ABSTRACT
In newborn dairy calves, it has been demonstrated that supranutritional maternal and colostral Se supplementation using Se yeast or sodium selenite, respectively, improves passive transfer of IgG. In beef cattle, agronomic biofortification with Se is a more practical alternative for Se supplementation, whereby the Se concentration of hay is increased through the use of Se-containing fertilizer amendments. It has been previously demonstrated that agronomic Se biofortification is an effective strategy to improve immunity and performance in Se-replete weaned beef calves. The objective of this experiment was to determine the effects of feeding beef cows Se-enriched alfalfa (Medicago sativa) hay during the last 8 to 12 wk of gestation on passive transfer of antibodies to calves. At 10 wk ± 16 d before calving, 45 cows were assigned to 1 of 3 treatment groups with 3 pens (5 cows/pen) per treatment: Control cows were fed non-Se-fortified alfalfa hay plus a mineral supplement containing 120 mg/kg Se from sodium selenite, Med-Se cows were fed alfalfa hay fertilized with 45.0 g Se/ha as sodium selenate, and High-Se cows were fed alfalfa hay fertilized with 89.9 g Se/ha as sodium selenate; both the Med-Se and the High-Se groups received mineral supplement without added Se. Colostrum and whole blood (WB) were collected from cows at calving, and WB was collected from calves within 2 h of calving and at 12, 24, 36, and 48 h of age. Concentrations of IgG1 and J-5 Escherichia coli antibody in cow colostrum and calf serum were quantified using ELISA procedures. Selenium concentrations linearly increased in WB (P < 0.001) and colostrum (P < 0.001) of cows and in WB of newborn calves (P < 0.001) with increasing Se concentration in alfalfa hay. Colostrum concentrations of IgG1 (P = 0.03) were increased in cows fed Se-biofortified alfalfa hay, but J-5 E. coli antibody (P = 0.43) concentrations were not. Calf serum IgG1 (P = 0.43) and J-5 E. coli antibody (P = 0.44) concentrations during the first 48 h of age were not affected by prior Se treatment of cows. These data suggest that feeding Se-biofortified alfalfa hay promotes the accumulation of Se and antibodies in colostrum but does not affect short-term serum antibody concentrations in calves.
Keywords: colostrum, newborn calves, passive absorption of immunoglobulin G1, pregnant beef cows, selenium, selenium-enriched alfalfa hay
INTRODUCTION
Selenium is an essential trace mineral important for immune function and overall health of cattle (Ammerman and Miller, 1975; Hefnawy and Tortora-Perez, 2010). Insufficient Se intake can predispose cattle and calves to subclinical diseases, resulting in poor livestock performance (Hefnawy and Tortora-Perez, 2010; Willshire and Payne, 2011; Ahsan et al., 2014). The role of Se in maintaining animal health is based primarily on the functions of selenocysteine-containing proteins, many of which have antioxidant activities (Fairweather-Tait et al., 2010).
In Se-deficient areas, several means of Se supplementation are available (e.g., organic Se yeast or inorganic sodium selenite can be added to the feed or mineral mixes for free-choice consumption; FDA, 2016). Agronomic Se biofortification is another alternative, whereby the Se concentration of forages is increased through the use of Se-containing fertilizer amendments (Whelan, 1989; Mäkelä et al., 1993; Broadley et al., 2006; Hall et al., 2011). It has been previously demonstrated that agronomic Se biofortification is an effective strategy to improve immunity and performance in Se-replete weaned beef calves; weaned beef calves had increased J-5 Escherichia coli antibody titers, lower mortality, and greater slaughter weights with Se biofortification (Hall et al., 2013a,b). In newborn dairy calves, Hall et al. (2014) have shown that supranutritional maternal and colostral Se supplementation, albeit using Se yeast or sodium selenite, respectively, improves passive transfer of IgG and, ultimately, the health of newborn calves.
The objective of this study was to determine the effects of feeding Se-replete beef cows Se-enriched alfalfa (Medicago sativa) hay during the last 8 to 12 wk of gestation on Se status and passive transfer of antibodies to their calves. The hypothesis is that feeding Se-replete beef cows Se-enriched alfalfa hay during the last 8 to 12 wk of gestation increases whole blood (WB) Se and serum IgG1 concentrations (an indicator of general immunity) and J-5 E. coli antibody titers (an indicator of specific immunity) in nursing beef calves.
MATERIALS AND METHODS
Animal Ethics Statement and Study Design
The experimental protocol was reviewed and approved by the Oregon State University Animal Care and Use Committee (Animal Care and Use Proposal number 4629). This was a prospective clinical trial of 14 wk duration (December 6, 2014, through March 9, 2015) involving 45 pregnant beef cows, primarily of Angus breeding. Multiple bulls had been initially used to establish pregnancy by AI; several bulls were then used to cover nonpregnant cows. The study design consisted of 3 treatment groups, with 3 pens of 5 cows/pen per treatment. The study was conducted at the Hogg Animal Metabolism barn on the Oregon State University campus (Corvallis, OR).
Corvallis is located at an elevation of 72 m within the Marine West Coast climate zone. Temperatures are mild year round, with warm, sunny summers and mild, wet winters with persistent overcast skies. Because of its close proximity to the coast range, temperatures dropping below freezing are uncommon. Rainfall total is 110.9 cm/yr. Typical distribution of precipitation includes about 50% of the annual total from December through February, lesser amounts in the spring and fall, and very little during the summer. Winter snow is uncommon, but if it occurs, it is usually in the form of heavy wet snow, ranging from a dusting to several centimeters that does not persist on the ground for more than a few days.
The cows ranged in age from 2 to 14 yr (4.0 yr ± 2.4) and originated from the Oregon State University beef ranch (Corvallis, OR). All cows had previously calved at least once. Body weights at 7 mo of pregnancy ranged from 420 to 757 kg (613.2 kg ± 95.5), and BCS ranged from 6 to 7 (1 to 9 scale). Routine farm management practices included the following vaccinations during Fall 2014: bovine rhinotracheitis virus diarrhea, parainfluenza 3, and respiratory syncytial virus vaccine, modified live virus, and Leptospira canicola, Leptospira grippotyphosa, Leptospira hardjo, Leptospira icterohemorrhagiae, and Leptospira pomona bacterin (Pyramid 10; Boehringer Ingelheim Vetmedica, St. Joseph, MO); Clostridium chauvoei, Clostridium septicum, Clostridium novyi, Clostridium haemolyticum, Clostridium sordelli, Clostridium tetani, and Clostridium perfringens types C and D bacterin–toxoid (Cavalry 9; Merck Animal Health, Madison, NJ); and bovine rotavirus and coronavirus vaccine, killed virus C. perfringens type C, and E. coli bacterin–toxoid (Scourguard 4kc; Zoetis, Kalamazoo, MI). Cows were also dewormed with Ivermectin (Ivomec; Merial Ltd., Duluth, GA).
At 10 wk ± 16 d before calving, 45 Angus and Angus-cross cows were blocked by BW, cow age, and projected calving date and assigned to 1 of 3 treatment groups of 15 cows each, using a randomized complete block design. Ear tags were used to identify cows. Cows were housed in 9 dry barn lots with continuous access to water, feed bunks, and shelter. Each pen housed 5 cows fed the same treatment. Pens provided 10 m2/cow of concrete flooring in open lots that were strip cleaned once weekly, 5 m2/cow of shavings in a loafing area, and 98 cm of feeder space/cow as concrete bunks. All measurements exceeded requirements (Midwest Plan Service, 1987).
Cows were fed alfalfa hay once daily in the evening. Alfalfa hay was readily consumed such that by the next morning, feed bunks were empty. There was no wastage, as alfalfa hay was kept swept into reach. Grass hay was fed in the morning. Cows were fed alfalfa hay at a rate of 2.5% BW/d. Based on DM nutrient analysis of hay samples (Table 1), cows were fed 70% alfalfa hay (17% CP and 58.7% TDN) and 30% grass [predominantly Schedonorus phoenix (Scop.) Holub and Agrostis capillaris] hay (6.8% CP) to achieve a ration CP of 13.9%. Three pens of 5 cows/pen were assigned to each treatment: Control cows were fed non-Se-fortified alfalfa hay as a major portion of the ration plus a mineral supplement (Table 2) containing 120 mg/kg Se (U.S. Food and Drug Administration, 2016) from sodium selenite, Med-Se cows were fed alfalfa hay harvested from a field fertilized with 45.0 g Se/ha as sodium selenate and fed a mineral supplement without added Se, and High-Se cows were fed alfalfa hay harvested from fields fertilized with 89.9 g Se/ha as sodium selenate and fed a mineral supplement without added Se; both the Med-Se and the High-Se groups received mineral supplement (Table 2) without added Se. Prior to this experiment, cows had free-choice access to the same mineral supplement (Table 2) containing 120 mg/kg Se from sodium selenite.
Table 1.
