Abstract
Individual hosts differ extensively in their competence for parasites, but traditional research has discounted this variation, partly because modeling such heterogeneity is difficult. This discounting has diminished as tools have improved and recognition has grown that some hosts, the extremely competent, can have exceptional impacts on disease dynamics. Most prominent among these hosts are the superspreaders, but other forms of extreme competence (EC) exist and others await discovery; each with potentially strong but distinct implications for disease emergence and spread. Here, we propose a framework for the study and discovery of EC, suitable for different host–parasite systems, which we hope enhances our understanding of how parasites circulate and evolve in host communities.
Keywords: disease, epidemic, infection, zoonosis
Highlights
A few members of host populations, so-called superspreaders, have disproportionate impacts on the risk of infectious disease emergence and spread.
Several other forms of EC exist; some of which might be exceptionally protective.
To discover and understand forms of EC, it is imperative to describe the distribution of, and covariation among, traits of individual hosts that mediate the many stages of host–parasite interactions.
Here, we provide a framework to do so, emphasizing how interplay among host traits related to parasite exposure behavior, susceptibility, replicability of parasites on/in hosts, and transmissibility, comprise host competence.
We hope this framework helps reveal new forms of EC and informs and improves management of disease risk.
Individual Hosts Contribute Unequally to Disease Dynamics
Most epidemiological theory has tended to discount intraspecific variation in host competence (see Glossary); the capacity for a host to cause an infection in another susceptible host or vector [1]. Only relatively circumscribed forms, such as variation among age classes or individuals with different levels of prior parasite exposure, have been well studied [2]. Heterogeneities in host competence have recently been recognized as integral to understanding and control of many outbreaks and epidemics 3, 4. In many systems, a small number of host individuals drive disease emergence and spread 5, 6. The best-known human example is Mary Mallon, a cook responsible for many cases of typhoid fever. Although superspreaders such as Mallon exist for many host–parasite combinations (Table 1 ), we still know little about what imbues some hosts with such EC [4]. Although extreme often connotes high in colloquial use, here we use its statistical form; both very high and very low values of competence constitute extreme in this paper. Presently, many forms of EC probably remain unknown, largely because of biases in the way we study many infections. This dearth of information potentiates a missed opportunity, as many diseases are most effectively controlled when these key hosts are targeted [6].
Table 1.
Examples of EC
| Type | Host species | Disease name and causative agenta | Parasite type | Primary route of transmission | Documented mechanisms | Relevant host traitsb | Strength of evidencec | Refs |
|---|---|---|---|---|---|---|---|---|
| Superspreader | Human | Severe acute respiratory syndrome (SARS)/SARS-Coronavirus (F: Coronaviridae, G: Betacoronavirus) | Virus | Direct contact (bodily fluids); indirect contact (aerosol, close-range) | High network centrality; comorbidity; high pathogen shedding | St, T | D | [6] |
| Superspreader | Human | Ebola virus disease/Ebola virus (F: Filoviridae, G: Ebolavirus) | Virus | Direct contact (blood, bodily fluids) | High network centrality (including postmortem); long infectious period | St, T | D | [10] |
| Superspreader | Human | Lassa hemorrhagic fever/Lassa virus (F: Arenaviridae, G: Arenavirus) | Virus | Direct contact (blood, bodily fluids) | High pathogen shedding | St, T | D | [6] |
| Superspreader | Human | Measles/rubeola virus (F: Paramyxoviridae, G: Morbillivirus) | Virus | Direct contact (respiratory fluids); indirect contact (aerosol, close-range) | High network centrality; high pathogen shedding; high exposure (travel) | E, St, T | D | [6] |
| Superspreader | Human | Rubella (German measles)/rubella virus (F: Togaviridae, G: Rubivirus) | Virus | Indirect contact (aerosol, close-range) | High network centrality (crowding event); high pathogen shedding | E, St, T | D | [6] |
| Superspreader | Human | Smallpox/variola virus (F: Poxviridae, G: Orthopoxvirus) | Virus | Direct contact (respiratory fluids); indirect contact (aerosol, close-range) | High exposure (travel); high network centrality (including postmortem); high pathogen shedding | E, St, T | D | [6] |
| Superspreader | Jackals (Canis mesomelas and Canis adustus) and Racoons (Procyon lotor) | Rabies/rabies virus (F: Rhabdoviridae, G: Lyssavirus) | Virus | Direct contact (saliva) | High connectivity between distant parts of contact network (nomads or dispersers) | T | H | [59] |
| Superspreader | Human | Typhoid fever/Salmonella enterica typhi | Bacterium | Fecal–oral contamination; direct contact | High pathogen shedding; high network centrality (food services); increased tolerance (subclinical carrier) | E, St, T | D | [60] |
