Abstract
The guidelines in this article provide veterinarians, veterinary technicians, and veterinary health care workers with an overview of evidence-based recommendations for the best practices associated with environmental cleaning and disinfection of a veterinary clinic that deals with small animals. Hospital-associated infections and the control and prevention programs necessary to alleviate them are addressed from an environmental perspective. Measures of hospital cleaning and disinfection include understanding mechanisms and types of contamination in veterinary settings, recognizing areas of potential concern, addressing appropriate decontamination techniques and selection of disinfectants, the management of potentially contaminated equipment, laundry, and waste management, and environmental surveillance strategies.
Keywords: Small animal, Veterinary clinic, Environmental contamination, Infection prevention, Surveillance
Key points
-
•
As in human medicine, hospital-associated infections (HAIs) exist in veterinary medicine and must be subject to control measures.
-
•
Environmental contamination with pathogens of concern is widespread in veterinary hospitals and should be an important target of proactive measures to prevent (limit) HAI.
-
•
For environmental cleaning and disinfection (C/D) to be effective, all stakeholders should be educated as to the need for appropriate C/D and the participation of all (at any level) should be encouraged to accomplish this goal.
-
•
Veterinary practices should seriously consider identifying personnel responsible for establishing infection control practices, establishing monitoring/audit procedures, and determining whether their practice situation warrants proactive environmental surveillance.
-
•
More research is required to identify the precise relationship between environmental contamination and HAI and to establish control and surveillance/monitoring procedures of direct relevance to veterinary medicine.
Background
The concept of infection control and prevention in veterinary medicine outside the surgical suite or epidemic disease control/eradication in livestock populations was more or less unheard of until the last 1 or 2 decades. During that time, there has been a paradigm shift, such that veterinary infection control is a growing discipline that is becoming part of the customary way in which veterinarians practice medicine. This shift is seen primarily in large academic teaching hospitals associated with specific veterinary schools and in specialty clinics dedicated to advanced diagnostics and care for animals. However, the nature of medicine and mission of veterinary hospitals are such that animals clinically affected by the agents that have the potential to spread among the hospital population, as well as subclinical carriers that may go unrecognized, are always likely to be present in veterinary medical facilities, regardless of size or specialty. The standard of care at every veterinary hospital should include a high level of hygiene, awareness of the dangers of transfer of infectious agents between both animals and people, and procedures to reduce infection risk wherever possible. Such infection control procedures are intended to prevent (limit) introduction and spread of infectious diseases within a group of patients and their human caregivers, thereby, protecting human, animal, and environmental health against biological threats. This article provides an overview of environmental considerations in infection control rather than an exhaustive review. There are numerous excellent resources that cover various aspects in greater detail, many of which are referenced in the following sections.
Health care–associated infections in human medicine
Nosocomial infection, otherwise known as hospital-acquired or more recently health care–associated infections (HAIs), are the subject of high-profile press coverage and government or internal regulation in human medicine. The latest published figures from human medicine in the United States suggest that in 2011, 722,000 patients contracted HAI in acute-care hospitals, more than half of which were acquired outside the intensive care unit (ICU).1 These infections resulted in 75,000 deaths and constitute the seventh leading cause of death in the United States.2 Although they may be artificial constructs, when these numbers are averaged out over time, they indicate that on any given day in the United States, 1 in 25 patients has at least 1 HAI, and every day of the year, 205 people die from HAIs. As shocking as these figures are, stringent control efforts instituted over the last 2 to 3 decades in human medicine, which were formalized in 2008,3, 4 seem responsible for an apparent decline in rates of HAI compared with the 1970s to 1990s, during which approximately 2 million HAIs were estimated to occur each year and were, in turn, associated with 100,000 annual deaths.2, 3, 4 In 2011, the most common HAIs included central line–associated bloodstream infections (54,500), catheter-associated urinary tract infections (30,100), surgical site infections (53,700), and Clostridium difficile infections (107,700).1, 2 The pathogens principally associated with these infections include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), Clostridium difficile, Acinetobacter, norovirus, and most recently, carbapenemase producing enterobactericeae.2 HAIs are estimated to account for $40 billion in excess health care costs each year.5
Environmental contamination in human hospitals
In the early 1990s, initial estimates of the sources of HAI among adult ICU patients suggested that endogenous microbiota accounted for 40% to 60% of infections, cross-infection from the hands of health care personnel for 20% to 40%, changes in the microbiota driven by antimicrobial drug use for 20% to 25%, and other factors, such as environmental contamination, for 20%.6 In the interim, much compelling evidence has accumulated to confirm an important role for the environment in pathogen transmission.7 Surfaces in the room of a patient colonized/infected with a hospital pathogen are frequently contaminated, pathogens can remain viable on hospital surfaces and equipment for extended periods, the hands, gloves, and other apparel of health care personnel are readily contaminated after being in contact with a contaminated environment, a person admitted to a room previously occupied by a patient colonized/infected with a hospital pathogen has an increased likelihood of being colonized/infected themselves, and improvements in terminal cleaning and disinfection (C/D) lead to decreased rates of infection.7, 8, 9 The organisms for which data implicating a role for environmental contamination are strongest include norovirus, Clostridium difficile, VRE, Acinetobacter spp, MRSA, and Pseudomonas aeruginosa.7, 9 It should be obvious that the list of organisms with the strongest links to environmental contamination is essentially the same as that for pathogens most closely related to HAI.
Health care–associated infections in veterinary medicine
In veterinary medicine, specific numbers and incidence of HAI are not so well documented, but 2 recently published studies10, 11 sought to estimate the occurrence of HAI using a standardized syndromic surveillance system in 2 clinical settings over a 12-week period at 5 veterinary teaching hospitals (VTHs); first, in hospitalized horses admitted for gastrointestinal disorders and second, in small animals in the critical care unit. Although there was variability between hospitals, when these differences were controlled for, of the 297 horses in the study population, 19.7% (95% confidence interval [CI], 14.5–26.7) were reported to have had at least 1 nosocomial event during hospitalization. Equivalent proportions for 1535 dogs were 16.3% (95% CI, 14.3–18.5), and of 416 cats, 12% (95% CI, 9.3–15.5) had at least 1 nosocomial event. In both horses and small animals, the most commonly reported syndrome was surgical site inflammation, with intravenous catheter site inflammation and urinary tract inflammation being the second most common in horses and small animals, respectively. In addition, in a published survey of biosecurity experts at 38 VTHs,12 31 (82%) hospitals reported the occurrence of a nosocomial disease outbreak in the 5 years before the survey interview. Although most of these outbreaks were associated with large animal facilities, there are also published reports of HAIs and outbreaks in small animal clinics.13, 14, 15, 16, 17, 18 There is more than enough evidence to indicate that, as in human medicine, HAIs in veterinary hospitals are a part of the world in which we live. Although certain issues, the principal pathogens, and thus the control measures may differ, it is nonsensical to imagine that reality for veterinary medicine is any different from human medicine, in that HAIs exist and must be subject to control measures.
Environmental contamination in veterinary hospitals
Many of the pathogens associated with HAI in humans are found in VTHs and can cause infections in animals. However, just as data on veterinary HAI are not readily available, the relationship of environmental contamination with most of these pathogens to HAI is not well defined. Nevertheless, as with human medicine, the link between HAIs and the environment of veterinary clinics is becoming more clear. There are numerous descriptions of environmental contamination associated with HAI in large animal hospitals,19, 20, 21, 22, 23 and at the University of Pennsylvania’s George D. Widener Hospital for Large Animals, where we conduct routine environmental surveillance for Salmonella, we have many examples directly correlating infection in animals (both community associated and HAI) with environmental contamination (Dr H. Aceto, unpublished observations, 2004). In addition, recently documented MRSA events in veterinary settings showed that environmental contamination ranged from 1% to 12%,24, 25, 26, 27 and there are reports of contamination of the environment and equipment in small animal hospitals with enterococci many of which were multidrug resistant (MDR).28, 29 One of these studies28 investigated the hypothesis that cage doors, stethoscopes, thermometers, and mouth gags used in participating hospitals would have bacterial contamination that could contribute to HAI. This investigation was accomplished by determining the prevalence of surface contamination with enterococci at 10 different veterinary hospitals. Because the locations within the hospitals that were sampled had direct patient contact, it is perhaps not surprising that they all yielded enterococci at 1 or more hospitals. To compare cleaning protocols with bacterial contamination, a veterinarian at each hospital was asked to complete a questionnaire. Results showed that only half of the hospitals had written standard operating procedures for hospital cleaning, and the wide variety of disinfectants used precluded examination of any relationship between cleaning and contamination. Moreover, 5 of 10 veterinarians surveyed reported almost never or never cleaning their stethoscopes, and there were also deficiencies in the cleaning of cage doors, thermometers, and mouth gags at some hospitals.
Despite the relative paucity of data, it should be apparent that veterinary hospitals are inherently contaminated and that any number of bacterial, viral, or fungal organisms may be harbored in the hospital environs. The nature of animals and the challenges they pose in terms of hygiene and containment almost guarantee contamination of a space. The range of species that may require hospitalization is large and varied, and each species may have distinct flora and different susceptibility risks.
As our understanding of HAI in veterinary medicine increases, medical staff and administrators alike are coming to realize the acute threat that HAIs pose to hospitalized veterinary patients and are looking toward proactive preventive measures rather than reactive damage control. The additional financial burden (eg, increased length of hospital stay, increased treatment costs, possible indemnification and legal costs, loss of future business) that HAI in general and outbreaks in particular can impose on a hospital is undoubtedly another motivating factor.19, 23, 30 The fact that many of the pathogens of importance to the health of hospitalized animals are also zoonotic is an equally important consideration, and all infection control programs should include measures to protect human health.31 In common with human medicine, it is becoming clear that the hospital environment is an important target of these proactive measures.
