materials and the corresponding Au catalysts were prepared using the co-precipitation method22,23 and deposition–precipitation method,23 respectively (see the Experimental Section for details). A series of characterizations, including X-ray diffraction (XRD), inductively coupled plasma atomic emission spectrometry (ICP–AES), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and CO2-temperature-programmed desorption (TPD), were conducted to obtain the structure and nature of supports and gold catalysts. The narrow, symmetric, and strong peaks of the support at 2θ angles (i.e., <25°) were observed, confirming its well-defined layered structure characteristics (Figure 1a). After calcination, the patterns of the mixed oxide reflected those of NiO because of the minor content of aluminum.23 No diffraction peaks belonging to Au were found for as-prepared gold nanocatalysts. It is related to the low loading or high dispersion of gold nanoparticles on the surface of NiAlO-2. The low content of as-prepared gold catalysts was also confirmed by ICP–AES (Table 1).
Figure 1.

XRD spectra of supports and Au catalysts: (a) NiAl-LDHs (Ni/Al = 2), (b) NiAlO-2-500 (Ni/Al = 2), (c) 0.15% Au/NiAlO-2-500, (d) 0.43% Au/NiAlO-2-500, (e) 0.68% Au/NiAlO-2-500, (f) 0.38% Au/NiAlO-3-500-SD, (g) 0.44% Au/NiAlO-4-500, and (h) 0.43% Au/NiAlO-2-500 (used).
Table 1. Selective Hydrogenation of 4-Nitrostyrene over Different Au Catalystsa.
| sel./%c |
|||||
|---|---|---|---|---|---|
| entry | catalystsb | con./%c | 1 | 2 | 3 |
| 1 | NiAlO-2-500 | 0.3 | 58.2 | 25.9 | 15.9 |
| 2 | 0.43% Au/NiAlO-2-500 | 98.4 | 98.1 | 0.2 | 1.7 |
| 3 | 0.38% Au/NiAlO-2-500-SD | 29.4 | 95.0 | 1.5 | 3.5 |
| 4 | 0.15% Au/NiAlO-2-500 | 18.9 | 82.9 | 16.2 | 2.9 |
| 5 | 0.68% Au/NiAlO-2-500 | 98.1 | 94.6 | 0.3 | 5.1 |
| 6 | 0.38% Au/NiAlO-3-500 | 40.5 | 63.6 | 35.3 | 1.1 |
| 7 | 0.44% Au/NiAlO-4-500 | 74.8 | 2.0 | 94.5 | 3.5 |
Reaction conditions: p(H2) = 1.0 MPa; T = 100 °C; catalyst 0.1 g; 4-nitrostyrene 100 μL; toluene 5 mL; stirring speed = 500 rpm; and reaction time 4 h.
The molar ratio of Ni to Al and the Au actual loading were determined by ICP–AES.
The conversion of 4-nitrostyrene and selectivity toward the products were determined by gas chromatography (GC) and GC–mass spectrometry (MS). No other byproducts were observed, and the carbon mass balance of about 96% was obtained by using o-xylene as an internal standard.
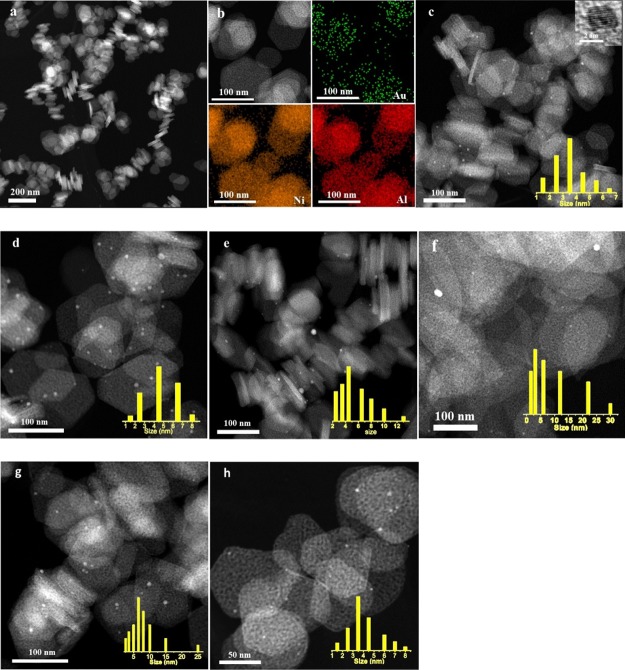
To obtain the size distributions of gold nanoparticles, high-angle annular dark-field imaging scanning TEM (HAADF-STEM) characterizations were conducted. As shown in Figure 2a, no gold nanoparticles were observed for 0.15% Au/NiAlO-2-500, but the elemental mappings showed Au, Ni, and Al (Figure 2b, O omitted). It indicated that this catalyst might had the very small size because of the low loading of gold (i.e., ultrasmall clusters and even single atoms). The average particle sizes of 0.43% Au/NiAlO-2-500 and 0.68% Au/NiAlO-2-500 catalysts were 3.5 and 4.8 nm, respectively, while the 0.38% Au/NiAlO-2-500-SD catalyst prepared using the sol deposition method possessed the larger average diameter of 6.1 nm along with the broader size distribution (Figure 2c–e). The HRTEM image of Au nanoparticles showed the obvious crystal lattices subjected to Au, with a crystal lattice spacing of 0.204 nm (inset of HAADF-STEM image of 0.43% Au/NiAlO-2-500), which is in accordance with the (200) planes of Au nanoparticles.23 It showed that the gold species on the support existed in the state of metal particles. The average particle sizes of 0.38% Au/NiAlO-3-500 and 0.44% Au/NiAlO-4-500 were 8.6 and 7.7 nm (Figure 2f–g), respectively. Although using the same preparation procedure and similar Au loadings, the mean size and size distributions of gold nanoparticles on supports with different compositions were different, indicating the different capabilities of supports to anchor gold nanoparticles.
