Highlights
-
•
This study was the first seroprevalence and genetic investigation of PCV3 in dogs in the Guangxi province, China.
-
•
This work is the first in the world to obtain the complete genome of dog PCV3.
-
•
These PCV3 strains from the Guangxi province help to determine that PCV3 from dog origin and pig origin are from different branches.
Keywords: Porcine circovirus type 3, Canine, Epidemiology, Phylogenetic analysis
Abstract
Porcine circovirus type 3 (PCV3) is an emerging circovirus species associated with several diseases. The study aimed to investigate the frequency of porcine circovirus 3 (PCV3) and its coinfection with canine parvovirus type 2 (CPV-2) in dogs in the Guangxi province from 2015 to 2017, China, and to examine the genome diversity of PCV3. Using polymerase chain reaction (PCR) amplification and sequencing, 96 of 406 (23.6%)samples were positive for PCV3, 38 out of 406 (9.4%) samples were coinfected with both PCV3 and CPV-2. The CPV-positive rate was significantly higher in the PCV3-positive samples than in the non-PCV3 samples, and the difference was extremely significant (P < 0.01). The complete genome (n=4) and ten capsid genes (n=10) of PCV3 were sequenced. Multiple sequence alignment results showed that these sequences shared 98.5–100% nucleotide similarity with the reference genome sequence and 97.5–100% nucleotide similarity with the reference capsid gene sequence. PCV3 was classified into two different genotypes, according to phylogenetic analysis based on the whole genome. These strains were clustered in PCV3a, showing a close relationship with PCV3-US/SD2016. Surprisingly, we separately analyzed these PCV3 strains from the Guangxi province and found that the dog and pig PCV3 are from different branches. In summary, this was the first seroprevalence and genetic investigation of PCV3 in dogs in the Guangxi province, China, and the first complete genome PCV3 from dogs obtained in the world. The results provide insights into the epidemiology and pathogenesis of this important virus.
1. Introduction
Circoviruses (family Circoviridae, genus Circovirus) are nonenveloped, circular, single-stranded DNA viruses and can infect various animals (including Homo sapiens, pigs, sheep, ducks, and dogs) (Li et al., 2010, 2011). Currently, the genus Circovirus includes 11 recognized species (Li et al., 2010).
Canine parvovirus type 2 (CPV-2) is a single-stranded, negative-sense, nonsegmented DNA virus with a genome length of 5.2 kb, belonging to the genus Parvoviridae of the family Parvovirus (Cotmore et al., 2014). CPV-2 is a common etiological agent that causes severe gastroenteritis in puppies. The virus has a high detection rate in clinical samples, and the most characteristic signs of this illness are diarrhea, emesis, and anorexia (Apaa et al., 2016). Multiple studies have confirmed that CPV-2 coinfects with more than one enteric pathogen, including Canine enteric coronavirus, Canine circovirus (CanineCV), and Canine distemper virus (Costa et al., 2014; Kotsias et al., 2019; Navarro et al., 2017). T. Thaiwong hypothesizes that the CPV-2 infection predisposes dogs to CanineCV infection and ultimately results in more severe disease (Thaiwong et al., 2016).
Porcine circovirus (PCV) is the smallest autonomously replicating virus with a genome length of 1.7 kb (Cao et al., 2018; Lv et al., 2014). The PCV genome contains two major open reading frames (ORFs). ORF1 encodes the replication-associated protein (Rep), which plays an important role in virus replication (Lekcharoensuk et al., 2004; Walia et al., 2014). ORF2 encodes the sole structural protein (capsid protein, Cap), which contains immunologically important epitopes associated with the virus neutralization (Cheung, 2012). Porcine circovirus type 2 (PCV2) infection causes a diverse range of diseases resulting in substantial economic loses to the porcine industry (Segales et al., 2005). It is worth noting that PCV2 could transmit to nonporcine hosts through cross-species transmission routes. Specifically, cattle, dogs and sheep can be infected with PCV2 (Li et al., 2011; Song et al., 2019; Wang et al., 2018; Zhai et al., 2017). Recently, a novel and genetically divergent circovirus, PCV3, was found in swine with PDNS, PMWS in the United States (Palinski et al., 2017). Subsequently, this virus has been detected in many countries and has an abnormally high positive rate (Costa et al., 2014; Deim et al., 2019; Faccini et al., 2017; Saraiva et al., 2019). In China, multiple epidemiological surveillance data indicated that this virus was extensively prevalent in pigs in many provinces and specific cities (Qi et al., 2019; Sun et al., 2018). Multiple studies have revealed that the virus was associated with several diseases (such as PDNS, multisystemic inflammation, reproductive failure) and coinfects with other viruses (for example, Torque teno sus virus, PCV2, porcine reproductive and respiratory syndrome virus) (Chen et al., 2019; Ha et al., 2018; Wen et al., 2018). Retrospective survey studies indicated that PCV3 infection could be traced back to 1996 (Sun et al., 2018). Recent studies confirmed that a PDNS-like disease is reproduced with PCV3 infection alone, and further research suggested that PCV3 is more pathogenic for piglets than PCV2 (Jiang et al., 2019).