Alfalfa and grass hay nutrient compositions (DM basis)1,2,3
| Alfalfa hay4 | ||||
|---|---|---|---|---|
| Nutrient | Control | Med-Se | High-Se | Grass hay |
| DM, g/kg | 874 | 874 | 878 | 871 |
| CP, g/kg | 170 | 183 | 157 | 68 |
| ADF, g/kg | 377 | 386 | 373 | 441 |
| NDF, g/kg | 402 | 417 | 430 | 640 |
| Nonfiber carbohydrates, g/kg | 316 | 289 | 286 | 195 |
| Fat, g/kg | 13.7 | 12.4 | 12.0 | 5.4 |
| Ash, g/kg | 98.3 | 98.6 | 115.0 | 91.6 |
| TDN, g/kg | 600 | 596 | 564 | 538 |
| Calcium, g/kg | 13.3 | 13.1 | 13.4 | 4.7 |
| Phosphorus, g/kg | 2.4 | 2.6 | 2.5 | 1.9 |
| Magnesium, g/kg | 3.2 | 3.5 | 3.4 | 2.4 |
| Potassium, g/kg | 23.3 | 23.3 | 22.5 | 15.9 |
| Sodium, g/kg | 0.98 | 1.14 | 1.11 | 1.35 |
| Copper, mg/kg | 10 | 11 | 11 | 4 |
| Iron, mg/kg | 292 | 447 | 261 | 613 |
| Manganese, mg/kg | 39 | 48 | 41 | 239 |
| Zinc, mg/kg | 20 | 19 | 20 | 24 |
| Selenium, mg/kg | 0.34 | 2.42 | 5.17 | 0.36 |
1Alfalfa hay samples were submitted to Cumberland Analytical Services, Maugansville, MD, for routine nutrient analysis and to Utah Veterinary Diagnostic Laboratory, Logan, UT, for Se analysis.
2Alfalfa hay DM determination was completed at a temperature of 105°C for 12 to 14 h in a forced draft oven. Methods for CP (990.03), ADF (973.18), ash (942.05), and minerals (985.01) were performed according to the AOAC International (AOAC, 2000). The NDF was determined according to Van Soest et al. (1991). Soluble protein was determined according to Krishnamoorthy et al. (1982). Fat was calculated by difference.
3Alfalfa hay samples were prepared for Se analysis as described by Davis et al. (2012), and Se was determined using inductively coupled argon plasma emission spectroscopy (ELAN 6000; PerkinElmer Inc., Shelton, CT).
4Control cows were fed non-Se-fortified alfalfa hay as a major portion of the ration plus a mineral supplement containing 120 mg/kg Se (U.S. Food and Drug Administration, 2016) from sodium selenite. Med-Se cows were fed alfalfa hay harvested from a field fertilized with 45.0 g Se/ha as sodium selenate and fed a mineral supplement without added Se. High-Se cows were fed alfalfa hay harvested from fields fertilized with 89.9 g Se/ha as sodium selenate and fed a mineral supplement without added Se; both groups received mineral supplement without added Se.
Table 2.
Mineral supplement compositions (DM basis)
| Mineral | Compositions |
|---|---|
| Calcium, g/kg | 57.0–64.0 |
| Phosphorus, g/kg | 30.0 |
| Magnesium, g/kg | 50.0 |
| Sodium chloride, g/kg | 503–553 |
| Cobalt, mg/kg | 50 |
| Copper, mg/kg | 2,500 |
| Manganese, mg/kg | 200 |
| Iodine, mg/kg | 200 |
| Zinc, mg/kg | 6,500 |
| Selenium, mg/kg | 0 or 120 |
1Loose granular form, provided by Wilbur-Ellis Company, Clackamas, OR.
After calving, calves were left with their mothers in individual pens for 48 h. Ear tags were used to identify calves. Body weights of all calves were recorded within 2 h of calving and after each nursing for 48 h. Calves were placed in a large weigh sling (Nasco, Fort Atkinson, WI) and weighed using a MAX digital weight indicator (Western Scale Co. Ltd., Port Coquitlam, BC, Canada) for precise mobile weighing.
Selenium-Fortified Alfalfa Hay
The soil was enriched with Se by mixing inorganic sodium selenate (RETORTE Ulrich Scharrer GmbH, Röthenbach, Germany) with water and spraying it onto the soil surface of an alfalfa field at application rates of 0, 45.0, or 89.9 g Se/ha immediately after the second cutting of hay in July 2014. The application rates were chosen based on a previous study (Hall et al., 2013a). Third-cutting alfalfa hay was harvested 40 d after Se application and then analyzed for nutrient and Se content (Table 1). Alfalfa yield was approximately 4.45 t/ha. A Penn State forage sampler was used to take 25 cores from random bales in each hay source (0, 45.0, or 89.9 g Se/ha). Samples were collected prior to beginning the feeding trial for each alfalfa hay source. Core samples were blended, and representative samples were selected for analysis. Alfalfa hay samples were submitted to commercial laboratories for analysis. Hay samples were prepared for Se analysis as described by Davis et al. (2012), and Se was determined using inductively coupled argon plasma emission spectroscopy (ELAN 6000; PerkinElmer Inc., Shelton, CT). Quantification of Se was performed by the standard addition method, using a 4-point standard curve. A quality-control sample (in a similar matrix) was analyzed after every 5 samples, and an analysis was considered acceptable if the Se concentration of the quality-control sample fell within ±5% of the standard/reference value for the quality control.
Blood and Colostrum Collection for Selenium Analyses of Cow and Calf Samples
Blood samples were collected from the jugular vein of cows at 10 wk ± 16 d before the expected average calving date (the baseline) into evacuated 2-mL EDTA tubes (final EDTA concentration of 2 g/L; Becton, Dickinson and Company, Franklin Lakes, NJ) and stored on ice until they were frozen at −20°C to measure WB Se concentrations at a later date. Whole-blood Se concentrations were measured in cows again after 4 wk of alfalfa hay consumption and at parturition. Whole-blood Se concentrations were similarly collected in calves within 2 h of birth (before colostrum feeding) and at 12, 24, 36, and 48 h after birth. Colostrum was collected within 2 h of calving.
Selenium concentrations in WB and colostrum were determined by a commercial laboratory (Utah Veterinary Diagnostic Laboratory, Logan, UT) using an inductively coupled argon plasma emission spectrometry method. Selenium was measured using an inductively coupled argon plasma emission spectroscopy (ELAN 6000) method as previously described (Hall et al., 2013a). In brief, quantitation of Se was performed by the standard addition method, using a 4-point standard curve. A quality-control sample (in a similar matrix) was analyzed after every 5 samples, and an analysis was considered acceptable if the Se concentration of the quality-control sample fell within ±5% of the standard/reference value for the quality control.
Blood and Colostrum Collection for IgG1 Analysis
Jugular venous blood was collected from calves into evacuated tubes without EDTA (10 mL; Becton, Dickinson and Company) for subsequent harvesting of serum. The tubes were centrifuged at 850 × g for 10 min at room temperature; serum was collected, transferred into 2.0-mL screw cap self-standing micro tubes (ISC BioExpress, Kaysville, UT), and stored at −20°C until IgG1 determination.
Concentrations of IgG1 in cow colostrum and calf serum were quantified using a direct sandwich ELISA procedure. The protocol was adapted from a commercially developed assay (Bethyl Laboratories, Montgomery, TX). In brief, 96-well microplates (Thermo Scientific Pierce, Fisher Scientific, Pittsburgh, PA) were coated with 100 μL of 1 μg/mL affinity purified sheep anti-bovine IgG1 (Bethyl Laboratories) diluted in 0.05 M carbonate–bicarbonate buffer (pH 9.6) and incubated for 1 h at room temperature. After incubation, plates were washed 3 times in Tween–Tris buffered saline (T-TBS; 50 mM Tris, 0.14 M NaCl, and 0.05% Tween-20, pH 8.0). For standards, purified bovine IgG1 (1 mg/mL; Bethyl Laboratories) was diluted in T-TBS from 3.75 to 500 ng/mL. Calf serum samples from all time points were diluted in T-TBS at 1:200,000 and 1:400,000. Cow colostrum samples were diluted 1:2,000,000 and 1:4,000,000. High and low IgG1 controls were included in each assay. All standards, samples, and controls were plated in duplicate at 100 μL per well and allowed to incubate for 30 min at room temperature. After incubation, plates were washed 3 times in T-TBS. Horseradish peroxidase conjugated sheep anti-bovine IgG1 (1 mg/mL; Bethyl Laboratories) was added at a 1:25,000 dilution and allowed to incubate for 30 min at room temperature. Plates were then washed 3 times in T-TBS, and 100 μL of 3,3',5,5'-tetramethylbenzidine (TMB; Sigma-Aldrich, St. Louis, MO) was added to each well. Plates were kept in the dark at room temperature and read at 655 nm until an absorbance of at least 0.650 optical density was reached in the 500-ng IgG1 standard well. The TMB reaction was then stopped by adding 100 μL of 1 N H2SO4, and the plate was read at 450 nm. Results are reported as milligrams per milliliter IgG1. The interassay CV for IgG1 was 5.6%.