Figure 2.
Representative HAADF-STEM images of Au catalysts: (a) 0.15% Au/NiAlO-2-500; (b) energy-dispersive X-ray spectroscopy elemental mapping of 0.15% Au/NiAlO-2-500; (c) 0.43% Au/NiAlO-2-500; (d) 0.68% Au/NiAlO-2-500; (e) 0.38% Au/NiAlO-2-500-SD; (f) 0.38% Au/NiAlO-3-500; (g) 0.44% Au/NiAlO-4-500; and (h) 0.43% Au/NiAlO-2-500 (used). Insets show a gold particle with the crystal lattice and the size distributions of gold nanoparticles.
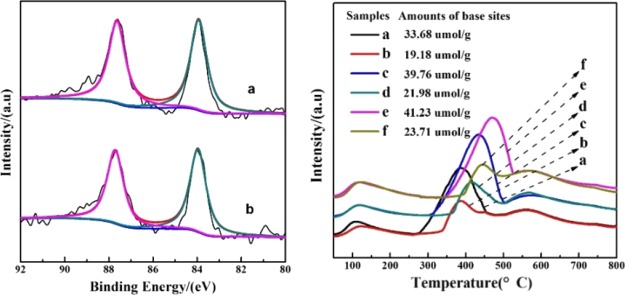
XPS were carried out to clarify the chemical states of gold nanocatalysts (Figure 3). Of note, the 0.15% Au/NiAl-2-500 only gave noisy spectra because of the low loading of gold (not shown). For 0.43% Au/NiAl-2-500 and 0.68% Au/NiAl-2-500, the binding energies of Au 4f7/2 were 83.9 eV, which are characteristic of 4f7/2 of the Au metallic (84.0 eV),24 which is in in accordance with the HRTEM results. CO2-TPD was conducted to measure the basicity of supports and the corresponding Au catalysts. As shown in Figure 3 (right), the desorption temperatures of CO2 (200–550 °C) and amounts of basic sites of NiAl oxides and the corresponding Au catalysts gradually increased with the increase in the molar ratio of Ni to Al. Among the observed samples, the NiAl-4-500 support and the 0.44% Au/NiAl-4-500 catalyst gave the strongest basicity, respectively.
Figure 3.
Left: Au 4f XPS spectra of typical Au catalysts: (a) 0.43% Au/NiAlO-2-500 and (b) 0.68% Au/NiAlO-2-500. Right: CO2-TPD spectra of supports and Au catalysts: (a) NiAlO-2-500; (b) 0.43% Au/NiAlO-2-500; (c) NiAlO-3-500; (d) 0.38% Au/NiAlO-3-500; (e) NiAlO-4-500; and (f) 0.44% Au/NiAlO-4-500.