Overall, these studies indicated that PCV3 is an important pathogen for pigs and is more pathogenic for piglets than PCV2. Surprisingly, recent studies indicated that dogs could be infected with PCV3 (Zhang et al., 2018). Our previous research also indicated that cattle could be infected with PCV3 (Wang et al., 2019). These results indicates that PCV3 is similar to PCV2 and could transmit to nonporcine hosts, possibly through cross-species transmission routes. However, knowledge about the infection rate and pathogenicity of this virus in dogs is limited. Therefore, we are very interested in investigating the presence of PCV3 in dogs in the Guangxi province, China.
2. Materials and methods
2.1. Sample collection
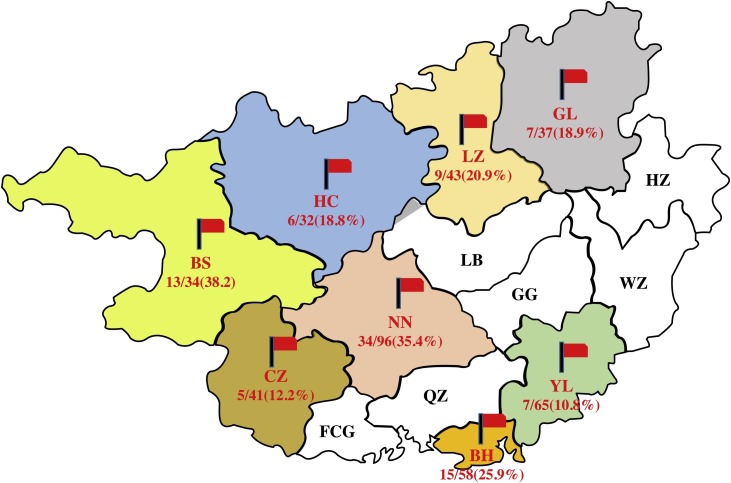
From 2015–2017, serum samples (n = 406) of dogs were collected from eight cities in the Guangxi, China (Including Nanning, Beihai, Guigang, Liuzhou, Yulin, Hechi, Baise and Guilin) (Fig. 1 ). This study received animal ethical approval (No. Xidakezi2000138) from Guangxi University (see Ethics approval and consent to participate).
Fig. 1.
Geographical distribution of porcine circovirus type 3 (PCV3) in Guangxi Province. The red flag represents the source of the sample and percentage represents positive rate. From which, Guilin, GL; Hezhou, HZ; Liuzhou, LZ; Laibin, LB; Wuzhou, WZ; Guigang, GG; Yulin, YL; Hechi, HC; Nanning, NN; Qinzhou, QZ; Beihai, BH; Baise, BS; Chongzuo, CZ; Fangchenggang, FCG.
2.2. DNA extraction and polymerase chain reaction (PCR)
The viral genome was extracted from canine serum using the EasyPure Viral DNA/RNA Kit (TransGen Biotech, Beijing, China) according to the manufacturer's instructions. Two primer pairs were designed based on the reference sequences of the PCV3/CN/Liaoning-23/2016 strain (NO. KX354055.1) and used published primers and protocols to detect CPV-2 (Table S1). The PCR mixture contained 2 μL of extracted DNA, 2 μL of primer pairs (10 μM), 25 μL of 2×Phanta Max Master Mix (Vazyme, Nanjing, China), and 21 μL of DNase/RNase-Free water. The PCR amplification conditions were as follows: predenaturation for 3 min at 95 °C, followed by 35 cycles of 15 s at 95 °C, 15 s at 62 °C, an extension for 1 min at 72 °C, then the final extension for 5 min at 72 °C. Subsequently, the PCR products were separated using 1.2% agarose gel electrophoresis of DNA and cloned into a pMD18-T vector (Takara Co. Dalian). The recombined vectors were amplified in Escherichia coli (E. Coli, DH5α) for sequencing.
