Abstract
Seizure disorders in young animals pose different considerations as to cause and therapeutic decisions compared with adult animals. Infectious diseases of the nervous system are more likely in puppies and kittens compared with adults. The diagnosis of canine distemper is often based on clinical signs. Idiopathic epilepsy typically occurs in dogs between 1 and 5 years of age; however, inflammatory brain diseases such as necrotizing encephalitis and granulomatous meningoencephalomyelitis also commonly occur in young to middle-aged small-breed dogs. The choice of which anticonvulsant to administer for maintenance therapy is tailored to each individual patient.
Keywords: Pediatric neurology, Dog, Cat, Seizures, Distemper, Neospora, Cryptococcus
Key points
-
•
Seizure disorders in young animals pose different considerations as to cause and therapeutic decisions compared with adult animals.
-
•
Infectious diseases of the nervous system are more likely in puppies and kittens compared with adults.
-
•
The diagnosis of canine distemper is often based on clinical signs, with the combination of neurologic abnormalities, particularly myoclonus, with extraneural signs leading to a high suspicion of canine distemper in dogs less than 1 year old.
-
•
Idiopathic epilepsy typically occurs in dogs between 1 and 5 years of age; however, inflammatory brain diseases such as necrotizing encephalitis and granulomatous meningoencephalomyelitis also commonly occur in young to middle-aged small-breed dogs.
-
•
The choice of anticonvulsant for maintenance therapy is tailored to each individual patient.
Seizures are the manifestation of abnormal synchronous electrical activity in the brain and are the most common neurologic disorder in dogs. Seizures may be categorized as generalized, when both cerebral hemispheres are involved, or partial, when cerebral involvement is focal. Generalized seizures result in loss of consciousness and the whole body is affected with signs such as paddling, convulsions, or increased tone. Salivation is common and some animals may urinate, defecate, or vomit at the time of the seizure. Partial seizures affect only a portion of the body. Consciousness is unimpaired during simple partial seizures and is altered during complex partial seizures. During psychomotor seizures, a complex partial seizure, the animal may appear panicked as it compulsively runs around, and may appear aggressive, hissing or growling. A preictal period before the seizure and a postictal period lasting minutes to hours after the seizure are common with generalized seizures. Preictal and postictal signs may include anxiety, attention seeking, panting, and pacing. The recognition of seizures can sometimes be difficult because vestibular episodes, dyskinesias, and syncopal episodes can appear seizurelike. A thorough history should be gathered including the potential for toxin exposure, familial history, previous events, activity during onset, time of onset, duration of the event, elimination during the event, and interictal behavioral or gait changes. In the pediatric patient, information regarding birth, litter mates, suckling, and weight gain is also important. Seizure disorders in young animals pose different considerations as to cause and therapeutic decisions compared with adult animals. Congenital, developmental, metabolic, toxic, infectious, and inflammatory causes are considered more likely in puppies and kittens.
A thorough evaluation for the underlying cause for seizures should be pursued to optimize anticonvulsant therapy. Patients with underlying causes other than idiopathic epilepsy may require medication in addition to anticonvulsant therapy. In addition, patients with secondary epilepsy often require anticonvulsant therapy sooner than those with primary/idiopathic epilepsy. Complete blood count (CBC), serum chemistry, and urinalysis should be done for all dogs and cats presenting for seizures. Hypoglycemia should be ruled out in all puppies and kittens. Bile acid testing is important in all puppies and small-breed dogs.
Portosystemic shunts
Portosystemic shunts (PSSs) are common in small-breed dogs (Yorkshire terrier, Maltese terrier, and miniature schnauzer) and are commonly diagnosed at less than 2 years of age. Congenital extrahepatic PSSs are usually single vessels that connect the portal venous system to the systemic circulation. They may arise from any vessel and account for most shunts. Portocaval shunts are more common than portoazygous shunts.1 PSS in large-breed dogs are typically intrahepatic in location. PSS often causes episodic encephalopathic signs such as mentation changes, head pressing, vocalization, ataxia, blindness, and seizures. Between 79% and 82% of dogs have central nervous system (CNS) signs associated with PSS.1, 2 Neurologic signs are typically worse after eating and often wax and wane. Other signs include vomiting, anorexia, polyuria, polydipsia, decreased weight, hematuria, and stranguria. The cause of hepatic encephalopathy is unclear, with several potential causes including hyperammonemia, altered tryptophan synthesis, false neurotransmitter synthesis, alterations in amino acid neurotransmitters, and increased cerebral endogenous benzodiazepine concentrations.3
Serum albumin and blood urea nitrogen are typically decreased in dogs with PSS. Preprandial and postprandial bile acids are increased in 95% and 98% of dogs with extrahepatic PSSs. Mean preprandial and postprandial bile acids were 139.9 and 248.2 μmol/L respectively in a study of 168 dogs with single extrahepatic shunts. Ammonium biurate or uric acid crystalluria is common.1 Hyperammonemia is also a common finding. Mean serum ammonia was 206 μmol/L in dogs and 295 μmol/L in cats with PSS in one study.4 The sensitivity of increased ammonia was 85% in dogs and 83% in cats. The specificity of ammonia was 86% in dogs and 76% in cats. The sensitivity of increased bile acids was 93% in dogs and 100% in cats. The specificity of bile acids was 67% in dogs and 71% in cats.4
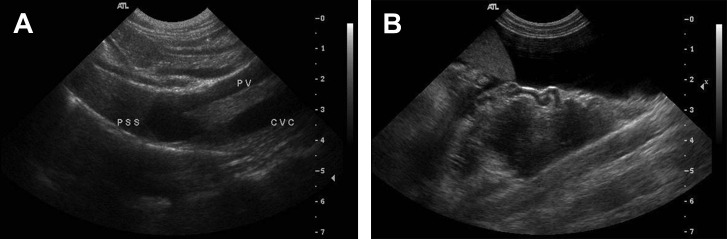
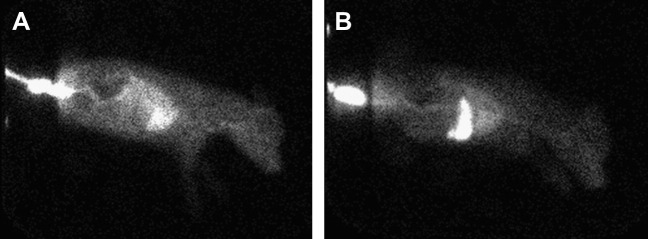
Abdominal radiographs typically indicate microhepatica. Confirmation of PSS may be done via abdominal ultrasound or nuclear scintigraphy. The overall sensitivity of ultrasonography was 92% and specificity was 98% in one study. Ultrasonography identified 38 of 42 (90%) extrahepatic PSSs in dogs and cats, and 11 of 11intrahepatic shunts. Ultrasonography correctly identified the extrahepatic location as portocaval or portoazygous 90% (34 of 38) of the time and correctly identified the location 11 of 11 times in intrahepatic shunts (Figs. 1 and 2 ).5
Fig. 1.
Abdominal ultrasound. (A) Intrahepatic portosystemic shunt. (B) Extrahepatic (tortuous) shunt vessel seen lateral to the spleen. Ascites is evident.
(Courtesy of Tom Baker, MS, Davis, CA.)
Fig. 2.
Nuclear scintigraphy. (A) The radioactive material bypasses the liver and is identified in the heart. (B) Negative for a shunt after surgical correction. The radioactive material is noted in the liver.
(Courtesy of Rich Larson, Davis, CA.)
Medical therapy for PSS consists of dietary modification, antibiotic therapy, and lactulose with the goal of decreasing NH3 production. A low-protein diet consisting of high-quality digestible protein is recommended. Commercially available liver disease diets or home-cooked diets with chicken, cottage cheese, rice, and pasta are typically fed. Feeding smaller portions more frequently may be beneficial. Ampicillin 22 mg/kg intravenously (IV) every 6 hours or metronidazole 7.5 to 10 mg/kg by mouth twice a day are commonly used to decrease NH3-producing bacteria. Lactulose acidifies colonic contents leading to conversion of NH3 to NH4. Lactulose also decreases fecal transit time, thereby speeding the elimination of bacteria and NH3. Lactulose is given to effect, to produce a soft stool. Gastrointestinal ulceration is an increased risk in patients with PSS. Thus, gastric protectants such as famotidine 0.5 mg/kg by mouth twice a day or omeprazole 0.5 to 1 mg/kg by mouth every 24 hours should be given.6 Anticonvulsant therapy is indicated if seizures are present or given perioperatively.
Surgical ligation/constriction is recommended when possible. Surgery redirects the flow of blood to the hepatic parenchyma. Various techniques are available, including surgical ligation or partial ligation, or the use of devices that gradually occlude the aberrant vessel, such as an ameroid constrictor or a cellophane band. The use of ameroid constrictors and cellophane bands has decreased mortality associated with surgery. Surgical therapy is typically considered superior to medical therapy; however, both therapies can be beneficial. A study comparing surgical therapy with medical therapy indicated a shunt-related mortality of 10.1% of dogs treated surgically versus a shunt-related mortality of 29.6% of dogs treated medically. Perioperative mortality was 4% in this study.7 Postoperative seizures are reported in 5% to 18% of dogs surgically and typically occur within 3 days of surgery.2, 8 Neurologic signs may occur from decreased circulating endogenous benzodiazepine ligands, imbalances in excitatory or inhibitory neurotransmitters,8 or hypoglycemia.2 Postoperative generalized seizures have been associated with high mortality, whereas dogs with postoperative partial seizures typically respond to therapy.1 Although phenobarbital therapy did not significantly reduce the incidence of seizures, it did seem to reduce the severity of seizures in a study.2 None of 42 dogs treated with levetiracetam 20 mg/kg by mouth 3 times a day had postoperative seizures in one study.8
Hydrocephalus
Hydrocephalus is an increase in cerebrospinal fluid (CSF) volume and should be considered in toy and brachiocephalic breeds. A dome-shaped head or persistent fontanelle may be present. Stenosis of the mesencephalic aqueduct is the most common cause of hydrocephalus. The stenosis typically results in fusion of the rostral colliculi.9 CSF accumulates rostral to the obstruction and cerebral cortical atrophy occurs. Tearing of ependymal cells and periventricular diverticula can occur from dilatation of the ventricular system.10 Hydrocephalus may also result from neoplasia or infection obstructing CSF flow. Hydrocephalus was reported secondary to a leiomyosarcoma in a 2-month-old Chihuahua.11 Cats may develop hydrocephalus secondary to infectious causes such as feline infectious peritonitis (FIP). Hydrocephalus can result from autosomal recessive inheritance in Siamese cats.12
The degree of clinical signs from hydrocephalus is variable. Most hydrocephalic animals are asymptomatic. Ventriculomegaly is a common incidental finding in toy and brachiocephalic breeds.13 Clinical signs can result from alterations in intracranial pressure, loss of cortical neurons or neuronal function, or periventricular edema secondary to abnormal CSF flow across ventricular walls. Clinical signs depend on the anatomic location most affected. Cerebral, vestibular, and cerebellar signs can be seen. A ventral and/or lateral strabismus can occur because of a skull or orbit deformity or from increased pressure on the mesencephalic tegmentum (Fig. 3 ).14 A thinned calvarium, decreased cerebral tissue, and changes in cerebral vascularity can cause hydrocephalic animals to be more sensitive to head trauma. A hemorrhagic event can lead to acute neurologic deterioration. Chronic subdural hematomas have been identified in a hydrocephalic dog.15
Fig. 3.

Chihuahua with hydrocephalus. Note the dome-shaped head and lateral strabismus oculus uterque (OU).
