Abstract
Background/Aims
Angiotensin-converting enzyme 2 (ACE2), its product, angiotensin-(1–7) and its receptor, Mas, may moderate the adverse effects of angiotensin II in liver disease. We examined the expression of these novel components of the renin angiotensin system (RAS) and the production and vasoactive effects of angiotensin-(1–7) in the bile duct ligated (BDL) rat.
Methods
BDL or sham-operated rats were sacrificed at 1, 2, 3 and 4 weeks. Tissue and blood were collected for gene expression, enzyme activity and peptide measurements. In situ perfused livers were used to assess angiotensin peptide production and their effects on portal resistance.
Results
Hepatic ACE2 gene and activity (P < 0.0005), plasma angiotensin-(1–7) (P < 0.0005) and Mas receptor expression (P < 0.01) were increased following BDL compared to shams. Perfusion experiments confirmed that BDL livers produced increased angiotensin-(1–7) (P < 0.05) from angiotensin II and this was augmented (P < 0.01) by ACE inhibition. Whilst angiotensin II increased vasoconstriction in cirrhotic livers, angiotensin-(1–7) had no effect on portal resistance.
Conclusions
RAS activation in chronic liver injury is associated with upregulation of ACE2, Mas and hepatic conversion of angiotensin II to angiotensin-(1–7) leading to increased circulating angiotensin-(1–7). These results support the presence of an ACE2-angiotensin-(1–7)-Mas axis in liver injury which may counteract the effects of angiotensin II.
Keywords: Liver fibrosis, ACE2, ACE, Angiotensin-(1–7), Angiotensin II, Mas receptor, Portal resistance
1. Introduction
The renin angiotensin system (RAS) and its effector molecule angiotensin II are now recognised to play a major role in tissue injury and fibrosis in a number of diseases including diabetes, vascular disease and chronic renal disease [1], [2], [3], [4]. More recently a possible role for the RAS in the pathogenesis of chronic liver diseases has been suggested by the finding that there is marked upregulation of intrahepatic RAS components in experimental liver injury [5], [6], [7], and that blockade of the RAS improves experimental hepatic fibrosis [8], [9], [10], [11].
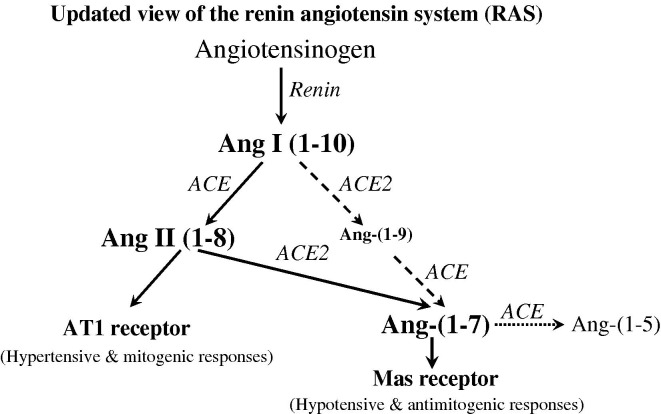
The identification of angiotensin converting enzyme 2 (ACE2), a homologue of ACE [12], [13], has highlighted that the RAS is more complex than previously thought (Fig. 1 ). ACE2 catalyses the conversion of angiotensin (Ang) II to Ang-(1–7) [14] and to a lesser extent Ang I to Ang-(1–9) [15], [16] which is converted to Ang-(1–7) by ACE [17]. Ang-(1–7) has a number of important actions that oppose those of Ang II including vasodilatation [18], [19] and inhibition of cell proliferation and tissue fibrosis [20], [21], [22], [23], [24], an effect that appears to be mediated through the Ang-(1–7) receptor Mas [21], [25], [26]. Ang-(1–7) is converted to the inactive peptide fragment Ang-(1–5) by the action of ACE, and use of ACE inhibitors has been shown to increase Ang-(1–7) levels in tissue and plasma [27], [28].
Fig. 1.
Schematic representation of the role of ACE2 in the renin angiotensin system (RAS) showing the predominant pathway in solid lines for the generation of hypotensive and anti-fibrotic peptide angiotensin-(1–7) (Ang-(1–7)) from the substrate Ang I via Ang II, catalysed by angiotensin converting enzyme (ACE) and ACE2, and its degradation by ACE into inactive peptide Ang-(1–5). AT1 receptor, Ang II type 1 receptor.
The role of ACE2 and its peptide products in liver disease is unknown. We have recently reported that hepatic ACE2 expression is increased in cirrhotic human livers and in a rat model of established hepatic fibrosis [6], suggesting that this enzyme could modulate hepatic and systemic angiotensin peptide production. The magnitude and significance of these possible effects are likely to depend on the relative levels of ACE2 and ACE expression and activity in different tissues [29], [30]. Although circulating Ang II levels are elevated in advanced liver disease [7], the expression of the ACE2 and ACE in the liver and other tissues during the development of liver disease and their effects on Ang II and Ang-(1–7) levels have not previously been studied.
We therefore used the bile duct ligation (BDL) model to examine the effects of chronic liver injury on the expression of these key mediators of the RAS. A time course study was performed to examine the sequential changes that occur in the liver, the circulation and other major sites of RAS expression and activity. In situ perfused rat liver experiments were also performed to determine whether the fibrotic liver has increased capacity to produce Ang-(1–7) from Ang II, and whether this could be altered by modifying the degradation of Ang-(1–7) with the ACE inhibitor, lisinopril. The effects of Ang-(1–7) and Ang II on portal resistance were also studied.
2. Materials and methods
2.1. Animal model of fibrosis
Experimental procedures were approved by the Animal Welfare and Ethics Committee of Austin Health and performed according to the NHMRC of Australia Guidelines for animal experimentation, and the principles of the Helsinki declaration. Eight-week-old male Sprague–Dawley rats (300–350 g) were housed (12 h light/dark) and fed standard rat chow (Norco, Lismore NSW, Australia) and water ad libitum. After 1 week acclimatization, BDL or sham operation was performed in rats as previously described [6]. Anaesthesia was achieved by intraperitoneal administration of pentobarbital (6 mg/100 g body weight, Boehringer Ingelheim, Artarmon, NSW, Australia).
2.2. Experimental protocol
2.2.1. Time course study
After surgery, BDL (n = 40) and sham (n = 40) rats were sacrificed at weeks 1, 2, 3 and 4 (n = 10 each time point) under lethal anaesthesia induced by intraperitoneal injection of pentobarbital (Boehringer Ingelheim, Australia). Cardiac blood was taken into tubes containing either heparin or an endopeptidase inhibitor mix (see below). Liver tissue samples were snap frozen in liquid nitrogen and stored at −80 °C and used for gene expression, autoradiography and hydroxyproline quantification. Liver tissue was fixed in 4% paraformaldehyde and paraffin embedded for histological assessment. In addition, tissue samples from the kidney, lung and small intestine at week 4 were taken for ACE and ACE2 activity assays.
2.2.2. In situ perfused rat liver preparation
A further group of rats underwent in situ perfused liver studies 4 weeks after BDL (n = 5) and age-matched healthy rats served as controls (n = 5) using standard techniques. Livers were perfused with oxygenated Krebs–Henseleit solution with 1% bovine serum albumin (BSA) and 0.1% dextrose. Portal flow was kept constant at 30 mL per minute in a non-recirculation system. Ang II was administered as a 60 pmol bolus injection and samples of effluent were collected continuously into separate tubes every 15 s from the subdiaphragmatic IVC. Two control samples were taken prior to Ang II injection. The experiment was repeated in an identical fashion following a 15-min pre-incubation with the ACE inhibitor lisinopril (10−6 mol/L). Samples of effluent were analysed for Ang-(1–7) using radioimmunoassay.
