Abstract

The metal salts of fatty acid (M-FA) are the most widely used metal precursors to colloidal semiconductor nanocrystals (NCs). They play a key role in controlling the composition, shape, and size of semiconductor NCs, and their purity is essential for attaining impeccable batch-to-batch reproducibility in the optical and electrical properties of the NCs. Herein, we report a novel, one-pot synthesis of a library of highly pure M-FAs at near-quantitative yields (up to 91%) using 1,8-diazabicyclo[5.4.0]undec-7-ene or the related nonionic/noncoordinating base as an inexpensive and ecofriendly catalyst in a green solvent medium. The method is highly general and scalable with vast academic and industrial potential. As a practical application, we also demonstrate the use of these high-quality M-FAs in the synthesis of the spectrum of colloidal semiconductor NCs (III–V, II–VI, IV–VI, I–VI, I–III–VI, and perovskite) having absorption/emission in visible to the near-infrared region.
1. Introduction
Because a seminal paper by Brus et al. was published in 1983,1 colloidal synthesis has emerged as a powerful and effective method to prepare high-quality inorganic nanocrystals (NCs).2,3 The metal salts of fatty acids (M-FAs) are the most common form of metal precursors in nearly all semiconductor NC syntheses based on the hot injection method in the noncoordinating solvent.4−11 In the colloidal synthesis of NCs, M-FA plays a crucial role in solubilizing the metal ions, controlling the precursor reaction, nucleation and growth processes, and passivating the surface and stabilizing the colloidal dispersion.12−16 Therefore, the role of M-FAs is decisive for the evolution of crystal phase,11 size,5 and shape17 and even the higher dimensional assembly of the NCs17,18 with unique physical and chemical properties. In addition to this, it is well-known that M-FAs are the key ingredients in a plethora of industrial and domestic products including cosmetics,19 lubricants,20−22 paints,23 biofuels,24,25 and rubber.26 Some of the common methods employed for the synthesis of M-FA precursors are the (1) vacuum method; (2) saponification method; (3) precipitation (double decomposition) method; and (4) direct reaction of fatty acids with metals. Conventionally, in the colloidal synthesis of semiconductor NCs, M-FAs are synthesized by the “vacuum method” where metal acetate and the desired fatty acid are vacuumed together at high temperature in high boiling solvents.4,5,8,11,13 For example, for the synthesis of InP NCs, the indium salt of fatty acid is prepared in situ by the vacuum method prior to the reaction. Typically, a mixture of In-acetate and fatty acid (myristic acid, palmitic acid, stearic acid, oleic acid or lauric acid) is mixed in a noncoordinating solvent [1-octadecene (1-ODE)]5 and are vacuumed at 110–130 °C for 1–2 h to obtain a “clear solution” of indium carboxylate.5,13 Unfortunately, there is no consensus about time, pressure, and temperature of this method with respect to purity and composition of the M-FA formed in situ, and this shortcoming may lead to batch-to-batch inconsistencies in the properties of the NCs. Except for a few special cases,6,27,28 where M-FAs have been fully characterized, the purity of the M-FA precursor in most cases were not confirmed prior to the colloidal synthesis and characterization. Therefore, given the sensitivity of these colloidal reactions and in view of the uncertainty involved with the purity of M-FAs prepared by the “vacuum method”, many of the kinetic studies and the conclusions reported therein may not be fully accurate, especially in quantitative studies. Recent reports have preferred pure M-FAs prepared ex situ for the synthesis and kinetic studies of NCs, emphasizing on the growing importance of the compositional purity of the precursor.6,29 For example, Owen and coworkers6 synthesized highly pure lead oleate directly from lead oxide and oleic acid in the presence of trifluoroacetic anhydride and triethylamine. They employed the pure lead oleate to study the kinetics of lead chalcogenide formation from a library of thiourea precursors. In another report, an iron oleate precursor was synthesized from ferric chloride and sodium oleate.29 This method is based on the first saponification of fatty acid with an inorganic base such as NaOH or KOH followed by the reaction of the alkali metal oleate with chloride salts of the desired metal ions.30,31 Other strategies for the synthesis of M-FA (M = Zn, Ca) include precipitation of M-FA via the double decomposition reaction32,33 and the direct reaction of fatty acids with some metals.34 However, most of these methods suffer from some pitfalls such as the presence of the free fatty acid impurity (e.g. the vacuum method), limited substrate scope (e.g. the precipitation method), poor atom economy, and lengthy reactions involving multiple steps (e.g. the saponification and double decomposition method). For example, the saponification and double precipitation methods first require fatty acids to be converted into alkali metal salts using the inorganic base, which will be subsequently purified, isolated, and then used for the preparation of other M-FAs. Clearly, there is a need for the development of a more general and simpler method to address the aforementioned drawbacks. Herein, we report a new approach to access a library of pure M-FA precursors in high yield by direct reaction of commercially available fatty acids with metal salts in the presence of a noncoordinating, sterically hindered organic base as an inexpensive and benign catalyst. The method is one-step and does not require an additional purification step.
2. Results and Discussion
We noted that the direct reaction of fatty acids with the corresponding metal chlorides essentially required an inorganic base to promote the reaction. However, the use of inorganic bases such as NaOH or KOH is not desirable in many cases because alkali metal ions compete with other intended metal ions in the reaction. This issue could be addressed by the use of nonionic, noncoordinating organic bases having a lower affinity toward metal ions. An ideal candidate would be a strong nitrogenous organic base, that is, accessible, nontoxic, and stable. In this context, initially, we screened 1,4-diazabicyclo[2.2.2]octane (DABCO) for the direct synthesis of M-FA from fatty acid and metal salt in one step. DABCO is an inexpensive, commercially available, and environmentally benign organic base.35 It is sufficiently basic (pKa conjugate acid ∼8.7) to abstract proton from fatty acids36 and is an effective catalyst in many organic transformations such as the Baylis–Hillman reaction,37 chlorination of alkenes via activation of N-chlorosuccinimide,38 and so forth. To access the feasibility of obtaining M-FA in a single step, we carried out a one-pot substitution reaction between 3.0 equiv of stearic acid (1a) and 1.0 equiv of indium trichloride (2a) in the presence of 4.0 equiv of DABCO, under reflux conditions in 7:4:3 ratio of hexane/ethanol/water as a green biphasic solvent mixture. We used DABCO as a base in slightly above stoichiometric amounts (4.0 equiv base compared to the fatty acid) in our initial experiments with the sole objective to promote complete deprotonation of fatty acids. The reaction yielded indium stearate (3a) in 72% yield in the pure form (Scheme 1).
Scheme 1. Reaction of Stearic Acid (1a) with Indium Trichloride (2a) to Obtain Indium Stearate (3a).
The biphasic nature of the solvent mixture, that is, the hexane/ethanol/water (7:4:3) system39 simplifies the isolation of the product which is extracted from the nonpolar hexane part, whereas the ionic impurities including the base and other byproducts remain in the aqueous/ethanol part, thus ensuring the high purity of the product. Figure 1 shows the characteristic asymmetric COO– stretching band centered around 1525 cm–1 for the intended product, indium stearate (3a),40 which is clearly distinguishable from the sharp C=O stretching peak at ∼1712 cm–1 for the free fatty acid (1a). Furthermore, the −OH (bending) and −COOH (bending) characteristic of 1a at 940 and 1296 cm–1 was also not detected in the product 3a. The thermogravimetric analysis (TGA) results show the ∼85% decrease in weight in the temperature range 150–500 °C consistent with the loss of three stearate groups (Figure S1). Above 500 °C, constancy in the weight loss exemplified the complete decomposition of indium stearate to afford In2O3. 1H and 13C NMR further confirmed the formation of 3a (Figures S2 and S3). The presence of three stearate groups in the product was further confirmed by 1H NMR analysis using 1,3,5-trimethoxybenzene as the internal standard (Figure S4). The measured melting point of the pure 3a is 145–148 °C.
Figure 1.
Fourier transform infrared (FTIR) spectrum of stearic acid (1a, black) and the indium stearate (3a, red) prepared from 1a. The characteristic stretching and bending signatures related to carboxylic and carboxylate groups are marked for clarity.
