Abstract
During recombinant Adeno-associated virus (AAV) production, a proportionately large amount of vectors is released in the culture supernatant, which is often discarded. It has been shown that these vectors often associate with vesiculated structures, such as exosomes. Exosome-associated AAV (vexosomes) represent an additional gene-delivery platform. The efficiency of such vexosomes in suicide gene therapy is unexplored. In the present study, we have generated AAV serotype 6 vexosomes containing an inducible caspase 9 (iCasp9) suicide gene by a differential ultracentrifugation-based protocol. We further tested the cytotoxic potential of these vexosomes in a human hepatocellular carcinoma (HCC) model in vitro and in vivo. The AAV6-iCasp9 containing vexosomes, when primed with a pro-drug (AP20187), demonstrated a significant loss in cell viability (57% ± 8% versus 100% ± 4.8%, p < 0.001) in comparison to mock-treated Huh7 cells. An intratumoral administration of AAV6-iCasp9 vexosomes and AP20187 in a murine xenograft model revealed a 2.3-fold increase in tumor regression in comparison to untreated animals. These findings were further corroborated by histological analysis and apoptosis assays. In conclusion, our data demonstrate the therapeutic potential of AAV6 vexosomes in a xenotransplantation model of HCC. Furthermore, the simplicity in production and isolation of vexosomes should further facilitate its application in other malignancies.
Keywords: vexosome, exosome, AAV6, suicide gene delivery
Graphical Abstract
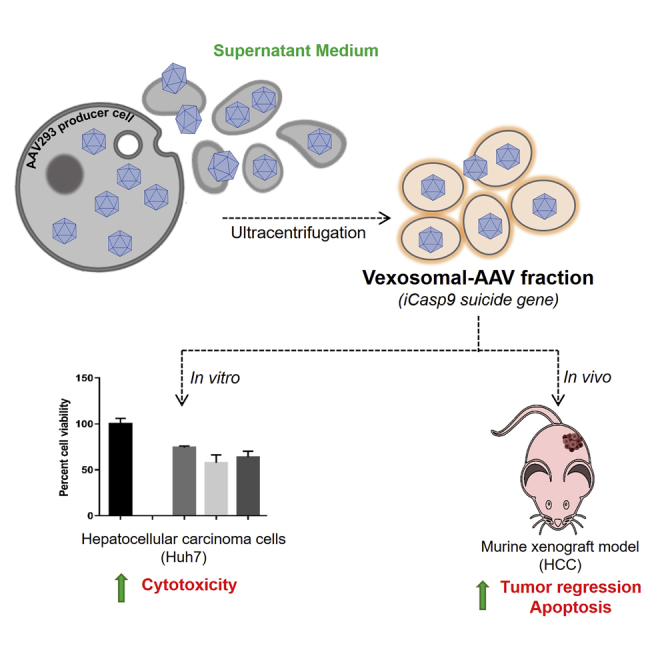
Exosome-associated Adeno-associated virus (AAV) vectors (vexosomes) have gained attention as a potent gene-delivery platform. Khan et al. demonstrate that AAV6 serotype-based vexosomes, containing an inducible caspase 9 gene, are cytotoxic in the presence of a pro-drug AP20187 and have a significant therapeutic potential for suicide gene therapy in hepatocellular carcinoma.
Introduction
Adeno-associated virus (AAV) vectors have been gaining importance as an efficient delivery system for in vivo gene transfer, owing to their long-term gene expression and broad tissue tropism.1,2 They have exhibited an excellent safety profile in clinical trials for hemophilia3 and Leber congenital amaurosis (LCA).4,5 However, the requirement of high vector doses (1012 vector genomes [vgs] per kilogram), particularly in the context of systemic gene transfer into humans,3,6 necessitates large-scale production of these vectors, thus limiting their widespread use. The arduous and intensive multistep purification protocol is one of the major factor contributing to the cost of this promising mode of gene therapy.7, 8, 9 Thus, further improvements to increase the yield and simplify the downstream purification process are pertinent to address the future needs in clinical application.