Chemoselective hydrogenation of 4-nitrostyrene was employed as a model reaction to evaluate catalytic properties of the as-prepared Au nanocatalysts, and the results are listed in Table 1. The plain NiAlO-2-500 support offered a negligible activity, while gold nanoparticles loaded on NiAlO-x-500 exhibited good catalytic properties, indicating that gold nanoparticles were catalytic active sites. It was previously reported that gold atoms active for H2 dissociation were neutral or had a net charge close to zero.25 According to our characterization results, the as-prepared Au nanoparticles existed at zero valence, confirming that they were indeed active sites. Furthermore, 0.43% Au/NiAlO-2-500 prepared by using the deposition–precipitation method showed higher catalytic performance than 0.38% Au/NiAlO-2-500-SD prepared by using the colloidal deposition method (entries 2 and 3). The former afforded the 98.4% conversion of 4-nitrostyrene and 98.1% selectivity to 4-aminostyrene, while the latter only afforded the 29.4% conversion and 95.0% selectivity. Therefore, the deposition–precipitation method was hereafter chosen and used to obtain Au nanocatalysts.
Next, the effects of preparation parameters such as Au loading amounts and calcination temperature were examined. When the Au actual loading was 0.43% and the calcination temperature was 500 °C, the corresponding 0.43% Au/NiAlO-2-500 catalysts showed the highest catalytic properties among the catalysts examined (entries 2–5). In gold-catalyzed hydrogenation reactions, the activation and dissociation of H2 is often the rate-limiting step. Small-sized gold nanocatalysts such as 0.43% Au/NiAlO-2-500 have more low-coordinated edge and corner atoms, being usually considered to be catalytic active sites for H2 dissociation, and then exhibit higher catalytic performance.19,25 It was reported that Au single atoms were not effective for hydrogenation reactions of the aldehydes and the nitro compounds because of the low activation capability of H2.26,27 Instead, Au nanoclusters with a large diameter showed high catalytic activity for hydrogenation of the nitro compounds.27 The poor activity of 0.15% Au/NiAlO-2-500 catalysts was supposed to be related to the ultrasmall size of gold species.
When the molar ratio of Ni to Al of the support changed from 2 to 4, the as-prepared Au catalysts exhibited the 74.8% conversion of 4-nitrostyrene and 94.5% selectivity toward 4-nitroethylbenzene under identical reaction conditions. The sizes of Au nanoparticles in 0.44% Au/NiAlO-4-500 are larger than those of 0.43% Au/NiAlO-2-500, thereby exhibiting the lower activity. The basicity of the catalysts was also reported to affect the reactivity for the hydrogenation of nitroaromatics.28,29 Tan et al.19 also found that the support with strong basicity (i.e., MgAl-hydrotalcite) afforded the low activity of the resultant Au nanocatalyst for chemoselective hydrogenation of 3-nitrostyrene. As shown in the CO2-TPD, NiAlO-4-500 as the support showed stronger basicity than NiAlO-2-500 and partially accounted for the low activity of the 0.44% Au/NiAlO-4-500 catalyst.
To clarify the different selectivity (hydrogenation of the nitro group vs the vinyl group) over Au nanoparticles loaded on supports with different compositions, control experiments were carried out using the styrene and the mixture of styrene and nitrobenzene as substrates under the same reaction conditions (Table 2). It showed that the styrene could be hardly consumed on the 0.43% Au/NiAlO-2-500 catalyst no matter if there was nitrobenzene in the substrate or not. Interestingly, the hydrogenation of the vinyl group was preferred over that of the nitro group using the 0.44% Au/NiAlO-4-500 catalyst. The evidence for different selectivity over aforementioned Au catalysts was provided by attenuated total reflection infrared (ATR-IR) spectroscopy (Figure 4). For the 0.43% Au/NiAlO-2-500 catalyst, the IR bands, assigned to the νas(NO2) and νs(NO2), were found at 1524 and 1347 cm–1, respectively, when 4-nitrostyrene or nitrobenzene was used as the probe molecule, but no ν(C=C) at 1631 cm–1 and δ(=C–H) at 1419 cm–1 were observed if styrene was applied instead. For the 0.44% Au/NiAlO-4-500 catalyst, nitrobenzene adsorption experiments showed that the νas(NO2) and νs(NO2) were absent, and the IR bands belonging to ν(C=C) and δ(=C–H) were observed in the styrene or 4-nitrostyrene adsorption experiments. It showed that the nitro group could selectively adsorb on the surface of the 0.43% Au/NiAlO-2-500 catalyst, while the vinyl group preferred to adsorb on the surface of the 0.44% Au/NiAlO-4-500 catalyst. For chemoselective hydrogenation of 4-nitrostyrene, 4-nitrostyrene was adsorbed on the surface of NiAl oxides, and the adsorption configuration of the nitro group or the vinyl group appeared over the 0.43% Au/NiAlO-2-500 or 0.44% Au/NiAlO-4-500 catalysts, respectively. Then, molecular hydrogen was first adsorbed on the surface of Au nanoparticles, and the resulting hydrogen atoms diffused to the surface of NiAl oxides around Au nanoparticles, followed by the interaction with the adsorbed 4-nitrostyrene and the generation of 4-aminostyrene or 4-nitroethylbenzene accordingly. Therefore, the different adsorption models of the nitro group and the vinyl group of 4-nitrostyrene over the 0.43% Au/NiAlO-2-500 and 0.44% Au/NiAlO-4-500 catalysts led to their distinct selectivity.