2.3. Multiple sequence alignment and Phylogenetic analysis
The ORF2 gene and genome sequences of PCV3 obtained in this study have been deposited in the GenBank under the accession numbers MH900457–MH900466 and MH916635-MH916638, respectively. In addition, a complete sequence (Guangxi-CZ/05, MH916639) obtained in our previous study was also analyzed in this study. The reference sequences for PCV3 was obtained from the GenBank. Multiple sequence alignments were carried out using Lasergene Package (DNA-STAR Inc.) within the Megalign program (DNAStar software), and the phylogenetic relationships were assessed with the MEGA software (version 7). Support for the phylogenetic relationships was determined by bootstrapping 1000 replicates. In the present study, the method described by Li et al. was used to divide the clades of PCV3 (Li et al., 2018).
3. Results
3.1. Screening for PCV3 prevalence in clinical samples
In this study, we investigated serum samples (n = 406) of dogs. The result indicated that PCV3 was detected in all the cities, 96 out of 406 (23.6%) samples were PCV3-positive samples, 115 of 406 (28.3%) samples were CPV-2 positive samples, 54 of 406 (13.3%) samples were coinfected with PCV3 and CPV-2 (Table 1 ). Interestingly, the positive rate of PCV3 ranged from 10.8 to 38.2%, with the highest rate recorded in Baise and the lowest in Yulin (Fig. 1). On the other hand, the highest positive rate of PCV3 ranged from 21.1 to 27.3%, with highest rate recorded in 2017 and the lowest in 2016 (Table S2). Then, the complete genome (n = 4) and the capsid gene (n = 12) were sequenced. Four complete sequences of PCV3 (Guangxi-BH/01, NN/02, LZ/03, GL/04) with a genome length of 2 kb, were similar to most of the reference sequences.
Table 1.
Frequency and distribution of CPV and PCV3 detected by PCR in samples from 8 cities in Guangxi.
| Prefecture | Numbers of samples tested | The positive rate of CPV | The positive rate of PCV3 | The positive rate of both CPV and PCV3 |
|---|---|---|---|---|
| Chongzuo | 41 | 9 | 5 | 2 |
| Guilin | 37 | 13 | 7 | 2 |
| Yulin | 65 | 11 | 7 | 3 |
| Nanning | 96 | 32 | 34 | 17 |
| Beihai | 58 | 13 | 15 | 5 |
| Hechi | 32 | 11 | 6 | 2 |
| Liuzhou | 43 | 15 | 9 | 3 |
| Baise | 34 | 11 | 13 | 4 |
| Total (%) | 406 | 115(28.3%) | 96(23.6%) | 38(9.4%) |
3.2. Multiple sequence alignment and analysis
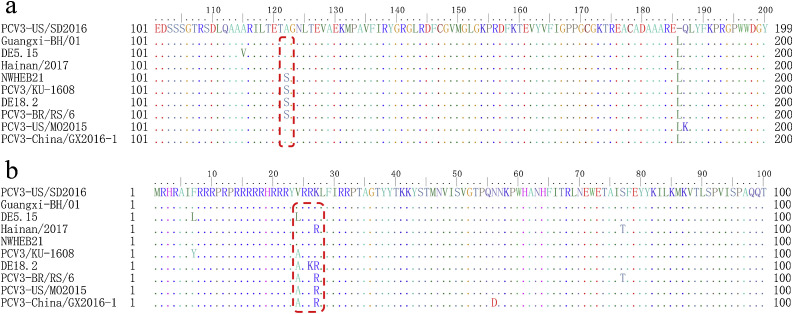
The multiple sequence alignment of these sequences showed 97.5–100% similarity with the reference capsid gene sequence and 98.5–100% similarity to the reference genome sequence, respectively. The comparison of the complete genome sequences revealed that these strains studied shared 99.7%–100% similarity with each other. In addition, all dog-origin PCV3 strains showed that they shared 98.3% to 100% nucleotide similarity with the ORF2 gene. Similar to previous reports, there are six variant sequences of amino acids 24 to 27 of the Cap (VRRR, VRRK, ARRK, ARRR, ARKR and LRRK), the strains in this study were Cap (24th VRRK 27th). In addition, a comparison of the amino acid sequences revealed a mutation from D124 to N124 in the Cap of Guangxi-C2 and Guangxi-C19 isolate, and a mutation from D124 to N124 in the Cap of Guangxi-NN/02 isolate. Notably, we detected two variant sequences of amino acids 122 of the Rep (V and A), these strains in this study were Rep (A 122th) (Fig. S1).