Blood and Colostrum J-5 Escherichia coli Antibody Titer Analysis
Passive transfer of specific antibody was assessed by measuring antibody titers in calves after vaccinating cows with a commercially available vaccine (Enviracor J-5 Escherichia coli Bacterin; distributed by Zoetis). Cows were immunized with bacterin after consuming Se-enriched alfalfa hay for 4 wk. Following manufacturer's instructions, 5 mL bacterin was subcutaneously administered in a 3-dose regimen, with each dose 2 wk apart. Concentrations of J-5 E. coli antibody in cow colostrum and calf serum were quantified by an indirect ELISA procedure described by Chaiyotwittayakun et al. (2004) with minor modification. The reporter compound TMB was used to react with horseradish peroxidase conjugated to sheep-anti-bovine-IgG1 (Bethyl Laboratories). After 100 μL of TMB was added to all wells, plates were incubated at room temperature in the dark in an Ultramark microplate reader (BioRad, Hercules, CA) until an absorbance of 0.6 was reached at 650 nm. Then, 100 μL of 1 N H2SO4 was added and absorbance at 450 nm was determined.
Pooled colostrum and pooled calf serum were used for standards. Ten microliters of colostrum from each cow and 10 μL of serum from each calf were pooled into single tubes and mixed. On every ELISA plate, a duplicate set of 8 dilutions from the respective pooled standards were assayed along with a set of colostrum dilutions for each individual cow or serum dilutions from each individual calf at each time point (12, 24, 36, and 48 h of age). The dilutions of pooled standards were used to normalize all ELISA plates, and ELISA absorbance readings were converted to arbitrary dilution units as follows. The pooled standards were curve fitted with a hyperbola curve using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). All standard curves had R2 values between 0.95 and 0.99. The regression lines were subsequently used to convert individual ELISA absorbance readings into arbitrary dilution units. The fold change in bacteria-reactive antibody was calculated from converted ELISA values for individual serum (diluted 1:1,000) or colostrum (diluted 1:20,000) divided by the pooled standard samples diluted to the same degree. Data were natural log transformed for subsequent analysis. Relative colostral J-5 E. coli antibody titers and the change in J-5 E. coli antibody titers from 12 h to 48 h in calf serum were determined using these fold multiples. The interassay CV for J-5 E. coli was 3.7%.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.4 (SAS Inst. Inc., Cary, NC), software. Mean values were used for each pen, as pen was the experimental unit. Homogeneity of variance among treatments was tested using the Levene test and graphical diagnostics of treatment box plots. Normal distribution was tested using the Shapiro–Wilks test and graphical diagnostics of the Quantile-Quantile plot. The colostral IgG1 concentrations lacked homogeneity of variance and, therefore, were analyzed with a t test using the Satterthwaite approximation for heterogeneous variance. The linear response of the dependent variables (Se forage content, colostral or WB Se concentrations in beef cows, and WB Se concentrations in beef calves) to the independent variable (Se fertilization rate) were evaluated using univariate regression in PROC REG. Pearson and Spearman correlation coefficients were calculated to examine the associations among variables. The effect of Se-fortified alfalfa on cow and calf WB Se concentrations, colostral and calf serum IgG1 concentrations and J-5 E. coli antibody titers, and calf BW were measured using PROC GLM for 1-time measurements and PROC MIXED and an unstructured variance–covariance structure for repeated measurements within animal. The unstructured variance–covariance matrix provided the most parsimonious variance–covariance matrix based on the lowest value by the Akaike information criterion. Fixed effects in the models were Se application rate (0, 45.0, and 89.9 g Se/ha) and, for repeated measurements, were time within animal and the time × Se application rate interaction. Linear and quadratic contrasts were constructed to evaluate the effects of Se application rate. All statistical tests were 2 sided. Data are reported as least squares means ± SEM unless otherwise noted. Statistical significance was declared at P ≤ 0.05 and a statistical tendency was declared at 0.05 < P ≤ 0.10.
RESULTS
Agronomic Biofortification
Fertilizing fields with increasing amounts of sodium selenate increased the Se concentration of third-cutting alfalfa hay from 0.34 mg Se/kg DM (nonfertilized control) to 2.42 and 5.17 mg Se/kg DM for sodium selenate application rates of 45.0 and 89.9 g Se/ha, respectively (Fig. 1). Calculated Se intake from dietary sources was 5.3, 27.6, and 57.5 mg Se/cow daily for cows consuming alfalfa hay with Se concentrations of 0.34 to 2.42 and 5.17 mg Se/kg DM in the alfalfa hay, respectively. Cows receiving nonfertilized control hay received an additional 3 mg Se/cow daily from the mineral supplement containing sodium selenite.
Figure 1.

Relationship between amount of Se applied by fertilization (g Se/ha) and observed forage Se content (g Se/kg DM). Fertilizing fields with increasing amounts of sodium selenate increased the Se concentration of third-cutting alfalfa hay from 0.34 mg Se/kg DM (nonfertilized control) to 2.42 and 5.17 mg Se/kg DM for sodium selenate application rates of 45.0 and 89.9 g Se/ha, respectively.
Cow Whole Blood: Selenium Concentrations
At the start of the experiment, cows had mean WB Se concentrations of 151 ng/mL (SEM 4; range 106–243 ng/mL), which were within the reference interval of adult cows (120–300 ng/mL; Hall et al., 2011). As shown in Fig. 2, WB Se concentrations linearly increased (P < 0.001) after feeding Se-fertilized alfalfa hay for 4 wk to 168 ± 6 (for 0 g Se/ha), 253 ± 6 (for 45.0 g Se/ha), and 339 ± 6 ng/mL (for 89.9 g Se/ha). Feeding Se-fertilized alfalfa hay for another 6 wk ± 16 d linearly increased (P < 0.001) WB Se concentrations to 193 ± 14 (for 0 g Se/ha), 345 ± 14 (for 45.0 g Se/ha), and 540 ± 14 ng/mL (for 89.9 g Se/ha) at parturition.
Figure 2.

Whole-blood Se concentrations (mean ± SEM) in pregnant beef cows consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha. Calculated Se intake from dietary sources was 5.3, 27.6, and 57.5 mg Se/cow daily for cows consuming alfalfa hay with Se concentrations of 0.34 to 2.42 and 5.17 mg Se/kg in the alfalfa hay DM, respectively. Cows receiving nonfertilized control hay received an additional 3 mg Se/cow daily from the mineral supplement containing sodium selenite. Blood was collected at baseline (10 wk ± 16 d before their expected calving date), after 4 wk consuming Se-biofortified hay, and at parturition (after approximately 10 wk ± 16 d consuming Se biofortified hay). There are Se treatment, time, and Se treatment × time interaction effects (all P < 0.001). A–DValues that differ at P < 0.05 have different letters; letters are alphabetically ordered from largest to smallest values.
Concentrations of WB Se did not differ (P = 0.68) after 10 wk ± 16 d in Med-Se cows (consuming 45.0 g Se/ha) compared with 4 wk in High-Se cows (consuming 89.9 g Se/ha) alfalfa hay. The regression equation between rate of Se application (g Se/ha) and observed WB Se concentration at calving (ng Se/mL) was y = 3.86 (±0.23) x + 186 (±13); (r2 = 0.976).
Cow Colostrum: Selenium, IgG1, and J-5 Escherichia coli Antibody Concentrations
As shown in Fig. 3, colostral Se concentrations linearly increased (P < 0.001) after feeding Se-fertilized alfalfa hay for 8 wk to 121 ± 13 (for 0 g Se/ha), 504 ± 13 (for 45.0 g Se/ha), and 1,339 ± 13 ng/mL (for 89.9 g Se/ha). In Control cows, Se concentrations in colostrum were less (−72 ng/mL; P = 0.005; Fig. 2 and 3) compared with WB Se concentrations measured at parturition. In Med-Se cows (45.0 g Se/ha application rate), Se concentrations in colostrum were enriched by 159 ng/mL (P < 0.001) compared with WB Se concentrations measured at parturition. Likewise, in High-Se cows (89.9 g Se/ha application rate), Se concentrations in colostrum were enriched by 799 ng/mL (P < 0.001) compared with WB Se concentrations measured at parturition (linear). The regression equation between rate of Se application (g Se/ha) of alfalfa hay and observed colostrum Se concentration (ng Se/mL) was y = 13.54 (±1.12) x + 46 (±65); (r2 = 0.955).
Figure 3.

