Abstract
Background
Safe food is central to social wellbeing. Coagulase-negative staphylococci (CNS) are a threat to food safety because they may harbor multiple enterotoxins and antimicrobial resistance (AMR) genes. CNS bacteria are an emerging nosocomial pathogen in public health. CNS also cause bovine mastitis with a significant economic loss in the dairy industry and may introduce toxins to the food supply chain resulting in foodborne illnesses. However, information on CNS and their AMR status are scarce in food animal production and processing lines in Ethiopia.
Methodology
This cross-sectional study evaluated the prevalence and AMR patterns of CNS in dairy farms and abattoirs using samples (n = 1001) from udder milk, beef carcass, personnel, and different abattoir and dairy equipment across five locations of central Oromia. The CNS isolates were identified via standard microbiological protocols and evaluated using disc diffusion test against 14 antimicrobials belonging to nine different broad classes. Uni-and-multivariable logistic regressions were used to analyze the association between potential risk factors (location, sample source, and sample type) and positivity to CNS.
Results
The overall prevalence of CNS in the five different geographic locations studied was 9.6% (range: 6.7–12.4%) and varied between abattoirs (11.3%) and dairy farms (8.0%). CNS were prevalent on the carcass, milk, equipment, personnel hands, and nasal samples. Of all CNS isolates, 7.1, 10.7, 7.1, 12.5, 17.9, 10.7, 12.5, 7.1, 1.8, 5.4, 1.8, and 5.4% exhibited AMR simultaneously to single, double, 3, 4, 5, 6, 7, 7, 8, 9, 10, 11, and 13 antimicrobials, respectively. Overall, the isolates displayed 51 different AMR phenotypic patterns in which 50% of the isolates exhibited quadruple-resistance simultaneously based on the nine broad antimicrobial classes tested using 14 representative antimicrobials. The prevalence of multidrug-resistant (MDR) CNS (i.e. ≥ 3 classes of antimicrobials) was significantly (p = 0.037) different between locations with 100, 57.1, 50, 86.7, and 76.9% in Addis Ababa, Adama, Assela, Bishoftu, and Holeta, respectively. However, the prevalence of MDR CNS was not significantly (p = 0.20) different between dairy farms (87.5%) and abattoirs (71.9%). We evaluated the effect of acquiring cefoxitin-resistance of the isolates on the efficacy (i.e. inhibition zone) of the rest antimicrobials using General Linear Model after adjusting geographical locations as a random effect. Isolates with cefoxitin-resistance significantly displayed resistance to eight antimicrobials of 14 tested including amoxicillin, penicillin, cloxacillin, chloramphenicol, nalidixic acid, nitrofurantoin, and tetracycline (p = 0.000), and erythromycin (p = 0.02). On the other hand, cefoxitin-resistant isolates were susceptible to gentamicin, ciprofloxacin, kanamycin, streptomycin, and sulphamethoxazone trimethoprim (p = 0.000). Thus, antimicrobials such as gentamicin and ciprofloxacin may be an alternative therapy to treat cefoxitin-resistant CNS, as 96.4% of CNS isolates were susceptible to these antimicrobials. Overall, 94.1 and 54.5% of the CNS isolates among cefoxitin-resistant and cefoxitin-susceptible, respectively, harbored resistance to 3 or more classes of antimicrobials i.e. MDR.
Conclusion
The overall prevalence of CNS in milk, meat, equipment, and food handlers in central Oromia was 9.6% but varied by location and sample source. Some specific niches such as equipment, hands, and nasal cavities of personnel are significant sites for the source of CNS. Most, but not all, MDR CNS isolates were cefoxitin-resistant. Overall, 78.6% of the CNS tested were MDR and 50% had resistance to four or more broad classes of antimicrobials. CNS in food animals (raw milk and meat), equipment, and food handlers can be the source of MDR to the public. Personnel safety and hygienic food handling practices are needed. In addition, further investigation into the risk factors for the transmission and mechanisms of resistance of the CNS is required for intervention.
Keywords: Microbiology, Microbial ecology of foods, Bacteria, Antibiotic resistance, Staphylococcus, Epidemiology, Antimicrobial resistance, CNS, Food hygiene, Food safety, Livestock, Prevalence, Multidrug resistance
Microbiology; Microbial ecology of foods; Bacteria; Antibiotic resistance; Staphylococcus; Epidemiology; Antimicrobial resistance, CNS, Food hygiene, Food safety, Livestock, Prevalence, Multidrug resistance.
1. Introduction
Ethiopia is the leading producer of livestock in Africa (Leta and Mesele, 2014). Livestock are a source of cash, draught power for crop cultivation in Ethiopia, and protein-rich food. However, food-producing animals are major sources of most foodborne pathogens (Heredia and García, 2018). Improper food-animal production and processing practices in farms and processing/supply lines may result in contamination of foods of animal origin with zoonotic pathogens for onward transmission to the consumers (Nunes et al., 2015; Fijałkowski et al., 2016; Cook et al., 2017).
CNS are important pathogens with food safety implications as they may contaminate food of animal origin including milk (Ruaro et al., 2013; Osman et al., 2015), cheese, sausage (Iacumin et al., 2006; Coton et al., 2010), and meat (Landeta et al., 2013; Bhargava and Zhang, 2014; Osman et al., 2016a, Osman et al., 2016b). CNS are frequently isolated from chicken carcasses, bulk tank milk, minced meat, and contact persons (Huber et al., 2011). CNS may also be a reservoir of different staphylococcal enterotoxins genes causing food poisoning (Nemati et al., 2008; Zell et al., 2008; Cunha et al., 2013; Rall et al., 2014; Nunes et al., 2015; Rodríguez et al., 2016). In addition, they may be a reservoir for multiple AMR genes (Bhargava and Zhang, 2012; Chajęcka-Wierzchowska et al., 2014, 2015; Nunes et al., 2015; Szczuka et al., 2016) including methicillin resistance genes (Otto, 2013; Bhargava and Zhang, 2014; Osman et al., 2015; Osman et al., 2016a, Osman et al., 2016b). Methicillin-resistant CNS strains generally exhibit a multidrug resistance phenotype (Osman et al., 2015). CNS are among the major etiologies of mastitis in dairy cows causing significant economic losses in the dairy industry (Taponen et al., 2008; Gillespie et al., 2009; Pyörälä and Taponen, 2009; Supré et al., 2011). CNS are also becoming emerging zoonotic pathogens (Davis et al., 2013). In this regard, CNS are currently the third major nosocomial pathogens next to Staphylococcus aureus and Pseudomonas aeruginosa (Spencer, 1996; Agvald-Öhman et al., 2003) causing diseases such as endocarditis, septicemia, urinary tract infection in humans.
Improving hygienic practices along the farm-to-fork supply continuum is key in preventing, reducing, or maintaining the quality and safety of food to safeguard the public health from foodborne pathogens (Lues and Van Tonder, 2007; Rani et al., 2017). Food hygiene and safety, however, is poorly controlled in developing countries as food for human consumption is approved based on visual inspection, if at all, without routine microbiological testing (Rani et al., 2017). For example, Ethiopia, the second-most populous country in Africa next to Nigeria, has a poorly developed infrastructure, surveillance system, and has few skilled personnel (Wamai, 2009). These limiting factors can promote foodborne and zoonotic pathogen transmission to humans due to strong human-animal interaction (Gumi et al., 2012), substandard food handling practices (Seleshe et al., 2014; Eshetie et al., 2018), and the common practice of consuming raw meat (Seleshe et al., 2014) and raw milk (Regassa et al., 2008). Moreover, the CNS population structure and AMR epidemiology along the farm-to-fork continuum have been poorly investigated in Ethiopia. To find effective targeted interventions, there should be a clear understanding of the local drivers of microbial beef and milk contamination, and the epidemiology (and AMR) of foodborne pathogens along the food production chain. Accordingly, this study evaluated the prevalence and AMR patterns of CNS isolated from beef, milk, related-equipment, and food handlers on dairy farms and abattoir settings in five geographical locations of central Oromia, Ethiopia.
2. Materials and methods
2.1. Study area
The study was conducted in Oromia, Ethiopia. Oromia is the largest region of the nine regions of Ethiopia in terms of livestock and human populations. Its capital is Addis Ababa (Tiwari, 2016). Five locations (cities) from central Oromia (Addis Ababa, Holeta, Bishoftu, Adama and Assela) were included in this study. Addis Ababa had a human population of 3.4 million in 2008. Holeta located 40 km west of Addis Ababa had a population of 25,000 in 2007. Adama is located 99 km east of Addis Ababa with a population of 300,000. Bishoftu is surrounded by seven lakes and a tourist city located 47 km to the southeast of Addis Ababa with a population of 171,000 in 2012 (Tiwari, 2016). Assela, population of 67,269 in 2007, is located at 175 km east from Addis Ababa (Tiwari, 2016).
