Summary
With concerns about the potential for the aerosol and airborne transmission of infectious agents, particularly influenza, more attention is being focused on the effectiveness of infection control procedures to prevent hospital-acquired infections by this route. More recently a number of different techniques have been applied to examine the temporal–spatial information about the airflow patterns and the movement of related, suspended material within this air in a hospital setting. Closer collaboration with engineers has allowed clinical microbiologists, virologists and infection control teams to assess the effectiveness of hospital isolation and ventilation facilities. The characteristics of human respiratory activities have also been investigated using some familiar engineering techniques. Such studies aim to enhance the effectiveness of such preventive measures and have included experiments with human-like mannequins using various tracer gas/particle techniques, real human volunteers with real-time non-invasive Schlieren imaging, numerical modelling using computational fluid dynamics, and small scale physical analogues with water. This article outlines each of these techniques in a non-technical manner, suitable for a clinical readership without specialist airflow or engineering knowledge.
Keywords: Aerosol, Airflow, Computational fluid dynamics, Mannequin, Schlieren, Transmission
Introduction
With the severe acute respiratory syndrome (SARS) outbreaks of 2003, the ongoing human avian influenza A(H5N1) infections and the more recent pandemic influenza A(H1N1/2009) cases in 2009, concerns about the aerosol or airborne transmission of infection have become topical and important.1, 2, 3, 4, 5 Such transmission of infectious agents may occur via short-range, large-droplet aerosols and long-range, smaller, airborne droplet nuclei.6, 7 The finding of viral nucleic acid in air samples, as well as the demonstration of viable viruses in exhaled air, have further reinforced concerns about the risk of infectious disease transmission.8, 9, 10, 11, 12, 13 With decisions to be made about the most appropriate personal protective equipment (PPE) to be used in infection control guidelines, perhaps one of the more controversial issues is which type of face mask (or respirator) to use, i.e. surgical or N95 types. To help to resolve this issue, it has become important to understand the proportion of infectious diseases potentially transmitted via the airborne route.
Other articles have described and discussed the different clinical situations in which such disease transmission via the airborne route can occur, as well as the various agents potentially transmitted and the evidence for the different virological and environmental factors affecting successful transmission via this route.6, 7, 14, 15, 16, 17, 18, 19 Related and important aspects such as airborne survival of infectious agents, droplet numbers, sizes and their formation, and potential infectious doses have also been covered elsewhere.6, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
The measurement and monitoring of airflow patterns as an indicator of the potential for aerosol and airborne transmission of infection has played an essential role in the appropriate design of containment and isolation systems for many years. Multiple infection control and laboratory biosafety guidelines refer to airflow measures as a surrogate marker of protection against aerosol or airborne infection. Examples include negative and positive isolation for infectious and immunosuppressed patients, respectively, ‘laminar’ and unidirectional airflow ventilation in operating theatres, regular airflow and pressure monitoring of biosafety cabinets in diagnostic and research laboratories, etc. It is only relatively recently that there has been a renewed interest in the detection and identification of airborne infectious agents, e.g. severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and influenza, by air-sampling and molecular detection of their nucleic acid through the polymerase chain reaction (PCR) and nucleic sequencing, or by detecting viable pathogens through direct culture methods.8, 9, 10, 11, 12, 13
Given the breadth and current relevance of this topic, this review article presents an overview of a range of airflow visualisation methods that can be used as surrogate markers of the potential for aerosol or airborne transmission of infectious agents and a discussion of recent developments in the field. The review is aimed at non-airflow specialists, and in particular those with a clinical background who may be involved with, or need to interpret, research using such techniques.
Airflow visualisation of simulated (with mannequins) or real human respiratory behaviour
The movement of the airborne particles around a person is partly governed by that person’s ‘microenvironment’ and partly by the airflow around the microenvironment. Typical respiratory processes that occur in an individual’s microenvironment may include: breathing (i.e. inhalation–exhalation), talking, laughing, coughing and sneezing. Each individual is surrounded by a thin layer of air or ‘boundary layer’ that adheres closely to the body surface. The temperature difference between the person and the room creates a characteristic ‘thermal plume’ above the person that then interacts with the room ventilation system.32, 33, 34, 35
Human respiration varies with the level of activity and can be characterised by specifying the breathing direction, frequency and tidal volume, with the latter two parameters contributing to the pulmonary ventilation rate of an individual. Other factors affecting this pulmonary ventilation rate include how much body heat is lost, the surrounding air temperature and the individual’s sex. All of these factors will influence the amount of air inhaled by an individual and therefore the potential amount of inhaled infectious agent (i.e. the potential level of exposure to that agent).36, 37, 38, 39, 40
Coughing, which is a physiological reflex designed to clear the respiratory tract of debris (including infectious material), may be one of the most efficient ways to initiate aerosol or airborne infection and may be a major, natural, physiological mechanism of cross-infection for some infectious agents.35, 41, 42, 43, 44, 45
The use of mannequins or human volunteers in airflow visualisation studies is an attempt to recreate realistic scenarios in which a range of useful analyses can be performed. These include the detailed analysis of flow and particle transport in the immediate microclimate surrounding the mannequin or human volunteer, which may be affected by the individual use of masks and respirators. This may also enhance the understanding of larger scale interactions between people and the bulk room air.
Methods using various types of mannequins
Resuscitation mannequins fitted with lung models have been used extensively by Hui et al. to demonstrate the production and dissemination of exhaled plumes from oxygen masks or ventilation systems that may potentially disseminate infectious agents from the wearer.46, 47, 48, 49, 50, 51 The air flow was visualised with smoke particles illuminated by a laser light sheet. The buoyant exhaled plumes moved an average horizontal distance of 0.4–0.5 m. These results have led to some recommendations for the use of such respiratory support procedures to be avoided, which has in turn led to some controversy about the relative risk:benefit ratio of applying a potentially life-saving intervention that may subsequently result in secondary infections in healthcare staff and other patients nearby.52, 53 Hui et al. do point out that their respiratory model has limitations and can only illustrate the movement of air. It cannot and does not attempt to estimate the actual number or sizes of particles that may carry potentially infectious organisms within such airflows. Therefore using these results to preclude the use of such potentially life-saving, respiratory support interventions is premature.
More sophisticated thermal mannequins have been designed to mimic human skin temperatures and create the thermal body plume, as well as to produce buoyant, warm exhalation flows as an accurate model of normal human breathing.54, 55, 56 These mannequins, originally developed for the study of advanced ventilation systems, have now been successfully adapted for the study of small particle and bioeffluent release from the body surface, e.g. the transport in the thermal plume above the person and exposure to other common, indoor, airborne contaminants.57, 58, 59 For infection control purposes, these thermal mannequins have also helped to define the most effective ventilation modes to be used on hospital wards in order to reduce the potential for the cross-transmission of infectious agents potentially carried in exhaled air, between patients on neighbouring beds.54, 55
These mannequins can be adapted to better understand the mechanisms involved with aerosol or airborne-transmitted infections. Such cross-infection is caused by the movement of airborne particles in the room airflow (particularly of droplet nuclei smaller than 5–10 μm) or by larger droplets settling at a short distance from the exhaling source. Droplet nuclei can be simulated with tracer gas released from the mannequin, e.g. CO2 or N2O, because the settling velocity is very low (i.e. these particles may stay suspended in the air for a considerable time, e.g. 5 μm droplet nuclei have a settling velocity of 1 m/h).53, 60, 61 A qualitative study of droplet nuclei movement can also be performed with a smoke tracer, as shown in Figure 1 .