Colostrum Se concentrations in beef cows, collected within 2 h of parturition. Cows had been consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d prior to calving. Calculated Se intake from dietary sources was 5.3, 27.6, and 57.5 mg Se/cow daily for cows consuming alfalfa hay with Se concentrations of 0.34 to 2.42 and 5.17 mg Se/kg in the alfalfa hay DM, respectively. Cows receiving nonfertilized control hay received an additional 3 mg Se/cow daily from the mineral supplement containing sodium selenite. There is a Se treatment effect (P < 0.001). A–CValues that differ at P < 0.05 have different letters; letters are alphabetically ordered from largest to smallest values.
As shown in Fig. 4A, colostral IgG1 concentrations increased after feeding Se-fertilized alfalfa hay for 8 wk to 97 ± 5 (for 0 g Se/ha), 168 ± 35 (for 45.0 g Se/ha), and 206 ± 52 ng/mL (for 89.9 g Se/ha) and were higher in Control cows compared with cows consuming Se-fertilized alfalfa hay (P = 0.03). Colostral IgG1 concentrations were correlated with WB Se concentrations (r = 0.68, P = 0.04) and tended to be associated with colostral Se concentrations (r = 0.63, P = 0.07).
Figure 4.
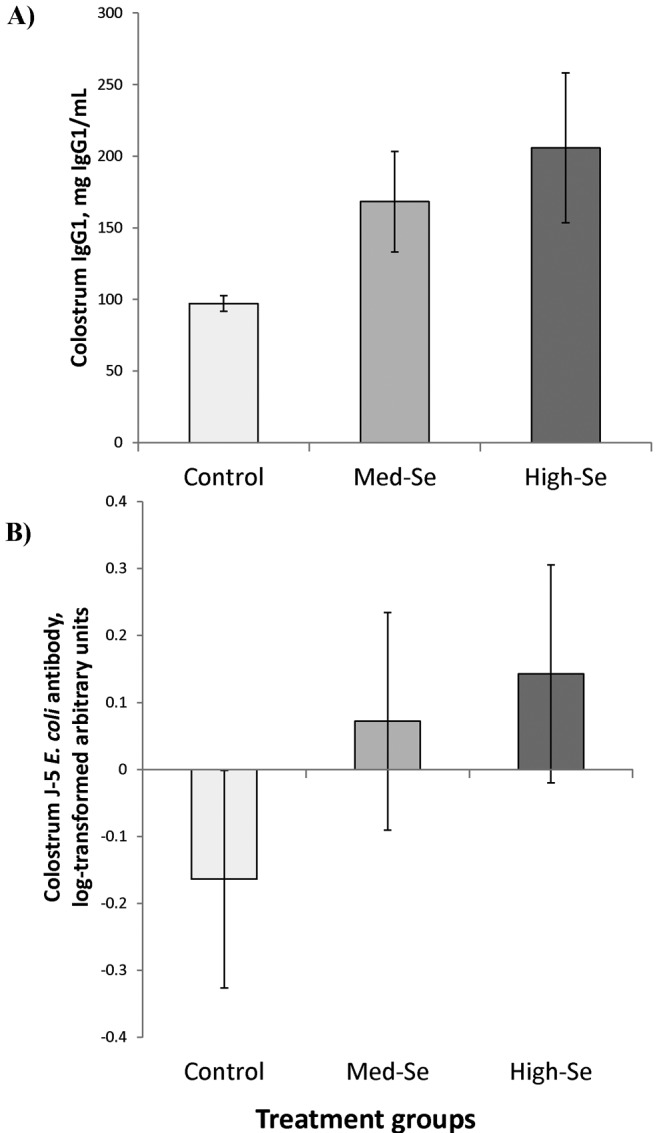
(A) Colostrum IgG1 concentrations in beef cows, collected within 2 h of parturition. Cows had been consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d prior to calving. Calculated Se intake from dietary sources was 5.3, 27.6, and 57.5 mg Se/cow daily for cows consuming alfalfa hay with Se concentrations of 0.34 to 2.42 and 5.17 mg Se/kg in the alfalfa hay DM, respectively. Cows receiving nonfertilized control hay received an additional 3 mg Se/cow daily from the mineral supplement containing sodium selenite. Immunoglobulin G1 concentrations were higher in cows consuming Se-fertilized alfalfa hay compared with cows consuming nonfertilized control alfalfa hay (P = 0.03). (B) Colostrum J-5 Escherichia coli antibody concentrations followed the same trend but did not differ among treatment groups. The fold change in bacteria-reactive antibody was calculated from converted ELISA values for individual colostrum (diluted 1:20,000) divided by the pooled set of samples diluted to the same degree, which are denoted as arbitrary units. Data in arbitrary units were natural log transformed for this analysis and are shown as such. There was no effect of Se treatment (P = 0.43).
As shown in Fig. 4B, colostral J-5 E. coli antibody concentrations followed the same trend as colostral IgG1 concentrations and were −0.16 ± 0.16 (for 0 g Se/ha), 0.07 ± 0.16 (for 45.0 g Se/ha), and 0.14 ± 0.16 (for 89.9 g Se/ha) natural log–transformed arbitrary units compared with the pooled set of samples but did not differ (P = 0.43) among treatment groups.
Calf Whole Blood: Selenium Concentrations
As shown in Fig. 5, WB Se concentrations in calves at birth linearly increased (P < 0.001) to 134 ± 30 ng/mL for Control cows (0 g Se/ha), 268 ± 30 ng/mL for Med-Se cows (45.0 g Se/ha), and 436 ± 30 ng/mL for High-Se cows (89.9 g Se/ha). The regression equation between amount of Se applied by fertilization (g Se/ha) and observed WB Se concentration (ng Se/mL) in calves at birth was y = 3.36 (±0.45) x + 128 (±26); (r2 = 0.888). The WB Se concentrations decreased in calves by 48 h to 116 ± 26 ng/mL for Control cows (0 g Se/ha), 258 ± 26 ng/mL for Med-Se cows (45.0 g Se/ha), and 389 ± 26 ng/mL for High-Se cows (89.9 g Se/ha). The regression equation between rate of Se applied by fertilization (g Se/ha) and observed WB Se concentration (ng Se/mL) in calves at 48 h of age was y = 3.04 (±0.38) x + 118 (±22); (r2 = 0.900). There were time (decreased from birth; P = 0.002), treatment (P = 0.003), and time × treatment interaction effects (P = 0.04).
Figure 5.
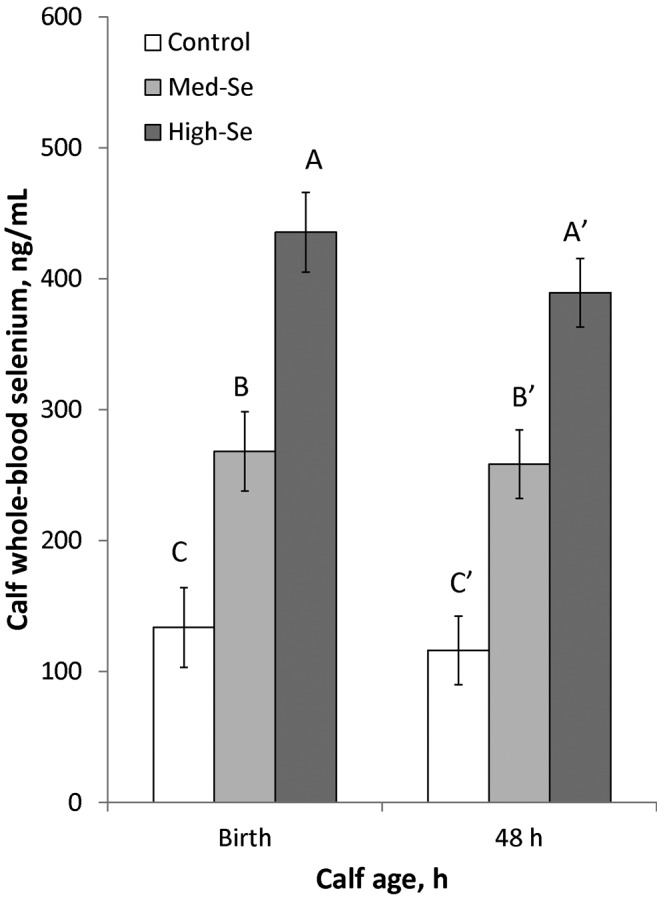
Whole-blood Se concentrations (mean ± SEM) in newborn calves born to mothers consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d before their expected calving date. Blood was collected within 2 h of calving and at 48 h of age. There were time (decreased from birth; P = 0.002), treatment (P = 0.003), and time × treatment interaction effects (time effect in High-Se calves and not in the other 2 treatment groups; P = 0.04). A–CValues that differ at P < 0.05 have different letters; A′ through C′ refers to time effects for treatments; letters are alphabetically ordered from largest to smallest values.
