Abstract
A new melatonin sulfonate derivative sodium 4-(3-(2-acetamidoethyl)-5-methoxy-1H-indol-1-yl) butane-1-sulfonate (MLTBS) with higher water solubility (695 times) and lower cytotoxicity than natural melatonin (MLT) was synthesized, yet with the same sleep aid function. The poor solubility of MLT in water has been improved with a simple chemical reaction, which solves the poor solubility of melatonin in water, overcoming the safety problem caused by adding organic reagents such as dimethyl sulfoxide (DMSO) and ethanol to increase the solubility. Moreover, the modified MLT still has the same sleep aid effect as the natural MLT and higher biological safety. As a novel potential drug for sleep aid, the new MLT derivative could also flourish the application and research of this molecule in medicine and biology.
Introduction
Melatonin (MLT), also known as the pineal hormone, is one of the hormones secreted by the brain pineal gland.1 It belongs to indole heterocyclic compounds with the chemical name N-acetyl-5-methoxytryptamine. MLT was discovered and synthesized by Dr. Aaronb Lerner, a dermatologist at Yale University, in 1953. This compound exists not only in primitive organisms as simple as prokaryotes but also in complex organisms as humans.2 MLT is an ancient molecule that exists in animals and plants. It has been extensively studied for its diverse biological effects.3 MLT is a kind of type II drug with low water solubility and high permeability. It has a very short half-life and limited bioavailability.4 Researchers found that the main function of MLT in the human body is to regulate the circadian rhythmicity5,6 and the secretion of the endocrine system.7 MLT can regulate human body clocks8 and induce physiological sleep.9 In recent years, scientists have focused on the antioxidant ability of MLT.10 Since MLT has a high antioxidant value and can be used as a skincare product to remove free radicals in the body, researchers have mixed MLT with Pickering cream to improve the stability of MLT and used it to protect the skin from the sun.11 On the other hand, there are more and more reports on the modification of natural MLT by chemical methods. Through the substitution modification of the MLT acetamide moiety, the molecule Neu-p11 (Figure S1) can be used to repair memory damage and is expected to be a potential drug for the treatment of Alzheimer’s disease.12 Meanwhile, Neu-11 has a neuroprotective mechanism in cerebral ischemia13 as well. The modified 5-hydroxy-2′-isobutyl-streptochlorin (HIS) (Figure S1) molecule on the indole ring of MLT has an anti-inflammatory function, which can inhibit the production of pro-inflammatory mediators and cytokines. The molecule is expected to be a leading drug in the treatment of various diseases mediated by inflammation.14 Substitution of the second position of the MLT indole ring by trifluoromethyl (MLTD, Figure S1) can significantly affect the sleep awakening cycle, which is expected to be used as a hypnotic drug in the treatment of sleep disorders.15 MLT can be used in many different fields. In addition to the field of sleep aid and antiaging agents, MLT has also been extensively studied in the field of anticancer.16,17 It can inhibit the growth of different types of cancer cells including human prostate cancer, colorectal cancer, melanoma, ovarian cancer, skin carcinoma, and breast cancer through a variety of mechanisms.18 For example, the substitution of the methoxyl group of MLT to afford UCM1037 (Figure S1) can effectively inhibit the production of LNCaP in prostate cancer cell lines.19 Brominated MLT (1-benzyl-2,4,6-tribromomelatonin, Figure S1) is also used in the treatment of bone marrow diseases.20
Results and Discussion
In our study, we found that the water solubility of MLT was very poor. MLT dissolved very little in water, and the solution was turbid. Such low solubility greatly limited the research and functional application of MLT. In the literature, organic solvents such as ethanol and dimethyl sulfoxide (DMSO) were usually added to increase its water solubility.19,21,22 However, these additives on the human body had certain stimulation and cytotoxicity on the human body. Yet, there are few reports on improving its water solubility in the literature. To deal with this problem, herein, we report a method to improve the water solubility of MLT by adding water-soluble groups, namely sulfonate. Sulfonate groups are often used to improve the solubility of hydrophobic drug molecules due to their good water solubility, low toxicity, and good stability.23−25 In this paper, the physiological functions of the modified MLT derivatives will be discussed.
