Abstract
Human Immunodeficiency Virus (HIV) infection remains a significant cause of mortality globally. Though antiretroviral therapy has significantly reduced AIDS-related morbidity and mortality, there are several drawbacks in the current therapy, including toxicity, drug–drug interactions, development of drug resistance, necessity for long-term drug therapy, poor bio-availability and lack of access to tissues and reservoirs. To circumvent these problems, recent anti-HIV therapeutic research has focused on improving drug delivery systems through drug delivery targeted specifically to host cells infected with HIV or could potentially get infected with HIV. In this regard, several surface molecules of both viral and host cell origin have been described in recent years, that would enable targeted drug delivery in HIV infection. In the present review, we provide a comprehensive overview of the need for novel drug delivery systems, and the successes and challenges in the identification of novel viral and host-cell molecules for the targeted drug delivery of anti-HIV drugs. Such targeted anti-retroviral drug delivery approaches could pave the way for effective treatment and eradication of HIV from the body.
Keywords: HIV, AIDS, Targeted drug delivery, Viral targets, Host targets
Graphical abstract
1. Introduction
Human Immunodeficiency Virus (HIV) is the primary cause of Acquired Immuno Deficiency Syndrome (AIDS) which still remains a significant cause of mortality globally [1]. HIV mainly targets immune cells i.e. CD4+ T cells, monocytes, macrophages, and dendritic cells. The decline in CD4+ T-cells in the more advanced stages of infection is responsible for the profound immune suppression that characterizes the advanced stage of AIDS. However, the advent of Highly Active Anti-Retroviral Therapy (HAART), a combination of drugs that inhibit HIV-1 replication, has led to reduced viremia and the onset of opportunistic infections in most patients and prolonged survival.
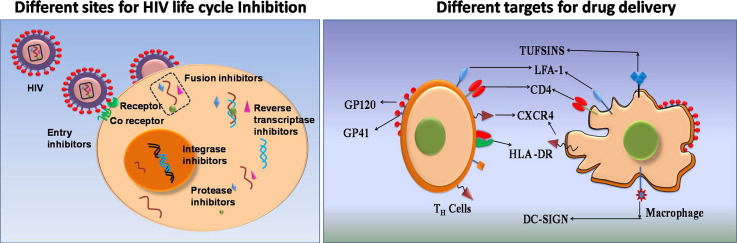
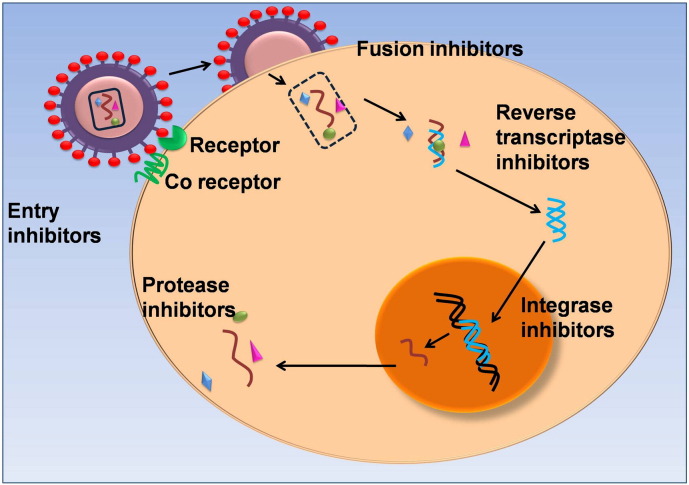
Chemotherapy has been the main mode of treatment of AIDS. The common classes of drugs that have been employed in the treatment of HIV infections are designed to inhibit a particular stage in the infectious cycle of HIV. Fig. 1 depicts the different targets for the various anti-retroviral drugs. Entry inhibitors comprising fusion inhibitors and co-receptor inhibitors prevent the entry of HIV into the host cell. While fusion inhibitors interact with the glycoprotein-41 (gp-41) on the viral envelope thereby blocking its fusion with the cell membrane, the co-receptor inhibitors interact with the CCR5 receptor in the host cell and prevent their interactions with the glycoprotein-120 (gp-120) of the virus [2]. Protease inhibitors (PIs) interfere with the proteolytic processing of viral proteins through binding interactions with the active site of the HIV protease [3]. The reverse transcriptase inhibitors form a major class of anti-retroviral drugs. These molecules disrupt the transformation of the single stranded RNA of the viral genome into a double stranded DNA by the referred to as nucleoside reverse transcriptase inhibitors (NRTIs) and nucleotide reverse transcriptase inhibitors (NtRTIs) respectively. These inhibitors binds directly to the reverse transcriptase enzyme and thus block the HIV life cycle. A related class of drugs known as non-nucleoside reverse transcriptase inhibitors (NNRTIs) binds to an allosteric site of reverse transcriptase resulting in inhibition of the enzyme activity. A recent addition to the repertoire of anti-retroviral drugs is the integrase inhibitors that interfere with the integration of the viral genome with the host cell. List of anti-HIV drugs are listed in Table 1 .
Fig. 1.
Sites of action of anti-retroviral drugs at different steps in the viral life cycle.
Table 1.
Different categories of anti-retroviral drugs.
| Drug category | Generic name |
|---|---|
| Nucleoside reverse transcriptase inhibitors | Zidovudine, Didanosine, Stavudine, Lamivudine, Abacavir, Emtrictabine |
| Non-nucleoside reverse transcriptase inhibitors | Nevirapine, Efavirenz, Etravirine, Rilpivirine |
| Nucleotide reverse transcriptase inhibitors | Tenofovir |
| Entry inhibitors | Maraviroc |
| Fusion inhibitor | Enfuvirtide |
| Integrase inhibitors | Raltegravir, Dolutegravir |
| Protease inhibitors | Saquinavir, Indinavir, Ritonavir, Nelfinavir, Atazanavir, Tipranavir, Darunavir |
2. Shortcomings of conventional anti-retroviral therapy
Anti-retroviral therapy (ARV) has significantly reduced AIDS-related morbidity and mortality. However there are several drawbacks in the current ARV therapy.
-
a)
Drug resistance: One other major problem with anti-HIV drug therapy is that of resistance. The process of HIV replication is rapid and error-prone (~ 10 billion viral particles are produced on a daily basis), while generating at least one mutation per genome. These genetic mutations enable the virus to develop resistance to anti-HIV drug therapy, especially when monotherapy is employed. An example is the emergence and selection of HIV subtype C virus in India, which contains multiple NF-kB (sites?) and strengthens the promoters of the virus.
-
b)
Long-term drug therapy: The nature of HIV infection, including viral persistence in reservoirs necessitates long-term uninterrupted multi-drug ARV therapy. Treatment compliance is critical regardless of whether a patient is treatment-naïve or treatment-experienced since poor patient compliance is often a factor in treatment failure and viral rebound. The lack of patient adherence to complicated drug administration regimens is further exacerbated by the cumulative costs of combined ARV therapy.
-
c)
Toxicity and drug–drug interactions: Prolonged treatment with ARV drugs has resulted in several side-effects, including constipation, fever, liver disorders, muscular dystrophy, metabolic disorders, and peripheral neuropathy. The use of a combination of drugs in the conventional therapy can also lead to undesirable drug–drug interactions thereby reducing the efficacy of the drugs. For instance, when a combination of the reverse transcriptase inhibitor nevirapine and protease inhibitor saquinavir was used to treat HIV infections, saquinavir levels in the plasma were found to drop rapidly. This is due to the induction of the liver cytochrome p450 by nevirapine leading to the metabolization of saquinavir and its subsequent elimination therefore reducing its availability and efficacy [4].
-
d)
Poor bio-availability: ARV drugs are burdened by poor pharmaco-kinetics. Most of the ARV drugs are formulated as solid dosage forms for oral route of administration. The oral dosage forms offer convenience, but the combined dose of compounds that make up a therapeutic regimen is usually high. High doses are preferred because the treatment objective is to completely inhibit viral proliferation, an effect which is proportional to drug concentrations. The delivery of drugs via oral route suffers from significant first-pass effect, variation of absorption and degradation in the gastrointestinal tract due to enzymes and extreme pH conditions leading to low and erratic bioavailability. For example, the expression of multidrug resistant efflux proteins (MRPs) such as P-glycoprotein (P-gp) on the gastrointestinal tract further decreases their oral bioavailability. The metabolism/elimination and transport barriers will substantially reduce the effective amount of anti-HIV drug reaching the target action site. The half-life for several ARV drugs is short, which then requires frequent administration of doses leading to poor patient compliance. The short residence time and reduced half-life of the drug in the plasma necessitate frequent administration of booster dosages as well as increased drug dosages contributing to the development of drug resistance. Some anti-retroviral drugs such as saquinavir possess poor bioavailability due to their metabolization by liver enzymes [5].
-
e)
Lack of access to tissues and reservoirs: Most of the anti-retroviral drugs cannot cross the blood–brain barrier (BBB) as a result of which they are ineffective in entering microglial cells such as astrocytes that hoards HIV particles [6]. A major factor is the high protein binding of most anti-HIV drugs that prevent their diffusion across the BBB. Also, it has been observed that the anti-retroviral drugs are unable to reach the lymphatic system that harbors HIV. Thus the conventional therapy is ineffective in annihilating viral reservoirs as less than 2% of the lymphatic cells are present in systemic circulation [7]. The cells of the lymphatic system like macrophages and dendritic cells are involved in the transmission of the virus to CD4+ TH cells (helper T lymphocytes) [8] and failure to target such reservoirs of HIV increases the risk of a viral relapse post-treatment.
3. Emergence of newer approaches to counter problems in conventional ARV therapy
To circumvent the problems mentioned above and effectively treat the HIV infection, recent anti-AIDS therapeutic development efforts have been focusing on improving drug delivery and not just on discovering new chemical entities. Efforts have been made to design novel drug delivery systems for anti-HIV agents to reduce the dosing frequency, to enhance the bioavailability, to improve the Central Nervous System (CNS) penetration, to inhibit the CNS efflux and to deliver them to the target cells selectively with minimal side effects.
4. Target molecules for drug delivery
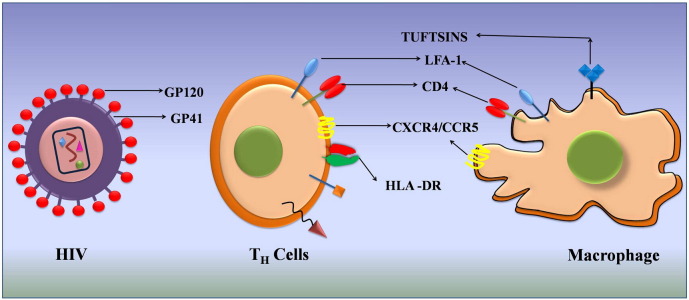
Among the recent approaches of novel drug delivery system for anti-HIV drugs, targeted/intracellular drug delivery only in host cells capable of getting infected with HIV or more specifically HIV-infected cells and reservoirs holds great promise. The main aim of drug targeting is to optimize a drug's therapeutic index by strictly localizing its pharmacological activity to the site or organ of action. The result of the targeting would be a significant reduction in drug toxicity, reduction of the drug dose, and increased treatment efficacy. During HIV infection, the virus infects only specific cells of the immune systems including CD4+ T-cells, macrophages and dendritic cells, which forms a strong rationale for targeting delivery of anti-HIV drugs specifically to these cells for increasing the efficacy of therapy. Drug delivery to HIV-infected tissues/cells with selectivity can be achieved by targeting surface markers on CD4+ T-cells, macrophages, dendritic cells and towards the CNS. Targeting these receptors can have two effects: i) prophylactic protection of the cell by occupying the receptor necessary for virus–cell interaction, and/or ii) improving the specific uptake of a nanocarrier loaded with anti-viral drugs. In this regard, several molecules of both viral and host cell origin have been described in recent years, that would enable targeted drug delivery in HIV infection. In the following review, we provide a brief overview of the recent developments in the identification of novel viral and host-cell molecules for the targeted drug delivery of anti-HIV drugs. A schematic representation of the different types of viral and host targets investigated for specifically targeting HIV-infected cells, thus far is shown in Fig. 2 .
Fig. 2.
Different virus-based and host cell-based targets for specific targeting of HIV infected cells.
4.1. Targeting virus moieties
The conserved regions in HIV envelope glycoproteins gp41 and gp120 are involved in binding of the virus to the host cells [9]. Many targeting strategies have focused on binding to these protein sequences to achieve specificity, though the high rates of mutation exhibited by the retrovirus make it challenging to identify a suitable targeting sequence on the envelope [10].