Importance of facility design
The physical environment can affect many facets of veterinary care, including patient comfort, patient stress, patient and staff safety, staff effectiveness, quality of care, and patient susceptibility to disease. Effective C/D are critical in preventing transmission of infectious agents between patients or from contaminated environments. To aid in this process, it is desirable that surfaces in animal housing and clinical spaces are cleanable and nonporous. This strategy can be as simple as ensuring that wood surfaces are properly sealed and painted or more complex by eliminating furniture and finishes that are not easily disinfected or, in the worst case, those that are essentially not cleanable by standard C/D methods. Furniture and finishes may include carpeting, upholstery, and unfinished or damaged wood surfaces. In a veterinary hospital, in which fecal material and respiratory or other secretions are abundant, frequently defy containment, are more likely to harbor pathogens, and may represent an HAI risk, cleanliness of treatment/procedures spaces, animal housing, and operating rooms is crucial. This necessity is particularly true in critical care and isolation units, where exacting evaluation of all surfaces, construction materials, and equipment is essential to maximize cleanability, environmental safety, and efficient operation of the area. Such considerations should influence the choice of materials and finishes for all kinds of surfaces (eg, floors, walls, benches and work surfaces, doors and frames); solid, nonporous surface materials that are highly durable and with as few seams as possible are best. For example, in personnel areas, such as nursing stations, seamless, highly cleanable and exceptionally durable poured epoxy floors, although more expensive, are preferable to materials such as vinyl tile. Wherever seams are present, regular maintenance to ensure their integrity or that of other interfaces between surfaces is essential. Block walls should be sealed, with urethane-based paints, for example. Although metal doors and frames are nonporous and may be the ideal, some composites are also suitable. Any wood surfaces must be properly sealed and painted. For all materials, regular inspection and maintenance are a must. To facilitate cleaning, there should be adequate provision of drains. Drains should be connected to the sanitary system, and, to limit future disruption for the conduct of work to replace corroded drains, use of stainless steel drain hubs should be considered to prolong the life of the drain in the face of water, cleaners, disinfectants, and animal urine. The choice of heating and ventilation systems and the location of heaters and air vents are all critical components in facility design. Although this factor may be most obvious for the control of pathogens classically considered to be transmitted via the airborne route, it may be less obvious that organisms normally thought of as fecal-oral can be greatly impacted by heating, ventilation, and air conditioning (HVAC) systems. Impacts can be both in terms of contaminating the system and in terms of the system facilitating pathogen spread via, for example, forced hot air systems that circulate contaminated particulates widely throughout a given space. It is imperative that engineers with knowledge about airborne transmission be involved in the design of HVAC systems for veterinary facilities, particularly isolation units.
Frequently unconsidered surfaces, such as in the vicinity of nursing stations, doorknobs and hardware, light switches, telephones, computers and their keyboards, are also becoming recognized hot spots of contamination.19, 22, 32, 33 When a hospital environment is not easy to both clean and disinfect, the potential for HAI increases, so critical evaluation of the hospital environment is essential.
Intentionally planning a space for improved safety and hygiene is an important concept in human medicine and an easy, although not necessarily inexpensive, idea to adapt to veterinary hospitals. Infection control and ease of disinfection should be essential considerations in newly designed veterinary facilities or those undergoing renovation. Hospital improvements must always consider the role that design plays in infection prevention. The architects and designers hired to plan a new health care facility or renovate current facilities are crucial in establishing design features that improve hospital safety and in identifying and using appropriate surfaces and substrates.34 The Center for Health Design’s internationally recognized Evidence-based Design Accreditation and Certification35 program awards credentials to individuals who show a thorough understanding of how to apply an evidence-based process to the design and development of health care settings. Evidence-based design is the process of basing decisions about the built environment on credible research to achieve the best possible outcomes; to this end, it also includes measuring and reporting results.
General concepts in environmental cleaning and disinfection
The goal of environmental C/D is not to completely sterilize the environment but rather to significantly decrease the pathogen load to a point at which disease transmission does not occur. Definitions of C/D terminology are given in Box 1 . At a minimum, C/D protocols should include the following steps:
-
1.
Detergent to remove organic debris (critical to the efficacy of most disinfectants)
-
2.
Rinsing
-
3.
Drying (optimum; or at a minimum water removal, because application of disinfectant to a water-logged area may result in dilution to the point of inefficacy)
-
4.
Disinfectant application at appropriate concentration, ensuring that the disinfectant remains wet on the surface for the required contact time.
Box 1. Basic definitions.
Cleaning: removal of foreign material (eg, contaminants, including dust, soil, chemical residues, pyrogens, large numbers of microorganisms) and the organic matter protecting them. Normally accomplished using water with detergents or enzymatic products. Thorough cleaning is required before high-level disinfection and sterilization, because inorganic and organic materials remaining on surfaces interfere with the effectiveness of these processes
Disinfection: the process that destroys most pathogenic microorganisms, especially the vegetative forms, but not necessarily bacterial spores, usually accomplished by use of liquid chemicals
Antisepsis: a special category of disinfection, referring to the inhibition or destruction of pathogenic microbes on the skin and mucous membranes
Biocides: distinct from disinfectants in that they are intended to destroy all forms of life, not just microorganisms
Sanitation: reduction of the number of bacterial contaminants to a safe level. Sanitizers are not concentrated enough or in contact with organisms long enough to effect disinfection
Terminal cleaning: the process carried out after a patient under isolation has been discharged, end of the day cleaning in areas such as operating rooms, or end of procedure cleaning in areas in which a patient known to be infected with a contagious disease has been handled
Sterilization: the process used to render an object free of all microorganisms, excluding prions
Veterinarians and staff should pay strict attention to the role that appropriate environmental C/D plays in the reduction and elimination of veterinary HAI.36, 37, 38 C/D processes are of utmost importance in ensuring patient, client, and staff safety and an uneventful hospital stay in terms of HAI. The steps in a typical practical C/D procedure of broad environmental application are shown in more detail in Box 2 , with an emphasis on critical concepts such as dilution rates and contact times for disinfectants. The steps provided are suitable for high-level C/D but are readily adaptable to more low-level C/D needs. Some, but not all, disinfectants have good cleaning in addition to disinfecting properties, so the basic number of steps can be reduced in noncritical areas (to determine whether a particular disinfectant product has adequate cleaning properties inspect the manufacturer’s label). However, where areas are grossly dirty, a separate detergent step is always required. In addition, some disinfectants require rinsing, because of the potential for toxicity or surface damage, but others may have residual activity, which might be negated by rinsing. The need for rinsing and claims for residual activity are also stated on the product label. Drying after C/D is always beneficial to pathogen control. Characteristics of each disinfectant product, including their compatibility with detergents and other chemicals, can be determined by careful inspection of the label, so it is important that individuals responsible for both choosing and using these products understand how to read the manufacturer’s label.36 More information on characteristics of disinfectants is covered in the section on choosing a disinfectant.
Box 2. Example of an effective, broad-application, environmental C/D protocol.
Have all material safety data sheet or product safety data sheets for C/D materials available and follow instructions for proper mixing, disposal, and personal protective equipment (eg, gloves, eye protection).
In the case of animal cages, remove all bedding (if intended for reuse, place in receptacle and send for laundering) and any organic material before cleaning. In other areas, remove any loose organic material (eg, feces, feed, hair, linens, bandage or other materials) before cleaning.
Clean surfaces with an anionic detergent. Scrubbing of surfaces is often necessary to remove biofilms and stubborn organic debris, especially in animal housing areas.
Rinse with clean water. For all rinsing and product application procedures, care must be exercised to avoid overspray. Unless equipment is moved to a dedicated cleaning area, high-pressure washing should generally be avoided. Higher pressures can help remove stubborn organic debris, but may also force debris and organisms into crevices or porous materials, from which they can later emerge, and they cause more aerosolization and overspray, which may spread organisms widely, even into previously uncontaminated areas. For methods not involving hoses see Box 3.
Allow to dry or at least ensure that the bulk of surface water is removed. If excess water remains, subsequently applied disinfectants may be diluted to the point of inefficacy
Apply disinfectant solution and allow the appropriate contact time. A dilute solution (1:25–1:50) of household (4%–5.25%) bleach with at least 15 minutes contact time is readily available and inexpensive but may not be the most effective choice. Many other options are available. Alternatives include accelerated hydrogen peroxide (eg, Accel TB [Virox Technologies Inc, Oakville Ontario, Canada]), quaternary ammonium disinfectants (eg, Roccal-D [Zoetis, Florham Park, NJ], Parvosol [Hess and Clark Inc, Randolph, WI]), peroxygen-based disinfectants (eg, Virkon-S [Sudbury, Suffolk, UK]/Trifectant [Vetoquinol, Fort Worth, TX]), or phenolics (eg, 1-Stroke Environ [Steris Corp, Mentor, OH], Tek-Trol [Bio-Tek Industries Inc, Atlanta GA]). Dilution rates and recommended contact times vary by product and are critical to efficacy; be sure to read the product label carefully and follow manufacturer’s instructions. Although not suitable for use in all areas, metered hose-end sprayers or foamers (eg, HydroFoamer/Sprayer [Hydro Systems Co, Cincinnati, OH]) are efficient delivery methods and generally ensure accurate dilution. Foamers might enhance surface contact.
Rinse thoroughly with clean water (although some disinfectants indicate that rinsing is unnecessary, it can prevent residue build-up over time).
Allow the treated area to dry as much as possible. Drying is important to achieve maximum effect; allow area to dry as much as possible (completely is preferred), before reintroducing animals or reusing the area. If postcleaning environmental samples are being collected, the area must be completely dry.
In known contaminated or high-risk areas, a second application of disinfectant with, for example, an accelerated hydrogen peroxide product should be considered as a final decontamination step. Ensure appropriate contact time, rinse with clean water, and allow the treated area to dry as much as possible, as stated above.
Several studies in human medicine have shown that less than 50% of hospital rooms are adequately cleaned and disinfected.39, 40 Less obvious environmental sites can be frequently overlooked. “Housekeeping, nurses, and aides will universally clean obviously soiled surfaces; many germ-infected sites go unnoticed.”41 Several methods are being used to improve C/D, including staff education, use of checklists, and hygiene assessment tools. Although there are few examples, hygiene assessments using luminometer readings33 and fluorescent tagging42 have been described in veterinary medicine. The Association for Professionals in Infection Control and Epidemiology and the Association for the Healthcare Environment are 2 human-based professional infection control societies that are using all of these approaches and are striving to improve the cooperation between medical staff and environmental services (housekeeping), with the singular goal of improved patient outcomes. Their educational campaign Clean Spaces, Healthy Patients incorporates educational resources and training materials. The initiative represents both an evolution and a revolution in infection prevention. Although for many years, the field focused on clean hands, there is now growing recognition that preventing HAIs is about clean hands touching clean equipment in clean environments. It also serves to bridge the gap and remove barriers to success between medical staff and environmental services.43 Although not all private veterinary practices have dedicated housekeeping personnel and C/D tasks are likely to be carried out by staff members who also have other duties, this training initiative in human medicine and the simple tools that it uses are equally applicable to veterinary medicine.