The standard method for generating AAV vectors involves the use of a producer cell line AAV293.10 During vector production, assembled AAV vector particles that accumulate inside the producer cells are harvested by cell lysis to release AAV particles, followed by different purification steps, such as ultracentrifugation and/or affinity-based purification methods.11,12 Recently, it has been reported that during vector production, a fraction of AAV vectors associated with microvesicles/endovesicles (exosome [exo]-associated AAVs or vexosomes) are naturally released into the supernatant fraction of the cell-culture media.13,14 These exo-AAV vectors seem to perform better than the conventionally purified AAV vectors15,16 in the setting of gene transfer to the retina,15 the nervous system,16 and the inner ear.17 Several studies have also demonstrated that exo-vectors not only have higher transduction efficiency but are also resistant to neutralizing antibodies.17,18 The latter feature may be of importance, particularly for therapeutic applications in vivo where endogenous anti-AAV antibodies often compromise the therapeutic efficacy of gene delivery.13
Whereas the efficiency of exo-AAV vectors during production of AAV serotypes, such as 1, 2, 5, 6, 7, 8, and 9, has been described13 and tested for replacement gene therapy of disorders, such as hemophilia,17 Leber congenital amaurosis,19 and hearing loss,18 its efficiency for suicide gene therapy is not known. We have recently demonstrated the potential of a AAV2-mediated inducible caspase 9 (iCasp9) gene delivery in a hepatocellular carcinoma (HCC) model.20 In the current study, we have evaluated the potential of exosome-associated AAV6 vectors (exo-AAV6 or AAV6 vexosomes) for their ability to deliver a suicide gene efficiently in an in vitro hepatic cancer model (Huh7 cells), as well as its therapeutic potential in the xenograft mice model of HCC.
Results
Size Distribution and Marker-Based Assessment of Vexosomes
In the initial set of investigations, cell-culture media from AAV6-producing AAV293 cells were subjected to ultracentrifugation, and a morphological characterization of the vexosome pellet was performed by transmission electron microscopy (TEM). Vexosomes isolated using the classical ultracentrifugation method showed a size distribution from 30 to 130 nm, with a median size of 50 nm (Figure 1A). These data are within the accepted size range for exosomes (<150 nm).21 Further quantification of the samples with an Exocet Exosome Quantitation Kit (System Biosciences, Mountain View, CA) for their exosomal yield showed that ∼5 × 107 vexosome particles were present per microliter of sample analyzed (data not shown).
Figure 1.
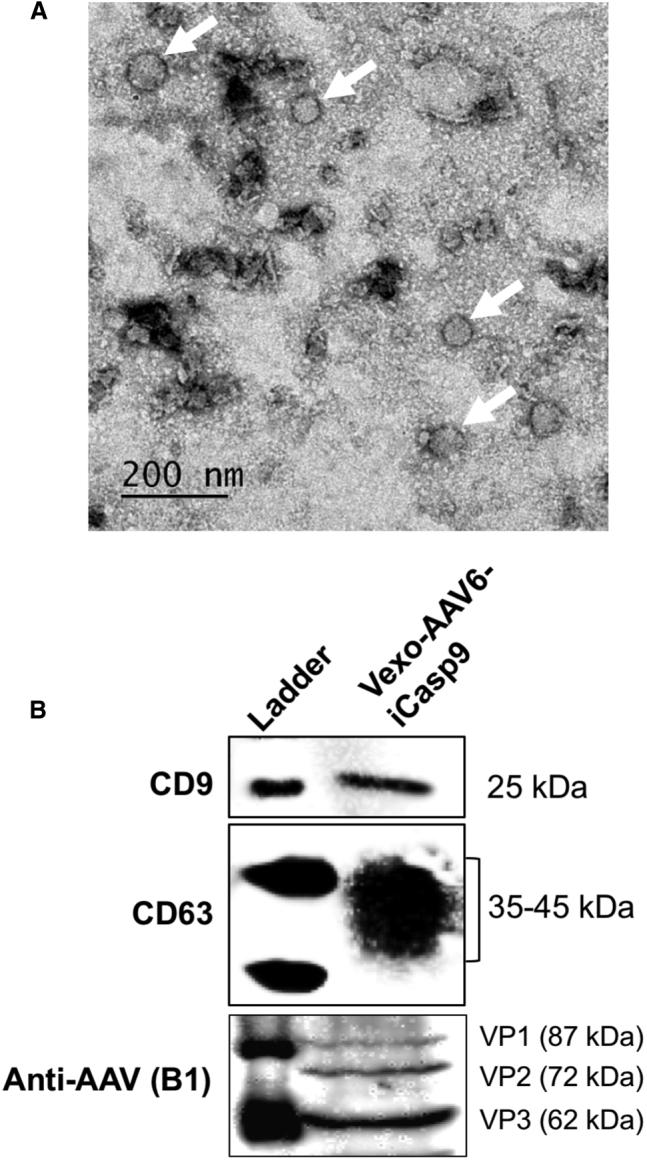
Characterization of Vexosomes Pelleted from AAV Vector-Producing AAV293 Cell-Culture Media
(A) Transmission electron microscopy- The representative image shows the presence of vexosomes of varying size. Arrows show the vexosomal membrane. Scale bar, 200 nm. (B) Immunoblotting- total cell proteins were harvested and resolved on a 10% SDS-PAGE gel. Western blotting was performed to detect specific exosomal marker proteins (CD9 and CD63) or AAV capsid proteins (VP1–3). The immune-reactive bands were detected by chemiluminescence imaging. Representative images from three biological replicates are shown.