In principle, other nonionic/uncharged nitrogenous bases with similar attributes (basicity, noncoordinating, and a good leaving group) as DABCO should also be effective for our reaction. In fact, we observed the formation of the desired product using other organic bases such as pyridine (aromatic amine), triethylamine (tertiary alkyl amine), and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) which is a sterically hindered base (entries 1–4, Table 1). Based on our screening studies, the best result was obtained with DBU which is a bicyclic amidine compound. The result obtained with DBU was better than with DABCO or other bases tested above which is possibly due to the higher basicity of DBU (pKa conjugate ∼13.5).41 It is well-known that the amidines are a stronger base than the tertiary amine or amides and are among the strongest nonionic/uncharged bases.42 DBU is a stable, inexpensive, nontoxic,43 and accessible base commonly used in organic transformation, therefore qualifying as an ideal candidate for further studies and optimization. Using the reaction between indium chloride and stearic acid in the presence of DBU as the model reaction, we studied the effect of the concentration of the base, temperature, and time on the reaction yield. In our studies so far, 4.0 equiv of the base (compared to a fatty acid) were used (entries 1–4, Table 1) to facilitate complete deprotonation of the carboxylic acid. To further understand the effect of the base on the reaction, we decreased the amount of DBU from 4.0 to 0.5 equiv in succession. The entries 4–6 shows that the yield of the desired product improved from an initial 78% (with 4.0 equiv DBU) to 85% (with 1.0 equiv DBU), when other conditions were constant. However, further lowering the amount of DBU to 0.5 equiv resulted in a decrease in the reaction yield to 66% (entry 7, Table 1). These results show that the role of DBU is catalytic in nature. To study the effect of temperature, we performed reactions at four different temperatures (25, 40, 60 °C, and reflux temperature) using 1.0 equiv of DBU (entries 6, 8–10, Table 1). While at room temperature, the reaction did not take place, the maximum yield was obtained under reflux conditions. Likewise, we investigated the effect of time for this transformation. When the reaction mixture was refluxed for a short period of time (entries 11 and 12, Table 1), the efficiency of the transformation was poor. Therefore, the reaction condition detailed in entry 6 was marked as the best-optimized reaction condition for our reaction. Furthermore, 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), a related amidine compound was also tested as a catalyst and as expected a comparable product yield was achieved (entry 13, Table 1), thus underscoring the effectiveness of analogous amidine compounds in general as a catalyst for this reaction. The summary of the optimization studies is tabulated below (Table 1).
Table 1. Optimization Studies for the One-Step Synthesis of Indium Stearate (3a)a.
| entry | base | equiv | temp (°C) | time (h) | yield (%) |
|---|---|---|---|---|---|
| 1 | DABCO | 4.0 | reflux | overnight | 72 |
| 2 | pyridine | 4.0 | reflux | overnight | 66 |
| 3 | triethylamine | 4.0 | reflux | overnight | 32 |
| 4a | DBU | 4.0 | reflux | overnight | 78 |
| 5b | DBU | 3.0 | reflux | overnight | 80 |
| 6c | DBU | 1.0 | reflux | overnight | 85 |
| 7d | DBU | 0.5 | reflux | overnight | 66 |
| 8e | DBU | 1.0 | 25 | overnight | nr |
| 9f | DBU | 1.0 | 40 | overnight | 22 |
| 10g | DBU | 1.0 | 60 | overnight | 60 |
| 11 | DBU | 1.0 | reflux | 6 h | 60 |
| 12 | DBU | 1.0 | reflux | 8 h | 58 |
| 13 | DBN | 1.0 | reflux | overnight | 82 |
Unless otherwise noted, all reactions were carried out with 4.0 equiv (with respect to a fatty acid) of the base under reflux conditions.
The reaction was performed with 3.0 equiv of the base.
The reaction was performed with 1.0 equiv of the base.
The reaction was performed with 0.5 equiv of the base.
nr denotes no reaction.
The reaction was performed at 40 °C.
The reaction was performed at 60 °C.
Next, under optimized conditions, we synthesized a large variety of M-FAs (3b–t) on reacting a series of metal salts (2a–p) with fatty acids (1a–e) in a single step using a catalytic amount of DBU (Scheme 2). All the substrates were well-tolerated under optimized reaction conditions, and the corresponding M-FAs (3b–t) were obtained in high yields (up to 91%, entries 1–19, Table 2). Table 2 presents an overview of the substrate scope of our reaction. In each case, the successful formation M-FAs was confirmed by the presence of characteristic −COO– asymmetric (∼1510–1650 cm–1)44 in FTIR (Figures S5–S23). For some M-FAs, we observe two asymmetric vibrations which are attributed to differences in the nature of the M–O bonds in the compound.45−47 These results show that the effect of coordination of the carboxylic ion with metal and the nature of the M–O bonding is reflected in COO– asymmetric frequencies. The characteristic C=O stretching vibration at slightly above 1700 cm–1 because of free fatty acids34 (Figure S24) is absent in our samples (Figures S5–S23), confirming their high purity. Furthermore, the TGA thermogram confirmed the weight % loss corresponding to the number of carboxylate groups per metal ion in each case (Figures S5–S23). For M-FAs containing diamagnetic metal ions, additional characterization was performed using 1H and 13C NMR (Figures S25–S50) to confirm the formation of the desired products. This method, thus, allows for the synthesis of highly pure M-FAs of diverse metal ions positioned in various groups of the periodic table. Specifically, we demonstrate the facile synthesis of M-FAs, where M = K (alkaline metal), Mg (alkaline earth metal), Mn, Fe, Co, Ni, Cu, Zn (first row transition metals), Ag, Cd (second row transition metals), Al, Ga, In (triels; group IIIA), Sn, Pb (tetrels, group IVA), and Gd (lanthanide). Especially, the carboxylates of group IIIA metals such as aluminum, gallium, indium, and so forth are in general considered difficult to synthesize under benign conditions because of the tendency of group IIIA metals to form a bond with a more covalent character.14 On the contrary, our method demonstrates excellent efficiency with nearly all metal ions.
Scheme 2. Synthesis of Metal Carboxylates (3) from Fatty Acid (1) and Metal Salt (2).
Table 2. Synthesis of Metal Carboxylates (3) from Fatty Acid (1) and Metal Salt (2).
| entry | fatty acid (1) | metal salt (2) | product (3) | yield (%) |
|---|---|---|---|---|
| 1 | stearic acid (1a) | GaCl3 (2b) | (C17H35CO2)3Ga (3b) | 90 |
| 2 | stearic acid (1a) | Pb(NO3)2 (2c) | (C17H35CO2)2Pb (3c) | 73 |
| 3 | stearic acid (1a) | AgNO3 (2d) | (C17H35CO2)Ag (3d) | 91 |
| 4 | stearic acid (1a) | AlCl3 (2e) | (C17H35CO2)3Al (3e) | 35 |
| 5 | stearic acid (1a) | FeCl3 (2f) | (C17H35CO2)3Fe (3f) | 90 |
| 6 | stearic acid (1a) | CuCl2 (2g) | (C17H35CO2)2Cu (3g) | 91 |
| 7 | stearic acid (1a) | CoCl2 (2h) | (C17H35CO2)2Co (3h) | 75 |
| 8 | stearic acid (1a) | ZnCl2 (2i) | (C17H35CO2)2Zn (3i) | 87 |
| 9 | stearic acid (1a) | SnCl2 (2j) | (C17H35CO2)2Sn (3j) | 90 |
| 10 | stearic acid (1a) | CdCl2 (2k) | (C17H35CO2)2Cd (3k) | 81 |
| 11 | stearic acid (1a) | KNO3 (2l) | (C17H35CO2)K (3l) | 85 |
| 12 | myristic acid (1b) | InCl3 (2a) | (C13H27CO2)3In (3m) | 90 |
| 13 | palmitic acid (1c) | InCl3 (2a) | (C15H31CO2)3In (3n) | 60 |
| 14 | lauric acid (1d) | InCl3 (2a) | (C11H23CO2)2In (3o) | 75 |
| 15 | oleic acid (1e) | Pb(NO3)2 (2c) | (C17H33CO2)2Pb (3p) | 90 |
| 16 | stearic acid (1a) | Mg(NO3)2 (2m) | (C17H35CO2)2Mg (3q) | 88 |
| 17 | stearic acid (1a) | MnCl2·4H2O(2n) | (C17H35CO2)2Mn (3r) | 70 |
| 18 | stearic acid (1a) | NiCl2·6H2O (2o) | (C17H35CO2)2Ni (3s) | 87 |
| 19 | stearic acid (1a) | GdCl3·6H2O (2p) | (C17H35CO2)3Gd(3t) | 45 |
3. Mechanism
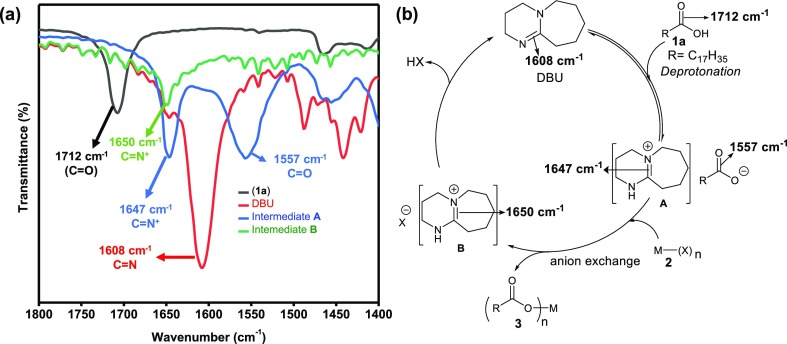
From optimization studies (entry 6, Table 1), it is clear that the DBU is highly efficient when used in a catalytic amounts. Unprotonated DBU has a characteristic C=N stretching at 1608 cm–1 (Figure 2a), and free stearic acid has C=O stretching at 1712 cm–1. When DBU and fatty acids (stearic acid) are mixed and heated close to the reaction temperature for 5 min, we observed a strong shift in C=N stretching to 1647 cm–1 which is attributed to the protonation of DBU. This observation, taken together with the detection of a shift of C=O stretching of fatty acid from 1712 to 1557 cm–1 unambiguously confirms the formation of the intermediate A (Figure 2) during the initial stage of the reaction. A similar observation has been reported for protonation of DBU by Pripol 1009, a commercially available bio-based fatty acid dimer.48 After the reaction is complete, we carefully analyzed the aqueous fraction of the biphasic solution system using FTIR. The extracted byproduct (aqueous fraction) exhibited C=N stretching at 1650 cm–1, a slight deviation from the intermediate A. In addition, we did not detect any carboxylate signature in this fraction, confirming the presence of the intermediate B which contains halide or nitrate as counter-ions. In other words, we detected the halide or nitrate salt of DBU in the solution after the reaction is completed (Figure 2a). Based on these observations, we propose a simple anion exchange mechanism involving DBU as a catalyst as depicted in Figure 2b.