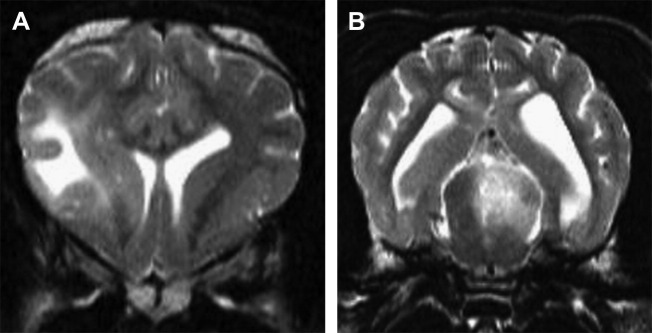
Diagnosis is made via cranial magnetic resonance imaging (MRI) or CT scan. Ultrasound through a persistent fontanelle may also be used in the diagnosis (Fig. 4 ). CT scan effectively evaluates the ventricular system. However, beam hardening artifact can obscure evaluation of the brainstem. The superior soft tissue imaging of MRI allows the most detailed evaluation of the disorder. MRI provides superior evaluation of the ventricular system and brainstem.14, 16 MRI can help rule out other causes for hydrocephalus such as infection and neoplasia and can evaluate for concurrent hemorrhage (Fig. 5 ). CSF analysis is important to rule out concurrent inflammatory or infectious disorders.
Fig. 4.
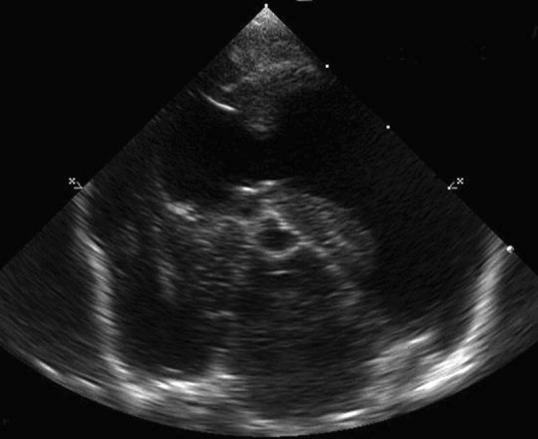
Transverse ultrasound through a persistent fontanelle at the level of the midbrain. The lateral ventricles are dilated.
Fig. 5.
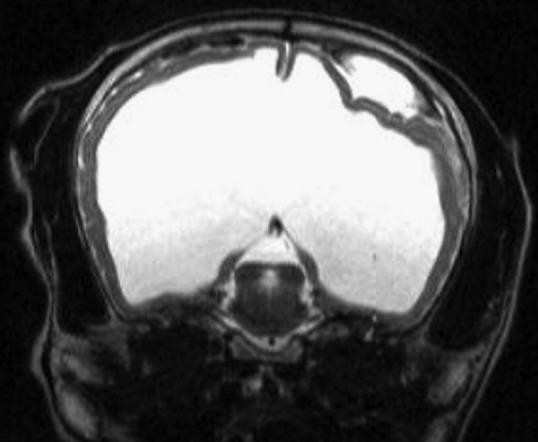
Transverse T2-weighted MRI. Severe hydrocephalus. An extra-axial hyperintense mass is present. MRI characteristics of the mass indicate a hemorrhage.
Medical therapy is indicated when hydrocephalus causes clinical signs. Medical therapy is directed at decreasing CSF production and decreasing periventricular edema. Glucocorticoids decrease CSF production by decreasing Na-K-ATPase activity17 and may be beneficial if periventricular edema is present. Prednisone is started at 0.5 mg/kg by mouth twice a day and is tapered to the lowest effective dose. Omeprazole also decreases CSF production and may be of benefit. The action of omeprazole action may be independent Na-K-ATPase inhibition.18 Acetazolamide, a carbonic anhydrase inhibitor, may be of benefit and is given at 10 mg/kg by mouth every 6 to 8 hours. Electrolytes should be monitored with acetazolamide therapy because it can cause hypokalemia.14, 16 Anticonvulsant therapy is indicated when hydrocephalus causes seizures.
Surgical placement of a ventriculoperitoneal shunt is considered for dogs with progressive signs not responsive to medical therapy (Fig. 6 ). Success with shunting is variable. Shunts have not been proved to be more effective than medical therapy. However, shunting does offer the possibility of long-term control of clinical signs.14 A study of ventriculoperitoneal shunting for congenital hydrocephalus in 36 dogs and cats indicated improved clinical signs in 72% of patients. Complications occurred in 22% of patients, typically within the first 3 months after shunt placement. Median follow-up in this study was 6 months, thus complications could be higher. Thirty-six percent of patients died of hydrocephalus-related complications or were euthanized. The 3-month postsurgical survival rate was 66% and the 18-month survival rate was 55%.19 Two smaller canine studies indicated postoperative complication rates of 25%20 and 29%.21
Fig. 6.
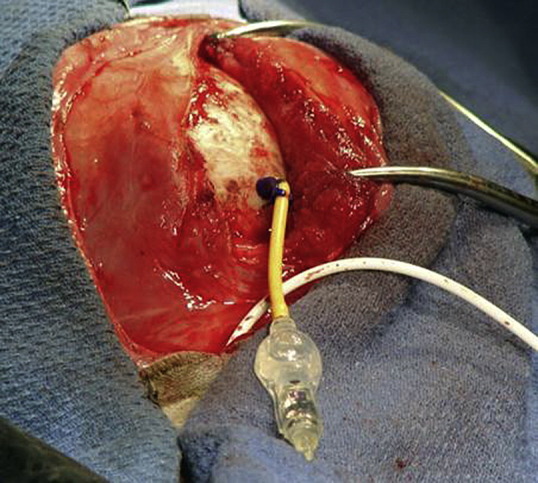
Surgical placement of a shunt in a coyote with severe hydrocephalus.
Potential complications of shunting include infection, occlusion of the shunt, undershunting, overshunting, shunt migration,19, 22 and valve fracture.21 The need for surgical revision is common in people. Shunt failure occurred in 177 of 344 hydrocephalic children. Only 48% of the shunts were still successful 1 year after shunt implantation. The most common reason for failure was shunt obstruction.22
L-2 hydroxyglutaric aciduria
A metabolic defect causing L-2 hydroxyglutaric aciduria has been recognized in Staffordshire bull terriers,23 the West Highland white terrier,24 and the Yorkshire terrier.25 L-2 hydroxyglutaric aciduria is an autosomal recessive disease in the Staffordshire bull terrier. The genetic mutation has been identified on exon 10 of chromosome 8 in the Staffordshire bull terrier.26 This mutation is different than the mutation in the Yorkshire terrier.25 Clinical signs of L-2 hydroxyglutaric aciduria include seizures, ataxia, stiff gait, decreased mentation, and head tremors. Signs occur from 4 months to 7 years of age, but are often identified at less than 1 year of age. Organic acid analysis of urine, CSF, and plasma are abnormal in affected dogs. MRI identified bilaterally symmetric, diffuse hyperintensity on T2 imaging in the gray matter throughout the brain. No effective treatment is known at this time, other than symptomatic anticonvulsant therapy. Dietary therapy may be of benefit. Cobalamin therapy may be beneficial in methylmalonic aciduria in people.23 A 6-month-old Cavalier King Charles spaniel was reported to have seizures secondary to a hexanoylglycine aciduria. l-Carnitine therapy is recommended in people with medium-chain acyl coenzyme A dehydrogenase deficiency and thus may be of benefit for hexanoylglycine aciduria.27
Lissencephaly
Lissencephaly is a congenital abnormality thought to occur from the arrest of neuronal migration to the cortical plate during fetal development. Lissencephaly results in a smooth cerebral cortical surface as gyri and sulci fail to develop. The cerebral cortex becomes thicker than normal. Fusion of the corpus callosum and internal capsule cause the corona radiata (white matter) to be absent.9, 28 Lissencephaly most commonly is reported in the Lhasa apso.9, 28, 29, 30 It has also been reported in the wire-haired fox terrier, the Irish setter and in Korat cats.9 Seizures are common and may be seen at 1 year of age. Behavioral changes, circling, and visual deficits may also be seen.9, 28, 30 Diagnosis is made via MRI (Fig. 7 ). Symptomatic anticonvulsant therapy is recommended.
Fig. 7.
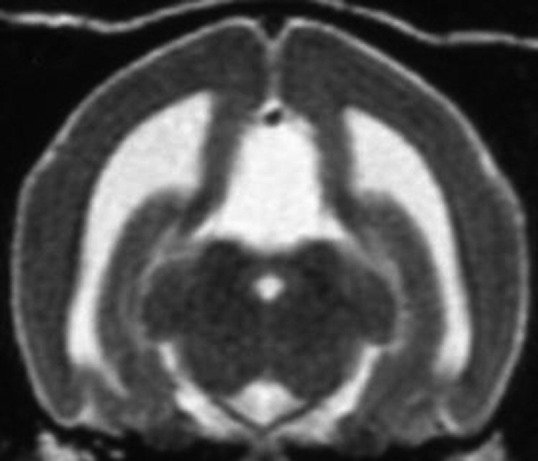
Transverse T2-weighted MRI in a Lhasa apso. The absence of gyri and sulci indicates lissencephaly.
(Courtesy of Gregg Kortz, DVM, Sacramento, CA.)
Granulomatous meningoencephalomyelitis
Granulomatous meningoencephalomyelitis (GME) accounts for 5% to 25% of all CNS disorders in dogs. Young to middle-aged small-breed dogs are most commonly affected. However, any age or breed may develop GME. On histology, GME is characterized by perivascular cuffs of primarily mononuclear cells within white matter. The accumulation of inflammatory cells is typically microscopic, but areas can coalesce to form a focal granulomatous mass. On histology, the brainstem is commonly involved. Multifocal disease is common, and all regions of the CNS (cerebrum, cerebellum, brainstem, and cervical spinal cord) may be affected. Ocular GME has also been reported. Clinical signs vary based on the region of the CNS involved. The onset may be acute or chronic.31
The cause of GME remains unclear. A T cell–mediated delayed-type hypersensitivity reaction with an autoimmune basis is suspected. Infectious causes have been explored and have not been identified. The combination of a nonspecific immunologic response and genetic and environmental factors are all considered likely to play roles in the cause of GME.32
Definitive diagnosis of GME can only be made via histopathology. However, a presumptive diagnosis is made antemortem, in the absence of brain biopsy, via a combination of MRI and CSF results. CBC, serum chemistry, and urinalysis are unremarkable. CSF is the single most useful test in inflammatory brain disease.33 CSF typically yields a mononuclear pleocytosis with increased protein; however, a mixed pleocytosis with an increased percentage of neutrophils is common. CSF results in GME can sometimes mimic the results of other inflammatory brain diseases. Brain imaging can be helpful in differentiating GME from other inflammatory brain disease such as necrotizing meningoencephalitis (NME) and necrotizing leukoencephalitis (NLE). NME and NLE have characteristic abnormalities on MRI that, if present, can help differentiate them from GME. In GME, MRI results often identify edema (hyperintense lesions on T2 and fluid-attenuated inversion recovery [FLAIR]) and contrast-enhancing lesions (Fig. 8 ). MRI abnormalities are typically located within white matter consistent with the histologic distribution of the disease. At times, patients with GME have normal MRI results.34
Fig. 8.
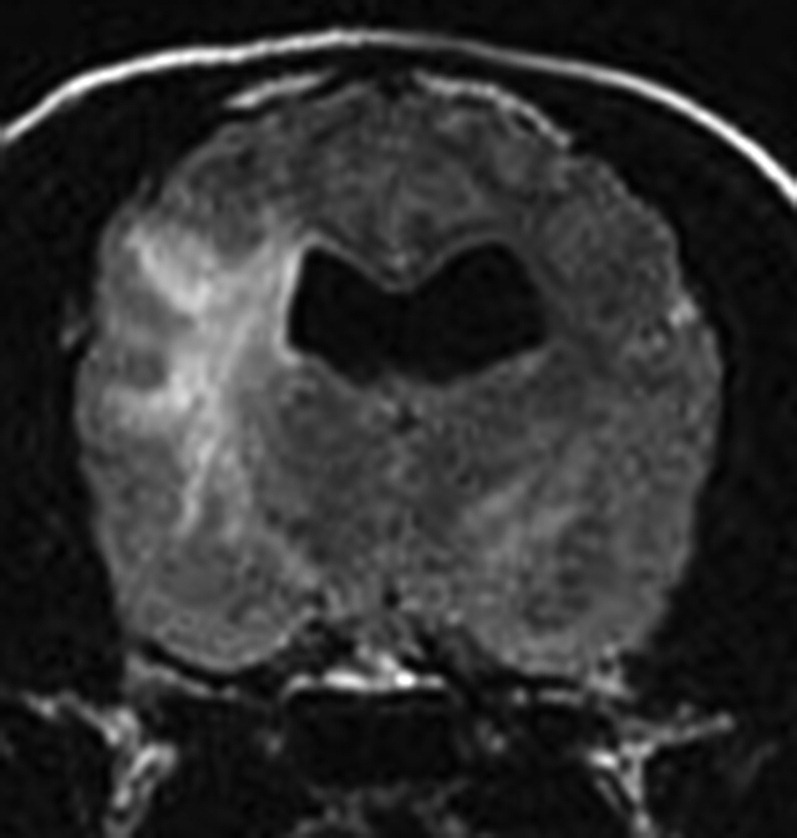
Transverse FLAIR MRI in a cockapoo. Note the significant hyperintense lesion in the white matter and a smaller hyperintense lesion in the opposite thalamus. CSF indicated a white blood cell count (WBC) of 668 cells/μL and a protein of 462 mg/dL. MRI and CSF findings are consistent with GME.