4.1.1. Glycoprotein-120 (gp120)
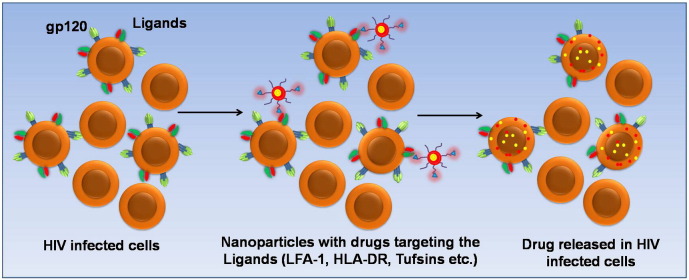
The glycoprotein-120 (gp120) present in the outer envelope of HIV enables the entry of the virus particles into CD4+ T-cells. It mainly binds to the CD4 receptor of the T-lymphocytes (TH cells) and macrophages and aids in the viral entry with the help of the fusion protein gp41 [11]. Upon infection, the gp120 is mainly exposed on the surface of the HIV infected cells and hence can be an attractive target for cell-specific delivery of anti-retroviral drugs. An immunoliposomal carrier encapsulating the protease inhibitor P11 and surface modified with anti-gp120 was reported to exhibit excellent specificity towards HIV infected cells due to binding of the anti-gp120 to the exposed domains of gp120 present on the surface of HIV infected cells [12]. Actinohivin, a prokaryotic lectin found in actinomycetes was identified to possess anti-HIV activity and was found to specifically target HIV infected cells. This lectin belongs to the family of carbohydrate binding agents (CBAs) and displays high affinity binding to the mannose residues of the D1 chains of high mannose-type glycans associated with gp120 of HIV [13]. In an earlier work, a glycosylphosphatidylinositol (GPI) moiety conjugated to gp120 was incorporated in liposomes and was found to selectively bind to CD4+ cells [14]. Comparison of the GPI-gp120 liposomes with liposomal vesicles formed from components of the viral envelope revealed differences in the intracellular localization in the two systems. As both carriers were internalized through CD4 mediated endocytosis, it was inferred that differences in the lipid composition in the two systems influenced their intracellular compartmentalization. This facet, however, remains largely unexplored in the context of targeted nanocarrier delivery of anti-retrovirals. Mesoporous silica nanoparticles with highly oriented and accessible pores were functionalized with an 18-mer fragment of CD4 designated as sCD4 either through amide conjugation or glycosyl linkage [15]. The functionalized nanoparticles exhibited excellent binding to gp120 protein. Recently, V3 loop of gp120 has been identified as the region involved in the initial entry of virus into the cell [16]. Antibodies against the V3 loop have been found to inhibit the entry of the virions [17]. Antibodies against the V3 loop can also be used to target HIV infected cells thereby conferring both target specificity and anti-viral activity. Another strategy involving the use of a tri-functional IgG-like bi-specific antibody with anti-gp120 and anti-C3d domains has been reported [18]. A tri-functional antibody contains two binding sites for different molecules and has been reported to display nearly 1000-fold enhancement in the targeting specificity when compared with the conventional antibodies that seek only a single target [18]. The bi-specific antibody reported against HIV infected cells was found to bind specifically with the gp120 fragment exposed in the infected cells as well as to the C3d, a component of the complement resulting in the activation of the complement-mediated lysis of the infected cells. A broad activity antibody against gp120, F105 has also been used to target the HIV infected cells [19]. F105 displays higher affinity to gp120 even at nanomolar concentrations. Crystal structure analysis of the binding fragment of F105 reveals a H-loop containing a unique sequence of serine and tyrosine residues and a phenyl alanine residue at the apex [19]. It is proposed that this phenyl alanine residue in F105 associates with the binding pocket of gp120 similar to the phenyl alanine residue (Phe43) from CD4 of the host cells. The serine and tyrosine residues in the H-loop stabilize the F105–gp120 binding through formation of hydrogen bonds [20]. In a novel approach, a CD4 mimetic peptide covalently linked with heparan sulfate was synthesized to inhibit HIV-1 entry into the host cell [21]. The CD4 mimetic peptide specifically binds to gp120 resulting in a conformation change that exposes the glycoprotein-binding domain of gp120, which interacts with the heparan sulfate moiety thereby blocking further interactions of the viral gp120 with the host cell receptors. A recombinant peptide expressing the CD4 binding region for gp120 designated as CD4(178)-PE40 and Pseudomonas toxin was developed to specifically target and destroy HIV infected cells. The peptide was found to specifically bind to the exposed regions of gp120 found on the surface of HIV infected cells [22]. A surfactant protein extracted from the lung surfactant, SP-D, which belongs to the lectin family has been found to display high affinity calcium-dependent binding to the glycosylated residues of gp120 [23]. The use of soluble CD4 and the immunoadhesin molecule CD4-IgG for targeting HIV infected cells has also been reported [24]. These molecules were found to bind to the HIV infected cells through the gp120 domain exposed on the surface of the infected cells. As conjugation of large antibodies to the surface of the nanoparticles is tedious and the risk of denaturation or loss of proper orientation conducive to ligand binding is high, smaller molecules that display similar specificity and affinity to the target antigen have been explored. Aptamers, a class of small molecules that can be either an oligonucleotide or a peptide, that exhibit high binding affinities to the target molecules can be a suitable alternative to antibodies [25]. A 2-fluoropyrimidine substituted RNA binding aptamer that specifically binds to the surface glycoprotein gp120, has been reported to display excellent target specificity [26]. Further studies on the use of such aptamer conjugated nanoparticle systems are warranted for improved therapy. Fig. 3 depicts a schematic representation of the concept of targeting HIV infected cells through gp120.
Fig. 3.
Targeting ligands on the surface of HIV infected cells through nano-conjugates loaded with the drug.
4.1.2. Glycoprotein-41 (gp41)
Glycoprotein-41 (gp41) is a trans-membrane protein present in the HIV envelope that facilitates the fusion of the virus with CD4+ cells [27]. The gp41 moiety is attached non-covalently to the larger glycoprotein120 (gp120), which is involved in the binding of the viral particles to CD4+ cells. The binding of gp120 to the CD4 receptors of the host cells causes conformational changes leading to the release of the stable gp41 [28]. The ectodomain of gp41 aids the fusion between the virus and host cell. The gp41 protein contains various subunits such as fusion peptides, N-terminal heptad repeats, C-terminal heptad repeats and membrane proximal extracellular region [29]. Similar to gp120, gp41 is also expressed on the surface of HIV infected cells and hence is an attractive target for therapeutic strategies involving selective delivery of the therapeutic agent to HIV infected cells. Antibodies against gp41 have been employed for targeting HIV infected cells [30]. In few cases, radio immunotherapy (RIT) using gp41 antibody labeled with 188Re (Rhenium) has been employed for targeting HIV infected cells containing viral particles budding on the cell [31]. The radioactive element causes the death of the HIV infected cells. This strategy was successfully demonstrated in vivo with about 99% reduction in the viral load reported. Recently, a new strategy has been reported for targeting both gp41 and gp120 using two antigenic determinants in a single antibody known as double variable domain immunoglobulins (DVDs). The use of DVDs promotes greater interactions with the two glycoprotein domains in the HIV infected cells resulting in higher uptake of the immunoconjugates and thereby leading to superior viral load reduction [32]. In a similar strategy, Lu et al. have reported a recombinant bivalent protein 2DLT, which contains the DID2 fragment of the CD4 and a peptide T1144 [33]. The DID2 domain of the 2DLT binds to the gp120 and then interacts with the NHR trimer repeat of the gp41 through T1144 resulting in the decay of the gp41 thus preventing further fusion of the HIV virions with other uninfected cells. Such recombinant proteins may also be used in conjugation with nanoparticles for targeting HIV infected cells, though such approaches have not been explored thus far. Many engineered peptides have been developed for targeting gp41. For instance, it has been identified that the peptide T20 binds specifically to the N-terminal heptad region of gp41 and inhibits infection by the virions due to prevention of conformational changes in gp41 responsible for viral entry [34]. The sequence of T20 is derived from the gp41. Similar peptides such as C34 and C37 that are also derived from gp41 have been designed to bind and inactivate the interactions between gp120 and the C-heptad repeat (C-HR) domain of gp41 leading to the inactivation of the env gene. These engineered peptides interact with specific exposed regions of the gp41 of HIV and prevent the entry of the virus into the cells [34]. The membrane proximal external region (MPER) in gp41 is also a highly conserved region containing 23 amino acids and antibodies against this domain have proved to be successful in targeting the HIV infected cells [34]. Surprisingly, though several molecules targeting conserved regions on the virus have been identified, integrating these targeting molecules with anti-retroviral drug containing nanoparticles has not been extensively investigated and remains in its infancy.
4.2. Targeting host cell moieties
Yet another interesting approach to target HIV-infected cells is to identify unique markers on the host cells that are also sought by the virus. Compared with virus-based targets, a relatively greater number of host cell-based targets have been identified. These include CD4 (receptor for HIV), chemokine receptors, CXCR4 and CCR5 (co-receptors for HIV), carbohydrate-binding antigens (CBAs), tuftsin, HLA-DR, DC-SIGN and LFA-1. However, recent studies indicate that the most promising targets include HLA-DR, DC-SIGN and LFA-1.
4.2.1. LFA-1
In addition to infection with cell-free virions, the importance of cell–cell spread across connecting membrane bridges and close cell–cell contacts referred to as virological synapse (VS) for HIV-1 propagation is increasingly being recognized as the predominant mechanism of HIV-1 propagation between T-cells. Assembly of the HIV-1 T-cell VS requires engagement of the HIV-1 Env surface subunit gp120, expressed on the effector cell, with its cellular receptors CD4 and CXCR4 on the target cell. Further recruitment of receptors and HIV-1 proteins to the conjugate interface is a cytoskeleton-dependent process in both target and effector T cells. Specifically, the VS is characterized by clustering of leukocyte function-associated antigen 1 (LFA-1, also known as αL β2 or CD11a/CD18) at the effector–target cell interface. The lymphocyte function associated antigen 1 (LFA-1) belongs to the integrin family of adhesion molecules and is hypothesized to contribute to the formation of a stable adhesive junction between the effector T-cell and the target T-cell. The major cognate ligand of LFA-1 on T cells is ICAM-1 (CD54). Furthermore, integrins have been implicated in cell–cell transmission of HIV-1 from dendritic cells (DCs) to T cells via LFA-1 and DC-SIGN (DC-specific intercellular adhesion molecule 3 [ICAM-3]-grabbing nonintegrin), and their probable role in this setting is to maintain robust DC–T-cell contacts. Since LFA-1 is a key molecule involved in transmission of HIV during the formation of the VS between T-cells and in DC–T-cell interaction, recent studies have targeted LFA-1 as a potential anti-HIV target. LFA-1 is present in T lymphocytes, B lymphocytes, neutrophils and macrophages and its expression is high during HIV infections. In addition, it has also been recognized that the HIV envelope glycoprotein gp120 induces high levels of LFA-1 expression [35].
Immunoliposomes modified with antibody targeting the LFA-1 integrin receptor were developed to deliver si-RNA against CCR5 gene and were evaluated for its efficacy in vitro and in vivo [36]. The antibody linked liposomes were able to deliver the si-RNA successfully into the infected cells. Cyclic peptides cIBR, cIBL and cIBC derived from ICAM-1 were able to bind and internalize into cells that express LFA-1 through the I-domain of LFA-1 and thus can be used to target HIV infected cells [37]. A monoclonal antibody AL-57 has also been demonstrated to exhibit high affinity to cells that express LFA-1 [38]. It was observed that the binding of AL-57 occurred with LFA-1 present only in lymphocytes activated by the HIV infection while the antibody binding did not occur in inactive cells thus displaying a high degree of selectivity towards HIV infected cells [39], [40]. An emerging paradigm in the use of monoclonal antibodies directed against LFA-1 is their ability to inhibit viral replication. In a recent study focused to understand the mechanism of viral replication inhibition by anti-LFA-1, it was found that the binding of the antibody to free virus did not influence its replication efficiency. However, binding of the antibody with the LFA-1 present in HIV infected cells resulted in the production of a soluble factor that interfered with the viral replication [40]. Hence, use of anti-LFA-1 could be doubly beneficial by ensuring specific targeting to HIV infected cells as well as inhibiting viral inhibition. This facet, however, needs to be explored in-depth with additional experiments in vivo. Fig. 3 represents the concept of targeting LFA-1 using anti-LFA-1 modified drug loaded nanoparticles. Though the above literature reports that studies in targeting LFA-1 with nanoparticles are few, the studies are promising and will surely open up avenues for more detailed studies on targeting LFA-1 as an anti-HIV target.
4.2.2. HLA-DR
The human leukocyte antigen (HLA) receptor belongs to the major histocompatibility class (MHC) of proteins and is involved in presenting of the antigens to the lymphocytes leading to the activation of the immune response [41]. Among the various MHC class II types, HLA-DR, HLA-DQ and HLA-DP are heterodimeric cell surface receptors found in macrophages, dendritic cells and B cells and present the antigen to the CD4+ T helper cells. Due to their localization on the surface of cells that serve as HIV reservoirs and their ability to attract CD4+ T helper cells, the HLA cell surface receptors are an attractive targeting moiety for HIV infected cells. Interestingly, HLA-DR molecule expression is increased during HIV infection along with CD25, a trans-membrane receptor of interleukin 2 [42].