Figure 2.
(a) FTIR spectra of the starting compound stearic acid (1a, black), the pristine DBU catalyst (red), a mixture of stearic acid and DBU heated to the reaction temperature for 5 min (intermediate A, blue) and the aqueous part of the reaction mixture after 13 h of reaction (intermediate B, green) and (b) proposed mechanistic pathway for the DBU-catalyzed synthesis of M-FAs.
Thus, the reaction proceeds via deprotonation of the carboxylic functionality of fatty acid (1a) in the presence of DBU under reflux conditions to generate the corresponding intermediate A (Figure 2), which further takes part in the anion exchange reaction with metal salt (2) to form intermediate B followed by the expulsion of halogen acid (HX) from B to generate DBU (the base catalyst) which further enters into another catalytic cycle. The driving force for the transformation of intermediate A to B in the presence of metal salt is the strong oxyphilic nature of the metal ions.49,50
4. Multigram Scale Synthesis
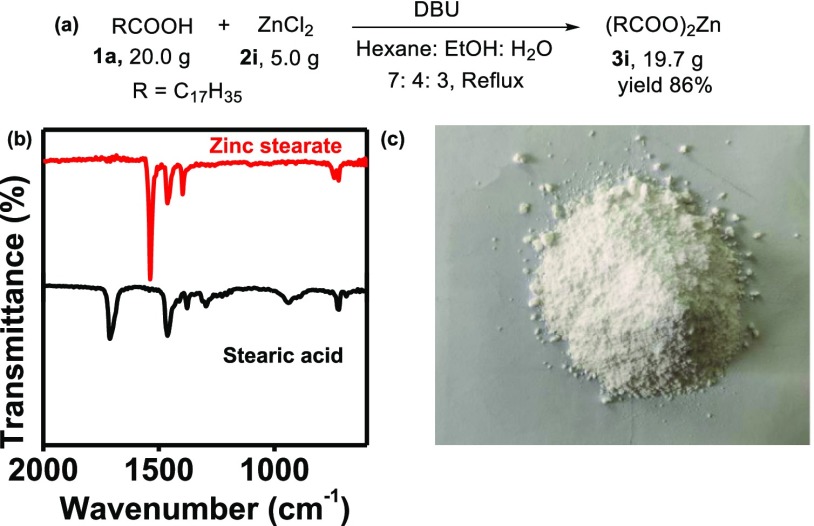
Because most of these M-FAs have potential commercial use, the scale-up of the reaction is desirable. We demonstrate the successful extension of our protocol to the multigram synthesis of M-FA. As a representative example, 5.0 g of ZnCl2 (2i) was reacted with 20.0 g of stearic acid (1a) in the presence of 5.5 mL DBU under optimized reaction conditions to obtain the corresponding zinc stearate (3i) in 19.7 g (86% yield, Figure 3a). The products were isolated in a pure form directly from the hexane fraction and characterized using FTIR (Figure 3b), NMR (Figures S33 and S34), and TGA (Figure S12). The measured melting point of the product obtained using the digital melting point apparatus was 128–130 °C, which is consistent with the literature value (130 °C).51 This further confirms the high purity of the product formed.
Figure 3.
(a) Scheme for reaction between ZnCl2 (2i) and stearic acid (1a) to yield zinc stearate (3i); (b) FTIR spectrum zinc stearate (3i, red) showing lower carbonyl stretching compared to the stearic acid (1a); and (c) photographic image of 19.7 g zinc stearate (3i).
5. Application
5.1. Synthesis of Colloidal Semiconductor NCs from the As-Prepared M-FA Precursors
The easy access to the library of highly pure M-FAs has tremendous importance in the growing field of colloidal semiconductor NCs or quantum dots exhibiting a unique optical and electrical property in the quantum confinement regime.52,53 In particular, we expect that our method will allow for the more precise study of reaction kinetics and efficient control of the properties of the NCs for meaningful application in many technologically important areas including optoelectronics,54 photovoltaics,55 bio-molecular imaging,56,57 and photocatalysis.58,59 We demonstrate the colloidal synthesis of different types of a semiconductor NCs such as CsPbBr3 perovskite, PbS (IV–VI),6 CdSe (II–VI),3 CuFeS2 (I–III–VI),60 Ag2S (I–VI),61 and InP (III–V)5 NCs using pure M-FAs obtained from this work (Table 3). All these materials are widely studied and applied for a range of applications such as photovoltaics (PbS and CuFeS2),62,63 light-emitting diodes (CdSe, InP, and CsPbBr3),64−66 bio-imaging in visible and near-infrared (InP and Ag2S)67,68 regions, and photocatalysis of organic transformation (CsPbBr3, CdSe, and InP).69−71 The respective M-FAs employed for the synthesis of these NCs are listed in Table 3, and their synthetic details are furnished in the Experimental Section. The NCs synthesized were highly stable as colloidal dispersion (Figure S51) and exhibited their characteristic optical properties (Figures 4 and S52). The powdered X-ray diffraction (PXRD) studies confirmed their crystal structure and high phase purity (Figure 5). The absorption and emission band widths expressed as half-width at half maximum (HWHM) or full-width at half maximum (fwhm) of the NCs were comparable to those reported for conventionally prepared NCs.72−75
Table 3. Various Semiconductor NCs Synthesized from the As-Prepared M-FA Precursors.
| entry | M-FA precursors | semiconductor type | semiconductor NCs |
|---|---|---|---|
| 1 | Pb(oleate)2 | perovskite | CsPbBr3 |
| 2 | Pb(oleate)2 | IV–VI | PbS |
| 3 | Cd(stearate)2 | II–VI | CdSe |
| 4 | Cu(stearate)2 and Fe(stearate)2 | I–III–VI | CuFeS2 |
| 5 | Ag(stearate) | I–VI | Ag2S |
| 6 | In(stearate)3 | III–V | InP |
Figure 4.