Figure 1.
(A) Airflow visualisation in the microenvironment around two life-size mannequins. The source mannequin is exhaling through the nose. Reprinted from Figure 2, Tang et al.6 with permission from Elsevier. (B) Demonstration of cross-infection in a simulated hospital ward. Exhalation flow from a life-size mannequin is indicated by a smoke tracer.
Airflow visualisation using tracer gas to simulate the smaller droplet nuclei, or particles used to simulate the larger droplets, has been performed in simulated hospital wards, where life-size, breathing, heated mannequins are placed in typical patient postures and smoke tracers are used to visualise the behaviour of their potential exhaled airflows.54, 55, 62, 63 In addition, two or more thermal mannequins with heated skin surfaces and simulated respiratory functions have been used to study the behaviour of airflows and the potential for cross-infection between people (Figure 1).32, 59, 60, 64
Thermal mannequins have also been used to design and test personalised ventilation systems that could be built into seats in aircrafts and trains. These systems produce a jet of air which can protect against ‘incoming’ air potentially carrying infectious agents. This is a new and promising research area that requires thermal mannequins with respiratory functions because of the detailed level of life-size, human anatomical realism required.65, 66, 67, 68, 69, 70, 71, 72 A specialised version of such a personalised ventilation system, designed for use in hospital beds, has also been tested with a thermal mannequin and tracer gas.55, 73, 74 The disadvantage of such systems is that they are only effective if patients remain in their beds, as such systems are not designed to be portable. Further work using such thermal, breathing mannequins is currently being conducted on the exhalation of suspended particles or droplets as a potential source of cross-infection in a hospital setting.61 Although short-range, large droplets (as opposed to long-range, small droplet nuclei) are often quoted as a major source and route of cross-infection in healthcare environments, this has been mainly inference and the actual visualisation of this phenomenon has not been performed convincingly.
Thermal mannequins are versatile and can be used with lasers or with tracer gas. They have advantages and also some disadvantages (Table I ). Their thermal profile may in some cases not exactly mimic that of humans and they are generally unmoving, although moving mannequins with a certain prescribed range of movements are now commercially available, mainly for shop-front or in-store advertising purposes.
Table I.
Advantages and disadvantages of different airflow visualisation techniques
| Technique | Advantages | Disadvantages |
|---|---|---|
| Real approaches: | ||
| Human volunteers | Realistic subjects and physiology, particularly with regard to thermal characteristics and thermal boundary layers | Safety is important. Human volunteers cannot be exposed to high intensity (e.g. laser) light or irritant or toxic tracer gases or particles |
| Hospital monitoring | Realistic situations and environments | Highly variable results, often obtained using non-standard techniques, making interpretation difficult, and therefore limiting any useful generalisation |
| Theoretical approaches: | ||
| Computational fluid dynamics (CFD) | Good spatial/temporal information. It is a standard modelling tool in the industry | Difficult to model moving bodies. Difficult to obtain accurate simulations due to required computing power and/or simulation time |
| Abstract approaches: | ||
| Physical analogues in scale model or in full scale (models) | Quick and relatively easy to build with reasonable spatial resolution. Able to test different hypotheses related to flow patterns in different geometries using a variety of flow-generating techniques/devices. Easy to work with tracer gas and airborne particles for the simulation of viruses and bacteria in full scale experiments with thermal mannequins |
Difficult to combine different contributions to bulk air flows in small scale, and difficult to work with movements of persons in full scale |
Methods using human volunteers
The use of realistic, human-like mannequins for simulating exhaled airflows has mostly required some kind of particulate tracer, such as smoke particles. However, using potentially irritant particulates precludes their use with actual human volunteers. Although mannequins may mimic human respiratory airflows to a certain extent, the use of human volunteers adds an additional realism that can further enhance the understanding of the behaviour of human exhaled flows. One method of doing this that has become popular recently is the use of Schlieren imaging.75, 76, 77, 78
The Schlieren imaging technique is not new and has been known to science and engineering for many years. It has been used previously in an infection control context, particularly in the UK, by Clark et al. starting in the late 1960s and continuing into the 1970s and 1980s.79, 80, 81 Clark et al. discovered by way of Schlieren imaging, for example, the escape of potentially contaminated air from neckline, cuffs, and ankles of surgical gowns and the backflow of air from microbiological safety cabinets (fume hoods) when switched off. Early work by Whyte and Shaw also used the technique to demonstrate the influence of the thermal plume generated by operating theatre lights.82
Schlieren imaging relies on thermal differences in the air to refract a light beam in order to visualise airflows. No invasive or potentially irritant tracers are necessary, and human volunteers are required only to stand in front of a concave mirror and breathe (or talk, cough or sneeze) across the illuminating light beam that is directed at the centre of the mirror, to produce a real-time, visible image of their exhaled airflows and thermal plume.
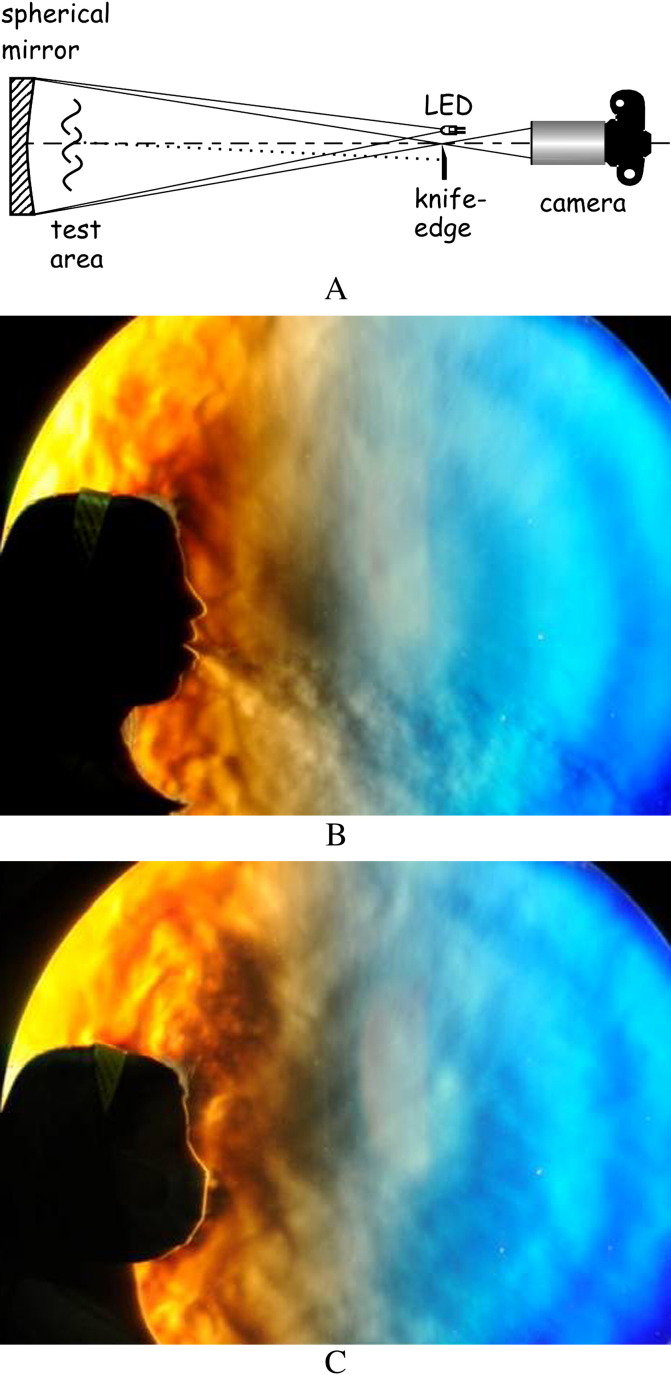
A simple embodiment of this instrument – actually the classical Foucault mirror test – is shown in Figure 2 . A small light source such as a light-emitting diode (LED) illuminates a precise, front-aluminised spherical mirror from its radius of curvature. The mirror is slightly offset to refocus the light below the LED. Beyond this focus a camera collects the beam to form an image which will be a uniform spot under ideal conditions. However, thermal disturbances in the air in front of the mirror – the test area – refract light rays such as the one shown by a dotted line in the diagram. By placing a knife-edge (razor blade) at the focus as shown, such refracted rays are stopped, and corresponding dark regions appear in the camera image. In this manner a Schlieren image is made up of illumination differences due to light refraction in the test area.76
Figure 2.
