Abstract
Background
The spread of pathogens via the airborne route is often underestimated, and little is known about the extent to which airborne microbial contamination levels vary throughout the day and night in hospital facilities.
Aims
To evaluate airborne contamination levels within intensive care unit (ICU) isolation rooms over 10–24-h periods in order to improve understanding of the variability of environmental aerial bioburden, and the extent to which ward activities may contribute.
Methods
Environmental air monitoring was conducted within occupied and vacant inpatient isolation rooms. A sieve impactor sampler was used to collect 500-L air samples every 15 min over 10-h (08:00–18:00 h) and 24-h (08:00–08:00 h) periods. Samples were collected, room activity was logged, and bacterial contamination levels were recorded as colony-forming units (cfu)/m3 air.
Findings
A high degree of variability in levels of airborne contamination was observed across all scenarios in the studied isolation rooms. Air bioburden increased as room occupancy increased, with air contamination levels highest in rooms occupied for the longest time during the study (10 days) (mean 104.4 cfu/m3, range 12–510 cfu/m3). Counts were lowest in unoccupied rooms (mean 20 cfu/m3) and during the night.
Conclusion
Peaks in airborne contamination were directly associated with an increase in activity levels. This study provides the first clear evidence of the extent of variability in microbial airborne levels over 24-h periods in ICU isolation rooms, and found direct correlation between microbial load and ward activity.
Keywords: Airborne, Contamination, Bacteria, Air sampling, Bioburden, Environment
Introduction
It is estimated that 10–33% of hospital-acquired infections (HAIs) are transmitted via the air [1]; however, the role of air as a vector in the spread of infection is less understood. Controversy surrounding particle size, transmission characteristics and associated infection risk has led to a lack of airborne infection control strategies in healthcare premises [2].
Airborne transmission is a route for many serious infectious organisms, such as norovirus, influenza, severe acute respiratory syndrome, meticillin-resistant Staphylococcus aureus (MRSA) and the highly contagious Mycobacterium tuberculosis, and multi-drug-resistant Acinetobacter spp. and Clostridium difficile have also been identified in hospital air [3]. Air quality standards exist for operating theatres [<180 colony-forming units (cfu)/m3 during an operation, <10 cfu/m3 during theatre commissioning and in ultraclean theatres] [4]; however, there are currently no accepted standards for other hospital areas, including intensive care units (ICUs) which house, arguably, the most vulnerable patients.
Micro-organisms originating from the human respiratory tract can become airborne by coughing, sneezing or exhaling, and remain suspended in the air for prolonged periods of time, sometimes indefinitely [5], [6], [7]. These infectious respiratory droplets can evaporate to droplet nuclei which have the ability to travel long distances on air currents, and can be easily dispersed throughout hospital buildings. As such, numerous studies have reiterated that environmental contamination should not be underestimated with regards to infection transmission directly from airborne dust, respiratory droplets or droplet nuclei, or indirectly once settled on to surfaces [8], [9], [10], [11].
The aim of this study was to assess, for the first time, continuous (10–24 h) monitoring of the levels of airborne micro-organisms in an ICU, and correlate changes in airborne contamination levels with room activity to improve understanding of the airborne microbial load in a hospital setting.
Methods
Setting
This study was conducted in isolation rooms of an ICU between May and December 2017. The ICU has three inpatient isolation rooms and a seven-bed open bay. Isolation rooms chosen for sampling tended to house serious burn trauma cases, critical postoperative care patients or potentially infectious patients. Air entering the ICU passes through high-efficiency particulate air filters. Both occupied and unoccupied isolation rooms, with an area of approximately 25–30 m2 (5 6 m), were sampled as part of the study. Rooms were maintained at positive pressure, with a temperature of approximately 20°C, and had no windows that could be opened. Rooms were cleaned daily; domestic staff cleaned the floor, sink, surfaces, bins and ledges, and nursing staff damp-dusted all frequently touched surfaces and equipment. Cleaning was monitored fortnightly by facilities staff, adhering to NHS Scotland National Cleaning Services Specifications. Glasgow Royal Infirmary infection control policies were adhered to throughout the study [12].