Recently, anti-HLA-DR molecule has been used to target the HLA-DR expressed on the HIV infected cells [43]. Immunoliposomes loaded with the protease inhibitor indinavir and surface modified with anti-HLA-DR were found to effectively bind to HIV infected cells and deliver the anti-retroviral drug leading to a significant reduction in the viral load [44]. In another study, the biodistribution of anti-HLA-DR conjugated immunoliposomes was evaluated using mice models and it was found that the immunoliposomes were concentrated in the secondary lymphoid organs like spleen and lymph nodes which are the main reservoirs of the HIV virions [101]. A unique advantage acquired through use of anti-HLA-DR modified liposomes was that it was able to bind both HIV infected cells and also the free HIV virions found entrapped in the follicular dendritic cell network [45]. It has also been reported that the other viral reservoirs such as monocytes, macrophages, and dendritic cells that are difficult to target due to the dormant nature of the viruses, can be easily targeted using the anti-HLA-DR due to the high levels of HLA-DR expression on these cells and can therefore reduce undesirable toxicity of the anti-retroviral drugs to normal cells. It was also observed that the liposomes with anti-HLA-DR preferentially accumulated in the lymph nodes rich in the HIV infected cells [46]. Immunoliposomes conjugated with anti-HLA-DR loaded with amphotericin B, an inhibitor of HIV replication, were also reported to target HIV infected cells and reduce the viral load [45]. Immunoliposomal systems encapsulating si-RNA against rev and tat genes of HIV that are involved in the replication process were successfully delivered to the target cells through conjugation of antibodies against HLA-B and HLA-C that belong to the MHC class I molecules. It is evident from the scan of literature that the anti-HLA antibodies conjugated to liposomes alone have been reported while other nanocarriers modified with HLA-DR are yet to be explored. One of the major challenges in HLA targeting lies in the design of an antibody with high specificity due to the presence of large number of serotypes for HLA.
Furthermore, HLA-DR CD38+ CD4+ T cells show not only increased susceptibility to HIV, but once infected also produce support higher viral replication levels compared to other cells [47]. The HIV peptides show high affinity to the HLA-DR moieties. A study has also demonstrated the high affinity of certain HIV-derived peptides to the HLA-DR on the cell surface [48]. Studies indicate that the HLA-DR is incorporated into the viral envelope at the budding stage of the HIV cycle and facilitates further infection of the other uninfected cells using the CD4 receptor as the receptor.
Taken together, these studies indicate that targeting of the HLA-DR will not only deliver the anti-retroviral drugs to the possible sites of HIV infection, but may also attenuate the HLA-DR-mediated spread of viral infection in the body [49].
4.2.3. DC-SIGN
The C-type lectin DC-SIGN (DC specific ICAM-3-grabbing nonintegrin) has been identified as a cell surface receptor on immature DCs that binds HIV and mediates transfer of virus to CD4+ permissive T cells. Contact of HIV-1 with DC-SIGN is thus the first event in the pathogenic cascade and, therefore, it is the primary target point for therapies aimed at HIV infection prevention. DC-SIGN binding to HIV results in internalization of virus to a non-endolysosomal compartment. From this compartment, internalized virus moves rapidly to synapses formed by infected DCs and CD4+ T cells. The transmission of virions is mainly due to the interaction of the CD4-DC-SIGN and followed by tetramer formation resulting in the higher affinity of the DC-SIGN to the gp120 residues thus resulting in the fusion of the membrane and capture and transfer of the HIV virions by the dendritic cells to the CD4 positive cell [50]. The silencing of the DC-SIGN expression in HIV infected cells has been shown to reduce the viral load, thus confirming the potential for DC-SIGN to function as a potential target [50]. Some of the molecules which bind DC-SIGN are important in targeting the HIV infected cells. These include polyman 19 which binds specifically to the DC-SIGN expressed on the HIV infected T cells. Thus DC-SIGN could be a critical target to combat early HIV infection.
4.2.4. Other targets
4.2.4.1. Cell surface glycoprotein CD4
The glycoprotein CD4 (cluster of differentiation 4) belongs to the immunoglobulin family and is expressed on the surface of several immune cells including T lymphocytes, monocytes, macrophages and dendritic cells [51]. CD4 elicits an immune response through interactions with the antigen presented by the major histocompatibility complex (MHC) molecule in the antigen presenting cells [52]. HIV hijacks CD4 as its primary receptor to invade its target cells. Therefore, targeting the CD4 moiety is an attractive stratagem to selectively deliver anti-HIV drugs to its target cells. The targeting of the CD4+ cells can be achieved through antibodies designed to bind specifically to any of the four domains D1 to D2 present in CD4. A liposomal carrier conjugated with antibodies against the D2 or D3 domains of CD4 was developed that specifically bound to the HIV infected cells [53]. Similarly, anti-CD4 conjugated liposomes were employed for the delivery of si-RNA to silence the rev gene that is implicated in the regulation and expression of virion proteins. A significant amount of viral inhibition was observed due to the effective targeting to CD4+ cells. In a recent work, lipid nanoparticles conjugated with two peptide sequences that were derived from the binding domains of CD4 designated as CD4-BP2 and CD4-BP4 were used for targeted delivery of the protease inhibitor indinavir to HIV infected CD4+ cells with high specificity [54]. One of the major challenges in targeting CD4+ cells is its possible interference with the normal functions of CD4 expressing cells. In normal conditions, CD4 is used by the T lymphocytes to bind to MHC (major histocompatibility complex) class II proteins for antigen recognition [55]. The binding domain of CD4 involved in its interactions with MHC class II overlaps with the binding domain involved in its interactions with gp120 of HIV, the differences being the higher contact area and stronger affinity of the gp120 with CD4 [56]. Therefore, design of peptidomimetics with greater affinity to CD4 has been explored that may also serve as competitive inhibitors of the gp120 binding to CD4 [57]. The use of such anti-CD4 peptides for targeting drug-loaded nanoparticles however has not been explored thus far. HIV-infected cell targeting has also been achieved using an engineered cytotoxic T lymphocyte (CTL) expressing a chimera CD4 receptor that employs the extracellular portion of CD4 as a targeting moiety. The specific binding of the engineered cell through the CD4 receptor with the CD4+ cells results in the activation of signaling pathways in CTL that causes the lysis of the HIV infected cells [58]. A novel concept that had been experimented during the late 90s was the development of ‘viraceuticals’ that involved engineered viruses of the Rhabdoviridae family devoid of their envelope proteins but expressing the co-receptors CD4 and CXCR4 that enables it to selectively seek gp120 bound cells and destroy them [59]. However, their successful demonstration in a clinical set-up is yet to be realized. In a departure from the conventional concept of targeting CD4+ cells for delivery of anti-retrovirals or inhibit viral entry, a recent report has employed anti-CD4 conjugated gold nanoparticles for visualizing the lymph nodes through X-ray computed tomography [60]. Such applications can be employed in the future for development of ‘theranostic’ systems that enable simultaneous visualization of the infected cells and their treatment using a co-administered therapeutic agent.
4.2.4.2. Chemokine receptors
The viral entry into the cell is facilitated by the presence of co-receptors CXCR4 and CCR5 that have also been employed for targeting HIV infected cells.
The CCR5 or C-C chemokine receptor type 5 belongs to the beta chemokine receptor family and acts as the receptor for many chemokines such as CCL5 and macrophage inflammatory proteins (MIPs) [61]. It is expressed by T cells, macrophages, microglial cells and dendritic cells [62]. It is also an important co-receptor recruited by the HIV virus to gain entry into the host cell. While CXCR4 is believed to be involved in the later stages of the viral infection, CCR5 is involved in the entry of virus during the early phases of the HIV infection [63]. As a result, CCR5 remains among the most extensively investigated chemokine receptor target for controlling the spread of HIV infections. A liposomal system covalently linked with CCR5 and encapsulating the model drug ethylene diaminetetraacetic acid (EDTA) was targeted to the HIV infected cells expressing gp120 along with soluble CD4 [63]. As CCR5 possesses affinity towards the HIV gp120, the CCR5-modified liposomes bind to the infected cells causing their selective death in the presence of soluble CD4 [64]. Monoclonal antibodies developed against CCR5 have also been employed for homing into HIV infected cells. The anti-CCR5 monoclonal antibody PRO140 can bind to multiple extracellular domains of CCR5 and therefore can selectively deliver anti-retroviral drugs efficiently to HIV infected cells on conjugation with nanoparticles [65]. The natural ligand for CCR5 is chemokine C-C ligand 5 (CCL5) also referred to as RANTES (regulated on activation, normal T cell expressed and secreted) can also be used to deliver the therapeutic moieties to HIV infected cells [65]. Synthetic peptides derived from the sequences in the extracellular loop of CCR5 that is involved in interactions with the viral proteins have also been employed for targeting HIV infected cells. These synthetic peptides have been demonstrated to bind to gp120 present in the surface of the virus infected cells [66]. A classic example of this category of peptides is the E51-derived peptide referred to as CCR5mim, which resembles the extracellular loop of CCR5 that has high affinity to gp120 and can therefore be used to target HIV infected cells [67]. In a novel approach, peptide nucleic acids (PNAs) with specificity towards the CCR5 gene were encapsulated in biodegradable nanoparticles of PLGA (poly(l-lactide-co-glycolide)) and delivered to HIV infected cells [68]. The PNAs were associated with the CCR5 gene through Hoogsteen bonding and resulted in the suppression of the gene expression leading to a condition akin to those found in cells carrying a CCR5-Δ32 mutation. These cells turn HIV resistant due to the absence of CCR5 expression on the cell surface thereby preventing the entry of HIV.
The CXCR4 (chemokine receptor type 4) also known as fusin, is a trans-membrane protein that belongs to the G protein coupled receptor family and is ubiquitously expressed by several cell types including T lymphocytes, endothelial cells and tumor cells [69]. The natural ligands for this receptor are the stromal derived factor-1alpha (SDF-1α) (CXCL12) that serves as a chemoattractant for lymphocytes [70] and ubiquitin that is implicated in mitigating pro-inflammatory molecules [71]. HIV-1 strains designated as T-tropic gain entry into the host cell through binding interactions with CD4 and CXCR4. It is believed that the primary binding with CD4 facilitates a conformation change in the viral protein that then binds to CXCR4. It is also found that several strains of HIV-2 are able to gain entry even in CD4− cells through the CXCR4 receptors suggesting that these viruses possess an envelope protein that binds specifically to the chemokine receptor [72]. A small basic bicyclam molecule AMD3100 has been synthesized that exhibits high affinity binding to CXCR4 [73]. This molecule has been found to be a useful target in cancer therapy and wound healing apart from potential anti-HIV properties [74]. However, its therapeutic use against HIV infections is limited as the viral load reduction post-administration of this drug was not significant. This is because in most cases the virus was able to gain entry through other chemokine receptors. Antibodies and peptide sequences directed against CXCR4 have been used to target the HIV infected cells. Peptide sequences derived from the viral macrophage protein vMIP-II designated as DV1, DV3 and DV1-K-(DV3) were investigated for their targeting affinity towards CXCR4 [75]. It was found that DV1 and DV3 exhibited moderate affinity while DV1-K-(DV3) displayed high affinity towards CXCR4 and hence can be explored further for targeting CXCR4 positive cells. In a recent study, a peptide N15P derived from the amino acid sequence 1–15 of the N-terminus of the viral macrophage inflammatory protein (vMIP-II) was encapsulated in a nanoliposomal carrier [76]. This liposomal system was found to inhibit the SDF-1 induced chemotactic activity of peripheral blood mononuclear cells through competitive binding to CXCR4. The antibody 12G5 against CXCR4 has also been used for targeting the HIV infected cells possibly through recognition of a specific sequence in the extracellular loop 2 (Ecl2) of CXCR4 [77]. Currently, a humanized anti-CXCR4 antibody designated as 515H7 is under preclinical trials to evaluate its targeting efficacy [78]. Stromal cell derived factor-1 (SDF-1) which is secreted by the bone marrow stromal cells has also been used for targeting HIV infected cells. Two types of SDF namely SDF-1α and SDF-1β have been investigated due to their high affinity and easy binding to the CXCR4 receptors thereby serving as promising targeting moieties towards CD4+ TH cells infected with HIV [79]. Conversely, CXCR4 can also be linked to the nanocarrier to target HIV infected cells due to its affinity for the viral gp120 that is present on the surface of HIV infected cells [80]. A trans-membrane protein, TM4 derived from CXCR4 has also been demonstrated to bind to CD4+ cells and can be used for targeting the HIV infected cells [81]. The synthetic pentapeptides 15K and 15D have been employed as inhibitory peptides for HIV infection and have been found to exhibit superior binding affinity towards CXCR4 co-receptor than the anti-CXCR4 monoclonal antibody 12G5 [82]. The selective binding of two synthetic antagonistic polypeptides TN140 and TN14003 to CXCR4 has been demonstrated in different cancer cell types and hence can be a promising moiety to target HIV infected cells [83], [84]. However, these peptides have not been evaluated in HIV infected cells yet. The recent emergence of ‘nanobodies’, single domain antibodies that contains a single chain of the variable domain binding to the antigen, has resulted in the development of ALX-0651 that displayed high affinity binding to CXCR4 comparable to AMD3100 [85]. This nanobody that binds to the Ecl2 loop of CXCR4 is currently in phase II clinical trials where its potential as a targeting ligand towards HIV infected cells is being evaluated.