Calf Whole Blood: Effect of Selenium Supplementation on IgG1 and J-5 Escherichia coli Antibody Concentrations from Birth to 48 Hours
As shown in Fig. 6, serum IgG1 concentrations in calves from dams fed their respective alfalfa hay treatments increased from 12 h (39.2 ± 2.7 mg/mL) to 24 h (47.5 ± 2.5 mg/mL; P < 0.001) and remained constant at 36 (47.5 ± 2.6 mg/mL; P = 0.99) and 48 h (45.4 ± 2.2 mg/mL; P = 0.13; overall time effect, P = 0.01). There was no effect of Se treatment on IgG1 concentrations (P = 0.43) nor a time × treatment interaction (P = 0.55).
Figure 6.
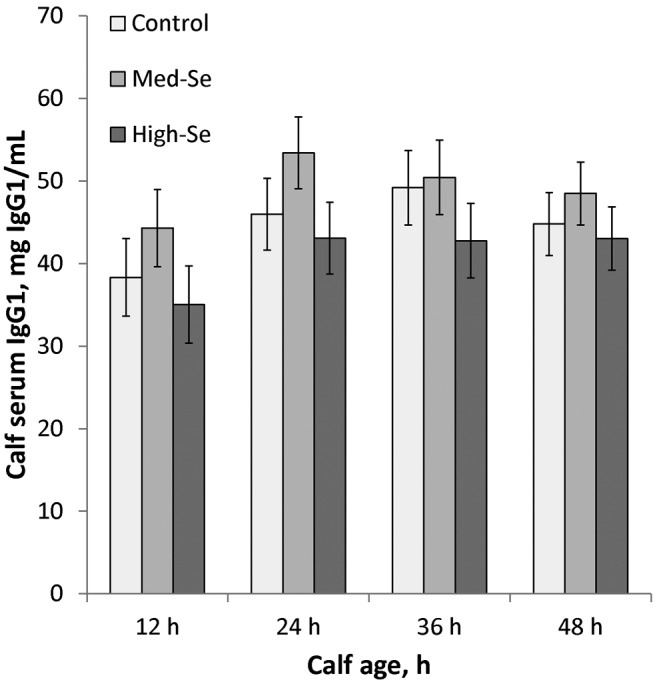
Serum IgG1 concentrations in calves born to mothers consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d before their expected calving date. Blood was collected at birth and at 12, 24, 36, and 48 h. There was a time effect (P = 0.01). Immunoglobulin G1 concentrations were not detectable at birth and increased from 12 h to 24 h (P < 0.001) and remained similar at 36 (P = 0.99) and 48 h (P = 0.13). There was no effect of Se treatment (P = 0.43) nor Se treatment × time interaction (P = 0.55).
As shown in Fig. 7, serum J-5 E. coli antibody concentrations followed a similar trend from 12 h (−0.43 ± 0.13 natural log–transformed arbitrary units) to 24 (−0.11 ± 0.55; P = 0.02), 36 (−0.18 ± 0.16; P = 0.04), and 48 h (−0.27 ± 0.18; P = 0.03; overall time effect, P = 0.04). There was no treatment effect (P = 0.44) nor treatment × time interaction (P = 0.25).
Figure 7.
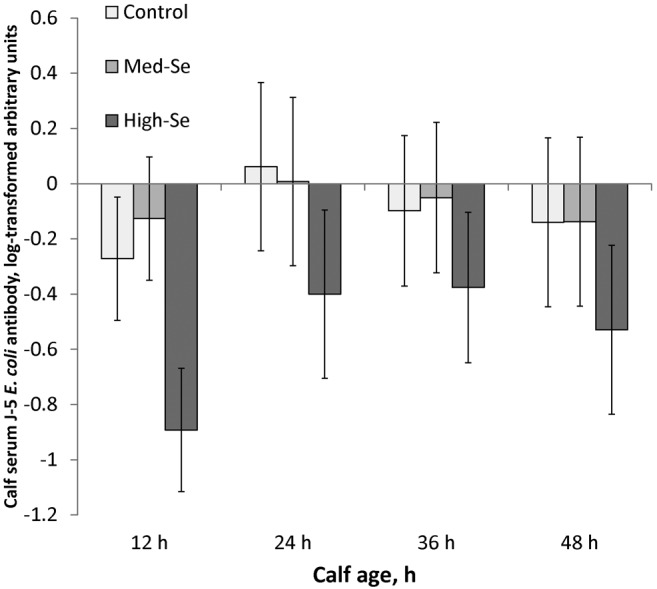
Serum J-5 Escherichia coli antibody concentrations in calves born to mothers consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d before their expected calving date. Blood was collected at birth and at 12, 24, 36, and 48 h. J-5 E. coli antibody concentrations followed a trend similar to that of serum IgG1 concentrations, with no treatment effect. The fold change in bacteria-reactive antibody was calculated from converted ELISA values for individual serum (diluted 1:1,000) divided by the pooled set of samples diluted to the same degree. Data were natural log transformed for this analysis. There was a time effect (P = 0.04). Concentrations of serum J-5 E. coli antibody increased from 12 h to 24 h (P = 0.02), remained constant from 24 h to 36 h (P = 0.22), and then decreased from 36 h to 48 h (P = 0.04). There was no effect of Se treatment (P = 0.44) nor Se treatment × time interaction (P = 0.25).
To further examine the relationship between colostral antibody concentrations and calf serum antibody concentrations, Pearson and Spearman correlation coefficients were calculated, and no correlations were detected between colostral and calf serum IgG concentrations at 12 (r = 0.14, P = 0.72), 24 (r = 0.01, P = 0.97), 36 (r = −0.16, P = 0.69), and 48 h (r = 0.08, P = 0.84) nor between colostral and calf serum J-5 E. coli antibody concentrations at 12 (r = −0.12, P = 0.75), 24 (r = 0.06, P = 0.87), 36 (r = 0.20, P = 0.61), and 48 h (r = 0.14, P = 0.72; the Spearman correlation coefficients are not shown).
As shown in Fig. 8, the amount of IgG1 ingested by calves via colostrum was calculated for the first 12, 24, and 48 h by multiplying increases in BW (g) after each nursing by dam IgG1 colostrum concentration. The dose of IgG1 ingested by calves over the 48-h time period from dams fed their respective alfalfa hay treatments were 533 ± 216 (for 0 g Se/ha), 842 ± 216 (for 45.0 g Se/ha), and 1,078 ± 216 g (for 89.9 g Se/ha), which did not differ (P = 0.28) among treatment groups. The dose of IgG1 ingested for the first 12 (P = 0.54) or 24 h (P = 0.46) also did not differ among treatment groups.
Figure 8.

The dose of IgG1 ingested by calves over the 48-h time period from dams fed their respective alfalfa hay treatments did not significantly differ among treatment groups. Calves were born to mothers consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 (High-Se cows) g Se/ha for 10 wk ± 16 d before their expected calving date. There was a time effect (P = 0.005). The dose of IgG1 ingested increased from 12 h to 24 h (P = 0.002) and then from 24 h to 48 h (P = 0.007). There was no effect of Se treatment (P = 0.39) nor Se treatment × time interaction (P = 0.42).
As shown in Fig. 9, the absorption efficiency of IgG1 from cow colostrum to calf serum was calculated by dividing the amount of IgG1 (g) in calf serum by the amount of IgG1 (g) ingested. The amount of IgG1 in calf serum was determined from BW multiplied by calf blood volume (0.0971) multiplied by calf serum IgG1 concentration. The absorption efficiency of IgG1 from cow colostrum to calf serum in the first 12 h from dams fed their respective alfalfa hay treatments were 105 ± 23 (0 g Se/ha), 69 ± 23 (45.0 g Se/ha), and 69 ± 23% (89.9 g Se/ha; P = 0.86). The absorption efficiency of IgG1 from cow colostrum to calf serum did not differ among treatment groups for the first 12 (P = 0.49), 24 (P = 0.48), and 48 h (P = 0.28).
Figure 9.
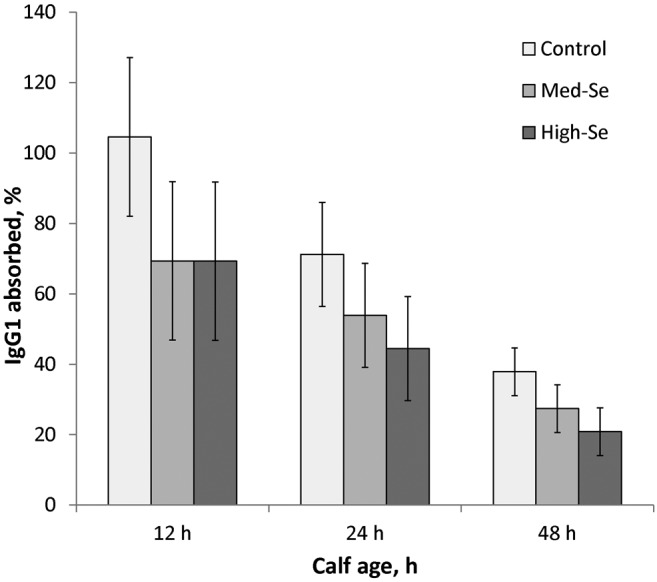
Absorption efficiency of IgG1 from cow colostrum to calf serum for the first 12, 24, and 48 h was not significantly different among treatment groups. Calves were born to mothers consuming 2.5% BW/d alfalfa hay grown in a field not fertilized with Se (0 g Se/ha; Control cows) or harvested from fields fertilized with sodium selenate at application rates of 45.0 (Med-Se cows) or 89.9 g Se/ha (High-Se cows) for 10 wk ± 16 d before their expected calving date. There was a time effect (P = 0.006). The absorption efficiency of IgG1 decreased from 12 h to 24 h (P = 0.04) and then from 24 h to 48 h (P = 0.001). There was no effect of Se treatment (P = 0.40) nor Se treatment × time interaction (P = 0.89).