Sample collection methods
Monitoring of airborne contamination was conducted using a Surface Air System Super-180 sieve impactor active air sampler (Cherwell Laboratories, Bicester, UK). The air sampler was situated in the corner of the isolation room, approximately 1–1.5m above the ground, and sampled the air by actively drawing a pre-set volume of air through the sampler. Five-hundred-litre air samples were collected every 15 min over 10-h (08:00–18:00 h) and 24-h (08:00–08:00 h) periods on to non-selective tryptone soya agar (TSA) plates (Oxoid Ltd, Basingstoke, UK), favourable for environmental sampling. An activity log was compiled to record room activity that may correlate with peaks in air contamination. After sampling, TSA plates were incubated at 37°C for 48 h and enumerated. The total number of microbial cfu on each plate was corrected for the statistical probability of multiple particles passing through the same hole by referring to correction tables supplied with the equipment [13]. The probable count was then used to calculate the cfu/m3 sampled using the equation:
where V is volume of air sampled, Pr is probable count, and X is cfu per 1 m3 of air.
Statistical analysis
Data were analysed using statistical control charts (Minitab v17) to determine data points classed as ‘out of control’ from the overall dataset of each case study based on rationale by previous work [14]. ‘Out-of-control’ observations (flagged in red) are data points >3 standard deviations above the mean, and are significantly greater than the mean of the dataset. Analysis of data between case studies was conducted using one-way analysis of variance at the 95% confidence level (Minitab v17).
Results
Airborne bioburden monitoring over 10 h in patient-occupied isolation rooms
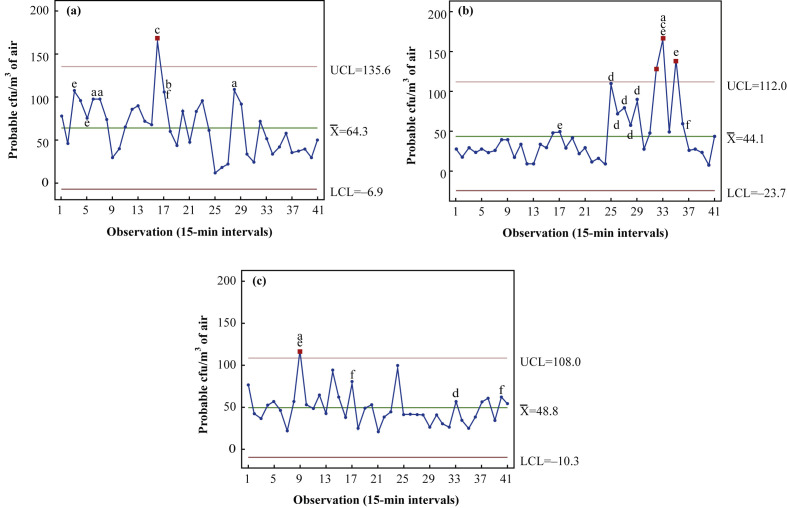
Ten-hour monitoring of patient-occupied isolation rooms took place on three separate sampling days from 08:00 to 18:00 h. The first case study (Figure 1 a) involved a 71-year-old male patient, who had undergone partial pancreatectomy for cancer and multi-organ failure, who occupied the room for 8 days prior to commencement of air monitoring. Results (Table I ) demonstrate a high degree of variability over the 10-h period, with a mean airborne bacterial load of 64.3 [standard deviation (SD) 31.8 cfu/m3], a minimum of 12 cfu/m3 and a maximum of 166 cfu/m3. This maximum (Observation 16 at 11:45 h) was statistically classified as ‘out of control’, and coincided with collection after fresh bed sheets were shaken in preparation of a bed change.
Figure 1.
Statistical control charts (Minitab v17) demonstrating levels of airborne bacteria over a 10-h period (08:00–18:00 h) in patient-occupied isolation rooms within an intensive care unit. Rooms were occupied by patients for differing periods prior to the commencement of air sampling: (a) 8 days, (b) 7 days and (c) 3 days. Each data point represents the probable colony-forming units (cfu)/m3 from air samples taken at 15-min intervals and incubated for 48 h. ‘Out-of-control’ data points are highlighted in red. ‘High-risk’ activities leading to increased airborne bioburden above the mean are identified as follows: a, presence of more than three staff; b, patient personal hygiene/turn; c, bed/sheet changes; d, visiting; e, movement of large equipment into/around room; f, cleaning. UCL, upper control limit; , mean; LCL, lower control limit. N=41.
Table I.