We designed and synthesized a new derivative of MLT by introducing a sulfonate group, namely sodium 4-(3-(2-acetamidoethyl)-5-methoxy-1H-indol-1-yl) butane-1-sulfonate (MLTBS) (Figure 1). This new derivative has better water solubility than MLT. MLTBS was synthesized from MLT by direct treatment with NaH and 1,4-butane sulfone in tetrahydrofuran (THF). We also confirmed that the newly synthesized MLT derivative has the original function of MLT in the sleep experiment of mice.
Figure 1.
Synthesis of MLTBS from MLT.
To evaluate the solubility of the new MLT derivative, we tested the solubility of MLT and MLTBS. The solubility of MLTBS in 100 mL of water was about 160 g, while that of MLT in 100 mL of water was 0.2 g, as inquired by SCCS (Scientific Committee on Consumer Safety, Opinion on MLT, 23 March 2010). Therefore, the solubility of MLTBS was 695 times that of MLT (Figure 2A). To make a more intuitive comparison of their difference of solubility, we added a certain amount of solid powder into the same volume of water and observed their dissolution in the water. As shown in Figure 2B, natural MLT was added into the water to form a turbid solution, while MLTBS could form a clear and transparent solution in the water. These results indicated that MLTBS has better water solubility.
Figure 2.
Solubility of MLT and MLTBS. (A) Histogram of MLT and MLTBS solubility. (B) Picture of MLT (5 mg) and MLTBS (50 mg) dissolved in 2 mL of water.
Loss of righting reflex (LORR) and recovery is a widely accepted method to evaluate rodent behavior. Researchers often use this harmless behavior pattern to assess whether the animals have retained their awareness of the surrounding environment or being influenced by the hypnotic effects of drugs.26,27 With the intent to verify that MLTBS has the same physiological activity as MLT, we established a mouse-sleeping model to test the sleep-promoting function of MLTBS. C57/B6 mice were purchased from the Animal Laboratory Center of Xiamen University and randomly divided into three groups. The sleep model of mice was simulated by an intraperitoneal injection of 1% pentobarbital sodium. Then, normal saline, ML, and MLTBS were injected, respectively. Since MLT dissolves poorly in water, 5% DMSO was added to the solution to help dissolving it (MLT and MLTBS were dissolved in saline containing 5% DMSO at a concentration of 86 μM). The sleep time was recorded starting from 1 min after the loss of righting reflex to the recovery of righting reflex. The results showed that MLTBS had the same effect as MLT. It could significantly prolong the sleep time of mice from 1 to 3 h (Figure 3A). However, the physiological content of MLT in the body is very low. MLT is mainly secreted at night. The amount of MLT secreted every night is between 10 and 80 μg.28 Studies have shown that to achieve an artificial MLT environment that produces physiological levels in the human body, the amount of exogenous MLT injected intravenously should be less than 100 μg.29 According to the dosage of MLTD in the previous literature,15 we reduced the concentration of MLT and MLTBS to 860 nM and then injected intraperitoneally. The experimental results are shown in Figure 3B. In the case of low MLTBS concentration, the same hypnotic effect as MLT could still be observed. Therefore, MLTBS has an important potential application in sleep aid.
Figure 3.
