Summary
Background
Healthcare workers (HCWs) may be the inadvertent interface between the healthcare setting and the community for infectious diseases transmission.
Aim
To investigate HCWs' contacts during a work day and compare these against working adults from the general population.
Methods
Prospective survey of contacts through 24 h self-reported diary in three public sector tertiary care hospitals and community-based working adults in Singapore. Participants were HCWs and working adults from the community.
Findings
In all, 211 HCWs and 1028 working adults reported a total of 4066 and 9206 contacts. HCWs reported more work-related contacts than community-based working adults (median of 13 versus 4), and more contacts that were neither household nor work-related (1 versus 0) but fewer household contacts (2 versus 3). HCWs reported more work-related contacts involving physical contacts, and more new contacts particularly with short duration (≤15 min) compared to community-based working adults. Among different HCW types, doctors reported the highest whereas ward-based nurses reported the lowest total work-related contacts. Around half of ward-based and clinic-based nurses' contacts involved physical touch. Work-related contacts reported by clinic-based nurses, doctors, and assorted HCWs were shorter than in ward-based nurses, with a substantial number effectively occurring with new contacts. Institutional effects significant on univariate analyses were much reduced and non-significant after adjusting for confounding by HCW type.
Conclusion
HCWs' contacts differ substantially from those of community-based working adults. HCWs may thus be at higher risk of acquiring and spreading contact-transmissible and respiratory infections due to the nature of their work. Whereas total number of contacts was fairly similar between HCW types, the characteristics of their contacts differed substantively.
Keywords: Healthcare workers, Community-based adults, Infectious diseases, Transmission, Nurses, Doctors
Introduction
Contact patterns can help us understand the dynamics of infectious diseases transmission and guide the design of infection control and prevention measures [1]. Models of transmission dynamics are increasingly applied to inform infectious disease control, but such models require relevant data on contact patterns as inputs [2]. Healthcare workers (HCWs) may be the inadvertent interface between the healthcare setting and the community for such infections. The HCWs' role as a vector for spreading pathogens to patients in the hospital setting is well recognized, and occupational infections among HCWs have been frequently documented, both for common pathogens circulating in the healthcare setting, as well as some newly emerged or re-emerging pathogens [3], [4], [5]. HCWs may thus contribute to disease transmission from the hospital to the community and vice versa [6], [7]. Moreover, over the past two decades, nosocomial outbreaks of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) coronavirus, and Ebola virus disease have emphasized how the healthcare setting may amplify the transmission of an infection that has newly emerged in a community [8], [9], [10]. Hence it is worthwhile to describe and compare the contact patterns which occur in the healthcare setting alongside those in the community among working adults in the general population.
A 24 h paper diary was previously used to compare nurses' daily contacts with those of matched controls from the general population in Germany [11]. Nurses' differed substantially from the general population in the number of total contacts, contacts where physical touch occurred, and in the duration of contacts. However, we found no other studies comparing the contacts of other types of HCWs with the general population. Contacts for other HCWs have been studied in specific units (e.g. general wards, emergency departments) within the healthcare setting, often using proximity-sensing technologies which focus on paired HCW–patient and HCW–HCW contacts [12], [13]. These studies found that nurses had more patient contacts than other HCWs. However, it remains unclear how contacts for other HCW types compare across different settings, and with individuals of similar age in the community, which may have implications for the transmission of pathogens into and out of the healthcare setting to the community. Moreover, modelling studies of disease transmission rely heavily on assumptions about contacts between key risk groups. In the absence of better data, some attempts to model transmission in healthcare settings have had to make simplifying assumptions about the nature of work-related contacts in HCWs relative to the general population, and neglect potential differences in risk that may arise from the variation in contacts among HCWs [14], [15].
We therefore conducted a multi-institutional study on HCW contacts in parallel with a large group of working adults from the general population. Using the 24 h contact diary, we describe the contacts of HCWs working in various settings, and show how the number, the locations and the characteristics of contacts differed, both when comparing with the general population, and between HCWs from different disciplines and settings. The characteristics include the periodicity, the duration of contacts, and the involvement of physical touch.
Methods
A prospective contact pattern survey of HCWs was conducted from three public sector tertiary care hospitals in Singapore: Tan Tock Seng Hospital (TTSH), National University Hospital (NUH), and Khoo Teck Puat Hospital (KTPH). This was then compared with data similarly collected from working adults recruited from the community (henceforth referred to as community-based working adults). In both surveys, a significant contact was defined as an interaction between two persons, either physical (involving skin-to-skin contact such as handshake, hug, kiss or contact sports) or non-physical (involving a two-way conversation with three or more words in the physical presence of another person, but no skin-to-skin contact). The surveys of HCWs and community-based working adults were approved by the ethics review boards of the National Healthcare Group and National University of Singapore respectively.