The mainstay of treatment is corticosteroids. Prednisone is used to treat CNS edema as well as to suppress the immune system. In rapidly deteriorating dogs or in dysphagic dogs, dexamethasone sodium phosphate 0.25 mg/kg IV or subcutaneously every 24 hours to twice a day is given. Prednisone at 1 mg/kg by mouth twice a day is later given. In severe or refractory cases, cytosine arabinoside may be given at 50 mg/m2 twice a day for 2 consecutive days and then repeated if necessary every 3 weeks. As an alternative, procarbazine at 25 mg/m2 by mouth every 24 hours35 or cyclosporine 3 to 15 mg/kg by mouth twice a day36 may be given. Routine monitoring of the CBC should be done when these drugs are given. Serial urinalysis with or without culture is also recommended in immunosuppressed dogs. Clinical signs and serial CSF evaluation are used to assess therapeutic success. Prednisone is tapered over a period of several months. Many dogs require long-term prednisone therapy. Adjunctive therapy such as cytosine arabinoside, cyclosporine, or procarbazine may lessen the dependence on corticosteroids and lead to a more favorable prognosis. The prognosis for GME has been considered guarded. The median survival time for dogs with focal signs was 114 days, whereas dogs with multifocal signs had a median survival time of 8 days in one study.31 Recent studies have shown a better prognosis. One study reported a median survival time of 14.0 months with procarbazine and prednisone treatment compared with 0.62 months for dogs not receiving treatment.37 Another study showed a median survival time of 930 days with use of cyclosporine or in combination with corticosteroids or ketoconazole.36 A study comparing the use of lomustine and prednisolone with prednisolone alone yielded a median survival time of 457 days compared with 329 days, respectively. The difference in survival time between groups was not statistically significant.38 A study evaluating the use of cytosine arabinoside and prednisone in 10 dogs with inflammatory brain disease of unknown cause indicated a median survival time of 531 days. The study included dogs with suspected GME as well as NME or NLE.39
Necrotizing encephalitis
NME is an inflammatory disease causing necrotic cystic lesions within the gray and white matter of the cerebrum. In pugs, Chihuahuas, Maltese terriers, shih tzu and papillon the necrotic condition is often adjacent to the cerebral gray-white matter junction. In the Yorkshire terrier, NLE causes necrotic white matter lesions in the cerebrum and typically inflammatory lesions in the brainstem and cerebellum. Because of the breed-specific variance in lesion location it is uncertain whether these diseases are variants of the same disease or different diseases.40 NME and NLE have also been reported in the Pekingese and French bulldog.40, 41 Male and female, young to middle-aged dogs are typically affected; however, dogs as old as 10 years of age have been reported. Seizures are common. Mentation changes, circling, and placing deficits may also be seen. Vestibular signs may predominate when the brainstem is affected in NLE. CSF typically indicates a mononuclear pleocytosis with increased protein. However, at times CSF is normal. MRI helps confirm the location and presence of cavitative lesions. MRI may also differentiate the disease from GME. Lesions with NME are multifocal, asymmetric, and present at the gray-white matter junction. They are typically hyperintense on T2 and FLAIR imaging and isointense or hypointense on T1 imaging. Contrast enhancement is variable (Fig. 9 ). NLE lesions are typically asymmetric, multifocal, and affect the subcortical white matter. The lesions are hyperintense on T2 and FLAIR imaging and isointense or hypointense on T1 imaging. Contrast enhancement is also variable (Fig. 10 ).32 Recommended treatment is prednisone at 1 mg/kg by mouth twice a day with a gradual reduction in dose if response to therapy occurs. The prognosis for necrotizing encephalitis is poor. Mean survival time with corticosteroid treatment was 97 days in one study.42 Dogs with necrotizing encephalitis may be less responsive to therapy than those treated for suspected GME.
Fig. 9.
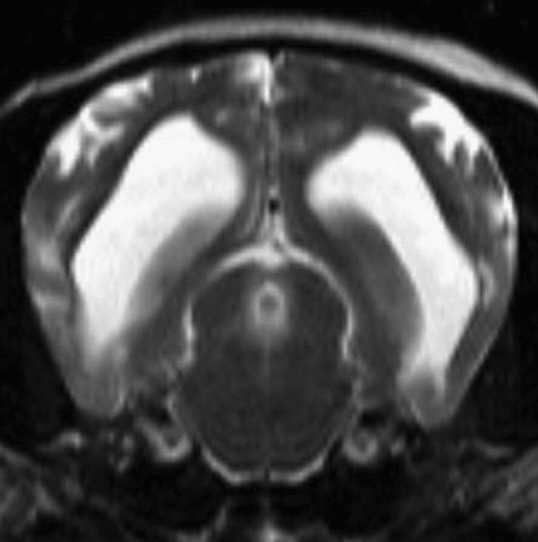
Transverse T2-weighted MRI of a Chihuahua. Note the multifocal strongly hyperintense lesions affecting the cerebral gray matter consistent with NME. CSF indicated a WBC of 1/μL and a protein of 45 mg/dL.
Fig. 10.
(A) Transverse T2-weighted MRI. Note the strongly hyperintense lesion in the cerebral white matter. (B) Transverse T2-weighted MRI. A second hyperintense lesion is present in the midbrain. MRI is consistent with NLE.
Infectious diseases
Infectious diseases of the nervous system are more likely in puppies and kittens compared with adults. Poor suckling leading to malnutrition and incomplete transfer of maternal antibodies, difficult parturition, environmental stresses, and concurrent disease processes such as parvovirus can weaken the immune system, increasing the chance of bacterial meningoencephalitis. Animals with bacterial meningoencephalitis may have a fever or leukocytosis. Lethargy and apparent back pain may also be present. Thorough examination, abdominal ultrasound, and thoracic radiographs can identify a nidus of infection. The infection can arise from a dermal lesion or a recent surgical procedure with hematogenous spread to the CNS. Otitis media interna can spread centrally into the brain leading to bacterial meningitis.
CSF results with bacterial meningoencephalitis are neutrophilic. However, severe neutrophilic pleocytosis can also be seen with noninfectious inflammatory diseases such as steroid-responsive meningitis arteritis. MRI may identify meningeal enhancement, abscess formation, or otitis media interna consistent with an infectious process, making steroid-responsive meningitis arteritis less likely (Figs. 11 and 12 ). Culturing the CSF and/or urine may help identify a bacterial cause. Visualization of bacteria in the CSF allows a more definitive diagnosis of bacterial meningoencephalitis. Staphylococcus, Escherichia coli, Streptococcus, and various anaerobes are typically present in bacterial meningoencephalitis.43 CNS penetration is often a consideration when choosing an antibiotic. At first, the compromised blood-brain barrier allows better penetration into the CNS. Once the blood barrier is intact, antibiotic penetration may be more difficult. Thus, a longer course of antibiotic therapy, higher dosages, or choosing an antibiotic that more reliably penetrates the CNS may be required. Chloramphenicol reaches high CNS concentrations, but is not typically used because of the potential adverse effects, particularly in neonates. Doxycycline penetrates the CNS, but its bacteriostatic nature and concerns for its use in young animals should be considered. Trimethoprim/sulfa also reaches high CNS concentrations, but concerns regarding potential adverse effects have also limited its use. Amoxicillin/clavulanic acid is broad spectrum, but reaches low CSF concentrations when the blood-brain barrier is intact. Fluoroquinolones are good choices for Staphylococcus and E coli, but have variable effects on Streptococcus and also reach low CSF concentrations.44
Fig. 11.
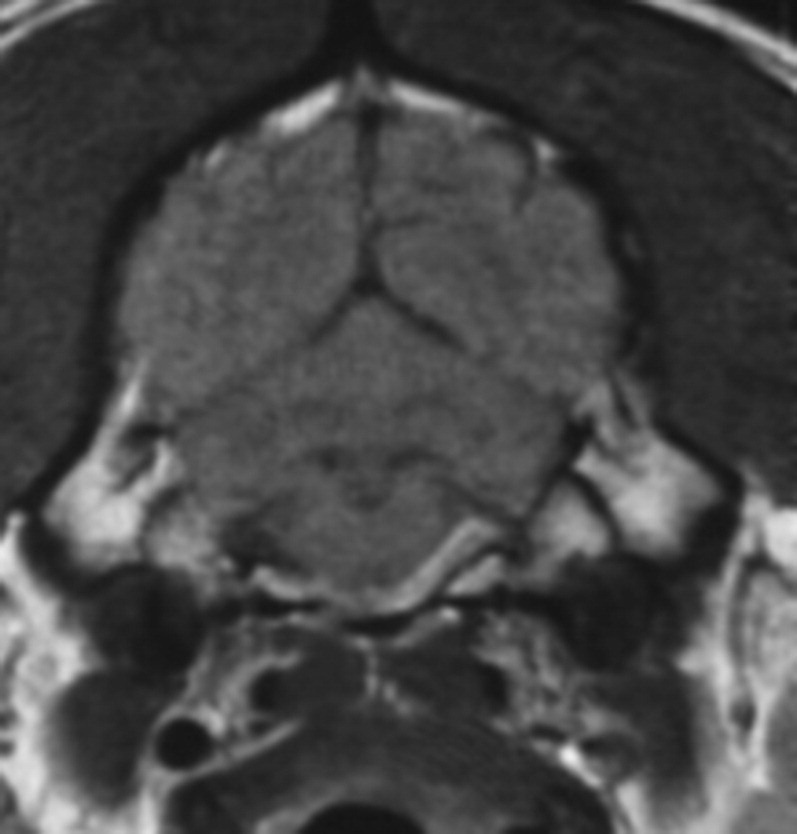
Transverse T1-weighted postcontrast MRI. Contrast enhancement is present in the ventrolateral brainstem consistent with an infectious process rather than steroid-responsive meningitis arteritis.
Fig. 12.