Typical large scale Schlieren imaging set-up (A), showing the spherical cross-sectional mirror, the test area, the light-emitting diode (LED) light source, the knife-edge that discriminates refracted from un-refracted light rays, and the camera that captures the schlieren image. Light from the LED is refracted through the exhaled air of a human subject in the test area, since that air is at a different temperature than the surroundings, creating Schlieren images such as (B), coughing and (C), coughing whilst wearing a surgical mask.
The most recent infectious disease experiments using the Schlieren technique have visualised airflows from human volunteers coughing, with and without wearing various face masks, in front of a large concave telescope mirror. These were designed as qualitative or semiquantitative visual aids to help infection control teams understand the patterns of such airflows and how they may contribute to the transmission of infectious agents from infected patients. Recent findings have demonstrated that the maximum airflow velocity in a human volunteer cough reached about 8 m/s, and also showed that both surgical and N95 face masks are effective in thwarting the escape of potentially infectious aerosols when worn by an infected individual.35, 44, 45 More specifically, in these experiments, airflow visualisation demonstrated to what extent the wearing of face masks could contain or limit (either by barrier, or redirection and deceleration effects) the potential airborne dissemination of infection, using airflow as a surrogate marker of the potential extent of such spread.35, 45
Figure 2 shows the basic Schlieren imaging set-up with images of a female volunteer coughing without a mask, then while wearing a surgical-type mask. The strong turbulent cough jet that penetrates forward into the room air without the mask is effectively stopped by the mask. Note that the expelled air is not contained, but is vented into the rising thermal plume of the subject. Thus the potential for contamination of the room air still exists (although there is likely to be a significant dilution of the infectious agent), but the direct threat of cough or sneeze plumes impinging on a nearby person is ameliorated.
Further experiments are planned (by Tang et al. in Singapore) to demonstrate how airflows behave during other everyday scenarios, e.g. quiet breathing, sneezing and talking between two people. Depending on the size of the Schlieren mirror available, other types of airflow environments can be simulated and examined, e.g. interactions among passengers in taxis, buses, trains and airplanes. In addition, it is possible in some circumstances to quantitatively measure the velocity and volume of exhaled airflows visualised using Schlieren imaging, which can be achieved by combining the principle of particle image velocimetry (PIV) with the Schlieren optics. In this case the ‘particles’ are actually turbulent eddies within the exhaled airflows, so again, no seeding with actual (potentially irritant) particles is therefore required.44, 83 This approach, which is still under development, requires flows that are both turbulent and refractive, as are the human cough and sneeze.
Although Schlieren imaging visualises airflows associated with human volunteers without the need for tracer gas or particles, safety concerns preclude the use of high-intensity laser lighting (Table I). From an analytical viewpoint, Schlieren images integrate three-dimensional flow information onto a single plane and also cannot be used in environments where there is insufficient temperature difference between the human exhalations and the surrounding air. Finally, the large, precise astronomical-quality mirror required for Schlieren imaging of airflows associated with humans is quite expensive.
Visualisation of ambient airflows in healthcare environments
Air moves around a room and between rooms as a consequence of: (a) ventilation (either forced or naturally ventilated); (b) the movement of people, equipment, furniture, doors;84 (c) buoyancy-driven flows generated by heat of people and equipment; (d) the disturbances created by human respiratory activities, such as breathing, talking and coughing.35, 44, 45, 85 The major difficulty in understanding how air laden with skin flakes, droplets and particles moves around is, first, the problem of making whole scale measurements of air velocities and bulk air movements over time in a large space, and second, how to interpret the results, particularly in a setting as complex as the hospital. This, together with the more biological questions (e.g. numbers of viable infectious agents exhaled and their threshold infectious doses for exposed individuals), is what makes the problem of understanding airborne transmission scientifically challenging. Yet, even solving only part of this question has a useful bearing on other problems. There are many techniques which can be applied to measure parameters such as air velocity, temperature and humidity at a single point in space. Since the mobile nature of any particles or droplets in air tends to result in their dilution over time, most sampling methods tend to integrate the information at a single point over time, e.g. by drawing air through sensors (such as laser counters) or to allow suspended, potentially infectious agents in the air to be inertially deposited on agar plates to allow for a subsequent quantitative analysis of colony counts.
More recently a number of different techniques have been applied to examine the temporal–spatial information about the airflow patterns and the movement of related, suspended material within this air in a hospital setting.
Computational models
Computational fluid dynamics (CFD) is a numerical method of calculating the movement of air (fluid) within open or closed spaces based on governing physical laws of mass, momentum and energy conservation (Figure 3 ). This technique has three main steps.
Figure 3.

A computational fluid dynamics (CFD) prediction of the temperature distribution in a room ventilated by displacement ventilation containing a single individual. The blue colour shows the cold supply flow and the red colour shows the heated air rising to the top of the room. The temperature distribution around the individual can be demonstrated using such a CFD prediction. Air heated in a layer close to the individual (by his/her natural body temperature) rises in a boundary layer, forming a thermal plume above the head. Exhalation flows are visible as horizontal heated flows emanating from the mouth. In the case of an infected person this flow will also contain a variable number of infected droplet nuclei and the plume rising above will also contain bioeffluents (like shed skin scales) released from the body surface. With certain assumptions, a direct prediction of the distribution of small droplet nuclei or larger droplets can also be made with this technique.