| Superspreader | Human | Tuberculosis/Mycobacterium spp. | Bacterium | Indirect contact (aerosol, close-range) | High network centrality (crowding event); long infectious period | T | D | [61] |
| Superspreader | Human | Mycoplasmosis/Mycoplasma pneumonia | Bacterium | Direct contact (bodily fluids); indirect contact (aerosol, close-range) | High network centrality (crowding event); high pathogen shedding | St, T | D | [6] |
| Superspreader | Great Reed Warblers (Acrocephalus arundinaceus); house sparrows (Passer domesticus) | Avian malaria/Plasmodium spp. and Leucocytozoon spp. | Protozoan | Vector (Culex and Aedes mosquitoes) | Genetic markers associated with presence/absence of infection | S, St | H | [29] |
| Superspreader | Domestic dogs (Canis lupus familiaris) | Chagas disease/Trypanosoma cruzi | Protozoan | Vector (Triatominae bugs) | Coinfection with worms (reduced immune response) | St | I | [62] |
| Superspreader | Human sickle-cell gene carrier (humans); Experimental mice strains (Mus musculus) | Rodent malaria/Plasmodium berghei | Protozoan | Vector (Anopheles mosquitoes) | Increased tolerance (subclinical carrier); long infectious period | S, St | H | [63] |
| Supershedder | Zebra finches (Taeniopygia guttata) | West Nile Virus/West Nile virus (F: Flaviviridae, G: Flavivirus) | Virus | Vector (mosquitoes) | High pathogen shedding (attractiveness to vectors) | E, St | D | [18] |
| Supershedder | Human | Skin infections (boils, impetigo, toxic shock syndrome, etc.)/Staphylococcus aureus | Bacterium | Indirect contact (aerosol, close-range); direct contact | High pathogen shedding (increased air dispersal caused by rhinovirus coinfection) | St, T | I | [64] |
| Supershedder | Domestic cattle (Bos taurus) | Gut infections (colonic escherichiosis, etc.)/Escherichia coli O157 | Bacterium | Indirect contact (food consumption, fomites); direct contact | High pathogen shedding; genetic variation in host tissue and pathogen strain causes reduced immunity | St, T | D | [65] |
| Supershedder | Mice (Mus musculus) | Salmonellosis/Salmonella enterica | Bacterium | Fecal–oral contamination, indirect contact (food consumption) | Physiological (changes in intestinal microbiota) | St | D | [66] |
| Supershedder | Water buffalo (Bubalus bubalis) | Brucellosis/Brucella abortus | Bacterium | Direct contact; Indirect contact (food consumption, fomites) | High pathogen shedding | St, T | D | [67] |
| Supertransmitter | Human | HIV/AIDS/Human immunodeficiency virus (F: Retroviridae, G: Lentivirus) | Virus | Direct contact (sexual contact, bodily fluids) | High connectivity (increased no. of sexual interactions) | E, T | D | [68] |
| Superblocker | Crimson rosellas (Platycercus elegans) | Psittacine beak and feather disease/beak and feather disease virus (F: Circoviridae, G: Circovirus) | Virus | Direct contact; indirect contact (fomites); vertical transmission | Host genetic variation; genotype rarity predicts lower viral load | S, St | H | [69] |
| Superreceiver | Meerkats (Suricata suricatta) | Bovine tuberculosis/Mycobacterium bovis | Bacterium | Direct contact; indirect contact (aerosol) | High exposure (lower ranking individuals; grooming and aggression) | E, S | I | [70] |
| Superattractor; Superreceiver | Domestic dogs (Canis lupus familiaris) | Canine leishmaniasis/Leishmania chagasi | Protozoan | Vector (Lutzomyia longipalpis and flies) | High exposure (attractiveness to vectors) | E | D | [5] |
| Superspreader; Supershedder | Bank voles (Myodes glareolus) | Piroplasmosis (Babesiosis)/Babesia microti | Protozoan | Vector (Ixodes scapularis ticks) | Long infectious period; high pathogen shedding | St | D | [71] |
| Superattractor; Superreceiver | Human | Human malaria/Plasmodium spp. | Protozoan | Vector (Anopheles mosquitoes) | High exposure (attractiveness to vectors) | E | D | [72] |
| Superreceiver; superspreader | House finches (Haemorhous mexicanus) | Mycoplasmosis/Mycoplasma gallisepticum | Bacterium | Direct contact; Indirect contact (aerosols, close-range and fomites) | High network centrality (frequent common feeder use) | T | H | [73] |
Table entries were selected to demonstrate the diversity of forms of extreme competence across host–parasite systems and mechanisms and represent the strongest available examples of each host–parasite pair.
aF, Family; G, genus.
bE, exposure; S, susceptibility; St, suitability; T, transmissibility.
cD, direct evidence; H, hypothetical, EC inferred by authors of present paper; I, inferred, EC inferred by authors of original paper.
Here, we have three goals. First, we describe the ecological importance of individual host-level EC and summarize some known examples from the literature (Table 1). Superspreading is by far the most common form, but others exist and yet others seem to await discovery. Second, we propose a framework for studying and revealing new forms of EC (Figure 1 ), which we apply to two distinct infections: malaria and lung nematode infection. Finally, we highlight a few promising research paths (Boxes 1 and 2 ) to reveal how hosts become so competent; a complement to other recent efforts 7, 8. We anticipate that this framework will be of value because it links disease processes within and among individual hosts for most host–parasite systems [4].
Figure I.
A Few Examples of How Host Tolerance Could Contribute to Host Competence.
Figure 1.
