Highlights
-
•
Non-progressive replication of foot and mouth disease virus was observed in mice peritoneal macrophages.
-
•
Macrophages turns to M1 type polarization in response to FMDV infection.
-
•
Upregulation of pro-inflammatory cytokines was peak by 8 h FMDV infection.
-
•
Type I IFN and viperin showed marked upregulation following FMDV infection in the macrophages.
Keywords: FMD, Virus, Macrophage, Polarization, Cytokines, Antiviral genes
Abstract
Despite the fact that macrophages link the innate and adaptive arms of immunity, it’s role in the early infection of foot and mouth disease virus (FMDV) is largely unknown. Recently, depletion of macrophages in vivo after vaccination has shown to drastically diminish the protection against FMDV challenge in mouse model. Even the ability of macrophages to reduce or resist FMDV infection is not known hitherto. Therefore, we examined the replication ability of FMDV in mice peritoneal macrophages and the responsiveness in terms of macrophage polarization and cytokine production. Negative strand specific RT-PCR indicated replication of FMDV RNA in macrophages. Absolute quantitation of FMDV transcripts, immunofluorescence studies and titre of the infectious progeny virus revealed that replication peaked at 12 hpi and significantly declined by 18 hpi indicating non-progressive replication in the infected macrophages. Further, significant up regulation of inducible nitric oxide synthase by 8 –12 hpi and increase of M1 specific CD11c + cells by 42.6 % after infection showed that FMDV induce M1 polarization. A significant up regulation of TNFα and IL12 transcripts at 8 hpi supported that M1 macrophages were functional. Further, we studied the expression of Type I to III interferons (IFN) and other antiviral molecules. The results indicate a marked up regulation of Type I IFNα and β by 9.2 and 11.2 fold, respectively at 8 hpi. Of the four IFN stimulated genes (ISG), viperin showed a significant up regulation by 286-fold at 12 hpi in the mice macrophages. In conclusion, the results suggest that replication of FMDV in mice peritoneal macrophages is non-progressive with up regulation of Type I IFN and ISGs. Further, FMDV induces M1 polarization in murine peritoneal macrophages.
1. Introduction
Macrophages, the differentiated mononuclear phagocytic leukocytes, are present in almost every tissue of the body. Upon activation by antigenic stimulus, they acquire pronounced ability to phagocyte and destroy a wide variety of viruses and other microorganisms (Lee and Jeon, 2005; Rigden et al., 2002). Macrophages not only detect and kill invading microorganisms, but when stimulated, they also secrete a mixture of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, IL-12 and Type I interferons that promote both innate and adaptive immune responses (Cheung et al., 2002; Kumagai et al., 2007). Together with dendritic cells, macrophages have a critical role in antigen processing and presentation to other immune cells that is critical for the initiation of adaptive immune responses (Kovacsovics-Bankowski et al., 1993).
During virus infection, initial virus-macrophage interaction can occur through low or high affinity receptors, leading to the activation of many signaling pathways in macrophages. Macrophages respond to various stimuli including cytokines, chemokines and microbial products that are rapidly generated following infection. Depending on the stimuli they encounter, tissue resident and circulatory macrophage populations can be directed to distinct phenotypic subsets in a process known as macrophage polarization. Macrophages can differentiate into classically activated M1 cells or alternatively activated M2 cells that differ in their surface receptor expression, cytokine and chemokine production (Benoit et al., 2008). M1 macrophages are microbicidal and pro-inflammatory, characterized by high production of nitric oxide (NO) and reactive oxygen intermediates while M2 macrophages are poorly microbicidal and have anti-inflammatory properties (Mosser and Edwards, 2008). Manipulating macrophage polarization is one of the important mechanisms employed by viruses to evade host immune responses. Most of the viruses induce infected monocytes/macrophages to display a unique M1/M2 polarization. For instance, respiratory syncytial virus (RSV) and chikungunya virus induce M1 polarity, while severe acute respiratory syndrome coronavirus (SARS-CoV) induces M2 polarity (Rivera-Toledo et al., 2015; Kumar et al., 2012; Page et al., 2012). In general, M2 polarized macrophages show high permissiveness for many viruses and optimal susceptibility in comparison to M1 cells (Poglitsch et al., 2012; Page et al., 2012). They, hence, prevent the inflammatory cytokine production and the polarization of macrophages toward the M1 phenotype in order to decrease the pro-inflammatory response. Hepatitis C virus (HCV) inhibits monocyte differentiation to either M1 or M2 macrophages (Zhang et al., 2016), whereas H3N2 influenza virus induces a mixed polarization, leading to dysfunction of both M1 and M2 macrophages (Hoeve et al., 2012). Indeed, changes in macrophage polarization represent a key strategy to swing between resistance or restricted virus replication and progressive virus replication (Cassol et al., 2009).
Foot and mouth disease (FMD) is a highly contagious and economically important viral disease of cloven hoofed domestic and wild animals. The economic loss due to FMD in India is estimated to be Rs. 120–140 billion per annum (Singh et al., 2013). The etiological agent, foot and mouth disease virus (FMDV) is a single-stranded, positive sense RNA virus belongs to the family Picornaviridae, genus Aphthovirus. The characteristic feature of FMDV is its antigenic and genetic diversity, which has been reflected by the presence of seven distinct serotypes (O, A, C, Asia 1 and South African Territories 1, 2 and 3) and constant emergence of variants within each serotype (Domingo et al., 2003; Knowles and Samuel, 2003). FMDV rapidly replicates and spreads among in-contact susceptible animals by aerosol. Though the disease is rarely fatal in adult animals, it is the most feared infectious animal disease owing to nearly 100 % morbidity, rapid spread and drastic fall in livestock productivity with calf mortality.
The virus elicits a rapid humoral response in either infected or vaccinated animals. In contrast to humoral immunity, relatively little is known about the contribution of cellular immunity, especially the role of macrophages in controlling FMDV infection. The relatively rapid reduction in viremia during FMD and the early initiation of adaptive immune responses would indicate that innate immune responses are robust. In acute virus infection, macrophages are recruited from the circulation in response to chemokines and serve as a powerful killer of invading pathogens with the secretion of inflammatory mediators and nitric oxide. Depletion of macrophages with clodronate liposomes drastically diminished the protection against FMDV challenge after vaccination in mouse model, thus indicating that this population plays a key role in early protection elicited by inactivated vaccine (Quattrocchi et al., 2011). It has been reported that FMDV can enter macrophages without aid from antibody, although the antibody will certainly increase the efficiency and rate of uptake, through the involvement of the cellular Fc receptors (McCullough et al., 1988). In another study, similar observations including viral RNA replication in macrophages were only found with antibody-opsonized FMDV (Baxt and Mason, 1995). Previous studies examining macrophage interactions in FMDV infection in vitro focused on infectivity, but not on macrophage activation and innate immune responses. A recent study has demonstrated that mouse macrophage cell line, RAW264.7 is an appropriate in vitro model for investigating the innate immune response to FMDV infection (Zhi et al., 2018). Unfortunately, monocyte/macrophage cell lines do not always simulate the true characteristics of primary cells (Sager et al., 1999). Indeed, the ability of FMDV to infect murine macrophages, its intracellular replicative potential, induce polarization and stimulate the innate immune responses is not studied hitherto. Here, we report that experimental infection of mice peritoneal macrophages with FMDV results in non-progressive replication of the virus, induction of M1 polarization and prominent up regulation of pro-inflammatory cytokines, Type I interferons (IFN) and host restriction factors such as viperin.