2.2. Dairy farms and abattoirs: sampling, sample types, and sample handling
The five locations were purposively selected based on their urbanization, high human settlement, and the following three criteria: (i) presence of intensive or semi-intensive dairy farms, (ii) presence of municipal or private-owned abattoirs for beef-meat supply, and (iii) a high number of people in the cities being served by these dairy farms and abattoirs.
At each location level, the samples for microbiological (i.e. CNS) screening was randomly sampled, except personnel samples that were collected based on their informed consent. In total, 1001 samples from the five locations were collected. The distribution of the samples by location were Assela (n = 181), Adama (n = 180), Bishoftu (n = 253), Addis Ababa (n = 193), and Holeta (n = 194). The type of samples collected from abattoirs and dairy farms of the studied five locations were described as follows. One abattoir from each location was used in the study. Overall, 487 samples were collected from five abattoirs, including meat/carcass, knife, slaughter line, butchers hand and nasal swabs based on informed consent. Male indigenous zebu cattle were predominantly slaughtered in the abattoirs. They came from extensive mixed crop-livestock farming systems although some of them came from distant pastoral areas to the abattoirs. In addition, 514 samples were collected from 53 dairy farms that had lactating dairy cows managed under intensive or semi-intensive production systems. The cows were crossbred of the Ethiopian indigenous zebu mainly with Holstein. Milk samples from cows' udder were collected after cleaning the teats with luke-warm water, drying and disinfecting with 70% alcohol. About 2mL of milk was collected from each cow. In addition, tank milk and swab samples were collected from buckets, tanks, milkers’ hands and nasal passages before milking using sterile cotton. Prior to sampling, we moistened the swab tips in buffered peptone water (BPW), and then rotated and rubbed them gently 8–10 times against the sampled surface of about 100-cm2 area. After completion of swabbing, each sample (swab) was put aseptically inside a sterile test tube containing 4mL of BPW using disposable gloves to avoid contamination. Then, the sampling bottles were capped, labeled, and stored in an ice-packed cool box for transport to the Microbiology laboratory at the College of Veterinary Medicine and Agriculture, Addis Ababa University laboratory for bacteriological analysis.
2.3. Bacteriological and biochemical tests for CNS bacteria isolation and identification
All samples were directly seeded onto 5% sheep blood agar and incubated aerobically at 37 °C for 24 h. Colonies suspected of being staphylococci were initially identified by their colony morphology followed by further sub-culturing on nutrient agar plates at 37 °C for 24 h to get a pure colony. We also performed a Gram stain and potassium hydroxide (KOH) test by mixing a drop (5–10μl) of 3% KOH with a loop-full bacteria on a slide. Development of a markedly viscid suspension or gels within 5–60s indicated the isolate was Gram-negative and if no gelling was observed, Gram-positive (Buck, 1982). For Gram-positive cocci, the catalase test was performed to distinguish catalase-negative (Streptococcus spp.) from catalase-positive (Staphylococcus spp.) by mixing a loop-full bacteria with a drop of 3% H2O2 on a clean glass slide. Coagulase-positive Staphylococcus spp. were identified by coagulase production (i.e. clotting/clumping) using the tube coagulase testing method. For the coagulase test, 0.5 ml of bacteria grown overnight in tryptone soya broth was mixed with 0.5 ml of fresh rabbit plasma and incubated for 24 h at 37 °C (Cunha et al., 2004). Mannitol fermentation using mannitol salt agar was used to identify the S. aureus (yellow colonies) and eliminate them from further study (Thakur et al., 2017). In addition to the colony morphology on culture, Gram stain, catalase, and coagulase test, we performed multiple sugar fermentation tests for CNS identification (Kloos and Schleifer, 1975; Harrigan, 1998; Becker et al., 2014).
2.4. Antimicrobial susceptibility testing
The susceptibility of the isolates was tested against 14 different antimicrobials (Oxoid, Hampshire, England) using the disk diffusion method as described by the clinical laboratory standard institute (CLSI, 2015). Briefly, 1–3 colonies were suspended in sterile saline, and the turbidity of the bacterial suspension was adjusted to a 0.5 McFarland standard. The standardized bacterial suspension was spread over Mueller Hinton agar and allowed the agar to dry. Antimicrobial coated discs were placed on the agar surface using sterile forceps and gently pressed with the point of a sterile forceps to ensure complete contact with the agar surface. The plates were incubated aerobically at 37 °C for 18 h as per manufacturers and CLSI recommendation (CLSI, 2015). Inhibition zone diameters (mm) were measured in millimeters. The isolates were classified as resistant or susceptible to an antimicrobial based on the susceptible zone diameter (mm) as per the manual of the manufacturer and CLSI. CNS isolates with intermediate breakpoints may become resistant overtime since there have been reports of weak regulations of antimicrobial use in Ethiopia (Beyene et al., 2015, 2016; Luseba and Rwambo, 2015; Suleman et al., 2016). Thus, we grouped the intermediate CNS isolates in the resistant group in this study. The 14 antimicrobials tested, their disc code, potency, and cut-off value for susceptible zone diameter (mm) were as follows: amoxicillin (25μg, ≥20mm), cefoxitin (30μg, ≥22mm), chloramphenicol (30μg, ≥18mm), ciprofloxacin (5μg, ≥21mm), cloxacillin (5μg, ≥18mm), erythromycin (15μg, ≥23mm), gentamicin (10μg, ≥15mm), kanamycin (30μg, ≥18mm), nalidixic acid (30μg, ≥19mm), nitrofurantoin (50μg, ≥17mm), penicillin G (10units, ≥29mm), streptomycin (10μg, ≥15mm), sulphamethoxazole trimethoprim (25μg, ≥16mm), and tetracycline (30μg, ≥19mm). These 14 antimicrobials belonged to nine different broad classes of antimicrobials, namely aminoglycosides, cephalosporins (b-lactam), folate pathway inhibitors, macrolides, nitrofurans, penicillins, phenicols, quinolones, and tetracyclines. Resistant isolates to three and more broad classes of antimicrobials in this study were recorded as multidrug-resistant (MDR) as described previously (Pesavento et al., 2007).
2.5. Data management and analysis
The farm and abattoir workers included in this study were healthy (no symptoms). Collection of hand and nasal swab samples had ethical approval (Ref. RD/LT/194/2013) and samples were taken with workers' consent. The aim of hand and nasal swabbing was to evaluate the hygiene and food safety practices of abattoir and dairy farmworkers in order to use the results as a risk indicator for the development of zoonoses and involvement of the workers in the dissemination of the pathogens on the farm and abattoirs to milk and meat destined to human consumption. The data were analyzed using SPSS software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY). Descriptive analysis (percentage) was used to summarize the proportion of bacterial isolates and AMR level (resistant or susceptible) in line with each potential risk factor. A comparison of the association between each potential risk factor (geographical location, sample source, and sample type) with the positivity of the sample was analyzed using the χ2 test (Fisher's exact test). Univariate and multivariate logistic regression analyses were performed to reveal the strength of association of the potential risk factors and prevalence. We evaluated whether cefoxitin resistance was correlated with the presence of AMR to other antimicrobials in order to use cefoxitin as a reliable predictor of the presence of MDR. Therefore, the number of antimicrobials resisted was compared in this study between cefoxitin-resistant versus cefoxitin-susceptible CNS subpopulations using General Linear (GLM) Model univariate after adjusting geographical locations as a random effect. In all the analysis, the significance level was set at α = 5% and a 95% confidence interval.
3. Results
3.1. Prevalence of CNS
The overall prevalence of CNS in central Oromia was 9.6%. The prevalence of CNS was ranged from 6.7-12.4% among the five geographical locations (χ2 = 5.3, p = 0.26) indicating some other spatial or non-spatial factors were playing a role for the variation (Table 1). The prevalence of CNS in the abattoir was higher than on dairy farms (χ2 = 3.1, p = 0.07). Moreover, some hotspots among the different niches within dairy farms and abattoirs seemed to exist for CNS. In this regard, the highest prevalence was detected in butchers' nasal passage (20.0%) and the least in milkers’ hand swabs (4.0%) or udder milk of cows (9.1%). Slaughter line swabs (18.9%) and hand swabs of butchers (13.5%) had higher contamination than carcass/meat swabs (10.5%) among the abattoir samples (Table 1). Although beef carcass and dairy cows (milk) were the sources of CNS, the higher prevalence of CNS isolates on the equipment and personnel indicated poor hygiene, but these prevalence values were not significantly (χ2 = 10.9, p = 0.36) different (Table 1).
Table 1.