Possible Forms and Mechanisms of Extreme Host Competence
For a Figure360 author presentation of Figure 1, see the figure legend at https://doi.org/10.1016/j.tree.2018.12.009
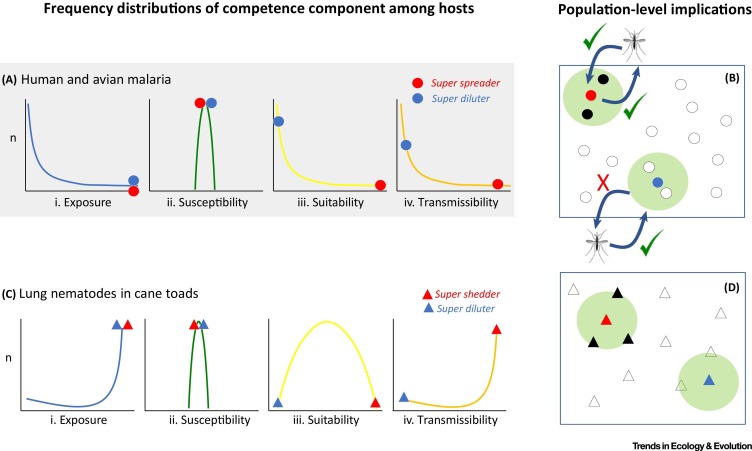
The four frequency distributions for two host–parasite interactions (A and C) depict variation among individual hosts in a population for: (i) exposure to parasites; (ii) susceptibility to parasites; (iii) suitability of a host for a parasite; and (iv) transmissibility of parasites once infected. The composite of these traits is host competence. Panel A depicts existing information on competence for human and avian malaria (Plasmodium and Haemoproteus). Exposure and transmissibility both depend on vector biting rates and are strongly right skewed in humans. By contrast, susceptibility is universally high. Data from a wild tropical avian community suggest that most infections are chronic with most individuals maintaining parasite burdens insufficient for transmission to vectors (i.e., low suitability). In panel A, a malaria (vector) superattractor has high exposure risk, but it is unknown whether such hosts tend to have high or low suitability and transmissibility and thus act as superspreaders or superdiluters. Red and blue circles denote traits of two different individuals in all four stages of the host–vector–parasite interaction. Panel B depicts that superattracting could have either superdiluting or superspreading consequences depending on relationships between traits within hosts. White-filled symbols depict uninfected hosts, black-filled symbols depict infected hosts, blue and red symbols reflect alternate forms of competence, and green-shaded circles reflect host impacts on local transmission. In panel C, frequency distributions reflect data from invasive populations of cane toads (Rhinella marina) and their lung nematodes (Rhabdias pseudosphaerocephala). Exposure rates are high, except at the leading edge of the geographic range of this host. Susceptibility is also high (100% success rates in experimental infections), yet suitability is variable with some hosts capable of clearing worms and others less so. Transmissibility is high for most hosts. Whether a host with a high burden has high transmissibility depends on parasite-mediated effects on factors determining the behavior during and duration of the period over which hosts shed parasites. Red and blue triangles denote traits of the two different individuals in all four stages of the host–parasite interaction. Panel D depicts the two possible outcomes of different trait combinations. White-filled symbols depict uninfected hosts, black-filled symbols depict infected hosts, blue and red symbols reflect alternate forms of competence, and green-shaded circles reflect local risk. Also see the supplemental information online regarding the supporting material for this figure.
Box 1. Parasite Tolerance and Host Competence.
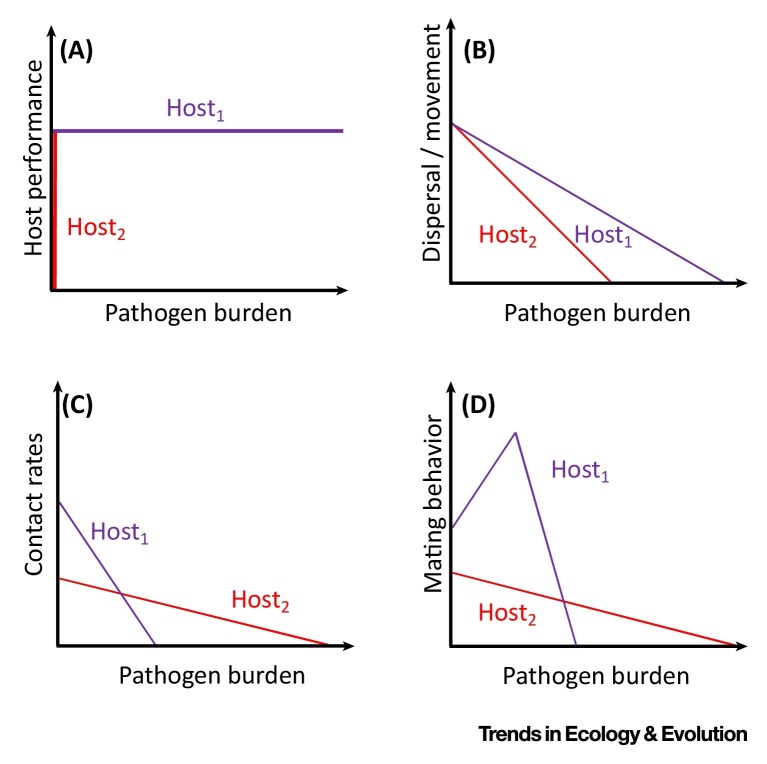
Parasite tolerance is a neglected aspect of host competence 14, 15, probably because it is such a new concept to disease ecology. Tolerance is typically quantified by plotting a reaction norm between host fitness, health or performance (y axis) and parasite burden (x axis). In this construct, a perfectly tolerant host will have zero slope (e.g., a reaction norm parallel to the x axis), whereas a completely intolerant host will have an infinite slope (i.e., its performance will be zero at any parasite burden; Figure I). Tolerance in a host population thus intervenes two extremes. The reaction norm framework produces at least three estimable parameters for quantification of competence: the intercept, slope, and area under the curve (AUC). The intercept captures host vigor in the absence of infection, the slope equates to host tolerance, and AUC represents the aggregate of host performance across the course of the infection, which could serve as a composite of suitability and transmissibility (Figure I).
Reaction norms are estimable for genotypes, individuals, or populations, but only with repeated measures of individuals across different parasite burdens can one definitively estimate the contribution of tolerance to competence as emphasized here. For instance, superspreaders should have the largest AUCs (Figure IA, Host1). In contrast, supersensitive hosts should have AUCs near zero; their performance declines abruptly at very low burden (Figure IA, Host2). Figure IB–D depicts other ways that tolerance could affect competence in a host population. In Figure IB, the reaction norm of dispersal is plotted for two host genotypes; Host1 has higher competence than Host2 because of higher tolerance and concomitantly greater AUC; it represents a supermover. In Figure IC, two hosts differ in tolerance and vigor. Host1 has higher performance than Host2 in the absence of infection, exposing it to greater parasite risk. However, the AUC for Host1 is also lower than that of Host2, making it a superattractor. Finally, in Figure ID, Host1 has higher performance than Host2 at the beginning of infection, with a positive reaction norm at early stages of infection followed by a sharp decline as parasite burden increases. In contrast, Host2 follows an average trajectory in the population. In this case, Host1 represents a superbreeder (terminal investor); its competence operates indirectly on a system by contributing new susceptible hosts, which could be more or less competent depending on inheritance of suitability or behavioral differences.