2. Materials and methods
2.1. Animals
Adult male Swiss albino mice of 8–12 weeks age (n = 12) served as the source of peritoneal macrophages (Invivo Biosciences, Bengaluru, India). Mice were maintained on pellet feed and water ad libitum. Experiment was approved by Institutional Animal Ethics Committee, IVRI, Bengaluru videF.8-56-Vol.II/RCSS/2018-19/2.
2.2. Isolation of peritoneal macrophages and culturing
Macrophages were collected from the peritoneal cavity in ice-cold PBS and centrifuged at 210 × g for 8 min at 4 °C. The cell pellet was re-suspended in RPMI 1640 supplemented with 10 % heat-inactivated fetal bovine serum (FBS; Hyclone, UK), 100 U/mL penicillin and 100 ug/mL streptomycin (Sigma-Aldrich, India). Adherent macrophage monolayers were obtained by seeding the cells in 12 well tissue culture plates (Corning, USA) at a concentration of 1 × 106 cells/well for 2 h at 37 °C in 5 % CO2. Non-adherent cells were removed by gentle washing and freshly prepared media with 10 % FBS was added for further culturing.
2.3. Virus infection
FMDV strain O/IND/R2/75 grown in BHK-21 cells was used in this study. Viral stocks were propagated on BHK-21 monolayer cells and titrated using the Reed and Muench method (Reed and Meunch, 1938) to derive multiplicity of infection (MOI). Cultured macrophages (1 × 106 cells/well) were washed twice with serum-free medium and were incubated with 2 MOI of FMDV in a final volume of 500 μL for 1 h at 37 °C in a humidified incubator with 5 % CO2. The cells were then washed twice and cultured further incubated in 1 ml medium with 3 % FBS in same conditions. Cells were collected at 4, 8, 12, 18 and 24 h post infection (hpi) and processed for RNA extraction. The experiment was repeated thrice with n = 3/time point.
2.4. Detection of FMDV replication by strand-specific RT-PCR
As a positive-strand RNA virus, FMDV produces an intermediate negative-strand RNA when it replicates. Thus, detection of negative-strand viral RNA by strand specific RT-PCR confirms the initiation of FMDV replication. In brief, total RNA was extracted from the infected cells at 0, 4, 8, 12, 18 and 24 hpi using Trizol reagent (Ambion, USA) according to the manufacturer's instructions. The negative strand viral RNA was reverse transcribed to cDNA using 1C97 F primer, which is the sense primer that primes the negative-strand FMDV RNA. The cDNA is then subjected to PCR to amplify a 249 bp region specific to FMDV serotype O using the primers, DHP13 F and NK61 R. All primers used in this study are listed in supplementary table 1.
2.5. Demonstration of FMDV antigen in infected macrophages by indirect immunofluorescence (IIF)
Macrophage cells grown in 24-well tissue culture plates were mock-infected or infected with FMDV O/ IND/R2/75 at 2 MOI for 4, 12 and 18 h. After the corresponding hpi, cells were washed and fixed with 4 % paraformaldehyde. Cells were permeabilized with 0.1 % Triton-X (v/v) for 10 min and then blocked with 3 % BSA for 30 min to prevent non-specific binding. Cells were then incubated with a monoclonal antibody raised against the 3AB protein of FMDV (1:100 dilution) for 1 h at room temperature followed by three cycles of 5 min washing with PBS containing 0.025 % Tween 20 (PBS-T). Bound antibodies were visualized using 1:1000 diluted anti-rabbit IgG conjugated with Alexa Fluor 488 after incubation for 1 h in dark at room temperature and washing thrice with PBS-T. Fluorescence images were acquired at 200 x using Nikon T100 fluorescence microscope with CCD camera and NIS software.
2.6. Viral RNA quantitation by real-time PCR
RNA extraction from the infected macrophages and cDNA preparation using Oligo dT primer were carried out as described in 2.7. For quantitation of virus in infected macrophages, absolute qPCR was performed using FMDV 3D polymerase specific primers (FMDV3DF and FMDV3DR) using ABI 7300 Real-time PCR system (Applied Biosystems, CA, USA). Virus copy number was determined based on the standard curve composed of five 10-fold serial dilutions of pcDNA3.1-FMDV3D plasmid. The copy number of virus in test samples was interpolated from the standard curve.
2.7. Infectious progeny virus detection and titration
Macrophages grown in 12-well tissue culture plates were infected with FMDV at 2 MOI. After 1 h incubation at 37 °C, cells were washed twice with PBS, pH 6, with 2 min interval. RPMI (1 mL) with 3 % FBS was added to the culture and incubated further at 37 °C in a humidified incubator under 5 % CO2. Supernatants and cells were collected at 4, 12 and 18 hpi and processed for checking infectious progeny virus and titration. Cells were washed with PBS and lysed in 1 ml GMEM media by repeated freeze thawing. Briefly, supernatants and cell lysates were centrifuged at 10,000 rpm for 10 min at 4 °C. Clarified supernatants were then used for checking infectious progeny virus and titration. To detect infectious progeny virus, 500 uL of clarified supernatant collected from cell lysate and supernatant was added on BHK-21 cell monolayers in 12-well tissue culture plates and incubated for 1 h at 37 °C. After 1 h, the media was removed and 1 ml fresh GMEM media was added to each well and incubated for 48 h at 37 °C in 5 % CO2. At the end of incubation period, the wells were observed for cytopathic effect (CPE). The titre of infectious progeny virus in FMDV infected cell lysates and supernatants was calculated by TCID50 method. Clarified supernatant was serially titrated on BHK-21 monolayers, in serum-free GMEM. After 48 h incubation TCID50 was calculated as reported elsewhere (Reed and Meunch, 1938). The experiment was repeated thrice with n = 3/time point.
2.8. RNA isolation and gene expression
The expression of the cellular genes including markers of macrophage polarization (iNOS and Arg1), cytokines (TNFα, IL12, IL10, IFNα, IFNβ, IFNγ and IFNλ3) and antiviral molecules (PKR, OAS1a, Mx1 and viperin) was analyzed by qRT-PCR. Total RNA was extracted from FMDV infected and uninfected peritoneal macrophages using Trizol reagent (Ambion, USA) according to the manufacturer's instructions. The purified RNA was further treated with DNase1 (Thermo Scientific, USA) for 60 min at 37 °C. One microgram of the total RNA from each sample was reverse transcribed into cDNA using Oligo(dT) primer and the M-MuLV Reverse Transcriptase (New England BioLabs, UK) according to the manufacturer's instructions. Gene expression analysis was performed by relative qPCR using SYBR Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer’s instructions. The expression of the target genes was normalized to GAPDH to generate ΔCt. Uninfected control (0 hpi) served as calibrator group to generate ΔΔCt. The relative fold was determined using 2−ΔΔCT as per Livak’s method (Livak and Schmittgen, 2001). Each biological sample (n = 3/time point) was run in triplicate and the mean was used for statistical analysis.