Study populations
Sample size calculation
The sample size needed to observe a difference of at least five in the mean number of contacts between HCWs and the community-based working adults would be 142 for each group, assuming a type I error of 5% and a type II error of 20%. This is with a standard deviation of 15 for the total numbers of daily contacts for each population, but assuming that the total number of daily contacts approximates a normal distribution. To compensate for the right-skewed distribution of contacts that we anticipated, we aimed to recruit at least 200 HCWs.
Healthcare workers
Recruitment of up to 100 HCWs was targeted from each of the three hospitals. To optimize manpower deployment, the study was rolled out sequentially from TTSH to NUH to KTPH from 2013 to 2016. A convenience recruitment strategy was used when enrolling the participants. In TTSH, nurses were recruited during their standard team meeting whereas non-nursing HCWs were recruited individually when they were working in the wards by study team members. HCWs in NUH were recruited through recruitment posters distributed via the hospital's intranet. Participants from KTPH were recruited during their standard ward-based team meeting. The study was explained to consenting HCWs, who were then asked to complete the diary with contacts that occurred during a 24 h period starting at 05:00 for the survey date. The study team would collect the completed contact diaries once informed by the participant. To avoid biases related to day-of-week effects on the contacts of HCW participants, the study team pre-assigned the day-of-week (using a random number generator), and instructed the participants to complete the survey for that pre-assigned day-of-week within two weeks of enrolment.
Community-based working adults
The community-based survey was originally designed to answer questions on interactions between different age groups and hence targeted a much larger age- and sex-stratified sample size of 3000 individuals. Participants were recruited from two previous cohort studies run by Saw Swee Hock School of Public Health, National University of Singapore [16], [17]. Field work for community-based recruitment occurred from June of 2013 to February of 2014. Consenting participants were to fill out two diaries, with one falling on a weekday and the other on a weekend, with participants allowed to choose any weekday and weekend day in the two weeks after enrolment. Participants were asked to complete the diary with contacts that occurred during a 24 h period starting at 05:00 for the survey date. Contact diaries could be returned by mail, e-mail or fax.
To facilitate a fair comparison with our HCWs, data were included only from community-based adults aged 20–64 years (approximately the age range for the HCWs in our study). Moreover, only one diary with at least one work-related contact was included for one community-based participant. For community-based working adults who returned two diaries with at least one-work-related contact, the diary with the higher number of work-related contacts was selected, to test our hypothesis that HCWs indeed had more work-related contacts than community-based working adults, even when compared against the day with a higher number of contacts.
Data collection
The contact diary used for both HCWs and community-based working adults was adapted from the version used in the POLYMOD study [2]. Demographic information including age, sex and the designation (for the HCWs) was collected. The diary was designed as a table in which participants recorded the following characteristics of their contacts for the whole day (24 h): age (or age range if participants were unsure of the exact age) and sex of the contact person; location where the contact occurred (with multiple locations allowed); indication of whether physical contact involved; contact duration (<5, 5–15, 15–60, and ≥60 min), and contact periodicity (whether they encountered this contact daily, weekly, monthly, or less). One contact person only occupied one row of the table. If a participant had repeated contact with an individual during the 24 h of data collection, the characteristics of all contacts with this person were aggregated.
Statistical analysis
The study investigated whether the total number of contacts, the location of contacts, as well as the distribution of three characteristics of contacts (physical versus non-physical contacts, contact duration and contact periodicity) differed by the type of participant. HCWs were compared with community-based working adults, and with HCWs working in various combinations of clinical settings and roles: ‘ward-based nurses’, ‘clinic-based nurses’, ‘doctors’ (who tend to cover both ward and clinic settings within a given workday), and other HCWs who did not fall into any of the prior types (‘assorted HCWs’).
Since the age and sex distribution of our HCWs differed substantially from the community-based sample of working adults, observations from the latter were weighted to give a modelled population similar in age and sex distribution to HCW participants; this was done by deriving an adjustment factor obtained from dividing the proportion of HCWs in each age–sex stratum by the proportion of community-based working adults in the corresponding strata detailed in Table I.
Table I.
Distribution of age and sex of community-based working adults and healthcare worker participants
| Age group (years) | Healthcare workers (N = 211)a |
Community-based working adults (N = 1028) |
Weights for community-based working adults |
|||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| All ages | 29 (13.7) | 182 (86.3) | 497 (48.3) | 531 (51.7) | NA | NA |
| 20–29 | 15 (7.1) | 107 (50.7) | 128 (12.5) | 130 (12.6) | 0.571 | 4.01 |
| 30–39 | 8 (3.8) | 57 (27.0) | 93 (9.0) | 110 (10.7) | 0.419 | 2.525 |
| 40–49 | 2 (0.9) | 14 (6.6) | 128 (12.5) | 133 (12.9) | 0.076 | 0.513 |
| ≥50 | 4 (1.9) | 4 (1.9) | 148 (14.4) | 158 (15.4) | 0.132 | 0.123 |
NA, not applicable.