4.2.4.3. Carbohydrate binding agents (CBAs)
The viral envelope contains many glycan residues that aid in specific binding to the host cells and also serve to evade immune recognition by masking the immune epitopes. Mannose residues are important binding moieties on the surface of the HIV glycoprotein gp120 [86]. Targeting the sugar moieties can be an effective strategy for specific homing onto HIV infected cells because of the numerous glycan residues that are exposed on the surface of the HIV infected cells. This concept has led to the emergence of carbohydrate binding agents (CBAs) that can be broadly classified either as lectins that belong to the protein family or as non-peptidic molecules with specificity towards a particular type of sugar moiety [87]. Apart from imparting the ability to selectively bind to the viral envelope thereby blocking its ability to interact with the host cell surface receptors, the repeated use of CBAs also induces the virus to delete the glycan residues thereby leading to unmasking of the hidden immune epitopes resulting in immune activation [88]. Most of the glycan residues are also involved in the folding of a protein to its native conformation. Deletion of the glycan residues by the virus in response to the treatment with CBAs can therefore indirectly hamper the function of the glycans in protein folding thereby reducing the virulence of the virus [89]. A host of peptidic CBAs, namely, lectins has been identified from different plant, microbial and mammalian sources. Apart from their origin, these lectins differ in their size and specificity towards the sugar residues that have an impact on their binding affinity as well as viral inhibition activity [90]. In general, it has been identified that lectins that display specificity towards α-1,2, α-1,3 and α-1,6-mannose oligomers exhibit better viral binding and inhibition when compared with lectins that exhibit specificity to other carbohydrate moieties [91]. An exception to this generalization is the lectin isolated from the stinging nettle Urtica dioica referred as UDA (U. dioica agglutinin) that displays specificity towards N-acetyl glucosamine moieties and has also displayed significant affinity towards the HIV glycan envelope [92]. The lectin cyanovirin-N (CV-N) extracted from the cyanobacterium Nostoc ellipsosporum [93] was found to bind to the α-1,2-mannose residues of gp120 with high affinity. Similarly, scyctovirin (SVN), a lectin extracted from the cyanobacterium Scytonema varium exhibited selectivity to α-1,2-α-1,6-mannose residues and was found to bind to HIV glycoproteins gp41 and gp120 present on the surface of the HIV infected cells [94]. The lectins from sources of eukaryotic origin GSA (Gerardia savaglia agglutinin) from the sea coral G. savaglia displays calcium dependent binding to the d-mannose residues in the HIV-1 glycoprotein envelope resulting in complete viral inhibition in in vitro conditions [95]. The lectin actinohivin isolated from the actinomycete Longisporum albida has also been identified as an important targeting moiety that binds to the mannose residues present in the gp120 at lower concentrations with an affinity greater than anti-gp120 antibodies thereby preventing the interaction of the viral glycoprotein with CXCR4 and CCR5 chemokine receptors [13]. Griffithsin, a lectin isolated from the red algae Griffithsia species has been identified to possess specific binding to mannose, glucose, fucose and N-acetyl glucosamine moieties thus conferring a broad spectrum of activity to this lectin towards different viral strains [96]. Griffithsin has been found to be effective in interfering with the interactions of the viral glycoproteins with CXCR4 as well as CCR5 [97]. It has also shown promise in inhibiting the SARS coronavirus by binding to the outer glycoprotein coat of SARS (severely compromised acute respiratory distress syndrome) virus. The lectin BCA (Boodlea coacta agglutinin) extracted from the green algae B. coacta shows structural similarities with the Galanthus nivalis agglutinin (GNA) isolated from the snowdrop plant and acts as a potent targeting moiety for the HIV infected cells [97], [98]. It has been shown to bind mainly to the α-1,2-mannose type N-glycans present in the HIV envelope glycoprotein gp120 with high affinity of about 3.7 × 108 M− 1 making it a promising targeting agent against HIV infected cells [97]. The binding affinity of BCA was found to increase with increasing number of mannose residues in the cluster. Other lectin molecules that have been identified to possess specific affinity towards the mannose residues of the HIV glycoproteins are CVL (Chaetopterus variapedatus lectin) isolated from the annelid worm C. variapedatus, NPA (Narcissus pseudonarcissus agglutinin) from the daffodil plant N. pseudonarcissus, SCL (Scilla campanulata lectin) from the bluebell plant S. campanulata, ConA (Concanavalin A) from the jack bean Canavalia ensiformis, jacalin from the jack fruit Artocarpus integrifolia, MBL (mannose binding lectin) that is found in the serum of mammalian systems and displays calcium-dependent binding to mannose residues, DC-SIGN (dendritic cell-specific intercellular adhesion molecule-grabbing non-integrin) expressed in dendritic cells of mammalian systems with specificity towards α-1,3 and α-1,6 mannotriose residues, etc. [98], [99]. A unique lectin referred to as mermaid, which is a C-type lectin expressed on the surface of the marine nematode Laxus oneistus was identified to possess a structure similar to DC-SIGN [100]. This lectin binds to gp120 with high affinity and has been found to inhibit the binding of the virus to the dendritic cells as well as to inhibit the transmission of the virus from dendritic cells to CD4+ T lymphocytes [100].
Yet another class of cationic cysteine-rich peptides expressed in the leukocytes is the defensins that possess anti-viral properties. Different isoforms of defensins namely α, β, and θ have been identified [101]. The defensins promote chemotaxis of the T lymphocytes and have also displayed the ability to inhibit viral entry and replication [102]. The anti-viral activities of α and β defensins have been mainly attributed to their ability to interfere with the PKC (protein kinase C) signaling in HIV infected CD4+ cells as well as enhancement in the C-C chemokine levels in the cells leading to competitive inhibition of the viral binding to the host cells [102]. The θ defensins also known as retrocyclins display similarity with lectins in binding specifically to the glycan residues of the HIV envelope and hence could serve as a glycan-targeting moiety. A recent attempt to encapsulate defensin peptides along with gp41 residue in biodegradable poly(l-lactide-co-glycolide) microparticles was reported [103]. The presence of defensin resulted in an elevated immunogenic response against HIV and hence it was proposed that such strategies could be effective as vaccines against HIV infections.
Early work on glycan targets have employed mannose as the targeting moiety towards the mannose receptors present in HIV infected cells like macrophages, dendritic cells and astrocytes resulting in increased uptake of the mannose-linked drug-loaded nanoparticles through receptor-mediated endocytosis leading to a decrease in the viral load [104], [105], [106]. Antibodies against the glycan residues of HIV have also been used as a targeting moiety. Monoclonal antibodies against the oligosaccharides in the glycoproteins gp120/gp41 were reported to bind to the exposed glycan residues on the surface of the HIV infected cells with high affinity [107]. One of the common monoclonal antibody employed to target the glycan residues is 2G12 that binds specifically to HIV gp120 through terminal mannose oligomers [107]. However, any mutation in this region resulting in the deletion of this residue might render the antibody ineffective. Such issues are not commonly encountered with the use of CBAs because they are capable of binding to multiple residues in the same glycoprotein sequence. As a result, numerous deletions and mutations have to be effected by the virus to achieve resistance against the CBAs [108]. This high genetic barrier achieved by the CBAs makes them attractive therapeutic moieties that can also be used to achieve target specificity. Among the non-peptidic CBAs, the antibiotics pradimicin (PRM-A) and nanomicin A (BNM-A) from actinomycete species have been found to exhibit calcium dependent binding to the terminal d-mannose of the viral glycoprotein forming a ternary complex [109], [110]. Concerted efforts to develop low molecular weight non-peptidic molecules with carbohydrate-specific binding are underway in many research laboratories. A liposomal carrier containing a C-type lectin with high binding specificity towards DC-SIGN was developed and was found to exhibit excellent target-specific delivery of its cargo, the fluorescent calcein suggesting that such carriers may be useful in the context of site-directed delivery of therapeutic agents [111]. The use of glycan residues for immuno-stimulatory purposes has also been attempted as a strategy to reduce viral infections. In order to achieve highly effective multivalent display of carbohydrate moieties to stimulate the immune system, novel structures such as glycoclusters and glycodendrimers have also been reported in literature [112]. The concept of using CBAs as part of the anti-HIV arsenal is however hampered by many factors. The high expense involved in the isolation and purification of the CBAs, storage and stability issues, mitogenic nature of the lectins, poor bioavailability and their inability to distinguish between pathogenic glycan targets from native host glycan residues remains to be addressed before these could be used in a clinical set-up.
4.2.4.4. Tuftsin
Tuftsin is a tetrapeptide derived from the immunoglobin IgG in the spleen and consists of the amino acid sequence Thr-Lys-Pro-Arg [113]. Since its discovery in 1970, tuftsin has found applications in immunotherapy due to its ability to bind to and activate macrophages and dendritic cells [113]. Binding of tuftsin to the tuftsin receptors expressed on the surface of the cells of the immune system activates their chemotaxis and phagocytosis. As these cells also serve as viral reservoirs during HIV infections, employing tuftsin to target the infected cells has been explored as a potential treatment strategy against AIDS. A drug–tuftsin conjugate was developed by covalently linking tuftsin to the reverse transcriptase inhibitor 3′-azido-3′-deoxythymidine (AZT) [114]. The conjugate was effectively used for targeting of macrophages, which hoards the HIV virions. A significant decrease in the viral load compared with the free drug was achieved thus illustrating the specific targeting potential of tuftsin. The tuftsin moiety also stimulated the release of the cytokine interleukin 1 (IL-1) from macrophages thereby increasing the immune response [114]. AG5poly(propylene imine) (PPI) dendrimer loaded with the reverse transcriptase inhibitor efavirenz was conjugated with tuftsin and evaluated for its anti-viral efficacy in vitro using macrophages [115]. The tuftsin-conjugated dendrimer significantly decreased the viral load when compared with the unconjugated PPI dendrimer suggesting that tuftsin conjugation enhances uptake in infected cells [115]. Tuftsin conjugated liposomes have also been extensively investigated for treatment of tuberculosis [116], malaria [116], leishmaniasis [117] and fungal infections [118]. However, apart from a few reports of tuftsin conjugated dendrimer systems, studies involving tuftsin-conjugated nanocarriers remain largely unexplored in the context of HIV therapy.
4.2.4.5. Transferrin
Transferrin is an important iron-binding glycoprotein present in blood that is primarily involved in the maintenance of iron levels in the biological system [119]. The affinity of iron to transferrin is extremely high at physiological pH but decreases with decrease in pH. Transferrin receptors are abundantly present in liver and brain in physiological conditions [120]. As the brain is an important sanctuary for HIV, it is essential to deliver anti-retroviral drugs across the blood–brain barrier. As the transferrin receptor expression is high in the brain, it is possible to deliver therapeutic agents into the brain through transferrin-conjugated nanocarriers that will be internalized using receptor-mediated endocytosis [121]. Transferrin-conjugated PLGA nanoparticles encapsulating the reverse transcriptase inhibitor nevirapine were successfully transported across the blood–brain barrier into human brain microvascular endothelial cells [122]. A similar strategy was reported to enable delivery of a HIV model antigen CN54gp140 through the epithelial barrier in the mucosal layer [123]. The transferrin-conjugated system was able to elicit higher immune response as evident from the high titers obtained for the antibodies IgG and IgA in the genital tract of female mice when compared with the unconjugated system. A specific antibody against the transferrin receptor namely OX26 that binds to the cells expressing the transferrin receptor was conjugated to a streptavidin moiety to deliver antisense oligonucleotides against the rev gene of HIV that was modified covalently with a biotin molecule. The biotinylation protected the oligonucleotides from exonuclease degradation and enabled its binding to the transferrin conjugated streptavidin. This system was found to increase the inactivation of the rev mRNA in the cerebral region when compared with the free oligonucleotides highlighting the ability of transferrin conjugated systems to cross the blood–brain barrier [124]. Albumin nanoparticles encapsulating the nucleoside reverse transcriptase inhibitor azidothymidine (AZT) were surface modified with transferrin and were found to cross the blood–brain barrier efficiently and decrease the viral load [125]. The biodistribution studies of the nanoparticulate system in Albino rats revealed selective enhancement in the uptake of the nanoparticles in the brain illustrating the targeting efficacy of transferrin. Investigations on the relation between iron levels and HIV infections have revealed that HIV infections lead to an increase in the oxidative stress in the cells and also cause the release of intracellular iron stores. As a result of the iron overload, it has been observed that the transferrin receptor expression in the infected cells is reduced. Hence, in the advanced stages of infection, it remains to be seen if transferrin receptor could still be an effective target. In a departure from conventional carriers, use of a modified transferrin as a drug carrier was reported as a proof of principle work. The authors had inserted a peptide sequence in transferrin that was recognized by the HIV protease enzyme. This system was successfully demonstrated to enter HIV infected cells and was lysed by the HIV protease enzyme and can be explored for drug delivery to HIV infected cells. Quantum rods comprising of a cadmium selenide core and a graded cadmium sulfide–zinc sulfide shell were conjugated with transferrin and complexed with saquinavir for treatment of neuro-AIDS [126]. These complexes were found to be extremely effective in transporting saquinavir across the blood–brain barrier.