2.9. Flow cytometric analysis of cell surface markers of macrophage polarization
Macrophages grown in 6-well tissue culture plates were infected with FMDV as described earlier and cells were harvested at 0, 4, 12 and 18 hpi. After treatment, cells were washed with PBS and detached from culture plates by adding PBS containing EDTA (5 mmol/L) and incubated at 4 °C for 10 min, before thorough pipetting. 1 × 106 cells per sample were used for analysis by Cytomics FC500 flow cytometer (Beckman Coulter, USA) and stained with the following antibodies: APC-conjugated anti-mouse F4/80 antibody, PE-conjugated anti-mouse CD11c and FITC-conjugated anti-mouse CD206 (all antibodies were from BioLegend, USA). Antibodies were diluted in FACS buffer (PBS containing 5 % FBS, pH 7.4). Each antibody was incubated at 4 °C for 15 min in the dark. Cells were washed twice with FACS buffer between each antibody incubation. After washing, cells were resuspended in FACS buffer and run on a Cytomics FC500 flow cytometer (Beckman Coulter, USA). M1 macrophages were identified as F4/80-positive/CD11c-positive while M2 macrophages were identified as F4/80-positive/CD206-positive. The data was analyzed by CXP Software Version 2.2 (Beckman Coulter, USA).
2.10. Statistical analysis
One-way Anova with Bonferroni post-hoc test was used to analyze the effect of hpi on virus titre of cell lysate, supernatant, relative fold change of each target gene and FACS data.
Virus titration result is expressed as mean ± standard deviation, while relative fold change of genes and FACS result are presented as mean ± standard error of the mean (SEM). Significance was set at 95 %. GraphPad Prism 5.0 software (San Diego, CA, USA) was used for statistical analysis and preparation of graphs.
3. Results
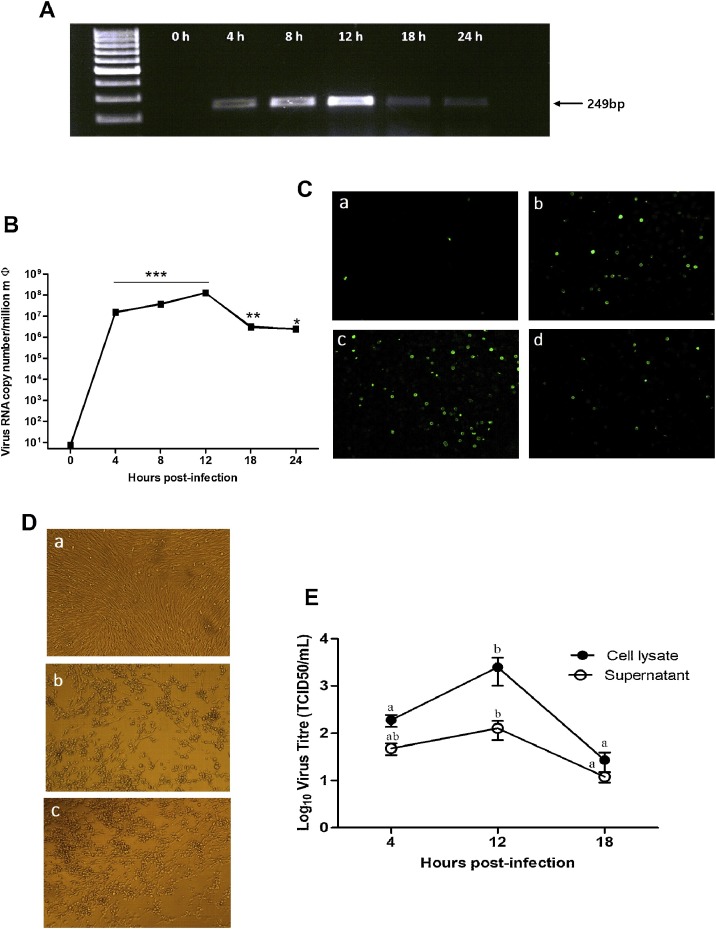
3.1. FMDV undergoes non-progressive replication in mice peritoneal macrophages
As replication of virus in a host cell is important for survival and propagation, we first demonstrated the presence of minus-stranded FMDV RNA in mice peritoneal macrophages by strand specific RT- PCR assay (Fig. 1 A) indicating initiation of replication cycle. Assessment of replication kinetics by absolute real-time PCR showed a significant increase in the copy number of viral RNA by 4 hpi as compared to 0 hpi (P < 0.001) and trend of increase continued till 12 hpi, where it reached peak. The copy number of viral RNA began to decline after 12 hpi and reached a 2-log difference at 18 hpi. The viral RNA at 24 hpi was still considerably lower than at 18 hpi (Fig. 1B). Since demonstration of the viral antigen within the cell provides evidence of synthesis of virus proteins, immunofluorescence was done. Fluorescence images of mice peritoneal macrophages stained for FMDV antigen showed that 40–50 % of cells were infected at 12 hpi, the time of peak virus production; however, by 18 hpi, only 15–20 % cells showed immunolocalization of FMDV (Fig. 1C). As the increase in viral RNA and the presence of non-structural proteins do not confer the proof of completion of viral replication cycle, virus titration of the infected cell lysate and supernatant was done using TCID50 method. Both cell lysate and supernatant were used as there was no typical CPE in FMDV infected macrophages. Confirmation of infectious progeny virus from infected macrophages was obtained by blind passage of infected cell lysates and supernatants on BHK-21 cell monolayers (Fig. 1D). Determination of virus titre in FMDV infected cell lysate showed a significant increase in the number of infectious progeny virus by 12 hpi as compared to 4 hpi (Fig. 1E). The virus titre began to decrease significantly (P < 0.05) after 12 h and reached a 2-log difference by 18 h. The trend of virus titre in the supernatant was comparable with that of cell lysate; however, it was non-significant (P > 0.05; Fig. 1E). There is congruence between the copy number of FMDV transcript and virus titre at 12 hpi. Overall, the replication was non- progressive suggesting the ability of macrophages to limit the propagation of FMDV in vitro.
Fig. 1.