(a) UV–vis (red) and PL spectra (blue) of CsPbBr3 perovskite NCs (excitation: 400 nm, emission: 515 nm, fwhm = 28 nm); (b) UV–vis spectrum of CuFeS2 NCs exhibiting a typical surface plasmon resonance band76 (HWHM: 156 nm); (c) UV–vis (red) and PL (blue) spectra of CdSe NCs (excitation: 480 nm, emission: 552 nm, fwhm: 33 nm); (d) UV–vis–NIR (red) and PL Spectra (blue) PbS NCs (excitation: 950 nm, emission: 1102 nm, fwhm: 149 nm); (e) UV–vis–NIR (red) and PL spectra (blue) of Ag2S NCs (excitation: 800 nm, emission: 1206 nm, fwhm: 186 nm); and (f) UV–vis spectra of InP NCs (HWHM: 37 nm).
Figure 5.
PXRD of (a) CsPbBr3 perovskite (cubic phase, JCPDS 00-054-0752); (b) CuFeS2 NCs (tetragonal, JCPDS 00-037-0471); (c) CdSe NCs (zinc blende, JCPDS 65-2891); (d) PbS NCs (rock salt, JCPDS 05-0592); (e) Ag2S NCs (monoclinic, JCPDS 00-014-0072); and (f) as-synthesized InP (pristine, red color) and corresponding spectrum (blue color) of the annealed (270 °C, 4 h) InP film (zinc blende, JCPDS 96-101-0147). Spectra in (a–e) have been obtained from the as-prepared samples without any annealing step to improve crystallinity.
6. Conclusions
In summary, we demonstrated an easy, scalable, and generalized methodology for the synthesis of highly pure M-FAs with metal ions from all blocks (s, p, d, and f blocks) of the periodic table. The sterically hindered, noncoordinating DBU and the analogous base were found to catalyze the formation of M-FAs via the abstraction of a proton from fatty acid to form fatty acid salt followed by anion exchange with a metal salt. The method was highly scalable, and the products were obtained on a multigram scale. The practical use of this methodology is also presented by using the as-synthesized M-FAs as the metal precursors for the formation of various semiconductor NCs such as CsPbBr3, InP, PbS, CdSe, CuFeS2, and Ag2S. We believe that our findings would be valuable for both academic and industrial settings with wide applications in the field of colloidal NCs, surfactants, emulsions, and so forth.
7. Experimental Section
7.1. General Information: Materials and General Considerations
InCl3 (99.99%), GaCl3 (99.99%), lauric acid (98%), palmitic acid (99%), Cs2(CO3) (99.9%), GdCl3·6H2O (99.9%), Br2 (≥99%), oleylamine (technical grade), 1-ODE (technical grade), Se (≥99.5%), trioctylphosphine (97%) 1, and 2-dimethoxyethane (99.5%) were all purchased from Sigma-Aldrich, India. Pb(NO3)2 (99%), FeCl3 (96%), CoCl2·6H2O (98%), NiCl2 (97%), MnCl2 (99%), Mg(NO3)2 (99%), and oleic acid (65–88%) were purchased from Merck, India. 1-Dodecanthiol (98%) was purchased from Loba Chemie. CuCl2·2H2O (99%), ZnCl2 (97%), CdCl2·H2O (98%), KNO3(99%), SnCl2·2H20, and stearic acid (90%) were purchased from Thomas Baker, India. Myristic acid (95%), AgNO3 (99.8%), and AlCl3 (96%) were purchased from SRL, Rankem, and Finar, respectively. All the FTIR spectra were acquired using Bruker ALPHA E, 200396; the TGA data were recorded on the TA Instrument Q-50 TGA. 1H NMR was obtained in CDCl3 and DMSO-d6 using Bruker ASCEND 400. The UV–visible spectra were collected using PerkinElmer (model: LS 55). The PL spectra were acquired using the HORIBA scientific spectrophotometer (model: PTI-QM 510), and PXRD was recorded on a PANalytical X-ray diffractometer using Cu Kα (λ = 1.54 Å) as the incident radiation (40 kV and 30 mA).
7.2. Preparation of M-FA Salts (Method A)
In a typical synthesis, 0.1 g of metal salt (1.0 equiv) was taken in a round bottom flask, and 3.0 equiv of fatty acid and 1.0 equiv of DBU were added to it. A mixture of hexane, ethanol, and water (ratio 7:4:3) was added to the above-mentioned mixture. It was then stirred for 2–3 min at room temperature. The reaction mixture was then refluxed overnight (13 h). In the following day, the product was extracted from the nonpolar hexane part and was dried under vacuum.
7.3. Preparation of CsPbBr3 NCs Using the As-Synthesized Lead Oleate (3p)
Colloidal CsPbBr3 NCs were synthesized following the reported method.11 Briefly, 0.077 g (0.1 mmol) of lead oleate, 30 μL (0.6 mmol), and 0.5 mL of oleylamine were mixed with 4 mL of 1-ODE and degassed for 30 min at room temperature followed by 30 min at 120 °C. The reaction vessel was back-filled with nitrogen and heated to 200 °C. At this temperature, cesium oleate solution (0.4 mL) was injected. After 10 s the reaction vessel was quenched by immersing in an ice bath. The purification was carried out through a centrifugation method, where 4 mL of crude solution was mixed with 4 mL of anhydrous toluene and centrifuged at 5000 rpm for 10 min. The supernatant was discarded, and the solid residue was washed again with anhydrous toluene at 5000 rpm for 5 min. Finally, the purified CsPbBr3 NCs were dispersed in anhydrous hexane.
7.4. Preparation of CdSe NCs Using the As-Synthesized Cadmium Stearate (3k)
Colloidal CdSe NCs were synthesized following the method reported earlier with slight modification.3,4 Briefly, 0.068 g (0.1 mmol) of cadmium stearate, 0.147 mL (0.5 mmol) of oleylamine, and 6 mL of 1-ODE were heated at 120 °C under vacuum for 1 h. After 1 h the flask was back-filled with nitrogen, and the reaction mixture was slowly heated to 150 °C. Separately, 0.15 g (1.9 mmol) of selenium powder was dissolved in 2 mL of trioctylphosphine. This solution was injected into the formerly prepared cadmium stearate solution to obtain CdSe NCs. The purification was done by taking 5 mL of crude solution and mixing with anhydrous toluene and anhydrous ethanol (1:4). The mixture was centrifuged at 5000 rpm for 10 min. The supernatant was discarded, and the solid residue was centrifuged again following the same procedure. After washing the CdSe NCs for three times, it was dispersed in anhydrous hexane.
7.5. Preparation of InP NCs Using the As-Synthesized Using Indium Stearate (3a)
Colloidal InP NCs were synthesized following the reported method with slight modification.5,77 Briefly, 0.097 g (0.1 mmol) of indium stearate was dissolved in 4 mL of 1-ODE by degassing it at 120 °C for 1 h. A clear solution was obtained which was heated under nitrogen in a Schlenk line until the temperature reached 270 °C. 12.5 mg (0.05 mmol, 14.5 μL) of tris(trimethylsilyl) phosphine in 0.5 mL 1-ODE was injected, and the reaction was carried out for 1 h. The purification was carried out through a centrifugation method, where 2 mL of crude solution was mixed with 2 mL of anhydrous hexane and 8 mL of anhydrous ethanol. The mixture was centrifuged at 5000 rpm for 10 min. The supernatant was discarded and the solid residue was washed again with (1:4) mixture of anhydrous hexane and anhydrous ethanol at 5000 rpm for 10 min. The purified NCs were dispersed in hexane.
7.6. Preparation of PbS Using As-Synthesized Lead Oleate (3p)
Colloidal PbS NCs were synthesized following the method reported earlier with slight modification.6 Briefly, 0.030 g (0.0389 mmol) of lead oleate was dissolved in 5 mL of 1-ODE at 120 °C for 30 min under vacuum. The flask was back-filled with nitrogen, and the temperature was brought down to 80 °C. Separately the 1,3-diphenyl thiourea prepared in the lab was dissolved in 1,2-dimethoxyethane and then injected in the reaction mixture. After the injection, the reaction was carried out for 15 min to obtain a black coloration of PbS NCs. For purification, the crude 4 mL of anhydrous toluene and 16 mL of anhydrous ethanol was added to 4 mL of crude PbS NCs, and the mixture was centrifuged at 5000 rpm for 10 min. The residue obtained was washed three times using the same method. The purified NCs were dispersed in hexane.