3.3. Phylogenetic analysis
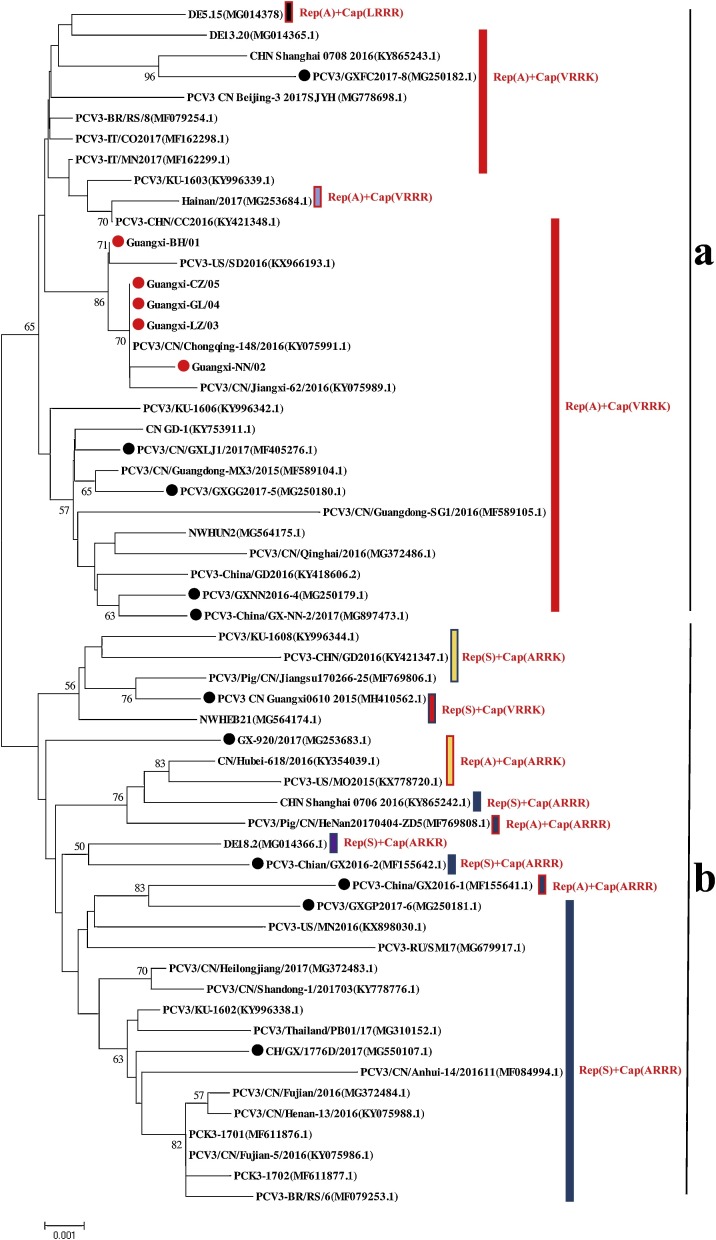
We used different methods (NJ, ML, and MCC) to reconstruct the phylogenies of the complete PCV3 genome, and different trees displayed similar stably structures in the division of PCV3 into different clades. The phylogenetic divergence analysis based on the complete genome sequences revealed that all the viruses are separated into two major genotypes (PCV3a and PCV3b), and these strains in the present study (Guangxi-BH/01, Guangxi-NN/02, Guangxi-LZ/03, Guangxi-GL/04 and Guangxi-CZ/05) were clustered in a branch representing PCV3a (Fig. 2 ). The dog-origin PCV3 had a closer relationship with the PCV3-US/SD-2016 strain, PCV3/CN/Chongqing-148/2016 strain, PCV3/CN/Jiangxi-62/2016 strain, and PCV3-BR/RS/8. In contrast, we separately analyzed these strains from Guangxi Province. The dog and pig PCV3 were from different branches.