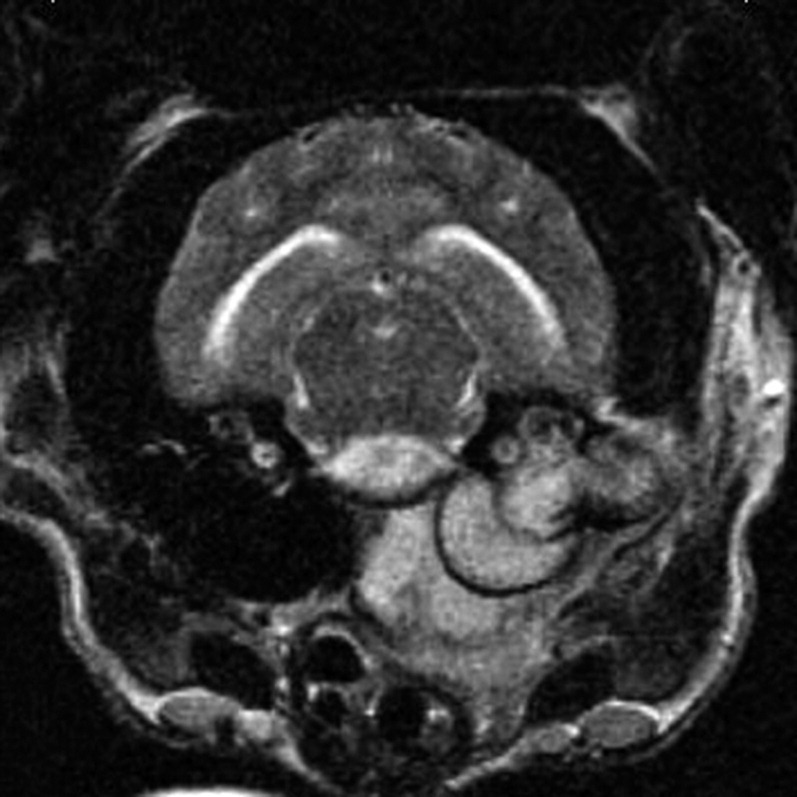
Transverse T2-weighted MRI. Hyperintense material is present in the tympanic cavity, in the soft tissue surrounding the tympanic bulla, and in the ventral brainstem. Otitis media interna has spread centrally into the brainstem.
Distemper
Canine distemper virus (CDV), a single-stranded RNA Morbillivirus, is typically spread through an aerosol route. CDV is epitheliotropic. The respiratory system is initially infected, with subsequent spread to the CNS, integument, bladder, and intestinal tract. CDV enters the nervous system through CSF or crosses the blood-brain barrier via infected mononuclear cells. Multifocal lesions are common. Primary demyelination often occurs in the white matter, although gray matter can also be affected. Areas of the brain that are commonly affected include the cerebellum, periventricular white matter, optic pathways, and spinal cord.45
Clinical signs are typically seen in dogs less than 1 year old. Persistent viral infection can lead to a chronic form, so-called old-dog distemper. Outbreaks may occur, particularly in animal shelters, caused by crowded conditions, diverse populations, and stress. A study in a Florida animal shelter indicated that only 43.2% of dogs admitted into the shelter had a protective antibody titer (PAT) for distemper. Age and whether a dog was neutered were associated with a PAT. Seventy-five percent of dogs more than 2 years old had a PAT compared with only 17.1% of dogs less than 1 year old. Neutered dogs were 8.3 times more likely to have a PAT than intact dogs.46 Vaccination has reduced the incidence, but 30% of cases occur in vaccinated dogs.47 Neurologic signs are typically focal despite multifocal lesions. Seizures and myoclonus are the most common neurologic signs. Brainstem, cerebellar, and myelopathic signs may also be seen.48 Myoclonus may be caused by hyperexcitability of the lower motor neuron. It may persist during sleep, but disappears with anesthesia.49
The diagnosis of CDV is often based on clinical signs. The combination of neurologic abnormalities, particularly myoclonus, with extraneural signs should lead to a high suspicion of CDV. Extraneural signs are common and include pneumonia, enteritis, conjunctivitis, rhinitis, discolored teeth, and hyperkeratosis of the nasal planum and foot pads.47 Pulmonary infiltrates are often identified by thoracic radiographs.48 CSF often identifies a mononuclear pleocytosis, but albuminocytologic disassociation or normal CSF analysis can occur.47 Serology is not typically helpful because of prior vaccination or previous subclinical infection. Severe CDV infection can sometimes have a low titer from immunosuppression. Direct immunofluorescence testing of conjunctival, vaginal, and nasal smears can confirm CDV only within 3 weeks of infection.50 Polymerase chain reaction (PCR) testing can be helpful. PCR testing may be done on CSF, blood, conjunctiva, or urine. Reverse transcriptase PCR (RTPCR) is considered to be the best antemortem method used to detect CDV. RTPCR detected CDV RNA in 88% of infected dogs via whole blood or CSF testing and in 86% of infected dogs via serum testing. In one study, 22 of 22 dogs tested positive when PCR testing was done on the urine. Twelve asymptomatic dogs tested negative. Vaccine virus can be identified for a couple of days after vaccination.51
The prognosis for distemper infections is variable. Severely affected dogs are likely to die, whereas mildly affected dogs may recover. Supportive care is the mainstay of therapy. Anticonvulsants should be used to treat seizures, antibiotics for pneumonia, and intravenous fluids to maintain hydration. Concurrent toxoplasma infection may occur and, if present, should be treated.52
Neosporosis
Neospora caninum, a protozoal parasite, is a primary CNS and neuromuscular pathogen in dogs. Pneumonia, encephalitis, myocarditis, hepatitis, and myonecrosis are the predominant lesions in neonates. Ninety-two percent of affected dogs are less than 1 year old. Transplacental transmission is the major route of infection. An ascending paralysis is typically the presenting sign with possible progression to the cervical, brainstem, and cerebellar regions. Polyradiculoneuritis and polymyositis may result in hyperextension of the pelvic limbs. Creatine kinase can be increased when a polymyositis is present. CSF abnormalities typically include a mononuclear pleocytosis. The diagnosis can be supported via serum immunofluorescence antibody (IFA) titers. Affected dogs have IFA titers greater than 1:200. However, clinically normal dogs can have titers as high 1:800. Tachyzoites can be identified in cells from CSF, dermal lesions, and bronchial lavage.53 PCR of the CSF was positive in 4 of 5 dogs in one report.54 Clindamycin 10 to 15 mg/kg by mouth every 12 hours for 4 weeks is typically used for neosporosis therapy,52 although trimethoprim sulfonamide 15 to 20 mg/kg by mouth every 12 hours may also be used alone or concurrently with clindamycin. Clindamycin is effective against tachyzoites, but is likely ineffective against encysted bradyzoites. Despite clinical improvement the N caninum infection is not cleared from the body.55 The prognosis is considered poor,53 but successful treatment is possible. Delayed therapy, muscle fibrosis, and pelvic limb hyperextension are considered poor prognostic indicators.54
Toxoplasmosis
Toxoplasma gondii is a protozoal parasite that is transmitted via ingestion of sporulated oocysts transplacentally or in undercooked meats.56 Cats are the definitive host. The most severe signs are caused by transplacental infection in kittens. Kittens may be stillborn or die within weeks. Pulmonary, CNS, cardiac, pancreatic, hepatic, and ocular lesions are common in clinically infected cats. Brain lesions are common, although neurologic signs are absent in many affected cats.57 Muscle involvement is uncommon in cats.58 Toxoplasma encephalitis occurs in up to 30% of people with acquired immune deficiency syndrome.56 Histopathologic brain lesions are common, with 53 of 55 histologically examined cats having lesions in one study. The most common lesions were disseminated glial histiocytic granulomas and mononuclear perivascular cuffing. Despite the frequency of histologic lesions, neurologic signs in the study were uncommon. Only 7 of 100 infected cats had clinical neurologic abnormalities. Eighty percent of brains examined brains had Toxoplasma organisms identified.57 Most clinical cases result from reactivated infections in cats older than 3 months of age.58
Positive serology can support the diagnosis. However 30% of cats and dogs have T gondii antibodies.55, 56 Positive immunoglobulin (Ig) M antibodies indicate recent infection and last for up to 12 weeks after inoculation. IgM antibodies typically are not seen in healthy cats and correlate more closely with disease. IgG antibodies support a more chronic infection.56 CSF analysis in CNS toxoplasmosis typically shows a mixed pleocytosis and increased protein.55 PCR testing can be done directly on CSF when toxoplasma is suspected. Clindamycin 10 to 12.5 mg/kg by mouth every 12 hours for 4 weeks is the treatment of choice.55
Cryptococcosis
Cryptococcus neoformans and Cryptococcus gatti are saprophytic yeastlike fungi. The characteristic heteropolysaccharide capsule provides virulence and resistance to desiccation.59 Eight molecular types have been described. VN I, a type of C neoformans, is the most common type in dogs. VG III, a type of C gatti, is most common in cats.60 Inhalation is the most likely route of infection. A predilection for the CNS occurs in both dogs and cats. Up to 55% of cats with cryptococcosis have CNS involvement.61 No age predilection is present in cats, whereas affected dogs are typically less than 4 years of age.62 CNS signs depend on lesion location. Cerebral involvement, particularly the olfactory bulb, is common, with seizures and obtundation being common signs. Vestibular involvement is common.59 Systemic abnormalities are also common, including chorioretinitis; nasal discharge; skin lesions; hilar lymphadenopathy; and renal, hepatic, and splenic involvement.59, 63 Therefore a thorough fundic examination, thoracic radiographs, and abdominal ultrasound are recommended when cryptococcosis is suspected.
Serum latex agglutination (LA) testing is highly sensitive and specific. LA testing is 91.6% to 98% sensitive64 and was 98% specific in one study.61 Neurologic signs and a positive serum LA test suggest CNS cryptococcosis. Identification of organisms in the CSF or positive CSF LA testing confirms the diagnosis. Negative serum LA testing with positive yeast identification or positive LA results in the CSF is possible.65 MRI results are variable. MRI may identify a gelatinous pseudocyst that is hypointense on T1 imaging and hyperintense on T2 imaging. The pseudocyst may have peripheral contrast enhancement. Meningeal, ependymal, or choroid plexus contrast enhancement may occur in other cases. An increased cellular/granulomatous response in dogs compared with cats may correlate with contrast enhancement patterns identified on MRI (Fig. 13 ).59, 66
Fig. 13.
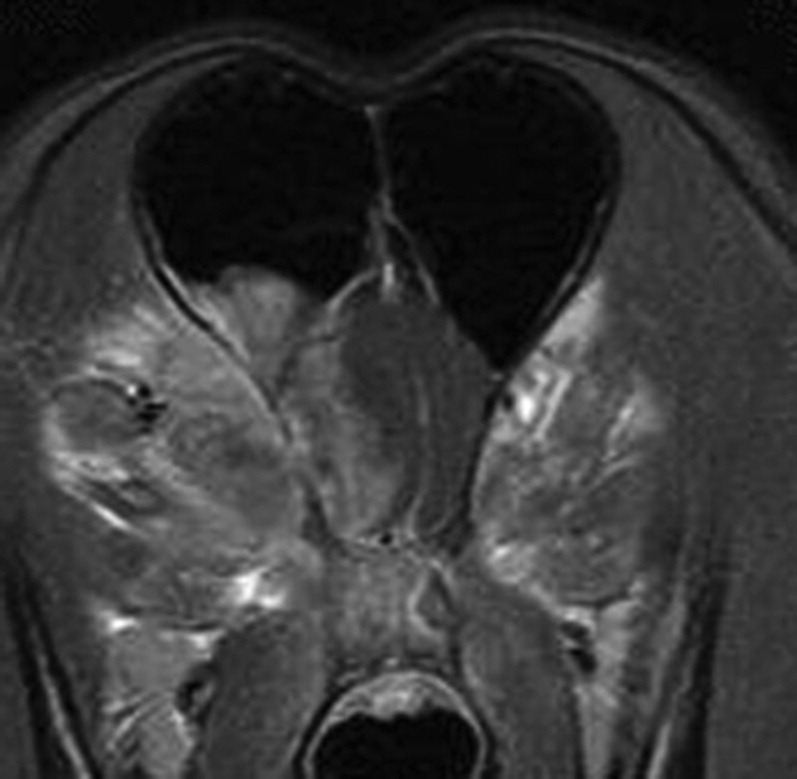
MRI of a 1.5-year-old German shepherd dog with CNS cryptococcosis in the olfactory bulb and nasal cavity. Transverse postcontrast T1-weighted image. Marked contrast enhancement is present with a midline shift of the falx cerebri.
