Abstract
The incidence of multi-drug-resistant Acinetobacter baumannii bloodstream infections (BSIs) increased two- to four-fold in three Israeli hospitals between 1997 and 2002, accounting for 3.5–18% of all hospital-acquired BSIs. This was associated with increasing carbapenem resistance reaching 35–54%, and by a dramatic increase in carbapenem consumption. In-hospital fatality rates ranged between 47% and 58% and were significantly higher than those seen with other nosocomial Gram-negative pathogens. A. baumannii was not restricted to intensive care units, but had spread to all hospital wards. Multi-drug-resistant A. baumannii has the potential to reach endemicity in hospitals and warrants more vigorous and innovative efforts to limit its spread.
Keywords: Acinetobacter baumannii, Nosocomial, Bacteraemia, Epidemiology, Drug resistance
Introduction
Acinetobacter spp. are ubiquitous in nature, present in virtually all water and soil samples, carried frequently on human skin and mucous membranes, and survive for extended periods of time in dry environments.1 Within the Acinetobacter genus, at least 32 genomic species have been identified, of which 17 have been given species names.1, 2 A. baumannii, genomic species 2, is closely related to species 1 (A. calcoaceticus) and to the unnamed genomic species 3 and 13TU, jointly referred to as the A. calcoaceticus–A. baumannii complex.1
A. baumannii has gained recognition in recent years as an increasingly prevalent nosocomial pathogen. The features of the genus that allow for its saprophytic existence assist in its spread in hospitals, namely the ability to survive in the inanimate environment and a striking capacity to develop antibiotic resistance.
A. baumannii comprised 1.3% of nosocomial bloodstream infections (BSIs) in the US SCOPE project between 1995 and 2002.3 In the SENTRY surveillance between 1997 and 1999, the prevalence of Acinetobacter spp. among nosocomial bacteraemias ranged from 0.7% in Canada to 4.3% in Latin America.4 In sources other than blood, the rates were higher. In Spain, rates of up to 9.6% have been described.5
In Israel, several hospitals have experienced a sharp rise in the prevalence of A. baumannii infections.6, 7, 8 We describe our experience with A. baumannii infections in three hospitals located in central Israel between 1997 and 2002.
Methods
The study hospitals were large, university hospitals with 1200 (hospital A), 900 (hospital B) and 850 (hospital C) beds. There were 20 general intensive care unit (ICU) beds in hospital A and 18 in hospital C. In hospital B, the number of beds increased from 12 before 2000 to 22 thereafter. All hospitals have burns and neurosurgery departments. Hospitals A and B also perform solid organ transplantations and cardiovascular surgery. Bone marrow transplantation is only performed in hospital A.
Fatality rates comprised in-hospital deaths from all causes. Hospital BSIs were defined as BSIs occurring more than 48 h after admission. Data were collected prospectively in hospital B, and through patient chart review in hospitals A and C. Multiple isolates within the same infectious episode were counted once in the analysis. Nosocomial A. baumannii BSI incidence is expressed as the number of BSIs per 10 000 patient-days in hospital. Carbepenem consumption data are given in defined daily doses (DDDs) per 100 patient-days (1 DDD meropenem=3 g; 1 DDD imipenem=2 g). Chi-squared test was used for contingency tables.
In hospital A, blood culture specimens were inoculated into BacT/Alert bottles (Organon Teknika, Durham, NC, USA). Identification of the isolates and susceptibility testing were performed using standard bacteriologic methods including an automated system (MicroScan, Baxter Healthcare Corporation, West Sacramento, CA, USA during 1997–2000, and Vitek II System, bioMérieux, Durham, NC, USA during 2001–2002). In hospitals B and C, blood culture specimens were inoculated into a 6B aerobic bottle and a 7A anaerobic bottle, and processed with the Bactec 9240 microbial detection system (Becton Dickinson, Franklin Lakes, NJ, USA). Susceptibility to antibiotics was tested by the disc diffusion agar method on Mueller–Hinton agar, according to the National Committee for Clinical Laboratory Standards procedures. The identification systems used may not distinguish between closely related genotypic strains of Acinetobacter sp., and thus some of the organisms referred to as A. baumannii may belong to closely related strains.