The first is to describe the geometry of the space that is being considered. This will inevitably involve some degree of simplification to focus on the major features of the space and to neglect any aspects that are small or unimportant. Typically furniture (except a bed) tends to be ignored and most researchers use simple geometrical forms for people either standing, sitting or lying down.85, 86, 87 Where the research interest is in the detail of flow in the vicinity of an object or a person, realistic representation of the feature becomes a more important consideration.88 Studies on personalised ventilation or the respiration process are examples of applications where CFD models need a detailed representation of the human form to properly simulate the interaction of airflows with the personal microclimate.89, 90
The three-dimensional geometrical space is then divided into discrete elements, known as the mesh, with the number of elements depending on the size of the space and required accuracy. As the mesh becomes finer, the number of elements increases and the calculations tend to become more accurate, but this comes at the price of significantly more computing power and simulation time (Table I). Most simulations will use variable mesh densities throughout the space with a finer mesh where properties of the flow (or other variables) vary significantly over short distances (such as near walls). It is difficult to state a typical resolution, but most relevant calculations run to resolutions of 1–3 million elements; for a typical isolation room (of 5 m × 4 m × 3 m) the mesh elements are therefore about the size of a house brick. As the resolution increases beyond about 2 million elements, computational requirement goes beyond the capabilities of a single computer, and would usually employ a cluster of computers or a supercomputer. For example, research currently underway at University College London involves running codes with 200–300 million nodes, employing a supercomputer (‘Legion’), which uses more than 2560 computer processors.
After the mesh has been chosen, the next key issue is establishing the physics that needs to be included in order to calculate the flow and properties. Flow is described by the Navier–Stokes equation, a mathematical description of the conservation of momentum (Newton’s second law of motion). Since room airflow is turbulent with scales or vortices smaller than the mesh elements, a degree of approximation is required to model these processes through the specification of a turbulence model. As rooms generally include people and equipment it is usually necessary to include equations of energy conservation to simulate the influence of heat sources and temperature gradients on air convection within the space.
The process of simulating airflow described so far is common to any indoor environment. Areas of interest in infection control primarily focus on the transport, deposition and survival of airborne micro-organisms and it is necessary to consider the models and approaches that are specific to this scenario. The behaviour of bioaerosols is generally represented in two ways: as a passive scalar (i.e. a tracer that moves with the airflow; Figure 1) or as discrete particles modelled using an equation of motion, which describes the balance of buoyancy and aerodynamic drag forces; Figure 3). Passive tracers tend to be more suited to respiratory pathogens and have been used in many studies, for example, evaluating how ventilation design and ward layout may reduce TB transmission risks, and showing that the predicted bioaerosol distribution in a hospital ward during a SARS outbreak correlated with the spatial pattern of infections. Particle models are used where pathogens may be carried on larger particles such as shed epithelial skin cells or where the evaporation or the deposition of particles is of interest.1, 85, 86, 91, 92, 93, 94 Both tracer approaches can also incorporate biological factors such as natural decay or inactivation due to decontamination processes. For example, CFD modelling of UV-C irradiation incorporating an experimentally derived decay function into the passive scalar tracer model has enabled the simulation of the behavior of UV disinfection devices at room scale and showed the importance of the interaction of the UV field with the airflow.95, 96
Application of such tracer models must also consider the characteristics of a potential pathogen source. In terms of spatial location, respiratory pathogens, released in the exhaled breath of a patient, are generally modelled using a small zone or point source, with the release characterised by specifying a velocity and pathogen concentration.45, 86, 90, 97, 98 Pathogens released through activity will be dispersed over a much wider area and hence should be released over a representative zone. This approach has been applied in studies of operating theatre ventilation, releasing tracers from the surfaces of people, to demonstrate the effect of factors such as the position of operating theatre lamps and people on the airborne contamination level.87, 99 Application to ward activities such as bed-making has also shown that the specification of the source has a major influence on the results.91 Duration of release is also an important consideration. In the real world, pathogens will not be released continuously into a space, but such transient (or periodic) analysis using CFD simulation adds considerably to the time and cost of the computations. As such, steady-state solutions are favoured by most researchers and the ‘worst case’ scenario of a continuous release is often presented. It is important that this simplification is recognised when evaluating the results of such simulations. After running the software to calculate the flow and movement of droplets/particles, the results are analysed using various visual and quantitative post-processing techniques. Care is taken to choose the most appropriate diagnostics as the amount of data generated by running such simulations is potentially enormous (Figure 3).
The main problem with applying CFD computations is that they tend to be under-resolved, due to the need to reduce the computational cost, which then competes with the desire to incorporate reality. The geometry of the problem is usually fixed, which means that processes, which may be important, such as the movement of people, doors opening and closing cannot be considered. More recent developments are seeking to address this with transient simulation, and studies incorporating the activity of people are becoming more widely used.81 This includes studies such as Brohus et al.’s model of operating room contamination that applied momentum sources within a fixed geometry to simulate movement of people, as well as workers such as Shih et al. who used a moving geometry approach to show the influence of healthcare worker movement and door opening on isolation room airflows.87, 98 The development of real-time CFD models is even on the horizon, with van Treeck et al.100 demonstrating the feasibility of such an approach to controlling thermal comfort in the operating theatre.
Physical analogue models
Analysing the movement of air within a hospital environment is extremely challenging, as airflow patterns are difficult to visualise in real time. In the engineering community, it is common practice to employ a physical analogue which has the same geometry as the problem under consideration but the analogue may be significantly smaller or larger. For this analogue to work, the characteristic flow patterns in the model and real-life flow patterns need to be the same or similar. For flows where buoyancy effects are not important, the key quantity is a dimensionless measure, the Reynolds number, which defines the relative strength of inertial to viscous forces in the flow. For sufficiently large Reynolds numbers, such as turbulent air mixing in a well-ventilated space, the mean flows in both the model and the real scenario will be equivalent. For situations where buoyancy forces are important, such as natural ventilation flows, the equivalence is acceptable, providing the Froude number (a dimensionless measure of the relative strength of inertial to buoyancy forces) is matched. When applied to studying flows in hospitals, it is usual to employ 1:10 scale-models constructed out of acrylic, using water as a working fluid (Figure 4 ).101 The advantage of using water as an air analogue is that the water can carry tracer particles (or dye) which can be illuminated and visualised relatively easily. Experiments can be recorded with one or more cameras to capture simultaneous, real-time images from different angles.101
Figure 4.

Example of a physical analogue in the form of a small scale model of a hospital isolation room with antechamber. Coloured food dyes can be used to visualise fluid flows with water simulating airflow in this type of model.
By combining a number of projected views it is possible to reconstruct how material moves in four dimensions. The advantage of this method is that it gives good spatial and temporal resolution of the movement of passive materials in flows which are similar to those occurring in the hospital environment. It is also easy to change the geometrical configuration of the scale-model. Hence, the use of such analogues can provide quantitative information about how contaminants move in both space and time (T.O. Robinson, L. Wilkinson, D. Koralege, unpublished data).101
Large scale flow measurements in real or mock rooms
Another approach is to take measurements in the actual hospital environment or a full-size mock-up of a hospital room (partially shown in Figure 1B). At the simplest level, the use of smoke can give qualitative information about air movement over time. Early applications included investigation of unidirectional airflows for infection control and visualisation of mixing and laminar operating theatre airflows to demonstrate overall flow characteristics.102, 103, 104 The method is used widely in the engineering community for checking flow paths and is recommended in UK’s Department of Health Guidelines (HTM03) for confirming correct pressure relationships in areas with specialist ventilation such as isolation rooms and operating theatres.105
The attainment of quantitative data requires direct measurement of air velocities at local points or evaluation using tracer gases. The former is most commonly accomplished using a single point hot wire anemometer to take a spot measurement or a series of comfort probe anemometers to determine velocity magnitude at a grid of points in space. The main limitation with this approach is the range of anemometer devices. Typical room air velocities are below the range of many devices and those that are capable of measurement generally only indicate magnitude rather than direction. The technique is widely used, ranging from practical applications such as confirming air velocities in ultraclean operating theatres and measuring flow rates at ventilation diffusers as part of outbreak assessment, through to validating other approaches such as CFD.85, 105 Neutrally buoyant helium bubble tracers have also been applied, particularly in early studies, to visualise and quantify the airflows in spaces such as operating theatre environments.104 This approach uses photographic techniques, relating the size of a bubble track image to camera exposure time to determine air velocities.