4.2.4.6. Aptamers
The challenges in conjugation of antibodies to carriers primarily due to changes in conformation induced by the chemical treatment steps result in a loss of target specificity. This has necessitated the development of novel small molecule ligands that retain the target specificity of antibodies but with greater structural stability. Aptamers are small molecules of single stranded oligonucleotides (DNA or RNA) or peptides that have excellent target specificity. Aptamers are developed using SELEX (systematic evolution of ligands by exponential enrichment) after a series of sequential steps that involve binding, separation, purification and amplification [127]. Aptamers also possess the ability to hybridize through Watson–Crick pairing and hence can be used to form chimeric structures with other molecules such as si-RNA and ribozymes that can be used for delivery into desired locations [128]. A chimeric aptamer–si-RNA system exhibiting specificity towards gp120 has been reported to enter HIV infected cells through gp120-mediated endocytosis [129]. The aptamer–si-RNA chimera is cleaved by dicer inside the cell, leading to the release of si-RNA, which binds to the mRNA of the HIV tat and rev genes and inhibits the HIV life cycle. Aptamers that bind specifically to HIV infected cells have been developed. A 2′F-RNA aptamer that binds to the gp120 of infected CD4+ cells has been reported and could be employed for targeting HIV infected cells [130]. Several aptamers with specificities towards the HIV reverse transcriptase, integrase, nucleoprotein, gp120, gag protein, tat protein, rev protein and transactivation response element (TAR) have been reported [131]. Novel aptamers with G-rich repeats that spontaneously form G-quadruplexes in the presence of divalent ions through Hoogsteen pairing have been developed for HIV integrase and gp120 through terminal modifications. These quadruplex aptamers display extraordinary stability and binding specificity to their target [132]. These aptamers have immense potential in the field of targeted delivery of anti-retrovirals that remain to be explored in-depth.
4.2.4.7. Low-density lipoprotein (LDL)
Low-density lipoprotein (LDL) is a type of lipoprotein that is involved in the transport of lipids into the cells [133]. LDL has the ability to attract macrophages and hence has been explored as a possible targeting moiety against HIV infected macrophages. LDL conjugated AZT (azidothymidine) was demonstrated to internalize into macrophages through LDL receptor-mediated endocytosis and deliver the drug [134]. Similar studies were carried out using LDL conjugated fluorothymidine, which binds to the macrophages infected with HIV and delivered the drug effectively causing a decrease in the viral load when compared with the free drug [135]. Another study had employed acetylated LDL loaded AZT for site-specific delivery to macrophages. The uptake of acetylated LDL modified carrier was achieved through scavenger receptors and resulted in a decrease in the viral load [136]. However, it was observed that HIV infections result in a decreased expression of the LDL receptor and hence targeting this receptor during advanced stages of the disease might not be an effective strategy.
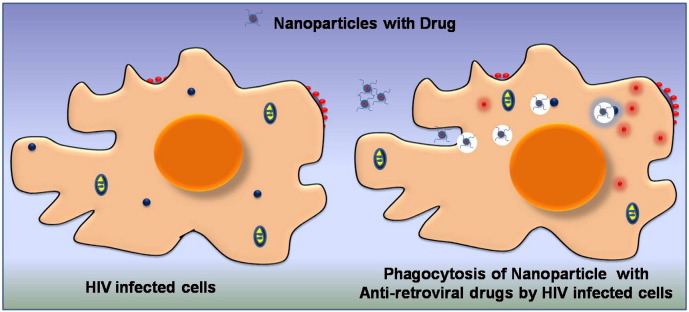
4.3. Passive targeting
Passive targeting does not involve any chemical modification of the carrier and its internalization into the desired location is mainly driven by specific property of the targeted cells. The concept of passive targeting has been well exploited in cancer therapeutics by utilizing the enhanced permeation and retention (EPR) property of cancer cells [137]. In case of HIV infections, the dendritic cells and macrophages having high amount of virions were found to transfer them to the CD4+ TH cells through virological synapses. These macrophages and dendritic cells have been observed to be highly active during HIV infections and exhibit higher phagocytic properties than uninfected cells [138]. This enhanced phagocytic activity of the macrophages can be harnessed to entrap anti-retroviral loaded carriers in the cells that are infected with HIV. This strategy of passive targeting was successfully demonstrated using indinavir encapsulated liposomal nanoparticles prepared using the synthetic phospholipids phosphatidyl choline, phosphatidyl ethanolamine and a stabilizing agent [139]. These nanoparticles exhibited good uptake in HIV infected macrophages and reduced the viral load significantly when compared with soluble indinavir. This difference between the encapsulated and free form of the drug was mainly attributed to the phagocytosis of the nanoparticles by macrophages. In another approach, a self-assembled dual drug conjugate of AZT (zidovudine) and didanosine (ddi) separated by adeoxycholyl spacer was synthesized [140]. This zidovudine–phosphoryl–deoxycholyl didanosine (ZPDD) conjugate was easily internalized by the phagocytic macrophages and resulted in the destruction of the virions. A schematic representation of nanoparticles internalized in infected cells through passive targeting is shown in Fig. 4 .
Fig. 4.
Schematic representation of a passive targeting strategy employing nanoparticles.
5. Emerging targets
Other targeting moieties for HIV positive cells that have been explored are thiamine [141], glutathione and albumin [142]. These moieties can easily pass through the blood–brain barrier and are efficient in delivering the drugs on conjugating with the nanocarriers. Yet another strategy that is still in the ‘proof of principle’ stage is the use of Pluronics® (tri-block copolymer of poly(ethylene oxide) and poly(propylene oxide)) micelle carriers to disable the efflux pumps in the HIV infected cells that are triggered by the infection to achieve multidrug resistance [143]. The Pluronics polymer and unimer alter the mitochondrial membrane fluidity thereby disrupting the electron transport chain. This impairs ATP production leading to the shutdown of the efflux pumps [144]. This strategy may enable sensitization of HIV infected cells to anti-retrovirals thereby enhancing their therapeutic efficiency.
Investigations using knockdown experiments and next generation sequencing to unravel complex interactions between HIV and human proteins have helped in identification of potential targets that could be explored in future for therapeutic applications. In a recent report, a novel viral accessory factor, Vif has been identified to recruit a human transcription factor CBF-β (core binding factor subunit beta) and uses the ubiquitin-ligase complex to degrade the restriction factors such as retroviral complementary DNA deaminase in the human system that can inactivate the viral replication [145]. Targeting CBF-β or Vif might offer better chances of curbing the viral replication. Another novel target could be Slit2N, a glycoprotein that has been found to inhibit the replication of both T tropic and M tropic HIVs probably through modification of the cytoskeleton dynamics [146]. This could also be explored in-depth as a therapeutic target for viral infections. In a novel approach, melittin, an active component of the bee toxin was encapsulated in liposome nanoparticles and evaluated for their anti-HIV activity [147]. Melittin is an activator of phospholipase enzyme and can destroy cells through disruption of the lipid coat. This cytolytic agent was found to selectively internalize to a greater extent in HIV infected cells where it destroyed the viral lipid coat. Though free melittin also was found to destroy the virus, its cytotoxicity towards normal cells was significantly high when compared to its nanoparticle counterpart, which displayed selective toxicity only to the virus. This system displayed toxicity towards both CXCR4 and CCR5 tropic viruses. As it attacked the lipid coat, the probability of the virus developing a resistance towards this molecule is remote. This system needs to be further evaluated and validated for clinical significance. RNA silencing has emerged as an important strategy to combat AIDS infections. Many new viral targets are being identified to be silenced using RNAi technology. Some of the targets that have been targeted through RNAi technology include the structural, regulatory, and accessory genes such as gag, pol, env, rev, tat, tar, vif, nef, vpx, vpr, etc. Attempts to silence factors triggered in the host cell as a result of HIV infection such as the Golgi transport proteins Rab6 and Vps53, karyopherin TnpO3, mediator complex Med28, Akt1, PrkaA1, Cd97, Neil3, Bmp2k and SerpinB6 have also been made to control HIV infection [148]. However, the success of RNAi depends on its complexation with a suitable nanocarrier as well as identification of a targeting moiety to selectively deliver these into the infected cells. Also, a new emerging paradigm is that it may be necessary to silence more than one gene target simultaneously to ensure total disruption of the HIV virions. But such studies are yet to be explored in-depth thereby leaving the field wide open for further research. Table 2 lists the numerous targeted nanocarriers that have been explored for HIV therapy, indicating the tremendous scope for further investigations employing a combination of therapeutic agents, nanocarriers and targeting agents.
Table 2.
List of targeted nanocarriers evaluated for HIV therapy.
| S. no | Nanocarrier | Targeting moiety | Therapeutic agent | Stage | Outcomes | Citation |
|---|---|---|---|---|---|---|
| 1 | Gelatin | Mannose | Didanosine | Preclinical | Enhanced uptake of the formulation in lymphoid organs | [105] |
| 2 | Gelatin | Mannan | Didanosine | Preclinical | Enhanced uptake of the formulation in liver, brain, spleen lymphoid organs | [149] |
| 3 | Albumin | Transferrin | AZT | Preclinical | Enhanced bio-distribution of drug in the brain cells compared with free drug | [125] |
| 4 | PLA | TAT | Ritonavir | Preclinical | Higher bioavailability and shows sustained release with lesser clearance in CNS | [150] |
| 5 | Liposome | Anti-HLA-DR | Indinavir | Preclinical | Lesser toxicity and immunogenicity compared with free drug | [151] |
| 6 | Liposome | LFA-1 | RNAi | Preclinical | Enhanced reduction in the viral load in case of the BLT-mice treated with nano-formulation. | [36] |
| 7 | Dendrimer | Mannose | Lamivudine, Efavirenz | Preclinical | Increase uptake in liver, spleen, kidney macrophages | [104], [152] |
| 8 | Lipid nanoparticles | CD4 binding peptide | Indinavir | Preclinical | Increased intracellular concentration of the drug in the lymph nodes and high antiviral activity-macaques | [153] |
| 9 | Liposome | Galactose | AZT | Preclinical | No hematological toxicity and higher accumulation and half life of the formulation in the liver of Sprague–Dawley rats | [154] |
| 10 | Liposome | Mannose | Zidovudine | Preclinical | Higher uptake of nano-formulation in spleen and lymph nodes | [155] |
| 11 | Liposome | Mannose | Stavudine | Preclinical | Drug concentrated in the liver, spleen and lungs and lesser clearance of the system | [156] |
| 12 | Liposome | Galactose | Stavudine | Preclinical | Higher concentration of nano-formulation in liver and lesser toxicity both liver and blood | [157] |
| 13 | Nano conjugate | ScFvCD7Cys-9R | SiRNA | Preclinical | Suppressed viremia in Hu-HSC mice | [158] |
| 14 | F105-p | ErbB2 single chain | SiRNA | Preclinical | Successful delivery of the siRNA into HIV infected cells of mice. | [159] |
| 15 | PLA | P24 | P24 | Preclinical | Increase humoral and cell mediated response stimulated by the p24 protein in PLA | [160] |
| 16 | Nanoparticle | Adjuvants | Plasmid DNA | Phase II clinical trails | Enhanced transport of the plasmid DNA to the Langerhans cells of the lymph nodes for maturation into dendritic cells for cellular immunity | [161] |
| 17 | Dendrimer | Tuftsin | Efavirenz | In vitro | – | [162] |
| 18 | Conjugate | Tuftsin | AZT | In vitro | – | [163] |
| 19 | PLGA | Transferrin | Nevirapine | In vitro | – | [122] |
| 20 | Liposome | Anti-HLA-DR | Amphotericin B | In vitro | – | [45] |
| 21 | Liposome | Anti-gp120 | P11 | In vitro | – | [164] |
| 22 | Liposome | CD4-IgG | – | In vitro | – | [24] |
| 23 | Liposome | CCR5 | EDTA | In vitro | – | [64] |
| 24 | Chimeric | Anti-gp120 aptamer | RNAi | In vitro | – | [165] |
6. Conclusion
There continues to be a vital need for newer agents to confront the emergence of drug resistance and various adverse effects with long-term use of ARV therapy. Also the half-life for several ARV drugs is short, requiring frequent administration of doses, which in turn leads to poor patient compliance. Therefore, the usage of novel drug delivery systems is a logical approach to overcome these problems and effectively treat HIV infection. Among the recent therapeutic approaches, a major thrust area is in developing effective drug delivery systems for the existing drugs, which have been tested and proven effective in reducing the viral load. In this review, the need for novel drug delivery, advantages, and recent developments in identification of viral and host surface molecules as markers for targeted drug delivery of antiretroviral drugs were discussed. These studies open new avenues for more in-depth studies on the effective use of these targeting strategies for HIV therapy. Such a comprehensive approach could ultimately prove effective not only in reducing viral load, but also in eradication of virus from the reservoirs.