Fig. 2.
Phylogenetic analyses of complete genome sequences from PCV3. The Maximum Likelihood (ML) trees were built using 1000 bootstraps replicates. Red circles indicate the strains detected in this study and black circles indicate strains isolated from Guangxi, China. In addition, a complete sequence (Guangxi-CZ/05, MH916639) obtained in our previous study was also analyzed in this study.
4. Discussion
CPV-2 is a common etiological agent that causes severe gastroenteritis in puppies and coinfects with more than one enteric pathogen (Zhou et al., 2016). T. Thaiwong hypothesizes that CPV-2 infection predisposed dogs to CaCV-1 infection and ultimately resulted in more severe diseases (Thaiwong et al., 2016). Multiple studies indicated that PCV3 is associated with multiple diseases in infected pigs (Jiang et al., 2019). Similar to PCV2, PCV3 also co-infects with other swine pathogens (such as PCV2, classical swine fever virus, PRRSV) (Chen et al., 2019; Zheng et al., 2018). Recent study indicated that dogs could be infected by PCV3 (Zhang et al., 2018), which was found in pig. The results indicated that PCV3 could transmit to non-porcine hosts, possibly through cross-species transmission routes. In this study, the molecular epidemiological investigation results confirmed that coinfection of PCV3 and CPV-2 was prevalent in dogs in the Guangxi province, China.
Multiple studies have confirmed that PCV3 is commonly co-infected with other pathogens (Chen et al., 2019; Ha et al., 2018), but the role of PCV3 in co-infection remains unclear. In the present study, nearly a quarter (23.6%) of clinical samples were PCV3-positive, and nearly one third (28.3%) of clinical samples were CPV-2-positive. Among them, 38 of 406 (9.36%) samples were coinfected with PCV3 and CPV-2. Interestingly, the CPV-positive rate was significantly higher in the PCV3-positive samples than non-PCV3 samples, and the difference was extremely significant (P < 0.01). Coinfection of pigs with PCV2 and other virus is known to exacerbate the disease severity (Segales et al., 2005; Yao et al., 2019). We identified the coinfection of PCV3 with CPV-2 in dogs and a possible synergy between PCV3 and CPV-2 coinfection with disease severity warrants further investigations.
Previous studies on PCV2 have shown that the amino acid mutation of Rep protein may affect the replication ability of the virus (Cheung, 2012), and the amino acid mutation of Cap protein may change the antigenicity of the virus (Lekcharoensuk et al., 2004). Consistent with previous reports, there are six variant sequences of amino acids 24 to 27 of the Cap (Sun et al., 2018). Several studies suggest that these four amino acids are associated with genetic variation and evolution of PCV3 (Ha et al., 2018; Qi et al., 2019). In addition, we also found mutations in other amino acids of the Cap (D124 to N124 and G160 to R160). However, the mutations in the 124th and 160th amino acid sequences may be unintentional, and these mutations are scattered variations rather than regular variation caused by co-infection (Table S3). Other researches also found co-infection between PCV3 and other pathogens. Unfortunately, they also did not find a relationship between PCV3 genetics and co-infection (Sun et al., 2018).
Currently, there is controversy about the classification of PCV3. In general, most scholars have divided PCV3 into two genotypes (Ha et al., 2018; Li et al., 2018), but there is no consensus on the best classification method. In the present study, we referred to the method described by Li et al, which also divided PCV3 into two different genotypes, PCV3a and PCV3b. Then, by combining genetic evolution analysis with amino acid sequence analysis, it was found that the Rep (A122th) and Cap (24th VRRK27th or 24th LRRR27th or 24th VRRR27th) were mainly in the PCV3a, and other strains were mainly in the PCV3b. Moreover, the tree displayed stably structures. Therefore, we suggested using the complete genome for PCV3 genotyping and divided PCV3 into a and b subtypes based on the aa codons in Rep (122 aa) and Cap (24aa to 27aa). Notably, all strains in this study were Cap (24th VRRK 27th) and were Rep (A 122th). This result indicates that there is no significant genetic difference in the PCV3 strains obtained in different regions in this study (Table S4). On the other hand, other studies have confirmed that there is no significant genetic difference in PCV3 strains in different regions.
