Abstract
Use of a fit-tested N95 or FFP2 mask is recommended to protect against transmission of airborne pathogens. This poses considerable logistic problems when preparing for, or dealing with, an epidemic. Some of these problems might be overcome by use of a compact reusable high-efficiency particulate air filtering mask that can be cut to size. We carried out a randomised controlled cross-over study to compare the efficacy of such a mask (Totobobo, Dream Lab One Pte Ltd, Singapore) with fit-tested N95 masks (1860 or 1860s or 1862; 3M, St Paul, MN, USA) in 22 healthy volunteers. The median (interquartile range) reduction in airborne particle counts was significantly higher [193-fold (145–200)] for N95 masks than for Totobobo masks [135-fold (83–184)] (P < 0.05). There was no statistically significant difference between the proportion of subjects achieving a reduction of ≥100-fold between N95 (19/22) and Totobobo (16/22) masks. We conclude that use of the Totobobo mask without fit testing cannot be recommended, but its performance is sufficiently promising to warrant further investigation.
Keywords: Disease transmission, Infection control, Occupational health, Patient-to-professional, Respiratory protective devices
Introduction
A fit-tested, disposable, negative pressure respirator of N95 (FFP2) standard or higher is considered a standard part of protective equipment for staff caring for patients with diseases spread by airborne particles.1, 2 Fit-testing of N95 masks is recommended, as the benefit of their high level of filtration is negated if the wearer inspires unfiltered gas around the edges of the mask.3, 4 The masks most likely to fit a population of staff will depend on the facial characteristics of that population.5 It may be possible to identify a small number of masks most likely to fit the population of at-risk staff by testing a larger range of masks on a sample of about 40 staff members.6 Nevertheless, not all staff will successfully fit the initial mask tested, and fit-testing takes 30 min per person on average. Repeat testing is recommended following a bodyweight change of more than 10% and annually.1 As a result, fit-testing requires considerable resources and considerable preparation in case of an epidemic.
Further problems related to the use of disposable N95 masks include the logistic problems of keeping large stocks of masks available and the need to stock multiple brands/models.
Recently, a compact reusable mask (Totobobo; Dream Lab One Pte Ltd, Singapore) has been produced. It is made of a plastic material which is trimmed to fit the user's face. Inspired air is filtered by disposable high-efficiency particulate air (HEPA) filters (Figure 1 ). If effective, this mask may address many of the problems related to use of disposable N95 masks. In particular, as the mask is trimmed to fit the user's face it may not be necessary to carry out fit-testing, and the fact that it is reusable may obviate the need to stock large quantities of masks. In view of the potential benefits of this new mask, we carried out a controlled cross-over pilot study to compare the in-vivo filtration capacity of trimmed but non-fit-tested Totobobo masks with a fit-tested N95 mask (1860 or 1860s or 1862; 3 M, St Paul, MN, USA).
Figure 1.
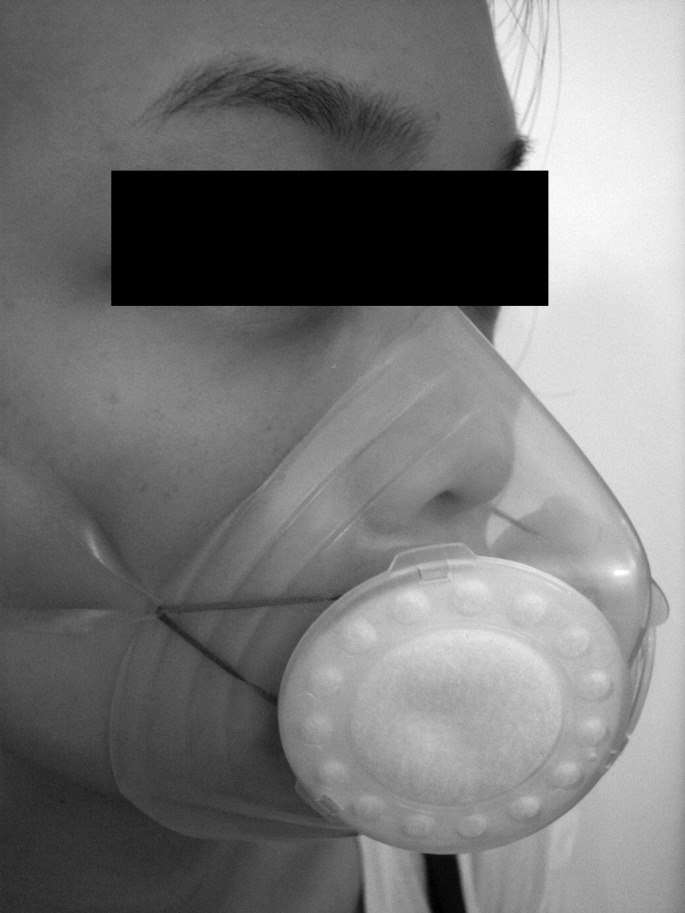
Totobobo mask. Inspired gas is filtered by high-efficiency particulate air (HEPA) filters. Plastic can be cut to fit the wearer's face. Transparency allows observation of contact between the plastic and the wearer's face.