Depending on size, caseload, and case type, veterinary hospitals should consider appointing a willing individual or a group that represents all relevant constituencies to oversee infection control issues. A preliminary step in establishing an infection control program should be an evaluation of the level of HAI risk and how risk averse you want to be in your practice. The risk evaluation process helps guide the nature and stringency of the infection control procedures to be adopted. Because infection control measures are generally associated with some cost, consideration should also be given to how the risk/benefit ratio of these procedures is assessed (the latter might require evaluation over time). Disinfection protocols, for example, should be frequently reviewed and if necessary altered based on evidence gathered through patient and, potentially, environmental surveillance. In addition, there is ample evidence that, in addition to antimicrobial resistance, microorganisms can be resistant to disinfectants, antiseptics, and sterilants, either constitutively, through acquired means, or by formation of biofilms.44 Keeping abreast of developments in antimicrobial resistance helps in determining the need for change.
When designing a C/D protocol, consideration should also be given to the effect of disinfectants (some of which are powerful oxidizers) on equipment, personnel, and materials in the environment. A particular disinfectant may be more costly at the outset but overall might be a prudent choice, because of minimal destruction of equipment or damage inflicted to surfaces over time. If prolonged use of a disinfectant is found to damage surfaces, an alternative should be sought, because loss of surface integrity defeats the object of maintaining sealed, cleanable surfaces in critical areas. The use of prepackaged wipes containing disinfectants such as accelerated hydrogen peroxide or quaternary ammonium compounds is a convenient means of disinfecting hand surfaces and certain types of delicate equipment. Clippers, clipper blades, bandage or suture scissors, thermometers, mouth gags, laryngoscopes, endoscopes, and all other equipment used on patients should be subject to appropriate C/D. There are differences between cleaning, disinfection, antisepsis, and terminal cleaning, as defined in Box 1. Many valuable resources cover all aspects of cleaning protocols, and the properties and use of disinfectants in significant detail.32, 33, 34, 35, 36, 37, 38, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55
Areas to address
When establishing cleaning protocols, it is important to appreciate that appropriate veterinary hospital C/D is not random. There are certain factors, such as risk, traffic flow, and the critical nature of a given space or item, that must be considered and other decisions that must be made; every aspect of the process, from what type of products and materials should be used, to the order of events, and frequency of cleaning, needs to be scrutinized and researched. In addition to the typical C/D protocol outlined in Box 2, steps in the 3 basic methods for performing environmental C/D are shown in Box 3 .
Box 3. Outline of basic cleaning methods.
-
1.Dry method
-
•Use of dust-retaining materials (ie, microfiber cloths; microfiber cloths and mops have shown superior microbe removal to other materials like cotton, particularly when used with a detergent cleaner) rather than traditional brooms or rags, to minimize the dispersion of contaminated dust in the environment
-
•When cloths are used for damp cleaning, only clean water should be used; no additional detergents are needed
-
•All cloths should be washed after every use
-
•
-
2.Wet method
-
•Double bucket technique: first bucket is for clean water and either detergent or disinfectant; second bucket is for clean rinse water
-
•Single bucket technique: solution must be changed as soon as it becomes dirty and before moving to any new area
-
•All mop buckets should be emptied, cleaned, and left to dry when not in use. Mop heads should be changed a minimum of once a day or sooner when visibly soiled
-
•
-
3.Terminal method
-
•Personnel conducting the process must use appropriate personal protective equipment (eg, gloves, disposable apron, eye protection) when indicated
-
•Discard all disposable items according to proper waste disposal regulations
-
•Remove portable equipment to dirty utility area for C/D or sterilization as needed
-
•Place all laundry into an appropriate bag, seal before removal from the space, and send for processing
-
•Dry dust patient area, beginning at the top and working toward the bottom (although walls are not considered particularly critical surfaces in human medicine, the fact that veterinary patients may regularly lick those surfaces as well as floors increases their importance in veterinary infection control)
-
•Wet dust patient area, beginning at top and working toward the bottom
-
•Wash mats, caging or animal housing areas, surgical and examination tables, and other static equipment with detergent and water, rinse, and dry
-
•If disinfection is required (mandatory for infectious patients), apply appropriate disinfectant solution to any/all areas, allow contact time, rinse, and dry
-
•Use special procedures for static equipment that cannot withstand treatment with water and detergents
-
•As needed, repeat washing and disinfection of floors
-
•
As part of the process of developing the most efficient cleaning protocols that are likely to be most effective in mitigating HAI, it is useful to consider the hospital environment divided into 2 principal groups:
-
1.
Surfaces that come into frequent contact with hands
-
2.
Surfaces that have minimal contact with hands
As a rule, hand-touch surfaces are of a larger concern than surfaces having minimal contact.43, 50 At the University of Pennsylvania’s large animal hospital, Salmonella has been used as an environmental biosensor for more than 10 years. When hand and floor surface samples are compared, of samples identified as positive for Salmonella over that period, approximately 60% were collected from hand areas (eg, stall door bolts, light switches, telephones, door knobs, drawer pulls, bench tops in records and preparation areas, refrigerator door handles, computer keyboards).
Picking and choosing which hospital surfaces and areas are of most concern and in most need of high-level C/D must also take other factors into consideration. In particular, the frequency of C/D necessary for an area should take into account:
-
•
Type of hospital unit (eg, high-risk units such as critical care, neonatal, and isolation units and surgical suites require frequent high-level C/D)
-
•
Potential for contamination with bodily fluids
-
•
Potential for contamination with dust, soil, or water
The National Specifications of Cleanliness for the UK National Health Service (NHS)54 suggest identifying hospital risk categories and the required level of service for each category, as outlined later. Veterinary hospitals should consider this approach and, as mentioned earlier, establish an infection control individual/group, which in the context of C/D ideally includes individuals responsible for C/D of the facilities, clinical representatives, and other relevant stakeholders. In addition to instituting C/D protocols, this group should be prepared to educate other staff to ensure buy-in at all levels and promote compliance. Although not widely adopted in veterinary medicine, auditing of C/D is a means of checking practice against a standard or desired outcome and it has been used to improve health service in human medicine.54, 56 The infection control team should be encouraged to develop and conduct audits of C/D. The NHS materials include some sample cleaning standards and audit score sheets that are eminently adaptable to veterinary use. Cleaning standards for a few selected items are shown in Table 1 . Elements for consideration of cleaning standards can be divided into 3 overlapping major groups: environment, direct contact, and patient equipment. In terms of the environment, there are several subgroups: floors; fixed assets; electrical appliances; toilets, sinks, and other washing facilities; and furnishings and fixtures; examples of which are indicated in Table 1. Issues to be considered when designing and implementing an audit process include frequency, personnel, methodology, sampling, scoring, and action. For human hospitals, personnel involvement and scoring systems can be complex. However, for most veterinary applications, audits could be conducted by an individual or, better yet, a small group, which comprises medical personnel and individuals responsible for C/D. Whoever conducts the audit should be able to competently judge what is acceptable in terms of cleanliness and infection prevention and control. Once the elements and standards criteria to be included in the audit have been identified, the scoring system could be as simple as acceptable (score 1) or unacceptable (score 0) for each element in a given space of a functional area (see later discussion), leading to an overall score for that area. Based on the number of elements scored, the area score can be converted into a percent acceptable. As a guideline, the NHS system uses the following percentages as targets for the 4 functional risk areas described later: very high, 98%; high, 95%; significant, 85%; low, 75%; areas with scores lower than this are considered for predetermined remedial action.
Table 1.
Examples of cleaning standards for selected elements found in the clinical environment
| Element | Standard |
|---|---|
| Floor: nonslip (environment/floors) | The complete floor, including all edges, corners, and main floor space, should have a uniform finish or shine and be visibly clean, with no blood and body substances, dust, dirt, debris, or spillages |
| Walls (environment/fixed assets) | All wall surfaces, including skirting, should be visibly clean, with no blood and body substances, dust, dirt, debris, adhesive tape, or spillages |
| All doors (environment/fixed assets) | All parts of the door structure should be visibly clean so that all door surfaces, vents, frames, and jambs have no blood or body substances, dust, dirt, debris, adhesive tape, or spillages |
| Switches, sockets, and data points (environment/fixed assets) | All wall fixtures (eg, switches, sockets, data points) should be visibly clean, with no blood and body substances, dust, dirt, debris, adhesive tape, or spillages |
| Sinks (environment/toilets sinks and other washing facilities) | The sink and wall-attached dispensers should be visibly clean, with no blood and body substances, dust, dirt, debris, lime scale, stains, or spillages. Plugholes and overflow should be free from build-up |
| Hand hygiene alcohol rub dispensers (environment/furnishings and fixtures) | All parts of the surfaces of hand hygiene alcohol rub dispensers should be visibly clean, with no blood and body substances, dust, dirt, debris, adhesive tape, or spillages. Dispensers should be kept stocked |
| Animal cages (environment/fixtures/direct contact) | All parts of the cage (including bars, interior walls, floor, and corners) should be visibly clean, with no blood and body substances, dust, dirt, debris, adhesive tape, or spillages |
| Tables (environment/furnishings and fixtures) | All parts of the table (including wheels, castors, and underneath) should be visibly clean, with no blood and body substances, dust, dirt, debris, adhesive tape, stains, or spillages |
| Waste receptacles (environment/furnishings and fixtures) | The waste receptacle should be visibly clean, including lid and pedal, with no blood and body substances, dust, dirt, debris, stains, or spillages. Receptacles should be emptied frequently and not allowed to overflow |
| Fridges and freezers (environment/furnishings and fixtures) | Fridges and freezers should be visibly clean, with no blood and body substances, dust, dirt, debris, spillages, food debris, or build-up of ice |
| Medical equipment connected to a patient (eg, intravenous infusion pumps, drip stand) (patient equipment/direct contact) | All parts, including underneath, should be visibly clean, with no blood and body substances, dust, dirt, debris, or spillages |
| Cleaning equipment (environment/fixtures, maybe electrical) | Cleaning equipment should be visibly clean, with no blood and body substances, dust, dirt, debris, or moisture |
Ideally, regular audits should form part of the quality assurance program of your clinic. Issues raised should be followed up according to their magnitude and location. Lead times should be identified for remediation. For example, a problem in the surgical area needs to be resolved immediately, whereas one in a storeroom for noncritical items may require checking within some reasonable time, such as during the next scheduled audit. Although it is important that deficiencies be identified and corrected, efforts should be made to avoid the perception that audits will be punitive in nature. Functional areas for evaluation can be categorized as follows:
-
1.Very high-risk functional areas
-
•Operating rooms, critical care units, departments in which invasive procedures are performed or in which immunocompromised patients are receiving care, isolation units
-
•Desired outcomes are achieved only through intensive and frequent cleaning
-