Summary of data generated from different case studies within an intensive care unit monitoring microbial air contamination levels over 10- and 24-h sampling periods
| Case study | Length of room occupancy (days) | Total mean [cfu/m3 (SD)] | Total range (cfu/m3) | P-value (95% CI) | Mean day (08:00–20:00 h) [cfu/m3 (SD)] | Mean night (20:00–08:00 h) [cfu/m3 (SD)] | P-value for day vs night (95% CI) | Activities which contributed to increased bioburden and consequent failing of control chart statistical tests (Observation No.) |
|---|---|---|---|---|---|---|---|---|
| Inpatient isolation room, 10-h studies | ||||||||
| Figure 1a | 8 | 64.3 (31.8) | 12–166 | 0.008 | - | - | Fresh bed sheets shaken (16) | |
| Figure 1b | 7 | 44.2 (36.1) | 8–166 | - | - | Increased staff presence from zero to two staff (9) | ||
| Figure 1c | 3 | 48.8 (20.5) | 20–116 | - | - | Patient helped out of bed (32,33) Bed removed from room (35) |
||
| Inpatient isolation room, 24-h studies | ||||||||
| Figure 2a | 10 | 104.4 (96.2) | 12–510 | <0.001 | 151.2 (111.9) | 56.6 (39.1) | <0.001 | Patient turn, patient physiotherapy, operation of mechanical hoist, high staff presence (14–18) Increased people traffic from one (visitor) to two (visitor + nurse) (33) |
| Figure 2b | 6 | 102.4 (68.8) | 5–355 | 113.6 (79.4) | 90.9 (54.3) | 0.080 | Increased people traffic from zero to two (visitor + nurse) (27–29) | |
| Figure 2c | 1 | 62.1 (82.4) | 0–398 | 86.9 (95.8) | 36.7 (56.3) | 0.002 | Increased staff presence from two to five staff (4) Ventilator change (9) Patient in bed taken for computed tomography scan followed by return (33,34) Patient turn (36) |
|
| Figure 2d | 0 | 20.0 (14.2) | 2–90 | 26.8 (16.3) | 13.0 (6.6) | <0.001 | Room cleaning (17) Brief open and close of door (31) Handover of sampler (49) |
|
cfu, colony-forming units; CI, confidence interval; SD, standard deviation.
Data were recorded in occupied and empty patient isolation rooms. For each study, details are also provided for the ward activities which were associated with the significant increases in airborne bioburden [the ‘out-of-control’ observations, as highlighted by the statistical process control charts (Figure 1, Figure 2)]. Mean and standard deviation were recorded for each 10-h case study (N=46), whilst 24-h studies were further analysed via day (08:00–20:00 h) and night (20:00–08:00 h) portions of the sample collection period (N=97).
The results of a second case study (Figure 1b) were generated in a room which housed a 37-year-old male patient with severe community-acquired pneumonia who had occupied the room for 7 days prior. A mean value of 44.1 (SD 36.1) cfu/m3 was recorded. The patient was mobile, talking and subsequently transferred from ICU after completion of the study. Airborne contamination levels remained low and consistent for most of the study (between 10 and 50 cfu/m3) from 08:00 to 14:00 h, during which time room activity was minimal. The number of people entering the room was low (0–2) as the patient did not require 1:1 care for most of the period. Bioburden levels increased from 10 to 110 cfu/m3 in response to the presence of a visitor at 14:00 h (Observation 25), and remained elevated until their departure (Observation 29 at 15:00 h). Significantly higher (‘out-of-control’) levels were observed when the patient was assisted out of bed, followed by the removal of the bed from the room. This group of activities occurred between 15:45 and 16:30 h (Observations 32–35) and resulted in an increase in air bioburden to 166 cfu/m3.
A third study (Figure 1c) was conducted in a room occupied by a 75-year-old female patient, admitted to ICU with pneumonia and multi-organ failure, for 3 days. A mean airborne bacteria load of 48.8 (SD 20.5) cfu/m3 was recorded, with a range of 20–116 cfu/m3. ‘Out-of-control’ levels occurred due to a high level of room activity during patient re-intubation, involving an increase from two to four staff within the room and a higher degree of physical movement around the patient's bed (Observation 9 at 10:00 h).
Overall, airborne bioburden data (Figure 1) demonstrate that there is significant variability (P=0.008) in airborne bacterial counts across the 10-h sampling period in all three independent case studies conducted in patient-occupied isolation room studies, regardless of patient scenario (Table I).