7.7. Preparation of Ag2S NCs Using the As-Synthesized Silver Stearate (3d)
Colloidal Ag2S NCs were synthesized following the reported method with slight modification.61,78 Briefly, 0.039 g (0.1 mmol) of silver stearate was mixed with 3 mL of 1-ODE and was vacuumed for 1 h at room temperature. After 1 h, the reaction temperature was increased to 100 °C and the degassing was continued for 30 min. This silver stearate solution was back-filled with nitrogen and the temperature was increased to 140 °C. Separately, 0.023 g (0.1 mmol) of 1,3-diphenyl thiourea and 0.3 mL of 1-DDT was vacuumed for 1 h at room temperature. This solution was swiftly injected into the silver stearate solution and was kept at 140 °C for 5 min. After 5 min, the reaction was allowed to come down to room temperature. The purification was carried out through a centrifugation method, where 3 mL of crude solution was mixed with 3 mL of anhydrous ethanol and centrifuged at 5000 rpm for 5 min. The supernatant was discarded, and the residue was washed again with anhydrous ethanol. This process was repeated two times. The final two washing was done with a mixture of anhydrous ethanol and anhydrous hexane (3:1) and centrifuged at 5000 rpm for 5 min. Finally, the purified Ag2S NCs were dispersed in anhydrous hexane.
7.8. Preparation of CuFeS2 NCs Using the As-Synthesized Iron Stearate (3f) and Copper Stearate (3g)
Colloidal CuFeS2 NCs were synthesized following the method reported earlier with slight modification.60 Briefly, 0.006 g (0.2 mmol) of sulfur was mixed with 2.5 mL of oleylamine and 1 mL of 1-ODE, and it was heated at 120 °C under vacuum for 30 min. Under nitrogen, the temperature was increased to 160 °C, and it was kept at this temperature. 0.090 g (0.1 mmol) of iron stearate and 0.063 g (0.1 mmol) of copper stearate was mixed with 3 mL of 1-ODE. The mixture was degassed for 1 h at 120 °C. The reaction flask was filled with nitrogen, and 1.5 mL of dodecanethiol was added. The temperature of the reaction was slowly increased to 180 °C. Sulfur solution was injected dropwise. After the addition of sulfur, the reaction was continued for another 10 min and then quenched in cold water. The purification was carried out through a centrifugation method, where 4 mL of crude solution was mixed with 4 mL of anhydrous toluene and 16 mL of anhydrous ethanol and centrifuged at 5000 rpm for 10 min. The supernatant was discarded, and the solid residue was washed again with a (1:4) hexane/ethanol mixture at 5000 rpm for 5 min. The NCs were finally centrifuged with the same solvent mixture at 5000 rpm for 3 min to obtain purified CuFeS2 NCs. The purified NCs were dispersed in hexane.
7.9. Spectral (NMR) Analyses, Melting Point, and Specification of Experimental Conditions
7.9.1. Indium(III) Stearate (3a)
It was prepared according to the general procedure discussed in method A. Indium chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained as a white powder in 85% yield. mp 145–148 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 28H), 1.60 (m, 2H), 2.33 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.12, 22.70, 24.72, 29.08, 29.26, 29.37, 29.45, 29.69, 31.93, 33.95, 179.35.
7.9.2. Gallium(III) Stearate (3b)
It was prepared according to the general procedure discussed in method A. Gallium chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 90% yield. 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 28H), 1.63 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.31, 22.88, 24.85, 29.24, 29.43, 29.56, 29.62, 29.79, 29.84, 32.11, 34.27, 180.46.
7.9.3. Lead(II) Stearate (3c)
It was prepared according to the general procedure discussed in method A. Lead nitrate was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 73% yield. 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.25 (m, 28H), 1.63 (m, 2H), 2.35 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.31, 22.88, 24.87, 29.24, 29.42, 29.54, 29.61, 29.77, 29.87, 32.10, 34.08, 179.40.
7.9.4. Silver(I) Stearate (3d)
It was prepared according to the general procedure discussed in method A. Silver nitrate was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 91% yield. mp 204–207 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.80 (m, 3H), 1.21 (m, 28H), 1.53 (m, 2H), 2.32 (m, 2H). 13C NMR (CDCl3, DMSO-d6, 3:1, 100 MHz): δ ppm 14.31, 22.54, 24.95, 28.99, 29.14, 29.17, 29.34, 29.46, 31.74, 34.12, 174.94.
7.9.5. Aluminum(III) Stearate (3e)
It was prepared according to the general procedure discussed in method A. Aluminum chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 35% yield. 1H NMR (CDCl3, 400MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 28H), 1.61 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.30, 22.88, 24.88, 29.26, 29.44, 29.55, 29.63, 29.79, 29.88, 30.02, 32.14, 34.23 179.98.
7.9.6. Ferric(III) Stearate (3f)
It was prepared according to the general procedure discussed in method A. Ferric chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 90% yield.
7.9.7. Copper(II) Stearate (3g)
It was prepared according to the general procedure discussed in method A. Copper chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 91% yield. mp 248–251 °C.
7.9.8. Cobalt(II) Stearate (3h)
It was prepared according to the general procedure discussed in method A. Cobalt chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 75% yield. mp 110–113 °C.
7.9.9. Zinc(II) Stearate (3i)
It was prepared according to the general procedure discussed in method A. Zinc chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 87% yield. mp 128–130 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.30 (m, 28H), 1.62 (m, 2H), 2.35 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100MHz): δ ppm 14.31, 22.88, 24.87, 29.24, 29.42, 29.55, 29.62, 29.77, 29.87, 32.11, 34.05, 179.21.
7.9.10. Tin Stearate (3j)
It was prepared according to the general procedure discussed in method A. Tin(II) chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 90% yield. 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.28 (m, 28H), 1.63 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.12, 22.70, 24.68, 29.07, 29.25, 29.37, 29.44, 29.69, 31.93, 34.02, 179.84.
7.9.11. Cadmium(II) Stearate (3k)
It was prepared according to the general procedure discussed in method A. Cadmium chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 81% yield. mp 132–135 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 28H), 1.61 (m, 2H), 2.35 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.31, 22.88, 24.86, 29.24, 29.42, 29.54, 29.77, 29.87, 34.12, 179.62.
7.9.12. Potassium Stearate (3l)
It was prepared according to the general procedure discussed in method A. Potassium nitrate was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 85% yield. 1H NMR (CDCl3, DMSO-d6, 3:1, 400 MHz): δ ppm 0.78 (t, 3H, J = 8 Hz), 1.16 (m, 28H), 1.49 (m, 2H), 2.16 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, DMSO-d6, 3:1 100 MHz): δ ppm 14.40, 22.76, 25.11, 29.26, 29.40, 29.43, 29.57, 29.73, 31.97, 34.39, 175.66.
7.9.13. Indium(III) Myristate (3m)
It was prepared according to the general procedure discussed in method A. Indium chloride was used as a metal precursor and myristic acid as a carboxylate precursor. The product was obtained in 90% yield. mp 142–145 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 20H), 1.61 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.28, 22.88, 24.94, 29.28, 29.47, 29.56, 29.66, 29.85, 32.11, 34.44, 180.93.
7.9.14. Indium(III) Palmitate (3n)
It was prepared according to the general procedure discussed in method A. Indium chloride was used as a metal precursor and palmitic acid as a carboxylate precursor. The product was obtained in 60% yield. 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.28 (m, 24H), 1.62 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.31, 22.88, 24.85, 29.24, 29.43, 29.56, 29.62, 29.78, 29.87, 32.12, 34.30, 180.63.
7.9.15. Indium(III) Laurate (3o)
It was prepared according to the general procedure discussed in method A. Indium chloride was used as a metal precursor and lauric acid as carboxylate precursor. The product was obtained in 75% yield. 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.27 (m, 16H), 1.61 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.28, 22.87, 24.92, 29.26, 29.45, 29.53, 29.64, 29.79, 32.09, 34.40, 180.91.
7.9.16. Lead(II) Oleate (3p)
It was prepared according to the general procedure discussed in method A. Lead nitrate was used as a metal precursor and oleic acid as a carboxylate precursor. The product was obtained in 90% yield. mp 91–94 °C; 1H NMR (CDCl3, 400 MHz): δ ppm 0.87 (t, 3H, J = 8 Hz), 1.27 (m, 18H), 1.62 (m, 2H), 2.01 (m, 1H), 2.34 (t, 2H, J = 8 Hz), 5.34 (dd, 1H, J = 4 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.29, 22.86, 25.00, 27.35, 29.20, 29.30, 29.39, 29.50, 29.70, 29.87, 32.08, 35.17, 129.88, 130.16, 180.67.