Values in parentheses are the percentage of the target population in that age and sex category.
The numbers of healthcare workers completing surveys from Tan Tock Seng Hospital, National University Hospital, and Khoo Teck Puat Hospital were 94, 62, and 55 respectively.
As the number of contacts was not normally distributed, non-parametric tests were used when comparing HCWs against community-based working adults. The square root of the number of contacts was used when comparing different types of HCWs and community-based working adults on the number and type of contacts, stratified by contact duration, periodicity, and whether physical touch occurred. In addition, univariate and multivariate linear regression were performed to determine whether other factors such as the institution, age, sex, and type of HCW participant were associated with the number of reported contacts (again using the square-root-transformed number of contacts, which substantially normalized the right-skewed distribution of contacts on visual inspection).
All data were analysed using Stata for Windows, version 11 (Stata Corp., College Station, TX, USA), with P < 0.05 (two-sided) considered statistically significant except for the comparison of the number of work-related contacts between HCWs and the community-based working adults (where one-sided P-values were used).
Results
Survey forms were returned from 249 HCWs; eight surveys were incomplete, and 30 reported no work-related contacts on the survey date submitted, leaving surveys from 211 HCWs for analysis. Of 2928 participants from the community, 2922 contributed surveys on two days, but only 1028 participants aged from 20 to 64 years had one survey with work-related contacts. Table I shows that, whereas both sexes and all age groups were evenly represented among community-based working adults, HCWs were predominantly female (86.3%) and aged between 20 and 39 years (88.6%).
Summary of reported contacts from community-based working adults and HCWs
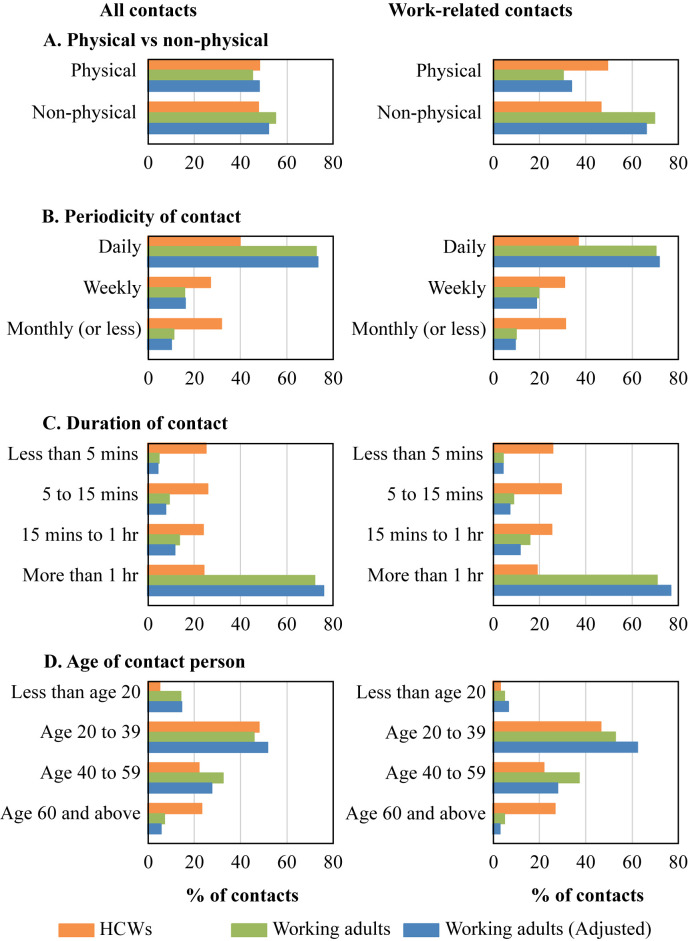
Healthcare workers and community-based working adults reported a total of 4066 and 9206 contacts respectively; 76.3% were work-related for the former compared to only 57.2% for the latter. In both groups, physical touch occurred with about half of all contacts (Figure 1 ). Persons they encountered daily contributed only 39.9% of HCWs' contacts but 73.3% of community-based working adults' contacts. For HCWs, about half the contacts lasted <15 min compared with <15% for community-based working adults; contacts aged <20 years were 5.3% for the former and 14.3% for the latter, whereas contacts aged ≥60 years were 23.3% and 7.3%, respectively.
Figure 1.
Distribution of all contacts and work-related contacts in healthcare worker participants and in community-based working adults from the community.