To investigate intrahepatic vasoactive responses to Ang-(1–7), in situ perfused livers from BDL rats 4 weeks after surgery (n = 4) and age-matched control rats (n = 6) were given varying bolus doses of Ang-(1–7). The procedure was repeated in another group of control livers (n = 5) after pre-constriction with phenylephrine. The changes in portal pressure were measured using an open vertical column attached to the portal vein. Vasoconstriction responses to Ang II were also compared in control (n = 5) and 4 weeks post BDL livers (n = 6).
Viability of all the preparations was determined by macroscopic and histological appearance of the liver, together with oxygen consumption.
2.3. Sampling protocol
2.3.1. Blood processing
One set of blood samples was collected into tubes containing heparin (15 IU/mL blood) for measurement of liver enzyme profiles and ACE and ACE2 activity. The second set of blood samples and the effluent samples from in situ perfused livers was collected into 20 μL/mL blood or effluent of an endopeptidase inhibitor mix (50 mmol Na2EDTA, 0.2 mol N-ethylmaleimide and 1–2 TIU/mL aprotinin) for measurement of angiotensin peptide levels.
2.3.2. Biochemical and histological assessment of liver injury and fibrosis
Plasma bilirubin, alanine aminotransaminase (ALT), alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) were measured by autoanalyser (Beckman Instruments, Fullerton, CA, US). Collagen content of the liver was determined by measurement of liver hydroxyproline by a colorimetric assay as previously described [31], [32].
2.3.3. Quantitative real time polymerase chain reaction (QPCR) analyses
All QPCRs were carried out using multiplexing where both the target gene and endogenous reference gene were amplified in a single well. The details of dual-fluorescent labelled oligonucleotide probes and primers are given in Table 1 . The probes and primers were designed using the Primer Express software program (PE Applied Biosystems, CA, USA). Pre-developed Taqman 18S ribosomal RNA kit was used as endogenous reference gene (PE Biosystems). Each sample was run and analysed in duplicate. The normalized values from sham tissues were used as the calibrator with a given value of 1 and the BDL groups compared with this calibrator.
Table 1.
Primer and probe sequences used for real time QPCR analysis
| Gene name | Probe/primer | Sequence |
|---|---|---|
| ACE2 | Probe | 5′-TTGTCTGCCACCCCACA-3′ |
| Forward | 5′-GCCAGGAGATGACCGGAAA-3′ | |
| Reverse | 5′-CTGAAGTCTCCATGTCCCAGATC-3′ | |
| Mas | Probe | 5′-CGGGATCCTCCTCTGG-3′ |
| Forward | 5′-CATCTCTCCTCTCGGCTTTGTG-3′ | |
| Reverse | 5′-CCTCATCCGGAAGCAAAGG-3′ | |
| ACE | Probe | 5′-CAACAAGACTGCCACCTGCTGGTCC-3′ |
| Forward | 5′-CACCGGCAAGGTCTGCTT-3′ | |
| Reverse | 5′-CTTGGCATAGTTTCGTGAGGAA-3′ | |
| AT1 | Probe | 5′-CTCATCGGCCAAAAAGCCTGCGT-3′ |
| Forward | 5′-CGGCCTTCGGATAACATGA-3′ | |
| Reverse | 5′-CCTGTCACTCCACCTCAAAACA-3′ |
2.3.4. In vitro ACE autoradiography
Frozen liver and kidney tissue sections (20 μm) at all four time points were cut on a cryostat (Microm, Germany) at −20 °C and thaw mounted onto slides. The specific radioligand 125I-MK351A was used for ACE autoradiography [33] as previously described [5]. Quantitation of binding density was determined by computerised densitometry analysis.
2.3.5. Tissue and plasma ACE2 and ACE activity assays
The activity of ACE2 in plasma and cell membrane preparations was determined using the quenched fluorescent substrate (7-methoxycoumarin-4-yl)acetyl-Ala-Pro-Lys(2,4-dinitrophenyl)-OH, (Mca-APK-(Dnp)-OH), as described previously [6], [15]. Fluorescence (λ = 320 nm, λ em = 405 nm) was measured using a Fluorstar Optima plate reader (BMG Technologies). The specific activity of ACE2 was determined using ACE2 Millennium inhibitor MLN4760 (gift from Dr. Natalie Dales, Millenium Pharmaceuticals, Cambridge, MA, USA) [34]. ACE substrate Hippuryl-His-Leu was used to determine ACE specific activity using a modification of a standard ACE assay [35].
2.3.6. Radioimmunoassay for angiotensin peptides
Plasma concentrations of Ang II and Ang-(1–7) were measured by radioimmunoassay. The antibodies for Ang II and Ang-(1–7) were raised in rabbit and guinea pig, respectively, against the natural peptide sequences of human conjugated to bovine thyroglobulin. The intra- and inter-assay coefficients of variation were 7.6% and 8.3%, and 4.5% and 10% for Ang II and Ang-(1–7), respectively.
2.4. Statistics
Means between groups were compared using ANOVA. Pearson correlation coefficient was used between the individual variables. When considerable variation was observed the data were log transformed to stabilize the variation before they were used in the analyses. Data are presented as the least-square mean ± SEM. ANOVA with repeated measures was performed on data derived from liver perfusion experiments, and the baseline-corrected mean values and baseline-corrected area under the Ang-(1–7) curves were determined. A P value of less than 0.05 was considered statistically significant. All statistical analyses were carried out using the SAS computer package (SAS, Statistics, Version 6.11, Cary, NC, USA).
3. Results
3.1. Hepatic fibrosis in the BDL model
Histological analysis showed either extensive fibrosis (weeks 1 and 2) or severe fibrosis (weeks 3 and 4) in the liver parenchyma following BDL. Plasma GGT, ALT, ALP and bilirubin levels were significantly elevated in BDL rats compared with the sham group at all four time points (P < 0.0005 to P < 0.05) (data not shown). BDL livers showed progressive increases in hydroxyproline content from week 1 to week 4 after surgery compared with sham rats with levels reaching 4 times higher by week 4 (P < 0.01) (data not shown).