•Initially audit at least once a week, until the lead cleaning manager and infection control team are satisfied that consistently high standards are being achieved, after which the audit frequency may be reduced to no less than twice monthly
-
•
-
2.High-risk functional areas
-
•Include examination rooms, general wards, sterile supplies, public thoroughfares, and public toilets
-
•Desired outcomes maintained by regular and frequent cleaning, with spot cleaning in between
-
•Initially audited at least once a month, until it is clear that consistently high standards are being achieved, after which the audit frequency may be reduced to no less than monthly
-
•
-
3.Significant-risk functional areas
-
•Pathology, outpatient areas, and laboratories
-
•Desired outcomes maintained by regular and frequent cleaning with spot cleaning in between
-
•Audited at least once every 3 months
-
•
-
4.Low-risk functional areas
-
•Administrative areas, nonsterile supply areas, record storage, and archives
-
•Desired outcomes maintained by regular and frequent cleaning with spot cleaning in between
-
•Audit at least twice a year
-
•
Choosing a disinfectant
There are numerous chemical agents available to disinfect health care facilities. These agents are mostly liquid based and fall into 9 broad categories: acids, alcohols, aldehydes, alkalis, biguanides, halogens (hypochlorites and iodine-based iodophors), oxidizing agents, phenolics, and quaternary ammonium compounds. Accelerated hydrogen peroxide products (eg, Accel, Virox Technologies, Oakville, ON, Canada), which claim virucidal, bactericidal, fungicidal, and tuberculocidal activity, have been introduced recently for disinfection of noncritical environmental surfaces and equipment. In addition, there are some newer disinfectant combinations that have synergistic actions (eg, Siloxycide, Preserve International, Reno, NV), a combination of hydrogen peroxide plus silver nitrate; the latter provides extended peroxide stability, and the combination claims increased efficacy compared with products containing only hydrogen peroxide; this combination is approved for use in health care settings. Quaternary ammonium/glutaraldehyde combinations (eg, Synergize, Preserve International; Reno, NV) were developed for use in livestock facilities and have been successful, but they are not approved for use in veterinary hospitals. There are also newer technologies, such as no-touch methods of surface disinfection.7, 48, 49 These methods include ultraviolet (UV) light and hydrogen peroxide dry-mist or vapor (HPV) fogging procedures.49 Both can be used for whole room decontamination and are more suited to occasional use either in response to a specific problem or as part of a program of thorough disinfection of specific spaces scheduled to occur at predetermined intervals. HPV is capable of killing a wide range of pathogens, including Cryptosporidium and ∼6 logs spores, whereas UV is reliably biocidal at 3 to 4 logs against vegetative bacteria. No animals or people can be present during the decontamination process. The use parameters for both are sensitive and require specialized equipment, representing a considerable capital investment, but unlike HPV, UV systems need no additional consumable products. Room decontamination is rapid with UV (∼15 minutes) compared with 3 to 5 hours for HPV. In addition, for HPV, the HVAC system must be disabled and the rooms sealed to prevent unwanted dilution of the vapor, which requires considerable staff time. HPV is uniformly distributed throughout the room via an automated dispersal system and is particularly useful for disinfecting temperature-sensitive or complex equipment and furniture. It has been speculated that over time HPV might cause damage to sensitive electronics as a result of microcondensation, but this should not be an issue with the dry-mist form. HPV leaves no toxic residues (aeration units convert HPV into oxygen and water), and it requires no rinsing. Similarly, UV systems generate no residues and are not associated with health and safety concerns, but the environmental penetration of these systems is probably less than HPV. Neither procedure removes dust or stains, so the area must be clean before decontamination. Although these systems have been used widely in human medicine, there are no data to show whether or not the incidence of HAI decreases.49 Other no-touch methods increasingly being used include self-disinfecting surfaces impregnated with heavy metals (eg, copper and silver) or germicides (eg, the bisphenolic compound triclosan). At our large animal hospital, we have successfully used silver-impregnated sticky mats (Dycem, Warwick, RI) in place of footbaths in main entry areas to our high-risk isolation and colic facilities as well as the entry way to the necropsy facility. Although we have not encountered specific problems with these mats, which are sampled weekly for the presence of Salmonella, it is well known that bacteria (particularly gram-negative organisms, including Escherichia coli) can be resistant to heavy metals by both intrinsic and, in some cases, acquired mechanisms. Five mechanisms of intrinsic resistance have been described that allow bacteria to survive in the presence of inhibitory or microbicidal concentrations of toxic metals44 Bacterial resistance to biocides such as triclosan can be intrinsic or acquired by mutation or plasmid or transposon acquisition and can be via several mechanisms: exclusion; efflux mechanisms; mutation to decrease target sensitivity; or target overproduction.44 The likelihood of resistance development should always be considered when choosing products, particularly because some items, such as the mats mentioned earlier, are expensive. Antimicrobial surface coatings based on nanoparticles are under development for both biomedical devices and environmental surfaces57, 58 and may have the advantage over microbicidal coatings based on metals or agents, such as triclosan, because they have prolonged activity, and some seem unlikely to be associated with the development of microbial resistance.57 Steam disinfection systems offer an alternative to liquid chemical disinfectants and can kill a diverse group of pathogenic organisms in seconds, but they are not suitable for all surfaces and introduce additional safety considerations.
Characteristics of the ideal disinfectant are shown in Box 4 . The possibility of achieving all of these qualities for all scenarios is unrealistic. Therefore, individuals responsible for developing C/D protocols must learn to discretionarily pick and choose disinfectants based on the properties of the disinfectant, the perceived challenges of the task, and desired results of the disinfection procedure. For example, the disinfectant that might be chosen for a footbath or foot mat is likely different from the agent picked for general purpose C/D, because the footbath must be quick kill and preferably stable in the face of organic debris. Quick kill and lack of surface damage tend to be mutually exclusive characteristics. Even in the case of routine surface disinfection, procedures should not make for slippery floor surfaces, cause films to build up over time, or damage surfaces (film build-up and surface damage preclude effective cleaning). Although footbaths are not used as commonly in small animal compared with large animal clinics, they may be effective in preventing the transmission of infectious agents.59, 60, 61, 62, 63 While opinions about the degree of efficacy vary, in the absence of other control methods, their use should certainly be considered in isolation areas, particularly if it is short-term, without attendant management problems.
Box 4. Characteristics of an ideal disinfectant.
-
1.
Broad spectrum: wide antimicrobial spectrum (including sporicidal), should have kill claims for the pathogens that are the common causes of HAIs and outbreaks
-
2.
Fast acting: high germicidal activity, rapid kill and short kill/contact time listed on the label
-
3.
Remains wet: surfaces stay wet long enough to meet listed kill/contact times with a single application or meet wet times recommended by evidence-based guidelines
-
4.
Not affected by environmental factors: stable and effective in the presence of organic matter (eg, blood, feces)
-
5.
Chemical compatibility: should be compatible with soaps, detergents, and other chemicals encountered in use, such that the effectiveness of neither chemical is affected, and that mixing does not result in toxicity, increased corrosiveness, or other reactivity
-
6.
Nontoxic: not irritating to user, other staff, clients, or patients. No induction of allergies (especially asthma and dermatitis). Disinfectant toxicity ratings are danger, warning, caution, and none. Ideally, choose products with the lowest toxicity rating
-
7.
Surface compatibility: be capable of penetration without destruction, proven compatible with common surfaces and equipment found in veterinary settings
-
8.
Persistence: sustained antimicrobial activity or residual postapplication antimicrobial effect
-
9.
Easy to use: ideally available in multiple forms (eg, wipes, sprays, concentrates); simple directions for use with information about PPE as required
-
10.
Acceptable odor: odor and aesthetics should be acceptable to users and others
-
11.
Economical: costs should not be prohibitively high but when considering the costs of a disinfectant product capabilities, cost per compliant use, and so on should also be considered
-
12.
Solubility: soluble in water
-
13.
Stability: stable in concentrate and use dilution
-
14.
Cleaner: good cleaning properties
-
15.
Nonflammable: flash point higher than 65.5°C (150°F)
There are numerous excellent resources36, 37, 45, 46, 47, 48, 49, 50, 51 that provide more information on chemical disinfectants, their classes, characteristics, and modes of activity. Iowa State’s Center for Food Safety and Public Health Web site46, 47 is a valuable and trusted resource in the veterinary community; it gives the spectrum of activity of the major disinfectant groups against various microorganisms and the characteristics of selected disinfectants in accessible readily understood tables.47 The susceptibility of various microorganisms and the level of disinfection required to kill them are summarized in Table 2 , and the characteristics of the most common disinfectants are shown in Table 3 . As mentioned earlier, for each chemical disinfectant, the manufacturer’s label36, 37 provides important information, including about microorganisms against which it has been shown to be effective, appropriate contact time, correct dilutions for the desired outcome (may be higher for bactericidal activity and lower for bacteriostatic claims, and different for parvicidal activity vs routine use), as well as other factors that may alter effectiveness of the agent. These other factors can include the length of time that a product remains effective when diluted; shelf-life of the concentrate; temperature range in which it is maximally effective; water hardness (because dilution rates of some disinfectants [eg, quaternary ammonium compounds] may vary depending on hardness of the water); interaction with detergents (which can alter the pH of a disinfectant and reduce its effectiveness); features of the surface to be disinfected (as previously mentioned, integrity of the surface, or lack thereof, can greatly affect disinfectant effectiveness); and organic load (Box 5 ).
Table 2.
Decreasing order of resistance of microorganisms to disinfection and sterilization and the required level of disinfection
| Microorganism | Examples | Disinfection Level Requireda | |
|---|---|---|---|
| More resistant | Prions | Scrapie, chronic wasting disease, bovine spongiform encephalopathy | Prion reprocessing |
 |
Bacterial spores | Bacillus, Clostridium | Sterilization |
| Protozoal oocysts | Cryptosporidium | High: can be classified as chemical sterilants, kill spores with prolonged contact times, shorter exposure periods kill all microorganisms except large numbers of bacterial spores | |
| Helminth eggs | Ancylostoma, Strongyloides, Trichuris | ||
| Mycobacteria/acid fast bacteria | Mycobacterium tuberculosis, Nocardia | ||
| Small nonenveloped (nonlipid) viruses | Calicivirus, circovirus, paramyxovirus, parvovirus | Intermediate: may be cidal for mycobacteria, vegetative bacteria, most viruses, and most fungi but do not necessarily kill bacterial spores | |
| Protozoal cysts | Giardia | ||
| Fungal spores | Aspergillus, Coccidioides, Microsporum canis, Trichophyton | ||
| Gram-negative bacteria | Acinetobacter, Escherichia, Pseudomonas, Salmonella | ||
| Vegetative fungi and algae | Aspergillus, Trichophyton, Candida, Malassezia | ||
| Vegetative helminths and protozoa | Ancylostoma, Strongyloides, Trichuris, Cryptosporidium, Giardia | ||
| Large, nonenveloped (nonlipid) viruses | Adenovirus, rhabdovirus, rotavirus | ||
| Gram-positive bacteria | Staphylococcus, Streptococcus, Enterococcus, MRSA, VRE | Low: may kill most vegetative bacteria, some fungi, and some viruses in a practical period ≤10 min | |
| Enveloped (lipid) viruses | Coronavirus, herpesvirus, influenza viruses | ||
| Less resistant | Mycoplasmas | Mycoplasma canis, M felis |
The designated disinfection levels were developed for medical and surgical materials and equipment, not the environment. They are included here because, for the most part, the concepts they convey are still valid considerations for environmental surfaces.