A higher proportion of HCWs' work-related contacts involved physical touch (49.5% versus 33.9% for community-based working adults). The proportion of contacts aged <20 years was similar, but HCWs had proportionately much more exposure to contacts aged ≥60 years than community-based working adults. Key differences persisted after using the weights from Table I to give a modelled population of community-based working adults similar in age and sex distribution to HCW participants.
Characteristics of reported contacts from community-based working adults and HCWs
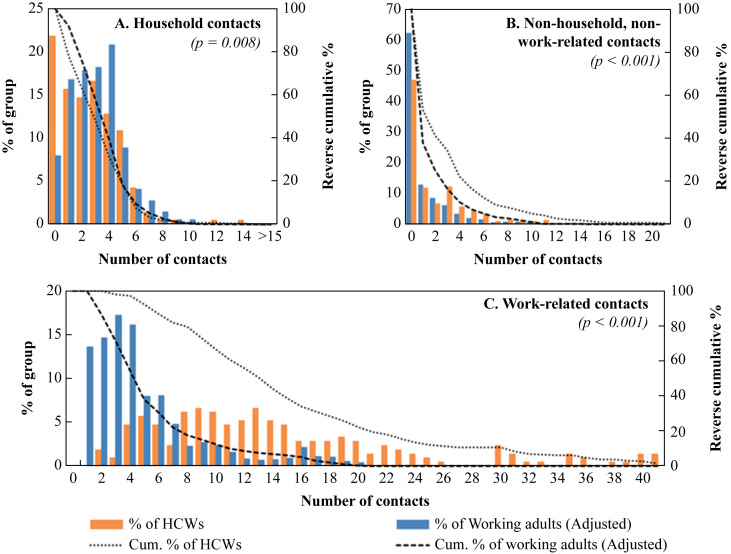
Healthcare workers had significantly fewer household contacts (median: 2 versus 3 for community-based working adults, P = 0.008; Figure 2 ), but more contacts that were neither household nor work-related (median: 1 versus 0 for community-based working adults; P < 0.001). HCWs reported 2 to 69 work-related contacts (median: 13), whereas community-based working adults had a right-skewed distribution of work-related contacts ranging from 1 to 24 (median: 4; P < 0.001).
Figure 2.
Number of contacts for community-based working adults and healthcare workers by location of contact.
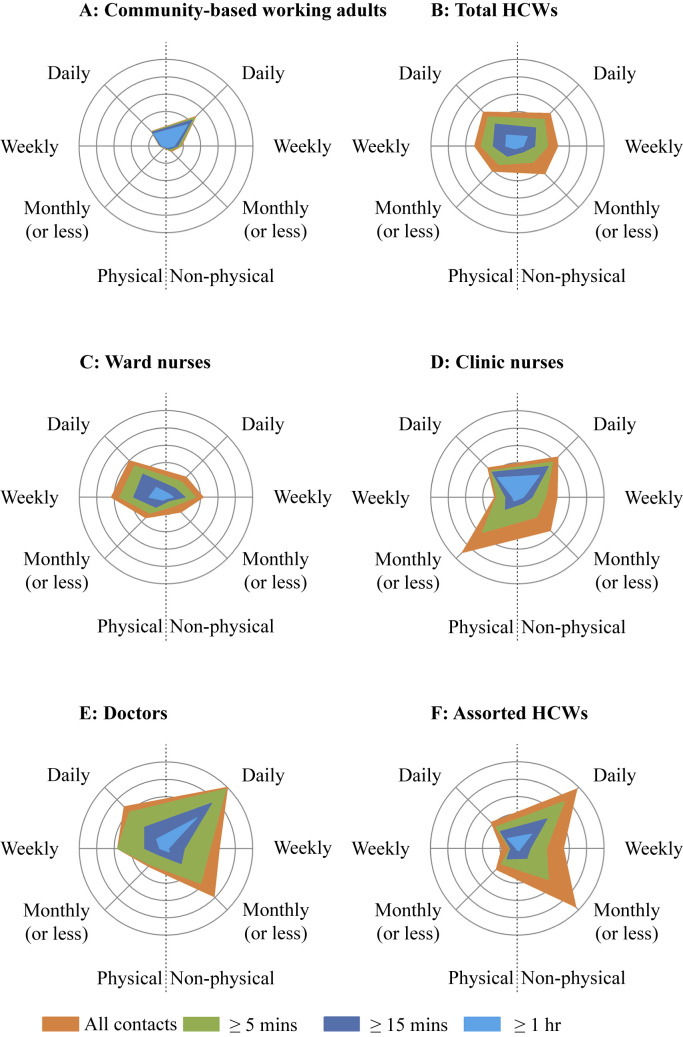
Figure 3 describes the average (square-root-transformed) number of work-related contacts per participant stratified by participant type and the three contact characteristics (physical versus non-physical contacts, contact duration and contact periodicity). Not only did community-based working adults have far fewer work-related contacts than HCWs, contacts were largely non-physical, though contact duration was long and mostly lasted ≥1 h, and was predominantly with individuals they met on daily basis. By contrast, HCWs reported an even spread between physical and non-physical contacts, as well as contacts of different durations (<5, 5–15, 15–60, and ≥60 min), and contacts they met daily, weekly and at a monthly/lesser frequency.