Prevalence of CNS in milk, meat, equipment, and personnel samples collected from dairy farm and abattoir settings in five locations of central Oromia, Ethiopia.
| Examined (n) | Negative (n) | Positive (n) | Prevalence (%) | χ2; p-value | ||
|---|---|---|---|---|---|---|
| Area | Addis Ababa | 193 | 169 | 24 | 12.4 | 5.3; p = 0.26 |
| Assela | 181 | 160 | 21 | 11.6 | ||
| Holeta | 194 | 175 | 19 | 9.8 | ||
| Bushoftu | 253 | 233 | 20 | 7.9 | ||
| Adama | 180 | 168 | 12 | 6.7 | ||
| Source | Abattoir | 487 | 432 | 55 | 11.3 | 3.1; p = 0.07 |
| Dairy farm | 514 | 473 | 41 | 8.0 | ||
| Sample type | Butchers' nasal swab | 15 | 12 | 3 | 20.0 | 10.9; p = 0.36 |
| Slaughter line swab | 37 | 30 | 7 | 18.9 | ||
| Butchers' hand swab | 37 | 32 | 5 | 13.5 | ||
| Milkers' nasal swab | 17 | 15 | 2 | 11.8 | ||
| Meat/Carcass swab | 361 | 323 | 38 | 10.5 | ||
| Udder milk | 297 | 270 | 27 | 9.1 | ||
| Tank swab | 50 | 46 | 4 | 8.0 | ||
| Bucket swab | 50 | 47 | 3 | 6.0 | ||
| Tank milk | 50 | 47 | 3 | 6.0 | ||
| Knife swab | 37 | 35 | 2 | 5.4 | ||
| Milkers' hand swab | 50 | 48 | 2 | 4.0 | ||
| Total | 1001 | 905 | 96 | 9.6 |
3.2. AMR prevalence in CNS
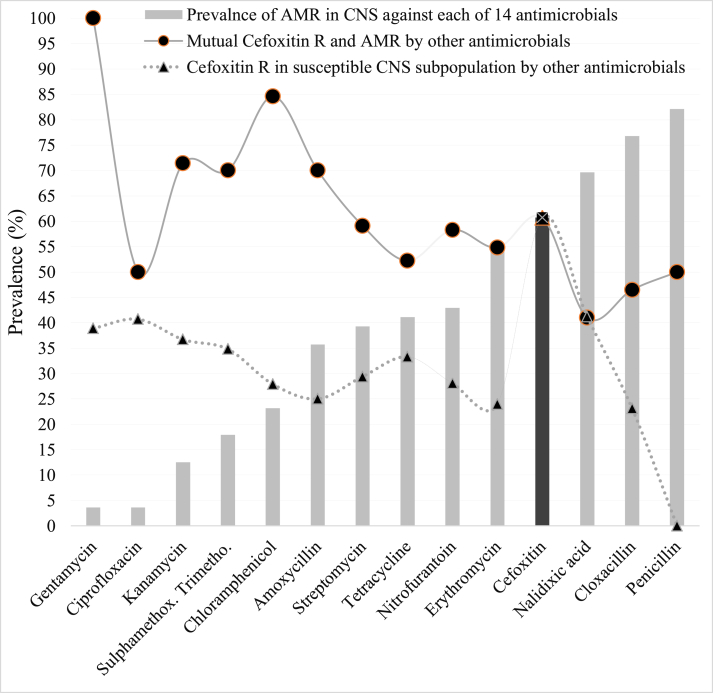
The prevalence of AMR among the 56 CNS isolates was gentamicin (3.6%), ciprofloxacin (3.6%), kanamycin (12.5%), sulphamethoxazole trimethoprim (17.9%), chloramphenicol (23.2%), amoxicillin (35.7%), streptomycin (39.3%), tetracycline (41.1%), nitrofurantoin (42.9%), erythromycin (55.4%), cefoxitin (60.7%), nalidixic acid (69.6%), cloxacillin (76.8%), and penicillin (82.1%) (Figure 1, light bar). Thirty-four (60.7%) of the 56 CNS isolates were cefoxitin-resistant (Figure 1, dark bar).
Figure 1.
Prevalence of AMR in CNS isolates (n = 56; bar) to 14 antimicrobials in central Oromia. Cefoxitin-resistance (dark bar) represents methicillin-resistance. Interestingly, cefoxitin-resistance was also prevalent within both the resistant (line with circle) and susceptible (dot-line with triangle) CNS subpopulations to the other 13 antimicrobials tested.
3.3. Association between cefoxitin-resistance/susceptibility and the rest 13 antimicrobials
Once the CNS isolates were categorized to the resistant or susceptible subpopulation groups based on resistance to 14 antimicrobials, an evaluation was conducted concerning the association between each of the 13 antimicrobials and cefoxitin-resistance/susceptibility data. Most of the isolates that had AMR to any of the other 13 antimicrobials also exhibited cefoxitin-resistance. Accordingly, the prevalence of cefoxitin-resistance was 100, 50, 71.4, 70, 84.6, 70, 59.1, 52.2, 58.3, 54.8, 41, 46.5, and 50% among CNS isolates resistant to gentamycin, ciprofloxacin, kanamycin, sulphamethoxazone trimethoprim, chloramphenicol, amoxicillin, streptomycin, tetracycline, nitrofurantoin, erythromycin, nalidixic acid, cloxacillin, and/or penicillin, respectively (Figure 1, line with circle mark; Supplementary 1). Cefoxitin-resistance was also detected in some CNS isolates despite they were susceptible to the rest 13 antimicrobials. In this regard, the prevalence of cefoxitin-resistance was 38.9, 40.7, 36.7, 34.8, 27.9, 25, 29.4, 33.3, 28.1, 24, 41.2, and 23.1% among CNS isolates susceptible to gentamycin, ciprofloxacin, kanamycin, sulphamethoxazone trimethoprim, chloramphenicol, amoxicillin, streptomycin, tetracycline, nitrofurantoin, erythromycin, nalidixic acid, cloxacillin, and penicillin, respectively (Figure 1, dotted line with triangle mark; Supplementary 1).
There was a significant association between cefoxitin-resistant isolates and acquisition of resistance to amoxicillin, chloramphenicol, cloxacillin, nalidixic acid, nitrofurantoin, penicillin, and tetracycline (χ2 = for each ranges 57.0 70.0; p = 0.000) and erythromycin (χ2 = 5.4; p = 0.02) suggesting resistance to cefoxitin might be a good reporter to predict resistance to these eight antimicrobials. However, cefoxitin-resistant isolates were significantly susceptible to gentamicin, ciprofloxacin, kanamycin, streptomycin, and sulphamethoxazone trimethoprim (χ2 = for each range 57.0–60.1; p = 0.000) (Supplementary 1), indicating gentamicin and ciprofloxacin may be used as an alternative therapy to treat cefoxitin-resistant superbugs. The relationship between cefoxitin (columns) vs. the remaining antimicrobial resistance (rows) was summarized in Supplementary 1.
3.4. Multidrug-resistant CNS on dairy farms, abattoirs, different sample types, and locations and its association with cefoxitin-resistance
The prevalence of MDR CNS (≥3 classes of antimicrobials) was significantly (χ2 = 9.80; p = 0.037) different between locations with 100.0, 57.1, 50.0, 86.7, and 76.9% being MDR in Addis Ababa, Adama, Assela, Bishoftu, and Holeta, respectively. The prevalence of MDR CNS between dairy farms (87.5%) and abattoirs (71.9%) was not significantly (χ2 = 1.99; p = 0.20) different.
After adjusting for geographical location as a random effect in the GLM model, the cefoxitin-resistant CNS isolates exhibited resistance on average to 7.7 antimicrobials (95% CI = 6.7–8.8 antimicrobials) using data of the 14 antimicrobials tested, while cefoxitin-susceptible groups exhibited resistance to 3.8 (95% CI = 2.9–4.7; p = 0.02) antimicrobials.