Table 3.
Characteristics of commonly used disinfectants
| Category | Acids | Alcohols | Aldehydes | Alkalis | Biguanides | Chlorine-Releasing Agents | Iodine Iodophors | Oxidizing Agents | Phenolic Compounds | Quaternary Ammonium Compounds |
|---|---|---|---|---|---|---|---|---|---|---|
| Examples | Acetic acid, citric acid, lactic acid | Ethanol, isopropanol, methanol | G, F, OPA | Sodium hydroxide (lye, caustic soda), calcium hydroxide (slaked lime), sodium carbonate (washing soda, soda ash), ammonium hydroxide | Chlorhexidine diacetate and gluconate (Nolvasan, Chlorhex, Virosan) | Sodium hypochlorite (bleach, Clorox), calcium hypochlorite, chlorine dioxide | Iodine solutions (tinctures) or iodophors (complex of iodine with neutral polymers, most commonly povidone-iodine, Betadine) | HP, AHP (Accel), PAA (Oxy-Sept 333), PMS (Virkon, Trifectant) | Various phenols (2-phenylphenol, benzylphenol, 4-chloro-3,5-dimethylphenol; One-Stroke Environ, Tek-Trol, Osyl, Lysol, Pine-Sol) | Various ammonium salts (benzalkonium chloride, benzethonium chloride, cetalkonium chloride, cetyl pyridinium chloride, tetraethylammonium bromide, cetyl trimethylammonium bromide, and domiphen bromide; Roccal-D, Parvosol, DiQuat, D-256) |
| Mechanism of action | Precipitate proteins, disrupt nucleic acids | Precipitate proteins, denature lipids, cell lysis | Denature proteins, alkylate nucleic acids | React with membrane lipids | Alter membrane permeability | Denature proteins | Denature proteins, disrupt nucleic acids | Denature proteins and lipids | Alter cell wall permeability, denature proteins | Disrupt cell membrane, denature proteins, inactivate enzymes |
| Suitable applications | Specialist applications mainly large animal, not recommended for general use | Limited surface disinfection, topical antiseptic, hand sanitizers (Purel) | Surface disinfection, fumigant (F), sterilization (G), high-level disinfectant (OPA) | Have been used for environmental disinfection but not recommended for general use | Surface disinfection, topical antiseptic | Surface disinfection, chlorine dioxide also fumigation and gas sterilization | Surface disinfection (Environmental Protection Agency–registered hard surface iodophors only), topical antiseptic (skin antiseptic iodophors) | Surface disinfection all, HP and AHP topical antiseptic, HP vapor sterilization, PAA fumigation, PMS aerosol fumigation | Surface disinfection | Surface disinfection |
| Efficacy with organic material | Poor, reduced | Poor, reduced | Moderate, reduced | Sodium hydroxide high, others low to moderate | Very poor, rapidly inactivated | Very poor, rapidly inactivated, except chlorine dioxide moderate | Slightly better than chlorine-releasing agents but still very poor, rapidly inactivated | Variable, HP low, AHP moderate, PMS and PAA high | High, effective | Poor to moderate, reduced |
| Efficacy with detergents/soap | ? | ? | Reduced | ? | Inactivated | Inactivated | Effective | ? | Effective | Inactivated |
| Efficacy with hard water | ? | ? | Reduced | ? | ? | Effective | ? | ? | Effective | Inactivated, but for some agents higher concentrations work, check label |
| Residual activity | Some | No | ? | ? | Yes (skin) | No | Some | Claimed for AHP | Yes | Some, brief |
| Advantages | Nontoxic, nonirritating at typical concentrations | Fast acting, no residues, overall low toxicity | Broad spectrum, relatively noncorrosive, relatively inexpensive, sporicidal in alkali solution | Ammonium hydroxide effective against coccidial oocysts, sodium hydroxide assists in prion destruction | Broad spectrum against bacteria, activity in aqueous alcohol solutions superior to aqueous only, relatively low toxicity | Broad spectrum, short contact time, inexpensive, sporicidal at higher concentration | Broad spectrum, stable in storage, relatively safe | Fast acting, broad spectrum, considered environmentally friendly, sporicidal | Broad spectrum, stable in storage, noncorrosive, effective over large pH range | Relatively broad spectrum (although variable between products), stable in storage, generally nonirritating to skin, effective at high temperatures and high pH (9–10) |
| Disadvantages | Change environmental pH, hazardous at high concentrations, corrosive, toxic | Rapid evaporation, flammable, irritation to injured skin | Toxic (F carcinogenic risk), irritating to mucous membranes and tissues, use in well-ventilated areas, toxic to fish | Very caustic, corrosive to metals, ammonium hydroxide intense pungent fumes, toxic to aquatic life | Limited activity against viruses, functions only in narrow pH range (5–7), toxic to fish (environmental concern), keratitis | Inactivated by sunlight and some metals, reduced activity at high pH and low temperatures, frequent application needed, surface to be disinfected must be clean and dry, corrosive to metals (not stainless steel) and some other materials, discolors fabrics, irritating to mucous membranes and skin, mixing with acids release toxic chlorine gas | Stains clothes, some surfaces and plastics, frequent application needed, corrosive, inactivated by quaternary ammonium compounds, contact sensitivity | Some (notably PMS, PAA) are damaging to plain metals, concrete, and some other surfaces, discolor fabrics, eye irritation | Toxic to animals, particularly cats and pigs, can cause skin and eye irritation, unpleasant odor, some have disposal restrictions, not recommended for food surfaces | Toxic to fish, lose activity at pH<3.5 and low temperatures |
| Gram-positive bacteria | + | + | + | + | + | + | + | + | + | + |
| Gram-negative bacteria | + | + | + | + | + | + | + | + | + | + |
| Mycobacteria | — | + | — | + | — | + | + | + | + | ± |
| Enveloped viruses | + | + | + | + | ± | + | + | + | ± | ± |
| Large nonenveloped viruses | — | ± | + | + | ± | + | ± | + | ± | — |
| Small nonenveloped viruses | ± | ± | + | ± | ± | + | ± | + | ± | — |
| Fungi | ± | ± | + | + | ± | + | + | ± | + | ± |
| Spores | ± | ± | + | ± | — | ± | ± | ± | — | — |
Box 5. Additional considerations in choosing cleaners and disinfectants.
A surface cannot be properly disinfected if it is not clean. A plain, anionic detergent should be chosen as a basic cleaner for animal housing and handling areas, including surgical suites.
Cost is important but should not be the only consideration; efficacy, ease of use, and potential deleterious effects must be part of the equation.
Make sure that the properties of all of the cleaners and disinfectants that you chose are compatible (eg, avoid combinations that lead to generation of chlorine gas).
Potential negative effects of disinfectants on equipment, personnel, and the environment. For example, prolonged use of some disinfectants, particularly powerful oxidizers such as the peroxygens, can damage surfaces (notably metals other than stainless steel, concrete, and tile). Loss of surface integrity defeats the object of maintaining sealed cleanable surfaces and makes cleaning more difficult.
Some disinfectants cause a surface film build-up over time, particularly around footbaths/mats but even with general use. Films can be slippery, can impede proper cleaning, and may promote biofilm formation. Although not widely used in small animal practice, footbaths/mats may be necessary in isolation units. Careful siting of footbaths/mats or changing disinfectant may avoid potential problems.
Prepackaged disinfectant wipes can be useful in C/D of delicate equipment and hard surfaces in sensitive areas. In general, accelerated hydrogen peroxide–based wipes are preferred over those containing quaternary ammonium compounds.
Laundry
Soiled hospital laundry and animal bedding may be considered a potential source of infection to both staff and patients and may cause cross-contamination of the environment. All reusable linens and bedding that have been contaminated with blood, urine, feces, or any other bodily fluids or exudates must be subjected to a decontamination process.64, 65 The following list of suggestions regarding laundry applies to all situations:
-
1.All workers involved in the collection, transport, sorting, or washing of soiled bedding must:
-
•Be appropriately trained
-
•Wear required personal protective equipment (PPE), including gloves of a sufficient thickness to minimize sharps injuries, face mask and eye protection when appropriate (eg, when there is a zoonotic risk in the handling of urine-soaked bedding from known or suspected cases of canine leptospirosis)
-
•Cover any exposed broken skin or lesions
-
•Have access to hand washing facilities
-
•
-
2.
Every effort should be made to eliminate the inadvertent disposal of harmful objects, such as sharps or instruments
-
3.
Animal bedding must be carefully shaken free of all loose debris and fecal matter before processing; such matter should be disposed of appropriately
-
4.
All soiled laundry must be held and transported in bins or bags impervious to liquids
-
5.
Clean and dirty laundry must have separate transport receptacles and storage facilities
In addition, it is worthwhile to sort and label used laundry into categories for processing. This practice is adopted to protect both workers, patients, and the environment from potentially infectious agents.
-
1.
General: bedding from patients not considered infectious or contagious
-
2.
Infectious: bedding contaminated with microorganisms that pose a hazard to workers who may have contact with it, the environment, or other animals; laundry associated with infected animals should be bagged and sealed before being removed to the laundry area
-
3.