Alt-text: Box 1
Box 2. Behavioral and Physiological Mediators of EC.
A few papers have summarized how EC, and particularly superspreading, might manifest physiologically and behaviorally [4], but several factors have yet to receive much attention. Behaviorally, for vertically transmitted infections, higher rates of host reproduction, quicker maturation, or long breeding seasons could foster superspreading [42]. Migratory disposition [43] through selection of stopover and breeding sites by infected individuals as well as local movements could affect EC depending on habitat quality [44]. More nomadic individuals can be better spreaders of feline influenza virus [45] and distemper [46], but particularly far-ranging individuals could dilute risk if their movements take them away from conspecifics until they become noninfectious or die.
Physiological drivers of EC are also numerous, particularly those involving the endocrine, nervous, and immune systems [47]. One of the best known involves responses to stressors [48]. Variation in stress responses affect multiple aspects of competence in most taxa, but few studies have yet considered whether EC can arise via stress 18, 19, 49. Symbionts that live on and within hosts might also affect EC [50], as these organisms sculpt host immune functions and behavior 51, 52. A final understudied but putative driver of EC is mitochondrial function. Among other activities, mitochondria regulate innate immune activation, adaptive immune cell differentiation [53], and detection of viruses through pathogen recognition receptors [54]. Some viruses and Toxoplasma gondii can hijack mitochondria to modulate host apoptosis [55] or meet parasite nutrient needs [56]. Collectively, we expect that major advances in understanding EC will occur when host defenses are studied in an organismal manner 57, 58.
Alt-text: Box 2
Forms of EC and Their Biological Significance
Central to most mathematical models for infectious diseases are estimates of R0, which quantify the number of new infections generated by the average infected host in a wholly susceptible population [2]. Typically, the mean values of host traits within species are used to estimate R0 and individual heterogeneity is ignored. This lack of inclusion of heterogeneity might explain why simple epidemiological models have been unable to explain or predict the behavior of some outbreaks [9]. Recently, researchers have demonstrated the value of using individual-level variants of R0 [10], such as ν [6], to model disease dynamics. One particular advantage of ν is that it can be decomposed into three elements: infectiousness, contact rate, and infectious period [4]. This empirical tractability facilitates linkage of within- and between-host processes in traditional mathematical frameworks.
When the distribution of ν is described in a population, it is often non-normal with only a few individuals having very high ν 6, 10. This distribution (k) is not altogether surprising; in many host–parasite systems, we have long known that 20% of hosts are responsible for 80% of new infections [5]. What remains obscure, though, is whether ν (or other proxies of R0) capture the true extent of heterogeneity in host competence that resides in populations; in particular, functionally exceptional forms. Superspreaders are often conspicuous behaviorally or in terms of their high parasite burdens or shedding rates [10], but more cryptic forms of EC might not be revealed by typical approaches to estimating ν. For instance, ν does not explicitly take into account that host behavior sometimes changes, often dramatically, in the presence and absence of infection. Parasites commonly alter host behavior for their own interests. Host sickness behaviors too are highly variable among individuals; some hosts become lethargic and seek refugia when infected but others behave almost as if uninfected, and still others become more active [11]. Consider that asymptomatic carriers of human dengue virus are responsible for up to 88% of new infections [12]; behaviorally, these individuals are not conspicuous. Also, a key aspect of ν, infectiousness, is often inferred from the parasite burdens of hosts. This proxy is sometimes representative of true infectiousness, but oftentimes it is not. Many hosts are much less competent than their parasite burdens would suggest [13]. Some hosts tolerate infections well and shed many parasites in the right places and times to infect other hosts 14, 15, but others, even those with high burdens, generate few infections because of particular behaviors or high mortality postinfection [9].
In Table 1, we list several published examples of EC, which represent the most distinct and best-supported examples we could find. Superspreading, unsurprisingly, is the most commonly documented form. Most likely, its apparent commonness is related to sampling bias; its importance in some high-profile cases and its relative conspicuousness facilitate its discovery. Notably, though, what comprises superspreading can vary depending on parasite taxonomy, mode of transmission, and several other factors (Table 1). Superspreading is thus not a monolithic phenomenon; it can arise by multiple combinations of host exposure, susceptibility, suitability, and transmissibility. There are several other forms of EC in Table 1, including supershedding, super-receiving, superattracting, and superdiluting that have received little attention. These forms of EC have different implications for host populations; best demonstrated through examples. Some genetic variants of cattle (Bos taurus) deposit more Escherichia coli into feedlots than others, making them putative supershedders [16]. Supershedding, unlike superspreading, could make some terrestrial habitats enduring hotspots for infection [17]. In aquatic systems, supershedding near currents could enhance parasite dispersal opportunities, making some supershedders supermovers too. Superattractors and superreceivers are often cast as variants of superspreaders, but their functional roles will depend on covariation between traits affecting (at least) suitability and transmission. In one series of experiments, physiologically stressed, West Nile virus-infected zebra finches (Taeniopygia guttata) attracted twice as many vectors as controls and were infectious to vectors, whereas controls were not 18, 19. There, superattractors were also superspreaders, but in other species low WNV suitability coupled with high vector attractiveness could produce superdiluters. For contact-transmitted infections, forms of network centrality can drive superspreading or superblocking (Table 1), depending on the traits of hosts at these key nodes and how such traits affect between-ness versus degree [20].