DISCUSSION
In cattle, IgG1 is the predominant immunoglobulin isotype in colostrum and is derived from serum (Butler, 1983; Baumrucker et al., 2010). Calves are born with a digestive tract that facilitates absorption of colostral proteins instead of degrading them (Hardy, 1969). The level of proteolytic activity is low and further decreased by trypsin inhibitors in colostrum (Quigley et al., 1995). Small intestinal epithelial cells absorb colostral IgG1 via pinocytosis, and IgG1 proteins pass through enterocytes into lacteals (Patt, 1977; Weaver et al., 2000). From the lacteals, colostral proteins reach the systemic circulation, which allows newborn calves to receive maternal IgG1 by passive transfer (Patt, 1977; Weaver et al., 2000). The period during which the intestine is permeable to IgG1 varies but is highest immediately after birth, declines after 6 h, and drops to relatively low levels by 24 h, signaling gut closure to passive transport (Quigley and Drewry, 1998). Peak serum IgG1 concentrations are normally reached 32 h postpartum because of ongoing transport of immunoglobulins across the enterocytes (Stott et al., 1979). After absorption ceases, concentrations of passively acquired IgG1 begin to decline through normal turnover processes (Chappuis, 1998). Calves that fail to adequately suckle possess low concentrations of IgG1 in their serum. Failure of passive transfer predisposes calves to infection, sepsis, and death (Patt, 1977; Weaver et al., 2000).
Data from the current experiment show that feeding pregnant beef cows Se-enriched alfalfa for 10 wk ± 16 d antepartum does not increase serum concentrations of IgG1 in their nursing calves compared with calves from Control cows fed non-Se-fertilized hay and an additional 3 mg Se/cow daily from the mineral supplement as sodium selenite. In calves, serum IgG1 concentrations generally, and serum J-5 E. coli antibody concentrations specifically, followed a similar pattern, increasing from 0 to 24 h and then remaining at plateau concentrations from 36 to 48 h.
Although benefits of supranutritional maternal organic Se supplementation (27.6, and 57.5 mg Se/cow daily) were not apparent, it is important to note that Control cows were Se replete and received the maximum U.S. Food and Drug Administration–allowed dose of sodium selenite (3 mg Se/cow daily) from the mineral supplement, in addition to 5.3 mg Se/cow daily from hay. Therefore, Control cows were not Se deficient, nor simply Se replete, but instead were also receiving supranutritional Se supplementation. It is possible that all calves in our study reached the physiologic limitation suggested by Besser et al. (1985) of the mass of immunoglobulin that can be absorbed to serum from a given volume of colostrum.
Feeding cows Se-biofortified alfalfa hay for 10 wk ± 16 d antepartum increased Se concentrations in cow WB and colostrum and in calf WB at parturition. Previously, researchers have shown that feeding cows forage fertilized with sodium selenate (known as agronomic biofortification) elevates WB Se content (Hall et al., 2011; Séboussi et al., 2016), whereas dietary supplementation with sodium selenite alone does not yield as high of WB Se concentrations (Hall et al., 2011). Others have shown that supplementation of organic (Se yeast) or inorganic (sodium selenate) Se to late gestation and early lactation beef cows has no effect on cow performance, milk production, or reproductive efficiency (Muegge et al., 2016). Feeding supranutritional amounts of Se yeast increased colostral Se concentrations in ewes (Stewart et al., 2012) and in dairy cows (Hall et al., 2014). The findings with Se-biofortified alfalfa hay corroborate these earlier organic Se yeast studies, as cows receiving Se-biofortified alfalfa hay (89.9 g Se/ha) had 192% greater WB Se and 1,023% greater colostral Se concentrations compared with Control cows. Colostral Se concentrations were enriched in cows receiving Se biofortified hay, which indicates that supranutritional Se may be incorporated at increased rates into colostral proteins for potential Se storage. In addition, colostral Se concentrations were less in Control cows compared with their corresponding WB Se concentrations, which indicates that although Control cows were Se replete, additional Se was not incorporated into colostral proteins. Future studies are needed to evaluate the ratio of colostral Se to WB Se concentrations with regard to overall Se status of the animal.
Similar to previous studies using organic Se yeast by Stewart et al. (2012) and Hall et al. (2014), newborn calves from cows receiving Se-biofortified alfalfa hay (89.9 g Se/ha) had 211% greater WB Se concentrations compared with Control calves at birth and 246% greater WB Se concentrations at 48 h. A subset of 24 of the 45 calves in the current study that were sampled 8 mo later had WB Se concentrations in calves from Control dams, Med-Se dams, or High-Se dams of 165 ± 27, 229 ± 15, and 251 ± 12 ng Se/mL, respectively, and the WB Se concentrations were greater (P = 0.03) in calves from cows consuming Se-biofortified forage compared with calves from Control cows, suggesting a long-term effect on calf Se status.
Exposure to higher Se concentrations antepartum resulted in greater IgG1 concentrations in bovine colostrum. In addition, colostral IgG1 and J-5 E. coli antibody concentrations tended to be associated with colostral Se concentrations. However, there was more variation in colostral IgG1 concentrations among cows in the Med-Se and High-Se groups compared with Control cows. Overall, colostral IgG1 concentrations were greater in cows consuming Se-fertilized alfalfa hay compared with cows consuming nonfertilized control alfalfa hay. The mechanism by which supranutritional Se supplementation increases colostral IgG1 concentration is unclear, and multiple factors may be involved. Potential explanations may be that supranutritional Se supplementation alters the number of specific nutrient transporters or growth and vascularization of mammary tissues as is hypothesized for intestinal tissues (Meyer et al., 2013).
Some researchers have shown that Se supplementation increases IgG1 colostral concentrations. Swecker et al. (1995) found that parenteral Se supplementation of pregnant beef cows (cows grazing Se-deficient pastures with marginal to deficient Se status; blood Se concentration of 50 ng/mL) with 120 mg of Se/kg of an inorganic salt mineral mix had increased IgG1 concentrations in their colostrum. Weekly drenching of ewes with organic Se yeast at supranutritional amounts for 1 yr resulted in greater colostral Se concentrations and greater colostral IgG concentrations than ewes receiving the maximum U.S. Food and Drug Administration–allowed concentration (Stewart et al., 2012, 2013). However, Lacetera et al. (1996) showed that percentages of colostral immunoglobulin did not differ between cows treated with vitamin E and Se injections at 3 and 1.5 wk before calving (sodium selenite; 5 mg/100 kg BW) compared with untreated Control cows. Similarly, Swanson et al. (2008) showed that Se supplementation (organically bound Se containing pellets; adequate Se, <0.5 mg of Se/kg of DM fed at 9.5 µg/kg BW, vs. high Se, 60 mg of Se/kg of DM fed at 81.8 µg/kg BW) had no effect on colostral IgG concentrations in pregnant ewe lambs. It is likely that the Se status of the cows, the form of Se administered (organic vs. inorganic), and the rate of Se supplementation (duration of feeding and concentration of Se in forage) are all important in determining whether an effect on colostral IgG concentration is observed. Based on the current results, forage Se provides a linear dose vs. time supplementation strategy for increasing IgG concentrations in colostrum.
Kamada et al. (2007) found that direct addition of 3 mg of sodium selenite/kg colostrum increased serum concentrations of IgG in neonatal dairy calves by 42% at 48 h but that the concentrations did not differ at 14 d. Similarly, adding 3 mg of sodium selenite/kg to colostrum increased serum IgG concentrations in calves by 32% at 48 h and by 33% at 14 d, whereas feeding colostrum from Se yeast–supplemented cows did not affect serum IgG concentrations in calves (Hall et al., 2014). A limitation of the current study is that serum concentrations of IgG1 and J-5 E. coli antibody concentrations at 14 d of age were not measured. Recent research shows that supranutritional Se supplementation has positive effects on the persistence of IgG1 in calf serum. Rodinova et al. (2008) found that Se supplementation (organically bound in the biomass of an alga; dosed at 180 µg Se/ewe daily) of pregnant ewes resulted in greater concentrations of serum IgG at 30 and 60 d of age compared with untreated controls. Hall et al. (2014) found that calves born to dairy cows receiving surpanutritional Se yeast supplementation during the last 8 wk of pregnancy had greater serum IgG concentrations at 48 h, 14 d, and 60 d of age. As such, Se may be more important for its long-term effects on maintaining IgG1 concentrations in calves vs. its short-term effects on maximizing passive transfer.