Results
Incidence
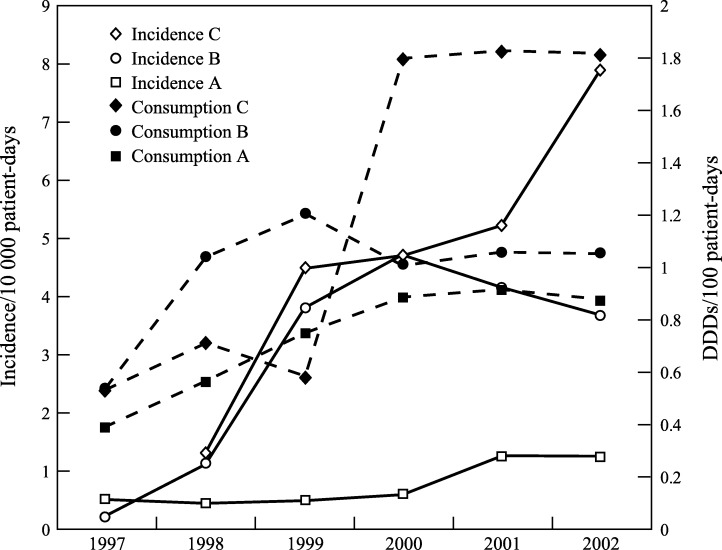
The number of A. baumannii nosocomial BSIs occurring between 1997 and 2002 was 177 in hospital A, 416 in hospital B, and 136 in hospital C. During the study years, the incidence of nosocomial A. baumannii BSIs increased substantially in all hospitals (Figure 1 ). The incidence was highest in hospital C, rising continuously from 1.3 to 7.8 BSIs per 10 000 patient-days. In hospital B, the incidence rose 19-fold from 0.25 in 1997 to 4.7 episodes per 10 000 patient-days in 2000, and declined thereafter (3.7 in 2002). In hospital A, the incidence was lower and the increase was only two-fold from 0.54 to 1.2 episodes per 10 000 patient-days. Within three years of its appearance in hospital B, A. baumannii became the single most common isolate among nosocomial BSIs at a rate of 15–18% of all nosocomial BSIs (Table I ). In hospitals A and C, the prevalence of A. baumannii among nosocomial BSIs ranged from 4 to 8% and from 3.5 to 12.3%, respectively. In hospital B, A. baumannii was the most common hospital-acquired Gram-negative BSI between 1999 and 2001.
Figure 1.
Incidence of Acinetobacter baumannii nosocomial bloodstream infections. Solid line, number of patient-unique episodes per 10 000 patient-days in hospitals A–C; dashed line, carbapenem (imipenem and meropenem) consumption in DDD per 100 patient-days in hospitals A–C.
Table I.
In-hospital fatality rates of hospital-acquired Gram-negative bloodstream infections (BSIs)
| Hospital A, 2001 |
Hospital B, 1999–2002 |
Hospital C, 1999–2002 |
||||
|---|---|---|---|---|---|---|
| N (%)a | Deaths (%)b | N (%)a | Deaths (%)b | N (%)a | Deaths (%)b | |
| Acinetobacter baumannii | 49 (8) | 23 (47) | 383 (16) | 223 (58) | 123 (9) | 58 (47) |
| Klebsiella pneumoniae | 75 (16) | 29 (39) | 209 (9) | 95 (45)c | 256 (18) | 91 (36)d |
| Pseudomonas aeruginosa | 102 (12) | 41 (40) | 211 (9) | 99 (47)c | 168 (12) | 67 (40) |
| 650 (100) | 2348 (100) | 1436 (100) | ||||
Percent of all nosocomial BSIs.
Percent of BSIs with the same pathogen.
Significant differences in fatality compared with A. baumannii, P<0.01.
Significant difference in fatality compared with A. baumannii, P=0.03.