3.2. Tissue ACE2 and ACE gene expression and activity
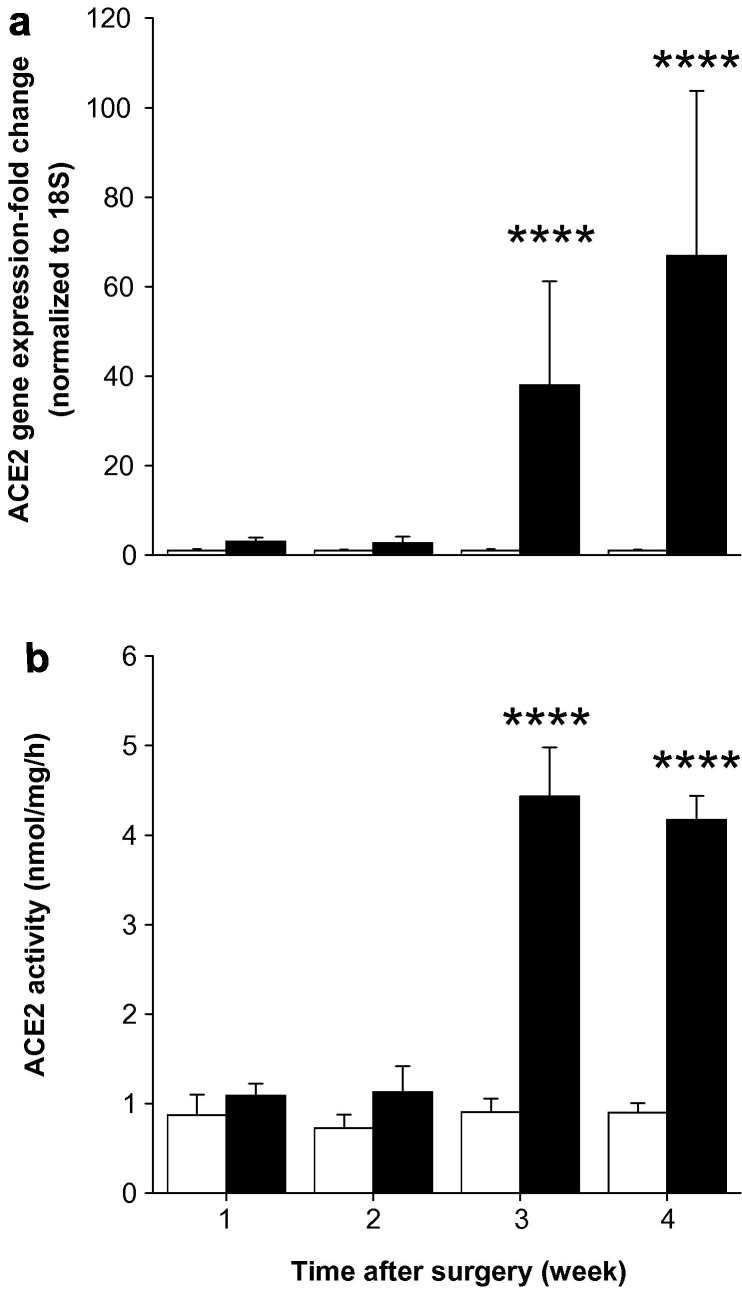
Bile duct ligation resulted in significant changes in hepatic ACE2 gene expression by week 3 with a 38-fold increase (P < 0.0005) compared with sham rats (Fig. 2 a). ACE2 expression increased further at week 4 to a level 67-fold higher (P < 0.0005) in BDL compared with sham rats. ACE2 activity followed a similar pattern to that of gene expression with higher activity (P < 0.0005) in BDL than shams (Fig. 2b).
Fig. 2.
Hepatic angiotensin converting enzyme 2 (ACE2) gene expression (a) and ACE2 activity (b) in sham-operated control (open bars) and bile duct ligated (BDL) (filled bars) rats. QPCR derived ACE2 gene expression values were normalized to ribosomal 18S and the shams were given a value of 1 at each time point. ACE2 activity was determined by measuring nmol of ACE2 substrate (Mca-APK-(Dnp)-OH) cleaved by solubilized membrane fractions. Each bar represents the mean ± SEM expression and activity from 8 to 10 rats (a) and from 7 to 8 rats (b), respectively, per treatment group. ∗∗∗∗P < 0.0005, BDL vs. sham.
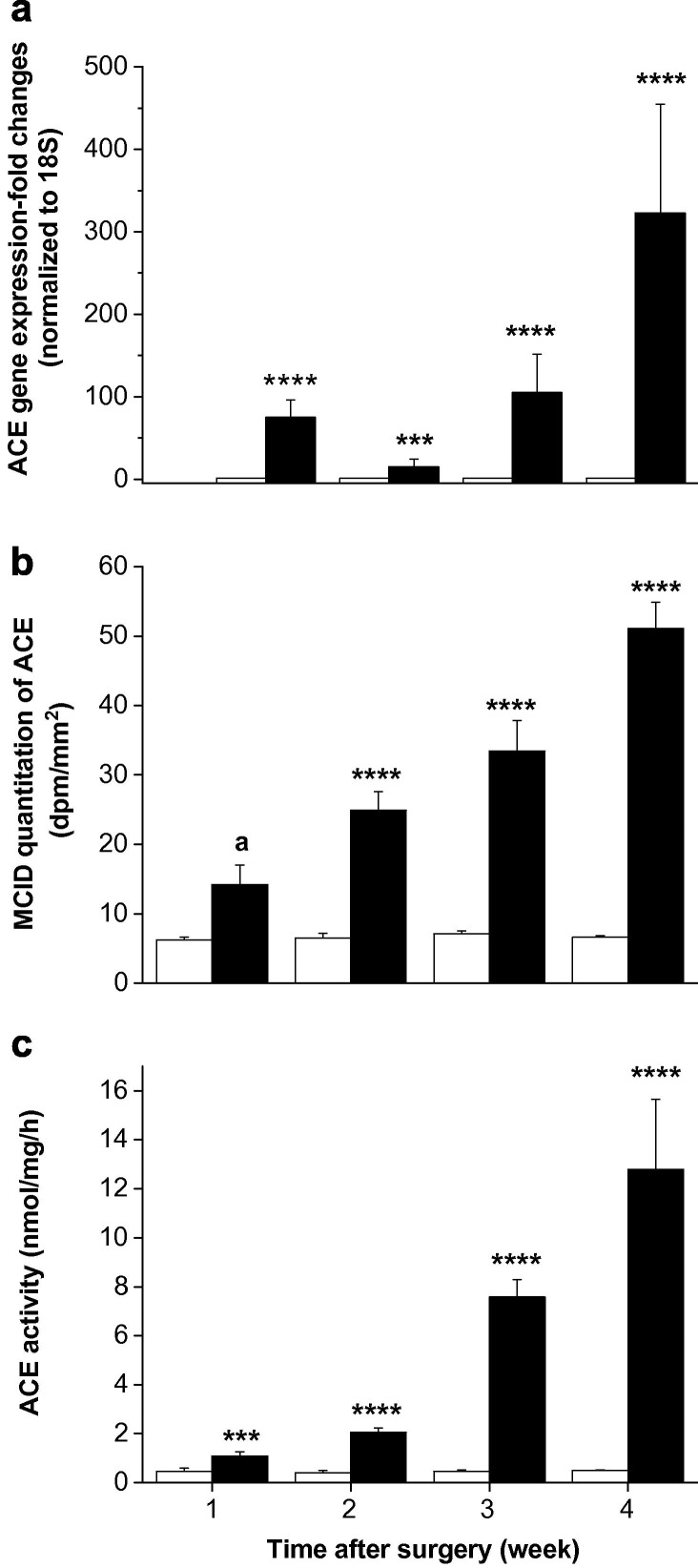
Hepatic ACE gene expression was 75-fold increased one week after BDL (P < 0.0005) and by week 4 it was increased 323-fold compared with shams (P < 0.0005) (Fig. 3 a). In vitro ACE autoradiography showed weak ACE labelling in sham livers while livers from BDL rats showed time-dependent (r = 0.89; P < 0.0005) increase in intensity and distribution of labelling, increasing progressively from week 1 to week 4 (P < 0.001) (Fig. 3b). Hepatic ACE activity mirrored the changes observed in gene expression (Fig. 3c).
Fig. 3.