More advanced techniques such as laser Doppler anemometry (LDA) systems and particle image velocimetry (PIV) offer a significantly more accurate approach, but are costly. They are derived from work on smaller scale flows and are only recently starting to be applied to room scale air flows.37 Applications tend to focus on acquiring data for CFD validation, such as Chao and Wan who used PIV data to measure velocities and turbulence parameters for a CFD model of particle motion in a clean room.106 While airflow measurement gives valuable data, it only gives information on the movement of the air itself and thus the behaviour of contaminants such as bioaerosols has to be inferred from the airflow patterns.
The application of tracer gas is the most common alternative approach that can be used to acquire quantitative data on ventilation rates and airflow, as well as having the capacity to be released in such a way as to mimic an infectious aerosolised source.107 Tracer gases (including CO2, N2O and SF6) have been employed in general indoor air studies as well as healthcare-specific scenarios. Among the many examples, studies have included assessing the performance of naturally ventilated tuberculosis (TB) isolation wards using a CO2 tracer, assessing the performance of isolation rooms and operating theatres using N2O and SF6 tracers, and evaluating the effect of the ventilation system on the risk of transmission between patients in ward environments.54, 103, 108, 109, 110, 111 Although this approach gives a better insight into contaminant transport, it has been acknowledged that tracer gases may not fully represent bioaerosols, particularly those released as larger droplets.103, 112 Pioneering work in healthcare ventilation showed that potassium iodide could be applied as a tracer with particles collected on membranes and subsquently visualised.112 Application of this approach to transfer between patients in an isolation ward indicated the need for better airflow control and good agreement with theoretical models.113, 114 Andersson et al. also used potassium iodide to study operating theatre ventilation, showing the role of activity on the airflow distribution in the room.103 Other early work applied in indoor and outdoor air used fluorescent particle tracers, enumerated visually or via electro-optical detection.115 More recent studies have used a range of particle tracers including sodium chloride and latex beads, enumerated directly using optical techniques such as laser particle counters rather than visual or chemical approaches.109, 116, 118 Direct comparison with bioaerosols has also been carried out, evaluating mixing and ventilation rates in an isolation room context measured using tracer gases, against NaCl particles and biological aerosols containing Bacillus subtilis or Staphylococcus aureus bacteria.109, 117 Results have shown good comparison across all tracers indicating that tracer gases and inert particles are both suitable surrogates, certainly for small (<2 μm) bioaerosols. An important consideration in real or mock-up studies is the safety of researchers and the public exposed to such tracers, which must be carefully assessed. While some tracer gases (CO2, N2O) have health implications at high concentrations, the ability to conduct experiments with live micro-organisms is generally much more challenging. Although some early studies dispersed bacteria in occupied indoor environments and outdoors as part of biological warfare programmes, modern work is restricted to specialist facilities or studies sampling the background microflora in an environment.102, 103, 104, 119, 120, 121, 122, 123
Since all of these full scale experiments are within a real or mock-up hospital environment, they provide some of the closest tests to reality that can be done. Such set-ups allow the possibility of obtaining measurements in environments that are perturbed by people and activities, rather than the idealised spaces inherent in CFD or physical analogue models.103, 122, 123 However, the techniques are not without their challenges. Many require extensive and costly equipment and realistic measurements can only be made at a limited number of points. They are also time consuming, with the need to reduce the tracer to background levels prior to the next experiment. It is therefore important that this is considered in the experimental design and acknowledged in analysing the results and final conclusions. Carrying out such experimental studies in conjunction with CFD simulations can offer a greater insight, enabling the validation of the modelling approach and the evaluation of aspects of the airflow that cannot be captured by the experiments alone.
Conclusions
Infectious agents in the air, carried by droplet nuclei, skin flakes and fine particles, move sufficiently slowly relative to the air that they are essentially carried by the airflow. Thus, to understand how infectious material spreads through the air it is important to use techniques that enable the air movement to be visualised and quantified in space and time. This rationale is already reflected in the many existing guidelines on general ward infection control, the construction and monitoring of infectious isolation rooms and laboratory biosafety cabinets. These techniques allow the tracking of air movements from potential sources of infection, e.g. human coughs, oxygen masks, door opening/closing. However, such techniques cannot answer the many other relevant questions related to aerosol or airborne infection, such as what proportion of transmission of an infection occurs via the aerosol or airborne route, what proportion of such suspended infectious agents are still viable, and what is the infectious dose to be inhaled to cause disease (not just infection) in any individual for any infectious agent. Yet, effective, accurate airflow visualisation techniques are an essential component to further the understanding of how aerosolised or airborne infection may be transmitted and, equally importantly, how they may be prevented or at least reduced.
Conflict of interest statement
None declared.
Funding source
During the writing of this review article, A. Nicolle, a postdoctoral research fellow, was supported under a grant from the Singapore National Medical Research Council (NMRC/1208/2009) to J.W. Tang. I. Eames is supported by a Senior Leverhulme Fellowship.
References
- 1.Wong T.W., Lee C.K., Tam W. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu I.T.S., Li Y., Wong T.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 3.Dawood F.S., Jain S., Finelli L. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.Smith G.J., Bahl J., Vijaykrishna D. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009;106:11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris J.S., Poon L.L., Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–173. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang J.W., Li Y., Eames I., Chan P.K., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Leung G.M., Tang J.W. Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 8.Booth T.F., Kournikakis B., Bastien N. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blachere F.M., Lindsley W.G., Slaven J.E. Bioaerosol sampling for the detection of aerosolized influenza virus. Influenza Other Respir Viruses. 2007;1:113–120. doi: 10.1111/j.1750-2659.2007.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blachere F.M., Lindsley W.G., Pearce T.A. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 11.Fabian P., McDevitt J.J., DeHaan W.H. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh K.N., Oliver B.G., Stelzer S., Rawlinson W.D., Tovey E.R. A new method for sampling and detection of exhaled respiratory virus aerosols. Clin Infect Dis. 2008;46:93–95. doi: 10.1086/523000. [DOI] [PubMed] [Google Scholar]
- 13.Stelzer-Braid S., Oliver B.G., Blazey A.J. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 14.Cole E.C., Cook C.E. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26:453–464. doi: 10.1016/S0196-6553(98)70046-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrel S.K., Molinari J. Aerosols and splatter in dentistry. J Am Dental Assoc. 2004;135:429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl. 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J.W. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;6(Suppl. 6):S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J.W., Lai F.Y., Wong F., Hon K.L. Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect. 2010;138:226–235. doi: 10.1017/S0950268809990410. [DOI] [PubMed] [Google Scholar]
- 20.Hemmes J.H., Winkler K.C., Kool S.M. Virus survival as a seasonal factor in influenza and poliomyelitis. Nature. 1960;188:430–431. doi: 10.1038/188430a0. [DOI] [PubMed] [Google Scholar]
- 21.Schaffer F.L., Soergel M.E., Straube D.C. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol. 1976;51:263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- 22.Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowen A.C., Steel J., Mubareka S., Palese P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–5652. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 25.Xie X., Li Y., Zhang T., Fang H.H. Bacterial survival in evaporating deposited droplets on a teflon-coated surface. Appl Microbiol Biotechnol. 2006;73:703–712. doi: 10.1007/s00253-006-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie X., Li Y., Chwang A.T., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisiting the Wells evaporation–falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Xie X., Li Y., Sun H., Liu L. Exhaled droplets due to talking and coughing. J R Soc Interface. 2009;6(Suppl. 6):S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan M.P., Chao C.Y. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J Biomech Engng. 2007;129:341–353. doi: 10.1115/1.2720911. [DOI] [PubMed] [Google Scholar]
- 29.Sze To G.N., Wan M.P., Chao C.Y.H. A methodology for estimating airborne virus exposures in indoor environments using the spatial distribution of expiratory aerosols and virus viability characteristics. Indoor Air. 2008;18:425–438. doi: 10.1111/j.1600-0668.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang S., Lee G.W., Chen C.M., Wu C.C., Yu K.P. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med. 2007;20:484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- 31.Franz D.R., Jahrling P.B., Friedlander A.M. Clinical recognition and management of patients exposed to biological warfare agents. J Am Med Assoc. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Li Y, Nielsen PV, Jensen RL, Litewnicki M, Zajas J. An experimental study of human exhalation during breathing and coughing in a mixing ventilated room. In: Bogucz EA, editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. Syracuse, NY, USA: 2009. Paper ID: 162.