Previous studies have shown that the high genetic stability of pig-origin PCV3 over the past 20 years (Sukmak et al., 2019; Sun et al., 2018). In the present study, the dog-origin PCV3 strain in this study share hight nucleic acid similarity (>95%) with reference sequences of pig-origin PCV3. In addition, compared with pig-origin PCV3 strain, there was no significant amino acid mutation in dog-origin PCV3 strain. Above all, the results of this study indicate that the high genetic stability of dog-origin PCV3. Surprisingly, we separately analyzed these PCV3 strains from the Guangxi Province and found that the dog and pig PCV3 are from different branches. Opriessnig proved that PCV2 in muscle and bone marrow was infectious and transmissible to pigs through the oral route (Opriessnig et al., 2007). The result indicated that PCV3 could transmit to nonporcine hosts by cross-species transmission routes. However, no evidence to prove that PCV3 in muscle and blood of pig was infectious and transmissible to dogs through the oral route. One guess is that PCV3 has been lurking in dogs for a long time.
Declaration of Competing Interest
The authors have no conflict of interest in regard to the research, authorship, and/or publication of this article.
Acknowledgements
This work was supported by the National Program on Key Research Project of China (grant number 2018YFD0500104 and 2018YFD0500803), Natural Science Foundation of Zhejiang Province (LQ19C180001) and National Natural Sciences Foundation of China (31802199) and Key Laboratory of Preventive Veterinary Medicine of Education Department in Guangdong Province of China (2014KTSPT037).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2019.197663.
Contributor Information
Huijun Lu, Email: huijun_lu@126.com.
Min Zheng, Email: zhgmn26@163.com.
Ningyi Jin, Email: ningyik@126.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Apaa T.T., Daly J.M., Tarlinton R.E. Canine parvovirus (CPV-2) variants circulating in Nigerian dogs. Vet. Rec. Open. 2016;3(1) doi: 10.1136/vetreco-2016-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Sun W., Lu H., Tian M., Xie C., Zhao G., Han J., Wang W., Zheng M., Du R., Jin N., Qian A. Genetic variation analysis of PCV1 strains isolated from Guangxi Province of China in 2015. BMC Vet. Res. 2018;14(1):43. doi: 10.1186/s12917-018-1345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Huang Y., Ye M., Li S., Xiao Y., Cui B., Zhu J. Co-infection status of classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circoviruses (PCV2 and PCV3) in eight regions of China from 2016 to 2018. Infect. Genet. Evol. 2019;68:127–135. doi: 10.1016/j.meegid.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Cheung A.K. Porcine circovirus: transcription and DNA replication. Virus Res. 2012;164(1–2):46–53. doi: 10.1016/j.virusres.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Costa E.M., de Castro T.X., Bottino Fde O., Garcia Rde C. Molecular characterization of canine coronavirus strains circulating in Brazil. Vet. Microbiol. 2014;168(1):8–15. doi: 10.1016/j.vetmic.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S.F., Agbandje-McKenna M., Chiorini J.A., Mukha D.V., Pintel D.J., Qiu J., Soderlund-Venermo M., Tattersall P., Tijssen P., Gatherer D., Davison A.J. The family Parvoviridae. Arch. Virol. 2014;159(5):1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deim Z., Dencso L., Erdelyi I., Valappil S.K., Varga C., Posa A., Makrai L., Rakhely G. Porcine circovirus type 3 detection in a Hungarian pig farm experiencing reproductive failures. Vet. Rec. 2019;185(3):84. doi: 10.1136/vr.104784. [DOI] [PubMed] [Google Scholar]
- Faccini S., Barbieri I., Gilioli A., Sala G., Gibelli L.R., Moreno A., Sacchi C., Rosignoli C., Franzini G., Nigrelli A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound. Emerg. Dis. 2017;64(6):1661–1664. doi: 10.1111/tbed.12714. [DOI] [PubMed] [Google Scholar]