Hepatic angiotensin converting enzyme (ACE) gene expression (a), ACE-specific radioligand 125I-MK351A binding (b) and ACE activity (c) in sham-operated control (open bars) and bile duct ligated (BDL) (filled bars) rats. QPCR derived ACE gene expression values were normalized to ribosomal 18S and the shams were given a value of 1 at each time point. ACE binding density was determined by computerised densitometry. ACE activity was determined by measuring nmol of ACE substrate (Hippuryl-His-Leu) cleaved by solubilized membrane fractions. Each bar represents the mean ± SEM expression, staining and activity from 8 to 10 rats (a), from 4 to 5 rats (b) and from 7 to 8 rats (c), respectively, per treatment group. ∗∗∗∗P < 0.0005, ∗∗∗P < 0.001, aP < 0.06, BDL vs. sham.
In contrast to the findings in the liver, there were no changes in ACE2 or ACE activity in lung and intestine, and ACE2 activity in the kidney of week 4 BDL animals compared to shams (data not shown). Similarly, ACE autoradiography results showed no difference between BDL and sham kidneys from week 1 to week 4 after surgery.
3.3. Hepatic Mas and angiotensin II type 1 (AT1) receptor expression
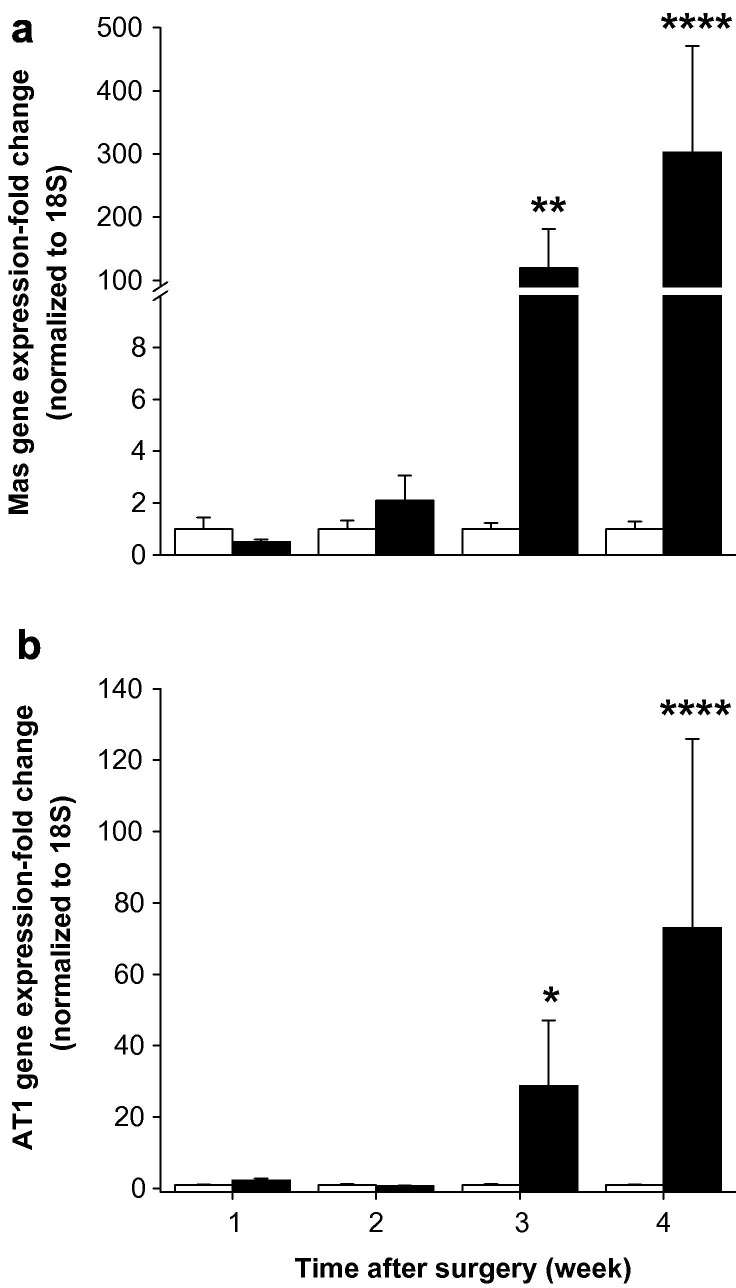
BDL did not cause changes in Mas gene expression during the first two weeks. At week 3 Mas expression increased abruptly by 120-fold (P < 0.01) and at week 4 it was 300-fold greater in BDL compared with sham rats (P < 0.0005) (Fig. 4 a). There was a close positive correlation between hepatic Mas gene and ACE2 gene expression (r = 0.98, P < 0.0005).
Fig. 4.
Hepatic angiotensin-(1–7) receptor Mas (a) and angiotensin II type I receptor, AT1 (b) gene expressions in sham-operated control (open bars) and bile duct ligated (BDL) (filled bars) rats. QPCR derived gene expression values were normalized to ribosomal 18S and the shams were given a value of 1 at each time point. Each bar represents the mean ± SEM expression from 9 to 10 rats (a) or from 8 to 10 rats (b) per treatment group. ∗∗∗∗P < 0.0005, ∗∗P < 0.01, ∗P < 0.05, BDL vs. sham.
BDL rats showed a significant increase in AT1 expression only at week 3 (P < 0.05) and week 4 (P < 0.0005) compared to sham rats (Fig. 4b).
3.4. Plasma ACE and ACE2 activity
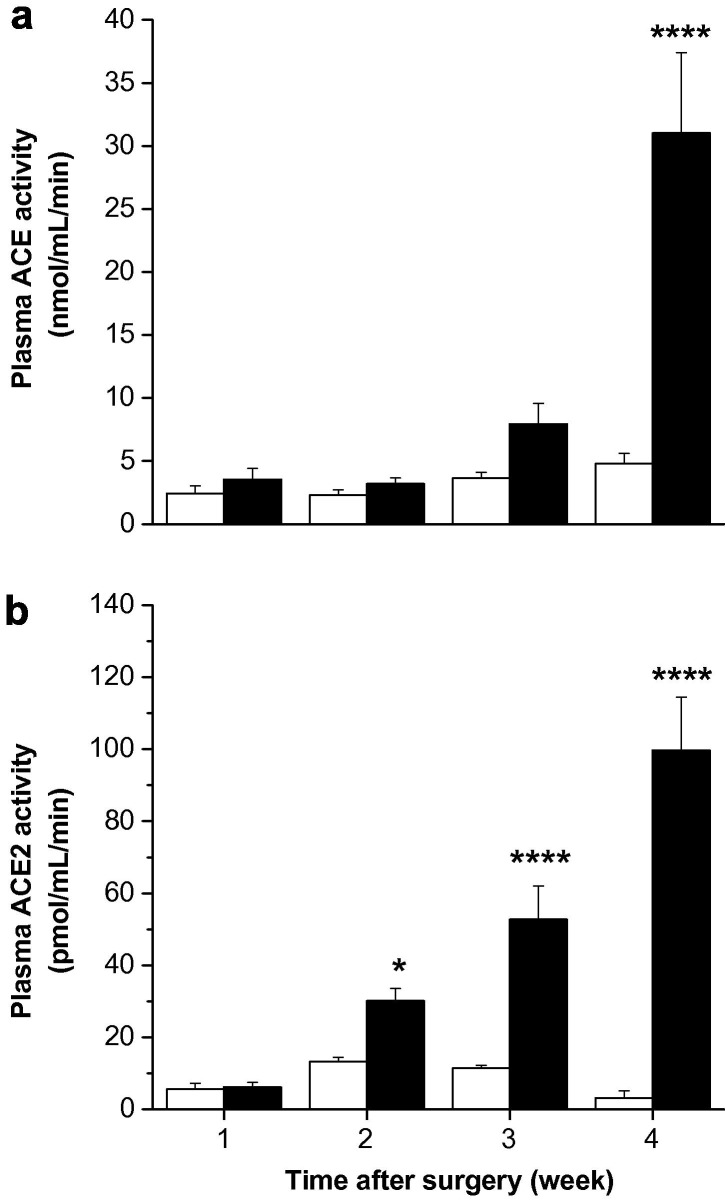
Plasma ACE activity was significantly increased by week 4 in BDL animals but did not change throughout the course of the experiment in sham animals (P < 0.0005 vs. sham) (Fig. 5 a). Plasma ACE2 activity was low in all sham groups and did not change over the experimental period. In parallel with the hepatic expression data, in BDL rats there was a major rise in plasma ACE2 activity with progression of liver injury (Fig. 5b), and there was a strong positive correlation between plasma ACE2 and liver ACE2 activity (r = 0.61, P < 0.0005).