Table 2. Control Experiments over 0.43% Au/NiAlO-2-500 and 0.44% Au/NiAlO-4-500a.
| conversion
(%) |
|||
|---|---|---|---|
| catalysts | styreneb | styrenec | nitrobenzenec |
| 0.43% Au/NiAlO-2-500 | 0.1 | 1.7 | 100.0 |
| 0.44% Au/NiAlO-4-500 | 97.2 | 51.0 | 4.4 |
Reaction conditions: p(H2) = 1.0 MPa; T = 100 °C; catalyst 0.1 g; toluene 5 mL; stirring speed = 500 rpm; and reaction time 4 h.
Substrate: 89.93 μL of styrene.
Substrate: 89.93 μL of styrene and 80.28 μL of nitrobenzene.
Figure 4.

ATR-IR spectra of adsorbed species on 0.43% Au/NiAlO-2-500 and 0.44% Au/NiAlO-4-500: (a) styrene; (b) 4-nitrostyrene; and (c) nitrobenzene.
Considering that the nitro group is one of the most easily reducible groups in transition metal-catalyzed reactions, the selective hydrogenation of the C=C double bond in the presence of the nitro group is also a great challenge.30,31 The controllable chemoselectivity toward the nitro and vinyl group of 3-nitrostyrene was obtained over heterogeneous Pt and Rh catalysts by changing the source of hydrogen, adding acid or basic organics into the reaction medium, and also using the carbon support with surface oxygen-containing groups and hetero P species.32−34 Recently, we reported that the switchable selectivity for the hydrogenation of the aldehyde and the nitro group of 4-nitrobenzaldehyde was also achieved by the use of ligand-on or ligand-off Au nanocluster catalysts.35 Even so, to our knowledge, our research is the first example to efficiently change the selectivity by tuning the composition of the support. It provides a novel and facile approach to design Au catalysts with high activity and tunable selectivity toward the hydrogenation of nitro compounds by modifying the composition of the support and adsorption behavior of different reducing groups on Au catalysts.
The distribution of the products with reaction time over the 0.43% Au/NiAlO-2-500 catalyst for the chemoselective hydrogenation of 4-nitrostyrene is monitored and shown in Figure 5. The yield of 4-aminostyrene increased with the increasing reaction time. After 180 min, the yield of 4-aminostyrene reached 96.5% and then remained almost unchanged. It showed that the 0.43% Au/NiAlO-2-500 catalyst was highly active and selective for chemoselective hydrogenation of 4-nitrostyrene.
Figure 5.
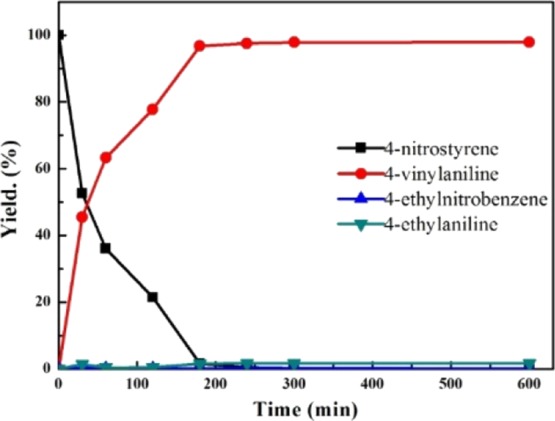
Evolution of the product distribution with reaction time over the 0.43% Au/NiAlO-2-500 catalyst. Reaction conditions: p(H2) = 1.0 MPa; T = 100 °C; catalyst 0.1 g; 4-nitrostyrene 100 μL; toluene 5 mL; stirring speed = 500 rpm; and reaction time 4 h.
The general applicability of the 0.43% Au/NiAlO-2.0-500 catalyst for chemoselective hydrogenation of a series of nitro compounds has been further investigated by extending the substrate scope, and the results are listed in Table 3. As seen in Table 3, good to excellent conversions (78.6–100.0%) and excellent selectivities (86.3–100.0%) were obtained in the hydrogenation of substrates except for 3,5-dimethylnitrobenzene. It showed that 0.43% Au/NiAlO-2.0-500 was a highly efficient catalyst for hydrogenation reactions of nitro compounds.
Table 3. Selective Hydrogenation of Different Substrates over 0.43% Au/NiAlO-2-500a.