- Ha Z., Xie C.Z., Li J.F., Wen S.B., Zhang K.L., Nan F.L., Zhang H., Guo Y.C., Wang W., Lu H.J., Jin N.Y. Molecular detection and genomic characterization of porcine circovirus 3 in pigs from Northeast China. BMC Vet. Res. 2018;14(1):321. doi: 10.1186/s12917-018-1634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang D., Wang J., Zhu S., She R., Ren X., Tian J., Quan R., Hou L., Li Z., Chu J., Guo Y., Xi Y., Song H., Yuan F., Wei L., Liu J. Induction of porcine dermatitis and nephropathy syndrome in piglets by infection with porcine circovirus type 3. J. Virol. 2019;93(4) doi: 10.1128/JVI.02045-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsias F., Bucafusco D., Nunez D.A., Lago Borisovsky L.A., Rodriguez M., Bratanich A.C. Genomic characterization of canine circovirus associated with fatal disease in dogs in South America. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekcharoensuk P., Morozov I., Paul P.S., Thangthumniyom N., Wajjawalku W., Meng X.J. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J. Virol. 2004;78(15):8135–8145. doi: 10.1128/JVI.78.15.8135-8145.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., He W., Zhu H., Bi Y., Wang R., Xing G., Zhang C., Zhou J., Yuen K.Y., Gao G.F., Su S. Origin, Genetic Diversity, and Evolutionary Dynamics of Novel Porcine Circovirus 3. Adv. Sci. Weinh. (Weinh) 2018;5(9) doi: 10.1002/advs.201800275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kapoor A., Slikas B., Bamidele O.S., Wang C., Shaukat S., Masroor M.A., Wilson M.L., Ndjango J.B., Peeters M., Gross-Camp N.D., Muller M.N., Hahn B.H., Wolfe N.D., Triki H., Bartkus J., Zaidi S.Z., Delwart E. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 2010;84(4):1674–1682. doi: 10.1128/JVI.02109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Shan T., Soji O.B., Alam M.M., Kunz T.H., Zaidi S.Z., Delwart E. Possible cross-species transmission of circoviruses and cycloviruses among farm animals. J. Gen. Virol. 2011;92(Pt 4):768–772. doi: 10.1099/vir.0.028704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q.Z., Guo K.K., Zhang Y.M. Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes. 2014;49(1):1–10. doi: 10.1007/s11262-014-1099-z. [DOI] [PubMed] [Google Scholar]
- Navarro R., Nair R., Peda A., Aung M.S., Ashwinie G.S., Gallagher C.A., Malik Y.S., Kobayashi N., Ghosh S. Molecular characterization of canine parvovirus and canine enteric coronavirus in diarrheic dogs on the island of St. Kitts: First report from the Caribbean region. Virus Res. 2017;240:154–160. doi: 10.1016/j.virusres.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T., Meng X.J., Halbur P.G. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 2007;19(6):591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- Palinski R., Pineyro P., Shang P., Yuan F., Guo R., Fang Y., Byers E., Hause B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017;91(1) doi: 10.1128/JVI.01879-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S., Su M., Guo D., Li C., Wei S., Feng L., Sun D. Molecular detection and phylogenetic analysis of porcine circovirus type 3 in 21 Provinces of China during 2015-2017. Transbound. Emerg. Dis. 2019;66(2):1004–1015. doi: 10.1111/tbed.13125. [DOI] [PubMed] [Google Scholar]
- Saraiva G.L., Vidigal P.M.P., Assao V.S., Fajardo M.L.M., Loreto A.N.S., Fietto J.L.R., Bressan G.C., Lobato Z.I.P., Almeida M.R., Silva-Junior A. Retrospective Detection and Genetic Characterization of Porcine circovirus 3 (PCV3) Strains identified between 2006 and 2007 in Brazil. Viruses. 2019;11(3) doi: 10.3390/v11030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J., Allan G.M., Domingo M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005;6(2):119–142. doi: 10.1079/ahr2005106. [DOI] [PubMed] [Google Scholar]