Fig. 5.
Plasma angiotensin converting enzyme (ACE) activity (a) and ACE2 activity (b) in sham-operated control (open bars) and bile duct ligated (BDL) (filled bars) rats. ACE and ACE2 activity were determined by measuring nmol of ACE substrate (Hippuryl-His-Leu) and pmol of ACE2 substrate (Mca-APK-(Dnp)-OH), respectively, cleaved by solubilized membrane fractions. Each bar represents the mean ± SEM activity from 9 to 10 rats per treatment group. ∗∗∗∗P < 0.0005, ∗P < 0.05, BDL vs. sham.
3.5. Plasma angiotensin-(1–7) and angiotensin II levels
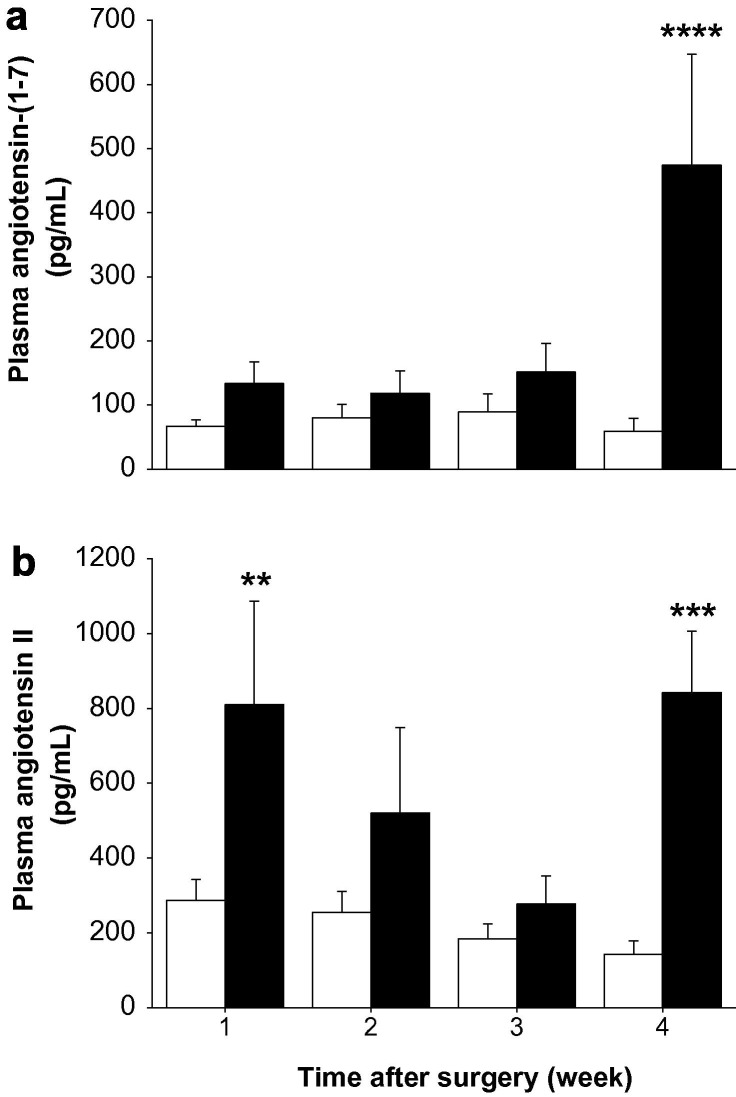
There was a non-significant trend for plasma Ang-(1–7) levels to be higher in the BDL groups than in the sham groups from week 1 through week 3. However, at week 4 in BDL rats there was a major increase (P < 0.001) in plasma Ang-(1–7), and at this time the levels in BDL rats were significantly (P < 0.0005) higher than in shams (Fig. 6 a).
Fig. 6.
Plasma concentrations of angiotensin-(1–7) (a) and angiotensin II (Ang II) (b) in sham-operated control (open bars) and bile duct ligated (BDL) (filled bars) rats. Angiotensin peptide levels were determined by radioimmunoassay. Each bar represents the mean ± SEM concentration from 9 to 10 rats per treatment group. ∗∗∗∗P < 0.0005, ∗∗∗P < 0.001, ∗∗P < 0.01, BDL vs. sham.
Plasma Ang II levels rose abruptly in the first week post BDL (P < 0.01) but then fell back to levels similar to sham at weeks 2 and 3 before rising to approximately 5 times sham levels at week 4 (P < 0.001) (Fig. 6b).
3.6. Angiotensin metabolism and vasoactive responses to angiotensin peptides in perfused control and BDL livers
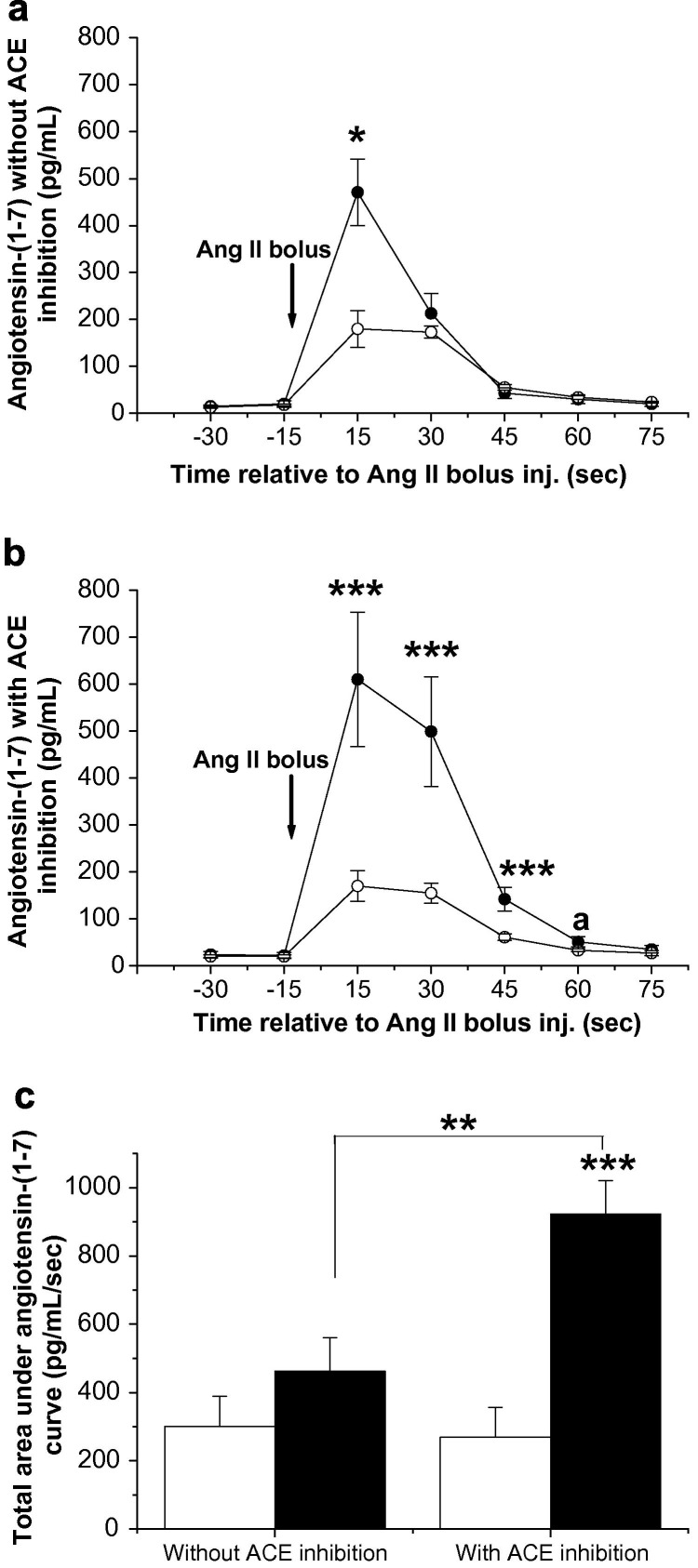
Following Ang II bolus injection, the level of Ang-(1–7) produced by the BDL livers was more than twice (P < 0.05) that in control livers at 15 s (Fig. 7 a). In control livers, following pre-treatment with the ACE inhibitor lisinopril, injection of Ang II produced low levels of Ang-(1–7) from the liver in an identical pattern to that observed without ACE inhibition (Fig. 7b). However, pre-treatment of BDL livers with lisinopril markedly increased Ang-(1–7) levels in the effluent, and the Ang-(1–7) levels at 15, 30 and 45 s post-injection were all significantly (P < 0.001) higher in the BDL than in the controls (Fig. 7b). This has led to a significantly (P < 0.001) elevated baseline-corrected total area under the Ang-(1–7) curve (Fig. 7c).