Figure 3.
Comparison of three contact characteristics between community-based working and different types of healthcare workers: physical and non-physical; periodicity of contacts (whether they met this contact daily, weekly, and monthly or less); and the duration of contact (≥1 h, ≥15 min, ≥5 min, and all contacts shown in light blue, dark blue, green and orange respectively). Each circle represents a square-root contact number of 0.5.
Among the different HCW types, doctors reported the highest whereas ward-based nurses the lowest total work-related contacts. Although around half of ward-based and clinic-based nurses' contacts involved physical touch, for the assorted HCWs, contacts involving physical touch were rare. Work-related contacts in clinic-based nurses, doctors, and assorted HCWs were relatively shorter than in ward-based nurses, with a substantial number effectively occurring with new contacts, i.e. persons they meet monthly or less frequently, or whom they never met before.
Factors associated with the number of work-related contacts for HCWs
The multivariate linear regression in Table II confirms that the combination of role and setting for the HCW was most strongly associated with the number of work-related contacts. Compared to the reference group (Assorted HCWs), ward-based nurses had fewer total contacts [β = –0.53; 95% confidence interval (CI): –1.00 to –0.06; P = 0.028]. By contrast, ward-based nurses had significantly more physical contacts than the reference Assorted HCWs group (β = 0.83; 95% CI: 0.38 to 1.27; P < 0.001), as did doctors (β = 1.15; 95% CI: 0.48 to 1.82; P = 0.001) and clinic-based nurses (β = 1.17; 95% CI: 0.60 to 1.75; P < 0.001). Male sex was associated with significantly fewer physical contacts (than females, β = –0.55; 95% CI: –1.01 to –0.09; P = 0.020). Institutional effects significant on univariate analyses were much reduced and non-significant after adjusting for confounding by HCW type.
Table II.
Effect of participant characteristics on number of work-related contacts, and number with physical contacts
| Regression coefficient, β (95% confidence intervals) |
||||
|---|---|---|---|---|
| Participant characteristics | All work-related contacts |
Work-related contacts with physical contact |
||
| Univariate | Multivariate | Univariate | Multivariate | |
| Institution (vs TTSH) | ||||
| KTPH | −0.18 (−0.56, 0.21) | 0.07 (−0.34, 0.48) | 0.10 (−0.28, 0.48) | −0.09 (−0.48, 0.30) |
| NUH | 0.42 (0.05, 0.79)∗ | 0.21 (−0.21, 0.62) | −0.28 (−0.64, 0.09) | −0.37 (−0.76, 0.02) |
| Age group (vs <30 years) | ||||
| 30–39 | 0.03 (−0.32, 0.38) | −0.06 (−0.41, 0.30) | 0.01 (−0.34, 0.35) | −0.07 (−0.41, 0.27) |
| 40–49 | 0.27 (−0.34, 0.87) | 0.25 (−0.35, 0.85) | −0.19 (−0.81, 0.42) | −0.28 (−0.87, 0.31) |
| ≥50 | 1.07 (0.24, 1.90)∗ | 0.84 (−0.04, 1.71) | −0.69 (−1.51, 0.13) | 0.16 (−0.68, 0.99) |
| Participant sex (vs female) | ||||
| Male | −0.02 (−0.48, 0.44) | −0.30 (−0.77, 0.18) | −0.70 (−1.15, −0.25)∗∗ | −0.55 (−1.01, −0.09)∗ |
| HCW group (vs Assorted HCWs) | ||||
| Ward-based nurses | −0.64 (−1.05, −0.23)∗∗ | −0.53 (−1.00, −0.06)∗ | 0.95 (0.56, 1.34)∗∗∗ | 0.83 (0.38, 1.27)∗∗∗ |
| Clinic-based nurses | −0.03 (−0.58, 0.52) | −0.1 (−0.70, 0.51) | 1.11 (0.58, 1.64)∗∗∗ | 1.17 (0.60, 1.75)∗∗∗ |
| Doctors | 0.20 (−0.48, 0.88) | 0.40 (−0.31, 1.11) | 1.02 (0.37, 1.67)∗∗ | 1.15 (0.48, 1.82)∗∗ |
TTSH, Tan Tock Seng Hospital; NUH, National University Hospital; KTPH, Khoo Teck Puat Hospital; HCW, healthcare worker.
∗0.01 ≤ P < 0.05.
∗∗0.001 ≤ P < 0.01.
∗∗∗P < 0.001.