Heat labile: bedding likely to be damaged by the normal laundering process
The average laundry process in a hospital setting is adequate to render bedding hygienically clean66 This level of cleaning is achieved through a combination of dilution with water, loosening with detergent, agitation, and heat (both in the washing and, just as importantly, drying processes). In regard to appropriate temperatures for the laundry cycle, it is suggested64, 65, 66 that the process should
-
•
Maintain 65°C for no less than 10 minutes or
-
•
Maintain 71°C for no less than 3 minutes or
-
•
Contain the addition of chemicals (ie, bleach) for heat-labile materials or lower temperatures1
Waste management
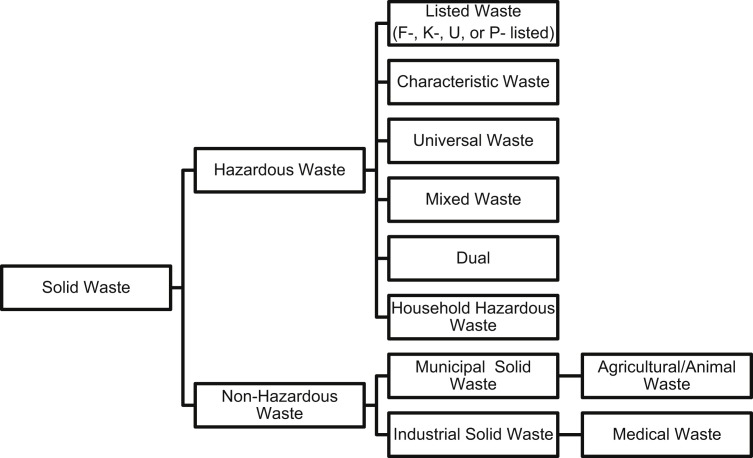
The handling of the waste generated by the veterinary industry is both complex and regulated. Waste is everything that no longer serves a purpose and needs a form of disposal. In professional settings such as veterinary clinics, there are specific guidelines in place regarding waste disposal. Most waste in the world is considered solid waste, regardless if it is in solid form. In a veterinary clinic, this waste may include but is not limited to animal tissues, bodily fluids and waste, carcasses, cleaning and laboratory chemicals, general medical waste, radiographic by-products, medications, chemotherapeutic agents, batteries, and solvents. The American Veterinary Medical Association (AVMA) has excellent resources available to guide the appropriate disposal of solid wastes generated by the veterinary industry and has developed categories for them (Fig. 1 ).67 Complete definitions of each of the categories are available at the AVMA Web site. Other jurisdictions may, of course, categorize wastes and the means of handling them differently, so veterinary hospitals should always check the regulations in their local jurisdiction.
Fig. 1.
Categories of solid waste.
(Data from AVMA. Available at: https://www.avma.org/PracticeManagement/Administration/Pages/Definitions-What-is-Waste.aspx. Accessed September 17, 2014. For full descriptions of the above categories visit the AVMA Web site.)
More complete information on appropriate waste management is available at the AVMA Web site.67 Bearing in mind the breadth of differences in both type of waste and regulatory body responsible for the different types (Table 4 ), it is important that there is a methodology associated with how a veterinary facility disposes of its waste. Decisions about waste disposal must include and may not be limited to:
-
•
Awareness of the options available for waste disposal in the physical area
-
•
Knowing which authorities have oversight so questions may be directed appropriately
-
•
Following specific material safety data sheet (MSDS) or product safety data sheet (PSDS) instructions for disposal
-
•
Specific practice policy and procedures regarding waste disposal
-
•
The need for training of individuals to ensure both safe handling and organizational compliance
Table 4.
Federal agencies that regulate veterinary waste disposal in the United States
| United States Federal Agency | Waste Regulated |
|---|---|
| Environmental Protection Agency | All waste with an environmental impact |
| Occupational Health and Safety Administration | Waste associated with potential employee exposure to hazardous substances |
| National Institute of Occupational Safety and Health | Workplace products that affect both human and public health |
| Drug Enforcement Agency | Disposal of controlled substances |
| Department of Transportation | Shipping of chemicals and specimens |
| Nuclear Regulatory Commission | Chemotherapeutic agents |
Data from AVMA. Available at: https://www.avma.org/PracticeManagement/Administration/Pages/Federal-Regulation-of-Waste-Disposal.aspx. Accessed September 17, 2014.
Although VTHs in academic institutions generally have their own offices of environmental health and safety, which can answer questions and assist in ensuring compliance with federal, state, local, and institutional policies, veterinary facilities without such resources should always keep in mind that overall, when in doubt, the default agency for questions and concerns regarding waste management is the Environmental Protection Agency (or country-specific equivalent) and direct enquires to their state office or the federal agency.
For the average veterinary clinic, a few specific guidelines for waste disposal and adequate staff training should be sufficient for compliance. The first step is categorizing waste. Traditional waste categories may include clinical, sharps, laboratory, pharmaceutical, infectious, and hazardous. What defines a category is both how the waste is handled at its point of collection and how it must be disposed of.53, 66, 68 Responsible medical waste producers should consider it their obligation to:
-
•
Describe the waste categories
-
•
Provide for safe collection and handling of waste by workers
-
•
Provide employee training to ensure safety
-
•
Establish a contract with a reputable waste disposal contractor
-
•
Investigate the waste disposal contractor for compliance
-
•
Pack the waste in accordance with regulations
-
•
Store waste properly on site before disposal
The adoption of a color-coded waste disposal system is another useful tool that could be implemented in the veterinary industry. Potential suggestions for waste color codes could be:
-
•
Red: biohazardous/infectious
-
•
Yellow: for incineration
-
•
Orange: biological/carcass/body parts
-
•
Purple: for incineration at a facility regulated to handle cytotoxic or chemotherapeutic agents
-
•
Green: recyclable
-
•
Black: general disposal/landfill
Environmental surveillance
Hospital surveillance exists in many forms, each with a precise goal.68 Some methods are culture based, meaning that a sample is taken and processed for results. Other methods are non–culture based, meaning that the activity relies on the observation of situations and their various outcomes. Either way, surveillance, when conducted properly, can be a useful and meaningful aspect of a complete hospital infection control program.10, 11, 12, 19, 22, 23 However, random surveillance of a hospital environment is not recommended. This advice is because random surveillance activities, either culture based or non–culture based, produce results, but they are results with no viable context.12 Therefore, when considering surveillance, always proceed methodically, with the intention of carrying out an associated and appropriate action. See the article elsewhere in this issue entitled “Veterinary hospital surveillance systems” by Burgess and Colleagues for more information.
Veterinary hospitals are inherently contaminated; any number of microorganisms might be harbored in its environs. Monitoring and surveillance are essentially the sensory mechanisms to any infection control program. Microbiological surveillance of the hospital environment can be a critical component of a successful infection control program.13, 14, 15, 16, 19, 22 Data to support this contention include findings indicating that many organisms of concern can survive for prolonged periods on surfaces and equipment.69 For example, Acinetobacter persists for 3 days to 5 months, Bordetella 3 to 5 days, Campylobacter jejuni up to 6 days, Chlamydophila psittaci 15 days, Clostridium difficile spores for 5 months, Enterococcus (including VRE) 5 days to 4 months, E coli as little as 1.5 hours to 16 months, Klebsiella 2 hours to 30+ months, canine parvovirus greater than 1 year, influenza viruses 1 to 2 days, norovirus up to 28 days, Pseudomonas aeruginosa 6 hours to 16 months, Staphylococcus aureus (including MRSA) 7 days to 7 months, and Salmonella enterica 1 day to years!69 Clearly, then, surveillance of the environment for pathogens associated with HAI is feasible and could be considered as part of an infection control program.
As mentioned earlier, environmental surveillance does not always imply microbiological evaluation, but it should certainly encompass monitoring/auditing to ensure proper hygiene and control of clutter that might impede effective cleaning. If undertaken, environmental sampling can be useful in determining which patient populations, traffic patterns, and protocols present a risk for hospital contamination, and in assessing how well containment efforts are performing.12, 19, 22, 33 Thus, if active environmental surveillance is undertaken, high traffic areas, critical spaces or equipment, treatment areas, and areas that house high-risk patients should be evaluated as the focus of that surveillance. Even in the absence of active surveillance of the environment, monitoring of the clinical status of patients can be a helpful indicator of when both patients and the environment require closer scrutiny.12 It should be the responsibility of designated infection control personnel to adjust the intensity or focus of surveillance (active or passive), based on developments in the hospital, the picture of disease prevalence in the referral area, literature, and so forth. In concert with these considerations, individual hospitals need to determine characteristics of their case population (size and type, critical care vs elective, both of which have been associated with increased risk),12 level of risk aversion, cost-benefit analysis, and so forth, either before initiating a surveillance program or, just as importantly, evaluating an ongoing infection control program. In either case, an end-all determination makes little sense, and periodic review is warranted.
A major difficulty with environmental surveillance is one of context if it is not conducted in a repeatable, methodical manner. For instance, with culture-based surveillance, at any point in time when surfaces are sampled19, 22 and cultured, bacteria may be found, but without a baseline point of reference, what does that positive finding mean? Establishment of a useful, active environmental sampling strategy might require collection of environmental and HAI baseline data, without which it is often not possible to assess the likelihood that particular infections are HAI, determine whether there has been an increase in such infections, or evaluate the efficacy of any interventions. However, in deciding whether or not to pursue a targeted surveillance program, even with good baseline data, it can be difficult to make such assessments on every occasion.
If the goal of hospital surveillance is to eliminate HAI, collecting data is futile if the process does not lead to an immediate action. Some of the potential actions associated with surveillance may be:
-
•
Establishment of baseline rates of infection or contamination
-
•
Provision of proof needed to convince practitioners to adopt best practices
-
•
Identification and control of outbreaks
-
•
Evaluation of the success of the infection control program
-
•
Drive potential changes to current policy and procedure
For the desired effect to be positively achieved, the collected information must be disseminated to individuals who can interpret and effect the desired change. In the case of HAIs and outbreak scenarios, surveillance can even be the foundation for a facility organizing and instituting or modifying an infection control program. If a facility is experiencing a suspected outbreak or is interested in introducing an infection control program, it is important for individuals to understand both the object of surveillance activities and various surveillances types (Box 6 ). However, in general, available reports suggest that most veterinary hospitals, including academic institutions, do not practice methodical collection and analysis of either patient or environmental data.12
Box 6. Potential surveillance types.
-
1.
Laboratory based: focused on the various types and numbers of concerning or alert (ie, likely to cause outbreaks or infection) organisms being isolated from the patient population
-
2.
Targeted: focused on high-risk areas, specific types of infections, procedures or a single organism
-
3.
Patient: focused on the potential for pre/post screenings at the times of entry and exit to the hospital to determine/prevent incidences of HAI
-
4.
Outbreak: focused on locating the source of a cluster of infection in various patients with the same organism
-
5.
Procedural: focused on determining the effectiveness of current C/D policies and procedures
-
6.