A particular goal of this paper is to advocate for research on forms of EC that would make some hosts exceptionally protective against infection risk in communities [7]. Such superdiluters might be just as pivotal to understanding and managing disease dynamics as superspreaders, but tailored investigations will be required to identify them. Unlike superspreaders, one cannot identify superdiluters by measuring parasite burden or even obvious behaviors, which might be why we found no examples of them in the literature. The existence of such hosts, however, is plausible. For water-borne infections, as in the Daphnia–Metschnikowia (host–fungal parasite) system [21], hosts can act as superdiluters by (i) consuming parasites (as food; superconsumers) or (ii) competing with hosts with higher suitability or transmissibility (reducing contact rates with susceptible hosts; superblockers) [22]. There is already indirect evidence for superdiluting with regards to human malaria: a single human genetic mutation is associated with increased phagocytosis of malaria parasites, which could make some individual hosts highly susceptible but unlikely to reach parasitemia sufficient for transmission [23]. Some individual hosts might also dilute local infection risk by maintaining protective levels of antibodies or other forms of immunity for exceptionally long periods (i.e., superrecallers).
A final plausible example of nonobvious EC, worthy of study, are superevolvers. This type of EC might be particularly applicable to some viruses, such as influenza virus, which is transmitted directly through respiratory fluids and/or fecal matter and is capable of circulating in a wide range of mammalian and avian species [24]. Most human influenza infections tend to resolve over the course of days, but sometimes they are chronic. Such chronically infected hosts might sometimes be able to generate genetically unique forms of viruses [25]. Only recently have the tools become precise enough to reveal genetic differentiation of viruses within single hosts, and for influenza virus, as many as ten variants per individual human infection have been observed [26]. The high mutation rate of influenza virus (and perhaps other RNA viruses), as well as their capacity to share nucleic acids horizontally (when the same host cells are coinfected by different strains; [24]), could enable some hosts to facilitate parasite evolution. Although most such variants will be eliminated before transmission because of strong purifying selection, genetic drift, bottleneck effects, and elimination by the host immune system, some few could manifest new forms of virulence or propensities to infect novel host cell types (via sialic acid receptors). It is too soon to know whether superevolvers are common, much less if they impact global infection dynamics [25]. However, the propensities of many viruses (e.g., HIV and hepatitis C virus) to hide out in tissues [14], as well as the heterogeneities among hosts in their abilities to cope with viral infections [25], should instigate efforts to identify them.
Case Studies of EC
In Figure 1, we provide a framework for decomposing host competence into four components – exposure, susceptibility, suitability and transmissibility – that should be amenable to studying EC, including revealing novel forms of EC if they exist. Although these aspects of competence are coarse and multifaceted, just as with terms that comprise ν, they are amenable to description at the individual level and even decomposable to their physiological bases 7, 8. So as to ground our framework in familiar territory, we collected data and plotted frequency distributions of all four aspects of host competence for two different infections: malaria parasites and lung nematodes (Figure 1). In one system (lung nematodes) we used data from one host species (cane toads); for malaria, we produced a composite of available data from humans and wild birds. A complete set of information was unavailable for either host group alone. For both examples, we encourage caution in overinterpreting apparent patterns, as our intention is simply to demonstrate the promise of the framework. The critical next step will be to collect data on the four dimensions of competence for the same host individuals in several host populations.
Malaria
Figure 1A depicts the distribution of traits from human and avian malaria hosts based on published and unpublished data. Although humans and birds harbor distinct malaria species, the biology of both systems is similar. The rate of bites from infected vectors per host is a good proxy for malaria exposure risk [27]; some hosts are more conspicuous to or favored by vectors than others are (Figure 1Ai). Susceptibility, defined in this system as successful new infections relative to the number of vector bites per individual per unit time, ranges between 47% and 63% in humans [27], indicating that susceptibility is generally high (Figure 1Aii). Experimental inoculations reveal that, although the likelihood of an infection establishing is dose dependent, only extremely low doses are uninfective [28]. With regard to host suitability, a survey of a community of tropical birds (L. Peacock and R.H. Clarke, unpublished data) revealed a preponderance of low parasitemia representing chronic infections (Figure 1Aiii). Under these conditions, only the small proportion of individuals with high parasitemia are likely to infect biting vectors. We know that suitability varies depending on host genetic and resistance factors, including immune defenses [29] and other aspects of host physiology and life history [30]. There is also growing evidence of the importance of host tolerance (Box 1) [31]; host genotypes that affect blood cell turnover (e.g., sickle cell anemia and β-thalassemia) can extend host lifespan postinfection [32]. In terms of transmissibility (Figure 1Aiv), although humans with low parasitemia (based on visual analysis of blood smears) are thought to sustain infections, the relationship between parasitemia and transmissibility is complex. For instance, high parasitemia often increases transmission probability, but exceptionally high parasitemia can negatively impact vector lifespans [33], meaning that intermediate parasite burden might be most transmissible. Malaria parasites can increase host CO2 and volatile compound output [34], making them more conspicuous to vectors. Altogether for malaria, then, the most competent host would be one that is attractive to infected vectors when uninfected, attractive to uninfected vectors when infectious, and able to generate and tolerate a high enough parasite burden that parasites are taken up in vector bites. In Figure 1B, we emphasize how one form of EC, superattracting, could lead to superspreading or superdiluting, depending on how attractiveness to vectors relates to suitability and transmissibility.