MLTBS can promote sleep in mice and prolong the sleep time. The mice were intraperitoneally injected with 1% sodium pentobarbital solution and then MLT and MLTBS solutions were intraperitoneally injected; physiological saline was used in the control group. MLT and MLTBS injection doses were 100 μL ((A) 86 μM; (B) 860 nM). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
MLT is one of the least recognized toxic substances. In human studies, high doses of MLT are well tolerated.30 No serious (grade 3) or life-threatening (grade 4) toxicity was shown. In animal experiments, the LD50 of MLT in mice was more than 800 mg/kg.31 We focused on verifying the toxicity of the synthesized MLTBS to cells and animals. Cell Counting Kit-8 (CCK-8) reagent was used to detect the effect of MLTBS on cell proliferation.32 CCK-8 mainly contains WST-8, which can be reduced by dehydrogenase in mitochondria to yellow formazan with high water solubility. The amount of formazan produced is proportional to the number of living cells. The number of living cells can be reflected by measuring the light absorption value at 450 nm wavelength by ELISA. B16F10 cell lines were inoculated into 96-well plates. After cell adherence, MLT and MLTBS solutions at different concentrations were added to each well in turn. The final concentrations were 10 μM, 100 μM, 1 mM, 5 mM, and 10 mM, respectively. After incubation for 24 h, the CCK-8 reagent was added into each well. The absorbance of the solutions was measured at 450 nm. The results showed that MLT and MLTBS had no effects on cell proliferation at low concentrations (Figure 4). At high concentrations of 5 and 10 mM, MLT affected the cell proliferation rate, while the effect of MLTBS was little, which is significantly different from that of MLT. Even at a higher concentration, the cytotoxicity of MLTBS on the cells was much smaller than that of MLT.
Figure 4.
Effects of MLTBS and MLT upon cell proliferation. Here, 100 μL of cell suspension (about 10 000 B16F10 cells) was added to each well of a 96-well plate. MLT and MLTBS were added with a final concentration of 10 mM, 5 mM, 1 mM, 100 μM, and 1 μM, respectively. The CCK-8 reagent was added. The OD450 values after 4 h were measured. The experiments were performed in triplicate three times. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
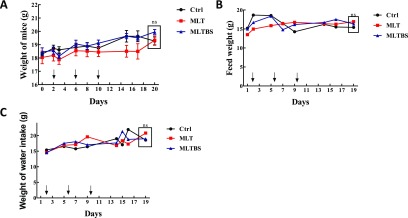
To test the safety of MLT in vivo, we injected MLT, MLTBS, and saline intraperitoneally into mice. We monitored the body weight and diet of the mice. The statuses of mice were observed. As shown in Figure 5, the injection of MLTBS had no significant effect on the body weight and diet of the mice compared with the control group and the MLT group. The results showed that MLTBS had good safety in vivo. In short, our results indicated that MLTBS has lower toxicity and good safety in vitro and in vivo.
Figure 5.
Effects of MLTBS on the growth and diet of mice. The control group was intraperitoneally injected with 200 μL of saline(black line). The MLT group was intraperitoneally injected with 200 μL of MLT solution (red line). The MLTBS group was intraperitoneally injected with 200 μL of MLTBS at a concentration of 10 mM on 2, 6, and 10 days, respectively. (A) Statistical curve of the mouse weight, (B) change in the feed weight, and (C) statistical curve of water intake in mice (ns, P > 0.05).
Conclusions
In summary, we have designed and synthesized a water-soluble MLT derivative, MLTBS. This new derivative retained the active structure of the MLT molecule and the original physiological function of MLT. Compared with MLT, MLTBS has an advantage of good water solubility, lower toxicity, and good safety, in vitro and in vivo. As a novel potential drug for sleep aid, MLTBS could also shed new light on the application and research of MLT in medicine and biology.
Experimental Section
Materials
Pentobarbital and CCK-8 were purchased from a local vendor, and C57/B6 mice were purchased from the Animal Laboratory Center of Xiamen University. B16F10 cells were obtained from American Type Culture Collection. The cells were grown at 37 °C under 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen) supplemented with 10% fetal bovine serum. All chemical reagents were purchased online from http://www.tansoole.com.