Clinical/ward liaison: focused on the activity of a medical health care worker visiting various hospital departments to evaluate patients, review records, attend rounds, or advise individuals about the most appropriate care and housing of patients to prevent HAI
If a decision is taken to initiate active surveillance for a particular organism(s), it might be useful to consider factors that went into the choice of Salmonella as the general biosensor most commonly used in large animal hospitals. Salmonella is among the most important cause of HAIs in large animal hospitals, it is a significant zoonotic threat, and it has been associated with numerous outbreaks at VTHs. In addition, the following characteristics favor Salmonella as an effective biosensor:
-
•
Survives well in the environment
-
•
Can spread readily on fomites
-
•
Relatively difficult to kill
-
•
Relatively easy to detect
-
•
Good indicator of effectiveness of infection control programs
-
•
Increase in environmental prevalence widely regarded as an early warning of problems
All of the same considerations should be part of the decision in choosing an environmental biosensor(s) for a small animal veterinary hospital, but this is easier said than done. Although evidence-based choices for large animal hospitals are straightforward, the options for small animal clinics are less clear. In this context, although still useful in evaluating the effectiveness of C/D, the value of measuring organisms like generic E coli to assess HAI and overall effectiveness of infection control programs is increasingly questioned. Potential targets include MDR pathogens of concern, microorganisms causing the most significant problem in your facility, or evidence from multi-institutional surveys, but further research is necessary to provide veterinarians with better guidance. For these same reasons, some advocate using non–culture-based techniques, such as fluorescent tagging, because they are not tied to specific pathogens.42 If specific pathogens are identified as targets, in addition to classic epidemiologic measures for the sensitivity, specificity, and predictive values of detection methods, another critical characteristic of testing that should be considered is speed to return of results. In terms of the environment, if it takes days to get results, there could be wide dissemination to patients and other parts of the hospital before corrective measures are initiated. These considerations explain the move away from traditional culture methods to more rapid techniques such as polymerase chain reaction, which often offer enhanced sensitivity and specificity, as well as greater speed.
Environmental surveillance can also be a useful tool for determining the effectiveness of C/D (Box 6).28, 33, 42, 68, 70 In this case, the methods and goals differ slightly from those associated with investigating HAIs or a potential disease cluster or outbreak. Basically, methods that evaluate the presence or absence of generic bacteria preferably with quantitation could suffice for C/D evaluation,33, 42 but the incorporation of specific indicator organisms increases the value of any such surveillance.68, 70 There are almost no studies of this type in veterinary medicine, but those that are available clearly indicate that environmental contamination exists and that like human medicine, although the cleaning of some surfaces (eg, examination tables, countertops) may just about be adequate, others are seriously deficient (eg, computer keyboards and other hand contact equipment).42 More research is needed to fully evaluate selected deficiencies, define such deficiencies in the context of C/D policies and practices, and further determine their relationship, if any, to HAIs.
Summary
Hospitalized animals are not the same as the general animal population; they are more likely to shed or acquire an infectious agent for a variety of reasons, including stress; immunosuppression; altered nutrition or disturbances to normal microbiota; administration of antimicrobials; being subject to procedures that are known risk factors for infection of various types; concentration close to other animals with similar risk factors. The standard of care at every veterinary hospital should therefore include a high standard of hygiene, awareness of the dangers of transfer of infectious agents between both animals and people, and procedures to reduce infection risk wherever possible. It is becoming increasingly clear that the environment of health care facilities is subject to contamination with pathogens and plays a role in at least some HAIs. Consequently, environmental management, with or without active surveillance, should be a component of risk reduction designed to limit HAIs. Effective C/D procedures are critical in preventing transmission of infectious agents between patients or from contaminated environments. Development of well-understood protocols and schedules for C/D, waste disposal, and environmental maintenance to ensure that surfaces remain sealed and cleanable is important. Education of veterinarians and all staff as to the need for thoroughness and vigilance when it comes to C/D is critical to successful mitigation of HAI risks.
The authors have nothing to disclose.
Low temperature (used to mitigate the cost of using high heat) laundry cycles rely heavily on bleach to reduce levels of microbial contamination. Regardless of the temperatures used for washing, the temperatures achieved in drying provide additional significant microbicidal action.66
References
- 1.Magill S.S., Edwards J.R., Bamberg W. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Centers for Disease Control and Prevention. Healthcare-associated infections. Atlanta (GA). Available at: http://www.cdc.gov/hai/. Accessed September 17, 2014.
- 3.US Department of Health and Human Services. National action plan to prevent health care–associated infections: road map to elimination. Available at: http://www.hhs.gov/ash/initiatives/hai/actionplan/. Accessed September 17, 2014.
- 4.Yokoe D.S., Anderson D.J., Berenholtz S.M. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol. 2014;35(8):967–977. doi: 10.1086/677216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott R.D. Division of Healthcare Quality Promotion; Centers for Disease Control and Prevention; Atlanta (GA): 2009. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention.http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf Available at: Accessed September 17, 2014. [Google Scholar]
- 6.Weinstein R.A. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med. 1991;91(Suppl 3B):179S–184S. doi: 10.1016/0002-9343(91)90366-6. [DOI] [PubMed] [Google Scholar]
- 7.Weber D.J., Anderson D., Rutala W.A. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 8.Morgan D.J., Rogawski E., Thom K.A. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40(4):1045–1051. doi: 10.1097/CCM.0b013e31823bc7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein R.A. Intensive care unit environments and the fecal patina: a simple problem? Crit Care Med. 2012;40(4):1333–1334. doi: 10.1097/CCM.0b013e318241012f. [DOI] [PubMed] [Google Scholar]
- 10.Ruple-Czerniak A.A., Aceto H., Bender J.B. Nosocomial infection rates in small animal referral hospitals: using syndromic surveillance to establish baseline rates. J Vet Intern Med. 2013;27(6):1392–1399. doi: 10.1111/jvim.12190. [DOI] [PubMed] [Google Scholar]
- 11.Ruple-Czerniak A.A., Aceto H., Bender J.B. Syndromic surveillance for evaluating the occurrence of healthcare-associated infections in equine hospitals. Equine Vet J. 2014;46(4):435–440. doi: 10.1111/evj.12190. [DOI] [PubMed] [Google Scholar]
- 12.Benedict K.M., Morley P.S., Van Metre D.C. Characteristics of biosecurity and infection control programs at veterinary teaching hospitals. J Am Vet Med Assoc. 2008;233:767–773. doi: 10.2460/javma.233.5.767. [DOI] [PubMed] [Google Scholar]
- 13.Weese J.S., Stull J. Respiratory disease outbreak in a veterinary hospital associated with canine parainfluenza virus infection. Can Vet J. 2013;54(1):79–82. [PMC free article] [PubMed] [Google Scholar]
- 14.Weese J.S., Armstrong J. Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J Vet Intern Med. 2003;17(6):813–816. doi: 10.1111/j.1939-1676.2003.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright J.G., Tengelsen L.A., Smith K.E. Multidrug-resistant Salmonella typhimurium in four animal facilities. Emerg Infect Dis. 2005;11(8):1235–1241. doi: 10.3201/eid1108.050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogeer-Gyles J.S., Mathews K.A., Boerlin P. Nosocomial infections and antimicrobial resistance in critical care medicine. J Vet Emerg Crit Care. 2006;16:1–18. [Google Scholar]
- 17.Sanchez S., McCrakin M.A., Stevenson C.R. Characterization of multidrug resistant Escherichia coli isolates associated with nosocomial infections in dogs. J Clin Microbiol. 2002;40(10):2586–2595. doi: 10.1128/JCM.40.10.3586-3595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene C.E. Environmental factors in infectious disease. In: Greene C.E., editor. Infectious diseases of the dog and cat. 3rd edition. Saunders Elsevier; St Louis (MO): 2006. pp. 991–1013. Chapter 94. [Google Scholar]
- 19.Dallap Schaer B.L., Aceto H., Rankin S.C. Outbreak of salmonellosis caused by Salmonella enterica serovar Newport MDR-ampC in a large animal veterinary teaching hospital. J Vet Intern Med. 2010;24:1138–1146. doi: 10.1111/j.1939-1676.2010.0546.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunowska M., Morley P.S., Traub-Dargatz J.L. Comparison of Salmonella enterica serotype Infantis isolates from a veterinary teaching hospital. J Appl Microbiol. 2007;102:1527–1536. doi: 10.1111/j.1365-2672.2006.03198.x. [DOI] [PubMed] [Google Scholar]
- 21.Ewart S.L., Schott H.C., II, Robison R.L. Identification of sources of Salmonella organisms in a veterinary teaching hospital and evaluation of the effects of disinfectants on detection of Salmonella organisms on surface materials. J Am Vet Med Assoc. 2001;218:1145–1151. doi: 10.2460/javma.2001.218.1145. [DOI] [PubMed] [Google Scholar]
- 22.Burgess B.A., Morley P.S., Hyatt D.R. Environmental surveillance for Salmonella enterica in a veterinary teaching hospital. J Am Vet Med Assoc. 2004;225(9):1344–1348. doi: 10.2460/javma.2004.225.1344. [DOI] [PubMed] [Google Scholar]
- 23.Ekiri A.B., Morton A.J., Long M.T. Review of the epidemiology of and infection control aspects of nosocomial Salmonella infections in hospitalized horses. Equine Vet Educ. 2010;22(12):631–641. [Google Scholar]
- 24.Weese J.S., DaCosta T., Button L. Isolation of methicillin-resistant Staphylococcus aureus from the environment in a veterinary teaching hospital. J Vet Intern Med. 2004;18:468–470. doi: 10.1892/0891-6640(2004)18<468:iomsaf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Hoet A.E., Johnson A., Nava-Hoet R.C. Environmental methicillin-resistant Staphylococcus aureus in a veterinary teaching hospital during a nonoutbreak period. Vector Borne Zoonotic Dis. 2011;11(6):609–615. doi: 10.1089/vbz.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller J., Armstrong S.K., Girvan E.K. Prevalence and distribution of methicillin-resistant Staphylococcus aureus within the environment and staff of a university veterinary clinic. J Small Anim Pract. 2009;50(4):168–173. doi: 10.1111/j.1748-5827.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 27.Loeffler A., Boag A.K., Sung J. Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J Antimicrob Chemother. 2005;56(4):692–697. doi: 10.1093/jac/dki312. [DOI] [PubMed] [Google Scholar]
- 28.KuKanich K.S., Ghosh A., Skarbek J.V. Surveillance of bacterial contamination in small animal veterinary hospitals with special focus on antimicrobial resistance and virulence traits of enterococci. J Am Vet Med Assoc. 2012;240(4):437–445. doi: 10.2460/javma.240.4.437. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh A., KuKanich K.S., Brown C.E. Resident cats in small animal veterinary hospitals carry multidrug resistant enterococci and are likely involved in cross-contamination of the hospital environment. Frontiers in Microbiol. 2012;3:1–14. doi: 10.3389/fmicb.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim L.M., Morley P.S., Traub-Dargatz J.L. Factors associated with Salmonella shedding among equine colic patients at a veterinary teaching hospital. J Am Vet Med Assoc. 2001;218(5):740–748. doi: 10.2460/javma.2001.218.740. [DOI] [PubMed] [Google Scholar]
- 31.Scheftel J.M., Elchos B.L., Cherry B. Compendium of veterinary standard precautions for zoonotic disease prevention in veterinary personnel: National Association of State Public Health Veterinarians Veterinary Infection Control Committee 2010. J Am Vet Med Assoc. 2010;237:1403–1422. doi: 10.2460/javma.237.12.1403. http://nasphv.org/Documents/VeterinaryPrecautions.pdf Available at: Accessed September 17, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Girling S.J., Fraser M.A. Bacterial carriage of computer keyboards in veterinary practices in Scotland. Vet Record. 2009;165(1):26–27. doi: 10.1136/vetrec.165.1.26. [DOI] [PubMed] [Google Scholar]
- 33.Bender J.B., Schiffman E., Hiber L. Recovery of staphylococci from computer keyboards in a veterinary medical centre and the effect of routine cleaning. Vet Record. 2012;170(16):414–416. doi: 10.1136/vr.100508. [DOI] [PubMed] [Google Scholar]
- 34.Lee D. Controlling infection through design. Long Term Living Magazine. 2012 http://www.ltlmagazine.com/article/controlling-infection-through-design Available at: Accessed September 17, 2014. [Google Scholar]
- 35.The Center for Health Design, Evidence-based design accreditation and certification (EDAC). Available at: http://www.healthdesign.org/edac. Accessed September 17, 2014.