Azole therapy is most commonly used for the treatment of cryptococcosis. Cryptococcosis is often treated successfully. Twenty-eight of 29 cats treated with fluconazole had successful outcomes.67 However, CNS involvement likely decreases the prognosis. Fluconazole has good CSF penetration and thus is recommended in CNS cryptococcosis. Fluconazole 25 to 50 mg by mouth twice a day is typically used in the cat and 2.5 to 10 mg/kg per day divided twice a day is used in the dog.59
Itraconazole 5 to 10 mg/kg per day may also be used. Successful outcomes occurred in 56% of cats and improvement was seen in 29% of cats treated with itraconazole in one study.68 Itraconazole is highly protein bound, insoluble in fluids, and is larger than fluconazole. It does not seem to cross the blood CSF barrier in detectable amounts.69 Itraconazole may successfully treat CNS cryptococcosis by crossing an inflamed blood-brain barrier or via drug accumulation within the highly lipid brain.70 In immunosuppressed people, initial treatment of cryptococcal meningitis has been a combination of intravenous amphotericin B and oral flucytosine. After 2 weeks of therapy, people are switched to azole therapy. Cryptococcus is susceptible to amphotericin B. However, the duration of use is limited because of poor CNS penetration and the potential for renal toxicity.71 Daily monitoring of renal values is recommended when administering amphotericin B. Lipid-complexed amphotericin B has increased efficacy and is less nephrotoxic than standard amphotericin B.59, 70 Flucytosine 50 mg/kg by mouth every 8 hours in cats44 penetrates well into the CSF, but should not be used as a single agent because resistance develops quickly.72 Flucytosine can cause dermal eruptions in dogs.62
The prognosis for dogs and cats with cryptococcosis is considered worse when the CNS is involved. Remission of greater than 1 year occurred in only 32% of cats and dogs in one study. Abnormal mentation was associated with a poor outcome. Single-drug therapy versus Multiple-drug therapy did not significantly affect the outcome. The use of glucocorticoids improved survival time within the first 10 days.66 CNS mycoses often cause inflammation and edema. Dying organisms can cause further inflammation and neurologic deterioration.73 Glucocorticoids decrease inflammation and edema within the CNS. Thus, when MRI indicates significant brain edema or when significant neurologic deterioration occurs, the use of glucocorticoids is indicated. Dexamethasone sodium phosphate 0.1 mg/kg IV or subcutaneously every 12 hours followed by prednisone 0.5 mg/kg by mouth twice a day is used. Prednisone should be tapered over 1 to 2 weeks if possible because high doses or long-term use of corticosteroids to avoid immunosuppression.59
FIP
FIP is a common and fatal infectious feline disease caused by a mutant form of feline enteric corona virus.74 A pyogranulomatous meningoencephalitis and meningomyelitis is seen with CNS involvement. The median age of cats with FIP affecting the CNS is 1 year.75 The diagnosis of FIP can often be challenging. Cats with neurologic FIP may have weight loss, mentation changes, seizures, ataxia, and hyperesthesia. Anterior uveitis, hyphema and retinal hemorrhage may be identified on ophthalmologic examination.74 Serum globulins are often increased.76 Seronegative FIP may be seen in cats with low titers, acute fulminant disease less than 10 days, and from immune complex consumption.77 MRI may identify periventricular changes consistent with ependymitis. The third and fourth ventricles are commonly affected. Secondary hydrocephalus may also be identified. CSF analysis indicates an increased protein value (mean 97.3 g/dL)74 and a neutrophilic pleocytosis (mean 28 cells/μL).75 CSF antibodies are likely of serum origin, but conflicting studies make the definitive antibody origin unknown.74, 75 A ratio of CSF antibodies/serum antibodies compared with CSF protein/serum total protein greater than 1 has typically suggested intrathecal antibody production.74, 75 However, a value greater than 1 does not necessarily imply active CNS infection.75 Prednisolone therapy is typically given at immunosuppressive dosages (1–2 mg/kg by mouth every 12 hours or typically 5 mg per cat by mouth every 12 hours) to cats with neurologic FIP to decrease CNS inflammation and for immunosuppression, but the prognosis is ultimately poor.
Epilepsy
Benign familial juvenile epilepsy has been reported in Lagotto Romagnolo dogs and is thought to be recessively inherited. Seizures are noted between 5 and 9 weeks of age. Seizures typically respond to anticonvulsant therapy. Discontinuation of anticonvulsants is often possible after several months of therapy. Seizure recurrence may occur in adulthood.78
Idiopathic epilepsy is classified as recurrent seizures without an apparent cause. Interictal neurologic examination is normal. Results of blood work, MRI, and CSF are all normal. Seizures typically are first noted between the ages of 1 and 5 years. Generalized tonic-clonic seizures are typically seen, although focal seizures are also possible. Idiopathic epilepsy is sometimes used to imply familial epilepsy because it is inherited in beagles, golden retrievers, Irish wolfhounds, English springer spaniels, Labrador retrievers, vizslas, Bernese mountain dogs, boxers, Belgian Tervurens, British Alsatians, Keeshonds, and standard poodles. Autosomal recessive inheritance is most common.79 Anticonvulsant therapy is typically started when seizure frequency increases, particularly when 2 or more seizures occur within 6 months. A baseline period is helpful to determine the natural seizure frequency and can help assess response to anticonvulsant therapy. However, this baseline period should not be too long because early antiepileptic treatment may lead to better seizure control.80 Patients with cluster seizures, status epilepticus, or secondary epilepsy should have anticonvulsant therapy started without delay. Anticonvulsant therapy is targeted to decrease the frequency and severity of seizures, increasing quality of life with as few adverse effects as possible.
Anticonvulsant choices
The choice of anticonvulsant for maintenance therapy is tailored to each individual patient. The patient’s age, medical history, potential for adverse effects, severity of the seizure disorder, and client concerns must all be considered. In patients with a history of cluster seizures, status epilepticus, or a progressive cause for seizures, the author typically chooses phenobarbital given its increased efficacy compared with other anticonvulsant options. Otherwise, in young patients with a less severe seizure disorder, the author often chooses either potassium bromide or levetiracetam given the decreased chance of hepatotoxicity. If a client is unable to consistently give medication multiple times a day, then starting with potassium bromide is reasonable. However, for dogs with a history of gastrointestinal issues, the author typically avoids potassium bromide. Although each medication has its benefits and risks, there is not a universal perfect anticonvulsant. Each medication may need to be adjusted for optimal seizure control or because of potential adverse effects. Monotherapy is typically preferred for initial therapy. However, when the seizure disorder is refractory to monotherapy, then multidrug therapy is indicated in the pursuit of adequate seizure control. It is important to educate clients about the goals of anticonvulsant therapy (improved seizure control with minimal to no adverse effects), potential adverse effects, and potential future need for multidrug therapy.
Phenobarbital
Phenobarbital historically has been the first-line treatment of seizures in dogs and cats. Phenobarbital is the most efficacious anticonvulsant, with one study reporting efficacy in 90% of suspected epileptic dogs.81 Phenobarbital increases the seizure threshold and decreases the spread to surrounding neurons. It enhances the inhibitory postsynaptic effects of gamma-aminobutyric acid (GABA), inhibits glutamate activity, and decreases calcium flux across neuronal membranes. Peak levels are achieved 4 to 6 hours after oral administration and its half-life (T1/2) is 1.5 to 3 days. Phenobarbital metabolism increases with chronic therapy.82 Phenobarbital is typically started at 2.5 to 3 mg/kg by mouth every 12 hours. The dose is then adjusted based on clinical response, adverse effects, and serum levels. Adverse effects include sedation, ataxia, polyphagia, polyuria/polydypsia (PU/PD), and increased liver enzymes. The potential for liver failure increases at serum levels of 35 to 40 μg/mL (therapeutic range, 15–40 μg/mL). Routine liver monitoring is recommended.82 Increases in alkaline phosphatase (ALP), alanine aminotransferase (ALT) and gamma glutamyl transferase (GGT) can result from enzyme induction rather than hepatic injury. Bile acid testing, bilirubin, and abdominal ultrasound were not affected by phenobarbital therapy in one study. Thus, bile acid testing, bilirubin, and abdominal ultrasound can help assess liver disease in dogs receiving phenobarbital.83 Larger increases in ALT compared with ALP are concerning because it also suggests hepatic disease. Hepatotoxicity can improve as the phenobarbital dose is lowered or withdrawn. However, with advanced liver disease, the effects can be irreversible and potentially fatal.80 Evaluating CBC and chemistry 2 to 4 weeks after starting therapy, 3 months later, and then every 6 months can be helpful. Bile acid testing should also be considered periodically or if hepatic enzymes are increasing. Phenobarbital’s shorter T1/2, compared with bromide, allows quicker manipulation of plasma levels, making it a good choice for dogs with frequent seizures.
Phenobarbital has been associated with rare congenital defects and bleeding problems in neonates. Although caution is recommended, phenobarbital is considered likely to be safer than other anticonvulsants. Small amounts are excreted into maternal milk and thus may affect nursing animals.44
Assessment of hypothyroidism is difficult in dogs receiving phenobarbital. Total T4 (TT4) and freeT4 (FT4) values may be significantly decreased in dogs receiving phenobarbital. Total T3 changes minimally and thyroid-stimulating hormone (TSH) increases after several months of phenobarbital therapy. Serum cholesterol also increases after phenobarbital administration. It is unclear whether the decrease in TT4 and FT4 is clinically significant. Increased thyroxine monoiodination to T3 at the cellular level may occur, compensating for hypothyroxinemia. If so, TT4, FT4, and TSH values are meaningless.84 TT4 values return to normal within 6 weeks of phenobarbital withdrawal. FT4 values may remain decreased for 10 weeks after cessation of phenobarbital therapy.85
Bromide
Bromide has also been a popular first-choice or second-choice anticonvulsant. Bromide was effective in 72% of epileptic dogs in one study86 and 74% in another study.81 Bromide hyperpolarizes the neuron through its replacement of chloride ions.82 Bromide has a T1/2 of 21 to 24 days and is excreted by the kidneys.80 Increased dietary salt intake increases renal excretion. Adverse effects of bromide include sedation, ataxia, PU/PD, polyphagia, gastrointestinal effects,82 and a possible association with pancreatitis.80 The lack of hepatic metabolism makes potassium bromide, 40 mg/kg/d in food, a good choice for young dogs. The therapeutic range is 1.0 to 3.0 mg/mL. If dogs are in status epilepticus or have cluster seizures, the long T1/2 of bromide can make it more difficult to use effectively. In these situations, a drug with a shorter T1/2 may be a better choice. A loading dose of potassium bromide can be given at 450 to 600 mg/kg by mouth total over the course of 5 days.82 Adverse effects are more common when using a loading dose. Bromide should not be given to cats because it causes pneumonitis in cats. The pneumonitis is typically reversible once bromide therapy is stopped.80 The reproductive safety of bromide has not been determined.44
Levetiracetam