We further characterized the vexosomes by immunoblotting for specific marker proteins. CD9 and CD63 are a set of specific tetraspanin markers that are enriched in exosomes.22 We observed that the exosomal fractions were positive for CD9 as well as CD63 markers (Figure 1B), as described earlier.17,23 Similarly, vexosomal lysates also demonstrated the presence of AAV capsid proteins (viral capsid protein [VP]1–3) (Figure 1B), highlighting the presence of AAV vectors in the vexosomal isolates. However, further immunolabeling of both the exosome membrane and AAV capsid and their detailed biophysical analysis may be necessary to quantitate the vector particles within the exosomes.
Distribution of AAV6 Vectors in Cell Lysates and Culture Media
It has been previously shown that the amount of exosome released into the supernatant media is serotype13,24 and time dependent.25,26 In order to determine the fraction of AAV6 vectors released into the supernatant media, cells and spent culture media were harvested on day 3, post-transfection (as described in Materials and Methods). DNase-resistant vector genomes in both cells and medium were assessed using the quantitative real-time PCR-based assay. Quantification of the AAV genome titers (Table 1) revealed that the overall yield of AAV vector harvested from the culture medium in this experiment was 1.22 × 1011 vgs. The vector yield obtained from vexosomes in culture medium was one-half of the yield to naked AAV6 vectors isolated, as described previously.13,17 It is possible that some of this loss could be attributed to the challenges associated with purification of vexosomes from a large volume of spent media.
Table 1.
Comparison of Conventional and Vexosome-Associated AAV6 Vectors Isolated for This Study
| Conventional AAV6 | Vexo-AAV6 | |
|---|---|---|
| Source | cell lysate | conditioned media |
| Titer (vg/mL) | 1.1 × 1012 | 4.9 × 1011 |
| Isolation | complex and time consuming | simple and quick |
| Method of isolation | iodixanol-gradient ultracentrifugation/column purification | ultracentrifugation |
| Composition | highly pure AAV vector | Vexo-AAV/associated host cellular proteins |
Vexosome (Vexo)-AAV6-iCasp9 Exhibits Enhanced Cytotoxicity In Vitro
To study the in vitro transduction efficiency of AAV6 vectors harvested from two distinct sources, Vexo-AAV6 and conventional AAV6 vectors were generated with the iCasp9 suicide gene. Huh7 cells were infected with either Vexo-AAV6 or conventional AAV6 vectors at a MOI of 5 × 104, and their cytotoxic effect was measured with an ATP based apoptosis assay. Our outcome data showed that the cell viability in the case of Vexo-AAV6-iCasp9-treated cells was substantially reduced to ∼57% when compared with mock-treated cells (57% versus 100%, p < 0.001) (Figure 2). Conventional AAV6-iCasp9 vectors also demonstrated similar cytotoxicity on Huh7 cells (cell viability 63% versus 100% in mock group, p < 0.001). The functional equivalence seen between the naked and vexosomal vectors here has been previously reported for other serotypes, such as AAV2.13
Figure 2.