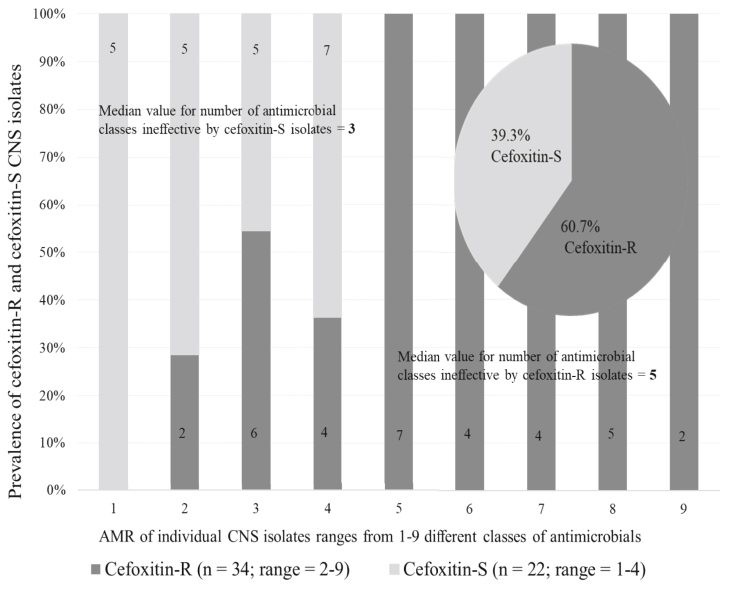
The 14 antimicrobials tested in this study were member of nine broad classes of antimicrobials. Since antimicrobial in the same class may have a relationship (i.e. similar effect), analyzing the susceptibility data of antimicrobials in the same class separately may be misleading (a duplication), resulting in sending false alarm to the public. Therefore, we regrouped the susceptibility data of the 14 antimicrobials to their respective nine broad classes and analyzed them further. Thus, after controlling the effect of sample source and location as a random effect in the GLM model, cefoxitin-resistant CNS isolates exhibited resistance on average to 5.1 classes of antimicrobials (95% CI = 4.4–5.8) whereas cefoxitin susceptible isolates had resistance to 2.6 (95% CI = 1.9–3.4; p = 0.007) of nine classes of antimicrobials tested (Table 2). Interestingly, the acquisition of the cefoxitin resistance phenotype was associated with AMR of the isolates to as many as nine classes of antimicrobials (median value of 5). Among the cefoxitin-susceptible CNS isolates, some isolates also exhibited MDR against multiple classes of antimicrobials (range 1–4 different antimicrobials with a median value of 3) (Figure 2).
Table 2.
Acquiring cefoxitin resistance increased the spectrum of AMR in CNS to several classes of antimicrobials after controlling the effect of location and sample source as a random effect in the GLM model.
| Variables | No. of classes∗ of antimicrobials ineffective (Mean) | 95% CI | F | p-value | |
|---|---|---|---|---|---|
| Source | Abattoir | 3.5 | 2.8–4.3 | 6.726 | 0.054 |
| Dairy farm | 5.0 | 4.2–5.7 | |||
| Area | Assela | 2.8 | 1.4–4.1 | 2.237 | 0.227 |
| Adama | 3.4 | 2.1–4.6 | |||
| Holeta | 3.7 | 2.7–4.7 | |||
| Bishoftu | 4.3 | 3.4–5.2 | |||
| Addis Ababa | 5.2 | 4.1–6.3 | |||
| Cefoxitin | Cefoxitin-S | 2.6 | 1.9–3.4 | 21.990 | 0.007 |
| Cefoxitin-R | 5.1 | 4.4–5.8 |
The 14 antimicrobials tested were regrouped into nine broad classes of antimicrobials. Table 2 showed the average number of ineffective antimicrobials of the nine antimicrobial classes tested against an individual CNS isolate from abattoir, dairy farm, five different locations, and by cefoxitin response status.
Figure 2.
Cefoxitin-resistance was associated with resistance to several classes of antimicrobials by individual CNS isolates. Cefoxitin-resistant (dark bar) and cefoxitin-susceptible isolates (light bar) exhibited AMR ranging from two to nine and one to four different classes of antimicrobials, respectively. 94.1% of 34 cefoxitin-resistant and 54.5% of 22 cefoxitin-susceptible CNS isolates harbored resistance to 3 or more classes of antimicrobials.
3.5. The spectrum of AMR ranges by individual isolates and AMR phenotypic patterns
A single CNS isolate had resistance to at least one and some isolates had AMR to several antimicrobials as many as to 13 antimicrobials (range = 1–13). Of all CNS isolates, 7.1, 10.7, 7.1, 12.5, 17.9, 10.7, 12.5, 7.1, 1.8, 5.4, 1.8, and 5.4% exhibited antimicrobial resistance to single, double, 3, 4, 5, 6, 7, 7, 8, 9, 10, 11, and 13 antimicrobials, respectively.
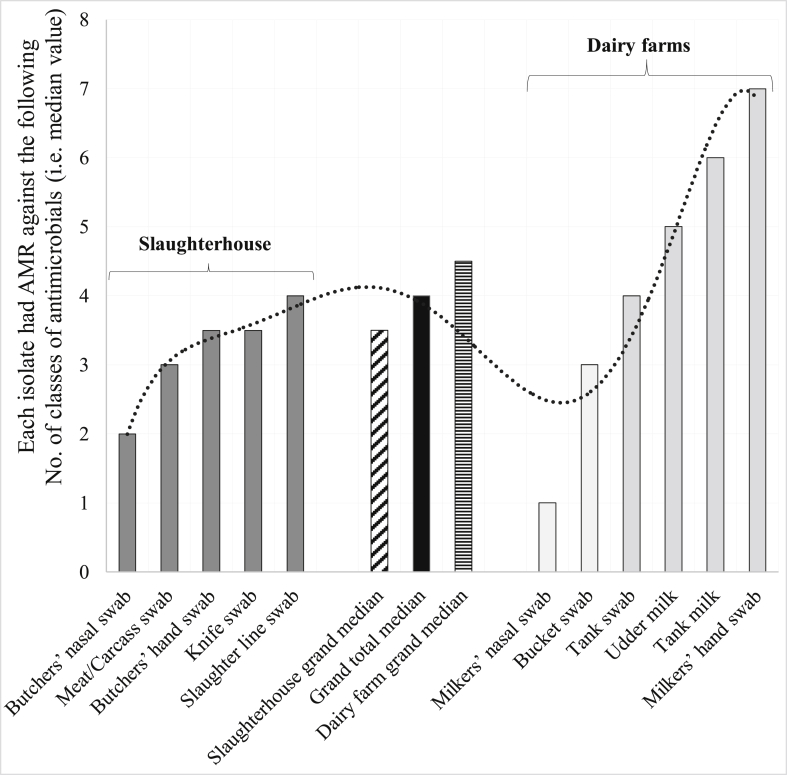
The overall prevalence of MDR in the study area of central Oromia was 78.6% based on the nine broad classes of 14 antimicrobials tested. The prevalence of MDR CNS isolates was not only high in different geographical locations (previous page), but also high in different sample sources except in nasal passages of milkers and butchers (Figure 3). Accordingly, 50% of CNS isolates from butchers nasal swab, meat/carcass, butchers hand, knife, slaughter line, milkers nasal swab, bucket, tank, udder milk, tank milk, and milkers hand exhibited AMR against 2, 3, 3.5, 3.5, 4, 1, 3, 4, 5, 6 and 7 classes of antimicrobials (i.e. median values), respectively (Figure 3).
Figure 3.
Of nine classes of antimicrobials tested, the number of classes of antimicrobials with poor efficacy against CNS isolates was summarized here using median value (50th percentile). The CNS isolates from different sample sources exhibited AMR resistance to three or more classes of antimicrobials (i.e. median values), except CNS isolates from nasal swabs of milkers and butchers. Overall, at least four classes of antimicrobials were ineffective against 50% of the isolates (dark bar in the middle). CNS isolates from dairy farms (horizontally serrated bar) exhibited more AMR to several antimicrobial classes than those from abattoirs (obliquely serrated bar). The dotted line showed trend line.
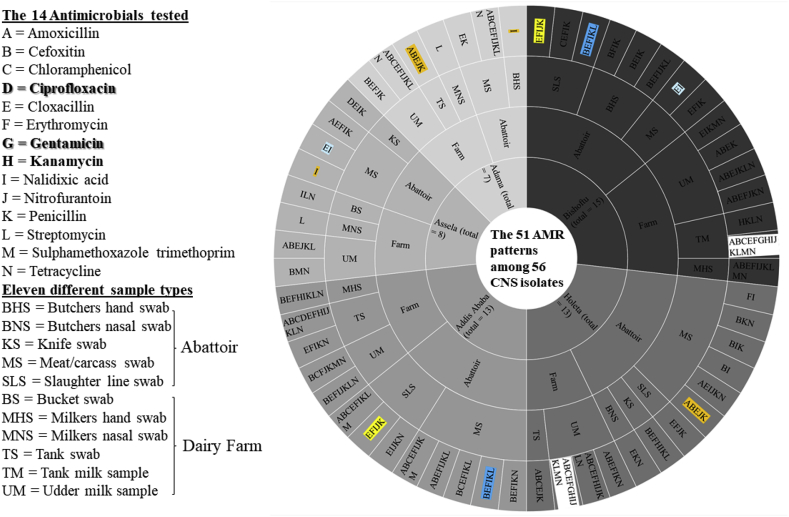
Further detailed analysis indicated that the 56 CNS isolates displayed 51 different AMR phenotypic patterns using 14 antimicrobial agents. The 32 abattoir isolates exhibited 28 different AMR patterns whereas the 24 dairy farm isolates displayed 23 different phenotypic patterns. Of the 56 isolates, four single drug-resistant isolates (7.1%) [milkers' nasal passages (2 isolates), 1 isolate from a meat swab, and 1 isolate from a butcher's hand swab] exhibited AMR to nalidixic acid and/or streptomycin as shown in Figure 4. Six isolates from the meat swab had double drug resistance. The remaining CNS isolates displayed heterogeneous AMR patterns ranging from three to 13 antimicrobials (Figure 4).