- 33.Liu L, Nielsen PV, Li Y, Jensen RL, Litewnicki M, Zajas J. Plume above a standing human body exposed to different air distribution strategies. In: Bogucz EA, editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. Syracuse, NY, USA: 2009. Paper ID: 605.
- 34.Nielsen PV, Jensen RL, Litewnicki M, Zajas J. Experiments on the microenvironment and breathing of a person in isothermal and stratified surroundings. In: Bogucz EA, editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. Syracuse, NY, USA: 2009. Paper ID: 374.
- 35.Tang J.W., Liebner T.J., Craven B.A., Settles G.S. A Schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(Suppl. 6):S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Höppe P. Temperature of expired air under variant climatic conditions. Int J Biometeor. 1981;25:127–128. doi: 10.1007/BF02184460. [DOI] [PubMed] [Google Scholar]
- 37.Marr D., Khan T., Glauser M., Higuchi H., Zhang J. On particle image velocimetry (PIV) measurements in the breathing zone of a thermal breathing mannequin. ASHRAE Trans. 2005;111:299–305. [Google Scholar]
- 38.Marr D.R., Spitzer I.M., Glauser M.N. Anisotropy in the breathing zone of a thermal mannequin. Exp Fluids. 2008;44:661–673. [Google Scholar]
- 39.Murthy R., Pavlidis I. Noncontact measurement of breathing function. IEEE Engng Med Biol Mag. 2006; May/June:57–67. doi: 10.1109/memb.2006.1636352. [DOI] [PubMed] [Google Scholar]
- 40.Gupta J.K., Lin C.H., Chenn Q. Characterizing exhaled airflow from breathing and talking. Indoor Air. 2010;20:31–39. doi: 10.1111/j.1600-0668.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 41.McCool F.D. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl.):48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 42.Gupta J.K., Lin C.H., Chenn Q. Flow dynamics and characterization of a cough. Indoor Air. 2009;19:517–525. doi: 10.1111/j.1600-0668.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 43.Khan T.A., Higuchi H., Marr D.R., Glauser M.N. Unsteady flow measurements of human micro environment using time resolved particle image velocimetry. In: Gameiro da Silva M.C., editor. 9th International conference on air distribution in rooms. 2004. p. 350. Coimbra, Portugal. [Google Scholar]
- 44.Tang J.W., Settles G.S. Coughing and aerosols. New Engl J Med. 2008;359:e19. doi: 10.1056/NEJMicm072576. [DOI] [PubMed] [Google Scholar]
- 45.Tang J.W., Settles G. Coughing and masks. N Engl J Med. 2009;361:e62. doi: 10.1056/NEJMicm0904279. [DOI] [PubMed] [Google Scholar]
- 46.Hui D.S., Ip M., Tang J.W. Airflows around oxygen masks: a potential source of infection? Chest. 2006;130:822–826. doi: 10.1378/chest.130.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui D.S., Hall S.D., Chan M.T. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest. 2006;130:730–740. doi: 10.1378/chest.130.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hui D.S., Hall S.D., Chan M.T. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132:540–546. doi: 10.1378/chest.07-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui D.S., Chow B.K., Chu L.C. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135:648–654. doi: 10.1378/chest.08-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hui D.S., Chow B.K., Ng S.S. Exhaled air dispersion distances during noninvasive ventilation via different respironics face masks. Chest. 2009;136:998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ip M., Tang J.W., Hui D.S. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35:684–689. doi: 10.1016/j.ajic.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCracken J. The consequences of withholding noninvasive ventilation during an epidemic. Respir Care. 2009;54:1412. [PubMed] [Google Scholar]
- 53.McCracken J. Should noninvasive ventilation be considered a high-risk procedure during an epidemic? Can Med Assoc J. 2009;181:663–664. doi: 10.1503/cmaj.081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian H., Li Y., Nielsen P.V., Hyldgaard C.E., Wong T.W., Chwang A.T. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air. 2006;16:111–128. doi: 10.1111/j.1600-0668.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen P.V. Control of airborne infectious diseases in ventilated spaces. J R Soc Interface. 2009;6(Suppl. 6):S747–S755. doi: 10.1098/rsif.2009.0228.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantelic J., Sze-To G.N., Tham K.W., Chao C.Y., Khoo Y.C. Personalized ventilation as a control measure for airborne transmissible disease spread. J R Soc Interface. 2009;6(Suppl. 6):S715–S726. doi: 10.1098/rsif.2009.0311.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brohus H., Nielsen P.V. Personal exposure in displacement ventilated rooms. Indoor Air. 1996;6:157–167. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 58.Hyldgaard C.E. Humans as a source of heat and air pollution. In: Mierzwinski S., editor. 4th international conference on air distribution in rooms. Roomvent 1994. 1994. pp. 413–433. Krakow, Poland. [Google Scholar]
- 59.Bjørn E., Nielsen P.V. Dispersal of exhaled air and personal exposure in displacement ventilated rooms. Indoor Air. 2002;12:147–164. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen P.V., Buus M., Winter F.V., Thilageswaran M. Contaminant flow in the microenvironment between people under different ventilation conditions. ASHRAE Trans. 2008;114:632–638. [Google Scholar]
- 61.Nielsen PV, Li Y, Buus M, Winther FV, Qian H. Cross infection in a hospital ward and deposition of particles exhaled from a source mannequin. In: 5th international workshop on energy and environment of residential buildings and 3rd international conference on build environment and public health, proceedings. Guilin, China: 2009. 162–169.