Methods
This was a prospective unblinded study of healthy Chinese volunteers using two different protective devices: a Totobobo mask (Dream Lab One Pte. Ltd, Singapore) and a fitted N95 filtering facepiece respirator (1860 or 1860s or 1862, 3M, St Paul, MN, USA). Approval was obtained from the joint Chinese University of Hong Kong/New Territories East Cluster Clinical Research Ethics Committee. Written informed consent was obtained from all participants and the study was carried out in accordance with International Conference on Harmonisation–Good Clinical Practice standards and the Declaration of Helsinki.
Twenty-two healthy volunteers who had previously passed a fit test with an 1860, 1860s or 1862 N95 filtering facepiece respirator were recruited. New masks were used for each subject. Prior to testing the N95 mask, the subject adjusted the mask and performed a user seal test. Prior to testing the Totobobo mask the investigator trimmed the mask according to the manufacturer's instructions and following training by the inventor. The investigator also visually checked the fit of both types of mask.
Volunteers underwent a standard mask-fitting protocol. In brief, the tests consisted of comparisons of particle counts inside and outside the protective device during a series of activities: normal breathing, deep breathing, turning the head from side to side, flexing and extending the head, talking loudly and bending over followed by normal breathing again. The sampling probe (TSI Incorporated, St Paul, MN, USA) for sampling the mask particle count was inserted through the fabric or plastic of the protective device. Air samples for measuring the ambient particle count were taken from just outside the mask about 3 cm from the sampling probe.
A PortaCount Plus (TSI Incorporated) connected to a computer running FitPlus for Windows software (TSI Incorporated) was used to count particles and calculate the ratio of ambient:device particle counts. This device counts all particles sized between 0.02 and 1 μm diameter. It calculates a fit factor, which is the average ratio of atmospheric:device particle concentrations.
To ensure an adequate ambient particle count throughout the testing, the 8026 Particle Generator (TSI Incorporated) was used to generate saline particles throughout the testing procedures.
All subjects were asked which mask they found more comfortable after completion of testing.
Statistical analysis
The primary end-point was the median ratio of ambient:mask particle counts. These ratios were compared using the Wilcoxon signed ranks test. P < 0.05 was considered significant. The sample size was calculated to achieve a power of 80% based on an effect size of a probability of 0.24 that the filtration factor using one mask is less than the filtration factor using the other mask and an α-value of 0.05 (two-tailed). The secondary end-point was the proportion of patients achieving a ratio of ≥100:1. This proportion was compared using Fisher's exact test.
Results
The median (interquartile range) filtration factor was significantly higher [193 (145–200)] for N95 masks compared to Totobobo masks [135 (83–184)] (P < 0.05). However, there was no statistically significant difference between the proportion of subjects achieving a ratio of ≥100 between N95 (19/22) and Totobobo (16/22) masks. Of the 20 subjects who gave an opinion on comfort, 13 [65%, confidence interval (CI): 44–85%] found the Totobobo mask more comfortable.