Discussion
Using a 24 h contact diary, our study highlights key differences in the contacts between various hospital-based HCWs and community-based working adults, as well as differences between HCWs in various settings which have important implications for infectious disease transmission. We compared total numbers of work-related contacts, but we also explored three aspects which characterize contacts: duration, whether physical touch occurred, and the contact periodicity, the last aspect having largely been neglected in other studies.
The higher frequency of work-related contacts for HCWs as compared to community-based working adults was also reported in a previous study which used contact diaries to compare contacts for nurses versus matched controls from the general population (median: 34 versus 4) [11]. We showed that this is also true for other HCW types, who on the average had even more total work-related contacts than nurses. Furthermore, we found that many of the interactions involving HCWs are effectively new contacts, i.e. persons they do not meet daily or even weekly. By contrast, community-based working adults largely work with the same set of individuals on a daily basis. The right-skewed distribution for community-based working adults (Figure 2C) suggests that a small minority have much higher numbers of work-related contacts, including new contacts; these may represent individuals in other service industries. However, while severely ill individuals are less likely to use services such as retail and food-and-beverage in the community, they will almost always present to – and moreover have physical interactions at – health facilities. The large number of new work-related physical contacts increases HCWs' risk of encountering and hence acquiring an infectious disease, especially because they provide healthcare services to patients who potentially present with an infection. Moreover, the large number of new contacts reported by HCWs implies that non-HCW contacts encountered at health facilities by an infectious individual would also mostly be new contacts. This may be what makes the healthcare setting a potent amplifier for certain types of infection. HCWs been reported to have a higher infection rate for influenza, measles, and tuberculosis [18], [19], [20]. In recent years, the possible role of the health facility in amplifying transmission for emerging infections such as SARS, MERS-CoV, and Ebola virus disease has also been highlighted, with nosocomial transmission being implicated in several large outbreaks [8], [9], [10], [21], [22]. We believe that nosocomial amplification arises partially because of the high numbers of new contacts among HCWs, and other patients and their visitors, which potentially allows the infection to spread beyond the immediate family members of an infectious individual [8], [23].
However, among HCWs, our study also revealed important differences which may affect their potential to spread various infectious diseases. Besides the type of HCW, other factors including the institution, age, and sex did not substantially affect HCWs' work-related contacts. Previous studies suggest that nursing staff had more work-related contacts in the ward environment, but we found that, considering their scope of work at the hospital level over an entire work day, doctors had the highest number of work-related contacts [24], [25]. However, both ward-based and clinic-based nurses reported high numbers of physical contacts; ward-based nurses reported the fewest contacts, but had the highest number of longer-duration contacts (≥15 min) where physical touch occurred, and clinic-based nurses had high numbers of short duration (<15 min) contacts, most of which were effectively new contacts. HCWs in different settings may hence vary in their risk for acquiring and spreading pathogens with different dominant modes of transmission. For instance, physical contact has been confirmed to play a key role in the transmission of antibiotic-resistant bacteria such as meticillin-resistant Staphylococcus aureus [26]. However, it is unclear how the risk arising from the inpatient environment, with more physical contacts of longer duration, might compare with clinic environments with higher numbers of new contacts. And although ward-based nurses had fewer total and new contacts, they seemed to be at higher risk of infection compared to other HCW types during the SARS outbreak in Singapore in 2003 [8], [27]. We suggest that longer contact durations may be needed for transmitting infections with less or intermittent pathogen shedding. Whereas the duration of contacts has been described elsewhere, there have been few studies integrating both the number and duration of contacts into a model to describe their combined impact on the potential pathways of transmission for different infections [2], [28], [29], [30]. Our study provides some of the key parameters needed to model such transmission, by re-examining the interaction between various contact characteristics, and incorporating information on whether the contacts are new (which increases the probability of encountering an infectious case) or recurrent (which effectively increases the duration of exposure). Although this has previously been neglected in some infectious disease transmission models, it is what will inform us on the relative vulnerabilities of types of HCW and setting when preventing different nosocomial infections.
Our study has several limitations. First, we used convenience sampling determined by the participating institutions to facilitate recruitment. Consequently, we are uncertain as to the representativeness of our study, and we had a great diversity in the types of HCW which was difficult to control for. Even within the broad groupings, we acknowledge that our analyses in some categories were limited to relatively small numbers. Second, our study was based on paper contact diaries, which have been shown to suffer from underreporting of contacts, and overestimation of contact durations [31]. Underreporting is of particular concern for short contacts, with longer contacts being better reported, and, despite our reminders to participants to report all their contacts, our study remains subject to such biases. Moreover, it is challenging to independently verify the overall accuracy of such diary-based surveys of contacts. Third, since we standardized the format of the paper diary for both the HCWs and the general population, this limited the recording of more detailed contacts among HCWs such as the classes of persons contacted (i.e. nurses, doctors, allied HCWs, patients or visitors). Also, the format of the survey meant that we were unable to collect information on individual contact episodes with each person they had contact with, and hence we were unable to quantify the extent to which personal protective equipment (such as gloves, gowns and masks) was used in the interactions with patients. To do so without interfering with the HCWs' routines would have required the use of other methods such as those based on direct observation of HCW–patient interactions [32], [33]. Finally, our definition of a ‘significant contact’, though similar to previous contact diary surveys, may not capture all instances where transmission could occur, for instance for respiratory pathogens where transmission could potentially arise through being in the same physical space for a prolonged period, even without physical touch or conversation [34].