Antimicrobial susceptibility and consumption
A. baumannii isolates were resistant to multiple antibiotics. More than 50% of isolates were susceptible to amikacin, imipenem, meropenem, ampicillin-sulbactam and colistin. Overall, 40% of isolates were resistant to amikacin. During the study years, resistance to imipenem increased progressively from 10% to 34% in hospital A, from 42% to 54% in hospital B, and from 5% to 34% in hospital C. Susceptibility testing for ampicillin-sulbactam was performed in hospitals A and B from 2000. Twenty-three percent of isolates in hospital A and 10% of isolates in hospital B were resistant to ampicillin-sulbactam. Susceptibility to colistin was tested in hospitals B and C, and nearly all isolates were susceptible.
Concurrently, carbapenem consumption increased from 0.53 DDDs per 100 patient-days in hospitals B and C to 1.2 in hospital B and 1.82 in hospital C. In hospital A, consumption was lower, ranging between 0.4 and 0.9 DDDs per 100 patient-days.
Fatality
In-hospital fatality rates following A. baumannii nosocomial BSIs were higher than fatality rates following nosocomial BSIs due to Pseudomonas aeruginosa or Klebsiella pneumoniae (Table I). In hospital B, 58% (223/383) of patients with A. baumannii BSIs died in hospital, compared with 47% of patients with P. aeruginosa bacteraemia (99/211, P<0.01) and 45% with K. pneumonia bacteraemia (95/209, P<0.01). The respective mortalities in hospital C were 47% (58/123 for A. baumannii) vs 40% (67/168 for P. aeruginosa, NS) and 36% (91/256 for K. pneumoniae, P=0.03).
Place of acquisition and source of infection
In all hospitals, the majority of A. baumannii BSIs were acquired outside the ICU, mainly in the medical departments (46–67% of all BSIs). Incidence, however, was highest in the ICU. In hospital B, 21% of the BSIs were acquired in the ICU, but the incidence of ICU-acquired A. baumannii BSIs was 60–72 per 10 000 patient-days compared with 2.7–4.0 outside the ICU.
Data on the source of BSIs were available from hospital B (prospective surveillance). Pneumonia accounted for 31.9% of A. baumannii BSIs, abdominal and postoperative infections accounted for 8.9%, and catheter-related infections accounted for 8.1%. Only 3.1% of BSIs were associated with urinary tract infections (UTIs). The source of infection remained unknown in 40% of patients. Fatality was highest with unknown source of infection and pneumonia (65% and 54%, respectively), and lowest with UTI (25%). BSIs caused by P. aeruginosa (211 episodes) and K. pneumoniae (209 episodes) were less commonly associated with pneumonia (7.6% and 18.2% respectively), and were more commonly associated with abdominal infections (23.9%, 16.6%), catheter-related infections (12.5%, 18%) and UTIs (9%, 18.2%).
Discussion
BSIs caused by A. baumannii have increased dramatically in three Israeli hospitals, comprising an increasingly significant percentage of hospital-acquired BSIs. Isolates were resistant to all cephalosporins and increasingly resistant to carbapenems. Fatality rates were consistently higher than those seen with other, notoriously life-threatening, Gram-negative infections in the hospital. Interestingly, infections were not limited to the ICUs but were acquired in multiple locations in the hospitals, mainly in the medical departments.
The results in our study are of major concern since both the prevalence and resistance rates of A. baumannii in these Israeli centres are higher than those reported from other locations. Reports from the USA up to 1998 and from Austria up to 2003 describe no resistance to imipenem in bloodstream isolates.9, 10 In Latin America between 1997 and 2001, 5.7–17.2% of bloodstream isolates were resistant to imipenem.11 Resistance is prevalent in Spain, reaching 28% in 1998.12 In comparison, resistance rates to imipenem in the last years of our study were 34–54%.
Crude mortality rates similar to those described in our study (47–58%) have been reported previously from Israel (54%).7 In the SCOPE project, 34% of patients with A. baumannii BSI died compared with 27% for all BSIs.3 A provocative matched cohort study claimed that A. baumannii BSIs are not associated with increased mortality rates in critically ill patients.13 In this study, crude mortalities were 42.2% in Acinetobacter cases vs 34.4% among controls. It is possible that the high mortality rate in our study can be explained by more severe underlying diseases in our patient population. Another possible explanation is the association with pneumonia as the source of infection, which was two to four times more frequent in A. baumannii compared with other Gram-negative nosocomial pathogens. We did not assess the appropriateness of empirical antibiotic treatment, clonality or virulence.