Synthesis of the Melatonin Derivative MLTBS
Melatonin (100 mg) was dissolved in tetrahydrofuran (10 mL), and then NaH (69 mg) was added to the above mixture in an ice bath. The reaction mixture was stirred for 30 min in the ice bath, and then, 1,4-butane sulfone (88 mg) was added dropwise into the above mixture. After that, the ice bath was removed, and the reaction was stirred overnight at room temperature. To quench the reaction, ice water (30 mL) was added. The reaction solution was extracted with ethyl acetate, and the water phase was obtained. Then, the water was removed by rotating evaporation under vacuum, and the residues were purified by column chromatography on silica gel to give a crude product, which is then purified with high-performance liquid chromatography (HPLC) to afford MLTBS sodium 4-(3-(2-acetamidoethyl)-5-methoxy-1H-indol-1-yl)butane-1-sulfonate (100 mg; yield, 59.5%), as a white solid. 1H NMR (500 MHz, DMSO): δ 7.97 (t, J = 5.6 Hz, 1H), 7.31 (d, J = 8.8 Hz, 1H), 7.12 (s, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.81–6.67 (m, 1H), 4.12–3.99 (m, 2H), 3.76 (s, 3H), 3.37–3.23 (m, 2H), 2.76 (t, J = 7.4 Hz, 2H), 2.47–2.38 (m, 2H), 1.81 (s, 3H), 1.80–1.71 (m, 2H), 1.60–1.48 (m, 2H). 13C NMR (126 MHz, DMSO): δ 169.63, 153.51, 131.74, 128.39, 127.11, 111.42, 111.27, 110.90, 101.02, 55.87, 51.39, 49.07, 45.75, 29.65, 25.59, 23.16, 22.97. Electrospray ionization-mass spectrometry (ESI-MS) m/z: [M + Na+] = 412.9.
Determination of Melatonin Solubility
The solubility of MLTBS was measured by the saturated solution method. First, 0.1 mL of distilled water was taken and placed in a 25 °C environment. Then, MLTBS was added slowly into the water and oscillated at the same time until MLTBS had precipitated. After standing for a period of time, the precipitate was filtered out. Then, we calculated the mass of MLTBS dissolved in water to be 160 mg. Therefore, at 25 °C, the solubility of MLTBS in water is 160 g/100 mL.
Effects of Melatonin on Sleep in Mice
We injected 300 μL of 1% sodium pentobarbital solution into mice to establish sleep models. Then, the mice were divided into three groups, with five in each group. The first group was injected with 100 μL of normal saline to act as the control group, the second group was injected with natural unmodified melatonin (MLT) solution (86 μM or 860 nM, 100 μL), and the third group was injected with modified melatonin (MLTBS) solution of the same concentration and volume. The sleep time was recorded 1 min after the loss of righting reflex, and the timer ended when the righting reflex returned.
Cell Proliferation Assays
B16F10 cell lines were inoculated into 96-well plates. After cell adherence, 10 μL of MLT and MLTBS solutions at different concentrations was added to each well in turn. The final concentrations were 10 μM, 100 μM, 1 mM, 5 mM, and 10 mM. After incubation for 24 h, the medium was replaced with fresh cell culture medium, and 10 μL of CCK-8 reagent was added into each hole and cultured in the incubator for 4 h, and the absorbance was measured at 450 nm.
Effects of MLTBS on the Growth and Diet of Mice
C57/B6 mice (about 18 g) were used and randomly divided into three groups: control group, MLT group, and MLTBS group. Then, 200 μL of normal saline, MLT, and MLTBS (MLT and MLTBS were dissolved in saline containing 5% DMSO, 10 mmol) were intraperitoneally injected, respectively, three times on 2, 6, and 10 days, and the changes in mice body weight and diet were recorded.
Acknowledgments
We are grateful to the School of Pharmaceutical Sciences Xiamen University for assistance with NMR detection and Xiamen Nuokangde Biological Technology Co., Ltd. (China) for financial support of our work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04120.
Structure of melatonin and derivatives (Figure S1); NMR and mass spectrum of MLTBS (Figure S2–S4) (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. J.Z. and X.Y. contributed equally to this work. Z.L. conceived the concept of water-soluble melatonin derivatives and supervised the project. J.Z. and X.Y. performed the main part of the experiment, Y.T., W.L., Q.L., H.W., and Y.L. performed the experiments. Z.L. and T.W. contributed to experimental design and wrote the manuscript.
Xiamen Nuokangde Biological Technology Co., Ltd. is the initiator and funding provider of this work (2019NKD0001).
The authors declare the following competing financial interest(s): Xiamen Nuokangde Biological Technology Co., Ltd is the initi-ator and funding provider of this work; Dr. Zhu Li is chairman of the company and surperises the project. The company is cooperated with Xiamen University, Sun Yat-Sen University, Mingguang peoples hospital and University Affiliated Keji High School to complete the proof-of-concept of the project and integrate all data into an article for publication. The intel-lectual property of the project is owned by Xiamen Nuokangde Biological Technology Co., Ltd and the patent of the project has been applied. (Patent application number: 201811622426.5).