Fig. 7.
In situ perfused rat liver and angiotensin-(1–7) production in response to angiotensin II (Ang II) bolus injection by bile duct ligated (BDL) rat liver (closed circles) and control rat liver (open circles). Livers were perfused with oxygenated Krebs Henseleit solution (1% BSA) for 25 min, two outflow effluent samples were collected and Ang II bolus injection (60 pmol) was given, followed by sample collection for 75 s (a). The same livers were perfused again with Krebs for 5 min, and then with angiotensin-converting enzyme (ACE) inhibitor lisinopril (10−6 mol/L) throughout sample collection (b). Baseline corrected total area under the angiotensin-(1–7) curve for BDL livers (filled bars) and control livers (open bars) with or without ACE inhibitor is shown in panel (c). Each circle/bar represents the mean ± SEM concentration from 4 to 5 rats per treatment group. ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, aP = 0.05, baseline-corrected BDL vs. control.
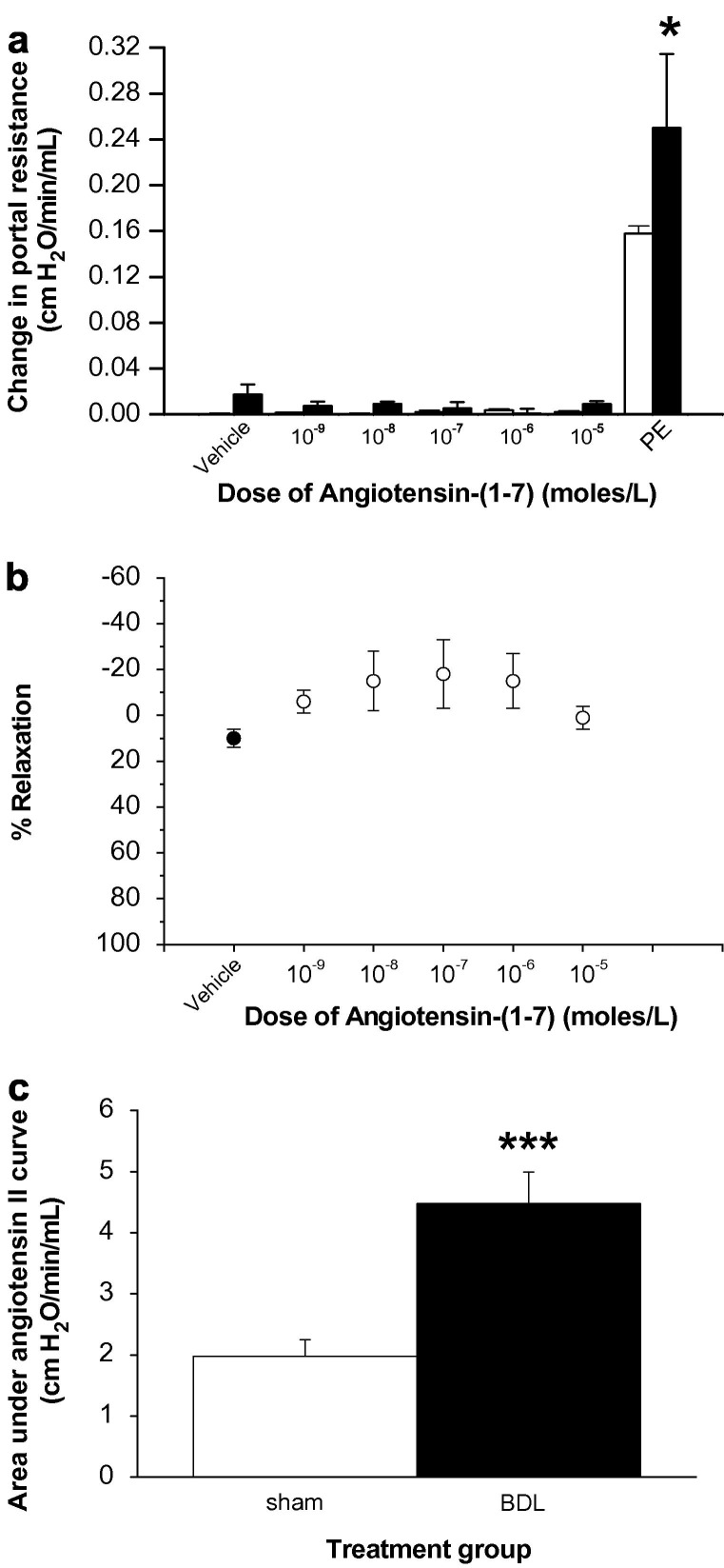
The portal pressure was not altered in response to 10−9, 10−8, 10−7, 10−6 and 10−5 M/L doses of Ang-(1–7) in either control or cirrhotic liver (Fig. 8 a). The responsiveness of these livers to vasoactive agents was confirmed at the end of each experiment by the administration of a bolus dose of phenylephrine (Fig. 8a). Furthermore, Ang-(1–7) also failed to alter portal pressure in in situ perfused control livers that had been pre-constricted with phenylephrine (Fig. 8b). In contrast, there was a marked vasoconstriction response to Ang II in both groups and cirrhotic livers were hyper-responsive to Ang II (P < 0.005) compared to those from healthy rats (Fig. 8c).
Fig. 8.