Replication of foot and mouth disease virus in mice peritoneal macrophages. (A) Detection of replicative negative-strand viral RNA by strand specific RT-PCR in mice macrophages after experimental infection with FMDV O/ IND/R2/75 at 2 MOI. Macrophages were collected at different time points from 0 to 24 h; total RNA was extracted and cDNA was prepared using a primer specific to the negative-strand RNA of FMDV. PCR revealed an amplicon of 249 bp specific to the virus. (B) Absolute quantitation of FMDV RNA in the peritoneal macrophages by real−time PCR. Total RNA was extracted from FMDV infected and uninfected (0 h) macrophages and quantitated by qRT−PCR using virus specific primers. The copy number of viral RNA in test samples was interpolated from the standards. The experiment was repeated thrice with n=3/time point. Representative data of a single experiment is presented. * indicates a p value < 0.05, ** indicates a p value < 0.01 and ***indicate a p value < 0.001. (C) Immunofluorescence demonstration of FMDV antigen in the peritoneal macrophages post−infection. Macrophage cells grown in 24-well tissue culture plates were (a) mock−infected and infected with FMDV for (b) 4 (c) 12 and (d) 18 h. After that cells were washed and fixed with 4 % paraformaldehyde and permeabilized with triton X100. Cells were incubated with a monoclonal antibody raised against the 3AB protein of FMDV. Anti−rabbit Alexa Fluor−488 conjugated IgG was used as secondary antibody. (D) Demonstration of virus progeny from infected macrophages. BHK−21 cells grown in 12−well tissue culture plates were (a) mock−infected or passaged with (b) cell lysate and (c) supernatant from 12 h FMDV infected macrophages. Appearance of CPE in (b) and (c) indicates the presence of infectious progeny virus in FMDV infected macrophages and supernatant. (E) Virus titre of FMDV infected cell lysate and supernatant at 4, 12 and 18 hpi. Supernatants and cells were collected separately at different time points and serially titrated on BHK−21 monolayers. The CPE was recorded after 2 days of incubation and the titre (TCID50) of infectious progeny virus in cell lysates and supernatants were calculated by Reed and Muench method. The results are from three independent experiments, expressed as the mean ± SD. Values with different lowercase superscripts (a, b) within a line are significantly different (P < 0.05).
3.2. Peritoneal macrophages differentiate into the M1 phenotype after infection with FMDV
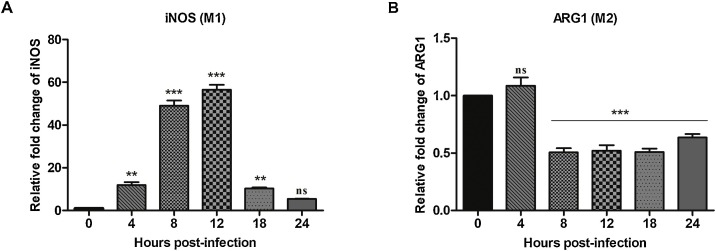
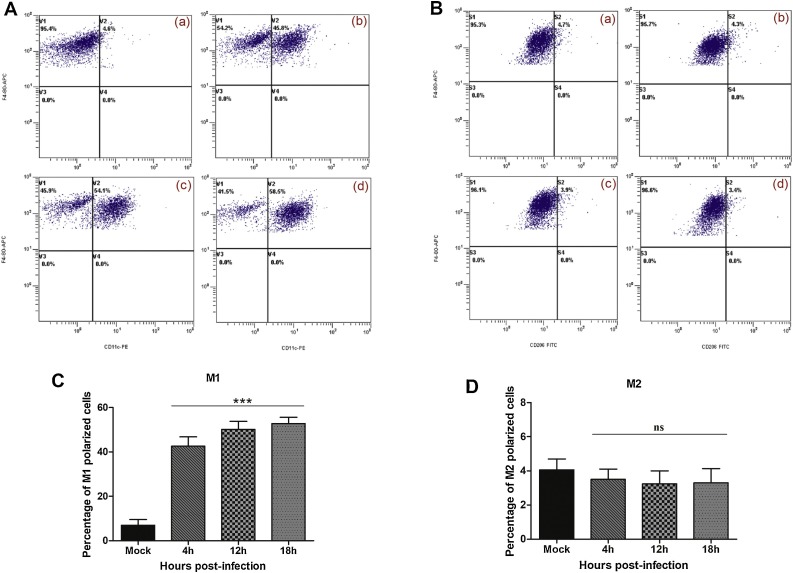
We next investigated whether infection of peritoneal macrophages with FMDV could induce phenotypic differentiation into either M1 or M2. Inducible nitric oxide synthase (iNOS), a key regulator of the M1 phenotype, was up regulated in peritoneal macrophages infected with FMDV (Fig. 2 A). After 4 hpi, 11.9 fold increase in iNOS was seen, which was statistically significant (P < 0.01) as compared to uninfected control (0 hpi). The expression peaked by 56.5 fold increase at 12 hpi (P < 0.001) and declined abruptly by 18 hpi. The relative expression of iNOS at 24 hpi was comparable with that of uninfected control (P > 0.05). In contrast, FMDV infection of mice peritoneal macrophages revealed a significant down-regulation of M2 phenotype specific arginase 1 (Arg1) by 50 % at 8 hpi that was maintained till 24 hpi (Fig. 2B; P < 0.001). To corroborate the real-time PCR results of FMDV induced polarization of mice peritoneal macrophages, flow cytometry was done (Fig. 3 A and B). The fraction of polarized cells before and after FMDV infection is shown in Fig. 3C and D. At 4 hpi, 42.6 % M1 specific CD11c expressing cells showed highly significant increase (P < 0.001), while it was only 6.9 % in the uninfected group (Fig. 3C). However, there was no further increase in the M1 polarized cells till 18 hpi. Unlike Arg1 expression, M2 specific CD206 expressing cells (%) was unaltered following FMDV infection (Fig. 3D). Only 3.3 % macrophages from FMDV infected macrophages were positive for CD206 by 18 hpi, which was similar to that of uninfected control group (Fig. 3D). Taken together, our results suggest that infection of mice peritoneal macrophages with FMDV induces M1 polarization.
Fig. 2.
Expression of markers specific to macrophage polarization in FMDV infected mice peritoneal macrophages. qRT-PCR assay for the expression of (A) M1 specific inducible nitric oxide synthase (iNOS) and (B) M2 specific arginase1 (ARG1) mRNA in FMDV infected (4, 8, 12, 18 and 24 h) and uninfected (0 h) peritoneal macrophages. mRNA expression levels were normalized to GAPDH to generate ΔCt. Relative fold change (2−ΔΔCt) was calculated using uninfected 0 h as calibrator group. The fold change was analysed by one-way ANOVA. When the F ratio was significant, orthogonal contrast was done with Bonferroni post-hoc test using 0 h as control. The experiment was repeated thrice with n = 3/time point. Representative data of a single experiment is presented. Each bar represents mean ± SEM (n = 3). ** indicates a p value < 0.01 and ***indicate a p value < 0.001, ns = non-significant.
Fig. 3.