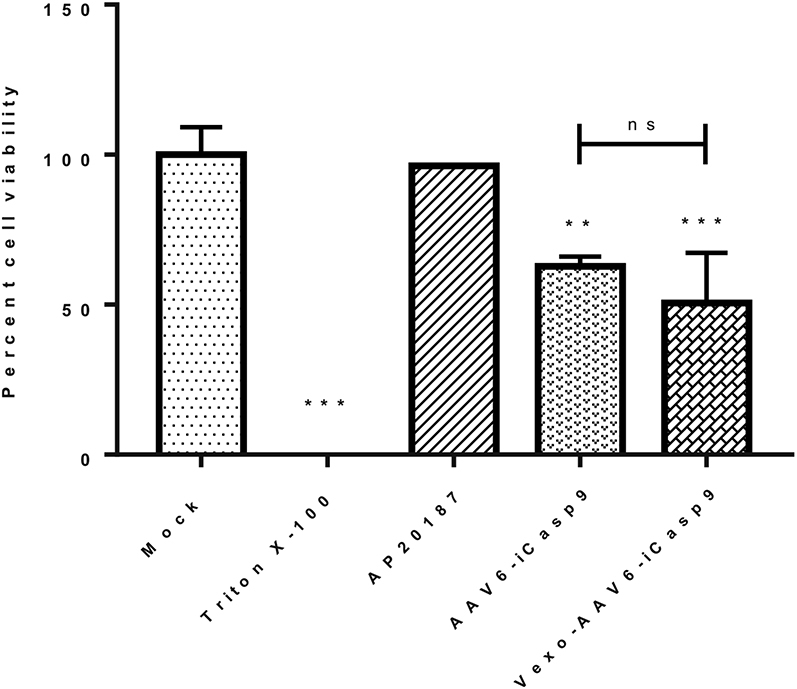
Vexosomes Are Cytotoxic to Hepatocellular Carcinoma, Huh7 Cells In Vitro
Huh7 cells were infected with either AAV6 or Vexo-AAV6-iCasp9 vectors, at a multiplicity of infection (MOI) of 5 × 104 vgs/cell, followed by treatment with AP20187. The viability of Huh7 cells treated with a combination of vector and dimerizer drug, after 72 h of vector infection, was determined using an ATP assay (Promega) and depicted in comparison to mock-treated cells. Triton X-100, positive control; AP20187, drug-only control. Data are mean ± SD from two independent experiments (n = 3 replicates each condition per experiment, ∗∗p < 0.01, ∗∗∗p < 0.001 in comparison to mock-treated cells). ns, not significant.
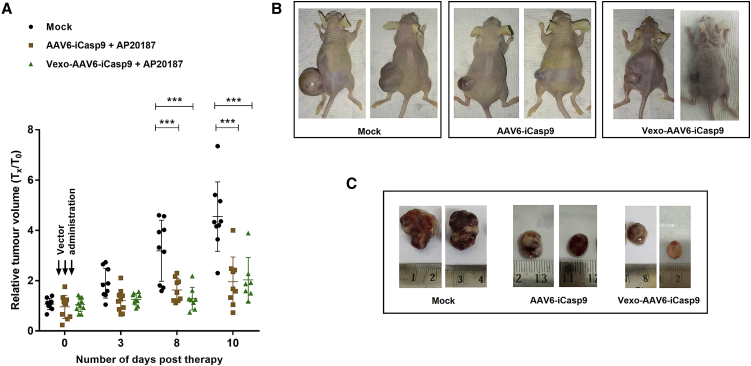
Vexo-AAV6 Vectors Mediate Significant Tumor Regression in a HCC Xenotransplantation Model
To validate the therapeutic utility of Vexo-AAV vectors, we utilized a xenotransplantation model of HCC, as described in Materials and Methods. About 15 to 20 days after Huh7 cell transplantation, mice developed tumor nodules. At this time point, we injected the tumor-bearing mice with an equivalent vector dose (2 × 1010 vg) of AAV6-iCasp9 or Vexo-AAV6-iCasp9 vectors. The control group of mice received PBS injections. A day later, we administered the dimerizer drug (AP20187, 1 mg/kg body weight) intraperitoneally, as described earlier.27 The experimental animals were then followed up for ∼10 days to limit the tumor burden in untreated mice, as described earlier.28 Some animals in treatment group were also lost during follow-up period, thus possibly requiring further dose-optimization. We observed a significant regression pattern in the tumor volumes of animals that were challenged with Vexo-AAV6-iCasp9- but not in the animals of the mock-treated group (Figure 3). The rate of tumor inhibition was ∼2.4-fold for the Vexo-AAV6-iCasp9 group on day 8, whereas it was ∼2-fold for the AAV6-iCasp9-administered group. The regression rate further increased to ∼2.35-fold in the case of AAV6-iCasp9, whereas it was ∼2.3-fold for the Vexo-AAV6-iCasp9 group by day 10 (Figure 3A). These data demonstrate that Vexo-AAV6 vectors are therapeutic in a xenotransplantation model of HCC and appear to be functionally equivalent to conventional AAV6 vectors in terms of suicide gene transfer efficiency both in vitro and in vivo.