Although colostral IgG1 concentrations were greater in cows consuming Se-fertilized alfalfa hay compared with cows consuming nonfertilized control alfalfa hay, there was not a correlation between colostral and calf serum IgG concentrations or between colostral and calf serum J-5 E. coli antibody concentrations. Furthermore, the absorption efficiencies of IgG1 from cow colostrum to calf serum across time were not different among treatment groups.
Other studies have also found that increased colostral IgG1 concentrations do not correlate with increased serum IgG1 concentration in calves. Besser et al. (1985) found a negative correlation between absorption efficiency and the mass of IgG1 fed. Moeini et al. (2011) found that antepartum Se supplementation (sodium selenite and vitamin E injections administered 4 and 2 wk prior to calving) resulted in increased colostral IgG1 but no difference in serum IgG1 concentration in calves. Bender (2009) investigated if and to what extent serum concentrations are correlated with antibody concentrations in colostral milk from birth to 10 d and concluded that individually diverse resorption patterns are in place that cannot be characterized by only immunoglobulin measurements.
Overall, the current experiment demonstrates that supranutritional Se supplementation leads to increased IgG1 concentrations in colostrum and, therefore, serves as a potential management tool for cattle production. If one were to feed pregnant cattle supranutritional concentrations of Se, their colostrum could be collected and administered to calves deemed too weak to suckle to minimize losses resulting from failure of passive transfer. Likewise, if a weak calf consumed only a small amount of colostrum from its mother, colostrum from a cow with high concentrations of IgG1 would be beneficial because increased IgG1 ingestion is the primary means to prevent failure of passive transfer.
Even though some calves did not ingest large quantities of milk during the first 12 h (1 Control calf, 1 Med-Se calf, and 5 High-Se calves drank ≤ 0.45 kg), all calves had serum IgG1 concentrations greater than recommended concentrations (8 to 16 mg/mL) by 48 h (Dewell et al., 2006; range in our study: 16 to 75 mg/mL). Serum IgG1 concentrations peaked at 24 h in calves from Control cows and after 36 h in calves from cows receiving Se-biofortified hay. This trend could indicate that calves from High-Se cows have delayed gut closure (Hall et al., 2014).
Variation in milk consumption was related to birth weight in that some small (<32 kg) and some large (>41 kg) calves did not ingest as much milk during the first 12 h. In future investigations, using 1 bull would help minimize birth weight variation and, therefore, variation in milk consumption. Another limitation of this study was that cows were of mixed genetics, with both purebred and crossbred cows. Having a more uniform population of cows would also reduce variability in birth weights.
In plants, Se is incorporated into methionine as selenomethionine, and when forage is consumed by livestock, Se from selenomethionine is converted into and then incorporated as selenocysteine into selenoproteins. The linear relationship between Se fertilizer application rate and forage Se concentration observed in this study is similar to what we have previously reported (Hall et al., 2009, 2013a). Plants, including alfalfa, absorb sodium selenate from the soil and synthesize selenoamino acids, with selenomethionine being the major selenocompound in forage legumes (Whanger, 2002). Plant species, chemical source of Se applied, and soil pH, sulfur, and iron content may alter Se availability for plant uptake as reviewed by the NRC (1983, 2007). These results suggest that Se content of soil, determined by Se application rate, is the primary determinant of Se content of common forage species. Future studies are needed to evaluate in greater detail the Se flow between fertilizer, soil, and plant. In the current experiment, there was a linear relationship between Se fertilizer application rate (g Se/ha) and forage Se concentration (mg Se/kg DM), which extends to cow WB, colostrum, and calf WB Se concentrations (ng/mL). Future experiments are needed to determine whether these mathematical relationships are consistent between experiments. Because of the linear relationship, another interesting finding was that cow WB Se concentrations were similar after 10 wk ± 16 d in Med-Se cows (consuming 45.0 g Se/ha) compared with 4 wk in High-Se cows (consuming 89.9 g Se/ha) alfalfa hay. Therefore, it may be possible to predict desired WB Se concentrations based on forage Se content and duration of feed consumption.
In summary, supranutritional Se supplementation using Se-biofortified alfalfa hay in beef cows during the last 10 wk ± 16 d of pregnancy resulted in increased WB Se concentrations in cows and calves and increased colostral Se and IgG1 concentrations but had no effect on serum IgG1 concentrations in calves in the first 48 h of age. The lack of change in serum IgG1 concentrations may result from a physiologic limitation of small intestinal epithelial cells to absorb additional IgG1.
Footnotes
Funded in part by a grant from the Agriculture Research Foundation (J.A. Hall and G.J. Pirelli, principal investigators), Oregon State University, Corvallis, OR 97331.
LITERATURE CITED
- Ahsan U., Kamran Z., Raza I., Ahmad S., Babar W., Riaz M. H., Iqbal Z. 2014. Role of selenium in male reproduction: A review. Anim. Reprod. Sci. 146:55–62. doi: 10.1016/j.anireprosci.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Ammerman C. B., Miller S. M. 1975. Selenium in ruminant nutrition: A review. J. Dairy Sci. 58:1561–1577. doi: 10.3168/jds.S0022-0302(75)84752-7 [DOI] [PubMed] [Google Scholar]
- AOAC 2000. Official methods of analysis. 17th ed.AOAC Int., Arlington, VA. [Google Scholar]
- Baumrucker C. R., Burkett A. M., Magliaro-Macrina A. L., Dechow C. D. 2010. Colostrogenesis: Mass transfer of immunoglobulin G1 into colostrum. J. Dairy Sci. 93:3031–3038. doi: 10.3168/jds.2009-2963 [DOI] [PubMed] [Google Scholar]
- Bender P. 2009. Determination of IgG and IgM levels in sera of newborn calves until the 10th day of life by ELISA and description of their correlation to total plasma protein concentration and GGT activity. Dtsch. Tieraerztl. Wochenschr. 116:44–52. [PubMed] [Google Scholar]
- Besser T. E., Garmedia A. E., McGuire T. C., Gay C. C. 1985. Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves. J. Dairy Sci. 68:2033–2037. doi: 10.3168/jds.S0022-0302(85)81065-1 [DOI] [PubMed] [Google Scholar]
- Broadley M. R., White P. J., Bryson R. J., Meacham M. C., Bowen H. C., Johnson S. E., Hawkesford M. J., McGrath S. P., Zhao F. J., Breward N., Harriman M., Tucker M. 2006. Biofortification of UK food crops with selenium. Proc. Nutr. Soc. 65:169–181. doi: 10.1079/PNS2006490 [DOI] [PubMed] [Google Scholar]
- Butler J. E. 1983. Bovine immunoglobulins: An augmented review. Vet. Immunol. Immunopathol. 4:43–152. doi: 10.1016/0165-2427(83)90056-9 [DOI] [PubMed] [Google Scholar]
- Chaiyotwittayakun A., Burton J. L., Weber P. S., Kizilkaya K., Cardoso F. F., Erskine R. J. 2004. Hyperimmunization of steers with J5 Escherichia coli bacterin: Effects on isotype-specific serum antibody responses and cross reactivity with heterogeneous Gram-negative bacteria. J. Dairy Sci. 87:3375–3385. doi: 10.3168/jds.S0022-0302(04)73473-6 [DOI] [PubMed] [Google Scholar]
- Chappuis G. 1998. Neonatal immunity and immunisation in early age: Lessons from veterinary medicine. Vaccine 16:1468–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. Z., Stegelmeier B. L., Panter K. E., Cook D., Gardner D. R., Hall J. O. 2012. Toxicokinetics and pathology of plant-associated acute selenium toxicosis in steers. J. Vet. Diagn. Invest. 24:319–327. doi: 10.1177/1040638711435407 [DOI] [PubMed] [Google Scholar]