Flow cytometric analysis of macrophage polarization in FMDV infected mice peritoneal macrophages. Representative scatter plots of flow cytometric analysis of (A) M1 and (B) M2 polarization in the mice peritoneal macrophages infected with FMDV. In brief, cells grown in 6-well tissue culture plates were (a) mock infected or infected with FMDV at 2 MOI for (b) 4 h, (c) 12 h and (d) 18 h for counting the polarized cells by flow cytometry. We first gated out the F4/80-positive cells. In this subgroup, we used CD11c and CD206 as markers to identify M1 or M2 macrophages. F4/80+, CD11c + cells are marked as M1 positive cells, F4/80+, CD206+ cells are marked as M2-positive cells. Panels (C) and (D) show mean positive population (%) plus standard error of the mean for M1 and M2 polarized cells in FMDV infected and mock-infected samples. The experiment was repeated thrice with n = 3/time point. Representative data of a single experiment is presented. Each bar represents mean ± SEM (n = 3). ***indicate a p value < 0.001 as calculated by one-way ANOVA with Bonferroni post-hoc test, ns = non-significant.
3.3. FMDV infection induces expression of pro-inflammatory but not anti-inflammatory cytokines in macrophages
We next investigated the expression of selected pro- and anti-inflammatory cytokine expression in peritoneal macrophages after FMDV infection in vitro (Fig. 4 ). Pro-inflammatory, but not anti-inflammatory cytokines were strongly induced. After 4 hpi, a statistically significant up regulation in the expression of TNFα by 19.4-fold was observed in macrophages, which peaked at 8 hpi (38.6-fold; P < 0.001); however, it declined to 1.9 fold by 18 hpi (Fig. 4A) and was comparable with uninfected control and 24 hpi (P > 0.05). Further, a statistically significant up regulation in the expression of IL12 was recorded from 4 to 18 hpi (P < 0.01) that declined to basal expression at 24 hpi (Fig.4B). The maximum increase in the expression of IL12 occurred at 8 h post virus infection, representing a 6.8-fold increase compared to uninfected control (P < 0.01). In terms of relative fold change, the up regulation of TNFα was nearly 5 times higher than that of IL12 at 8 hpi, when the peak expression of both pro-inflammatory cytokines was noticed. The expression of anti-inflammatory cytokine, IL10, was not modulated (P > 0.05) and ranged from 1 to 1.5-fold (Fig. 4C). These results indicate that infection of mice peritoneal macrophages with FMDV induces the expression of pro-inflammatory cytokines supporting M1 polarization.
Fig. 4.
Pro and anti-inflammatory cytokine mRNA expression in FMDV infected mice peritoneal macrophages.Total RNA was isolated from FMDV infected and uninfected peritoneal macrophages and then analyzed by qRT-PCR to detect the pro-inflammatory cytokines (A) TNFα and (B) IL12 and the anti-inflammatory cytokine (C) IL10. Cytokine mRNA expression levels were normalized to GAPDH. Relative fold change (2−ΔΔCt) was calculated using uninfected 0 h as calibrator group. The fold change was analyzed by one-way ANOVA. When the F ratio was significant, orthogonal contrast was done with Bonferroni post-hoc test using 0 h as control. The experiment was repeated thrice with n = 3/time point. Representative data of a single experiment is presented. Each bar represents mean ± SEM (n = 3). ** indicate a p value < 0.01, *** indicate a p value < 0.001, ns = non-significant.
3.4. Infection of macrophages with FMDV upregulates the expression of host restriction factors
We next investigated the expression of a number of host restriction factors in FMDV infected macrophages. Expression pattern of Type I IFN in terms of magnitude and time-dependent modulation was comparable sensulato (Fig. 5 A). A significant up regulation of IFNα was observed as early as 4 hpi (P < 0.01). The peak expression was observed at 8 hpi, representing a 9.2-fold increase compared to uninfected 0 h control (Fig. 5A). Similarly, a 4.7-fold increase in IFNβ expression (Fig. 5B) was detected at 4 hpi, which peaked (11.2-fold) at 8 hpi, returning to pre-infection level by 24 hpi. The expression of Type II IFNγ and Type III IFNλ3 was not modulated as compared to Type I IFN. The maximum up regulation of IFNλ3 occurred at 8 hpi, which was significant as compared to uninfected control (P < 0.05; Fig. 5D). Infection of mice macrophages with FMDV did not modulate the expression of IFNγ (P > 0.05; 5C).
Fig. 5.
The expression of interferons in mice peritoneal macrophages infected with FMDV. Total RNA was isolated from macrophages after infection with or without FMDV and then analysed by qRT-PCR to detect Type I interferons (A) IFNα and (B) IFNβ, Type II interferon (C) IFNγ and Type III interferon (D) IFNλ3. mRNA expression levels were normalized to GAPDH to generate ΔCt. Relative fold change (2−ΔΔCt) was calculated using uninfected 0 h as calibrator group. The fold change was analysed by one-way ANOVA. When the F ratio was significant, orthogonal contrast was done with Bonferroni post-hoc test using 0 h as control. The experiment was repeated thrice with n = 3/time point. Representative data of a single experiment is presented. Each bar represents mean ± SEM (n = 3). * indicates a p value < 0.05, ** indicates a p value < 0.01 and ***indicate a p value < 0.001, ns = non-significant.
To monitor antiviral status, we also evaluated the mRNA expression of IFN stimulated antiviral genes (ISG) such as PKR, OAS1a, Mx1 and viperin in FMDV infected mice peritoneal macrophages. Overall, FMDV infection strongly induced the expression of ISGs at 12 hpi with marked up regulation of viperin (Fig. 6 A–D). A highly significant (P < 0.001) up regulation of PKR expression was observed from 4 to 18 hpi (Fig. 6A). The peak of PKR mRNA induction (47.3-fold increase) was observed at 12 hpi and the level decreased to 29-fold by 18 hpi. We also found a statistically significant (P < 0.01) increase of 7.4-fold in the expression of OAS1a at 4 hpi (Fig. 6B). The induction was significantly high (P < 0.001) from 8 to 24 hpi (Fig. 6B). Macrophages infected in vitro with FMDV exhibited an initial increase in Mx1 expression to 27.3-fold by 4 hpi which lasted until 12 hpi before a significant reduction of 9.8-fold by 18 hpi (Fig. 6C). Interestingly, the relative abundance of viperin mRNA in FMDV infected macrophages during the initial hours of infection were more than 200-fold higher compared to uninfected control. At 12 hpi, the up regulation of viperin reached a maximum of 286 fold (P < 0.001). The viperin gene induction was significantly higher (37.8-fold) even after 24 h post-virus infection. Altogether, FMDV significantly up regulated Type I IFN and ISGs in the mice peritoneal macrophages.
Fig. 6.