Airborne bioburden monitoring over 24 h in patient-occupied isolation rooms
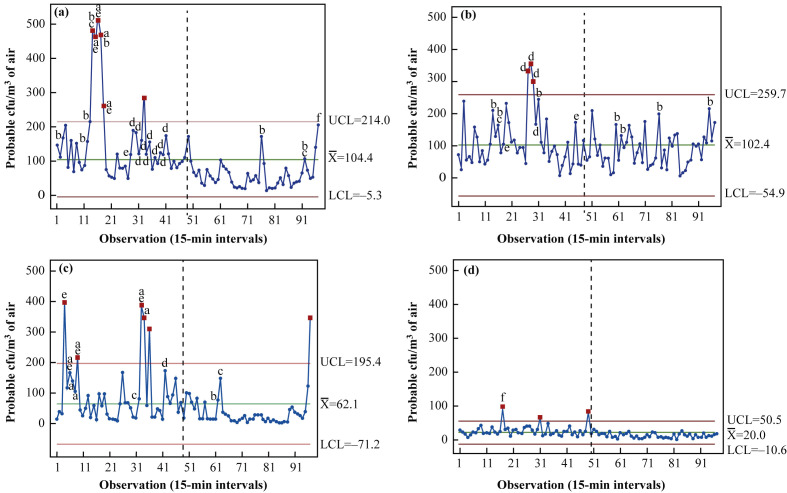
The first 24-h case study was conducted in a room occupied for 10 days by a 70-year-old female with respiratory failure on a background of gastroenteritis and Clostridium difficile infection (Figure 2 a). Over the 24-h period, the mean air bioburden was 104.4 (SD 96.2) cfu/m3, with minimum and maximum recorded values of 12 and 510 cfu/m3, respectively. When the dataset was divided into ‘day’ and ‘night’ (08:00–20:00 h and 20:00–08:00 h, respectively), the mean airborne day count was 151.2 cfu/m3 and the mean night count was 56.6 cfu/m3 (P<0.001). The ‘out-of-control’ levels collected at 11:15–11:45 h (Observations 14–16) were a direct result of a high degree of room activity in which increased staff presence (from one to five) aided the movement of the patient from a bed via a mechanical hoist. Additionally, footfall in and out of the room was substantially higher during these samples, leading to a peak count of 510 cfu/m3, the highest level of air bioburden recorded across the entire set of case studies.
Figure 2.
Statistical control charts (Minitab v17) demonstrating levels of airborne bacteria over a 24-h period (08:00–08:00 h) in occupied and unoccupied inpatient isolation rooms of an intensive care unit. In patient-occupied rooms, rooms were occupied by patients for differing periods prior to the commencement of air sampling: (a) 10 days, (b) 6 days and (c) 1 day. Monitoring of an empty patient room was also included for comparison (d). For analysis, periods of day and night were categorized as 08:00–20:00 h and 20:00–08:00 h, respectively. Each data point represents the probable colony-forming units (cfu)/m3 from air samples taken at 15-min intervals and incubated for 48 h. ‘Out-of-control’ data points are highlighted in red. High-risk activities leading to increased airborne bioburden above the mean are identified as follows: a, presence of more than three staff; b, patient personal hygiene/turn; c, bed/sheet changes; d, visiting; e, movement of large equipment into/around room; f, cleaning. UCL, upper control limit; , mean; LCL, lower control limit. N=97.
Figure 2b displays the air monitoring results in a room occupied for 6 days by a male patient with Guillian-Barre demyelinating disease and widespread muscle weakness. Air contamination levels varied substantially across 24 h, with ‘out-of-control’ levels occurring during visiting hours. The mean air bioburden across the 24-period was 102.4 (SD 68.8) cfu/m3, with a minimum value of 5 cfu/m3 recorded at 04:45 h and a maximum value of 355 cfu/m3 recorded at 14:45 h. The mean values for day and night were 113.6 and 91.0 cfu/m3, respectively (P=0.080) (Table I).