Notes
Dr. Z.L. is the chairman of the company and supervises the project. The company is co-operated with Xiamen University, Sun Yat-Sen University, and Mingguang People’s Hospital and University Affiliated Keji High School to complete the proof of concept of the project and integrate all data into an article for publication. The intellectual property of the project is owned by Xiamen Nuokangde Biological Technology Co., Ltd., and the patent of the project has been applied (Patent application number: 201811622426.5).
Supplementary Material
References
- Pala D.; Lodola A.; Bedini A.; Spadoni G.; Rivara S. Homology Models of Melatonin Receptors: Challenges and Recent Advances. Int. J. Mol. Sci. 2013, 14, 8093–8121. 10.3390/ijms14048093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla-Neto J.; Amaral F. G.; Afeche S. C.; Tan D. X.; Reiter R. J. Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 2014, 56, 371–381. 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- Salehi B.; Sharopov F.; Fokou P. V. T.; Kobylinska A.; Jonge Ld.; Tadio K.; Sharifi-Rad J.; Posmyk M. M.; Martorell M.; Martins N.; Iriti M. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681 10.3390/cells8070681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Zhao X.; Zu Y.; Wang L.; Wu W.; Deng Y.; Zu C.; Liu Y. Melatonin-loaded silica coated with hydroxypropyl methylcellulose phthalate for enhanced oral bioavailability: Preparation, and in vitro-in vivo evaluation. Eur. J. Pharm. Biopharm. 2017, 112, 58–66. 10.1016/j.ejpb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- LeGates T. A.; Fernandez D. C.; Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–54. 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy A.; Wehr T.; Goodwin F.; Newsome D.; Markey S. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Rawashdeh O.; de Borsetti N. H.; Roman G.; Cahill G. M. Melatonin Suppresses Nighttime Memory Formation in Zebrafish. Science 2007, 318, 1144–1146. 10.1126/science.1148564. [DOI] [PubMed] [Google Scholar]
- Winfree A. T. Human body clocks and the timing of sleep. Nature 1982, 297, 23–27. 10.1038/297023a0. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal S. R.; Trakht I.; Spence D. W.; Srinivasan V.; Dagan Y.; Cardinali D. P. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat. Clin. Pract. Neurol. 2008, 4, 436–447. 10.1038/ncpneuro0847. [DOI] [PubMed] [Google Scholar]
- Rodriguez C.; Mayo J. C.; Sainz R. M.; Antolín I.; Reiter R. J.; et al. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. 10.1046/j.1600-079X.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Marto J.; Ascenso A.; Gonçalves L. M.; Gouveia L. F.; Manteigas P.; Pinto P.; Oliveira E.; Almeida A. J.; Ribeiro H. M. Melatonin-based pickering emulsion for skin’s photoprotection. Drug Delivery 2016, 23, 1594–1607. 10.3109/10717544.2015.1128496. [DOI] [PubMed] [Google Scholar]
- He P.; Ouyang X.; Zhou S.; Yin W.; Tang C.; Laudon M.; Tian S. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm. Behav. 2013, 64, 1–7. 10.1016/j.yhbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Buendia I.; Gómez-Rangel V.; González-Lafuente L.; Parada E.; León R.; Gameiro I.; Michalska P.; Laudon M.; Egea J.; López M. G. Neuroprotective mechanism of the novel melatonin derivative Neu-P11 in brain ischemia related models. Neuropharmacology 2015, 99, 187–195. 10.1016/j.neuropharm.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Shim D. W.; Shin H. J.; Han J. W.; Ji Y. E.; Jang C. H.; Koppula S.; Kang T. B.; Lee K. H. A novel synthetic derivative of melatonin, 5-hydroxy-2′-isobutyl-streptochlorin (HIS), inhibits inflammatory responses via regulation of TRIF-dependent signaling and inflammasome activation. Toxicol. Appl. Pharmacol. 2015, 284, 227–235. 10.1016/j.taap.