Reaction conditions: p(H2) = 1.0 MPa; T = 100 °C; catalyst 0.1 g; 4-nitrostyrene 100 μL; toluene 5 mL; stirring speed = 500 rpm; and reaction time 4 h.
The conversion of 4-nitrostyrene and selectivity for the products were determined by GC and GC–MS.
Reaction time 165 min.
Others include aniline.
Finally, the reusability of the 0.43% Au/NiAlO-2.0-500 catalyst was examined. No apparent loss of conversion and selectivity was observed when it was consecutively recycled six times in the chemoselective hydrogenation of 4-nitrostyrene (Figure 6). Subsequently, the used catalyst was examined by XRD, HAADF-STEM, and ICP–AES (Figures 1 and 2). XRD results showed no obvious changes in the used catalyst. HAADF-STEM results confirmed that the average size and distribution of gold nanoparticles did not change obviously, and the gold loading of the used catalyst was almost the same as that of the fresh one, as determined by ICP–AES. These results demonstrate that the 0.43% Au/NiAlO-2.0-500 catalyst was a highly active and stable catalyst for the chemoselective hydrogenation of nitro compounds.
Figure 6.

Recyclability of 0.43% Au/NiAlO-2-500 for selective hydrogenation of 4-nitrostyrene. Reaction conditions: p(H2) = 1.0 MPa; T = 100 °C; catalyst 0.1 g; 4-nitrostyrene 100 μL; toluene 5 mL; stirring speed = 500 rpm; and reaction time 4 h.
3. Conclusions
The preparation conditions had great influence on the sizes of supported Au nanoparticles, nature of support and then their performance in nitro compounds chemoselective hydrogenation. The deposition–precipitation method instead of the colloidal deposition method, low Au loading, and low Ni/Al molar ratio were beneficial for the formation of small-sized Au nanoparticles. Excellent catalytic performance of the as-prepared Au catalysts depended on the small sizes of Au nanoparticles and basicity of supports. The 0.43% Au/NiAlO-2-500 catalyst with NiAlO-2 as the support afforded 98.4% conversion and 98.1% selectivity toward 4-aminostyrene for the hydrogenation of 4-nitrostyrene. It also showed good versatility for other substrates containing nitro groups and good recyclability. Surprisingly, using NiAlO-4-500 as the support instead, the 0.44% Au/NiAlO-4-500 catalyst afforded 74.8% conversion and 94.5% selectivity for 4-nitroethylbenzene. For the first time, the switchable selectivity for hydrogenation of the nitro group and the vinyl group was achieved over Au catalysts by changing the composition of supports. The change in selectivity was connected with the different adsorption configuration of the nitro and vinyl group on the surface of the support. The fine regulation of preparation parameters such as small sizes of Au nanoparticles and nature of support can afford the Au nanocatalysts with excellent activity and tunable selectivity for hydrogenation of nitro compounds.
4. Experimental Section
4.1. Preparation of NiAl-LDHs
The NiAl-LDH was obtained using the co-precipitation method.22,23 An aqueous solution of NaOH and Na2CO3 and a mixed aqueous solution containing nickel nitrate and aluminum nitrate were separately dropped to 50 mL of deionized water at room temperature under stirring. The pH of the solution was maintained at around 9.0 during the process. The as-prepared material was transferred to an autoclave and heated at 180 °C for 48 h. The resultant suspension was separated by filtration, washed with deionized water, and dried at 80 °C for 12 h. Finally, NiAl-LDH-x in diverse compositions (x = 2.0, 3.0, and 4.0; x refers to the molar ratio of nickel to aluminum) were prepared by changing the molar ratio of nickel nitrate to aluminum nitrate.
4.2. Preparation of Au Nanocatalysts
The Au/NiAlO-x catalysts were produced by a deposition–precipitation method.23 Typically, 1.0 g of NiAl-LDH-x was added to 80 mL of deionized water, and the as-obtained mixture was stirred for 1 h. Then, 2.0 mL of aqueous HAuCl4 (0.025 mol/L) was then added and stirred for 2 h. After 0.3 g of urea was added, temperature of the resulting mixture was increased to 80 °C and maintained for a period of time. The as-prepared suspension was centrifugated, thoroughly washed with deionized water, and dried at 60 °C for 12 h. Finally, the solid was calcined under an Ar atmosphere at 500 °C for 2 h, denoted as Au/NiAlO-x-500.