The final case study (Figure 2c) was conducted in an isolation room occupied for 1 day by a 56-year-old immunocompromised female patient with respiratory failure and a background of rheumatoid arthritis. The overall mean value across the 24-h case study was 62.1 (SD 82.4) cfu/m3, with a range of 0–398 cfu/m3. An initial surge in airborne bacteria to the maximum value of 398 cfu/m3 occurred in response to an increase in staff presence in order to assist patient intubation. A significantly high air bioburden of 214 cfu/m3 was also observed when a ventilator was changed (Observation 9 at 10:00 h). Contamination levels peaked again at Observations 33–36 (16:00–17:00 h), when the patient was wheeled out of the room for a computed tomography scan, resulting in air counts of 300–400 cfu/m3. The mean day time value was 86.9 cfu/m3, followed by relatively low and consistent values during the night (36.7 cfu/m3) (P=0.002). Counts increased from Observation 94 to Observation 97 (07:15–08:00 h) during morning handover.
As a baseline control for comparison, monitoring was also conducted in an empty isolation room (Figure 2d). Airborne bacteria levels were low and consistent across the 24-h period; however, average values between day and night still varied from 26.8 cfu/m3 (08:00–20:00 h) to 13.0 cfu/m3 (20:00–08:00 h) (P<0.001). An overall mean value of 20.0 (SD 14.2) cfu/m3 was recorded. Significant (‘out-of-control’) levels occurred within this dataset during cleaning of the empty room.
Correlation of high air bioburden levels and room activity
Table II details specific room activities which were consistently linked to high levels of air contamination across all studies, based on the collated activity logs. Increases in air bioburden as a result of each activity were calculated as a percentage increase from the sample mean of the corresponding study to allow a fair comparison. The two highest risk activities for increasing bioburden were: (i) the movement/operation of large pieces of equipment; and (ii) an increased number of staff in the room. The movement or operation of large equipment into and around patient rooms (e.g. x-ray scanners, mechanical hoists, trolleys) resulted in an increase in air bioburden of 197.6%, with a range of 3.1–540.9% (N=16). An increase in staff numbers within patient rooms caused similar peaks in contamination levels. When more than three staff were present in the room, air counts increased by an average of 197.1% (N=15) from the sample mean. Percentage increase values ranged from 18.2% to 518.4%. When this scope was widened to include staff numbers greater than two, the average increase in airborne bacteria was 154.7% (N=43), with a range of 1.5–540.9%. The highest recorded number of staff in a patient isolation room at a given time across all case studies was nine. Other high-risk activities included bed changes (+145.3%), patient personal hygiene/turn (+103.9%), visiting hours (+83.8%) and cleaning (+56.6%).
Table II.
Overview of the high-risk ward activities which contributed to increases in airborne microbial bioburden
| Activity | Average % increase from sample mean | Range (%) |
|---|---|---|
| Presence of more than three staff | 197.1 | 18.2–518.4 (N=15) |
| Personal patient hygiene/turn | 103.9 | 1.5–359.8 (N=16) |
| Bed/sheet changes | 145.3 | 1.5–276.4 (N=7) |
| Visiting | 83.8 | 5.4–247.3 (N=23) |
| Movement of large equipment into/around room | 197.6 | 3.1–540.9 (N=16) |
| Cleaning | 56.6 | 27.1–95.4 (N=5) |
Activities which consistently correlated with high air counts were selected, and percentage increases in colony-forming units/m3 were calculated from the sample mean of the corresponding case study. The overall average percentage increase is given, alongside the sample size (N).
Discussion
Understanding the route and transmission of infectious micro-organisms plays a key role in infection prevention. Recently, the role of the environment as a source of infection within clinical establishments has been documented increasingly [15]. However, to date, few studies have characterized levels of airborne micro-organisms within an ICU over extended time periods. Previous clinical air studies have focused on short time periods or specific activities of interest [14], [16], [17]. This study has significantly expanded this information by successfully demonstrating the levels and fluctuations of airborne bacteria within an ICU during different patient and environmental scenarios over 10- and 24-h periods.
Airborne microbial counts were shown to vary considerably across the 10- or 24-h sampling periods during all case studies (Table I), and this variation was expected given the extremely dynamic nature of an ICU. Results also enabled peaks in airborne bacterial load to be correlated with specific activities, and particular activities to be statistically classified as ‘out of control’, but it is important to bear in mind that these ‘out-of-control’ peaks are relative only to the dataset as a whole in which they were recorded.
Mean bioburden levels recorded in this study are lower than those from other ICU studies which have reported levels between 350 and 450 cfu/m3 [18], [19], and higher than those from a more recent study (<40 cfu/m3) [20]. The differences are likely due to confounding factors including differences in air change ventilation rates, number of medical staff and patients, patient conditions and, importantly, the sample number and collection times. The degree of variation evidenced in the different case studies in the present work demonstrates that mean levels will be significantly different if different sampling periods and/or lower sample numbers are used.