7. Future directions
We envisage that future directions in the field will involve a multi-pronged strategy to target HIV at various stages of infection. These include the transmission of the virions from dendritic cells and macrophages to the CD4+ T cells with potential targets being HLA-DR and DC-SIGN. Furthermore, cell-to-cell transmission of HIV between CD4+ T cells through the virological synapses is also a critical stage of viral transmission in the body. In case of cell-to-cell transmission, LFA-1 is likely to emerge as a potential target for targeted anti-HIV therapy. Another key area should involve countering the challenges in targeting latent HIV in multiple reservoirs. In this regard, strategies that combine the release the HIV virions from latently infected cells such as activation of the protein kinase pathway along with anti-HIV drug delivery hold tremendous potential. In summary, recent scientific advances in the development of targeted drug delivery of anti-HIV drugs hold great promise for the development of improved treatment strategies and should certainly pave the way towards global eradication of HIV/AIDS.
Acknowledgments
The authors gratefully acknowledge the financial support from the Indian Council for Medical Research (ICMR, Grant No. 35/9/2009-BMS) and infrastructural support from SASTRA University.
References
- 1.Piot P., Bartos M., Ghys P.D., Walker N., Schwartländer B. The global impact of HIV/AIDS. Nature. 2001;410:968–973. doi: 10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T., Trkola A., Thompson D.A., Cormier E.G., Kajumo F.A., Maxwell E., Lin S.W., Ying W., Smith S.O., Sakmar T.P. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr A., Samaras K., Chisholm D.J., Cooper D.A. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 4.Gerber J.G. Using pharmacokinetics to optimize antiretroviral drug–drug interactions in the treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 2000;30:S123–S129. doi: 10.1086/313857. [DOI] [PubMed] [Google Scholar]
- 5.Eagling V., Wiltshire H., Whitcombe I., Back D. CYP3A4-mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in vitro. Xenobiotica. 2002;32:1–17. doi: 10.1080/00498250110085845. [DOI] [PubMed] [Google Scholar]
- 6.Schweighardt B., Atwood W.J. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res. Hum. Retrovir. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- 7.Gunaseelan S., Gunaseelan K., Deshmukh M., Zhang X., Sinko P.J. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv. Drug Deliv. Rev. 2010;62:518–531. doi: 10.1016/j.addr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felts R.L., Narayan K., Estes J.D., Shi D., Trubey C.M., Fu J., Hartnell L.M., Ruthel G.T., Schneider D.K., Nagashima K. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc. Natl. Acad. Sci. 2010;107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissenhorn W., Dessen A., Harrison S., Skehel J., Wiley D. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal-Gamse C., Lee F.-H., Haggarty B., Jordan A.P., Yi Y., Lee B., Collman R.G., Hoxie J.A., Doms R.W., Laakso M.M. Adaptive mutations in a human immunodeficiency virus type 1 envelope protein with a truncated V3 loop restore function by improving interactions with CD4. J. Virol. 2009;83:11005–11015. doi: 10.1128/JVI.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myszka D.G., Sweet R.W., Hensley P., Brigham-Burke M., Kwong P.D., Hendrickson W.A., Wyatt R., Sodroski J., Doyle M.L. Energetics of the HIV gp120–CD4 binding reaction. Proc. Natl. Acad. Sci. 2000;97:9026–9031. doi: 10.1073/pnas.97.16.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton R., Ohagen A., Nicol F., Del Vecchio A.M., Jonckers T.H., Goethals O., Van Loock M., Michiels L., Grigsby J., Xu Z. Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antiviral Res. 2009;84:142–149. doi: 10.1016/j.antiviral.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Hoorelbeke B., Huskens D., Férir G., François K.O., Takahashi A., Van Laethem K., Schols D., Tanaka H., Balzarini J. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob. Agents Chemother. 2010;54:3287–3301. doi: 10.1128/AAC.00254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreier H., Moran P., Caras I. Targeting of liposomes to cells expressing CD4 using glycosylphosphatidylinositol-anchored gp120. Influence of liposome composition on intracellular trafficking. J. Biol. Chem. 1994;269:9090–9098. [PubMed] [Google Scholar]
- 15.Cheng K., El-Boubbou K., Landry C.C. Binding of HIV-1 gp120 glycoprotein to silica nanoparticles modified with CD4 glycoprotein and CD4 peptide fragments. ACS Appl. Mater. Interfaces. 2011;4:235–243. doi: 10.1021/am2013008. [DOI] [PubMed] [Google Scholar]
- 16.Rizzuto C.D., Wyatt R., Hernández-Ramos N., Sun Y., Kwong P.D., Hendrickson W.A., Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 17.Wu L., Gerard N.P., Wyatt R., Choe H., Parolin C., Ruffing N., Borsetti A., Cardoso A.A., Desjardin E., Newman W. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 18.Jia L., Xu Y., Zhang C., Wang Y., Chong H., Qiu S., Wang L., Zhong Y., Liu W., Sun Y. Hypothesis A novel trifunctional IgG-like bispecific antibody to inhibit HIV-1 infection and enhance lysis of HIV by targeting activation of complement. Virol. J. 2010;7:142. doi: 10.1186/1743-422X-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton R., Ohagen A., Goethals O., Smets A., Van Loock M., Michiels L., Kennedy-Johnston E., Cunningham M., Jiang H., Bola S. Binding kinetics, uptake and intracellular accumulation of F105, an anti-gp120 human IgG1κ monoclonal antibody, in HIV-1 infected cells. J. Virol. Methods. 2007;139:17–23. doi: 10.1016/j.jviromet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson R.A., Piscitelli C., Teintze M., Cavacini L.A., Posner M.R., Lawrence C.M. Structure of the Fab fragment of F105, a broadly reactive anti-human immunodeficiency virus (HIV) antibody that recognizes the CD4 binding site of HIV type 1 gp120. J. Virol. 2005;79:13060–13069. doi: 10.1128/JVI.79.20.13060-13069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connell B.J., Baleux F., Coic Y.-M., Clayette P., Bonnaffé D., Lortat-Jacob H. A synthetic heparan sulfate-mimetic peptide conjugated to a mini CD4 displays very high anti-HIV-1 activity independently of coreceptor usage. Chem. Biol. 2012;19:131–139. doi: 10.1016/j.chembiol.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Berger E.A., Clouse K.A., Chaudhary V.K., Chakrabarti S., FitzGerald D.J., Pastan I., Moss B. CD4-Pseudomonas exotoxin hybrid protein blocks the spread of human immunodeficiency virus infection in vitro and is active against cells expressing the envelope glycoproteins from diverse primate immunodeficiency retroviruses. Proc. Natl. Acad. Sci. 1989;86:9539–9543. doi: 10.1073/pnas.86.23.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen J., Gaiha G.D., Palaniyar N., Dong T., Mitchell D.A., Clark H.W. Surfactant protein D modulates HIV infection of both T-cells and dendritic cells. PLoS One. 2013;8:e59047. doi: 10.1371/journal.pone.0059047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flasher D., Konopka K., Chamow S.M., Dazin P., Ashkenazi A., Pretzer E., Düzgüneş N. Liposome targeting to human immunodeficiency virus type 1-infected cells via recombinant soluble CD4 and CD4 immunoadhesin (CD4-IgG) Biochim. Biophys. Acta Biomembr. 1994;1194:185–196. doi: 10.1016/0005-2736(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 25.Brody E.N., Gold L. Aptamers as therapeutic and diagnostic agents. Rev. Mol. Biotechnol. 2000;74:5–13. doi: 10.1016/s1389-0352(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Dey A.K., Griffiths C., Lea S.M., James W. Structural characterization of an anti-gp120 RNA aptamer that neutralizes R5 strains of HIV-1. RNA. 2005;11:873–884. doi: 10.1261/rna.7205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veronese F.D., DeVico A.L., Copeland T.D., Oroszlan S., Gallo R.C., Sarngadharan M. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985;229:1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- 28.Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai L., Gochin M., Liu K. Biochemistry and biophysics of HIV-1 gp41 — membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design. Curr. Top. Med. Chem. 2011;11:2959–2984. doi: 10.2174/156802611798808497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purtscher M., Trkola A., Gruber G., Buchacher A., Predl R., Steindl F., Tauer C., Berger R., Barrett N., Jungbauer A. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 31.Dadachova E., Patel M.C., Toussi S., Apostolidis C., Morgenstern A., Brechbiel M.W., Gorny M.K., Zolla-Pazner S., Casadevall A., Goldstein H. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 2006;3:e427. doi: 10.1371/journal.pmed.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig R.B., Summa C.M., Corti M., Pincus S.H. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS One. 2012;7:e46778. doi: 10.1371/journal.pone.0046778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L., Pan C., Li Y., Lu H., He H., Jiang S. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology. 2012;9:104. doi: 10.1186/1742-4690-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahle K.M., Steger H.K., Root M.J. Asymmetric deactivation of HIV-1 gp41 following fusion inhibitor binding. PLoS Pathog. 2009;5:e1000674. doi: 10.1371/journal.ppat.1000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hioe C.E., Tuen M., Vasiliver-Shamis G., Alvarez Y., Prins K.C., Banerjee S., Nadas A., Cho M.W., Dustin M.L., Kachlany S.C. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA) PLoS One. 2011;6:e23202. doi: 10.1371/journal.pone.0023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.-S., Peer D., Kumar P., Subramanya S., Wu H., Asthana D., Habiro K., Yang Y.-G., Manjunath N., Shimaoka M. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 2009;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phongpradist R., Chittasupho C., Intasai N., Siahaan T.J., Berkland C.J., Charoenkwan P., Anuchapreeda S., Ampasavate C. Biodegradable nanoparticles surface modification techniques with cIBR peptide targeting to LFA-1 expressing leukemic cells, Journal of nanotechnology in engineering and medicine. 2012;3 [Google Scholar]
- 38.Shimaoka M., Kim M., Cohen E.H., Yang W., Astrof N., Peer D., Salas A., Ferrand A., Springer T.A. AL-57, a ligand-mimetic antibody to integrin LFA-1, reveals chemokine-induced affinity up-regulation in lymphocytes. Proc. Natl. Acad. Sci. 2006;103:13991–13996. doi: 10.1073/pnas.0605716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peer D., Zhu P., Carman C.V., Lieberman J., Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl. Acad. Sci. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsubota H., Lord C.I., Watkins D.I., Morimoto C., Letvin N.L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J. Exp. Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehra N., Taneja V. HLA, immune response and disease. Indian J. Pediatr. 1989;56:171–179. doi: 10.1007/BF02726601. [DOI] [PubMed] [Google Scholar]
- 42.Biancotto A., Iglehart S.J., Vanpouille C., Condack C.E., Lisco A., Ruecker E., Hirsch I., Margolis L.B., Grivel J.-C. HIV-1-induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray C.M., Lawrence J., Schapiro J.M., Altman J.D., Winters M.A., Crompton M., Loi M., Kundu S.K., Davis M.M., Merigan T.C. Frequency of class I HLA-restricted anti-HIV CD8 + T cells in individuals receiving highly active antiretroviral therapy (HAART) J. Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 44.Muthu M.S., Singh S. Targeted nanomedicines: effective treatment modalities for cancer. AIDS and brain disorders. Nanomedicine. 2008;4(1):105–118. doi: 10.2217/17435889.4.1.105. [DOI] [PubMed] [Google Scholar]
- 45.Bestman-Smith J., Désormeaux A., Tremblay M.J., Bergeron M.G. Targeting cell-free HIV and virally-infected cells with anti-HLA-DR immunoliposomes containing amphotericin B. AIDS. 2000;14:2457–2465. doi: 10.1097/00002030-200011100-00006. [DOI] [PubMed] [Google Scholar]
- 46.Dufresne I., Désormeaux A., Bestman-Smith J., Gourde P., Tremblay M.J., Bergeron M.G. Targeting lymph nodes with liposomes bearing anti-HLA-DR Fab′ fragments. Biochim. Biophys. Acta Biomembr. 1999;1421:284–294. doi: 10.1016/s0005-2736(99)00137-6. [DOI] [PubMed] [Google Scholar]
- 47.Kestens L., Vanham G., Gigase P., Young G., Hannet I., Vanlangendonck F., Hulstaert F., Bach B.A. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–798. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Jurcevic S., Praud C., Coppin H.L., Bertrand A., Ricard S., Thomsen M., Lakhdar-Ghazal F., De Preval C. Role of polymorphic residues of human leucocyte antigen-DR molecules on the binding of human immunodeficiency virus peptides. Immunology. 1996;87:414–420. doi: 10.1046/j.1365-2567.1996.458547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawn S.D., Butera S.T. Incorporation of HLA-DR into the envelope of human immunodeficiency virus type 1 in vivo: correlation with stage of disease and presence of opportunistic infection. J. Virol. 2000;74:10256–10259. doi: 10.1128/jvi.74.21.10256-10259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin W., Li C., Du T., Hu K., Huang X., Hu Q. DC-SIGN plays a stronger role than DCIR in mediating HIV-1 capture and transfer. Virology. 2014;458–459:83–92. doi: 10.1016/j.virol.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Sweet R.W., Truneh A., Hendrickson W.A. CD4: its structure, role in immune function and AIDS pathogenesis, and potential as a pharmacological target. Curr. Opin. Biotechnol. 1991;2:622–633. doi: 10.1016/0958-1669(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 52.Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., van Rooijen N. MHC class II-dependent basophil–CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slepushkin V.A., Salem I.I., Andreev S.M., Dazin P., Düzgüneş N. Targeting of liposomes to HIV-1-infected cells by peptides derived from the CD4 receptor. Biochem. Biophys. Res. Commun. 1996;227:827–833. doi: 10.1006/bbrc.1996.1592. [DOI] [PubMed] [Google Scholar]
- 54.Endsley A.N., Ho R.J. Enhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticles. JAIDS J. Acquir. Immune Defic. Syndr. 2012;61:417–424. doi: 10.1097/QAI.0b013e3182653c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porcelli S., Brenner M.B., Greenstein J.L., Terhorst C., Balk S.P., Bleicher P.A. Recognition of cluster of differentiation 1 antigens by human CD4–CD8- cytolytic T lymphocyte. Nature. 1989;341(6241):447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 56.Pollard S.R., Rosa M.D., Rosa J.J., Wiley D. Truncated variants of gp120 bind CD4 with high affinity and suggest a minimum CD4 binding region. EMBO J. 1992;11:585. doi: 10.1002/j.1460-2075.1992.tb05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neffe A.T., Bilang M., Grüneberg I., Meyer B. Rational optimization of the binding affinity of CD4 targeting peptidomimetics with potential anti HIV activity. J. Med. Chem. 2007;50:3482–3488. doi: 10.1021/jm070206b. [DOI] [PubMed] [Google Scholar]
- 58.B. Seed, B. Banapour, C. Romeo, W. Kolanus, Targeted cytolysis of HIV-infected cells by chimeric CD4 receptor-bearing cells, in, Google Patents, 2004.