The Au/NiAlO-2-500-SD catalysts were prepared as follows. Typically, 0.3 mL of aqueous HAuCl4 (0.025 mol/L) and 0.4 g of polyvinyl pyrrolidone were added to 20.0 mL of deionized water under stirring. After 5.0 mL of deionized water containing 0.004 g NaBH4 was added dropwise, the resultant mixture was stirred for 2.0 h. Then, 0.2 g of NiAl-LDH-2.0 was added, and the as-obtained mixture was stirred at room temperature for 2.0 h. The suspension was centrifugated, thoroughly washed with deionized water, and dried at 60 °C for 12 h. Finally, the solid was calcined under atmosphere of Ar at 500 °C for 2 h, denoted as Au/NiAlO-2-500-SD.
4.3. Characterizations of Supports and Catalysts
XRD patterns were obtained on a Shimadzu XRD-6000 using Cu Kα radiation operated at 40 kV and 30 mA. STEM images were recorded on a JEOL JEM2100F S/TEM in a high-angle annular dark-field STEM mode at an operating voltage of 200 kV. The gold content and molar ratio of nickel to aluminum of catalysts were measured by ICP–AES. XPS were performed under ultrahigh vacuum (<10–6 Pa) on a Thermo Scientific ESCALAB 250Xi spectrometer with an Al anode (Al Kα = 1486.6 eV). All binding energies were calibrated using contaminant carbon (C 1s = 284.6) as the reference. CO2-TPD was examined on a micromeritics chemisorb 2720 instrument with a thermal conductivity detector. An ATR-IR spectroscope was equipped with a DTGS detector, and the spectrum was recorded with a Nicolet 5700 spectrometer.
4.4. Evaluation of Catalytic Properties of Au Nanocatalysts
The chemoselective hydrogenation of 4-nitrostyrene was carried out in a stainless steel autoclave equipped with a pressure gauge under magnetic stirring. The 5.0 mL of toluene, 100 μL of 4-nitrostyrene, and 0.1 g of Au catalysts were introduced into the autoclave. After sealing, the autoclave was flushed 4 times with hydrogen and then pressurized at 1.0 MPa. To initiate the reaction, the reactor was heated to 100 °C and maintained at this temperature under stirring. After reaction, the mixture was cooled to room temperature and analyzed by GC/MS.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (21576248, 21671178, and 21571159) and the research fund from the doctoral program of Zhengzhou University of Light Industry (2014BSJJ007).
The authors declare no competing financial interest.
References
- Tafesh A. M.; Weiguny J. A review of the selective catalytic reduction of aromatic nitro compounds into aromatic amines, isocyanates, carbamates, and ureas using CO. Chem. Rev. 1996, 96, 2035. 10.1021/cr950083f. [DOI] [PubMed] [Google Scholar]
- Corma A.; Serna P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 2006, 313, 332. 10.1126/science.1128383. [DOI] [PubMed] [Google Scholar]
- Jagadeesh R. V.; Surkus A.-E.; Junge H.; Pohl M.-M.; Radnik J.; Rabeah J.; Huan H.; Schunemann V.; Bruckner A.; Beller M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073. 10.1126/science.1242005. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Jin R. Heterogeneous catalysis by gold and gold-based bimetal nanoclusters. Nano Today 2018, 18, 86. 10.1016/j.nantod.2017.12.009. [DOI] [Google Scholar]
- Burawoy A.; Critchley J. P. Electronic spectra of organic molecules and their interpretation V: Effect of terminal groups containing multiple bonds on the K-bands of conjugated systems. Tetrahedron 1959, 5, 340. 10.1016/0040-4020(59)80025-9. [DOI] [Google Scholar]
- Makosch M.; Lin W.-I.; Bumbálek V.; Sá J.; Medlin J. W.; Hungerbühler K.; van Bokhoven J. A. Organic thiol modified Pt/TiO2 catalysts to control chemoselective hydrogenation of substituted nitroarenes. ACS Catal. 2012, 2, 2079. 10.1021/cs300378p. [DOI] [Google Scholar]