Vasoactive responses of angiotensin peptides in normal and cirrhotic rat livers. The livers were perfused as described in Section 2.2.2. Panel (a) depicts the change in portal resistance in in situ perfused livers from rats 4-weeks after bile duct ligation (BDL) (filled bars, n = 4) or age-matched controls (open bars, n = 6) in response to varying bolus doses of angiotensin-(1–7). The responsiveness of the liver to angiotensin-(1–7) was confirmed at the end of each experiment by the administration of a bolus dose of phenylephrine (PE). Panel (b) depicts the percentage of relaxation of pre-constricted control livers (n = 5) in response to a vehicle administration (closed circle) followed by varying bolus doses of angiotensin-(1–7) (open circles). Panel (c) depicts the vascular response of sham (open bar, n = 5) and BDL (filled bar, n = 6) livers in response to a bolus dose (10−8 M/L) of angiotensin II. Each circle/bar represents the mean ± SEM concentration. ∗∗∗P < 0.005, ∗P < 0.05, BDL vs. sham.
4. Discussion
The traditional view of the RAS in which the primary focus has been on the vasoconstrictor properties of Ang II has been challenged in recent years with the discovery of ACE2 [12], [13]. It has been suggested that ACE2 plays a counter-regulatory role to ACE, and several possible beneficial effects of ACE2 and its peptide product Ang-(1–7) have been reported in animal models of hypertension and cardiac disease [19], [24], [29], [30], [36], [37] and following lung injury caused by SARS coronavirus infection [38], [39].
The effects of liver disease on the novel RAS components, ACE2, Ang-(1–7) and the Ang-(1–7) receptor, Mas, had not previously been investigated. The current studies showed that as rats developed advanced biliary fibrosis, increasing expression of components of the classic RAS such as ACE, AT1 receptor and angiotensin II [5], [6], [7] was accompanied by increased hepatic and plasma ACE2 activity, increased Mas expression in the liver and a major rise in plasma levels of Ang-(1–7) levels. Indeed by week 4 post BDL plasma concentrations of this peripheral vasodilatory peptide with anti-proliferative and tissue protective properties [18], [19], [20], [21], [22], [23], [24] approached those of the vasoconstrictor Ang II.
Our studies provided a number of lines of evidence to suggest that the diseased liver itself contributed significantly to the increased production of Ang-(1–7) following BDL. ACE2 activity (expressed per mg of tissue) was not increased in other sites of ACE2 expression, including lung, kidney and intestine, whilst it was increased more than 4-fold in the liver, so that when the mass of individual organ is taken into account, following BDL the liver was the organ of greatest ACE2 activity. We have shown previously that ACE2 is proteolytically cleaved from membrane by metalloproteases to release a soluble form with catalytic activity [40], [41]. Thus, in the present study, the diseased liver was the likely source of the major increase in plasma ACE2 activity. In situ perfused rat liver experiments confirmed that following administration of Ang II, greater amounts of Ang-(1–7) were produced by diseased livers than controls. Importantly, the increase in hepatic ACE2 expression in BDL livers was accompanied by upregulation of ACE expression and activity. The finding that Ang-(1–7) release from BDL, but not sham livers, was greatly increased by ACE inhibition indicates that this high level of hepatic ACE activity facilitated the breakdown of Ang-(1–7), reducing Ang-(1–7) release (Fig. 1). This confirmed that BDL livers have a much greater capacity to metabolize exogenously administered Ang II into Ang-(1–7), but that much of this Ang-(1–7) is subsequently broken down by ACE. These findings are in keeping with the concept that the net tissue production of Ang-(1–7)/Ang II is determined by the relative activity of ACE2 and ACE [19].
Whilst the primary purpose of these studies was to examine the effects of liver injury on the novel ACE2-Ang-(1–7)-Mas axis of the RAS, we also documented for the first time the progressive changes that occur in ACE and Ang II levels as fibrosis develops in this model. The current studies demonstrated bi-model changes in plasma Ang II levels during the course of the experiment with an initial marked rise at week one followed by a fall in levels at weeks 2 and 3 and then a significant rise at week 4. These changes are likely to reflect a balance between influences of multiple factors including an acute response to the initial liver injury following BDL surgery, increases in hepatic and serum ACE activity, Ang II degradation by hepatic and serum ACE2 and activation of the systemic RAS in response to the development of portal hypertension and vasodilatation [7], [42].
Recently, the G protein-coupled receptor Mas has been reported as being the principal functional receptor for Ang-(1–7) [25], [26]. However, there is limited understanding of the factors controlling Mas expression and its relationship to ACE2. In the present study, we showed that the transcription of Mas gene in the liver was very closely linked to ACE2 gene expression. This provides support for the concept of the development of an hepatic ACE2-Ang-(1–7)-Mas axis as liver fibrosis progresses in which increased Mas is available to transduce Ang-(1–7) signal [26]. Furthermore, Ang-(1–7) has been shown to be a vasodilator in a number of vascular beds [6], [43], [44], [45] and thus it might be expected to reduce intrahepatic tone in cirrhotic livers; an effect that might help counterbalance vasoconstriction mediated by locally generated vasoactive hormones such as endothelin 1 and Ang II. However, despite marked upregulation of Mas receptor expression, the present data showed that Ang-(1–7) does not elicit an intrahepatic vasodilator response in perfused BDL rat livers. Thus it appears that whilst the breakdown of locally produced Ang II by ACE2 may help protect the cirrhotic liver from Ang II mediated vasoconstriction and tissue injury, there is no detectable direct effect of Ang-(1–7) on sinusoidal resistance, possibly reflecting the known general impairment of NO dependent vasodilatation in the cirrhotic liver [46]. In contrast BDL livers were hyper-responsive to Ang II compared to those from healthy rats suggesting that our in situ perfusion model is appropriate to measure pressure changes in response to angiotensin peptides. This latter finding is also of interest given that the relative importance of Ang II as mediator of increased portal resistance has been questioned [47] based on a study of hepatic hemodynamics in isolated perfused cirrhotic rat livers which suggested that Ang II mediated vasoconstriction is attenuated in the cirrhotic liver [48].
In conclusion, this study shows that in the BDL rat, Ang-(1–7) levels are markedly increased as chronic liver injury progresses and suggest that this is likely to reflect increased ACE2 activity in liver and plasma, and conversion of Ang II to Ang-(1–7) by the diseased liver. The changes in ACE2 expression were confined to the liver indicating that they represent a local response to liver injury and/or upregulation of the ACE-AT1 receptor axis of the RAS. These findings raise the possibility that upregulation of hepatic ACE2 and Mas, and the generation of Ang-(1–7) represent a counter-regulatory response to RAS-mediated liver injury. Whether, as in other organs [19], [29], manipulating this axis may affect tissue injury in chronic liver disease remains to be determined.
Acknowledgement
This work was supported by the National Health and Medical Research Council of Australia.
Associate Editor: A. Geerts
Footnotes
The authors received funding from National Health and Medical Research Council of Australia which enabled them to carry out their study.