Extensive variation in air counts was observed in 10-h patient-occupied isolation room studies, and mean values reflected the length of room occupation, with one exception (Figure 2b). In this study, the patient occupied the room for 7 days, but the mean airborne bacterial load was only 44.1 cfu/m3. This correlated well with room activity as, in this case, the patient was conscious and required little 1:1 care.
Results from 24-h monitoring also indicated that the longer the patient occupied the room, the greater the mean air bioburden and additionally, the mean day airborne counts were significantly different from the equivalent night levels (P<0.001). This observation reflected the reduced activity in the unit overnight. However, it was interesting to observe that a patient turn activity, which resulted in a significant peak in air bioburden during the day (Figure 2c, Observations 34 and 75), had minimal effect when carried out at night. This potentially indicates that the activity of the ICU as a whole contributes to airborne contamination even within individual isolation rooms, highlighting how easily airborne micro-organisms are dispersed through the ICU in general. Studies in burns units have demonstrated the ease with which bacteria are liberated from the patient into the air [21]. One study showed that 31% of dressing changes on MRSA-positive burns patients liberated the organism into the air [22]. A similar finding was observed in the present study, whereby an average increase in air bioburden of 103.9% (N=16) was recorded during patient personal hygiene/turn activities involving bed bathing and physical movement of the patient.
A number of patient-care-related activities contributed to peaks in air contamination levels, most of which are centred on an increase in people traffic. It is estimated that each individual disperses approximately 104 particles while walking, many of which are viable and some pathogenic, meaning the more people present in a room, the greater the chance of dispersing biological particles which may have the potential to cause harm [23], [24]. This is relevant to the present study where an average percentage increase in air bioburden of 197.1% was generated as a result of more than three staff members present in the isolation room. Bed sheet changes have also been implicated in the increase in aerial dispersal of bacteria. In the present study, this caused an average increase in air bioburden of 145.3% (N=7). Previous studies have recorded similar results whereby mean counts of airborne MRSA from infected patients increased from 4.7 cfu/m3 to 116 cfu/m3 during bed sheet changes, and remained elevated for some time after the event [25]. Similarly, air counts of up to 2614 cfu/m3 were recorded in response to bed changes in a burns unit, with elevated levels persisting for up to 60 min [14].
A previous study monitored variations in airborne bioaerosols in a hospital ward in response to general ward activities; however, the longest period of air sampling was 8 h, with no account of overnight activity and air data [16]. Results agree with the present study in terms of bioaerosol-generating activities and increased dispersal during early mornings when ward activity was high. A strong correlation between increased viable counts and increased Staphylococcus spp. was also observed, indicating the likelihood of increased dispersal of S. aureus when peaks in air contamination occurred. Most high-risk activities identified have been linked to high airborne bacterial levels previously, with one exception. The movement of large medical equipment into/within patient rooms caused the highest overall average increase in air bioburden at 197.6% (N=16). This could be due to movement of large air volumes already containing viable organisms, or may implicate equipment as significant environmental reservoirs of micro-organisms within the ICU.
Surfaces have been well implicated in the cross-infection of patients by acting as reservoirs for the transmission of micro-organisms, but uncertainty remains regarding the degree of contribution of the airborne route to the overall spread of infection. However, pneumonia and respiratory tract infections were the second largest group of HAIs, and accounted for 22.4% of the total HAIs in Scotland in 2016 [26]. All airborne microbes ultimately end up depositing on to surrounding surfaces, and so can contribute indirectly to infection transmission via direct surface contact. A recent study aimed to establish a correlation between air and surface microbes in the critical care environment, further emphasizing this phenomenon [20]. Their research found a strong association between passive air sampling counts and surface counts, and made the important point that surface bacteria will include a portion of airborne bacteria after settling. Settle plate standards were also proposed in 2000, as the ‘index of microbial air contamination’, a passive form of air sampling in which microbial contamination from the air is evaluated after it has settled on to the surface of agar plates [27]. Using settle plates as part of routine environmental screening for HAI risk from airborne contaminants could be a positive addition to infection control strategies; however, as shown in the present study through active air sampling, biologically active particles are present at all times in the air of the ICU, even in unoccupied rooms. Therefore, if using passive sampling methods, care should be taken to ensure that counts are not underestimated due to the potential for droplet nuclei to remain suspended for prolonged periods [6].