Figure 3.
Vexo-AAV6-iCasp9 Vectors Inhibit Tumor Growth in a Huh7 Cell Xenograft Model
(A) HCC tumor-bearing mice were injected with PBS (Mock) intratumorally or with AAV6-iCasp9 or Vexo-associated AAV6-iCasp9 vectors (2 × 1010 vgs/animal). Animals from both the AAV6-iCasp9 and Vexo-AAV6-iCasp9 group demonstrated significant tumor regression when compared to mock-injected animals (data are mean ± SD, n = 7–10 per group, ∗∗∗p < 0.001). (B and C) Representative images of animals (B) and their tumors after enucleation (C).
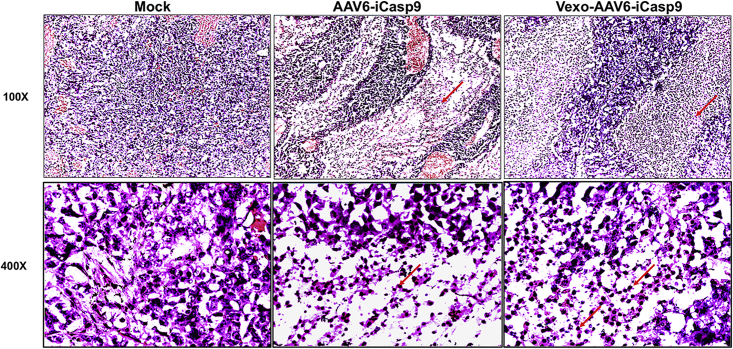
Administration of Vexo-AAV6 Enhances Apoptosis in the Recipient Tumor Tissue
Tumor-bearing animals from mock or treated groups were humanely euthanized at the end of the experiment, and the subcutaneous tumors were harvested 10 days after gene transfer. For morphological characterization in tissue sections, we performed hematoxylin and eosin staining. Animals from the mock-administered group showed a higher number of mitotically active, large proliferating cells (Figure 4). In the case of tissue sections obtained from the Vexo-AAV6-iCasp9 vector-treated group, where a significant tumor regression was seen, a significant number of small, condensed apoptotic cells with a pyknosis of their nuclei were observed (Figure 4).
Figure 4.
Histological Analysis of Huh7 Xenograft Tumor Tissue
Excised tumors were sectioned and stained with hematoxylin and eosin at the end of the experiment. Representative images are shown. A notable pyknosis (red arrow) and apoptosis are seen with Vexo-AAV6-iCasp9 vector + AP20187-administered animals (magnifications: top, 100×; bottom, 400×).
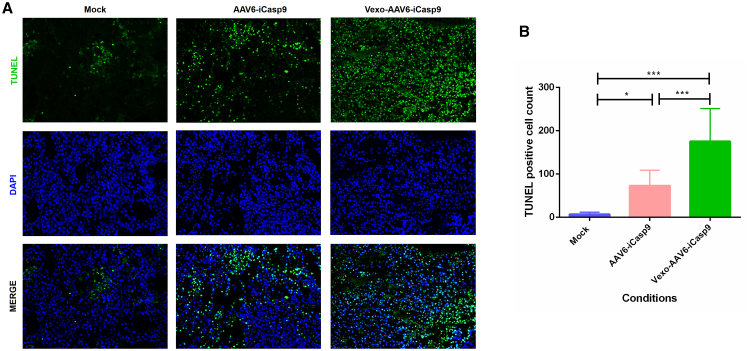
In order to confirm these observations further, a terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate (dUTP) nick-end labeling (TUNEL) assay was performed.29, 30, 31 The tumor sections from the Vexo-AAV6-iCasp9- or standard AAV6-iCasp9-treated group showed marked presence of proapoptotic cells (green color) in comparison to sections prepared from the control animal group (Figure 5A). A quantitative assessment of the extent of DNA damage by ImageJ analysis (Figure 5B) revealed a significant proportion of TUNEL-positive cells in the case of the Vexo-AAV6-iCasp9 vector-administered group (175 ± 75) in comparison to either the conventional AAV vector-administered (72 ± 35, p < 0.001) or the mock-administered group (7 ± 4, p < 0.001). These data also highlight certain differences in the proportion of TUNEL-positive cells observed here to the phenotypic outcome (tumor volume) seen between Vexo-AAV6-iCasp9- versus AAV6-iCasp9-treated groups (Figure 3). This could possibly be due to a late burst in apoptosis in animals treated with Vexo-AAV6 -iCasp9 vectors. Thus, a further long-term follow-up (>10 days) may be required to assess the complete impact of suicide gene transfer with AAV6 vexosomes.