The expression of interferon stimulated antiviral genes in FMDV infected mice peritoneal macrophages. Total RNA was isolated from FMDV infected and uninfected macrophages and then analysed by qRT-PCR to detect IFN stimulated antiviral molecules (A) PKR, (B) OAS1a, (C) Mx1 and (D) viperin. mRNA expression levels were normalized to GAPDH to generate ΔCt. Relative fold change (2−ΔΔCt) was calculated using uninfected 0 h as calibrator group. The fold change was analysed by one-way ANOVA. When the F ratio was significant, orthogonal contrast was done with Bonferroni post-hoc test using 0 h as control. The experiment was repeated thrice with n = 3/time point. Representative data of a single experiment is presented. Each bar represents mean ± SEM (n = 3). * indicates a p value < 0.05, ** indicates a p value < 0.01 and ***indicate a p value < 0.001.
4. Discussion
The role of macrophages in the pathogenesis of picorna virus infections, including FMDV infection of animals, remains incompletely understood. FMDV can infect porcine macrophages either directly or with the aid of antibody, though the latter mode augmented the virus uptake through Fc receptors (McCullough et al., 1988; Baxt and Mason, 1995; Rigden et al., 2002). A recent study demonstrated that FMDV can directly infect the mouse macrophage cell line RAW264.7 without the aid of antibodies as in BHK-21 cells and replicate progressively (Zhi et al., 2018). However, little is known on the effect of FMDV infection on the polarization of macrophages and activation of innate immune response in any species. In this study, we provide experimental evidence that FMDV O/IND/R2/75 directly infects mice peritoneal macrophages in vitro and replicates till 12 hpi. Demonstration of negative stranded RNA by specific RT-PCR indicated that viral replication was initiated. The virus RNA copy number which increased up to 12 hpi began to reduce after 12 hpi and reached a 2-log difference at 18 hpi as indicated by real time quantification result (Fig. 1B). The result is supported by the immunofluorescence detection of FMDV antigen in the infected macrophages up to 18 hpi (Fig. 1C). A significant increase in the virus titre at 12 hpi with a subsequent decrease at 18 hpi in the clarified cell lysate indicates replication of FMDV within the murine macrophages. Though a similar trend was observed in the virus titre of clarified supernatant, the time dependent variation was non-significant, which indicates that the mature virions are not released. This is supported by the lack of classical CPE in the FMDV infected macrophages. Terminal release of progeny virus by cytolytic process might be affected in the replication cycle of FMDV infected murine macrophages as restrictive replication is reported for a few enveloped viruses that are released by budding. For instance, mice macrophages restrict the replication of herpes simplex virus 1 (HSV-1) by inhibiting viral macromolecular synthesis (Sarmiento, 1988) and caprine arthritis encephalitis virus undergoes restrictive replication in the ovine fibroblast culture due to abnormal cleavage of glycoprotein of the envelope (Chebloune et al., 1996). Abortive infection of Influenza virus in murine dendritic cells is also reported due to defective release of viral progeny from the cell surface (Ioannidis et al., 2012). Our results suggest that FMDV replication is non progressive in murine macrophages as they did not support classical viral replication.
Although polarized macrophages contribute to the pathogenesis of various viral diseases, little is known on the macrophage polarization following FMDV infection. FMDV infection induced M1 differentiation of mice peritoneal macrophages from the basal M0 state, which was evident by the significant up-regulation of M1 specific marker, CD11c (Fig. 3C) and increased expression of iNOS (Fig. 2A). Viruses can manipulate the polarization between M1 and M2 according to the infectious process. Human cytomegalovirus (HCMV) is a typical example which induces a classical M1 differentiation and pro-inflammatory state during the early infection phase that helps virus dissemination and later skewed to M2 to avoid virus clearance by restricting the inflammatory response (Stevenson et al., 2014). In our study, we observed a consistent M1 status in FMDV infected macrophages till 18 hpi (Fig. 3C); however, the iNOS expression was down-regulated markedly after 12 hpi, suggesting that the NO production was reduced along with marked decline in virus copy number as excess NO production can induce cell damage due to oxidative stress (Gross and Wolin, 1995). M1 polarized macrophages secrete pro-inflammatory cytokines and produce elevated levels of NO and reactive oxygen intermediates and exhibit marked phagocytic activity (Mosser and Edwards, 2008). A significantly higher expression of pro-inflammatory cytokines such as TNFα and IL12 in the FMDV infected murine macrophages (Fig. 4) might have limited the intracellular replication of virus in M1 polarized cells. On the other hand, anti-inflammatory IL-10 elaborated from dendritic cells induce immunosuppressive milieu to allow the expansion of FMDV in the pigs (Díaz-San Segundo et al., 2009).
It is reported that type I (including IFN-α, β), Type II (IFNγ) and Type III (IFNλ) IFNs had certain antiviral activities against FMDV (Wu et al., 2003; Moraes et al., 2007; Perez-Martin et al., 2012; Diaz-San Segundo et al., 2011). In our study, we demonstrated that Type I IFNs, both IFN α and β shows a significant up regulation following FMDV infection, and the up regulation was observed as early as 4 hpi. Type I IFNs are primarily responsible for initiating direct antiviral actions in virus infected cells and do so with more efficiency than Type II IFN (Tan et al., 2005). Pre-treatment of cells with IFN surprisingly inhibited the replication of major serotypes of FMDV (Chinsangaram et al., 2001). Swine pretreated with replication-defective human adenovirus type 5 (Ad5) vector expressing Type I and II IFNs are completely protected when challenged with FMDV (Chinsangaram et al., 2003; Diaz-San Segundo et al., 2011). Similarly, Type I and II IFNs delay and reduce the clinical signs in cattle challenged with FMDV (Wu et al., 2003; Perez-Martin et al., 2012). However, the expression of IFNγ and IFNλ3 was not modulated as compared to Type I IFNs in our experiments.
Virus infections rapidly induce Type I IFN production, which, in turn, stimulates a vast range of ISG through paracrine and autocrine signaling (Der et al., 1998). Antiviral effectors such as double-stranded RNA-dependent protein kinase (PKR), 2΄, 5΄-oligoadenylate synthetase 1a (OAS1a), Mx1 and viperin are some of the well-studied ISGs in the context of infection with RNA virus (Langland et al., 2006; Silverman, 2007; Haller et al., 2007; Seo et al., 2011). Every stage of the virus life cycle is a possible target for ISG intervention including virus entry, viral genome replication, viral protein synthesis and release of new virion (Goujon et al., 2013; Munir and Berg, 2013; Durfee et al., 2010; Wang et al., 2007). In our study, macrophages abundantly express ISGs against FMDV infection at 12 hpi, which would be critical for inhibiting FMDV replication beyond 12 h. Acting in a concerted manner, several of ISGs restrict virus propagation through the induction of direct antiviral activity and the modulation of multiple cellular immune responses. However, like other viruses, FMDV counteracts the innate immune responses by blocking the IFN response (Chinsangaram et al., 1999; de los Santos et al., 2006). FMDV is highly sensitive to the inhibitory effects of type I IFN that involves at least three IFN-stimulated-gene products: PKR, OAS1a and Mx1 (Chinsangaram et al., 2001; de los Santos et al., 2006; Cai et al., 2013). Upon sensing viral RNA, OAS activates latent endoribonuclease (RNaseL) to cleave the viral RNA and limit the virus replication (Chakrabarti et al., 2011). In addition to OAS, PKR also act as a sensor for foreign RNA. Activation of PKR finally leads to the expression of a subset of ISGs such as viperin (Munir and Berg, 2013). We also found a highly significant (P < 0.01) increase in OAS1a and PKR expression by infected macrophages from 4 to 18 h post-FMDV infection. In addition, Mx1 proteins inhibit the replication of FMDV in BHK-21 and PK-15 cells, supporting the antiviral activity against FMDV (Cai et al., 2013; Yuan et al., 2015). Up regulation of Mx1 transcripts from 4 to 12 hpi coincides with the decline in the copy number of FMDV after 12 hpi (Fig. 1B and Fig. 6C).