A limitation of this study was that it was not possible to identify the collected micro-organisms. It is important to note that although certain activities resulted in high levels of air bioburden, this does not necessarily correlate with a high level of pathogenic organisms. Recently, it was shown that environmental bioburden measured by total colony count did not predict the presence of clinically relevant pathogenic organisms [28]. Additionally, viral collection was not possible with this methodology. Future consideration should be given to identification and correlation of airborne micro-organisms with strains originating from the patients housed in the environment; however, the scope of the present study was to assess overall variability of airborne bacteria and changes in response to key activities.
In conclusion, this study successfully recorded, for the first time, environmental air contamination levels in an ICU across 24-h time periods. Bioaerosol counts varied significantly across sampling periods; however, peaks were a direct result of room activity, particularly during the presence of increased numbers of medical staff and/or use of large equipment. Various other factors contributed to increased levels of air contamination, predominantly length of room occupation and people traffic. Although these results are specific to this ICU setting, this study provides insight into the typical background levels of airborne micro-organisms in the critical care setting, and how they change in response to the everyday operation of this dynamic environment. A greater understanding of the airborne transmission route and the clinical airborne microflora is required to understand the role of airborne pathogens in the spread of HAIs more fully, with the aim of establishing more direct and continuous infection control strategies.
Acknowledgements
The authors wish to thank the staff and patients at Glasgow Royal Infirmary ICU for their support and patience during this study. The authors also wish to thank The Robertson Trust for their support. Due to the nature of the study, data underpinning this publication cannot be made openly available, but are stored securely on the University of Strathclyde KnowledgeBase at: https://doi.org/10.15129/ee8f296b-2eeb-41f5-ae55-f527fd5ca1bf.
Conflict of interest statement
None declared.
Funding sources
LRD was supported by an Engineering and Physical Sciences Research Council Doctoral Training Grant (Reference EP/M508159/1).
References
- 1.House of Commons Public Accounts Committee . Authority of the House of Commons; London: 2009. Reducing healthcare-associated infections in hospitals in England. Fifty-second report of Session 2008–9.https://publications.parliament.uk/pa/cm200809/cmselect/cmpubacc/812/812.pdf Available at: [last accessed July 2017] [Google Scholar]
- 2.Hobday R., Dancer S. Roles of sunlight and natural ventilation for controlling infection: historical and current perspectives. J Hosp Infect. 2013;84:271–282. doi: 10.1016/j.jhin.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirhoseini S.H., Nikaeen M., Shamsizadeh Z., Khanahmad H. Hospital air: a potential route for transmission of infections caused by β-lactam-resistant bacteria. Am J Infect Control. 2016;44:898–904. doi: 10.1016/j.ajic.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health . Specialised ventilation for healthcare premises. Part A: design and validation. The Stationery Office; London: 2007. Health technical memorandum HTM 03e01. [Google Scholar]
- 5.Fitzgerald D., Haas D.W. Mycobacterium tuberculosis. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. 6th edition. Churchill Livingstone; Philadelphia: 2005. pp. 2852–2886. [Google Scholar]
- 6.Beggs C.B. The airborne transmission of infection in hospital buildings: fact or fiction? Indoor Built Environ. 2003;12:9–18. [Google Scholar]
- 7.Morowaska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 8.Dancer S.J. Importance of the environment in MRSA acquisition: the case for hospital cleaning. Lancet Infect Dis. 2008;8:101–113. doi: 10.1016/S1473-3099(07)70241-4. [DOI] [PubMed] [Google Scholar]
- 9.King M.F., Noakes C.J., Sleigh P.A., Camargo-Valero M.A. Bioaerosol deposition in single and two-bed hospital rooms: a numerical and experimental study. Build Environ. 2013;59:436–447. [Google Scholar]