Levetiracetam has become increasing popular in the past decade. A few studies with limited power have indicated 56% to 64% response to adjunctive levetiracetam therapy for epilepsy87, 88 as well as for emergency treatment of status epilepticus and cluster seizures.89 Levetiracetam binds synaptic vesicular protein SV2A. Levetiracetam may prevent hypersynchronization of burst firing and seizure propagation. The T1/2 is 4 hours in dogs and 5 hours in cats. Initial dosing was recommended at 10 to 20 mg/kg by mouth 3 times a day. However, a honeymoon effect was noted. After several months of therapy, seizure control was lost.87 This honeymoon phenomenon and pharmacokinetic evaluation has led to a recommended starting dose of 20 mg/kg by mouth 3 times a day.90 An extended-release formulation is now also available and is dosed at 20 mg/kg by mouth twice a day. The extended-release tablets are not suitable for small dogs or cats because the tablets should not be cut. The adverse effects of levetiracetam seem minimal with sedation/ataxia occurring uncommonly. Sixty-six percent of the drug is excreted unchanged through the kidneys. The cytochrome P 450 system does not seem to be involved. However, concurrent phenobarbital administration has been shown to decrease levetiracetam T1/2 and decreases levetiracetam blood levels. Thus dogs on both phenobarbital and levetiracetam concurrently may require higher dosages of levetiracetam.91 Levetiracetam is excreted into maternal milk and so should be used with caution in nursing animals. Levetiracetam should be used with caution in pregnant animals because high dosages have increased embryofetal mortality in rabbits and rats.44
Zonisamide
Zonisamide is a sulfonamide-based anticonvulsant drug that has also become popular in the past decade. As with levetiracetam, a few reports with limited patient numbers have been published. One study indicated that 9 of 11 refractory epileptic dogs responded to zonisamide therapy in the first 8 months of therapy. However, only 6 of 11 responded after 17 months of therapy.92 Another study indicated that 7 of 12 refractory epileptic dogs responded to zonisamide therapy.93 Zonisamide monotherapy was effective in 6 of 10 epileptic dogs in another report.94 Zonisamide blocks voltage-dependent sodium channels and T-type calcium channels. Its T1/2 is about 15 hours in the dog and 35 hours in cats. Zonisamide is metabolized by hepatic microsomal enzymes and is also renally excreted. Its T1/2 is reduced with concurrent phenobarbital administration. Zonisamide is administered 5 to 10 mg/kg by mouth twice a day in dogs, with the higher dose range for dogs also receiving phenobarbital. Cats are given 5 to 10 mg/kg by mouth every 24 hours, because of the longer T1/2. Zonisamide is typically well tolerated, with anorexia, sedation, and ataxia being the most common adverse effects.80 Acute hepatopathy within 3 weeks of starting zonisamide therapy has recently been reported in 2 dogs.95, 96 Renal tubular acidosis has also been reported recently. Acid-base changes resolved after discontinuation of zonisamide.97 When zonisamide was given to pregnant dogs, ventricular septal defects, cardiomegaly, and valvular and arterial anomalies occurred. Thus zonisamide is not recommended for use in pregnant animals. It is unknown whether zonisamide is excreted into maternal milk.44
Gabapentin
Gabapentin binds voltage-gated calcium channels and decreases intracellular calcium influx. It is excreted unchanged by the kidneys with about 30% to 40% hepatic metabolism in dogs.98 Gabapentin’s maximum absorption occurs within 2 hours of administration. The T1/2 is 3 to 4 hours in dogs99 and 3 hours in cats.100 The initial dose is 10 mg/kg by mouth 3 times a day. Six of 11 refractory epileptic dogs responded to gabapentin therapy in one study.99 Sedation and ataxia are the most common adverse effects.80 The liquid formulation contains xylitol and thus should not be given to dogs. Dogs requiring liquid or small doses should have the drug compounded so that it does not contain xylitol. Gabapentin has been teratogenic in mice, rats, and rabbits and should thus be used with caution or avoided in pregnant animals. Gabapentin is excreted through maternal milk. However, the amount that is excreted is not thought to be clinically significant.44
Pregabalin
Pregabalin is a GABA analogue structurally similar to gabapentin. Pregabalin has a higher affinity for the alpha-2-delta subunit of neuronal voltage-gated calcium channels than does gabapentin. The T1/2 of pregabalin is 7 hours in dogs98 and 10.4 hours in cats.101 Pregabalin is administered at 2 to 4 mg/kg by mouth 2 to 3 times a day. Seven of 11 refractory epileptic dogs responded to pregabalin therapy in one study. Adverse effects included sedation and ataxia and were common. Thus, a starting dose of 2 mg/kg by mouth 2 to 3 times a day with a gradual increase in dose by 1 mg/kg is recommended in dogs.98 A dose of 1 to 2 mg/kg by mouth twice a day is recommended in cats.101 Very high dosages of pregabalin given to pregnant rats and rabbits caused skeletal malformations in offspring. Pregabalin is also excreted into maternal milk.44
Felbamate
Felbamate enhances the inhibitory effects of GABA, blocks voltage-dependent sodium channels, and blocks N-methyl-d-aspartate receptors.80, 102 The T1/2 in dogs is 5 to 6 hours and is administered at a starting dose of 15 mg/kg80 to 20 mg/kg102 by mouth 3 times a day. Doses as high as 70 mg/kg by mouth 3 times a day may be required in some dogs. Felbamate can cause aplastic anemia and fatal hepatopathy in people, thus its use is limited. In dogs, about 30% of felbamate undergoes hepatic metabolism with the rest excreted unchanged in the urine. Adverse effects in dogs include hepatic effects, mild thrombocytopenia, and leukopenia.80, 102 Blood dyscrasias were reversible with stopping felbamate therapy.102 Felbamate is excreted into maternal milk. Although no overt teratogenic effects have been documented, caution is recommended in pregnant animals.44
Benzodiazepines
Benzodiazepines are potent anticonvulsants that enhance the inhibitory effects of GABA. The T1/2 of diazepam is 3.2 hours in dogs and about 15 to 20 hours in cats. The longer T1/2 of diazepam in cats makes it a possible candidate for long-term anticonvulsant therapy. However, the use of oral diazepam is limited because of the potential risk of severe/fatal liver disease in cats. Strict monitoring is required if diazepam is used orally in cats. Tolerance develops with maintenance use of benzodiazepines. Thus the short duration of action in dogs and the development of tolerance limit the use of benzodiazepines to the emergency setting.80, 82 Diazepam at 0.5 mg/kg IV or 1 to 2 mg/kg rectally is recommended for immediate treatment of seizures. The absorption of benzodiazepines when administered rectally is variable, resulting in questionable efficacy. Concurrent phenobarbital administration increases the metabolism of diazepam. Thus, the higher dose range is recommended for rectal diazepam when dogs are concurrently receiving phenobarbital therapy. Diazepam 0.5 mg/kg/h may be administered as a constant-rate infusion (CRI). The dose is then titrated based on seizure control and sedation.80 Midazolam is water soluble and is a good choice for emergency treatment when a CRI is needed through a peripheral catheter or via intramuscular injection. Midazolam’s T1/2 in dogs is 77 minutes.103 A dose of 0.07 to 0.2 mg/kg is recommended as an intravenous or intramuscular bolus for immediate seizure control and a dosage of 0.05 to 0.5 mg/kg/h when given as a CRI. When a benzodiazepine has been used to stop the acute seizure, it is important to also administer a longer acting anticonvulsant to prevent further seizures.80
Diazepam causes congenital abnormalities when given in the first trimester of pregnancy and should be avoided if possible in pregnant animals. Benzodiazepines and metabolites are excreted into maternal milk and can thus affect nursing animals.44
Clorazepate is metabolized to nordiazepam and has a T1/2 of 4 to 6 hours in dogs. Tolerance does not develop as readily compared with diazepam. Clorazepate may be helpful in short-term control of breakthrough seizures.82 A dose of 0.5 to 1 mg/kg by mouth 2 to 3 times a day is recommended.44, 82 Clorazepate increases phenobarbital levels. Serum phenobarbital levels should be monitored with its use 2 and 4 weeks after starting clorazepate therapy.82 Clorazepate should be used with extreme caution in aggressive animals. The metabolite nordiazepam is distributed into milk and thus may affecting nursing animals.44
The use of acepromazine in dogs with a history of seizures has commonly not been recommended because of a possible decrease in the seizure threshold with its use. However, evidence to warrant a contraindication for the use of acepromazine in patients with seizures is lacking. The use of acepromazine in patients with seizures is unlikely to result in seizures. A retrospective study evaluated 36 dogs with a history of seizures that were given acepromazine for sedation or as a preanesthetic. None of the 36 dogs had seizures within 16 hours of use.104
Therapeutic monitoring
Therapeutic levels and toxic levels are guidelines extrapolated from people. These ranges are based on population statistics and may not accurately reflect whether an individual canine or feline patient is controlled. Nonetheless, they can be helpful in optimizing anticonvulsant therapy, assessing risk factors of therapy, and helping to gauge when a second anticonvulsant may be needed. Determining anticonvulsant levels is most beneficial when (1) a dose change is needed because of inadequate seizure control or toxicity. The formula (desired blood level/current blood level) times the current dose equals the newly recommended dose (2) A refractory patient becomes controlled, which allows the identification of a therapeutic level for that patient, provided the seizure disorder does not worsen. (3) The patient is sensitive to the toxic effects of the anticonvulsant (most commonly bromide). Obtaining a blood level as soon as clinical signs of toxicity abate identifies the toxic level for that patient. Evaluation of blood levels may not be important when patients are currently and historically well controlled and free of adverse effects. Patients receiving phenobarbital should ideally have a level obtained once they reach steady state given the risk of hepatotoxicity at levels greater than 35 μg/mL. In addition, serial phenobarbital levels every 6 to 12 months can be of benefit if hepatic metabolism changes. Peak and trough phenobarbital levels were recommended in the past. However, only 9% of patients receiving phenobarbital had a clinically significant (>30%) change between peak and trough levels in one study. Thus a single sample is typically sufficient.105 Identifying the timing of sample collection relative to dosing can be helpful when the level is in the high reference range so that toxicity can be avoided. Therapeutic levels for levetiracetam, zonisamide, and pregabalin are unknown for dogs at this time. Thus, routine monitoring of levels is typically not done with usage of these drugs. Bromide levels are affected by Cl−. Increased dietary Cl− or intravenous fluids containing Cl− increase renal excretion of bromide and thus lower serum bromide levels. A patient receiving bromide therapy may lose seizure control as a result of a change in diet or intravenous fluids. In contrast, a decrease in dietary Cl− may lead to toxicity (sedation and ataxia) as renal bromide excretion is reduced and serum bromide levels subsequently increase. Thus, dietary changes should be minimized in patients receiving bromide therapy.
When transitioning from one anticonvulsant to another, several factors may influence the recommendations for the transition. If significant adverse effects are not apparent, then it is reasonable to add the additional drug while continuing the initial anticonvulsant at the maintenance dose. If the initial anticonvulsant drug is to be withdrawn it should be tapered over the course of several months because it is often difficult to definitively know how effective the initial drug was. Weaning the initial drug over several months may help prevent withdrawal seizures. If sedation or ataxia is noted with the addition of the new anticonvulsant, either drug may be tapered to limit adverse effects. If seizure control is successful without adverse effects, maintenance therapy with multiple drugs may be warranted and thus the initial drug may not be withdrawn. If a second or third drug is implemented because of adverse effects, tapering the drug with the most likely adverse effects is recommended. If adverse effects are of mild to moderate concern then tapering a drug such as phenobarbital may be done every 2 to 4 weeks by 25% each time. Potassium bromide may be reduced more quickly at times because of its longer T1/2. Rapid tapering or withdrawal is necessary when adjusting medications because of severe adverse effects from existing therapy. In this situation, the existing therapy should be stopped immediately or over the course of a few days. It is important to concurrently start another anticonvulsant with a shorter T1/2 so that steady state is rapidly achieved. Furthermore, the additional drug should have a different profile of adverse effects.