- Serna P.; Boronat M.; Corma A. Tuning the behavior of Au and Pt catalysts for the chemoselective hydrogenation of nitroaromatic compounds. Top. Catal. 2011, 54, 439. 10.1007/s11244-011-9668-z. [DOI] [Google Scholar]
- Vilé G.; Almora-Barrios N.; López N.; Pérez-Ramírez J. Structure and reactivity of supported hybrid platinum nanoparticles for the flow hydrogenation of functionalized nitroaromatics. ACS Catal. 2015, 5, 3767. 10.1021/acscatal.5b00885. [DOI] [Google Scholar]
- Wei H.; Liu X.; Wang A.; Zhang L.; Qiao B.; Yang X.; Huang Y.; Miao S.; Liu J.; Zhang T. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634. 10.1038/ncomms6634. [DOI] [PubMed] [Google Scholar]
- Lin L.; Yao S.; Gao R.; Liang X.; Yu Q.; Deng Y.; Liu J.; Peng M.; Jiang Z.; Li S.; Li Y.-W.; Wen X.-D.; Zhou W.; Ma D. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 2019, 14, 354. 10.1038/s41565-019-0366-5. [DOI] [PubMed] [Google Scholar]
- Haruta M.; Kobayashi T.; Sano H.; Yamada N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405. 10.1246/cl.1987.405. [DOI] [Google Scholar]
- Zhao J.; Liu H.; Ye S.; Cui Y.; Xue N.; Peng L.; Guo X.; Ding W. Half-encapsulated Au nanoparticles by nano iron oxide: promoted performance of the aerobic oxidation of 1-phenylethanol. Nanoscale 2013, 5, 9546. 10.1039/c3nr01468a. [DOI] [PubMed] [Google Scholar]
- Villa A.; Dimitratos N.; Chan-Thaw C. E.; Hammond C.; Prati L.; Hutchings G. J. Glycerol oxidation using gold-containing catalysts. Acc. Chem. Res. 2015, 48, 1403. 10.1021/ar500426g. [DOI] [PubMed] [Google Scholar]
- Li G.; Abroshan H.; Liu C.; Zhuo S.; Li Z.; Xie Y.; Kim H. J.; Rosi N. L.; Jin R. Tailoring the electronic and catalytic properties of Au25 nanoclusters via ligand engineering. ACS Nano 2016, 10, 7998. 10.1021/acsnano.6b03964. [DOI] [PubMed] [Google Scholar]
- Sun X.; Li F.; Shi J.; Zheng Y.; Su H.; Sun L.; Peng S.; Qi C. Gold nanoparticles supported on MgOx-Al2O3 composite oxide: An efficient catalyst for selective hydrogenation of acetylene. Appl. Surf. Sci. 2019, 487, 625. 10.1016/j.apsusc.2019.05.152. [DOI] [Google Scholar]
- Zhong R.-Y.; Sun K.-Q.; Hong Y.-C.; Xu B.-Q. Impacts of organic stabilizers on catalysis of Au nanoparticles from colloidal preparation. ACS Catal. 2014, 4, 3982. 10.1021/cs501161c. [DOI] [Google Scholar]
- Zhao J.; Ge L.; Yuan H.; Liu Y.; Gui Y.; Zhang B.; Zhou L.; Fang S. Heterogeneous gold catalysts for selective hydrogenation: From nanoparticles to atomically precise nanoclusters. Nanoscale 2019, 11, 11429. 10.1039/c9nr03182k. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhang J.; Wang H.; Shao Y.; Liu X.; Wang Y.-Q.; Lewis J. P.; Xiao F.-S. Activity and selectivity in nitroarene hydrogenation over Au nanoparticles on the edge/corner of anatase. ACS Catal. 2016, 6, 4110. 10.1021/acscatal.6b00530. [DOI] [Google Scholar]
- Tan Y.; Liu X. Y.; Zhang L.; Wang A.; Li L.; Pan X.; Miao S.; Haruta M.; Wei H.; Wang H.; Wang F.; Wang X.; Zhang T. ZnAl-hydrotalcite-supported Au25 nanoclusters as precatalysts for chemoselective hydrogenation of 3-nitrostyrene. Angew. Chem., Int. Ed. 2017, 56, 2709. 10.1002/anie.201610736. [DOI] [PubMed] [Google Scholar]
- Wang L.; Guan E.; Zhang J.; Yang J.; Zhu Y.; Han Y.; Yang M.; Cen C.; Fu G.; Gates B. C.; Xiao F. S. Single-site catalyst promoters accelerate metal catalyzed nitroarene hydrogenation. Nat. Commun. 2018, 9, 1362. 10.1038/s41467-018-03810-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.; Liu X. Y.; Li L.; Kang L.; Wang A.; Zhang T. Effects of divalent metal ions of hydrotalcites on catalytic behavior of supported gold nanocatalysts for chemoselective hydrogenation of 3-nitrostyrene. J. Catal. 2018, 364, 174. 10.1016/j.jcat.2018.05.007. [DOI] [Google Scholar]