The spread of A. baumannii to almost all hospital departments within a few years, as described in our study, is another cause of major concern. The potential of A. baumannii to persist in the environment and spread effectively among patients has defeated the traditional infection control measures in the hospitals. Infection control measures implemented against A. baumannii infections point to environmental cleaning as important in the control of acinetobacter outbreaks.14, 15 However, most successful reports describe small outbreaks or control measures implemented in small units.
We report these findings to highlight the potential of multi-drug-resistant A. baumannii to establish endemicity in a hospital. Similar to the recent severe acute respiratory syndrome (SARS) outbreak, this is another alert for the possible emergence of new pathogens with unprecedented abilities to spread in hospitals and increase the already high burden of nosocomial infections.
References
- 1.Bergogne-Berezin E., Towner K.J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dessel H., Dijkshoorn L., van der Reijden T. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol. 2004;155:105–112. doi: 10.1016/j.resmic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Gales A.C., Jones R.N., Forward K.R., Linares J., Sader H.S., Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999) Clin Infect Dis. 2001;32(Suppl. 2):S104–S113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 5.Velasco E., Byington R., Martins C.S., Schirmer M., Dias L.C., Goncalves V.M. Bloodstream infection surveillance in a cancer centre: a prospective look at clinical microbiology aspects. Clin Microbiol Infect. 2004;10:542–549. doi: 10.1111/j.1469-0691.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 6.Simhon A., Rahav G., Shazberg G., Block C., Bercovier H., Shapiro M. Acinetobacter baumannii at a tertiary-care teaching hospital in Jerusalem, Israel. J Clin Microbiol. 2001;19:389–391. doi: 10.1128/JCM.39.1.389-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolyakov R., Borer A., Riesenberg K. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hosp Infect. 2003;54:32–38. doi: 10.1016/s0195-6701(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 8.Abbo A., Navon-Venezia S., Hammer-Muntz O., Krichali T., Siegman-Igra Y., Carmeli Y. Acinetobacter baumannii. Emerg Infect Dis. 2005;11:22–29. doi: 10.3201/eid1101.040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisplinghoff H., Edmond M.B., Pfaller M.A., Jones R.N., Wenzel R.P., Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31:690–697. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 10.Daxboeck F., Assadian O., Blacky A., Koller W., Hirschl A.M. Resistance of gram-negative non-fermentative bacilli causing bloodstream infection, Vienna, 1996–2003. Eur J Clin Microbiol Infect Dis. 2004;23:415–416. doi: 10.1007/s10096-004-1118-4. [DOI] [PubMed] [Google Scholar]
- 11.Tognim M.C., Andrade S.S., Silbert S., Gales A.C., Jones R.N., Sader H.S. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis. 2004;8:284–291. doi: 10.1016/j.ijid.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Bano J., Pascual A., Galvez J. Acinetobacter baumannii bacteremia: clinical and prognostic features. Enferm Infecc Microbiol Clin. 2003;21:242–247. doi: 10.1016/s0213-005x(03)72930-9. [DOI] [PubMed] [Google Scholar]
- 13.Blot S., Vandewoude K., Colardyn F. Nosocomial bacteremia involving Acinetobacter baumannii in critically ill patients: a matched cohort study. Intens Care Med. 2003;29:471–475. doi: 10.1007/s00134-003-1648-8. [DOI] [PubMed] [Google Scholar]
- 14.Denton M., Wilcox M.H., Parnell P. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J Hosp Infect. 2004;56:106–110. doi: 10.1016/j.jhin.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Melamed R., Greenberg D., Porat N. Successful control of an Acinetobacter baumannii outbreak in a neonatal intensive care unit. J Hosp Infect. 2003;53:31–38. doi: 10.1053/jhin.2002.1324. [DOI] [PubMed] [Google Scholar]