- 62.Qian H., Nielsen P.V., Li Y., Hyldgaard C.E. Airflow and contaminant distribution in hospital wards with a displacement ventilation system. In: Hou X.S., Yand J.L., Tian L.W., Zheng C., editors. Built environment and public health – Proceedings of BEPH 2004. China Environmental Science Press; Beijing: 2004. pp. 355–364. [Google Scholar]
- 63.Qian H., Li Y., Nielsen P., Huang X. Spatial distribution of infection risk of SARS transmission in a hospital ward. Build Environ. 2009;44:1651–1658. [Google Scholar]
- 64.Nielsen PV, Olmedo I, Ruiz de Adana M, Grzelecki P, Jensen RL. Airborne cross-infection risk between two people in a displacement ventilated room. ASHRAE IAQ (in press).
- 65.Jacobs P., de Gids W.F. The aircraft seat as indoor air quality and temperature control system. In: Yang X.D., Zhao B., Zhao R., editors. Proceedings of indoor air 2005. Tsinghua University Press; Beijing: 2005. Paper ID: I(1)–426. [Google Scholar]
- 66.Melikov A. Personalized ventilation. Indoor Air. 2004;14(Suppl. 7):157–167. doi: 10.1111/j.1600-0668.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen P.V., Hyldgaard C.E., Melikov A., Andersen H., Soennichsen M. Personal exposure between people in a room ventilated by textile terminals – with and without personalized ventilation. HVAC&R Res. 2007;13:635–643. [Google Scholar]
- 68.Nielsen PV, Bartholomaeussen NM, Jakubowska E, et al. Chair with integrated personalized ventilation for minimizing cross infection. In: Proceedings of the 10th international conference on air distribution in rooms. Roomvent 2007. Helsinki, Finland: 2007. Paper ID: 1078.
- 69.Nielsen P.V., Barszcz E., Czarnota T. The influence of draught on a seat with integrated personalized ventilation. In: Strøm-Tejsen P., Olesen B.W., Wargocki P., Zukowska D., Toftum J., editors. Indoor air 2008: proceedings of the 11th international conference on indoor air quality and climate. 2008. Copenhagen, Denmark. Paper ID: 247. [Google Scholar]
- 70.Khalifa H.E., Janos M.I., Dannenhoffer J.F. Energy-neutral personal ventilation. In: Strøm-Tejsen P., Olesen B.W., Wargocki P., Zukowska D., Toftum J., editors. Indoor air 2008: proceedings of the 11th international conference on indoor air quality and climate. 2008. Copenhagen, Denmark. Paper ID: 159. [Google Scholar]
- 71.Halvonova B., Melikov A.K. Performance of “ductless” personalized ventilation in conjunction with displacement ventilation: impact of walking occupant. In: Bogucz E.A., editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. 2009. Syracuse, NY, USA. Paper ID: 520. [Google Scholar]
- 72.Dygert R.K., Dang T.Q. Mitigation of cross-contamination in an aircraft cabin via localized suction removal. In: Kim K.W., Yoon D.W., Yeo M.S., Moon H.J., Park C.S., editors. Proceedings of the 11th international conference on air distribution in rooms. Roomvent 2009. 2009. pp. 1675–1682. Busan, South Korea. [Google Scholar]
- 73.Nielsen P.V., Jiang H., Polak M. Proceedings of the 10th International conference on air distribution in rooms. Roomvent 2007. 2007. Bed with integrated personalized ventilation for minimizing cross infection. Helsinki, Finland. Paper ID: 1077. [Google Scholar]
- 74.Nielsen P.V., Polak M., Jiang H., Li Y., Qian H. Protection against cross infection in hospital beds with integrated personalized ventilation. In: Strøm-Tejsen P., Olesen B.W., Wargocki P., Zukowska D., Toftum J., editors. Indoor air 2008: proceedings of the 11th international conference on indoor air quality and climate. 2008. Copenhagen, Denmark. Paper ID: 244. [Google Scholar]
- 75.Settles G.S. Visualizing full-scale ventilation airflows. ASHRAE J. 1997;39:19–26. [Google Scholar]
- 76.Settles G.S. Springer; Berlin: 2001. Schlieren and shadowgraph techniques. Visualising phenomena in transparent media. [Google Scholar]
- 77.Settles G.S. Fluid mechanics and homeland security. Annu Rev Fluid Mech. 2006;38:87–110. [Google Scholar]
- 78.Craven B.A., Settles G.S. A computational and experimental investigation of the human thermal plume. J Fluids Engng. 2006;128:1251–1258. [Google Scholar]
- 79.Lewis H.E., Foster A.R., Mullan B.J., Cox R.N., Clark R.P. Aerodynamics of the human microenvironment. Lancet. 1969;293:1273–1277. doi: 10.1016/s0140-6736(69)92220-x. [DOI] [PubMed] [Google Scholar]
- 80.Clark R.P., Edholm O.G. Arnold; London: 1985. Man and his thermal environment. [Google Scholar]
- 81.Clark R.P., de Calcina-Goff M.L. Some aspects of the airborne transmission of infection. J R Soc Interface. 2009;6(Suppl. 6):S767–S782. doi: 10.1098/rsif.2009.0236.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whyte W., Shaw B.H. The effect of obstructions and thermals in laminar flow systems. J Hyg. 1974;72:415–423. doi: 10.1017/s0022172400023652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonassen D.R., Settles G.S., Tronosky M.D. Schlieren “PIV” for turbulent flows. Optics Lasers Engng. 2006;44:190–207. [Google Scholar]
- 84.Tang J.W., Eames I., Li Y. Door-opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: the importance of vorticity and buoyancy airflows. J Hosp Infect. 2005;61:283–286. doi: 10.1016/j.jhin.2005.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;152:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 86.Noakes C.J., Sleigh P.A., Escombe A.R., Beggs C.B. Use of CFD analysis in modifying a TB ward in Lima, Peru. Indoor Built Environ. 2006;151:41–47. [Google Scholar]
- 87.Brohus H., Balling K.D., Jeppesen D. Influence of movements on contaminant transport in operating room. Indoor Air. 2006;16:356–372. doi: 10.1111/j.1600-0668.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 88.Nielsen P.V. Computational fluid dynamics and room air movement. Indoor Air. 2004;14:134–143. doi: 10.1111/j.1600-0668.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 89.Gao N., Nui J. CFD study on micro-environment around human body and personalised ventilation. Build Environ. 2004;39:795–805. [Google Scholar]
- 90.Gao N., Niu J. Transient CFD simulation of the respiration process and inter-person exposure assessment. Build Environ. 2006;41:1214–1222. doi: 10.1016/j.buildenv.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hathway E.A., Noakes C.J., Sleigh P.A. CFD modelling of a hospital ward: assessing risk from bacteria produced from respiratory and activity sources. In: Strøm-Tejsen P., Olesen B.W., Wargocki P., Zukowska D., Toftum J., editors. Indoor air 2008: proceedings of the 11th international conference on indoor air quality and climate. 2008. Copenhagen, Denmark. Paper ID: 45. [Google Scholar]
- 92.Zhang Z., Chen Q. Experimental measurements and numerical simulations of particle transport and distribution in ventilated rooms. Atmos Environ. 2006;40:3396–3408. [Google Scholar]
- 93.Zhao B., Yang C., Yang X., Liu S. Particle dispersion and deposition in ventilated rooms: testing and evaluation of different Eulerian and Lagrangian models. Build Environ. 2008;43:388–397. [Google Scholar]