- Song T., Hao J., Zhang R., Tang M., Li W., Hui W., Fu Q., Wang C., Xin S., Zhang S., Rui P., Ren H., Ma Z. First detection and phylogenetic analysis of porcine circovirus type 2 in raccoon dogs. BMC Vet. Res. 2019;15(1):107. doi: 10.1186/s12917-019-1856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukmak M., Thanantong N., Poolperm P., Boonsoongnern A., Ratanavanichrojn N., Jirawattanapong P., Woonwong Y., Soda N., Kaminsonsakul T., Phuttapatimok S., Wajjwalku W. The retrospective identification and molecular epidemiology of porcine circovirus type 3 (PCV3) in swine in Thailand from 2006 to 2017. Transbound. Emerg. Dis. 2019;66(1):611–616. doi: 10.1111/tbed.13057. [DOI] [PubMed] [Google Scholar]
- Sun J., Wei L., Lu Z., Mi S., Bao F., Guo H., Tu C., Zhu Y., Gong W. Retrospective study of porcine circovirus 3 infection in China. Transbound. Emerg. Dis. 2018;65(3):607–613. doi: 10.1111/tbed.12853. [DOI] [PubMed] [Google Scholar]
- Thaiwong T., Wise A.G., Maes R.K., Mullaney T., Kiupel M. Canine Circovirus 1 (CaCV-1) and Canine Parvovirus 2 (CPV-2): Recurrent Dual Infections in a Papillon Breeding Colony. Vet. Pathol. 2016;53(6):1204–1209. doi: 10.1177/0300985816646430. [DOI] [PubMed] [Google Scholar]
- Walia R., Dardari R., Chaiyakul M., Czub M. Porcine circovirus-2 capsid protein induces cell death in PK15 cells. Virology. 2014;468–470:126–132. doi: 10.1016/j.virol.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Wang W., Sun W., Cao L., Zheng M., Zhu Y., Li W., Liu C., Zhuang X., Xing J., Lu H., Luo T., Jin N. An epidemiological investigation of porcine circovirus 3 infection in cattle in Shandong province, China. BMC Vet. Res. 2019;15(1):60. doi: 10.1186/s12917-019-1793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li W., Xu X., Wang W., He K., Fan H. Phylogenetic analysis of two goat-origin PCV2 isolates in China. Gene. 2018;651:57–61. doi: 10.1016/j.gene.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Wen S., Sun W., Li Z., Zhuang X., Zhao G., Xie C., Zheng M., Jing J., Xiao P., Wang M., Han J., Ren J., Liu H., Lu H., Jin N. The detection of porcine circovirus 3 in Guangxi, China. Transbound. Emerg. Dis. 2018;65(1):27–31. doi: 10.1111/tbed.12754. [DOI] [PubMed] [Google Scholar]
- Yao J., Qin Y., Zeng Y., Ouyang K., Chen Y., Huang W., Wei Z. Genetic analysis of porcine circovirus type 2 (PCV2) strains between 2002 and 2016 reveals PCV2 mutant predominating in porcine population in Guangxi. China. BMC veterinary research. 2019;15(1):118. doi: 10.1186/s12917-019-1859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S.L., Zhou X., Lin T., Zhang H., Wen X.H., Zhou X.R., Jia C.L., Tu D., Zhu X.L., Chen Q.L., Wei W.K., Lv D.H. Reappearance of buffalo-origin-like porcine circovirus type 2 strains in swine herds in southern China. New Microbes New Infect. 2017;17:98–100. doi: 10.1016/j.nmni.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu Z., Zou Y., Zhang N., Wang D., Tu D., Yang L., Deng Z., Yang Y., Jiang P., Wang N. First molecular detection of porcine circovirus type 3 in dogs in China. Virus Genes. 2018;54(1):140–144. doi: 10.1007/s11262-017-1509-0. [DOI] [PubMed] [Google Scholar]
- Zheng S., Shi J., Wu X., Peng Z., Xin C., Zhang L., Liu Y., Gao M., Xu S., Han H., Yu J., Sun W., Cong X., Li J., Wang J. Presence of Torque teno sus virus 1 and 2 in porcine circovirus 3-positive pigs. Transbound. Emerg. Dis. 2018;65(2):327–330. doi: 10.1111/tbed.12792. [DOI] [PubMed] [Google Scholar]
- Zhou L., Tang Q., Shi L., Kong M., Liang L., Mao Q., Bu B., Yao L., Zhao K., Cui S., Leal E. Full-length genomic characterization and molecular evolution of canine parvovirus in China. Virus Genes. 2016;52(3):411–416. doi: 10.1007/s11262-016-1309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.