References
- 1.Mehl M.L., Kyles A.E., Hardie E.M. Evaluation of ameroid ring constrictors for treatment for single extrahepatic portsosystemic shunts in dogs: 168 cases (1196-1991) J Am Vet Med Assoc. 2005;226:2020–2030. doi: 10.2460/javma.2005.226.2020. [DOI] [PubMed] [Google Scholar]
- 2.Tisdall P.L., Hunt G.B., Youmans K.R. Neurological dysfunction in dogs following attenuation of congenital extrahepatic portosystemic shunts. J Small Anim Pract. 2000;41:539–546. doi: 10.1111/j.1748-5827.2000.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 3.Podell M. Neurologic manifestations of systemic disease. In: Ettinger S.J., editor. Textbook of veterinary internal medicine. 6th edition. Elsevier; St Louis (MO): 2005. pp. 798–802. [Google Scholar]
- 4.Ruland K., Fischer A., Hartmann K. Sensitivity and specificity of fasting ammonia and serum bile acids in the diagnosis of portosystemic shunts in dogs and cats. Vet Clin Pathol. 2010;39:57–64. doi: 10.1111/j.1939-165X.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Anjou M.A., Pennick D., Cornejo L. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Vet Radiol Ultrasound. 2004;45:424–437. doi: 10.1111/j.1740-8261.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- 6.Berent A.C., Weisse C. Hepatic vascular anomalies. In: Ettinger S.J., editor. Textbook of veterinary internal medicine. 7th edition. Elsevier; St Louis (MO): 2010. pp. 1649–1672. [Google Scholar]
- 7.Greenhalgh S.N., Dunning M.D., McKinley T.J. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. J Am Vet Med Assoc. 2010;236:1215–1220. doi: 10.2460/javma.236.11.1215. [DOI] [PubMed] [Google Scholar]
- 8.Fryer K.J., Levine J.M., Peycke L.E. Incidence of postoperative seizures with and without levetiracetam pretreatment in dogs undergoing portosystemic shunt attenuation. J Vet Intern Med. 2011;25:1379–1384. doi: 10.1111/j.1939-1676.2011.00819.x. [DOI] [PubMed] [Google Scholar]
- 9.Summers B.A., Cummings J.F., De Lahunta A. Veterinary neuropathology. Mosby; St Louis (MO): 1995. Malformations of the central nervous system; pp. 68–94. [Google Scholar]
- 10.Wunschmann A., Oglesbee M. Periventricular changes associated with canine hydrocephalus. Vet Pathol. 2001;38(1):67–73. doi: 10.1354/vp.38-1-67. [DOI] [PubMed] [Google Scholar]
- 11.Zabka T.S., Lavely J.A., Higgins R.J. Primary intra-axial leiomyosarcoma with obstructive hydrocephalus in a young dog. J Comp Pathol. 2004;131:334–337. doi: 10.1016/j.jcpa.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Hoskins J.D. Clinical evaluation of the kitten: from birth to eight weeks of age. Compend Contin Educ Pract Vet. 1990;12(9):1215–1225. [Google Scholar]
- 13.Saito M., Olby N.J., Spaulding K. Relationship among basilar artery resistance index, degree of ventriculomegaly, and clinical signs in hydrocephalic dogs. Vet Radiol Ultrasound. 2003;44(6):687–694. doi: 10.1111/j.1740-8261.2003.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Harrington M.L., Bagley R.S., Moore M.P. Hydrocephalus. Vet Clin North Am Small Anim Pract. 1996;26(4):843–856. doi: 10.1016/s0195-5616(96)50108-7. [DOI] [PubMed] [Google Scholar]
- 15.Nykamp S., Scrivani P., De Lahunta A. Chronic subdural hematomas and hydrocephalus in a dog. Vet Radiol Ultrasound. 2001;42(6):511–514. doi: 10.1111/j.1740-8261.2001.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavely J.A. Pediatric neurology of the dog and cat. Vet Clin North Am Small Anim Pract. 2006;36:475–501. doi: 10.1016/j.cvsm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Lindvall-Axelsson M., Hedner P., Owman C. Corticosteroid action on choroids plexus: reduction in Na-K ATPase activity, choline transport capacity, and rate of CSF formation. Exp Brain Res. 1989;77:605–610. doi: 10.1007/BF00249613. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S., Corbett W.S., Simbartl L.A. Different effects of omeprazole and Sch 28080 on canine cerebrospinal fluid production. Brain Res. 1997;754:321–324. doi: 10.1016/s0006-8993(97)00175-3. [DOI] [PubMed] [Google Scholar]
- 19.Biel M., Kramer M., Forterre F. Outcome of ventriculoperitoneal shunt implantation for treatment of congenital internal hydrocephalus in dogs and cats: 36 cases (2001–2009) J Am Vet Med Assoc. 2013;242:948–958. doi: 10.2460/javma.242.7.948. [DOI] [PubMed] [Google Scholar]
- 20.Shihab N., Davies E., Kenny P.J. Treatment of hydrocephalus with ventriculoperitoneal shunting in twelve dogs. Vet Surg. 2011;40:477–484. doi: 10.1111/j.1532-950X.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 21.de Stefani A., de Risio L., Platt S.R. Surgical technique, postoperative complications and outcome in 14 dogs treated for hydrocephalus by ventriculoperitoneal shunting. Vet Surg. 2011;40:183–191. doi: 10.1111/j.1532-950X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 22.Kestle J., Drake J., Milner R. Long term follow-up data from the shunt design trial. Pediatr Neurosurg. 2000;33:230–236. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 23.Abramson C.J., Platt S.R., Jakobs C. L-2 hydroxyglutaric aciduria in Staffordshire bull terriers. J Vet Intern Med. 2003;17:551–556. doi: 10.1111/j.1939-1676.2003.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 24.Garosi L.S., Penderis J., McConnell J.F. L-2 hydroxyglutaric aciduria in a West Highland white terrier. Vet Rec. 2005;156:145–147. doi: 10.1136/vr.156.5.145. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Masian D.F., Artuch R., Mascort J. L-2-hydroxyglutaric aciduria in two female Yorkshire terriers. J Am Anim Hosp Assoc. 2012;48(5):366–371. doi: 10.5326/JAAHA-MS-5967. [DOI] [PubMed] [Google Scholar]
- 26.Penderis J., Calvin J., Abramson C. L-2-hydroxyglutaric aciduria: characterization of the molecular defect in a spontaneous canine model. J Med Genet. 2007;44:334–340. doi: 10.1136/jmg.2006.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt S., McGrotty Y.L., Abramson C.J. Refractory seizures associated with an organic aciduria in a dog. J Am Anim Hosp Assoc. 2007;43:163–167. doi: 10.5326/0430163. [DOI] [PubMed] [Google Scholar]
- 28.Saito M., Sharp N.J., Kortz G.D. Magnetic resonance imaging features of lissencephaly in 2 Lhasa apsos. Vet Radiol Ultrasound. 2002;43(4):331–337. doi: 10.1111/j.1740-8261.2002.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 29.Zaki F.A. Lissencephaly in Lhasa apso dogs. J Am Vet Med Assoc. 1976;169:1165–1168. [PubMed] [Google Scholar]
- 30.Greene C.E., Vandevelde M., Braund K. Lissencephaly in two Lhasa apso dogs. J Am Vet Med Assoc. 1976;169(4):405–410. [PubMed] [Google Scholar]
- 31.Muñana K.R., Luttgen P.J. Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982-1996) J Am Vet Med Assoc. 1998;212(2):1902–1906. [PubMed] [Google Scholar]
- 32.Schatzberg S.J. Idiopathic granulomatous and necrotizing inflammatory disorders of the canine central nervous system. Vet Clin North Am Small Anim Pract. 2010;40:101–120. doi: 10.1016/j.cvsm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Lamb C.R., Croson P.J., Cappello R. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid. Vet Radiol Ultrasound. 2005;46(1):17–22. doi: 10.1111/j.1740-8261.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 34.Cherubini G.B., Platt S.R., Anderson T.J. Characteristics of magnetic resonance images of granulomatous meningoencephalomyelitis in 11 dogs. Vet Rec. 2006;159:110–115. doi: 10.1136/vr.159.4.110. [DOI] [PubMed] [Google Scholar]
- 35.Cuddon PA, Coates JR, Murray M. New treatments for granulomatous meningoencephalomyelitis. In Proceedings 20th ACVIM. Dallas (TX): 2002. p. 319–21.
- 36.Adamo P.F., Rylander H., Adams W.M. Ciclosporin use in multi-drug therapy for meningoencephalomyelitis of unknown aetiology in dogs. J Small Anim Pract. 2007;48(9):486–496. doi: 10.1111/j.1748-5827.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 37.Coates J.R., Barone G., Dewey C.W. Procarbazine as adjunctive treatment of dogs with presumptive antemortem diagnosis of granulomatous meningoencephalomyelitis: 21 cases (1998-2004) J Vet Intern Med. 2007;21:100–106. doi: 10.1892/0891-6640(2007)21[100:paatft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Flegel T., Boettcher I.C., Matiasek K. Comparison of oral administration of lomustine and prednisolone or prednisolone alone as treatment for granulomatous meningoencephalomyelitis or necrotizing encephalitis in dogs. J Am Vet Med Assoc. 2011;238:337–345. doi: 10.2460/javma.238.3.337. [DOI] [PubMed] [Google Scholar]
- 39.Zarfoss M., Schatzberg S., Venator K. Combined cytosine arabinoside and prednisone therapy for meningoencephalitis of unknown aetiology in 10 dogs. J Small Anim Pract. 2006;47:588–595. doi: 10.1111/j.1748-5827.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 40.Higgins R.J., Dickinson P.J., Kube S.A. Necrotizing meningoencephalitis in 5 Chihuahua dogs. Vet Pathol. 2008;45(3):336–346. doi: 10.1354/vp.45-3-336. [DOI] [PubMed] [Google Scholar]
- 41.Park E.S., Uchida K., Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME) Vet Pathol. 2012;49:682–692. doi: 10.1177/0300985811429311. [DOI] [PubMed] [Google Scholar]
- 42.Levine J.M., Fosgate G.T., Porter B. Epidemiology of necrotizing meningoencephalitis in pug dogs. J Vet Intern Med. 2008;22:961–968. doi: 10.1111/j.1939-1676.2008.0137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin P.J., Parry B.W. Streptococcal meningoencephalitis in a dog. J Am Anim Hosp Assoc. 1999;35:417–422. doi: 10.5326/15473317-35-5-417. [DOI] [PubMed] [Google Scholar]
- 44.Plumb D.C. 7th edition. Wiley Blackwell; Ames (IA): 2011. Veterinary drug handbook. [Google Scholar]
- 45.Vandevelde M., Zurbriggen A. Demyelination in canine distemper virus infection: a review. Acta Neuropathol. 2005;109:56–68. doi: 10.1007/s00401-004-0958-4. [DOI] [PubMed] [Google Scholar]
- 46.Lechner E.S., Crawford P.C., Levy J.K. Prevalence of protective antibody titers for canine distemper virus and canine parvovirus in dogs entering a Florida animal shelter. J Am Vet Med Assoc. 2010;236:1317–1321. doi: 10.2460/javma.236.12.1317. [DOI] [PubMed] [Google Scholar]
- 47.Koutinas A.F., Polizopoulou Z.S., Baumgaertner W. Relation of clinical signs to pathological changes in 19 cases of canine distemper encephalomyelitis. J Comp Pathol. 2002;126:47–56. doi: 10.1053/jcpa.2001.0521. [DOI] [PubMed] [Google Scholar]
- 48.Moritz A., Frisk A.L., Baumgärtner W. The evaluation of diagnostic procedures for the detection of canine distemper virus infection. European Journal of Companion Animal Practice. 2000;10:38–47. [Google Scholar]
- 49.Inada S. Electromyographic analysis of canine distemper myoclonus. Electromyogr Clin Neurophysiol. 1989;29:323–331. [PubMed] [Google Scholar]
- 50.Jozwik A., Frymus T. Comparison of the immunofluorescence assay with RT-PCR and nested PCR in the diagnosis of canine distemper. Vet Res Commun. 2005;29:347–359. doi: 10.1023/b:verc.0000048528.76429.8b. [DOI] [PubMed] [Google Scholar]
- 51.Saito T.B., Alfieri A.A., Wosiacki S.R. Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Res Vet Sci. 2006;80:116–119. doi: 10.1016/j.rvsc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Dubey J.P., Lappin M.R. Toxoplasmosis and neosporosis. In: Greene C.E., editor. Infectious diseases of the dog and cat. 2nd edition. WB Saunders; Philadelphia: 1998. pp. 493–509. [Google Scholar]
- 53.Ruehlmann D., Podell M., Oglesbee M. Canine neosporosis: a case report and literature review. J Am Anim Hosp Assoc. 1995;31:174–183. doi: 10.5326/15473317-31-2-174. [DOI] [PubMed] [Google Scholar]
- 54.Garosi L., Dawson A., Couturier J. Necrotizing cerebellitis and cerebellar atrophy caused by Neospora caninum infection: magnetic resonance imaging and clinicopathologic findings in seven dogs. J Vet Intern Med. 2010;24:571–578. doi: 10.1111/j.1939-1676.2010.0485.x. [DOI] [PubMed] [Google Scholar]
- 55.Dubey J.P., Lappin M.R. Toxoplasmosis and neosporosis. In: Greene C.E., editor. Infectious diseases of the dog and cat. 4th edition. Elsevier Saunders; St Louis (MO): 2012. pp. 806–827. [Google Scholar]
- 56.Vollaire M.R., Radecki S.V., Lappin M.R. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am J Vet Res. 2005;66(5):874–877. doi: 10.2460/ajvr.2005.66.874. [DOI] [PubMed] [Google Scholar]
- 57.Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases. J Am Vet Med Assoc. 1993;203(1):1556–1566. [PubMed] [Google Scholar]
- 58.Dickinson P.J., LeCouteur R.A. Feline neuromuscular disorders. Vet Clin North Am Small Anim Pract. 2004;34:1307–1359. doi: 10.1016/j.cvsm.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Lavely J., Lipsitz D. Fungal infections of the central nervous system in the dog and cat. Clin Tech Small Anim Pract. 2005;20:212–219. doi: 10.1053/j.ctsap.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Singer L.M., Meyer W., Thompson G.R. Molecular epidemiology and antifungal susceptibility among Cryptococcus isolates from North American dogs and cats. J Vet Intern Med. 2012;26(3):788–789. [Google Scholar]
- 61.Lester S.J., Kowalewich N.J., Bartlett K.H. Clinicopathologic features of an unusual outbreak of cryptococcosis in dogs, cats, ferrets, and a bird: 38 cases (January to July 2003) J Am Vet Med Assoc. 2004;225:1716–1722. doi: 10.2460/javma.2004.225.1716. [DOI] [PubMed] [Google Scholar]
- 62.Kerl M.E. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721–747. doi: 10.1016/s0195-5616(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 63.Berthelin C.F., Legendre A.M., Bailey C.S. Cryptococcosis of the nervous system in dogs, part 2: diagnosis, treatment, monitoring, and prognosis. Progr Vet Neurol. 1994;5:136–146. [Google Scholar]
- 64.O’Toole T.E., Sato A.F., Rozanski E.A. Cryptococcosis of the central nervous system in a dog. J Am Vet Med Assoc. 2003;222:1722–1726. doi: 10.2460/javma.2003.222.1722. [DOI] [PubMed] [Google Scholar]
- 65.Stevenson TL, Dickinson PJ, Sturges BK, et al. Magnetic resonance imaging of intracranial cryptococcosis in dogs and cats. Proceedings ACVIM. Minneapolis (MN): 2004.