Lung Nematode Infection
Nematodes either have a free-living life stage before infecting a definitive host species, or they require both intermediate and definitive hosts to complete their life cycles. Nematodes (and macroparasites generally) do not replicate on or in their hosts; burden is increased by exposure and decreased immunologically or through grooming. In Figure 1C, we plotted the distributions of aspects of host competence among introduced Australian cane toads and the lung nematode, Rhabdias pseudosphaerocephala [35]. Parasite exposure is high for most individual hosts (Figure 1Ci), especially when toads congregate seasonally around dwindling water supplies [36]. Most toads can also be readily infected (experimentally) with lung nematodes [37], suggesting that susceptibility is generally high (Figure 1Cii). In the case of suitability (Figure 1Ciii), toads differ in their capacity to maintain infections [37]. Finally, transmission is highly dependent on host density [38]; hosts from low-density areas (i.e., range edges) have low infection rates but hosts in the range core have high prevalence (Figure 1Civ). Parasite-driven changes in behavior postinfection can further impinge on transmissibility; lung nematodes can increase host body temperature and moisture content of feces as well as choice of defecation sites [39]. This toad–nematode system presents an opportunity to discern whether high parasite burden equates to supershedding or superdiluting. Many occurrences of supershedding are known for other macroparasites and parasites generally (Table 1). In Figure 1D, we highlight how the within-individual relationship between suitability and transmissibility might lead to different forms of EC in toad hosts.
Concluding Remarks
The literature is replete with examples of superspreading and their ecological and evolutionary ramifications [40]. However, there are data to implicate other forms of EC; many of which will have distinct consequences for epidemic risk and dynamics [41]. Going forward, perhaps the greatest need is to describe trait distributions for host competence in populations, including description of what covariation structure exists among traits within individuals [3]. We might aspire to refine our lexicon as well, asking whether it is effective to recognize both individuals and species as EC when the former is a nested element of the latter. As the components and consequences of EC within species vary, we think it sensible to study EC at the level of individuals, scaling up to species-level composites when appropriate (see Outstanding Questions). We might also resolve whether the prefix ‘super’ connotes the form of EC that individuals manifest or the effects that such hosts have on their communities. Finally, for many human diseases (including zoonoses), we have artificial mechanisms (e.g., education, prophylaxis, and institutional and social forms of hygiene) to control some infections. These conditions could make efforts to exploit knowledge about ECs difficult but often worthwhile to implement.
Outstanding Questions.
Syndromes between host physiology and behavior are well known, but multiple aspects of host competence (Figure 1) are rarely quantified in the same individuals. Descriptions of trait distributions in host populations (Figure 1), and covariance thereof, would resolve whether ‘super’ refers to the magnitude of effects of a particular individual on a system, its relative rarity, or both.
We lack viable proxies of EC, but the use of experimental, laboratory organisms could provide some tools for difficult-to-study wild-animal and human systems (e.g., infection duration, exposure risk, and transmission success). Some forms of EC (e.g., superdiluters) warrant attention in laboratory studies, as field methods tend not to be amenable to their discovery. Laboratory studies are also more likely to reveal how different exposure doses and route of transmissions impinge on EC.
Dose dependency of susceptibility and parasitemia transmission thresholds warrant more experimental attention, as they will modify multiple aspects of host competence.
EC variants affect communities differently depending upon niche and spatiotemporal overlap among hosts, parasite and vectors, and the quality of environments in which interactions occur. Studies on the context dependency of EC will be critical.
The advent of tools such as therapeutic interfering particles, which inhibit the growth of pathogens [59], have shown promising signs for lowering HIV/AIDS prevalence more effectively than vaccines or drugs alone. These tools might be impactful, as they directly target traits of EC hosts.
This paper emphasizes hosts, but EC in vectors and parasites also warrants investigation. Clearly, parasite and vector strains differ in virulence, infectivity, and even mutability, but how much individual heterogeneity in these traits exists is little known.
Glossary
- Competence
the propensity of one host to cause an infection in another host or vector.
- Exposure
the contribution of individual host behavioral, ecological and life history traits that affect contact with an infected host, vector, or environment.
- Infectiousness
the propensity for an infected individual to enter the transmissibility phase of competence including relapses during chronic infections.
- R0
the number of new infections generated by the average infected host in a wholly susceptible population.
- Resistance
the ability of a host to limit parasite burden after exposure to a parasite.
- Suitability
the propensity of a host to permit parasite survival long enough for the parasite to produce viable offspring, including the number of such offspring.
- Superattractor
a more conspicuous and/or less behaviorally defended host (to vectors).
- Superblocker
a host with high connectivity, but low suitability, in a social network context.
- Superbreeder
a host that makes strong terminal investments upon parasite exposure or infection.
- Superconsumer
a host that consumes and digests many parasites or vectors.
- Superdiluter
a host that greatly reduces risk of infection for other hosts.
- Superevolver
a host that facilitates transmissible evolutionarily change in a parasite.
- Supermover
a host that moves a parasite across a large physical distance.
- Superrecaller
an individual host that remains protected against reinfection for a long period post-parasite exposure and recovery.
- Superreceiver
a host having a high number of contacts with infected hosts, vectors, or sites in an environment; functionally, resembles a superattractor but is agnostic in regard to mode of transmission.
- Supershedder
a host that passes a large parasite burden into the environment.
- Superspreader
a host that greatly increases risk of infection for other hosts.
- Susceptibility
the propensity of a host to become infected upon exposure to a parasite.
- Tolerance
the relationship between parasite burden and host fitness, performance or health (Box 1).
- Transmissibility
the propensity of a host to transfer parasites to another susceptible host or vector, including the sensitivity of said host to manipulative effects of parasites.
- ν
individual reproductive number, as opposed to the average reproductive number in R0, for an infection.
Footnotes
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tree.2018.12.009.