Figure 5.
TUNEL Staining in Huh7 Xenograft Tumor Tissue
(A) Representative images showing massive apoptosis within the tumors harvested from the Vexo-AAV6-treated group as compared to the mock group (magnification, 200×). Nuclei stained with the TUNEL assay are green. Sections were counterstained with DAPI. (B) Data from (A) (9 sections per condition) were quantified by ImageJ analysis and expressed as mean ± SD (∗p < 0.05, ∗∗∗p < 0.001).
Discussion
Exosomes are small extracellular vesicles that are secreted from a cell for communication32, 33, 34 and are known to maintain tissue homeostasis.35,36 Exosomes are secreted out from the cell after the fusion of multivesicular bodies (MVBs) to the cell membrane.37 Recently, exosomes have gained attention because of the diversity of the cargo capacity they carry (mRNA, microRNA [miRNA], proteins, DNA)38, 39, 40 and immune-evasion41 properties. Several DNA and RNA viruses have been previously shown to be associated with exosomes,42, 43, 44 suggesting a plausible evolutionary conserved mechanism.45 Furthermore, the role of exosomes in gene delivery in general46 and with vexosomes harvested during AAV production has been explored in different disease settings, such as in hemophilia,17 LCA,19 or neurological diseases.47 The efficacy AAV2- based vexosomes with enhanced transduction of the retinal cells upon intravitreal delivery has been known.19 Other studies that have employed vexosomes derived from AAV serotypes, such as AAV1, -6, -7, -8, or -9, have mostly analyzed the rate of formation and distribution of vectors in the cell lysate and culture media.24,25 To the best of our knowledge, this is the first study to demonstrate the therapeutic potential of the Vexo-associated AAV serotype 6 vectors in a murine model of HCC.
HCC is associated with profound mortality and/or morbidity, even after conventional interventions, such as surgical resection or use of tyrosine kinase inhibitors.48,49 We have recently developed a AAV2-based, iCasp9-based suicide gene therapy as an alternative approach to treat HCC.20 Due to the fact that frequency of high titer neutralizing antibodies to AAV2 in the general population can be as high as ∼70%,50 we wished to evaluate an alternate AAV6 serotype for its potential in delivering the iCasp9 suicide gene. Interestingly, the AAV6 serotype can efficiently target liver cells, including Huh7 cells.51,52 Furthermore, the feasibility and attractiveness of harvesting vexosome-associated AAV6 vectors from spent media, which otherwise are routinely discarded during vector production, motivated us to test the utility of this system for therapeutic suicide gene therapy. It must be highlighted that the vexosomes for our study were isolated from routine packaging conditions of AAV generation without incorporating additional modifications, such as use of exosome-specific media (2% bovine exosome-free fetal bovine serum [FBS]), as described earlier.16 Our data demonstrate that AAV6 vexosomes thus generated from routine AAV packaging had a significant therapeutic efficacy when a combination of AAV6-iCasp9 suicide gene-containing vexosomes and AP20187 was tested (Figure 3). However, our data also showed that the efficacy of Vexo-associated-AAV6 was similar to that of AAV6 naked vectors. Previous studies with AAV serotypes 1, 8, and 9, carrying a bi-bicistronic expression cassette consisting of the human serum protein α1 antitrypsin (AAT) and green fluorescent protein (GFP), demonstrated equivalent protein expression after administration of vector preparations from cell lysates and culture fluids for all three serotypes.25 These data highlight that the efficiency of vexosome-based gene therapy can be disease and context specific. It further stands to reason that the efficacy of the vexosomes generated will be dependent on the source and type of packaging cells (human versus insect cells),53 physical parameters of vector packaging (media pH, composition, etc.), AAV serotype employed,13 and size difference in the expression cassettes packaged.26 All of these factors imply a recombinant vector-dependent processing mechanism that determines the AAV/vexosome distribution in media and lysate. Further detailed mechanistic studies are required to understand their impact in generating AAV6 vexosomes.