In conclusion, our study demonstrated that HCWs differ substantially from community-based working adults in the total number of daily contacts, but also the nature of the contacts, with HCWs far more likely to have physical contacts and new contacts; this may enhance their chances of encountering, acquiring, and spreading contact-transmissible and respiratory infections. Whereas doctors had the most contacts, nurses had more skin-to-skin touch and contacts of a longer duration, which would increase their risk for acquiring and spreading infections where physical contact or prolonged exposure is important for transmission. These observations may explain why health facilities are vulnerable to amplifying transmission of emerging infections, and the differential risk of various types of HCWs. The findings also provide critical inputs for the development and validation of infectious disease models for informing control and prevention within the healthcare setting.
Acknowledgements
We would like to acknowledge HCWs from TTSH, NUH, and TTSH, and all community participants for supporting our study.
Conflict of interest statement
None declared.
Funding source
This work was funded by the National Medical Research Council of Singapore (NMRC/CSA/011/2009).
References
- 1.Wallinga J., Edmunds W.J., Kretzschmar M. Perspective: human contact patterns and the spread of airborne infectious diseases. Trends Microbiol. 1999;7:372–377. doi: 10.1016/s0966-842x(99)01546-2. [DOI] [PubMed] [Google Scholar]
- 2.Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttunen R., Syrjänen J. Healthcare workers as vectors of infectious diseases. Eur J Clin Microbiol Infect Dis. 2014;33:1477–1488. doi: 10.1007/s10096-014-2119-6. [DOI] [PubMed] [Google Scholar]
- 4.Jagger J. Caring for healthcare workers: a global perspective. Infect Control Hosp Epidemiol. 2007;28:1–4. [Google Scholar]
- 5.Suwantarat N., Apisarnthanarak A. Risks to healthcare workers with emerging diseases: lessons from MERS-CoV, Ebola, SARS, and avian flu. Curr Opin Infect Dis. 2015;28:349–361. doi: 10.1097/QCO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 6.Coltart C.E.M., Johnson A.M., Whitty C.J.M. Role of healthcare workers in early epidemic spread of Ebola: policy implications of prophylactic compared to reactive vaccination policy in outbreak prevention and control. BMC Med. 2015;13:271. doi: 10.1186/s12916-015-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salgado C.D., Farr B.M., Hall K.K., Hayden F.G. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2:145–155. doi: 10.1016/s1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishna G., Choo P., Leo Y.S., Tay B.K., Lim Y.T., Khan A.S. SARS transmission and hospital containment. Emerg Infect Dis. 2004;10:395–400. doi: 10.3201/eid1003.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S.H., Kim Y.-S., Jung Y., Choi S.Y., Cho N.H., Jeong H.W. Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in Daejeon, South Korea. Infect Chemother. 2016;48:99–107. doi: 10.3947/ic.2016.48.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohuabunwo C., Ameh C., Oduyebo O., Ahumibe A., Mutiu B., Olayinka A. Clinical profile and containment of the Ebola virus disease outbreak in two large West African cities, Nigeria, July–September 2014. Int J Infect Dis. 2016;53:23–29. doi: 10.1016/j.ijid.2016.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard H., Fischer R., Mikolajczyk R.T., Kretzschmar M., Wildner M. Nurses' contacts and potential for infectious disease transmission. Emerg Infect Dis. 2009;15:1438–1444. doi: 10.3201/eid1509.081475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oussaid N., Voirin N., Régis C., Khanafer N., Martin-Gaujard G., Vincent A. Contacts between health care workers and patients in a short-stay geriatric unit during the peak of a seasonal influenza epidemic compared with a nonepidemic period. Am J Infect Control. 2016;44:905–909. doi: 10.1016/j.ajic.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Lowery-North D.W., Hertzberg V.S., Elon L., Cotsonis G., Hilton S.A., Vaughns C.F., 2nd Measuring social contacts in the emergency department. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee V.J., Chen M.I. Effectiveness of neuraminidase inhibitors for preventing staff absenteeism during pandemic influenza. Emerg Infect Dis. 2007;13:449–457. doi: 10.3201/eid1303.060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Dool C., Bonten M.J.M., Hak E., Wallinga J. Modeling the effects of influenza vaccination of health care workers in hospital departments. Vaccine. 2009;27:6261–6267. doi: 10.1016/j.vaccine.2009.07.104. [DOI] [PubMed] [Google Scholar]
- 16.Lim W.-Y., Chen C.H.J., Ma Y., Chen M.I., Lee V.J., Cook A.R. Risk factors for pandemic (H1N1) 2009 seroconversion among adults, Singapore, 2009. Emerg Infect Dis. 2011;17:1455–1462. doi: 10.3201/eid1708.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singapore Population Health Studies, NUS – Saw Swee Hock School of Public Health. Available at: https://www.sph.nus.edu.sg/research/sphs [last accessed October 2017].