7.9.17. Magnesium(II) Stearate (3q)
It was prepared according to the general procedure discussed in method A. Magnesium nitrate was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 88% yield. mp 118–122 °C 1H NMR (CDCl3, 400 MHz): δ ppm 0.88 (t, 3H, J = 8 Hz), 1.29 (m, 28H), 1.62 (m, 2H), 2.34 (t, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ ppm 14.31, 22.88, 22.89, 24.89, 29.25, 29.44, 29.55, 29.63, 29.78, 29.86, 32.11, 34.15, 179.52.
7.9.18. Manganese(II) Stearate (3r)
It was prepared according to the general procedure discussed in method A. Manganese chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 70% yield. mp 108–111 °C.
7.9.19. Nickel(II) Stearate (3s)
It was prepared according to the general procedure discussed previously. Zinc chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 87% yield. mp 84–87 °C.
7.9.20. Gadolinium(III) Stearate (3t)
It was prepared according to the general procedure discussed in method A. Gadolinium chloride was used as a metal precursor and stearic acid as a carboxylate precursor. The product was obtained in 45% yield. mp 107–110 °C.
Acknowledgments
S.T., S.P., and K.B. acknowledge the SERB-DST, Government of India for research funding (EEQ/2016/000751 and EMR/2016/002505). S.B. would like to thank the Department of Science and Technology, Government of India (DST/INSPIRE/03/2016/001207) [IF160689] for financial support under the DST-INSPIRE Scheme. A.P. acknowledges the SERB-DST (EEQ/2016/000685) and the DST-Inspire (DST/INSPIRE/04/2015/002674), for financial assistance. The authors thank Nimuka Tamang and Subas Chandra Mohanta for help with synthesis of NCs.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04448.
NMR spectra, FTIR spectra, and TGA spectra of metal carboxylates. Photoluminescence excitation spectrum of CsPbBr3 NCs and photographs of colloidal solutions of different NCs (PDF)
Author Contributions
S.B. carried out syntheses, characterization, and mechanistic studies of M-FAs; he also contributed to data analysis and paper writing. S.B. and K.B. contributed in the conceptualization, synthesis, and characterization of NCs. S.P., A.P., and S.T. contributed to experiment design, data analysis, explanation of results, and paper writing. S.T. verified the results and drafted the paper. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Rossetti R.; Nakahara S.; Brus L. E. Quantum Size Effects in the Redox Potentials, Resonance Raman Spectra, and Electronic Spectra of CdS Crystallites in Aqueous Solution. J. Chem. Phys. 1983, 79, 1086–1088. 10.1063/1.445834. [DOI] [Google Scholar]
- Brust M.; Walker M.; Bethell D.; Schiffrin D. J.; Whyman R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid-Liquid System. J. Chem. Soc., Chem. Commun. 1994, 801–802. 10.1039/c39940000801. [DOI] [Google Scholar]
- Murray C. B.; Norris D. J.; Bawendi M. G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. 10.1021/ja00072a025. [DOI] [Google Scholar]
- Yu W. W.; Peng X. Formation of High-Quality CdS and Other II-VI Semiconductor Nanocrystals in Noncoordinating Solvents:Tunable Reactivity of Monomers. Angew. Chem., Int. Ed. 2002, 41, 2368–2371. . [DOI] [PubMed] [Google Scholar]
- Battaglia D.; Peng X. Formation of High Quality InP and InAs Nanocrystals in a Noncoordinating Solvent. Nano Lett. 2002, 2, 1027–1030. 10.1021/nl025687v. [DOI] [Google Scholar]
- Hendricks M. P.; Campos M. P.; Cleveland G. T.; Jen-La Plante I.; Owen J. S. A Tunable Library of Substituted Thiourea Precursors to Metal Sulfide Nanocrystals. Science 2015, 348, 1226–1230. 10.1126/science.aaa2951. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman Q. A.; Martínez-Sarti L.; Goldoni L.; Imran M.; Baranov D.; Bolink H. J.; Palazon F.; Manna L. Molecular Iodine for a General Synthesis of Binary and Ternary Inorganic and Hybrid Organic–Inorganic Iodide Nanocrystals. Chem. Mater. 2018, 30, 6915–6921. 10.1021/acs.chemmater.8b03295. [DOI] [Google Scholar]
- Reiss P.; Protière M.; Li L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. 10.1002/smll.200800841. [DOI] [PubMed] [Google Scholar]
- Imran M.; Caligiuri V.; Wang M.; Goldoni L.; Prato M.; Krahne R.; De Trizio L.; Manna L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. 10.1021/jacs.7b13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa S.; Bhardwaj K.; Basel S.; Pradhan S.; Eling C. J.; Adawi A. M.; Bouillard J.-S. G.; Stasiuk G. J.; Reiss P.; Pariyar A.; Tamang S. Long-Term Ambient Air-Stable Cubic CsPbBr3 Perovskite Quantum Dots Using Molecular Bromine. Nanoscale Adv. 2019, 1, 3388–3391. 10.1039/c9na00486f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary D. C.; Cossairt B. M. Role of Acid in Precursor Conversion During InP Quantum Dot Synthesis. Chem. Mater. 2013, 25, 2463–2469. 10.1021/cm401289j. [DOI] [Google Scholar]
- Li L.; Reiss P. One-Pot Synthesis of Highly Luminescent InP/ZnS Nanocrystals without Precursor Injection. J. Am. Chem. Soc. 2008, 130, 11588–11589. 10.1021/ja803687e. [DOI] [PubMed] [Google Scholar]
- Tamang S.; Lincheneau C.; Hermans Y.; Jeong S.; Reiss P. Chemistry of InP Nanocrystal Syntheses. Chem. Mater. 2016, 28, 2491–2506. 10.1021/acs.chemmater.5b05044. [DOI] [Google Scholar]
- Reiss P.; Carrière M.; Lincheneau C.; Vaure L.; Tamang S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. 10.1021/acs.chemrev.6b00116. [DOI] [PubMed] [Google Scholar]
- Thanh N. T. K.; Maclean N.; Mahiddine S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- Almeida G.; Goldoni L.; Akkerman Q.; Dang Z.; Khan A. H.; Marras S.; Moreels I.; Manna L. Role of Acid-Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals. ACS Nano 2018, 12, 1704–1711. 10.1021/acsnano.7b08357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliehe C.; Juarez B. H.; Pelletier M.; Jander S.; Greshnykh D.; Nagel M.; Meyer A.; Foerster S.; Kornowski A.; Klinke C.; et al. Ultrathin PbS Sheets by Two-Dimensional Oriented Attachment. Science 2010, 329, 550–553. 10.1126/science.1188035. [DOI] [PubMed] [Google Scholar]
- Dasgupta B. R.; Huang L.; Sanzgiri V. R.; Sethna S. D.; Shah P. C.. Novel Cosmetic Cream Composition. WO 2008104591 A2, PCT/EP2008/052433, 2008.
- Sahoo R. R.; Biswas S. K. Frictional Response of Fatty Acids on Steel. J. Colloid Interface Sci. 2009, 333, 707–718. 10.1016/j.jcis.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Gregory J. N.; Spink J. A. Lubricating Properties of Molecular Layers of Stearic Acid and Calcium Stearate on Metal Surfaces. Nature 1947, 159, 403. 10.1038/159403a0. [DOI] [PubMed] [Google Scholar]
- Li J.; Wu Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21–43. 10.3390/lubricants2010021. [DOI] [Google Scholar]
- Hermans J. J.; Keune K.; Van Loon A.; Iedema P. D. An Infrared Spectroscopic Study of the Nature of Zinc Carboxylates in Oil Paintings. J. Anal. At. Spectrom. 2015, 30, 1600–1608. 10.1039/c5ja00120j. [DOI] [Google Scholar]
- Lappi H.; Alén R. Pyrolysis of Vegetable Oil Soaps-Palm, Olive, Rapeseed and Castor Oils. J. Anal. Appl. Pyrolysis 2011, 91, 154–158. 10.1016/j.jaap.2011.02.003. [DOI] [Google Scholar]
- Pratiwi M.; Neonufa G. F.; Prakoso T.; Soerawidjaja T. H. The Synthesis of Magnesium Soaps as Feed for Biohydrocarbon Production. MATEC Web Conf. 2018, 156, 03001. 10.1051/matecconf/201815603001. [DOI] [Google Scholar]
- Rakhmatullina A. P.; Akhmed’yanova R. A.; Liakumovich A. G.; Portnoi T. B.; Mokhnatkina E. G.; Il’yasov R. S. Active Processing Additives Based on Zinc and Calcium Salts of Stearic and Oleic Acids and Their Mixtures. Int. Polym. Sci. Technol. 2004, 31, 29–32. 10.1177/0307174x0403101209. [DOI] [Google Scholar]
- Wang F.; Yu H.; Li J.; Hang Q.; Zemlyanov D.; Gibbons P. C.; Wang L.; Janes D. B.; Buhro W. E. Spectroscopic Properties of Colloidal Indium Phosphide Quantum Wires. J. Am. Chem. Soc. 2007, 129, 14327–14335. 10.1021/ja074049h. [DOI] [PubMed] [Google Scholar]
- Franke D.; Harris D. K.; Xie L.; Jensen K. F.; Bawendi M. G. The Unexpected Influence of Precursor Conversion Rate in the Synthesis of III-V Quantum Dots. Angew. Chem., Int. Ed. 2015, 54, 14299–14303. 10.1002/anie.201505972. [DOI] [PubMed] [Google Scholar]
- Park J.; An K.; Hwang Y.; Park J.-G.; Noh H.-J.; Kim J.-Y.; Park J.-H.; Hwang N.-M.; Hyeon T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- Wolfgang S. M.; Heider T. P.. Stearate Composition and Method of Production Thereof. U.S. Patent 7,456,306 B2, 2008.