In addition, previous reports with vexosomes derived from other AAV serotypes, such as AAV8, have known to confer phenotypic correction in hemophilia mice and an increased resistance to neutralizing anti-AAV antibodies.17 This is likely due to the fact that Vexo-AAV vectors are naturally enveloped with exosomal membranes and thus, possibly have a propensity to shield the viral vectors from neutralizing antibodies. In the case of the AAV6 vexosomes, a similar outcome may be expected in the setting of neutralizing antibody positivity, but further evaluation of this phenomenon is required in suitable murine models in vivo.
In conclusion, we have demonstrated the utility of AAV6-based vexosomal vectors at considerably low doses (2 × 1010 vgs) for suicide gene therapy of HCC. The attractiveness of our approach is deriving these highly efficient Vexo-AAV6 vectors from media discarded during routine AAV packaging. Apart from the ease of harvesting these vexosomes, the overall protocol for vexosome isolation is completed in ∼2.5 h, as described earlier,19 adding significantly to the appeal of this mode of gene delivery. This approach can be also potentially applicable for the treatment of other solid malignancies, such as breast or head/neck cancer. However, concerted efforts are needed to understand the safety and immunogenicity of vexosomes in multiple settings and further standardize the methods to scale up the production of vexosomes for clinical use.
Materials and Methods
Cell Culture
Human AAV293 packaging cells were purchased from Stratagene (San Diego, CA, USA) and the human hepatocellular carcinoma (Huh7) cell line was a kind gift from Dr. Saumitra Das (Indian Institute of Science, Bangalore). The cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM), supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), ciprofloxacin (HiMedia Laboratories, Mumbai, India), and piperacillin (MP Biomedicals, Irvine, CA, USA) at 10 μg/mL in a humidified atmosphere, supplemented with 5% CO2 at 37°C.
Vector Preparation
Conventional AAV vectors were generated as described previously.10 Briefly, AAV293 cells, expanded in 15 cm2 dishes (n = 40), were cotransfected using polyethylenimine (PEI; Polysciences, Warrington, PA, USA) with an equimolar concentration of plasmids carrying the rep/cap plasmid (p.AAVR2/C6), an inducible caspase 9 transgene (p.AAV-CBa-iCasp9)20 and adenoviral helper plasmids (p.Helper). Transfected cultures were maintained in IMDM, supplemented with 10% FBS. About 68 h after transfection, cells were collected by centrifuging the suspension at 1,000 g for 5 min. After three freeze-thaw cycles (–80°C, 37°C), AAV particles were purified from the cell lysates by iodixanol gradient centrifugation. The gradient fraction containing AAV vectors was further purified by column chromatography (HiTrap Q column; GE Healthcare Life Sciences, Chicago, IL, USA). Purified vectors were finally concentrated using Amicon Ultra-15 centrifugal filtration devices (Millipore, Bedford, MA, USA). The supernatant culture medium from the same 40, 15 cm plates (800 mL), 68 h post-transfection, was pooled. This conditioned medium fraction was transferred into 50 mL tubes and stored at −80°C for vexosome isolation.
Vexosome Isolation
Vexosomes were collected from the conditioned media (800 mL) of AAV293 cells cultured for 68 h. The vexosomes were purified by differential centrifugation of the conditioned media, as described previously.13,25 The supernatant media were first centrifuged at 800 g for 10 min to sediment the cells and centrifuged at 20,000 g for 1 h (Optima L-100K Ultra Centrifuge; Beckman Coulter, Brea, CA, USA) to remove the cellular debris. Vexosomes were separated from the supernatant by further centrifugation at 100,000 g for 1.5 h. The pellet was then resuspended in 100 μL of PBS (vexosome fraction).
Quantitative Polymerase Chain Reaction
The AAV genome titers in both the standard AAV6 and vexosome preparation were measured by a quantitative real-time PCR, as described earlier.54 Samples were treated with DNase before quantitative real-time PCR in order to eliminate nonencapsidated DNA. The primers were targeted to the polyadenylation (PolyA) signal in the encapsidated genome, and the quantitative real-time PCR performed on a CFX96 real-time PCR instrument (Bio-Rad, Hercules, CA, USA) using SYBR Green (Promega, Madison, WI, USA). The titers were generated from two replicate analyses and are represented as vgs per milliliter.