- 59.Triggle D.J. Drug development in the 21st century: medicines, man and receptors. Med. Chem. Res. 2004;13:238–248. [Google Scholar]
- 60.Eck W., Nicholson A.I., Zentgraf H., Semmler W., Bartling S.n. Anti-CD4-targeted gold nanoparticles induce specific contrast enhancement of peripheral lymph nodes in X-ray computed tomography of live mice. Nano Lett. 2010;10:2318–2322. doi: 10.1021/nl101019s. [DOI] [PubMed] [Google Scholar]
- 61.McNicholl J.M., Smith D.K., Qari S.H., Hodge T. Host genes and HIV: the role of the chemokine receptor gene CCR5 and its allele. Emerg. Infect. Dis. 1997;3:261. doi: 10.3201/eid0303.970302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee B., Sharron M., Montaner L.J., Weissman D., Doms R.W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He J., Chen Y., Farzan M., Choe H., Ohagen A., Gartner S., Busciglio J., Yang X., Hofmann W., Newman W. 1997. CCR3 and CCR5 are Co-receptors for HIV-1 Infection of Microglia. [DOI] [PubMed] [Google Scholar]
- 64.Bronshtein T., Toledano N., Danino D., Pollack S., Machluf M. Cell derived liposomes expressing CCR5 as a new targeted drug-delivery system for HIV infected cells. J. Control. Release. 2011;151:139–148. doi: 10.1016/j.jconrel.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 65.Trkola A., Ketas T.J., Nagashima K.A., Zhao L., Cilliers T., Morris L., Moore J.P., Maddon P.J., Olson W.C. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 2001;75:579–588. doi: 10.1128/JVI.75.2.579-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agrawal L., VanHorn-Ali Z., Berger E.A., Alkhatib G. Specific inhibition of HIV-1 coreceptor activity by synthetic peptides corresponding to the predicted extracellular loops of CCR5. Blood. 2004;103:1211–1217. doi: 10.1182/blood-2003-08-2669. [DOI] [PubMed] [Google Scholar]
- 67.Kwong J.A., Dorfman T., Quinlan B.D., Chiang J.J., Ahmed A.A., Choe H., Farzan M. A tyrosine-sulfated CCR5-mimetic peptide promotes conformational transitions in the HIV-1 envelope glycoprotein. J. Virol. 2011;85:7563–7571. doi: 10.1128/JVI.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schleifman E.B., McNeer N.A., Jackson A., Yamtich J., Brehm M.A., Shultz L.D., Greiner D.L., Kumar P., Saltzman W.M., Glazer P.M. Site-specific genome editing in PBMCs with PLGA nanoparticle-delivered PNAs confers HIV-1 resistance in humanized mice. Mol. Ther. Nucleic Acids. 2013;2:e135. doi: 10.1038/mtna.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herbein G., Mahlknecht U., Batliwalla F., Gregersen P., Pappas T., Butler J., O'Brien W.A., Verdin E. Apoptosis of CD8+; T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 70.Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D., Zhang J., Ratajczak J., Ratajczak M. CXCR4–SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 71.Bach H.H., IV, Saini V., Baker T.A., Tripathi A., Gamelli R.L., Majetschak M. Initial assessment of the role of CXC chemokine receptor 4 after polytrauma. Mol. Med. 2012;18:1056. doi: 10.2119/molmed.2011.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Endres M.J., Clapham P.R., Marsh M., Ahuja M., Turner J.D., McKnight A., Thomas J.F., Stoebenau-Haggarty B., Choe S., Vance P.J. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 73.Donzella G.A., Schols D., Lin S.W., Esté J.A., Nagashima K.A., Maddon P.J., Allaway G.P., Sakmar T.P., Henson G., DeClercq E. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 74.Gonda T.A., Varro A., Wang T.C., Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin. Cell Dev. Biol. 2010;21:2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y., Duggineni S., Espitia S., Richman D.D., An J., Huang Z. A synthetic bivalent ligand of CXCR4 inhibits HIV infection. Biochem. Biophys. Res. Commun. 2013;435:646–650. doi: 10.1016/j.bbrc.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., Sun H., Li X., Mo X., Zhang G. Anti-HIV effect of liposomes bearing CXCR4 receptor antagonist N15P. Trop. J. Pharm. Res. 2013;12:503–509. [Google Scholar]
- 77.BouHamdan M., Strayer D., Wei D., Mukhtar M., Duan L., Hoxie J., Pomerantz R. Inhibition of HIV-1 infection by down-regulation of the CXCR4 co-receptor using an intracellular single chain variable fragment against CXCR4. Gene Ther. 2001;8:408–418. doi: 10.1038/sj.gt.3301411. [DOI] [PubMed] [Google Scholar]
- 78.C. Klinguer-hamour, Novel antibodies for the treatment of HIV, in, Google Patents, 2010.
- 79.Hesselgesser J., Liang M., Hoxie J., Greenberg M., Brass L.F., Orsini M.J., Taub D., Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J. Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- 80.Carnec X., Quan L., Olson W.C., Hazan U., Dragic T. Anti-CXCR4 monoclonal antibodies recognizing overlapping epitopes differ significantly in their ability to inhibit entry of human immunodeficiency virus type 1. J. Virol. 2005;79:1930–1933. doi: 10.1128/JVI.79.3.1930-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J., He L., Combs C.A., Roderiquez G., Norcross M.A. Dimerization of CXCR4 in living malignant cells: control of cell migration by a synthetic peptide that reduces homologous CXCR4 interactions. Mol. Cancer Ther. 2006;5:2474–2483. doi: 10.1158/1535-7163.MCT-05-0261. [DOI] [PubMed] [Google Scholar]
- 82.Chertov O., Zhang N., Chen X., Oppenheim J.J., Lubkowski J., McGrath C., Sowder R.C., II, Crise B.J., Malyguine A., Kutzler M.A. Novel peptides based on HIV-1 gp120 sequence with homology to chemokines inhibit HIV infection in cell culture. PLoS One. 2011;6:e14474. doi: 10.1371/journal.pone.0014474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang Z., Wu T., Lou H., Yu X., Taichman R.S., Lau S.K., Nie S., Umbreit J., Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 84.Tamamura H., Omagari A., Hiramatsu K., Gotoh K., Kanamoto T., Xu Y., Kodama E., Matsuoka M., Hattori T., Yamamoto N. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg. Med. Chem. Lett. 2001;11:1897–1902. doi: 10.1016/s0960-894x(01)00323-7. [DOI] [PubMed] [Google Scholar]
- 85.Ramsey D.M., McAlpine S.R. Halting metastasis through CXCR4 inhibition. Bioorg. Med. Chem. Lett. 2013;23:20–25. doi: 10.1016/j.bmcl.2012.10.138. [DOI] [PubMed] [Google Scholar]
- 86.Ji X., Gewurz H., Spear G.T. Mannose binding lectin (MBL) and HIV. Mol. Immunol. 2005;42:145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Pollicita M., Schols D., Aquaro S., Peumans W.J., Van Damme E.J.M., Perno C.F., Balzarini J. Carbohydrate-binding agents (CBAs) inhibit HIV-1 infection in human primary monocyte-derived macrophages (MDMs) and efficiently prevent MDM-directed viral capture and subsequent transmission to CD4+ T lymphocytes. Virology. 2008;370:382–391. doi: 10.1016/j.virol.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 88.Mathys L., Balzarini J. Exposure of HIV-1 to a combination of two carbohydrate-binding agents markedly delays drug resistance development and selects for virus strains with compromised fitness. J. Antimicrob. Chemother. 2014;69(3):582–593. doi: 10.1093/jac/dkt414. [DOI] [PubMed] [Google Scholar]
- 89.Balzarini J., Van Laethem K., Hatse S., Froeyen M., Peumans W., Van Damme E., Schols D. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: a new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 2005;280:41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 90.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Hoorelbeke B., Van Damme E.J., Rougé P., Schols D., Van Laethem K., Fouquaert E., Balzarini J. 2011. Differences in the Mannose Oligomer Specificities of the Closely Related Lectins from Galanthus nivalis and Zea mays Strongly Determine Their Eventual Anti-HIV Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balzarini J., Neyts J., Schols D., Hosoya M., Van Damme E., Peumans W., De Clercq E. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 93.Hu Q., Mahmood N., Shattock R.J. High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology. 2007;368:145–154. doi: 10.1016/j.virol.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McFeeters H., Gilbert M., Wood A., Haggenmaker C., Jones J. Scytovirin engineering improves carbohydrate affinity and HIV-1 entry inhibition. Biochem. Physiol. S. 2013;2:2. [Google Scholar]
- 95.KljajiĆ Z., SchrÖDer H.C., Rottmann M., CuperloviĆ M., Movsesian M., Uhlenbruck G., GasiĆ M., Zahn R.K., MÜLler W.E.G. A d-mannose-specific lectin from Gerardia savaglia that inhibits nucleocytoplasmic transport of mRNA. Eur. J. Biochem. 1987;169:97–104. doi: 10.1111/j.1432-1033.1987.tb13585.x. [DOI] [PubMed] [Google Scholar]
- 96.Mori T., O'Keefe B.R., Sowder R.C., Bringans S., Gardella R., Berg S., Cochran P., Turpin J.A., Buckheit R.W., McMahon J.B. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 97.Sato Y., Hirayama M., Morimoto K., Yamamoto N., Okuyama S., Hori K. High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007;5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Botos I., Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog. Biophys. Mol. Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Bulgheresi S., Schabussova I., Chen T., Mullin N.P., Maizels R.M., Ott J.A. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 2006;72:2950–2956. doi: 10.1128/AEM.72.4.2950-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang T.L., Klotman M.E. Defensins: natural anti-HIV peptides. AIDS Rev. 2004;6:161. [PubMed] [Google Scholar]
- 102.Klotman M.E., Chang T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 103.Mohan T., Sharma C., Bhat A.A., Rao D.N. Modulation of HIV peptide antigen specific cellular immune response by synthetic α- and β-defensin peptides. Vaccine. 2013;31:1707–1716. doi: 10.1016/j.vaccine.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 104.Dutta T., Jain N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta Gen. Subj. 2007;1770:681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Jain S.K., Gupta Y., Jain A., Saxena A.R., Khare P., Jain A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine Nanotechnol. Biol. Med. 2008;4:41–48. doi: 10.1016/j.nano.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B: Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Calarese D.A., Scanlan C.N., Zwick M.B., Deechongkit S., Mimura Y., Kunert R., Zhu P., Wormald M.R., Stanfield R.L., Roux K.H. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 108.Pikora C., Wittish C., Desrosiers R.C. Identification of two N-linked glycosylation sites within the core of the simian immunodeficiency virus glycoprotein whose removal enhances sensitivity to soluble CD4. J. Virol. 2005;79:12575–12583. doi: 10.1128/JVI.79.19.12575-12583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oki T., Konishi M., Tomatsu K., Tomita K., Saitoh K., Tsunakawa M., Nishio M., Miyaki T., Kawaguchi H. Pradimicin, a novel class of potent antifungal antibiotics. J. Antibiot. 1988;41:1701. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 110.Takeuchi T., Hara T., Naganawa H., Okada M., Hamada M., Umezawa H., Gomi S., Sezaki M., Kondo S. New antifungal antibiotics, benanomicins A and B from an actinomycete. J. Antibiot. 1988;41:807. doi: 10.7164/antibiotics.41.807. [DOI] [PubMed] [Google Scholar]
- 111.Gieseler R.K., Marquitan G., Hahn M.J., Perdon L.A., Driessen W.H.P., Sullivan S.M., Scolaro M.J. DC-SIGN-specific liposomal targeting and selective intracellular compound delivery to human myeloid dendritic cells: implications for HIV disease. Scand. J. Immunol. 2004;59:415–424. doi: 10.1111/j.0300-9475.2004.01431.x. [DOI] [PubMed] [Google Scholar]