Viperin is a potent host restriction factor that is highly inducible by both Type I and Type II IFNs (Der et al., 1998). The remarkable up regulation of viperin transcript against FMDV infection at 12 hpi in the murine macrophages (Fig. 6D) is consistent with its inhibitory effect on many RNA viruses (Wang et al., 2012; Helbig et al., 2013). Over expression of viperin could inhibit Hepatitis C virus replication by interacting with viral non-structural protein 5A (Helbig et al., 2011). Viperin effectively inhibited the replication of dengue virus and Zika virus in human monocyte-derived macrophages and human hepatoma cell line Huh-7, respectively (Helbig et al., 2013; Van der Hoek et al., 2017).
In conclusion, FMDV directly infects the murine peritoneal macrophages in vitro; however, the replication is non-progressive. Further, FMDV up regulates TNFα, IL12, Type I IFN and ISG including viperin in a time-dependent manner and induces phenotypic and functional M1 polarization. Similar studies in the cattle will enhance our understanding the role of macrophages during early FMDV infection.
Declaration of Competing Interest
The authors declare no potential conflicts of interest concerning the research, authorship, publication of this article, and/or financial and personal relationships that could inappropriately influence this work.
Acknowledgements
The authors thank the Director, IVRI, Bareilly and Joint Director, IVRI, Bangalore for providing necessary facilities for carrying out this research work. We thank Dr. M. Hossamani, IVRI, Bangalore for kindly providing the monoclonal anti-FMDV 3AB antibody for immunofluorescence work. The authors thank Kerala Veterinary and Animal Sciences University, Pookode, Kerala for providing external deputation at IVRI for the first author.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2020.197906.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Baxt B., Mason P.W. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor- mediated adsorption. J. Virol. 1995;207:503–509. doi: 10.1006/viro.1995.1110. [DOI] [PubMed] [Google Scholar]
- Benoit M., Desnues B., Mege J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Cai K.J., Meng Q.L., Qiao J., Huang J., Zhang Z.C., Wang G.C., Wang J.W., Chen C.F. Expression of bovine Mx1 protein inhibits the replication of foot-and-mouth disease virus in BHK-21 cells. Acta Virol. 2013;57(4):429–434. doi: 10.4149/av_2013_04_429. [DOI] [PubMed] [Google Scholar]
- Cassol E., Cassetta L., Rizzi C., Alfano M., Poli G. M1 and M2A polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Jha B.K., Silverman R.H. New insights into the role of RNase L in innate immunity. J. Interferon Cytokine Res. 2011;31:49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebloune Y., Sheffer D., Karr B.M., Stephens E., Narayan O. Restrictive type of replication of ovine/caprine lentiviruses in ovine fibroblast cell cultures. Virology. 1996;222:21–30. doi: 10.1006/viro.1996.0394. [DOI] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Lau A.S., Luk W., Lau Y.L., Shortridge K.F., Gordon S., Guan Y., Peiris J.S. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease. Lancet. 2002;360(9348):1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Chinsangaram J., Piccone M.E., Grubman M.J. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 1999;73(12):9891–9898. doi: 10.1128/jvi.73.12.9891-9898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Koster M., Grubman M.J. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 2001;75(12):5498–5503. doi: 10.1128/JVI.75.12.5498-5503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsangaram J., Moraes M.P., Koster M., Grubman M.J. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 2003;77(2):1621–1625. doi: 10.1128/JVI.77.2.1621-1625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos T., de Avila Botton A., Weiblen R., Grubman M.J. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J. Virol. 2006;80(4):1906–1914. doi: 10.1128/JVI.80.4.1906-1914.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der S.D., Zhou A., Williams B.R., Silverman R.H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U.S.A. 1998;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-San Segundo F., Weiss M., Pérez-Martín E., Koster M.J., Zhu J., Grubman M.J., de los Santos T. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology. 2011;413(2):283–292. doi: 10.1016/j.virol.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Díaz-San Segundo F., Rodríguez-Calvo T., de Avila A., Sevilla N. Immunosuppression during acute infection with foot-and-mouth disease virus in swine is mediated by IL-10. PLoS One. 2009;4(5):e5659. doi: 10.1371/journal.pone.0005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Escarmis C., Baranowski E., Ruiz-Jarabo C., MCarrillo E., Nunez J.I., Sobrino F. Evolution of foot-and-mouth disease virus. Virus Res. 2003;91:47–63. doi: 10.1016/s0168-1702(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Durfee L.A., Lyon N., Seo K., Huibregtse J.M. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol. Cell. 2010;38(5):722–732. doi: 10.1016/j.molcel.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon C., Moncorge O., Bauby H., Doyle T., Ward C.C., Schaller T., Hué S., Barclay W.S., Schulz R., Malim M.H. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature. 2013;502(7472):559–562. doi: 10.1038/nature12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S.S., Wolin M.S. Nitric oxide: pathophysiological mechanisms. Annu. Rev. Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Haller O., Stertz S., Kochs G. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect. 2007;9(14–15):1636–1643. doi: 10.1016/j.micinf.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Helbig K.J., Eyre N.S., Yip E., Narayana S., Li K., Fiches G., McCartney E.M., Jangra R.K., Lemon S.M., Beard M.R. The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology. 2011;54(5):1506–1517. doi: 10.1002/hep.24542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig K.J., Carr J.M., Calvert J.K., Wati S., Clarke J.N., Eyre N.S., Narayana S.K., Fiches G.N., McCartney E.M., Beard M.R. Viperin is induced following dengue virus type-2 (DENV-2) infection and has anti-viral actions requiring the C-terminal end of viperin. PLoS Negl. Trop. Dis. 2013;7(4):e2178. doi: 10.1371/journal.pntd.0002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeve M.A., Nash A.A., Jackson D., Randall R.E., Dransfield I. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis L.J., Verity E.E., Crawford S., Rockman S.P., Brown L.E. Abortive replication of influenza virus in mouse dendritic cells. J. Virol. 2012;86(10):5922–5925. doi: 10.1128/JVI.07060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N.J., Samuel A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80. doi: 10.1016/s0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K.L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. U.S.A. 1993;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y., Takeuchi O., Kato H., Kumar H., Matsui K., Morii E., Aozasa K., Kawai T., Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Kumar S., Jaffar-Bandjee M.C., Giry C., de Kerillis L.C., Merits A., Gasque P., Hoarau J.J. Mouse macrophage innate immune response to chikungunya virus infection. J. Virol. 2012;9:313. doi: 10.1186/1743-422X-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland J.O., Cameron J.M., Heck M.C., Jancovich J.K., Jacobs B.L. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119:100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Jeon Y.J. Macrophage activation by polysaccharide isolated from Astragalus membranaceus. Int. Immunopharmacol. 2005;5(7–8):1225–1233. doi: 10.1016/j.intimp.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCullough K.C., Parkinson D., Crowther J.R. Opsonization enhanced phagocytosis of foot-and-mouth disease virus. Immunology. 1988;65:187–191. [PMC free article] [PubMed] [Google Scholar]