Figure 4.
AMR phenotypic patterns of CNS isolated from different sample types obtained from dairy farms and abattoirs in five locations of central Oromia, Ethiopia. Four isolates harbored single drug-resistance that was to either nalidixic acid (I) or streptomycin (L). They were from Adama and Assela. Overall, the CNS isolates displayed 51 different AMR phenotypic patterns using 14 different antimicrobials.
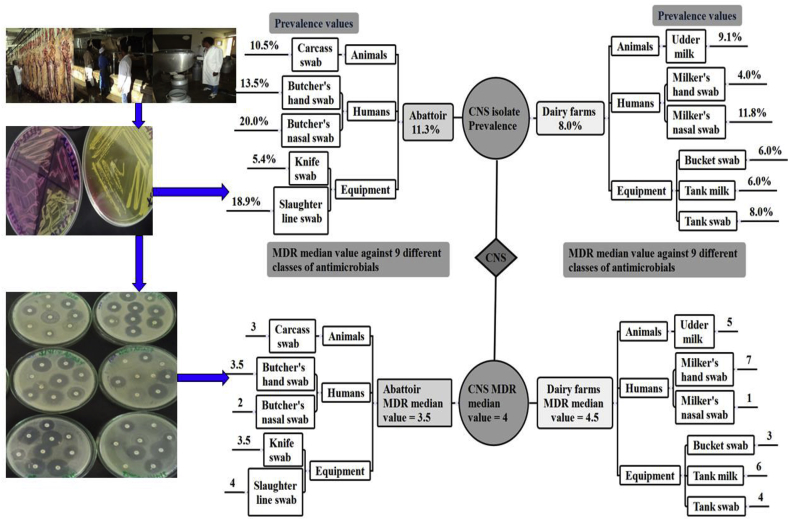
The overall distribution of CNS bacteria isolates in different niches and their AMR patterns in central Oromia is summarized in Figure 5.
Figure 5.
Summary of the overall distribution of CNS bacteria isolates in different niches and their AMR patterns in central Oromia, Ethiopia. The overall prevalence of CNS in abattoirs and dairy farms was 11.3 and 8.0%, respectively. The overall number of classes of antimicrobials ineffective among CNS isolated from abattoir and dairy farm were 3.5 and 4.5, respectively.
4. Discussion
CNS may cause mastitis in dairy cows (Taponen et al., 2008), produce biofilm (Szczuka et al., 2016), and serve as reservoirs for enterotoxin (Cunha et al., 2013) and AMR genes (Bhargava and Zhang, 2012, Bhargava and Zhang, 2014; Otto, 2013; Verraes et al., 2013; Osman et al., 2015). Thus, the presence of CNS on food, equipment, and food handlers can be food safety and public health risks, but epidemiological information regarding the important pathogens is scarce in Ethiopia. This study provides updated information on the prevalence of CNS (and their AMR patterns) in the milk, beef, food handlers and associated equipment on dairy farms and abattoir settings in five locations of central Oromia, Ethiopia.
In this study, the overall prevalence of CNS in central Oromia was 9.6%. It varied among the five geographical locations ranging from 6.7-12.4%. Similar to our finding, geographical variation in CNS prevalence has been reported in several other studies from around the world (Taponen et al., 2008; Gillespie et al., 2009; Pyörälä and Taponen, 2009; Supré et al., 2011). However, we didn't investigate whether spatial or bacteria genetic factors were playing a role in the variation in the prevalence. Some studies indicated that certain genotypes were highly virulent causing repeated infection and were more prevalent (Gillespie et al., 2009), which may explain the spatial variation in prevalence in the current study.
The prevalence of CNS was higher (>13.5%) on equipment and personnel than on carcasses (10.5%) in the abattoir setting. On dairy farms, CNS prevalence was higher in milkers' nasal swab samples (11.8%) than the udder milk (9.1%). These findings suggest that equipment/tool and personnel hygiene (i.e. poor washing and handling practices) may play a major role in food contamination. CNS has been reported as a causative agent of bovine mastitis, with a prevalence ranging from 1.7-41% in several studies with a significant variation around the world (van Loosdrecht et al., 1987; Taponen et al., 2008; Gillespie et al., 2009; Pyörälä and Taponen, 2009; Supré et al., 2011; Klibi et al., 2018). CNS has also been reported in meat (beef, chicken, and turkey) (Bhargava and Zhang, 2014). In the current study, CNS was ubiquitously present in different samples including in udder, tank milk, bucket, milk tank as well as from beef, knife, slaughter lines, and from the hands and nasal cavities of abattoir and farm personnel. These findings suggest that CNS is a highly resilient and adaptive organism able to grow (or survive) in different conditions. Thus, it may serve as an indicator of substandard hygienic, food handling practices, and food safety issues during milking, slaughtering, and storage.
In this study, the prevalence of AMR varied among the 14 antimicrobials tested. AMR against penicillin was the highest (82.1%) followed by cloxacillin (71.4%). Very high prevalence to penicillin (94.2%) in China, 77.8% in Pakistan (Syed et al., 2018) and 70.6% in Tunisia (Klibi et al., 2018) have been reported among CNS isolates from different animal species including humans. Overall, we noticed that ≥50% of all CNS isolates in central Oromia displayed AMR to four different classes of antimicrobials (i.e. the median number) with resistance ranges of 1–9 classes of antimicrobials. These resistant isolates were collected from milk, beef, equipment, and food handlers indicating widespread distribution MDR CNS isolates. The reasons for widespread MDR isolates in Ethiopia may be due to overuse of antimicrobials (Abera et al., 2014; Yadesa et al., 2015; Gebretekle and Serbessa, 2016), abuse in veterinary clinics (Beyene et al., 2015, 2016; Luseba and Rwambo, 2015; Suleman et al., 2016), and the unregulated movement (smuggling) and mishandling of antimicrobials (Suleman et al., 2016). That said, the majority of (96.4%) of the CNS isolates studied were susceptible to both gentamicin and ciprofloxacin, even the cefoxitin-resistant isolates. Contrary to the 3.6% prevalence among CNS isolates in the current study, a study elsewhere reported higher (18.4%) gentamycin resistance (Ma et al., 2011). This may suggest that the use, prescription or distribution of gentamycin and ciprofloxacin might be the reason for the variation. The use of both antimicrobials in veterinary practice seems low in Ethiopia since information in the literature is scarce on the sensitivity of CNS isolates from animals to gentamicin and ciprofloxacin.
A widely accepted indicator of MDR is methicillin-resistance as both traits may be linked to a single genetic element that confers resistance to methicillin and the most commonly prescribed class of antimicrobials (Grundmann et al., 2006; Osman et al., 2015). Because we didn't have access to methicillin disks, we used cefoxitin (30μg) as it is a reliable predictor of the presence of methicillin resistance (MR) gene i.e. mecA (Fernandes et al., 2005; Swenson and Tenover, 2005; Broekema et al., 2009; Loomba et al., 2010). Cefoxitin has been suggested for predicting MDR to all beta-lactam agents (Tan et al., 2009; Polsfuss et al., 2011). Accordingly, cefoxitin testing has been suggested as an indicator for detecting resistance to a number of antimicrobials such as penicillins, aminopenicillins (e.g. ampicillin, amoxicillin), cephalosporins, carbapenems and monobactams (Kong et al., 2009; Dallenne et al., 2010). The reason for reliability of cefoxitin testing is due to its ability to predict the acquisition of mecA and/or mecC genes that result in the acquisition of penicillin-binding enzymes/proteins (PBP 2a or PBP 2′) by bacteria and these enzymes make all beta-lactam antibiotics ineffective (Dien Bard et al., 2014). In this study, 60.7% of CNS isolates displayed cefoxitin resistance, but we did not test for the mecA or C genes. Methicillin/cefoxitin-resistant CNS isolates have been widely reported but the prevalence values vary by host and geographical sources (Otto, 2013). The prevalence of methicillin-resistant CNS isolates in studies from elsewhere ranges from 13-30% in animals (Vanderhaeghen et al., 2012; Argudín and Butaye, 2016), 8.7% in retail meat (beef, chicken, and turkey) (Bhargava and Zhang, 2014), 20.6% in milk (Klibi et al., 2018), 19–60% in humans from different parts of the world (Barbier et al., 2010; Ma et al., 2011; Talebi et al., 2015; Syed et al., 2018), and 47% in humans in Ethiopia (Deyno et al., 2017).