- 10.Otter JA, Yezli S, Salkeld JAG, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control;41:S6–11. [DOI] [PubMed]
- 11.Bogusz A., Stewart M., Hunter J., Yip B., Reid D., Robertson C. How quickly do hospital surfaces become contaminated after detergent cleaning? Healthcare Infect. 2013;18:3–9. [Google Scholar]
- 12.NHS Greater Glasgow and Clyde . NHS Greater Glasgow and Clyde; Glasgow: 2012. National infection prevention and control manual.https://www.nhsggc.org.uk/your-health/infection-prevention-and-control/# Available at: [last accessed April 2019] [Google Scholar]
- 13.International PBI S.p.A. International PBI S.p.a; Milan: 2005. SAS super 100/180”, “DUO SAS super 360”, “SAS isolator” - code n. 18198/19121, 24584, 43216/43217 instruction manual. Revision 5; pp. 1–53. [Google Scholar]
- 14.Bache S.E., Maclean M., Gettinby G., Anderson J.G., MacGregor S.J., Taggart I. Airborne bacterial dispersal during and after dressing and bed changes on burns patients. Burns. 2015;41:39–48. doi: 10.1016/j.burns.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Dancer S.J. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hathway E.A., Noakes C.J., Fletcher L.A., Sleigh P.A., Clifton I., Elliot M.W. The role of nursing activities on the bioaerosol production in hospital wards. Indoor Built Environ. 2013;22:410–421. [Google Scholar]
- 17.Andersen B.M., Rasch M., Kvist J., Tollefsen T., Lukkassen R., Sandvik L. Floor cleaning: effect on bacteria and organic materials in hospital rooms. J Hosp Infect. 2009;71:57–65. doi: 10.1016/j.jhin.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Huang P.Y., Shi Z.Y., Chen C.H., Den W., Huang H.M., Tsai J.J. Airborne and surface-bound microbial contamination in two intensive care units of a medical centre in central Taiwan. Aerosol Air Qual Res. 2013;13:1060–1069. [Google Scholar]
- 19.Bauer T.M., Ofner E., Just H.M., Just H., Daschner F.D. An epidemiological study assessing the relative importance of airborne and direct contact transmission of microorganisms in a medical intensive care unit. J Hosp Infect. 2009;15:301–309. doi: 10.1016/0195-6701(90)90087-5. [DOI] [PubMed] [Google Scholar]
- 20.Smith J., Adams C.E., King M.F., Noakes C.J., Robertson C., Dancer S.J. Is there an association between airborne and surface microbes in the critical care environment? J Hosp Infect. 2018;100:e123–e129. doi: 10.1016/j.jhin.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Bache S.E., Maclean M., Gettinby G., Anderson J.G., MacGregor S.J., Taggart I. Quantifying bacterial transfer from patients to staff during burns dressing and bed changes: implications for infection control. Burns. 2013;39:220–228. doi: 10.1016/j.burns.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Dansby W., Purdue G., Hunt J., Arnoldo B., Phillips D., Moody B. Aerolization of methicillin-resistant Staphylococcus aureus during an epidemic in a burn intensive care unit. J Burn Care Res. 2008;29:331–337. doi: 10.1097/BCR.0b013e3181667583. [DOI] [PubMed] [Google Scholar]
- 23.Noble W.C. Dispersal of skin microorganisms. Br J Dermatol. 1975;93:477–485. doi: 10.1111/j.1365-2133.1975.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 24.Sadrizadeh S., Tammelin A., Ekolind P., Holmberg S. Influence of staff number and internal constellation on surgical site infection in an operating room. Particuology. 2014;13:42–51. [Google Scholar]
- 25.Shiomori T., Miyamoto H., Makishima K., Yoshida M., Fujiyoshi T., Udaka T. Evaluation of bedmaking-related airborne and surface methicillin-resistant Staphylococcus aureus contamination. J Hosp Infect. 2002;50:30–35. doi: 10.1053/jhin.2001.1136. [DOI] [PubMed] [Google Scholar]
- 26.Health Protection Scotland . HPS; Glasgow: 2017. Scottish national point prevalence survey of healthcare associated infection and antimicrobial prescribing.https://www.hps.scot.nhs.uk/pubs/detail.aspx?id=3236/ Available at: [last accessed May 2018] [Google Scholar]
- 27.Pasquarella C., Pitzurra O., Svino A. The index of microbial air contamination. J Hosp Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 28.Widmer F., Frei R., Romanyuk A., Tschudin Sutter S., Widmer A. Overall bioburden by total colony count does not predict the presence of pathogens with high clinical relevance in hospital and community environments. J Hosp Infect. 2019;101:240–244. doi: 10.1016/j.jhin.2018.11.014. [DOI] [PubMed] [Google Scholar]