- 94.Lu W., Howarth A.T. Numerical analysis of indoor aerosol particle deposition and distribution in two-zone ventilation system. Build Environ. 1996;31:41–50. [Google Scholar]
- 95.Noakes C.J., Fletcher L.A., Beggs C.B., Sleigh P.A., Kerr K.G. Development of a numerical model to simulate the biological inactivation of airborne microorganisms in the presence of ultraviolet light. J Aerosol Sci. 2004;354:489–507. [Google Scholar]
- 96.Noakes C.J., Beggs C.B., Sleigh P.A. Modelling the performance of upper room ultraviolet germicidal irradiation devices in ventilated rooms: comparison of analytical and CFD methods. Indoor Built Environ. 2004;136:477–488. [Google Scholar]
- 97.Wan M.P., Chao C.Y.H., Ng Y.D., Sze To G.N., Yu W.C. Dispersion of expiratory droplets in a general hospital ward with ceiling mixing type mechanical ventilation system. Aerosol Sci Tech. 2007;413:244–258. [Google Scholar]
- 98.Shih Y.-C., Chiu C.-C., Wang O. Dynamic airflow simulation within an isolation room. Build Environ. 2007;429:3194–3209. doi: 10.1016/j.buildenv.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chow T.T., Yang X.Y. Performance of ventilation system in a non-standard operating room. Build Environ. 2003;38:1401–1411. [Google Scholar]
- 100.van Treeck C., Pfaffinger M., Wenisch P., Frisch J., Yue Z., Egger M., Rank E. Towards computational steering of thermal comfort assessment. In: Strøm-Tejsen P., Olesen B.W., Wargocki P., Zukowska D., Toftum J., editors. Indoor air 2008: proceedings of the 11th international conference on indoor air quality and climate. 2008. Copenhagen, Denmark. Paper ID: 762. [Google Scholar]
- 101.Eames I., Shoaib D., Klettner C., Taban V. Movement of airborne contaminants in an isolation room. J R Soc Interface. 2009;6:757–766. doi: 10.1098/rsif.2009.0319.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lidwell O.M., Towers A.G. Protection from microbial contamination in a room ventilated by a uni-directional air flow. J Hyg. 1969;67 doi: 10.1017/s0022172400041474. p. 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andersson P.A., Hambraeus A., Zettersten U., Ljungqvist B., Neikter K., Ransjö U. A comparison between tracer gas and tracer particle techniques in evaluating the efficiency of ventilation in operating theatres. J Hyg. 1983;91:509–519. doi: 10.1017/s0022172400060551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whyte W., Shaw B.H., Freeman M.A.R. An evaluation of a partial-walled laminar-flow operating room. J Hyg. 1974;73:61–76. doi: 10.1017/s0022172400023858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Department of Health, UK . Stationery Office; London: 2007. Health Technical Memorandum HTM 03-01: Specialised ventilation for healthcare premises, Part A: design and validation. [Google Scholar]
- 106.Chao C.Y.H., Wan M.P. A study of the dispersion of expiratory aerosols in unidirectional downward and ceiling-return type airflows using a multiphase approach. Indoor Air. 2006;16:296–312. doi: 10.1111/j.1600-0668.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 107.Lidwell O.M. The evaluation of ventilation. J Hyg. 1960;58:297–305. doi: 10.1017/s0022172400038419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Escombe A.R., Oeser C.C., Gilman R.H. Natural ventilation for the prevention of airborne contagion. PLoS Medicine. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Booth W., Beato B., Noakes C., Fletcher L., Sleigh A., Tomlinson N. Characterisation of the protection provided by the ventilation strategy in hospital isolation rooms. In: Bogucz E.A., editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. 2009. Syracuse NY, USA. Paper ID: 685. [Google Scholar]
- 110.Rydock J.P., Eian P.K. Containment testing of isolation rooms. J Hosp Infect. 2004;57:228–232. doi: 10.1016/j.jhin.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 111.Lidwell O.M., Williams R.E.O. The ventilation of operating theatres. J Hyg. 1960;58:449. doi: 10.1017/s0022172400038584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Foord N., Lidwell O.M. The control by ventilation of bacterial transfer between hospital patients and its assessment by means of a particle tracer. I. An airborne particle tracer for cross infection studies. J Hyg. 1972;70:279–287. doi: 10.1017/s0022172400022336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lidwell O.M. The control by ventilation of bacterial transfer between hospital patients and its assessment by means of a particle tracer. II. Ventilation in subdivided isolation units. J Hyg. 1972;70:279–287. doi: 10.1017/s0022172400022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hambraeus A., Sanderson H.F. The control by ventilation of bacterial transfer between hospital patients and its assessment by means of a particle tracer. III. Studies with an airborne-particle tracer in an isolation ward for burned patients. J Hyg. 1972;70:299–312. doi: 10.1017/s002217240002235x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldberg L.J. Application of the microaerofluorometer to the study of dispersion of a fluorescent aerosol into a selected atmosphere. J Appl Meteorol. 1968;7:68–72. [Google Scholar]
- 116.Grinshpun S.A., Adhikari A., Honda T. Control of aerosol contaminants in indoor air: combining the particle concentration reduction with microbial inactivation. Environ Sci Tech. 2007;41:606–612. doi: 10.1021/es061373o. [DOI] [PubMed] [Google Scholar]
- 117.Noakes C.J., Fletcher L.A., Sleigh P.A., Booth W.B., Beato-Arribas B., Tomlinson N. Comparison of tracer techniques for evaluating the behaviour of bioaerosols in hospital isolation rooms. In: Bogucz E.A., editor. Proceedings of the healthy buildings 2009, 9th international conference and exhibition. 2009. Syracuse, NY, USA. Paper ID: 504. [Google Scholar]
- 118.Johnson D.L., Lynch R.A. An efficient analytical method for particle counting in evaluating airborne infectious isolation containment using fluorescent microspheres. J. Occup Environ Hyg. 2008;5:271–277. doi: 10.1080/15459620801935056. [DOI] [PubMed] [Google Scholar]
- 119.Riley R.L., Permutt S., Kaufman J.E. Convection, air mixing and ultraviolet air disinfection in rooms. Arch Environ Health. 1971;22:200–207. doi: 10.1080/00039896.1971.10665833. [DOI] [PubMed] [Google Scholar]
- 120.Christopher G.W., Cieslak T.J., Pavlin J.A., Eitzen E.M. Biological warfare, a historical perspective. J Am Med Assoc. 1997;278:412–417. [PubMed] [Google Scholar]
- 121.Clark R.P., Reed P.J., Seal D.V., Stephenson M.L. Ventilation conditions and air-borne bacteria and particles in operating theatres: proposed safe economies. J Hyg. 1985;95:325–335. doi: 10.1017/s0022172400062744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts K., Hathway A., Fletcher L.A., Beggs C.B., Elliott M.W., Sleigh P.A. Bioaerosol production on a respiratory ward. Indoor Built Environ. 2006;15:35–40. [Google Scholar]
- 123.Roberts K., Smith C.F., Snelling A.M. Aerial dissemination of Clostridium difficile spores. BMC Infect Dis. 2008;8:7. doi: 10.1186/1471-2334-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]