- Moraes M.P., de los Santos T., Koster M., Turecek T., Wang H., Andreyev V.G., Grubman M.J. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. J. Virol. 2007;81(13):7124–7135. doi: 10.1128/JVI.02775-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir M., Berg M. The multiple faces of protein kinase R in antiviral defense. Virulence. 2013;4:85–89. doi: 10.4161/viru.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C., Goicochea L., Matthews K., Zhang Y., Klover P., Holtzman M.J., Hennighausen L., Frieman M. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection. J. Virol. 2012;86(24):13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin E., Weiss M., Diaz-San Segundo F., Pacheco J.M., Arzt J., Grubman M.J., de los Santos T. Bovine type III interferon significantly delays and reduces the severity of foot-and-mouth disease in cattle. J. Virol. 2012;86(8):4477–4487. doi: 10.1128/JVI.06683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglitsch M., Weichhart T., Hecking M., Werzowa J., Katholnig K., Antlanger M., Krmpotic A., Jonjic S., Horl W.H., Zlabinger G.J., Puchhammer E., Saemann M.D. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am. J. Transplant. 2012;12(6):1458–1468. doi: 10.1111/j.1600-6143.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- Quattrocchi V., Langellotti C., Pappalardo J.S., Olivera V., Di Giacomo S., van Rooijen N., Mongini C., Waldner C., Zamorano P.I. Role of macrophages in early protective immune responses induced by two vaccines against foot and mouth disease. Antiviral Res. 2011;92(2):262–270. doi: 10.1016/j.antiviral.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Meunch H. A simple method for estimating fifty percent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Rigden R.C., Carrasco C.P., Summerfield A., McCullough K.C. Macrophage phagocytosis of foot-and-mouth disease virus may create infectious carriers. Immunology. 2002;106:537–548. doi: 10.1046/j.1365-2567.2002.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Toledo E., Torres-González L., Gómez B. Respiratory syncytial virus persistence in murine macrophages impairs IFN-β response but not synthesis. Viruses. 2015;7(10):5361–5374. doi: 10.3390/v7102879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager H., Davis W.C., Jungi T.W. Bovine monocytoid cells transformed to proliferate cease to exhibit lineage-specific functions. Vet. Immunol. Immunopathol. 1999;68(2–4):113–130. doi: 10.1016/s0165-2427(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Sarmiento M. Intrinsic resistance to viral infection. Mouse macrophage restriction of herpes simplex virus replication. J. Immunol. 1988;141(8):2740–2748. [PubMed] [Google Scholar]
- Seo J.Y., Yaneva R., Cresswell P. Viperin: a multifunctional, interferon-inducible protein that regulates virus replication. Cell Host Microbe. 2011;10:534–539. doi: 10.1016/j.chom.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R.H. Viral encounters with 2’,5’-Oligoadenylate synthetase and RNase l during the interferon antiviral response. J. Virol. 2007;81(23):12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Prasad S., Sinha D.K., Verma M.R. Estimation of economic losses due to foot and mouth disease in India. Indian Journal of Animal Science. 2013;83(9):964–970. [Google Scholar]
- Stevenson E.V., Collins-McMillen D., Kim J.H., Cieply S.J., Bentz G.L., Yurochko A.D. HCMV reprogramming of infected monocyte survival and differentiation: a goldilocks phenomenon. Viruses. 2014;6:782–807. doi: 10.3390/v6020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Derrick J., Hong J., Sanda C., Grosse W.M., Edenberg H.J., Taylor M., Seiwert S., Blatt L.M. Global transcriptional profiling J. demonstrates the combination of type I and type II interferon enhances antiviral and immune responses at clinically relevant doses. J. Interferon Cytokine Res. 2005;25(10):632–649. doi: 10.1089/jir.2005.25.632. [DOI] [PubMed] [Google Scholar]
- Van der Hoek K.H., Eyre N.S., Shue B., Khantisitthiporn O., Glab-Ampi K., Carr J.M., Gartner M.J., Jolly L.A., Thomas P.Q., Adikusuma F., Jankovic-Karasoulos T., Roberts C.T., Helbig K.J., Beard M.R. Viperin is an important host restriction factor in control of Zika virus infection. Sci. Rep. 2017;7:4475. doi: 10.1038/s41598-017-04138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hinson E.R., Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe. 2007;2(2):96–105. doi: 10.1016/j.chom.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Wang S., Wu X., Pan T., Song W., Wang Y., Zhang F., Yuan Z. Viperin inhibits hepatitis C virus replication by interfering with binding of NS5A to host protein hVAP-33. J. Gen. Virol. 2012;93:83–92. doi: 10.1099/vir.0.033860-0. [DOI] [PubMed] [Google Scholar]
- Wu Q., Brum M.C., Caron L., Koster M., Grubman M.J. Adenovirus-mediated type I interferon expression delays and reduces disease signs in cattle challenged with foot-and-mouth disease virus. J. Interferon Cytokine Res. 2003;23(7):359–368. doi: 10.1089/107999003322226014. [DOI] [PubMed] [Google Scholar]
- Yuan B., Fang H., Shen C., Zheng C. Expression of porcine Mx1 with FMDV IRES enhances the antiviral activity against foot-and-mouth disease virus in PK-15 cells. Arch. Virol. 2015;160(8):1989–1999. doi: 10.1007/s00705-015-2473-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang Y., Zhai N., Song H., Li H., Yang Y., Li T., Guo X., Chi B., Niu J., Crispe I.N., Su L., Tu Z. HCV core protein inhibits polarization and activity of both M1 and M2 macrophages through the TLR2 signaling pathway. Sci. Rep. 2016;6:36160. doi: 10.1038/srep36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X., Lv J., Wei Y., Du P., Chang Y., Zhang Y., Gao Y., Wu R., Guo H. Foot-and-mouth disease virus infection stimulates innate immune signaling in the mouse macrophage RAW 264.7 cells. Can. J. Microbiol. 2018;64(2):155–166. doi: 10.1139/cjm-2017-0348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.