Cefoxitin-resistant CNS isolates tend to be resistant to an average of 7.7 antimicrobials (95% CI = 6.7–8.8 antimicrobials) including penicillin, cloxacillin, and other antimicrobials in the current study. CNS isolates have been reported with both methicillin-resistance and multidrug-resistant in Europe (Stefani and Varaldo, 2003). Such methicillin-resistant with MDR phenotypes have been detected from animals (Osman et al., 2015) and humans (Agvald-Öhman et al., 2003). In the current study, 94.1 and 54.5% of cefoxitin-R and cefoxitin-S CNS isolates, respectively, harbored resistance to 3 or more classes of antimicrobials (i.e. were MDR). The MDR carrier state can make CNS bacteria the reservoirs and source of multiple AMR genes that commensal or pathogenic bacteria can pick up (Agvald-Öhman et al., 2003; Bhargava and Zhang, 2012, Bhargava and Zhang, 2014; Otto, 2013; Verraes et al., 2013; Osman et al., 2015). The finding is alarming for Ethiopia due to the high frequency of intense human-animal interaction, the widespread consumption of raw meat and milk and substandard food handling practices (Seleshe et al., 2014; Eshetie et al., 2018). Overall, 78.6% of CNS isolates in the current study area exhibited MDR using the nine broad classes of antimicrobial agents. The findings of others have indicated that the emergence of MDR is correlated with the use patterns of antimicrobials on farm (Mouton et al., 1990), inclusion of metals in the feed (Argudín and Butaye, 2016), and the distribution of AMR gene encoding plasmids in bacteria population (Lopatkin et al., 2017). However, we did not investigate the social, plasmid, spatial factors, and antimicrobial use patterns in central Oromia that contributed to the MDR among CNS isolates. Antimicrobial use patterns such as intensive cloxacillin and cephalosporin consumption have been reported for promoting the emergence of methicillin-resistant or MDR CNS through selection (Mouton et al., 1990).
In this study, we hypothesized that cefoxitin resistance could serve as an indicator of MDR CNS; however, some cefoxitin-susceptible CNS isolates also harbored multidrug-resistance. Moreover, some cefoxitin-resistant CNS isolates were susceptible to most or all of the other antimicrobials tested in the current study (Supplementary 1). In addition, some cefoxitin-susceptible CNS isolates also harbored MDR suggesting that cefoxitin testing alone has limitations and may not capture all resistance mechanisms circulating in Ethiopia. To complement the cefoxitin biomarker, developing an economically and technically feasible test for antimicrobial resistance is essential to protect the public. Some researchers have suggested testing two or three combinations of antimicrobials rather than testing bacteria isolates against a single or whole range of antimicrobials within diverse antimicrobial classes for determining susceptibility/resistance status of foodborne pathogens. These researchers have suggested combined test of cefoxitin and cloxacillin (Tan et al., 2009; Polsfuss et al., 2011), penicillin and cefoxitin (Dien Bard et al., 2014) or vancomycin (Schwalbe et al., 1987; Srinivasan et al., 2002; Cremniter et al., 2010; Das et al., 2011; Martins et al., 2013; Gardete and Tomasz, 2014; Hasan et al., 2016).
The 56 CNS isolates tested in this study displayed 51 different AMR phenotypic patterns using 14 antimicrobial agents. In the literature, information is scarce on the total number of AMR phenotypic patterns displayed by CNS. We recently identified 21 AMR patterns in similar Staphylococci (i.e. bovine S. aureus in the USA), which were clustered into nine genetically distinct groups (Abdi et al., 2018). We did not investigate whether the CNS isolates in the 51 different AMR phenotypic patterns were genetically different from each other, but we predict that such phenotypically heterogeneous isolates may also have genetic heterogeneity based on findings from elsewhere. A study in the USA indicated that CNS isolates from different sources are highly heterogeneous and genetically unrelated, but those from the same body site during repeated sampling are genetically similar (Gillespie et al., 2009), the similarity may be due to persistence.
5. Conclusions
The prevalence of CNS in central Oromia is 9.6% varied between the five locations and between dairy farms and abattoirs. Some specific niches such as equipment, hands, and nasal passages of personnel are significant hotspots for CNS. Poor hygienic practices of equipment and personnel who handle milk and meat are the possible source for onward contamination of food and transmission to the consumers. The majority of CNS (78.6%) in central Oromia were MDR (i.e. ≥ 3 antimicrobials). Phenotypically, these CNS populations displayed 51 different AMR phenotypes. The prevalence of methicillin/cefoxitin-resistance in food-borne pathogens are a potential threat to public health in Ethiopia. Therefore, we recommend farm and abattoir workers safety, food hygiene, and proper cooking practices, prudent drug use, regular monitoring and tracing of CNS phenotypes, genotypes, and AMR. The mechanisms of resistance of CNS at play in Ethiopia requires further study. There is a lack of information on veterinary antimicrobial use patterns and possible transmission of CNS from animals to humans in Ethiopia. Since livestock may be reservoirs, we suggest that control of CNS infections in livestock will be an important step in controlling the transmission of AMR CNS isolates to humans.
Declarations
Author contribution statement
Fikru Gizaw: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tolera Kekeba, Fikadu Teshome, Matewos Kebede, Tekeste Abreham, Halefom Hayishe: Performed the experiments.
Hika Waktole: Contributed reagents, materials, analysis tools or data.
Takele Beyene Tufa, Fufa Abunna, Ashenafi Feyisa Beyi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Bedaso Mammo Edao, Dinka Ayana: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Reta Duguma Abdi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Office of Research and Technology Transfer of the Addis Ababa University, Ethiopia, with grant ID (RD/LT/194/2013).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank dairy farm and abattoir workers for their cooperation.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abdi R.D., Gillespie B.E., Vaughn J. Antimicrobial resistance of Staphylococcus aureus isolates from dairy cows and genetic diversity of resistant isolates. Foodborne Pathog Dis. 2018;15:449–458. doi: 10.1089/fpd.2017.2362. [DOI] [PubMed] [Google Scholar]
- Abera B., Kibret M., Mulu W. Knowledge and beliefs on antimicrobial resistance among physicians and nurses in hospitals in Amhara Region, Ethiopia. BMC Pharmacol. Toxicol. 2014;15:26. doi: 10.1186/2050-6511-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agvald-Öhman C., Lund B., Edlund C. Multiresistant coagulase-negative staphylococci disseminate frequently between intubated patients in a multidisciplinary intensive care unit. Crit. Care. 2003;8:R42–R47. doi: 10.1186/cc2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argudín M.A., Butaye P. Dissemination of metal resistance genes among animal methicillin-resistant coagulase-negative staphylococci. Res. Vet. Sci. 2016;105:192–194. doi: 10.1016/j.rvsc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Barbier F., Ruppé E., Hernandez D. Methicillin-resistant coagulase-negative staphylococci in the community: high homology of SCCmec IVa between Staphylococcus epidermidis and major clones of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2010;202:270–281. doi: 10.1086/653483. [DOI] [PubMed] [Google Scholar]
- Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene T., Endalamaw D., Tolossa Y., Feyisa A. Evaluation of rational use of veterinary drugs especially antimicrobials and anthelmintics in Bishoftu, Central Ethiopia. BMC Res. Notes. 2015;8:482. doi: 10.1186/s13104-015-1466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene T., Assefa S., Ayana D., Beyene T.J., Tadesse F., Woldemichael D.N. Assessment of rational veterinary drugs use in livestock at Adama district veterinary clinic, central Ethiopia. J. Vet. Sci. Technol. 2016;7:319–326. [Google Scholar]
- Bhargava K., Zhang Y. Multidrug-resistant coagulase-negative staphylococci in food animals. J. Appl. Microbiol. 2012;113:1027–1036. doi: 10.1111/j.1365-2672.2012.05410.x. [DOI] [PubMed] [Google Scholar]