Supplemental Information
The following is Supplemental information to this article:
References
- 1.Gervasi S.S. The context of host competence: a role for plasticity in host–parasite dynamics. Trends Parasitol. 2015;31:419–425. doi: 10.1016/j.pt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heesterbeek H. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347 doi: 10.1126/science.aaa4339. aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez-Prokopec G.M. Coupled heterogeneities and their impact on parasite transmission and control. Trends Parasitol. 2016;32:356–367. doi: 10.1016/j.pt.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanderWaal K.L., Ezenwa V.O. Heterogeneity in pathogen transmission: mechanisms and methodology. Functional Ecol. 2016;30:1606–1622. [Google Scholar]
- 5.Woolhouse M.E.J. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. U. S. A. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Smith J.O. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron D. Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Curr. Opin. Behav. Sci. 2015;6:35–40. [Google Scholar]
- 8.Martin L.B. Host competence: an organismal trait to integrate immunology and epidemiology. Integr. Comp. Biol. 2016;56:1225–1237. doi: 10.1093/icb/icw064. [DOI] [PubMed] [Google Scholar]
- 9.Handel A., Rohani P. Crossing the scale from within-host infection dynamics to between-host transmission fitness: a discussion of current assumptions and knowledge. Phil. Trans. R. Soc. B. 2015;370 doi: 10.1098/rstb.2014.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau M.S.Y. Spatial and temporal dynamics of superspreading events in the 2014–2015 West Africa Ebola epidemic. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2337–2342. doi: 10.1073/pnas.1614595114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelman J., Martin L. Vertebrate sickness behavior: an adaptive and integrated neuroendocrine immune response. Integr. Comp. Biol. 2009;49:202–214. doi: 10.1093/icb/icp028. [DOI] [PubMed] [Google Scholar]
- 12.Quirine A. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han B.A. Host species composition influences infection severity among amphibians in the absence of spillover transmission. Ecol. Evol. 2015;5:1432–1439. doi: 10.1002/ece3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adelman J.S., Hawley D.M. Tolerance of infection: a role for animal behavior, potential immune mechanisms, and consequences for parasite transmission. Horm. Behav. 2017;88:79–86. doi: 10.1016/j.yhbeh.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Burgan S.C., Gervasi S.S., Johnson L.R., Martin L.B. How individual variation in host tolerance affects host competence to transmit parasites. Physiol. Biochem. Zool. 2019;92:49–57. doi: 10.1086/701169. [DOI] [PubMed] [Google Scholar]
- 16.Stein R.A., Katz D.E. Escherichia coli, cattle and the propagation of disease. FEMS Microbiol. Lett. 2017;364 doi: 10.1093/femsle/fnx050. fnx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paull S.H. From superspreaders to disease hotspots: linking transmission across hosts and space. Front. Ecol. Environ. 2011;10:75–82. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gervasi S.S. Stress hormones predict a host superspreader phenotype in the West Nile virus system. Proc. R. Soc. B. 2017 doi: 10.1098/rspb.2017.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gervasi S.S. Host stress hormones alter vector feeding preferences, success, and productivity. Proc. R. Soc. B. 2016 doi: 10.1098/rspb.2016.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWaal K.L. Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis) J. Anim. Ecol. 2014;83:406–414. doi: 10.1111/1365-2656.12137. [DOI] [PubMed] [Google Scholar]
- 21.Cáceres C.E. Physical structure of lakes constrains epidemics in Daphnia populations. Ecology. 2006;87:1438–1444. doi: 10.1890/0012-9658(2006)87[1438:psolce]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Strauss A.T. Linking host traits, interactions with competitors and disease: mechanistic foundations for disease dilution. Funct. Ecol. 2018;32:1271–1279. [Google Scholar]
- 23.Willcocks L.C. A defunctioning polymorphism in FCGR2 B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostafa A. Zoonotic potential of influenza A viruses: a comprehensive overview. Viruses. 2018;10:497. doi: 10.3390/v10090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue K.S. Within-host evolution of human influenza virus. Trends Microbiol. 2018;26:781–793. doi: 10.1016/j.tim.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue K.S. Parallel evolution of influenza across multiple spatiotemporal scales. Elife. 2017;6 doi: 10.7554/eLife.26875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D.L. A quantitative analysis of transmission efficiency versus intensity for malaria. Nat. Commun. 2010;1:108. doi: 10.1038/ncomms1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindblade K.A. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev. Anti Infect. Ther. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 29.Bonneaud C. Major histocompatibility alleles associated with local resistance to malaria in a passerine. Evolution. 2006;60:383–389. [PubMed] [Google Scholar]
- 30.Samuel M.D. The dynamics, transmission, and population impacts of avian malaria in native Hawaiian birds: a modeling approach. Ecol. Appl. 2011;21:2960–2973. [Google Scholar]
- 31.Boutlis C.S. Malaria tolerance − for whom the cell tolls? Trends Parasitol. 2006;22:371–377. doi: 10.1016/j.pt.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong L. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar. J. 2013;12:317. doi: 10.1186/1475-2875-12-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawes E.J. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malar. J. 2009;8 doi: 10.1186/1475-2875-8-228. 228–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson A. Plasmodium-associated changes in human odor attract mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E4209–E4218. doi: 10.1073/pnas.1721610115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubey S., Shine R. Origin of the parasites of an invading species, the Australian cane toad (Bufo marinus): are the lungworms Australian or American? Mol. Ecol. 2008;17:4418–4424. doi: 10.1111/j.1365-294X.2008.03922.x. [DOI] [PubMed] [Google Scholar]