- Wang L.; Lü Z.; Li F.; Duan X. Study on the mechanism and kinetics of the thermal decomposition of Ni/Al layered double hydroxide nitrate. Ind. Eng. Chem. Res. 2008, 47, 7211. 10.1021/ie800609c. [DOI] [Google Scholar]
- Zhao J.; Yu G.; Xin K.; Li L.; Fu T.; Cui Y.; Liu H.; Xue N.; Peng L.; Ding W. Highly active gold catalysts loaded on NiAl-oxide derived from layered double hydroxide for aerobic alcohol oxidation. Appl. Catal., A 2014, 482, 294. 10.1016/j.apcata.2014.05.040. [DOI] [Google Scholar]
- Park E. D.; Lee J. S. Effects of pretreatment conditions on CO oxidation over supported Au catalysts. J. Catal. 1999, 186, 1. 10.1006/jcat.1999.2531. [DOI] [Google Scholar]
- Boronat M.; Illas F.; Corma A. Active sites for H2 adsorption and activation in Au/TiO2 and the role of the support. J. Phys. Chem. A 2009, 113, 3750. 10.1021/jp808271y. [DOI] [PubMed] [Google Scholar]
- Ohyama J.; Hayashi Y.; Ueda K.; Yamamoto Y.; Arai S.; Satsuma A. Effect of FeOx modification of Al2O3 on Its supported Au catalyst for hydrogenation of 5-hydroxymethylfurfural. J. Phys. Chem. C 2016, 120, 15129. 10.1021/acs.jpcc.6b01542. [DOI] [Google Scholar]
- Ohyama J.; Esaki A.; Koketsu T.; Yamamoto Y.; Arai S.; Satsuma A. Atomic-scale insight into the structural effect of a supported Au catalyst based on a size-distribution analysis using Cs-STEM and morphological image-processing. J. Catal. 2016, 335, 24. 10.1016/j.jcat.2015.11.021. [DOI] [Google Scholar]
- Liu X.; Li H.-Q.; Ye S.; Liu Y.-M.; He H.-Y.; Cao Y. Gold-catalyzed direct hydrogenative coupling of nitroarenes to synthesize aromatic Azo compounds. Angew. Chem., Int. Ed. 2014, 53, 7624. 10.1002/anie.201404543. [DOI] [PubMed] [Google Scholar]
- Shimizu K.-i.; Miyamoto Y.; Kawasaki T.; Tanji T.; Tai Y.; Satsuma A. Chemoselective hydrogenation of nitroaromatics by supported gold catalysts: mechanistic reasons of size- and support-dependent activity and selectivity. J. Phys. Chem. C 2009, 113, 17803. 10.1021/jp906044t. [DOI] [Google Scholar]
- Gupta A.; Haque A.; Vankar Y. D. Zeolite (H-ZSM 5)-catalysed reduction of conjugated nitroalkenes with sodium cyanoborohydride. Chem. Commun. 1996, 1653. 10.1039/cc9960001653. [DOI] [Google Scholar]
- Jourdant A.; González-Zamora E.; Zhu J. Wilkinson’s Catalyst Catalyzed Selective Hydrogenation of Olefin in the Presence of an Aromatic Nitro Function: A Remarkable Solvent Effect. J. Org. Chem. 2002, 67, 3163. 10.1021/jo025595q. [DOI] [PubMed] [Google Scholar]
- Beier M. J.; Andanson J.-M.; Baiker A. Tuning the chemoselective hydrogenation of nitrostyrenes catalyzed by ionic liquid-supported platinum nanoparticles. ACS Catal. 2012, 2, 2587. 10.1021/cs300529y. [DOI] [Google Scholar]
- Huang L.; Luo P.; Pei W.; Liu X.; Wang Y.; Wang J.; Xing W.; Huang J. Selective hydrogenation of nitroarenes and olefins over rhodium nanoparticles on hydroxyapatite. Adv. Synth. Catal. 2012, 354, 2689. 10.1002/adsc.201200330. [DOI] [Google Scholar]
- Wu Q.; Zhang B.; Zhang C.; Meng X.; Su X.; Jiang S.; Shi R.; Li Y.; Lin W.; Arai M.; Cheng H.; Zhao F. Significance of surface oxygen-containing groups and heteroatom P species in switching the selectivity of Pt/C catalyst in hydrogenation of 3-nitrostyrene. J. Catal. 2018, 364, 297. 10.1016/j.jcat.2018.05.025. [DOI] [Google Scholar]
- Zhao J.; Li Q.; Zhuang S.; Song Y.; Morris D. J.; Zhou M.; Wu Z.; Zhang P.; Jin R. Reversible control of chemoselectivity in Au38(SR)24 nanocluster-catalyzed transfer hydrogenation of nitrobenzaldehyde derivatives. J. Phys. Chem. Lett. 2018, 9, 7173. 10.1021/acs.jpclett.8b02784. [DOI] [PubMed] [Google Scholar]