- 112.Wang S.-K., Liang P.-H., Astronomo R.D., Hsu T.-L., Hsieh S.-L., Burton D.R., Wong C.-H. Targeting the carbohydrates on HIV-1: interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc. Natl. Acad. Sci. 2008;105:3690–3695. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tzehoval E., Segal S., Stabinsky Y., Fridkin M., Spirer Z., Feldman M. Tuftsin (an Ig-associated tetrapeptide) triggers the immunogenic function of macrophages: implications for activation of programmed cells. Proc. Natl. Acad. Sci. 1978;75:3400–3404. doi: 10.1073/pnas.75.7.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jain K., Kesharwani P., Gupta U., Jain N. Dendrimer toxicity: let's meet the challenge. Int. J. Pharm. 2010;394(1):122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 115.Dutta T., Garg M., Jain N.K. Targeting of efavirenz loaded tuftsin conjugated poly (propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 116.Agarwal A., Kandpal H., Gupta H., Singh N., Gupta C. Tuftsin-bearing liposomes as rifampin vehicles in treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 1994;38:588–593. doi: 10.1128/aac.38.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guru P.Y., Agrawal A.K., Singha U.K., Singhal A., Gupta C.M. Drug targeting in Leishmania donovani infections using tuftsin-bearing liposomes as drug vehicles. FEBS Lett. 1989;245:204–208. doi: 10.1016/0014-5793(89)80222-4. [DOI] [PubMed] [Google Scholar]
- 118.Owais M., Ahmed I., Krishnakumar B., Jaina R.K., Bachhawat B.K., Gupta C.M. Tuftsin-bearing liposomes as drug vehicles in the treatment of experimental aspergillosis. FEBS Lett. 1993;326:56–58. doi: 10.1016/0014-5793(93)81760-w. [DOI] [PubMed] [Google Scholar]
- 119.Banjoko S.O., Oseni F.A., Togun R.A., Onayemi O., Emma-Okon B.O., Fakunle J.B. Iron status in HIV-1 infection: implications in disease pathology. BMC Clin. Pathol. 2012;12:26. doi: 10.1186/1472-6890-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ali S.A., Joao H.C., Hammerschmid F., Eder J., Steinkasserer A. Transferrin trojan horses as a rational approach for the biological delivery of therapeutic peptide domains. J. Biol. Chem. 1999;274:24066–24073. doi: 10.1074/jbc.274.34.24066. [DOI] [PubMed] [Google Scholar]
- 121.Qian Z.M., Li H., Sun H., Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 122.Kuo Y.-C., Lin P.-I., Wang C.-C. Targeting nevirapine delivery across human brain microvascular endothelial cells using transferrin-grafted poly (lactide-co-glycolide) nanoparticles. Nanomedicine. 2011;6:1011–1026. doi: 10.2217/nnm.11.25. [DOI] [PubMed] [Google Scholar]
- 123.Mann J., Stieh D., Klein K., de Stegmann D., Cranage M., Shattock R., McKay P. Transferrin conjugation confers mucosal molecular targeting to a model HIV-1 trimeric gp140 vaccine antigen. J. Control. Release. 2012;158:240–249. doi: 10.1016/j.jconrel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boado R.J., Tsukamoto H., Pardridge W.M. Drug delivery of antisense molecules to the brain for treatment of Alzheimer's disease and cerebral AIDS. J. Pharm. Sci. 1998;87:1308–1315. doi: 10.1021/js9800836. [DOI] [PubMed] [Google Scholar]
- 125.Mishra V., Mahor S., Rawat A., Gupta P.N., Dubey P., Khatri K., Vyas S.P. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J. Drug Target. 2006;14:45–53. doi: 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- 126.Mahajan S.D., Roy I., Xu G., Yong K.-T., Ding H., Aalinkeel R., Reynolds J.L., Sykes D.E., Nair B.B., Lin E.Y. Enhancing the delivery of anti retroviral drug “saquinavir” across the blood brain barrier using nanoparticles. Curr. HIV Res. 2010;8:396. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sefah K., Shangguan D., Xiong X., O'Donoghue M.B., Tan W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010;5:1169–1185. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- 128.Burnett J.C., Rossi J.J. RNA-based therapeutics: current progress and future prospects. Chem. Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou J., Swiderski P., Li H., Zhang J., Neff C.P., Akkina R., Rossi J.J. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37:3094–3109. doi: 10.1093/nar/gkp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J., Li H., Li S., Zaia J., Rossi J.J. Novel dual inhibitory function aptamer–siRNA delivery system for HIV-1 therapy. Mol. Ther. 2008;16:1481–1489. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nielsen M.H., Pedersen F.S., Kjems J. Molecular strategies to inhibit HIV-1 replication. Retrovirology. 2005;2:10. doi: 10.1186/1742-4690-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li H., Yuan G., Du D. Investigation of formation, recognition, stabilization, and conversion of dimeric G-quadruplexes of HIV-1 integrase inhibitors by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2008;19:550–559. doi: 10.1016/j.jasms.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 133.Stangl H., Hyatt M., Hobbs H.H. Transport of lipids from high and low density lipoproteins via scavenger receptor-BI. J. Biol. Chem. 1999;274:32692–32698. doi: 10.1074/jbc.274.46.32692. [DOI] [PubMed] [Google Scholar]
- 134.Mankertz J., Von Baeyer H., Rokos K., Nündel M., Pauli G., Riedel E. Cell specific uptake of antiretroviral drugs: AZT coupled to LDL inhibits HIV replication in human macrophages. Int. J. Clin. Pharmacol. Ther. 1995;33:85–88. [PubMed] [Google Scholar]
- 135.Mankertz J., Matthes E., Rokos K., von Baeyer H., Mankertz J., Matthes E., Rokos K., von Baeyer H., Pauli G., Riedel E. Selective endocytosis of fluorothymidine and azidothymidine coupled to LDL into HIV infected mononuclear cells. Biochim. Biophys. Acta Mol. Basis Dis. 1996;1317:233–237. doi: 10.1016/s0925-4439(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 136.Hu J., Liu H., Wang L. Enhanced delivery of AZT to macrophages via acetylated LDL. J. Control. Release. 2000;69:327–335. doi: 10.1016/s0168-3659(00)00319-9. [DOI] [PubMed] [Google Scholar]
- 137.Greish K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: are we there yet? Drug Discov. Today Technol. 2012;9:e161–e166. doi: 10.1016/j.ddtec.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 138.Löbenberg R., Kreuter J. Macrophage targeting of azidothymidine: a promising strategy for AIDS therapy*. AIDS Res. Hum. Retrovir. 1996;12:1709–1715. doi: 10.1089/aid.1996.12.1709. [DOI] [PubMed] [Google Scholar]
- 139.Dou H., Morehead J., Destache C.J., Kingsley J.D., Shlyakhtenko L., Zhou Y., Chaubal M., Werling J., Kipp J., Rabinow B.E. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358:148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 140.Jin Y., Xin R., Tong L., Du L., Li M. Combination anti-HIV therapy with the self-assemblies of an asymmetric bolaamphiphilic zidovudine/didanosine prodrug. Mol. Pharm. 2011;8:867–876. doi: 10.1021/mp100457d. [DOI] [PubMed] [Google Scholar]
- 141.Misra A., Ganesh S., Shahiwala A., Shah S.P. Drug delivery to the central nervous system: a review. J. Pharm. Pharm. Sci. 2003;6:252–273. [PubMed] [Google Scholar]
- 142.Bender A., Schäfer V., Steffan A.M., Royer C., Kreuter J., Rübsamen-Waigmann H., von Briesen H. Inhibition of HIV in vitro by antiviral drug-targeting using nanoparticles. Res. Virol. 1994;145:215–220. doi: 10.1016/s0923-2516(07)80025-2. [DOI] [PubMed] [Google Scholar]
- 143.Dos Santos C.A., Ribeiro G.B., Knirsch M.C., Junior A.P., Vessoni Penna T.C. Influence of Pluronic® F68 on ceftazidime biological activity in parenteral solutions. J. Pharm. Sci. 2011;100:715–720. doi: 10.1002/jps.22287. [DOI] [PubMed] [Google Scholar]
- 144.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 145.Yang G., Xiong X. Viral infectivity factor: a novel therapeutic strategy to block HIV-1 replication. Mini Rev. Med. Chem. 2013;13:1047–1055. doi: 10.2174/1389557511313070008. [DOI] [PubMed] [Google Scholar]
- 146.Anand A.R., Zhao H., Nagaraja T., Robinson L.A., Ganju R.K. 2013. N-terminal Slit2 Inhibits HIV-1 Replication by Regulating the Actin Cytoskeleton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Falco A., Barrajón-Catalán E., Menéndez-Gutiérrez M.P., Coll J., Micol V., Estepa A. Melittin-loaded immunoliposomes against viral surface proteins, a new approach to antiviral therapy. Antiviral Res. 2013;97:218–221. doi: 10.1016/j.antiviral.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 148.Vlachakis D., Tsiliki G., Pavlopoulou A., Roubelakis M.G., Tsaniras S.C., Kossida S. Antiviral stratagems against HIV-1 using RnA interference (RnAi) technology. Evol. Bioinformatics Online. 2013;9:203. doi: 10.4137/EBO.S11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaur A., Jain S., Tiwary A. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta Pharma. 2008;58:61–74. doi: 10.2478/v10007-007-0045-1. [DOI] [PubMed] [Google Scholar]
- 150.Rao K.S., Reddy M.K., Horning J.L., Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gagné J.-F., Désormeaux A., Perron S., Tremblay M.J., Bergeron M.G. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim. Biophys. Acta Biomembr. 2002;1558:198–210. doi: 10.1016/s0005-2736(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 152.Wan L., Zhang X., Pooyan S., Palombo M.S., Leibowitz M.J., Stein S., Sinko P.J. Optimizing size and copy number for PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophages. Bioconjug. Chem. 2007;19:28–38. doi: 10.1021/bc070066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kinman L., Bui T., Larsen K., Tsai C.-C., Anderson D., Morton W.R., Hu S.-L., Ho R.J. Optimization of lipid-indinavir complexes for localization in lymphoid tissues of HIV-infected macaques. JAIDS J. Acquir. Immune Defic. Syndr. 2006;42:155–161. doi: 10.1097/01.qai.0000214822.33905.87. [DOI] [PubMed] [Google Scholar]
- 154.Garg M., Jain N.K. Reduced hematopoietic toxicity, enhanced cellular uptake and altered pharmacokinetics of azidothymidine loaded galactosylated liposomes. J. Drug Target. 2006;14:1–11. doi: 10.1080/10611860500525370. [DOI] [PubMed] [Google Scholar]
- 155.Kaur C.D., Nahar M., Jain N.K. Lymphatic targeting of zidovudine using surface-engineered liposomes. J. Drug Target. 2008;16:798–805. doi: 10.1080/10611860802475688. [DOI] [PubMed] [Google Scholar]
- 156.Garg M., Asthana A., Agashe H.B., Agrawal G.P., Jain N.K. Stavudine-loaded mannosylated liposomes: in-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. J. Pharm. Pharmacol. 2006;58:605–616. doi: 10.1211/jpp.58.5.0005. [DOI] [PubMed] [Google Scholar]
- 157.Garg M., Dutta T., Jain N.K. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. Eur. J. Pharm. Biopharm. 2007;67:76–85. doi: 10.1016/j.ejpb.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 158.Eguchi A., Meade B.R., Chang Y.-C., Fredrickson C.T., Willert K., Puri N., Dowdy S.F. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat. Biotechnol. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song E., Zhu P., Lee S.-K., Chowdhury D., Kussman S., Dykxhoorn D.M., Feng Y., Palliser D., Weiner D.B., Shankar P., Marasco W.A., Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 160.Aline F., Brand D., Pierre J., Roingeard P., Séverine M., Verrier B., Dimier-Poisson I. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 161.Lori F., Calarota S., Lisziewicz J. Nanochemistry-based immunotherapy for HIV-1. Curr. Med. Chem. 2007;14:1911–1919. doi: 10.2174/092986707781368513. [DOI] [PubMed] [Google Scholar]
- 162.Dutta T., Garg M., Jain N.K. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Fridkin M., Tsubery H., Tzehoval E., Vonsover A., Biondi L., Filira F., Rocchi R. Tuftsin–AZT conjugate: potential macrophage targeting for AIDS therapy. J. Pept. Sci. 2005;11:37–44. doi: 10.1002/psc.587. [DOI] [PubMed] [Google Scholar]
- 164.Clayton R., Ohagen A., Nicol F., Del Vecchio A.M., Jonckers T.H.M., Goethals O., Van Loock M., Michiels L., Grigsby J., Xu Z., Zhang Y.P., Gutshall L.L., Cunningham M., Jiang H., Bola S., Sarisky R.T., Hertogs K. Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antiviral Res. 2009;84:142–149. doi: 10.1016/j.antiviral.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 165.Zhou J., Shu Y., Guo P., Smith D.D., Rossi J.J. Dual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 inhibition. Methods. 2011;54:284–294. doi: 10.1016/j.ymeth.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]