- Dewell R. D., Hungerford L. L., Keen J. E., Laegreid W. W., Griffin D. D., Rupp G. P., Grotelueschen D. M. 2006. Association of neonatal serum immunoglobulin G1 concentration with health and performance in beef calves. J. Am. Vet. Med. Assoc. 228:914–921. doi: 10.2460/javma.228.6.914 [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait S. J., Collings R., Hurst R. 2010. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 91:1484S–1491S. doi: 10.3945/ajcn.2010.28674J [DOI] [PubMed] [Google Scholar]
- Hall J. A., Bobe G., Hunter J. K., Vorachek W. R., Stewart W. C., Vanegas J. A., Estill C. T., Mosher W. D., Pirelli G. J. 2013a. Effect of feeding selenium-fertilized alfalfa hay on performance of weaned beef calves. PLoS One 8:e58188. doi: 10.1371/journal.pone.0058188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. A., Bobe G., Vorachek W. R., Estill C. T., Mosher W. D., Pirelli G. J., Gamroth M. 2014. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy Sci. 97:4379–4391. doi: 10.3168/jds.2013-7481 [DOI] [PubMed] [Google Scholar]
- Hall J. A., Bobe G., Vorachek W. R., Hugejiletu, Gorman M. E., Mosher W. D., Pirelli G. J. 2013b. Effects of feeding selenium-enriched alfalfa hay on immunity and health of weaned beef calves. Biol. Trace Elem. Res. 156:96–110. doi: 10.1007/s12011-013-9843-0 [DOI] [PubMed] [Google Scholar]
- Hall J. A., Harwell A. M., Van Saun R. J., Vorachek W. R., Stewart W. C., Galbraith M. L., Hooper K. J., Hunter J. K., Mosher W. D., Pirelli G. 2011. Agronomic biofortification with selenium: Effects on whole blood selenium and humoral immunity in beef cattle. Anim. Feed Sci. Technol. 164:184–190. doi: 10.1016/j.anifeedsci.2011.01.009 [DOI] [Google Scholar]
- Hall J. A., Van Saun R. J., Nichols T., Mosher W., Pirelli G. 2009. Comparison of selenium status in sheep after short-term exposure to high-selenium-fertilized forage or mineral supplement. Small Rumin. Res. 82:40–45. doi: 10.1016/j.smallrumres.2009.01.010 [DOI] [Google Scholar]
- Hardy R. N. 1969. Proteolytic activity during the absorption of 131-I-gamma-globulin in the new-born calf. J. Physiol. 205:453–470. doi: 10.1113/jphysiol.1969.sp008977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefnawy A. E., Tortora-Perez J. L. 2010. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 89:185–192. doi: 10.1016/j.smallrumres.2009.12.042 [DOI] [Google Scholar]
- Kamada H., Nonaka I., Ueda Y., Murai M. 2007. Selenium addition to colostrum increases immunoglobulin G absorption by newborn calves. J. Dairy Sci. 90:5665–5670. doi: 10.3168/jds.2007-0348 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy U., Muscato T. V., Sniffen C. J., Van Soest P. J. 1982. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 65:217–225. doi: 10.3168/jds.S0022-0302(82)82180-2 [DOI] [Google Scholar]
- Lacetera N., Bernabucci U., Ronchi B., Nardone A. 1996. Effects of selenium and vitamin E administration during a late stage of pregnancy on colostrum and milk production in dairy cows, and on passive immunity and growth of their offspring. Am. J. Vet. Res. 57:1776–1780. [PubMed] [Google Scholar]
- Mäkelä A.-L., Näntö V., Mäkelä P., Wang W. 1993. The effect of nationwide selenium enrichment of fertilizers on selenium status of healthy Finnish medical students living in south western Finland. Biol. Trace Elem. Res. 36:151–157. doi: 10.1007/BF02783174 [DOI] [PubMed] [Google Scholar]
- Meyer A. M., Neville T. L., Reed J. J., Taylor J. B., Reynolds L. P., Redmer D. A., Hammer C. J., Vonnahme K. A., Caton J. S. 2013. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J. Anim. Sci. 91:2628–2639. doi: 10.2527/jas.2012-5953 [DOI] [PubMed] [Google Scholar]
- Moeini M. M., Kiani A., Mikaeili E., Shabankareh H. K. 2011. Effect of prepartum supplementation of selenium and vitamin E on serum Se, IgG concentrations and colostrum of heifers and on hematology, passive immunity and Se status of their offspring. Biol. Trace Elem. Res. 144:529–537. doi: 10.1007/s12011-011-9148-0 [DOI] [PubMed] [Google Scholar]
- Muegge C. R., Brennan K. M., Schoonmaker J. P. 2016. Supplementation of organic and inorganic selenium to late gestation and early lactation beef cows effect on cow and preweaning calf performance. J. Anim. Sci. 94:3399–3408. doi: 10.2527/jas.2015-0226 [DOI] [PubMed] [Google Scholar]
- Midwest Plan Service 1987. Beef housing and equipment handbook. 4th ed.Iowa State University, Ames, IA. [Google Scholar]
- NRC 1983. Selenium in nutrition. Rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 2007. Nutrient requirements of small ruminants: Sheep, goats, cervids, and New World camelids. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Patt J. A. 1977. Factors affecting duration of intestinal permeability to macromolecules in newborn animals. Biol. Rev. Camb. Philos. Soc. 52:411–429. doi: 10.1111/j.1469-185X.1977.tb00855.x [DOI] [Google Scholar]
- Quigley J. D., 3rd, Drewry J. J. 1998. Nutrient and immunity transfer from cow to calf pre- and postcalving. J. Dairy Sci. 81:2779–2790. doi: 10.3168/jds.S0022-0302(98)75836-9 [DOI] [PubMed] [Google Scholar]
- Quigley J. D., Martin K. R., Dowlen H. H. 1995. Concentrations of trypsin-inhibitor and immunoglobulins in colostrum of Jersey cows. J. Dairy Sci. 78:1573–1577. doi: 10.3168/jds.S0022-0302(95)76780-7 [DOI] [PubMed] [Google Scholar]
- Rodinova H., Kroupova V., Travnicek J., Stankova M., Pisek L. 2008. Dynamics of IgG in the blood serum of sheep with different selenium intake. Vet Med-Czech 53:260–265. [Google Scholar]
- Séboussi R., Tremblay G. F., Ouellet V., Chouinard P. Y., Chorfi Y., Bélanger G., Charbonneau É. 2016. Selenium-fertilized forage as a way to supplement lactating dairy cows. J. Dairy Sci. 99:5358–5369. doi: 10.3168/jds.2015-10758 [DOI] [PubMed] [Google Scholar]
- Stewart W. C., Bobe G., Vorachek W. R., Pirelli G. J., Mosher W. D., Nichols T., Van Saun R. J., Forsberg N. E., Hall J. A. 2012. Organic and inorganic selenium: II. Transfer efficiency from ewes to lambs. J. Anim. Sci. 90:577–584. doi: 10.2527/jas.2011-4076 [DOI] [PubMed] [Google Scholar]
- Stewart W. C., Bobe G., Vorchek W. R., Stang B. V., Pirelli G. J., Mosher W. D., Hall J. A. 2013. Organic and inorganic selenium: IV. Passive transfer of immunoglobulin from ewe to lamb. J. Anim. Sci. 91:1791–1800. doi: 10.2527/jas.2012-5377 [DOI] [PubMed] [Google Scholar]
- Stott G. H., Marx D. B., Menefee B. E., Nightengale G. T. 1979. Colostral immunoglobulin transfer in calves. 1. Period of absorption. J. Dairy Sci. 62:1632–1638. doi: 10.3168/jds.S0022-0302(79)83472-4 [DOI] [PubMed] [Google Scholar]
- Swanson T. J., Hammer C. J., Luther J. S., Carlson D. B., Taylor J. B., Redmer D. A., Neville T. L., Reed J. J., Reynolds L. P., Caton J. S., Vonnahme K. A. 2008. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J. Anim. Sci. 86:2415–2423. doi: 10.2527/jas.2008-0996 [DOI] [PubMed] [Google Scholar]
- Swecker W. S., Jr, Thatcher C. D., Eversole D. E., Blodgett D. J., Schurig G. G. 1995. Effect of selenium supplementation on colostral IgG concentration in cows grazing selenium-deficient pastures and on postsuckle serum IgG concentration in their calves. Am. J. Vet. Res. 56:450–453. [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA) 2016. Title 21. Food and drugs: Food additive permitted in feed and drinking water of animals. Section 21CFR573.920 Selenium. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr = 573.920. (Accessed March 22, 2016.)
- Van Soest P. J., Robertson J. B., Lewis B. A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Weaver D. M., Tyler J. W., VanMetre D. C., Hostetler D. E., Barrington G. M. 2000. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 14:569–577. doi: 10.1111/j.1939-1676.2000.tb02278.x [DOI] [PubMed] [Google Scholar]
- Whanger P. D. 2002. Selenocompounds in plants and animals and their biological significance. J. Am. Coll. Nutr. 21:223–232. doi: 10.1080/07315724.2002.10719214 [DOI] [PubMed] [Google Scholar]
- Whelan B. R. 1989. Uptake of selenite fertilizer by subterranean clover pasture in Western Australia. Aust. J. Exp. Agric. 29:517–522. doi: 10.1071/EA9890517 [DOI] [Google Scholar]
- Willshire J. A., Payne J. H. 2011. Selenium and vitamin E in dairy cows: A review. Cattle Pract. 19:22–30. [Google Scholar]