- Phanstiel O.; Dueno E.; Wang Q. X. Synthesis of Exotic Soaps in the Chemistry Laboratory. J. Chem. Educ. 1998, 75, 612. 10.1021/ed075p612. [DOI] [Google Scholar]
- Gönen M.; Ozturk S.; Balkose D.; Okur S.; Ulku S. Preparation and Characterization of Calcium Stearate Powders and Films Prepared by Precipitation and Langmuir-Blodgett Techniques. Ind. Eng. Chem. Res. 2010, 49, 1732–1736. 10.1021/ie901437d. [DOI] [Google Scholar]
- Gönen M.; Balköse D.; Inal F.; Ulku S. Zinc Stearate Production by Precipitation and Fusion Processes. Ind. Eng. Chem. Res. 2005, 44, 1627–1633. 10.1021/ie049398o. [DOI] [Google Scholar]
- Dou Q.; Ng K. M. Synthesis of Various Metal Stearates and the Corresponding Monodisperse Metal Oxide Nanoparticles. Powder Technol. 2016, 301, 949–958. 10.1016/j.powtec.2016.07.037. [DOI] [Google Scholar]
- Baghernejad B. 1,4-Diazabicyclo[2.2.2]Octane (DABCO) as a Useful Catalyst in Organic Synthesis. Eur. J. Chem. 2010, 1, 54–60. 10.5155/eurjchem.1.1.54-60.2. [DOI] [Google Scholar]
- Aggarwal V. K.; Emme I.; Fulford S. Y. Correlation between PKa and Reactivity of Quinuclidine-Based Catalysts in the Baylis-Hillman Reaction: Discovery of Quinuclidine as Optimum Catalyst Leading to Substantial Enhancement of Scope. J. Org. Chem. 2003, 68, 692–700. 10.1021/jo026671s. [DOI] [PubMed] [Google Scholar]
- Faltin C.; Fleming E. M.; Connon S. J. Acrylamide in the Baylis-Hillman Reaction: Expanded Reaction Scope and the Unexpected Superiority of DABCO over More Basic Tertiary Amine Catalysts. J. Org. Chem. 2004, 69, 6496–6499. 10.1021/jo0490907. [DOI] [PubMed] [Google Scholar]
- Pimenta L. S.; Gusevskaya E. V.; Alberto E. E. Intermolecular Halogenation/Esterification of Alkenes with N-Halosuccinimide and Acetic Acid Catalyzed by 1,4-Diazabicyclo[2.2.2]Octane. Adv. Synth. Catal. 2017, 359, 2297–2303. 10.1002/adsc.201700117. [DOI] [Google Scholar]
- Röhlich C.; Wirth A. S.; Köhler K. Suzuki Coupling Reactions in Neat Water as the Solvent: Where in the Biphasic Reaction Mixture Do the Catalytic Reaction Steps Occur?. Chem.—Eur. J. 2012, 18, 15485–15494. 10.1002/chem.201201266. [DOI] [PubMed] [Google Scholar]
- Luo S.; Feng J.; Ng K. M. Large Scale Synthesis of Nearly Monodisperse, Variable-Shaped In2O3 Nanocrystals via a One-Pot Pyrolysis Reaction. CrystEngComm 2014, 16, 9236–9244. 10.1039/c4ce01223b. [DOI] [Google Scholar]
- Kaupmees K.; Trummal A.; Leito I. Basicities of Strong Bases in Water: A Computational Study. Croat. Chem. Acta 2014, 87, 385–395. 10.5562/cca2472. [DOI] [Google Scholar]
- Shekouhy M.; Khalafi-Nezhad A. Polyethylene Glycol-Bonded 1,8-Diazabicyclo[5.4.0]Undec-7-Ene (PEG-DBU) as a Surfactant-Combined Base Catalyst for the Application of Nucleosides as Reagents in Multi-Component Syntheses of 8-Substituted Pyrido[2,3-d]Pyrimidine-6-Carbonitriles in Water. Green Chem. 2015, 17, 4815–4829. 10.1039/c5gc01448d. [DOI] [Google Scholar]
- Shieh W.-C.; Lozanov M.; Loo M.; Repič O.; Blacklock T. J. DABCO- and DBU-Accelerated Green Chemistry for N-, O-, and S-Benzylation with Dibenzyl Carbonate. Tetrahedron Lett. 2003, 44, 4563–4565. 10.1016/s0040-4039(03)00992-4. [DOI] [Google Scholar]
- Bronstein L. M.; Huang X.; Retrum J.; Schmucker A.; Pink M.; Stein B. D.; Dragnea B. Influence of Iron Oleate Complex Structure on Iron Oxide Nanoparticle Formation. Chem. Mater. 2007, 19, 3624–3632. 10.1021/cm062948j. [DOI] [Google Scholar]
- Jóna E.; Ondrušová D.; Pajtášová M.; Šimon P.; Michálek J. A Study of Curative Interactions in the Presence of Cobalt(II) Stearate. J. Appl. Polym. Sci. 2001, 81, 2936–2943. 10.1002/app.1744. [DOI] [Google Scholar]
- Roy P. K.; Surekha P.; Rajagopal C.; Choudhary V. Effect of Cobalt Carboxylates on the Photo-Oxidative Degradation of Low-Density Polyethylene. Part-I. Polym. Degrad. Stab. 2006, 91, 1980–1988. 10.1016/j.polymdegradstab.2006.02.007. [DOI] [Google Scholar]
- Gönen M.; Egbuchunam T. O.; Balköse D.; İnal F.; Ülkü S. Preparation and Characterization of Magnesium Stearate, Cobalt Stearate, and Copper Stearate and Their Effects on Poly(Vinyl Chloride) Dehydrochlorination. J. Vinyl Addit. Technol. 2015, 21, 235–244. 10.1002/vnl.21384. [DOI] [Google Scholar]
- Torron S.; Hult D.; Pettersson T.; Johansson M. Tailoring Soft Polymer Networks Based on Sugars and Fatty Acids toward Pressure Sensitive Adhesive Applications. ACS Sustainable Chem. Eng. 2017, 5, 2632–2638. 10.1021/acssuschemeng.6b02978. [DOI] [Google Scholar]
- Bunting J. W.; Thong K. M. Stability Constants for Some 1: 1 Metal-Carboxylate Complexes. Can. J. Chem. 1970, 48, 1654. 10.1139/v70-273. [DOI] [Google Scholar]
- Bala T.; Prasad B. L. V.; Sastry M.; Kahaly M. U.; Waghmare U. V. Interaction of Different Metal Ions with Carboxylic Acid Group: A Quantitative Study. J. Phys. Chem. A 2007, 111, 6183–6190. 10.1021/jp067906x. [DOI] [PubMed] [Google Scholar]
- Lide D. R.; Baysinger G.. CRC Handbook of Chemistry and Physics; CRC Press, Taylor & Francis Group: Boca Raton, Fl, 2007. [Google Scholar]
- Wise F. W. Lead Salt Quantum Dots: The Limit of Strong Quantum Confinement. Acc. Chem. Res. 2000, 33, 773–780. 10.1021/ar970220q. [DOI] [PubMed] [Google Scholar]
- Luo X.; Lai R.; Li Y.; Han Y.; Liang G.; Liu X.; Ding T.; Wang J.; Wu K. Triplet Energy Transfer from CsPbBr3 Nanocrystals Enabled by Quantum Confinement. J. Am. Chem. Soc. 2019, 141, 4186–4190. 10.1021/jacs.8b13180. [DOI] [PubMed] [Google Scholar]
- Talapin D. V.; Lee J.-S.; Kovalenko M. V.; Shevchenko E. V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. 10.1021/cr900137k. [DOI] [PubMed] [Google Scholar]