References
- 1.Rosenberg M.E., Smith L.J., Correa-Rotter R., Hostetter T.H. The paradox of the renin-angiotensin system in chronic renal disease. Kidney Int. 1994;45:403–410. doi: 10.1038/ki.1994.52. [DOI] [PubMed] [Google Scholar]
- 2.Pagliaro P., Penna C. Rethinking the renin-angiotensin system and its role in cardiovascular regulation. Cardiovasc Drugs Ther. 2005;19:77–87. doi: 10.1007/s10557-005-6900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston C.I. Tissue angiotensin converting enzyme in cardiac and vascular hypertrophy, repair, and remodeling. Hypertension. 1994;23:258–268. doi: 10.1161/01.hyp.23.2.258. [DOI] [PubMed] [Google Scholar]
- 4.Cooper M.E., Tikellis C., Thomas M.C. Preventing diabetes in patients with hypertension: one more reason to block the renin-angiotensin system. J Hypertens Suppl. 2006;24:S57–S63. doi: 10.1097/01.hjh.0000220408.91987.eb. [DOI] [PubMed] [Google Scholar]
- 5.Paizis G., Cooper M.E., Schembri J.M., Tikellis C., Burrell L.M., Angus P.W. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 6.Paizis G., Tikellis C., Cooper M.E., Schembri J.M., Lew R.A., Smith A.I. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataller R., Gabele E., Parsons C.J., Morris T., Yang L., Schoonhoven R. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–1055. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson J.R., Clouston A.D., Ando Y., Kelemen L.I., Horn M.J., Adamson M.D. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148–155. doi: 10.1053/gast.2001.25480. [DOI] [PubMed] [Google Scholar]
- 9.Paizis G., Gilbert R.E., Cooper M.E., Murthi P., Schembri J.M., Wu L.L. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376–385. doi: 10.1016/s0168-8278(01)00146-5. [DOI] [PubMed] [Google Scholar]
- 10.Yoshiji H., Kuriyama S., Fukui H. Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumour Biol. 2002;23:348–356. doi: 10.1159/000069792. [DOI] [PubMed] [Google Scholar]
- 11.Yoshiji H., Kuriyama S., Noguchi R., Ikenaka Y., Yoshii J., Yanase K. Amelioration of liver fibrogenesis by dual inhibition of PDGF and TGF-beta with a combination of imatinib mesylate and ACE inhibitor in rats. Int J Mol Med. 2006;17:899–904. [PubMed] [Google Scholar]
- 12.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 13.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 14.Santos R.A., Campagnole-Santos M.J., Andrade S.P. Angiotensin-(1–7): an update. Regul Pept. 2000;91:45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 15.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 16.Ferrario C.M., Chappell M.C., Tallant E.A., Brosnihan K.B., Diz D.I. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 17.Burrell L.M., Johnston C.I., Tikellis C., Cooper M.E. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marangoni R.A., Carmona A.K., Passaglia R.C., Nigro D., Fortes Z.B., de Carvalho M.H. Role of the kallikrein-kinin system in Ang-(1–7)-induced vasodilation in mesenteric arterioles of Wistar rats studied in vivo–in situ. Peptides. 2006;27:1770–1775. doi: 10.1016/j.peptides.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario C.M., Trask A.J., Jessup J.A. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tallant E.A., Clark M.A. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1–7) Hypertension. 2003;42:574–579. doi: 10.1161/01.HYP.0000090322.55782.30. [DOI] [PubMed] [Google Scholar]
- 21.Tallant E.A., Ferrario C.M., Gallagher P.E. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher P.E., Tallant E.A. Inhibition of human lung cancer cell growth by angiotensin-(1–7) Carcinogenesis. 2004;25:2045–2052. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 23.Iwata M., Cowling R.T., Gurantz D., Moore C., Zhang S., Yuan J.X. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:H2356–H2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 24.Grobe J.L., Mecca A.P., Mao H., Katovich M.J. Chronic angiotensin-(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–H2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 25.Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos R.A., Castro C.H., Gava E., Pinheiro S.V., Almeida A.P., Paula R.D. Impairment of in vitro and in vivo heart function in angiotensin-(1–7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 27.Iyer S.N., Chappell M.C., Averill D.B., Diz D.I., Ferrario C.M. Vasodepressor actions of angiotensin-(1–7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 28.Iyer S.N., Ferrario C.M., Chappell M.C. Angiotensin-(1–7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- 29.Ferrario C.M. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 30.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 31.Bergman I., Loxley R. New spectrophotometric method for the determination of proline in tissue hydrolyzates. Anal Chem. 1970;42:702–706. doi: 10.1021/ac60289a036. [DOI] [PubMed] [Google Scholar]
- 32.Andrade Z.A., Cox T.M., Cheever A.M. Regression of hepatic lesions after treatment of Schistosoma mansoni or Schistosoma japonicum infection in mice: a comparative study. Am J Trop Med Hyg. 1993;49:1–9. doi: 10.4269/ajtmh.1993.49.1. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y., Mendelsohn F.A. Angiotensin converting enzyme inhibition in heart, kidney, and serum studied ex vivo after administration of zofenopril, captopril, and lisinopril. J Cardiovasc Pharmacol. 1991;18:478–486. doi: 10.1097/00005344-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Dales N.A., Gould A.E., Brown J.A., Calderwood E.F., Guan B., Minor C.A. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc. 2002;124:11852–11853. doi: 10.1021/ja0277226. [DOI] [PubMed] [Google Scholar]
- 35.Barton M., Carmona R., Morawietz H., d’Uscio L.V., Goettsch W., Hillen H. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension. 2000;35:329–336. doi: 10.1161/01.hyp.35.1.329. [DOI] [PubMed] [Google Scholar]
- 36.Huentelman M.J., Grobe J.L., Vazquez J., Stewart J.M., Mecca A.P., Katovich M.J. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 37.Simoes e Silva A.C., Pinheiro S.V., Pereira R.M., Ferreira A.J., Santos R.A. The therapeutic potential of Angiotensin-(1–7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603–609. doi: 10.2174/138955706776876203. [DOI] [PubMed] [Google Scholar]
- 38.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 41.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asbert M., Jimenez W., Gaya J., Gines P., Arroyo V., Rivera F. Assessment of the renin-angiotensin system in cirrhotic patients. Comparison between plasma renin activity and direct measurement of immunoreactive renin. J Hepatol. 1992;15:179–183. doi: 10.1016/0168-8278(92)90033-l. [DOI] [PubMed] [Google Scholar]
- 43.Sampaio W.O., Nascimento A.A., Santos R.A. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 44.Neves L.A., Williams A.F., Averill D.B., Ferrario C.M., Walkup M.P., Brosnihan K.B. Pregnancy enhances the angiotensin (Ang)-(1–7) vasodilator response in mesenteric arteries and increases the renal concentration and urinary excretion of Ang-(1–7) Endocrinol. 2003;144:3338–3343. doi: 10.1210/en.2003-0009. [DOI] [PubMed] [Google Scholar]
- 45.Neves L.A., Averill D.B., Ferrario C.M., Aschner J.L., Brosnihan K.B. Vascular responses to Angiotensin-(1–7) during the estrous cycle. Endocrine. 2004;24:161–165. doi: 10.1385/ENDO:24:2:161. [DOI] [PubMed] [Google Scholar]
- 46.Hendrickson H., Chatterjee S., Cao S., Morales Ruiz M., Sessa W.C., Shah V. Influence of caveolin on constitutively activated recombinant eNOS: insights into eNOS dysfunction in BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G652–G660. doi: 10.1152/ajpgi.00143.2003. [DOI] [PubMed] [Google Scholar]
- 47.Rockey D.C. Vasoactive agents in intrahepatic portal hypertension and fibrogenesis: implications for therapy. Gastroenterology. 2000;118:1261–1265. doi: 10.1016/s0016-5085(00)70379-9. [DOI] [PubMed] [Google Scholar]
- 48.Ballet F., Chretien Y., Rey C., Poupon R. Differential response of normal and cirrhotic liver to vasoactive agents. A study in the isolated perfused rat liver. J Pharmacol Exp Ther. 1988;244:283–289. [PubMed] [Google Scholar]