- 66.Sykes J.E., Sturges B.K., Cannon M.S. Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system cryptococcosis from California. J Vet Intern Med. 2010;24:1427–1438. doi: 10.1111/j.1939-1676.2010.0633.x. [DOI] [PubMed] [Google Scholar]
- 67.Malik R., Wigney D.I., Muir D.B. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol. 1992;30:133–144. doi: 10.1080/02681219280000181. [DOI] [PubMed] [Google Scholar]
- 68.Medleau L., Jacobs G.J., Marks A.M. Itraconazole for the treatment of Cryptococcus in cats. J Vet Intern Med. 1995;9:39–42. doi: 10.1111/j.1939-1676.1995.tb03270.x. [DOI] [PubMed] [Google Scholar]
- 69.Perfect J.R., Savani D.V., Durack D.T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and Candida pyelonephritis in rabbits. Antimicrobial Agents Chemother. 1986;29:579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grooters A.M., Taboada J. Update on antifungal therapy. Vet Clin North Am Small Anim Pract. 2003;33:749–758. doi: 10.1016/s0195-5616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 71.Davis L.E. Fungal infections of the central nervous system infections. Neurol Clin. 1999;17:761–781. doi: 10.1016/s0733-8619(05)70165-1. [DOI] [PubMed] [Google Scholar]
- 72.Slavoski L.A., Tunkel A.R. Therapy of fungal meningitis. Clin Neuropharmacol. 1995;18:95–112. doi: 10.1097/00002826-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Tiches D., Vite C.H., Dayrell-Hart B. A case of canine central nervous system cryptococcosis: management with fluconazole. J Am Anim Hosp Assoc. 1998;34:145–151. doi: 10.5326/15473317-34-2-145. [DOI] [PubMed] [Google Scholar]
- 74.Foley J.E., Lapointe J.M., Koblik P. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med. 1998;12:415–423. doi: 10.1111/j.1939-1676.1998.tb02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boettcher I.C., Steinberg T., Matiasek K. Use of anti-coronavirus antibody testing of cerebrospinal fluid for diagnosis of feline infectious peritonitis involving the central nervous system in cats. J Am Vet Med Assoc. 2007;230(2):199–205. doi: 10.2460/javma.230.2.199. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann K., Binder C., Hirschberger J. Comparison of different tests to diagnose feline infectious peritonitis. J vet Intern Med. 2003;17:781–790. doi: 10.1111/j.1939-1676.2003.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foley J.E. Feline infectious peritonitis and feline enteric coronavirus. In: Ettinger S., Feldman E., editors. Textbook of veterinary internal medicine. 6th edition. Elsevier; St Louis (MO): 2005. pp. 663–666. [Google Scholar]
- 78.Jokinen T.S., Metsahonkala L., Bergamasco L. Benign familial juvenile epilepsy in lagotto romagnolo dogs. J Vet Intern Med. 2007;21:464–471. doi: 10.1892/0891-6640(2007)21[464:bfjeil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 79.Licht B.G., Lin S., Luo Y. Clinical characteristics and mode of inheritance of familial focal seizures in standard poodles. J Am Vet Med Assoc. 2007;231(10):1520–1528. doi: 10.2460/javma.231.10.1520. [DOI] [PubMed] [Google Scholar]
- 80.Thomas W.B. Idiopathic epilepsy in dogs and cats. Vet Clin North Am Small Anim Pract. 2010;40:161–179. doi: 10.1016/j.cvsm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Boothe D.M., Dewey C., Carpenter D.M. Comparison of phenobarbital with bromide as a first choice antiepileptic drug for treatment of epilepsy in dogs. J Am Vet Med Assoc. 2012;240(9):1073–1083. doi: 10.2460/javma.240.9.1073. [DOI] [PubMed] [Google Scholar]
- 82.Boothe D.M. Anticonvulsant therapy in small animals. Vet Clin North Am Small Anim Pract. 1998;28(2):411–448. doi: 10.1016/s0195-5616(98)82011-1. [DOI] [PubMed] [Google Scholar]
- 83.Müller P.B., Taboada J., Hosgood G. Effects of long-term phenobarbital treatment on the liver on dogs. J Vet Intern Med. 2000;14:165–171. doi: 10.1111/j.1939-1676.2000.tb02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Müller P.B., Wolfsheimer K.J., Taboada J. Effects of long term phenobarbital treatment on the thyroid and adrenal axis and adrenal function tests in dogs. J Vet Intern Med. 2000;14:157–164. doi: 10.1892/0891-6640(2000)014<0157:eolpto>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 85.Gieger T.L., Hosgood G., Taboada J. Thyroid function and serum hepatic enzyme activity in dogs after phenobarbital administration. J Vet Intern Med. 2000;14(3):277–281. doi: 10.1111/j.1939-1676.2000.tb01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trepanier L.A., Van Schoick A., Schwark W.S. Therapeutic serum drug concentrations in epileptic dogs treated with potassium bromide alone or in combination with other anticonvulsants: 122 cases (1992-1996) J Am Vet Med Assoc. 1998;213(10):1449–1453. [PubMed] [Google Scholar]
- 87.Volk H.A., Matiasek L.A., Feliu-Pascual A.L. The efficacy and tolerability of levetiracetam in pharmacoresistant epileptic dogs. Vet J. 2008;176:310–319. doi: 10.1016/j.tvjl.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Muñana K.R., Thomas W.B., Inzana K.D. Evaluation of levetiracetam as adjunctive treatment for refractory canine epilepsy: a randomized, placebo-controlled, crossover trial. J Vet Intern Med. 2012;26:341–348. doi: 10.1111/j.1939-1676.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 89.Hardy B.T., Patterson E.E., Cloyd J.M. Double-masked, placebo-controlled study of intravenous levetiracetam for the treatment of status epilepticus and acute repetitive seizures in dogs. J Vet Intern Med. 2012;26:334–340. doi: 10.1111/j.1939-1676.2011.00868.x. [DOI] [PubMed] [Google Scholar]
- 90.Moore S.A., Muñana K.R., Papich J.A. Levetiracetam pharmacokinetics in healthy dogs following oral administration of single and multiple doses. Am J Vet Res. 2010;71:337–341. doi: 10.2460/ajvr.71.3.337. [DOI] [PubMed] [Google Scholar]
- 91.Moore S.A., Muñana K.R., Papich J.A. The pharmacokinetics of levetiracetam in healthy dogs concurrently receiving phenobarbital. J Vet Pharmacol Ther. 2011;34(1):31–34. doi: 10.1111/j.1365-2885.2010.01188.x. [DOI] [PubMed] [Google Scholar]
- 92.Von Klopmann T., Rambeck B., Tipold A. Prospective study of zonisamide therapy for refractory idiopathic epilepsy in dogs. J Small Anim Pract. 2007;48:134–138. doi: 10.1111/j.1748-5827.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 93.Dewey C.W., Guiliano R., Boothe D.M. Zonisamide therapy for refractory idiopathic epilepsy in dogs. J Am Anim Hosp Assoc. 2004;40:285–291. doi: 10.5326/0400285. [DOI] [PubMed] [Google Scholar]
- 94.Chung J.Y., Hwang C.Y., Chae J.S. Zonisamide monotherapy for idiopathic epilepsy in dogs. N Z Vet J. 2012;60(6):357–359. doi: 10.1080/00480169.2012.680855. [DOI] [PubMed] [Google Scholar]
- 95.Miller M.L., Center S.A., Randolph J.F. Apparent acute idiosyncratic hepatic necrosis associated with zonisamide administration in a dog. J Vet Intern Med. 2011;25:1156–1160. doi: 10.1111/j.1939-1676.2011.00783.x. [DOI] [PubMed] [Google Scholar]
- 96.Schwartz M., Muñana K.R., Olby N.J. Possible drug-induced hepatopathy in a dog receiving zonisamide monotherapy for treatment of cryptogenic epilepsy. J Vet Med Sci. 2011;73(11):1505–1508. doi: 10.1292/jvms.11-0164. [DOI] [PubMed] [Google Scholar]
- 97.Cook A.K., Allen A.K., Espinosa D. Renal tubular acidosis associated with zonisamide therapy in a dog. J Vet Intern Med. 2011;25:1454–1457. doi: 10.1111/j.1939-1676.2011.00801.x. [DOI] [PubMed] [Google Scholar]
- 98.Dewey C.W., Cerda-Gonzalez S., Levine J.M. Pregabalin as an adjunct to phenobarbital, potassium bromide, or a combination of phenobarbital and potassium bromide for treatment of dogs with suspected idiopathic epilepsy. J Am Vet Med Assoc. 2009;235:1442–1449. doi: 10.2460/javma.235.12.1442. [DOI] [PubMed] [Google Scholar]
- 99.Platt S.R., Adams V., Garosi L.S. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. 2006;159:881–884. [PubMed] [Google Scholar]
- 100.Siao K.T., Pypendop B.H., Ilkiw J.E. Pharmacokinetics of gabapentin in cats. Am J Vet Res. 2010;71(7):817–821. doi: 10.2460/ajvr.71.7.817. [DOI] [PubMed] [Google Scholar]
- 101.Cautela MA, Dewey CW, Schwark WS, et al. Pharmokinetics of oral pregabalin in cats after single dose administration. In Proceedings ACVIM Forum. Anaheim (CA): 2010. p. 739.
- 102.Ruehlmann D., Podell M., March P. Treatment of partial seizures and seizure-like activity with felbamate in SUL dogs. J Small Anim Pract. 2001;42:403–408. doi: 10.1111/j.1748-5827.2001.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 103.Read M.R. Midazolam. Compendium. 2002;24:774–777. [Google Scholar]
- 104.Tobias K.M., Marioni-Henry K., Wagner R. A retrospective study on the use of acepromazine maleate in dogs with seizures. J Am Anim Hosp Assoc. 2006;42:283–289. doi: 10.5326/0420283. [DOI] [PubMed] [Google Scholar]
- 105.Levitski R.E., Trepanier L.A. Effect of timing of blood collection on serum phenobarbital concentrations in dogs with epilepsy. J Am Vet Med Assoc. 2000;217:200–204. doi: 10.2460/javma.2000.217.200. [DOI] [PubMed] [Google Scholar]