Discussion
Our results indicate that the performance of a Totobobo mask cut to fit the subject's face, but not fit-tested, is inferior to the performance of a fit-tested N95 mask. Furthermore the filtration factor was >100 in only 16 of 22 subjects when wearing the Totobobo mask. These data suggest that the use of Totobobo masks, even when cut to fit the subject's face, does not obviate the need for a mask-fitting programme. Our failure to demonstrate a significant difference in the proportion of subjects achieving a filtration factor ≥100 may be due to the relatively small number of subjects in our study, which was not powered to show a difference in a binary outcome.
However, our finding does not mean that the Totobobo mask is not worthy of further evaluation, only that it should not be used without fit-testing. Data on the performance of different N95 masks in a population that has not previously been assessed for mask-fit demonstrate that the proportion of subjects achieving an adequate fit (filtration factor ≥100) with an individual mask varies from 0 to 95%, with the majority of masks fitting <40% of the test population.4, 6, 7, 8 In this context the performance of the Totobobo mask (73% of subjects achieved filtration factor ≥100) is good. If this finding is replicated, particularly in a population with different facial characteristics, then using the Totobobo mask in the panel of masks to be tested may reduce the number of masks that need to be tested before an adequate fit is found. This potential advantage, however, will need to be balanced against the time taken to trim the Totobobo mask to size. Furthermore, in situations where there is insufficient time to carry out fit-testing, use of the Totobobo mask may be a useful interim measure.
In addition to this, the fact that the Totobobo mask is designed to be reusable may provide significant advantage. This would minimise the problems associated with the need to stockpile disposable masks and the difficulty of obtaining supplies in an epidemic.1 Prior to concluding that the mask is reusable, it is necessary to test the effect of sterilisation processes on the mask. It is conceivable that such processes may make the mask less pliable and the fit less good. Moreover, it is important to ensure that the process of changing the HEPA filters does not contaminate the user with infectious particles.
In terms of comfort, 65% (CI: 44–85%) of subjects preferred the Totobobo mask to the N95 mask (1860, 1860s; 3M). Although the CI straddles 50%, this may be due to our small sample size. Comfort is an important issue when masks need to be worn for long periods of time.9 Greater comfort may translate into greater compliance with proper use of the mask and hence greater protection in clinical use. The experience of our intensive care unit during the severe acute respiratory syndrome epidemic suggests that compliance is an important factor in determining the risk of occupational infection.10
In theory, all subjects should have achieved a filtration factor ≥100 with the N95 mask, as they had previously passed a fit test with the same model of N95 mask. The lower than expected pass rate may reflect the fact that subjects did not undergo a regular fit-testing programme. The US Occupational Safety and Health Administration, Centers for Disease Control and Protection recommends that workers using N95 respirators should have repeated fit-testing on a regular basis. Although this imposes a considerable logistic and financial burden and is controversial, our data suggest that a significant proportion of subjects may not be adequately protected without regular testing.11
The results of a recent randomised controlled trial conducted in emergency departments and medical and paediatric wards suggest that surgical masks provide a similar level of protection to N95 masks against transmission of seasonal influenza.12 However, we would be cautious concerning extrapolation of these data to all healthcare environments and to patients with influenza A H1N1 (2009) infection. The risk of healthcare worker infection relates to a number of factors including number of infectious particles produced by patients and the number of organisms that constitutes an infectious dose.13 If the infectious dose is very small or the number of infectious particles produced is very high, then the benefit of the much higher filtration efficacy of N95 respirators should be greater.14 These values vary with the organism and are currently unknown for influenza A H1N1. Furthermore, in certain areas, such as intensive care units, the frequent use of aerosol-generating procedures will increase the number of infectious particles produced and therefore the likely benefit of N95 respirators.
There are a number of weaknesses in our study. First, the investigator responsible for cutting the masks received direct training from the inventor of the mask. It is possible that the performance of the mask might not be as good if this training were conducted by an individual with less insight into the functioning of the mask. Second, our study was conducted in an exclusively Chinese group of subjects. Facial characteristics may vary between racial groups and it is possible that our results will not be replicated in other racial groups. Third, the sample size was too small to adequately assess the effect of type of mask on the proportion of subjects with an adequate fit or comfort. Fourth, the comfort of the masks was only superficially tested. However, our study was a pilot study designed to determine if the mask was worthy of more extensive investigation, and in this respect we believe it has generated useful data. Based on our findings we feel that a larger multi-racial study that assesses comfort as well as fit is warranted.