- Bhargava K., Zhang Y. Characterization of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in retail meat. Food Microbiol. 2014;42:56–60. doi: 10.1016/j.fm.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Broekema N.M., Van T.T., Monson T.A., Marshall S.A., Warshauer D.M. Comparison of cefoxitin and oxacillin disk diffusion methods for detection of mecA-mediated resistance in Staphylococcus aureus in a large-scale study. J. Clin. Microbiol. 2009;47:217–219. doi: 10.1128/JCM.01506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J.D. Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl. Environ. Microbiol. 1982;44:992. doi: 10.1128/aem.44.4.992-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajęcka-Wierzchowska W., Zadernowska A., Nalepa B., Sierpińska M., Łaniewska-Trokenheim Ł. Retail ready-to-eat food as a potential vehicle for staphylococcus spp. harboring antibiotic resistance genes. J. Food Protect. 2014;77:993–998. doi: 10.4315/0362-028X.JFP-13-466. [DOI] [PubMed] [Google Scholar]
- Chajęcka-Wierzchowska W., Zadernowska A., Nalepa B., Sierpińska M., Łaniewska-Trokenheim Ł. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin – phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015;46:222–226. doi: 10.1016/j.fm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- CLSI . 2015. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, CLSI Supplement VET01S. Wayne, Pennsylvania, USA; p. 240. [Google Scholar]
- Cook E.A.J., de Glanville W.A., Thomas L.F., Kariuki S., de Clare Bronsvoort B.M., Fèvre E.M. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Publ. Health. 2017;17:14. doi: 10.1186/s12889-016-3923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coton E., Desmonts M.-H., Leroy S., Coton M., Jamet E., Christieans S., Donnio P.-Y., Lebert I., Talon R. Biodiversity of coagulase-negative staphylococci in French cheeses, dry fermented sausages, processing environments and clinical samples. Int. J. Food Microbiol. 2010;137:221–229. doi: 10.1016/j.ijfoodmicro.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Cremniter J., Slassi A., Quincampoix J.-C. Decreased susceptibility to teicoplanin and vancomycin in coagulase-negative staphylococci isolated from orthopedic-device-aAssociated infections. J. Clin. Microbiol. 2010;48:1428–1431. doi: 10.1128/JCM.02098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha M., Calsolari R.A.O., Júnior J.P.A. Detection of enterotoxin and toxic shock syndrome toxin 1 genes in staphylococcus, with emphasis on coagulase-negative staphylococci. Microbiol. Immunol. 2013;51:381–390. doi: 10.1111/j.1348-0421.2007.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Cunha MdLRS., Sinzato Y.K., Silveira L.V.A. Comparison of methods for the identification of coagulase-negative staphylococci. Memórias do Inst. Oswaldo Cruz. 2004;99:855–860. doi: 10.1590/s0074-02762004000800012. [DOI] [PubMed] [Google Scholar]
- Dallenne C., Da Costa A., Decré D., Favier C., Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- Das M.K., Pathengay A., Shah G.Y., Koday N.K. Vancomycin-resistant coagulase negative Staphylococcus endophthalmitis following cataract surgery. J. Cataract Refract. Surg. 2011;37:1908–1909. doi: 10.1016/j.jcrs.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Davis M.F., Cain C.L., Brazil A.M., Rankin S.C. Two coagulase-negative staphylococci emerging as potential zoonotic pathogens: wolves in sheep's clothing? Front. Microbiol. 2013;4:123. doi: 10.3389/fmicb.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyno S., Fekadu S., Astatkie A. Resistance of Staphylococcus aureus to antimicrobial agents in Ethiopia: a meta-analysis. Antimicrob. Resist. Infect. Contr. 2017;6:85. doi: 10.1186/s13756-017-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien Bard J., Hindler J.A., Gold H.S., Limbago B. Rationale for eliminating staphylococcus breakpoints for β-lactam agents other than penicillin, oxacillin or cefoxitin, and ceftaroline. Clin. Infect. Dis. 2014;58:1287–1296. doi: 10.1093/cid/ciu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshetie T., Hussen K., Teshome T., Mekonnen A. Meat production, consumption and marketing tradeoffs and potentials in Ethiopia and its effect on GDP growth: a review. J. Market. Consum. Res. 2018;42:228–233. [Google Scholar]
- Fernandes C.J., on behalf of the Australian Group on Antimicrobial R. Fernandes L.A., on behalf of the Australian Group on Antimicrobial R. Collignon P., on behalf of the Australian Group on Antimicrobial R Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2005;55:506–510. doi: 10.1093/jac/dki052. [DOI] [PubMed] [Google Scholar]
- Fijałkowski K., Peitler D., Karakulska J. Staphylococci isolated from ready-to-eat meat – identification, antibiotic resistance and toxin gene profile. Int. J. Food Microbiol. 2016;238:113–120. doi: 10.1016/j.ijfoodmicro.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Gardete S., Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Invest. 2014;124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebretekle G.B., Serbessa M.K. Exploration of over the counter sales of antibiotics in community pharmacies of Addis Ababa, Ethiopia: pharmacy professionals’ perspective. Antimicrob. Resist. Infect. Contr. 2016;5:2. doi: 10.1186/s13756-016-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie B.E., Headrick S.I., Boonyayatra S., Oliver S.P. Prevalence and persistence of coagulase-negative staphylococcus species in three dairy research herds. Vet. Microbiol. 2009;134:65–72. doi: 10.1016/j.vetmic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- Gumi B., Schelling E., Berg S. Zoonotic transmission of tuberculosis between pastoralists and their llvestock in South-East Ethiopia. EcoHealth. 2012;9:139–149. doi: 10.1007/s10393-012-0754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan W.F. Academic Press; San Diego: 1998. Laboratory Methods in Food Microbiology. [Google Scholar]
- Hasan R., Acharjee M., Noor R. Prevalence of vancomycin resistant Staphylococcus aureus (VRSA) in methicillin resistant S. aureus (MRSA) strains isolated from burn wound infections. Tzu Chi Med. J. 2016;28:49–53. doi: 10.1016/j.tcmj.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia N., García S. Animals as sources of food-borne pathogens: a review. Anim. Nutr. 2018;4:250–255. doi: 10.1016/j.aninu.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Ziegler D., Pflüger V., Vogel G., Zweifel C., Stephan R. Prevalence and characteristics of methicillin-resistant coagulase-negative staphylococci from livestock, chicken carcasses, bulk tank milk, minced meat, and contact persons. BMC Vet. Res. 2011;7:6. doi: 10.1186/1746-6148-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacumin L., Comi G., Cantoni C., Cocolin L. Ecology and dynamics of coagulase-negative cocci isolated from naturally fermented Italian sausages. Syst. Appl. Microbiol. 2006;29:480–486. doi: 10.1016/j.syapm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Klibi A., Maaroufi A., Torres C., Jouini A. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents. 2018;52:930–935. doi: 10.1016/j.ijantimicag.2018.07.026. [DOI] [PubMed] [Google Scholar]
- Kloos W.E., Schleifer K.H. Simplified scheme for routine identification of human staphylococcus species. J. Clin. Microbiol. 1975;1:82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K.-F., Schneper L., Mathee K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. cta Pathologica, Microbiologica et Immunologica Scandinavica. 2009;118:1–36. doi: 10.1111/j.1600-0463.2009.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeta G., Curiel J.A., Carrascosa A.V., Muñoz R., de las Rivas B. Characterization of coagulase-negative staphylococci isolated from Spanish dry cured meat products. Meat Sci. 2013;93:387–396. doi: 10.1016/j.meatsci.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Leta S., Mesele F. Spatial analysis of cattle and shoat population in Ethiopia: growth trend, distribution and market access. SpringerPlus. 2014;3:310. doi: 10.1186/2193-1801-3-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba P.S., Taneja J., Mishra B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J. Global Infect. Dis. 2010;2:275–283. doi: 10.4103/0974-777X.68535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatkin A.J., Meredith H.R., Srimani J.K., Pfeiffer C., Durrett R., You L. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat. Commun. 2017;8:1689. doi: 10.1038/s41467-017-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lues J.F., Van Tonder I. The occurrence of indicator bacteria on hands and aprons of food handlers in the delicatessen sections of a retail group. Food Contr. 2007;18:326–332. [Google Scholar]
- Luseba D., Rwambo P. Review of the policy, regulatory and administrative framework for delivery of livestock health products and services in Eastern and Southern Africa. GALVmed. 2015. https://assets.publishing.service.gov.uk/media/5aa66defed915d4f563b6e92/48_PLS_ESA_report_March2015.pdf pp.52.