- Dayal S.; Kopidakis N.; Olson D. C.; Ginley D. S.; Rumbles G. Photovoltaic Devices with a Low Band Gap Polymer and CdSe Nanostructures Exceeding 3% Efficiency. Nano Lett. 2010, 10, 239–242. 10.1021/nl903406s. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Hong G.; Zhang Y.; Chen G.; Li F.; Dai H.; Wang Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second near-Infrared Window. ACS Nano 2012, 6, 3695–3702. 10.1021/nn301218z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X.; Pinaud F. F.; Bentolila L. .; Tsay J. M.; Doose S.; Li J. J.; Sundaresan G.; Wu A. M.; Gambhir S. S.; Weiss S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-B.; Tung C.-H.; Wu L.-Z. Semiconducting Quantum Dots for Artificial Photosynthesis. Nat. Rev. Chem. 2018, 2, 160–173. 10.1038/s41570-018-0024-8. [DOI] [Google Scholar]
- Pal A.; Ghosh I.; Sapra S.; König B. Quantum Dots in Visible-Light Photoredox Catalysis: Reductive Dehalogenations and C-H Arylation Reactions Using Aryl Bromides. Chem. Mater. 2017, 29, 5225–5231. 10.1021/acs.chemmater.7b01109. [DOI] [Google Scholar]
- Bhattacharyya B.; Pandey A. CuFeS2 Quantum Dots and Highly Luminescent CuFeS2 Based Core/Shell Structures: Synthesis, Tunability, and Photophysics. J. Am. Chem. Soc. 2016, 138, 10207–10213. 10.1021/jacs.6b04981. [DOI] [PubMed] [Google Scholar]
- Jiang P.; Tian Z.-Q.; Zhu C.-N.; Zhang Z.-L.; Pang D.-W. Emission-Tunable Near-Infrared Ag2S Quantum Dots. Chem. Mater. 2012, 24, 3–5. 10.1021/cm202543m. [DOI] [Google Scholar]
- Liu M.; Voznyy O.; Sabatini R.; García de Arquer F. P.; Munir R.; Balawi A. H.; Lan X.; Fan F.; Walters G.; Kirmani A. R.; et al. Hybrid Organic-Inorganic Inks Flatten the Energy Landscape in Colloidal Quantum Dot Solids. Nat. Mater. 2017, 16, 258–263. 10.1038/nmat4800. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Zhou B.; Yang C.; Liao S.; Zhang W.-H.; Li C. CuFeS2 Colloidal Nanocrystals as an Efficient Electrocatalyst for Dye Sensitized Solar Cells. Chem. Commun. 2016, 52, 11488–11491. 10.1039/c6cc06241e. [DOI] [PubMed] [Google Scholar]
- Son D. I.; Kim H. H.; Hwang D. K.; Kwon S.; Choi W. K. Inverted CdSe-ZnS Quantum Dots Light-Emitting Diode Using Low-Work Function Organic Material Polyethylenimine Ethoxylated. J. Mater. Chem. C 2014, 2, 510–514. 10.1039/c3tc31297f. [DOI] [Google Scholar]
- Li D.; Kristal B.; Wang Y.; Feng J.; Lu Z.; Yu G.; Chen Z.; Li Y.; Li X.; Xu X. Enhanced Efficiency of InP-Based Red Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 34067–34075. 10.1021/acsami.9b07437. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Wang Z.-K.; Zhuo M.-P.; Tian Q.-S.; Jin Y.; Liao L.-S. Self-Assembled High Quality CsPbBr3 Quantum Dot Films toward Highly Efficient Light-Emitting Diodes. ACS Nano 2018, 12, 9541–9548. 10.1021/acsnano.8b05185. [DOI] [PubMed] [Google Scholar]
- Stasiuk G. J.; Tamang S.; Imbert D.; Poillot C.; Giardiello M.; Tisseyre C.; Barbier E. L.; Fries P. H.; De Waard M.; Reiss P.; et al. Cell-Permeable Ln(III) Chelate-Functionalized InP Quantum Dots as Multimodal Imaging Agents. ACS Nano 2011, 5, 8193–8201. 10.1021/nn202839w. [DOI] [PubMed] [Google Scholar]
- Tang R.; Xue J.; Xu B.; Shen D.; Sudlow G. P.; Achilefu S. Tunable Ultrasmall Visible-to-Extended near-Infrared Emitting Silver Sulfide Quantum Dots for Integrin-Targeted Cancer Imaging. ACS Nano 2015, 9, 220–230. 10.1021/nn5071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Lin Y.; Sun Y.; Beard M. C.; Yan Y. Lead-Halide Perovskites for Photocatalytic α-Alkylation of Aldehydes. J. Am. Chem. Soc. 2019, 141, 733–738. 10.1021/jacs.8b08720. [DOI] [PubMed] [Google Scholar]
- Harris C.; Kamat P. V. Photocatalytic Events of CdSe Quantum Dots in Confined Media. Electrodic Behavior of Coupled Platinum Nanoparticles. ACS Nano 2010, 4, 7321–7330. 10.1021/nn102564x. [DOI] [PubMed] [Google Scholar]
- Chakraborty I. N.; Roy S.; Devatha G.; Rao A.; Pillai P. P. InP/ZnS Quantum Dots as Efficient Visible-Light Photocatalysts for Redox and Carbon-Carbon Coupling Reactions. Chem. Mater. 2019, 31, 2258–2262. 10.1021/acs.chemmater.9b00086. [DOI] [Google Scholar]
- Huang X.; Parashar V. K.; Gijs M. A. M. Nucleation and Growth Behavior of CdSe Nanocrystals Synthesized in the Presence of Oleylamine Coordinating Ligand. Langmuir 2018, 34, 6070–6076. 10.1021/acs.langmuir.7b01337. [DOI] [PubMed] [Google Scholar]
- Hou B.; Cho Y.; Kim B. S.; Hong J.; Park J. B.; Ahn S. J.; Sohn J. I.; Cha S.; Kim J. M. Highly Monodispersed PbS Quantum Dots for Outstanding Cascaded-Junction Solar Cells. ACS Energy Lett. 2016, 1, 834–839. 10.1021/acsenergylett.6b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman F. D.; Hocaoglu I.; Ozturk D. G.; Gozuacik D.; Kiraz A.; Yagci Acar H. Highly Luminescent and Cytocompatible Cationic Ag2S NIR-Emitting Quantum Dots for Optical Imaging and Gene Transfection. Nanoscale 2015, 7, 11352–11362. 10.1039/c5nr00189g. [DOI] [PubMed] [Google Scholar]
- Curley J. J.; Slugocki T.; Hotz C.. Use of Heteroleptic Indium Hydroxides as Precursors for InP Nancrystals. U.S. Patent 20,170,137,360 A1, 2017.
- Sugathan A.; Bhattacharyya B.; Kishore V. V. R.; Kumar A.; Rajasekar G. P.; Sarma D. D.; Pandey A. Why Does CuFeS2 Resemble Gold?. J. Phys. Chem. Lett. 2018, 9, 696–701. 10.1021/acs.jpclett.7b03190. [DOI] [PubMed] [Google Scholar]
- Gary D. C.; Cossairt B. M. Role of Acid in Precursor Conversion during InP Quantum Dot Synthesis. Chem. Mater. 2013, 25, 2463–2469. 10.1021/cm401289j. [DOI] [Google Scholar]
- Ji C.; Zhang Y.; Zhang X.; Wang P.; Shen H.; Gao W.; Wang Y.; Yu W. W. Synthesis and Characterization of Ag2SxSe1–x Nanocrystals and Their Photoelectrochemical Property Changyin. Nanotechnology 2017, 28, 065602. 10.1088/1361-6528/aa523c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.