- 18.Kuster S.P., Shah P.S., Coleman B.L., Lam P.P., Tong A., Wormsbecker A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steingart K.R., Thomas A.R., Dykewicz C.A., Redd S.C. Transmission of measles virus in healthcare settings during a communitywide outbreak. Infect Control Hosp Epidemiol. 1999;20:115–119. doi: 10.1086/501595. [DOI] [PubMed] [Google Scholar]
- 20.Tudor C., Van der Walt M., Margot B., Dorman S.E., Pan W.K., Yenokyan G. Tuberculosis among health care workers in KwaZulu-Natal, South Africa: a retrospective cohort analysis. BMC Public Health. 2014;14:891. doi: 10.1186/1471-2458-14-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowell G., Abdirizak F., Lee S., Lee J., Jung E., Nishiura H. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M.I.C., Loon S.-C., Leong H.-N., Leo Y.-S. Understanding the super-spreading events of SARS in Singapore. Ann Acad Med Singapore. 2006;35:390–394. [PubMed] [Google Scholar]
- 23.Fukutome A., Watashi K., Kawakami N., Ishikawa H. Mathematical modeling of severe acute respiratory syndrome nosocomial transmission in Japan: the dynamics of incident cases and prevalent cases. Microbiol Immunol. 2007;51:823–832. doi: 10.1111/j.1348-0421.2007.tb03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isella L., Romano M., Barrat A., Cattuto C., Colizza V., Van den Broeck W. Close encounters in a pediatric ward: measuring face-to-face proximity and mixing patterns with wearable sensors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhems P., Barrat A., Cattuto C., Pinton J.F., Khanafer N., Régis C. Estimating potential infection transmission routes in hospital wards using wearable proximity sensors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plipat N., Spicknall I.H., Koopman J.S., Eisenberg J.N. The dynamics of methicillin-resistant Staphylococcus aureus exposure in a hospital model and the potential for environmental intervention. BMC Infect Dis. 2013;13:595. doi: 10.1186/1471-2334-13-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M.I.C., Leo Y.-S., Ang B.S.P., Heng B.-H., Choo P. The outbreak of SARS at Tan Tock Seng Hospital – relating epidemiology to control. Ann Acad Med Singapore. 2006;35:317–325. [PubMed] [Google Scholar]
- 28.Ibuka Y., Ohkusa Y., Sugawara T., Chapman G.B., Yamin D., Atkins K.E. Social contacts, vaccination decisions and influenza in Japan. J Epidemiol Community Health. 2016;70:162. doi: 10.1136/jech-2015-205777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smieszek T. A mechanistic model of infection: why duration and intensity of contacts should be included in models of disease spread. Theor Biol Med Model. 2009;6:25. doi: 10.1186/1742-4682-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Cao E., Zagheni E., Manfredi P., Melegaro A. The relative importance of frequency of contacts and duration of exposure for the spread of directly transmitted infections. Biostatistics. 2014;15:470–483. doi: 10.1093/biostatistics/kxu008. [DOI] [PubMed] [Google Scholar]
- 31.Smieszek T., Castell S., Barrat A., Cattuto C., White P.J., Krause G. Contact diaries versus wearable proximity sensors in measuring contact patterns at a conference: method comparison and participants’ attitudes. BMC Infect Dis. 2016;16:341. doi: 10.1186/s12879-016-1676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L., Ng H.L., Ho H.J., Leo Y.S., Prem K., Cook A.R. Contacts of healthcare workers, patients and visitors in general wards in Singapore. Epidemiol Infect. 2017:1–11. doi: 10.1017/S0950268817002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan D.J., Pineles L., Shardell M., Graham M.M., Mohammadi S., Forrest G.N. The effect of contact precautions on healthcare worker activity in acute care hospitals. Infect Control Hosp Epidemiol. 2013;34:69–73. doi: 10.1086/668775. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone-Robertson S.P., Mark D., Morrow C., Middelkoop K., Chiswell M., Aquino L.D. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174:1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]