- 36.Caveney L. Guidelines for effective cleaning and disinfection. In: Caveney L., Jones B., editors. Veterinary infection prevention and control. 1st edition. Wiley-Blackwell John Wiley and Sons; Chichester (United Kingdom): 2012. pp. 107–127. Chapter 6. [Google Scholar]
- 37.Dvorak G., Petersen C.A. Sanitation and disinfection. In: Miller L., Hurley K., editors. Infectious disease management in animal shelters. 1st edition. Wiley-Blackwell John Wiley and Sons; Ames (IA): 2009. pp. 49–60. Chapter 4. [Google Scholar]
- 38.Center for Food Security and Public Health, Iowa State University. Infection control for veterinarians. Available at: http://www.cfsph.iastate.edu/Infection_Control/overview-of-infection-control-for-veterinarians.php. Accessed September 17, 2014.
- 39.Carling P.C., Parry F., von Beheren M. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:1–7. doi: 10.1086/524329. [DOI] [PubMed] [Google Scholar]
- 40.Goodman E.R., Platt R., Bass R. Impact of environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol. 2008;29:593–599. doi: 10.1086/588566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaVecchia-Ragone G. Where are the germs? Long Term Living Magazine. 2013 http://www.ltlmagazine.com/article/where-are-germs Available at: Accessed September 17, 2014. [Google Scholar]
- 42.Weese J.S., Lowe T., Walker M. Use of fluorescent tagging for assessment of environmental cleaning and disinfection in a veterinary hospital. Vet Rec. 2012;171(9):217–219. doi: 10.1136/vr.100796. [DOI] [PubMed] [Google Scholar]
- 43.Association for Professionals in Infection Control and Epidemiology (APIC) and the Association for the Healthcare Environment (AHE). Clean spaces healthy patients project. Available at: http://cleanspaces.site.apic.org/. Accessed September 17, 2014.
- 44.McDonnell G.E. Antisepsis, disinfection, and sterilization: types, action, and resistance. ASM Press; Washington, DC: 2007. Mechanisms of antimicrobial resistance; pp. 253–334. Chapter 8. [Google Scholar]
- 45.Perry K., Caveney L. Chemical disinfectants. In: Caveney L., Jones B., editors. Veterinary infection prevention and control. 1st edition. Wiley-Blackwell John Wiley and Sons; Chichester (United Kingdom): 2012. pp. 129–143. Chapter 7. [Google Scholar]
- 46.Dvorak G. Center for Food Security and Public Health, Ames, IA: Iowa State University; 2005. Disinfection 101.http://www.cfsph.iastate.edu/BRM/resources/Disinfectants/Disinfection101Feb2005.pdf Available at: Accessed September 17, 2014. [Google Scholar]
- 47.Center for Food Security and Public Health, Iowa State University. Disinfection. Available at: http://www.cfsph.iastate.edu/Disinfection/index.php. Accessed September 17, 2014.
- 48.Rutala W.A., Weber D.J. Selection of the ideal disinfectant. Infect Control Hosp Epidemiol. 2014;35(7):855–865. doi: 10.1086/676877. [DOI] [PubMed] [Google Scholar]
- 49.Rutala W.A., Weber D.J. Selection and use of disinfectants in healthcare. In: Mayhall C.G., editor. Hospital epidemiology and infection control. 4th edition. Lippincott Williams & Wilkins; Philadelphia: 2012. pp. 1180–1212. Chapter 80. [Google Scholar]
- 50.Damani N. Manual of infection prevention and control. 3rd edition. Oxford University Press; Oxford (United Kingdom): 2012. Disinfection and sterilization; pp. 59–95. Chapter 6. [Google Scholar]
- 51.McDonnell G.E. ASM Press; Washington, DC: 2007. Antisepsis, disinfection, and sterilization: types, action, and resistance. [Google Scholar]
- 52.Fraise A.P. Decontamination of the environment and medical equipment in hospitals. In: Fraise A.P., Maillard J.Y., editors. Russell, Hugo and Ayliffe’s principles and practice of disinfection, preservation and sterilization. 4th edition. Blackwell Publishing; Oxford (United Kingdom): 2004. pp. 563–585. Part 2, Practice. Chapter 18. [Google Scholar]
- 53.Fraise A.P., Bradley C. Decontamination of equipment, the environment and the skin. In: Fraise A.P., Bradley C., editors. Ayliffe’s control of healthcare-associated infection: a practical handbook. 5th edition. Hodder Arnold Publishing; London: 2009. pp. 107–149. Chapter 6. [Google Scholar]
- 54.The national specifications for cleanliness in the NHS: a framework for setting and measuring performance outcomes. 2007. p. 25–7. Available at: http://www.nrls.npsa.nhs.uk/resources/?entryid45=59818. Accessed September 17, 2014.
- 55.McLay C. Preventing/controlling the transmission of infectious agents. In: McLay C., editor. Infection prevention competency review guide. 4th edition. Association for Professionals in Infection Control and Epidemiology (APIC); Washington, DC: 2010. pp. 163–241. Chapter 3. [Google Scholar]
- 56.Khamis N., van Knippenberg-Gordebeke G. Audits in infection prevention and control. In: Friedman C., Newsom W., editors. Basic concepts of infection control. 2nd edition. International Federation of Infection Control (APIC); Northern Ireland (United Kingdom): 2011. pp. 71–80. Chapter 6. [Google Scholar]
- 57.Park D., Larson A.M., Klibanov A.M. Antiviral and antibacterial polyurethanes of various modalities. Appl Biochem Biotechnol. 2013;169(4):1134–1146. doi: 10.1007/s12010-012-9999-7. [DOI] [PubMed] [Google Scholar]
- 58.Varghese S., Elfakhri S., Sheel D.W. Novel antibacterial silver-silica surface coatings prepared by chemical vapour deposition for infection control. J Appl Microbiol. 2013;115(5):1107–1116. doi: 10.1111/jam.12308. [DOI] [PubMed] [Google Scholar]
- 59.Morley P.S., Morris S.N., Hyatt D.R. Evaluation of the efficacy of disinfectant footbaths as used in veterinary hospitals. J Am Vet Med Assoc. 2005;226:2053–2058. doi: 10.2460/javma.2005.226.2053. [DOI] [PubMed] [Google Scholar]
- 60.Amass S.F., Arighi M., Kinyon J.M. Effectiveness of using a mat filled with peroxygen disinfectant to minimize shoe sole contamination in a veterinary hospital. J Am Vet Med Assoc. 2006;228:1391–1396. doi: 10.2460/javma.228.9.1391. [DOI] [PubMed] [Google Scholar]
- 61.Dunowska M., Morley P.S., Patterson G. Evaluation of the efficacy of a peroxygen disinfectant-filled footmat for reduction of bacterial load on footwear in a large animal hospital setting. J Am Vet Med Assoc. 2006;228:1935–1939. doi: 10.2460/javma.228.12.1935. [DOI] [PubMed] [Google Scholar]
- 62.Stockton K.A., Morley P.S., Hyatt D.R. Evaluation of the effects of footwear hygiene protocols on nonspecific bacterial contamination of floor surfaces in an equine hospital. J Am Vet Med Assoc. 2006;228:1068–1073. doi: 10.2460/javma.228.7.1068. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann F.A., Dusick A.F., Young K.M. Impact of disinfectant-filled foot mats on mechanical transmission of bacteria in a veterinary teaching hospital. J Am Vet Med Assoc. 2013;242(5):682–688. doi: 10.2460/javma.242.5.682. [DOI] [PubMed] [Google Scholar]
- 64.McDonald L.L., Pugliese G. Textile processing service. In: Mayhall C.G., editor. Hospital epidemiology and infection control. 2nd edition. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 1031–1034. [Google Scholar]
- 65.Hoffman P. Laundry, kitchens and healthcare waste. In: Fraise A.P., Bradley C., editors. Ayliffe’s control of healthcare-associated infection: a practical handbook. 5th edition. Hodder Arnold Publishing; London: 2009. pp. 107–149. Chapter 6. [Google Scholar]
- 66.Damani N. Manual of infection prevention and control. 3rd edition. Oxford University Press; Oxford (United Kingdom): 2012. Support services; pp. 327–347. Chapter 18. [Google Scholar]
- 67.American Veterinary Medical Association, Veterinary practice waste disposal decision maker. Available at: https://www.avma.org/PracticeManagement/Administration/Pages/Veterinary-Practice-Waste-Disposal-Decision-Maker.aspx. Accessed September 17, 2014.
- 68.Sehulster L.M., Rose L.J., Noble-Wang J. Microbiologic sampling of the environment in healthcare facilities. In: Mayhall C.G., editor. Hospital epidemiology and infection control. 4th edition. Lippincott Williams & Wilkins; Philadelphia: 2012. pp. 1059–1075. Chapter 72. [Google Scholar]
- 69.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130–137. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dancer S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect. 2004;56:10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]