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Akanmu M. A.; Songkram C.; Kagechika H.; Honda K. A novel melatonin derivative modulates sleep-wake cycle in rats. Neurosci. Lett. 2004, 364, 199–202. 10.1016/j.neulet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Blask D. E.; Dauchy R. T.; Sauer L. A. Putting cancer to sleep at night. Endocrine 2005, 27, 179–188. 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Tian X.; Peng Y.; Sun Z.; Wang C.; Tang N.; Li B.; Jian Y.; Wang W.; Huo X.; Ma X. Mitochondrial cytochrome P450 (CYP) 1B1 is responsible for melatonin-induced apoptosis in neural cancer cells. J. Pineal Res. 2018, 65, e12478 10.1111/jpi.12478. [DOI] [PubMed] [Google Scholar]
- Mediavilla M. D.; Sanchez-Barcelo E. J.; Tan D. X.; Manchester L.; Reiter R. J. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 2010, 17, 4462–4481. 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- Calastretti A.; Gatti G.; Lucini V.; Dugnani S.; Canti G.; Scaglione F.; Bevilacqua A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1505. 10.3390/ijms19051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuo S.; Masanori S.; Azusa S.; Reiter R. J.; Atsuhiko H. Novel bromomelatonin derivatives as potentially effective drugs to treat bone diseases. J. Pineal Res. 2010, 45, 229–234. 10.1111/j.1600-079X.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- Jardim-Perassi B. V.; Arbab A. S.; Ferreira L. C.; Borin T. F.; Varma N. R.; Iskander A.; Shankar A.; Ali M. M.; de Campos Zuccari D. A. P. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS One 2014, 9, e85311 10.1371/journal.pone.0085311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida C. S.; Castrucci A. M. dL.; Lamy-Freund M. High melatonin solubility in aqueous medium. J. Pineal Res. 1994, 16, 198–201. 10.1111/j.1600-079X.1994.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Islam M. F.; Rojas E.; Bergey D. M.; Johnson A. T.; Yodh A. G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. 10.1021/nl025924u. [DOI] [Google Scholar]
- Nguyen H. L.; Kumar N.; Audibert J.-F.; Ghasemi R.; Lefevre J.-P.; Ha-Thi M.-H.; Mongin C.; Leray I. Water-soluble aluminium fluorescent sensor based on aggregation-induced emission enhancement. New J. Chem. 2019, 43, 15302–15310. 10.1039/C9NJ03532J. [DOI] [Google Scholar]
- Niu S. L.; Ulrich G.; Ziessel R.; Kiss A.; Renard P.-Y.; Romieu A. Water-Soluble BODIPY Derivatives. Org. Lett. 2009, 11, 2049–2052. 10.1021/ol900302n. [DOI] [PubMed] [Google Scholar]
- Wasilczuk A. Z.; Maier K. L.; Kelz M. B.. The Mouse as a Model Organism for Assessing Anesthetic Sensitivity. In Methods in Rnzymology; Academic Press: Elsevier, 2018; Vol. 602, pp 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P. Molecular targets underlying general anaesthesia. Br. J. Pharmacol. 2006, 147, S72–S81. 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claustrat B.; Brun J.; Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Geoffriau M.; Claustrat B.; Veldhuis J. Estimation of frequently sampled nocturnal melatonin production in humans by deconvolution analysis: evidence for episodic or ultradian secretion. J. Pineal Res. 1999, 27, 139–144. 10.1111/j.1600-079X.1999.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Andersen L. P. H.; Gögenur I.; Rosenberg J.; Reiter R. J. The safety of melatonin in humans. Clin. Drug Invest. 2016, 36, 169–175. 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- Taziki S.; Sattari M. R.; Dastmalchi S.; Eghbal M. A. Cytoprotective effects of melatonin against amitriptyline-induced toxicity in isolated rat hepatocytes. Adv. Pharm. Bull. 2015, 5, 329. 10.15171/apb.2015.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G.; Ji C.; Lan C.; Yan W.; Rong H. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009, 10, 10 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.