- 36.Tingley R., Shine R. Desiccation risk drives the spatial ecology of an invasive anuran (Rhinella marina) in the Australian semi-desert. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelehear C. Rhabdias pseudosphaerocephala infection in Bufo marinus: lung nematodes reduce viability of metamorph cane toads. Parasitology. 2009;136:919–927. doi: 10.1017/S0031182009006325. [DOI] [PubMed] [Google Scholar]
- 38.Phillips B.L. Parasites and pathogens lag behind their host during periods of host range advance. Ecology. 2010;91:872–881. doi: 10.1890/09-0530.1. [DOI] [PubMed] [Google Scholar]
- 39.Finnerty P.B. Survival of the feces: does a nematode lungworm adaptively manipulate the behavior of its cane toad host? Ecol. Evol. 2018;8:4606–4618. doi: 10.1002/ece3.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modlmeier A.P. The keystone individual concept: an ecological and evolutionary overview. Anim. Behav. 2014;89:53–62. [Google Scholar]
- 41.Gopinath S. Role of disease-associated tolerance in infectious superspreaders. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15780–15785. doi: 10.1073/pnas.1409968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fedorka K.M. Reproductive and immune system interactions in the context of life history and sexual selection theory. In: Malagoli D., Ottaviani E., editors. Eco-immunology: Evolutive Aspects and Future Perspectives. Springer; 2014. pp. 49–72. [Google Scholar]
- 43.Fritzsche McKay A., Hoye B.J. Are migratory animals superspreaders of infection? Integr. Comp. Biol. 2016;56:260–267. doi: 10.1093/icb/icw054. [DOI] [PubMed] [Google Scholar]
- 44.Beldomenico P.M., Begon M. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 2010;25:21–27. doi: 10.1016/j.tree.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Fountain-Jones N.M. Urban landscapes can change virus gene flow and evolution in a fragmentation-sensitive carnivore. Mol. Ecol. 2017;26:6487–6498. doi: 10.1111/mec.14375. [DOI] [PubMed] [Google Scholar]
- 46.Craft M.E. Disease transmission in territorial populations: the small-world network of Serengeti lions. J. R. Soc. Interface. 2011;8:776–786. doi: 10.1098/rsif.2010.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashley N.T., Demas G.E. Neuroendocrine-immune circuits, phenotypes, and interactions. Horm. Behav. 2017;87:25–34. doi: 10.1016/j.yhbeh.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin L.B. Stress and immunity in wild vertebrates: timing is everything. Gen. Comp. Endocrinol. 2009;163:70–76. doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Murone J. Exposure to corticosterone affects host resistance, but not tolerance, to an emerging fungal pathogen. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson P.T. Why infectious disease research needs community ecology. Science. 2015;349 doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osborne L.C. Virus–helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345:578–582. doi: 10.1126/science.1256942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biard C. Interpreting immunological indices: the importance of taking parasite community into account: An example in blackbirds Turdus merula. Methods Ecol. Evol. 2015;6:960–972. [Google Scholar]
- 53.Weinberg S.E. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koshiba T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta. 2013;1833:225–232. doi: 10.1016/j.bbamcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Neumann S. How do viruses control mitochondria-mediated apoptosis? Virus Res. 2015;209:45–55. doi: 10.1016/j.virusres.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawford M.J. Toxoplasma gondii scavenges host‐derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 2006;25:3214–3222. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tauber A.I. Oxford University Press; 2017. Immunity: The Evolution of an Idea. [Google Scholar]
- 58.Schmid Hempel P. Oxford University Press; 2011. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. [Google Scholar]
- 59.Russell C.A. Spatial control of rabies on heterogeneous landscapes. PLoS One. 2006;1 doi: 10.1371/journal.pone.0000027. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dougan G., Baker S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol. 2014;68:317–336. doi: 10.1146/annurev-micro-091313-103739. [DOI] [PubMed] [Google Scholar]
- 61.Curtis A.B. Extensive transmission of Mycobacterium tuberculosis from a child. N. Engl. J. Med. 1999;341:1491–1495. doi: 10.1056/NEJM199911113412002. [DOI] [PubMed] [Google Scholar]
- 62.Enriquez G.F. Is the infectiousness of dogs naturally infected with Trypanosoma cruzi associated with poly-parasitism? Vet. Parasitol. 2016;223:186–194. doi: 10.1016/j.vetpar.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira A. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 64.Bassetti S. Dispersal of Staphylococcus aureus into the air associated with a rhinovirus infection. Infect. Control Hosp. Epidemiol. 2005;26:196–203. doi: 10.1086/502526. [DOI] [PubMed] [Google Scholar]
- 65.Wang O. Host mechanisms involved in cattle Escherichia coli O157 shedding: a fundamental understanding for reducing foodborne pathogen in food animal production. Sci. Rep. 2017;7:7630. doi: 10.1038/s41598-017-06737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawley T.D. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capparelli R. Heterogeneous shedding of Brucella abortus in milk and its effect on the control of animal brucellosis. J. Appl. Microbiol. 2009;106:2041–2047. doi: 10.1111/j.1365-2672.2009.04177.x. [DOI] [PubMed] [Google Scholar]
- 68.Poundstone K.E. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol. Rev. 2004;26:22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 69.Eastwood J.R. Host heterozygosity and genotype rarity affect viral dynamics in an avian subspecies complex. Sci. Rep. 2017;7:13310. doi: 10.1038/s41598-017-13476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drewe J.A. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc. Biol. Sci. 2010;277:633–642. doi: 10.1098/rspb.2009.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes V.L., Randolph S.E. Testosterone increases the transmission potential of tick-borne parasites. Parasitology. 2001;123:365–371. doi: 10.1017/s0031182001008599. [DOI] [PubMed] [Google Scholar]
- 72.Guelbéogo W.M. Variation in natural exposure to anopheles mosquitoes and its effects on malaria transmission. eLife. 2018;7 doi: 10.7554/eLife.32625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adelman J.S. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. Biol. Soc. 2015;282 doi: 10.1098/rspb.2015.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure360: an author presentation of Figure 1