Cost analysis was not part of our study although it would be an important consideration if our results are confirmed in a larger multi-racial study. The approximate retail prices (without bulk discount or delivery) of the masks are US$20 for the reusable Totobobo mask, with replacement filters costing US$1.60, and US$0.97 for single-use 3M 1860 respirators. Other factors that would need to be considered in a costing exercise are the costs of storage of masks, cleaning of Totobobo masks and fit-testing as well as bulk purchase discounts, and the number of times that a Totobobo mask can be reused.
In conclusion, despite being cut to size, the Totobobo mask (non-fit-tested) does not perform as well as a fit-tested N95 mask. Its performance and design features are sufficiently promising to warrant further investigation of its use.
Conflict of interest statement
None declared.
Funding sources
This study was supported by a Direct Grant for Research from The Chinese University of Hong Kong. Totobobo masks were supplied free by the manufacturer.
References
- 1.Gomersall C.D., Tai D.Y.H., Loo S. Expanding ICU facilities in an epidemic: recommendations based on experience from the SARS epidemic in Hong Kong and Singapore. Intensive Care Med. 2006;32:1004–1013. doi: 10.1007/s00134-006-0134-5. [DOI] [PubMed] [Google Scholar]
- 2.Gomersall C.D., Loo S., Joynt G.M., Taylor B.L. Pandemic preparedness. Curr Opin Crit Care. 2007;13:742–747. doi: 10.1097/MCC.0b013e3282f1bafd. [DOI] [PubMed] [Google Scholar]
- 3.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Laboratory performance evaluation of N95 filtering facepiece respirators. Morb Mortal Wkly Rep. 1998;47:1045–1049. [PubMed] [Google Scholar]
- 5.Zhuang Z., Coffey C.C., Ann R.B. The effect of subject characteristics and respirator features on respirator fit. J Occup Environ Hyg. 2005;2:641–649. doi: 10.1080/15459620500391668. [DOI] [PubMed] [Google Scholar]
- 6.Lee K., Slavcev A., Nicas M. Respiratory protection against Mycobacterium tuberculosis: quantitative fit test outcomes for five type N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1:22–28. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]
- 7.McMahon E., Wada K., Dufresne A. Implementing fit testing for N95 filtering facepiece respirators: practical information from a large cohort of hospital workers. Am J Infect Control. 2008;36:298–300. doi: 10.1016/j.ajic.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey C.C., Lawrence R.B., Campbell D.L., Zhuang Z., Calvert C.A., Jensen P.A. Fitting characteristics of eighteen N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1:262–271. doi: 10.1080/15459620490433799. [DOI] [PubMed] [Google Scholar]
- 9.Radonovich L.J., Jr., Cheng J., Shenal B.V., Hodgson M., Bender B.S. Respirator tolerance in health care workers. J Am Med Assoc. 2009;301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- 10.Gomersall C.D., Joynt G.M., Ho O.M. Transmission of SARS to healthcare workers. The experience of a Hong Kong ICU. Intensive Care Med. 2006;32:564–569. doi: 10.1007/s00134-006-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellerman S.E., Tokars J.I., Jarvis W.R. The costs of healthcare worker respiratory protection and fit-testing programs. Infect Control Hosp Epidemiol. 1998;19:629–634. doi: 10.1086/647888. [DOI] [PubMed] [Google Scholar]
- 12.Loeb M., Dafoe N., Mahony J. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. J Am Med Assoc. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 13.Fennelly K.P., Nardell E.A. The relative efficacy of respirators and room ventilation in preventing occupational tuberculosis. Infect Control Hosp Epidemiol. 1998;19:754–759. doi: 10.1086/647719. [DOI] [PubMed] [Google Scholar]
- 14.Derrick J.L., Gomersall C.D. Protecting healthcare staff from severe acute respiratory syndrome. A study of the filtration capacity of multiple surgical masks. J Hosp Infect. 2005;59:365–368. doi: 10.1016/j.jhin.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