- Ma X.X., Wang E.H., Liu Y., Luo E.J. Antibiotic susceptibility of coagulase-negative staphylococci (CoNS): emergence of teicoplanin-non-susceptible CoNS strains with inducible resistance to vancomycin. J. Med. Microbiol. 2011;60:1661–1668. doi: 10.1099/jmm.0.034066-0. [DOI] [PubMed] [Google Scholar]
- Martins P.D., de Almeida T.T., Basso A.P., de Moura T.M., Frazzon J., Tondo E.C., Frazzon A.P.G. Coagulase-positive staphylococci isolated from chicken meat: pathogenic potential and vancomycin resistance. Foodborne Pathog Dis. 2013;10:771–776. doi: 10.1089/fpd.2013.1492. [DOI] [PubMed] [Google Scholar]
- Mouton R.P., Hermans J., Simoons-Smit A.M., Hoogkamp-Korstanje J.A.A., Degener J.E., Bv Klingeren. Correlations between consumption of antibiotics and methicillin resistance in coagulase negative staphylococci. J. Antimicrob. Chemother. 1990;26:573–583. doi: 10.1093/jac/26.4.573. [DOI] [PubMed] [Google Scholar]
- Nemati M., Hermans K., Vancraeynest D., De Vliegher S., Sampimon O.C., Baele M., De Graef E.M., Pasmans F., Haesebrouck F. Screening of bovine coagulase-negative staphylococci from milk for superantigen-encoding genes. Vet. Rec. 2008;163:740–743. [PubMed] [Google Scholar]
- Nunes R.S.C., Aguila E.M.D., Paschoalin V.M.F. Safety evaluation of the coagulase-negative staphylococci microbiota of salami: superantigenic toxin production and antimicrobial resistance. BioMed Res. Int. 2015;483548 doi: 10.1155/2015/483548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman K., Badr J., Al-Maary K.S., Moussa I.M.I., Hessain A.M., Girah Z.M.S.A., Abo-shama U.H., Orabi A., Saad A. Prevalence of the antibiotic resistance genes in coagulase-positive-and negative-staphylococcus in chicken meat retailed to consumers. Front. Microbiol. 2016;7:1846. doi: 10.3389/fmicb.2016.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman K.M., Abd El-Razik K.A., Marie H.S.H., Arafa A. Coagulase-negative staphylococci collected from bovine milk: species and antimicrobial gene diversity. J. Food Saf. 2015;36:89–99. [Google Scholar]
- Osman K.M., Amer A.M., Badr J.M., Helmy N.M., Elhelw R.A., Orabi A., Bakry M., Saad A.S.A. Antimicrobial resistance, biofilm formation and mecA characterization of methicillin-susceptible S. aureus and non-S. aureus of beef meat origin in Egypt. Front. Microbiol. 2016;7:222. doi: 10.3389/fmicb.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection. Bioesays. 2013;35:4–11. doi: 10.1002/bies.201200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento G., Ducci B., Comodo N., Nostro A.L. Antimicrobial resistance profile of Staphylococcus aureus isolated from raw meat: a research for methicillin resistant Staphylococcus aureus (MRSA) Food Contr. 2007;18:196–200. [Google Scholar]
- Polsfuss S., Bloemberg G.V., Giger J., Meyer V., Böttger E.C., Hombach M. Practical approach for reliable detection of AmpC beta-lactamase-producing Enterobacteriaceae. J. Clin. Microbiol. 2011;49:2798–2803. doi: 10.1128/JCM.00404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyörälä S., Taponen S. Coagulase-negative staphylococci—emerging mastitis pathogens. Vet. Microbiol. 2009;134:3–8. doi: 10.1016/j.vetmic.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Rall V.L.M., Miranda E.S., Castilho I.G., Camargo C.H., Langoni H., Guimarães F.F., Araújo Júnior J.P., Fernandes Júnior A. Diversity of staphylococcus species and prevalence of enterotoxin genes isolated from milk of healthy cows and cows with subclinical mastitis. J. Dairy Sci. 2014;97:829–837. doi: 10.3168/jds.2013-7226. [DOI] [PubMed] [Google Scholar]
- Rani Z.T., Hugo A., Hugo C.J., Vimiso P., Muchenje V. Effect of post-slaughter handling during distribution on microbiological quality and safety of meat in the formal and informal sectors of South Africa: a review. S. Afr. J. Anim. Sci. 2017;47:255–267. [Google Scholar]
- Regassa A., Medhin G., Ameni G. Bovine tuberculosis is more prevalent in cattle owned by farmers with active tuberculosis in central Ethiopia. Vet. J. 2008;178:119–125. doi: 10.1016/j.tvjl.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rodríguez A., Gordillo R., Andrade M.J., Córdoba J.J., Rodríguez M. Development of an efficient real-time PCR assay to quantify enterotoxin-producing staphylococci in meat products. Food Contr. 2016;60:302–308. [Google Scholar]
- Ruaro A., Andrighetto C., Torriani S., Lombardi A. Biodiversity and characterization of indigenous coagulase-negative staphylococci isolated from raw milk and cheese of North Italy. Food Microbiol. 2013;34:106–111. doi: 10.1016/j.fm.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Schwalbe R.S., Stapleton J.T., Gilligan P.H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N. Engl. J. Med. 1987;316:927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Seleshe S., Jo C., Lee M. Meat consumption culture in Ethiopia. Korean J. Food Sci. Animal. Res. 2014;34:7–13. doi: 10.5851/kosfa.2014.34.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R.C. Predominant pathogens found in the European prevalence of infection in intensive care study. Eur. J. Clin. Microbiol. Infect. Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Dick J.D., Perl T.M. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 2002;15:430–438. doi: 10.1128/CMR.15.3.430-438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani S., Varaldo P.E. Epidemiology of methicillin-resistant staphylococci in Europe. Clin. Microbiol. Infec. 2003;9:1179–1186. doi: 10.1111/j.1469-0691.2003.00698.x. [DOI] [PubMed] [Google Scholar]
- Suleman S., Woliyi A., Woldemichael K., Tushune K., Duchateau L., Degroote A., Vancauwenberghe R., Bracke N., Spiegeleer B.D. Pharmaceutical regulatory framework in Ethiopia: a critical evaluation of its legal basis and implementation. Ethiopian J. Health Sci. 2016;26:259–276. doi: 10.4314/ejhs.v26i3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supré K., Haesebrouck F., Zadoks R.N., Vaneechoutte M., Piepers S., De Vliegher S. Some coagulase-negative staphylococcus species affect udder health more than others. J. Dairy Sci. 2011;94:2329–2340. doi: 10.3168/jds.2010-3741. [DOI] [PubMed] [Google Scholar]
- Swenson J.M., Tenover F.C. Results of disk diffusion testing with cefoxitin orrelate with presence of mecA in staphylococcus spp. J. Clin. Microbiol. 2005;43:3818–3823. doi: 10.1128/JCM.43.8.3818-3823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed E., Satti F.N., Mubasher S., Rasheed F., Ilyas M., Anwar A.I., Wu Zaman. Coagulase-negative staphylococcus species; resistance and therapeutic decisions at the turn of the novel millennium. Prof. Med. J. 2018;25:764–769. [Google Scholar]
- Szczuka E., Jabłońska L., Kaznowski A. Coagulase-negative staphylococci: pathogenesis, occurrence of antibiotic resistance genes and in vitro effects of antimicrobial agents on biofilm-growing bacteria. J. Med. Microbiol. 2016;65:1405–1413. doi: 10.1099/jmm.0.000372. [DOI] [PubMed] [Google Scholar]
- Talebi M., Shafiee M., Sadeghi J., Moghadam N.A., Saifi M., Pourshafie M.R. Genotypic diversity of methicillin-resistant coagulase-negative staphylococci isolated from inpatients and outpatients. Microb. Drug Resist. 2015;22:147–154. doi: 10.1089/mdr.2014.0195. [DOI] [PubMed] [Google Scholar]
- Tan T.Y., Ng L.S.Y., He J., Koh T.H., Hsu L.Y. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob. Agents Chemother. 2009;53:146–149. doi: 10.1128/AAC.00862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taponen S., Björkroth J., Pyörälä S. Coagulase-negative staphylococci isolated from bovine extramammary sites and intramammary infections in a single dairy herd. J. Dairy Res. 2008;75:422–429. doi: 10.1017/S0022029908003312. [DOI] [PubMed] [Google Scholar]
- Thakur P., Nayyar C., Tak V., Saigal K. Mannitol-fermenting and tube coagulase-negative staphylococcal isolates: unraveling the diagnostic dilemma. J. Lab. Phys. 2017;9:65–66. doi: 10.4103/0974-2727.187926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A. Themes in urban infrastructure research in Ethiopian cities. In: Tiwari A., editor. Urban Infrastructure Research: A Review of Ethiopian Cities. Springer International Publishing; Cham: 2016. pp. 7–34. [Google Scholar]
- van Loosdrecht M.C., Lyklema J., Norde W., Schraa G., Zehnder A.J. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen W., Vandendriessche S., Crombé F., Nemeghaire S., Dispas M., Denis O., Hermans K., Haesebrouck F., Butaye P. Characterization of methicillin-resistant non-Staphylococcus aureus staphylococci carriage isolates from different bovine populations. J. Antimicrob. Chemother. 2012;68:300–307. doi: 10.1093/jac/dks403. [DOI] [PubMed] [Google Scholar]
- Verraes C., Van Boxstael S., Van Meervenne E. Antimicrobial resistance in the food chain: a review. Int. J. Environ. Res. Publ. Health. 2013;10:2643–2669. doi: 10.3390/ijerph10072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamai R.G. Reviewing Ethiopia’s health system development. J. Jpn. Med. Assoc. 2009;52:279–286. [Google Scholar]
- Yadesa T.M., Gudina E.K., Angamo M.T. Antimicrobial use-related problems and predictors among hospitalized medical in-patients in Southwest Ethiopia: prospective observational study. PloS One. 2015;10 doi: 10.1371/journal.pone.0138385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell C., Resch M., Rosenstein R., Albrecht T., Hertel C., Götz F. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int. J. Food Microbiol. 2008;127:246–251. doi: 10.1016/j.ijfoodmicro.2008.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.