Transmission Electron Microscopy
To characterize the vexosomes, ∼10 μL of either neat or diluted (1:2) vexosomes was adsorbed onto TEM copper grids (Ted Pella, Redding, CA, USA), stained (2% uranyl acetate). The TEM images were acquired in a transmission electron microscope (FEI Technai G2, Hillsboro, OR, USA). About 10–20 images of vexosomes from each grid were acquired and further quantified using ImageJ analysis (National Institutes of Health, Bethesda, MD, USA).
Immunoblotting
The vexosome pellet containing AAV vectors was isolated, as described above, and their protein fraction isolated by using radioimmunoprecipitation assay (RIPA) buffer (Pierce, Thermo Fisher Scientific, Waltham, MA, USA). The protein concentration in the isolated samples was determined by the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific), as per the manufacturer’s instructions. Subsequently, an equal amount of vexosomal protein (25–60 μg) was denatured with a 4× denaturation buffer (Bio-Rad) at 95°C for 10 min. The denatured samples were then separated in a 10% SDS-polyacrylamide gel by electrophoresis. The resolved proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane and further blocked in 2% skimmed milk to minimize nonspecific binding of antibodies. Immunoblotting was performed using anti-human CD9 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), anti-human CD63 antibody (Abcam, Cambridge, MA, USA), or AAV capsid-specific anti-AAV(B1) antibody (Fitzgerald, North Acton, MA, USA). The chemiluminescent signals were developed using the West PicoPlus enhanced chemiluminescence (ECL) substrate kit (Thermo Fisher Scientific) and captured using a ChemiDoc Imager (ECL; Thermo Fisher Scientific).
AAV6 Vexosome-Mediated Cytotoxicity Assays In Vitro
About ∼1.5 × 104 Huh7 cells per well in a 48-well plate were either mock (PBS) infected or infected (MOI: 5 × 104 vgs/cell) with AAV6-iCasp9 vectors or AAV6-iCasp9 vexosomes. 24 h postinfection, transfected cells were treated with AP20187 (10 nm; Ariad Pharmaceuticals, Cambridge, MA, USA). 2 days later, we measured the cell viability by an ATP assay, per the commercial protocol (CellTiter-Glo; Promega) and as previously reported.20
AAV6 Vexosome-Mediated Suicide Gene Delivery In Vivo
Our animal studies utilized athymic nude mice (8–10 weeks old) from a BALB/c genetic background (National Institute of Nutrition, Hyderabad, India). The animal use and experimentation were approved by the Institutional Animal Ethics Committee (IIT-Kanpur, India). For xenotransplantation, we subcutaneously administered ∼5 million Huh7 cells in a mixture of 25% Matrigel (Sigma-Aldrich, St. Louis, MO, USA) in 200 μL vol. When the animals developed palpable tumors (150–200 mm3 size), they were randomized into three groups: mock, AAV6-iCasp9, and vexosomal fraction (Vexo-AAV6-iCasp9). Animals from the treatment group received ∼2 × 1010 vgs of each of the vectors intratumorally. Subsequently, vector-injected animals were administered with AP20187 thrice and 2 days apart from each dose of 1 mg/kg body weight, as described earlier.20 The animals (n = 7-10 per group) were continuously evaluated and the tumor volumes calculated (0.5 × L [largest diameter] × W2 [shortest diameter in millimeters]), as described previously.55 At the end of experiment, animals were euthanized humanely and the tumor samples harvested, as previously described.20
Histology and In Situ TUNEL Assays
Tumors from each group were harvested, washed briefly with PBS, fixed, and cryosectioned (6 μm). The sections (n = 3 per animal; 3 animals per group) were then stained with hematoxylin and eosin. We also performed an in situ TUNEL, and counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific) was performed to detect apoptotic cells, according to the manufacturer’s protocol (Invitrogen). Images were captured in a Leica DM5000B microscope (Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
All values are expressed as mean ± standard deviation (SD). Statistical analysis was performed using analysis of variance (ANOVA) tests using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA). A p value of <0.05 between the control and test groups was considered to be statistically significant.
Author Contributions
N.K., S.M., and S.B. conducted the experiments. G.R.J. supervised the study. N.K. and G.R.J. wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Ms. Vijayata Singh, IIT-Kanpur for preparation of AAV6 vexosomes. This work was supported by a Nanomission grant (SR/NM/NS-1084/2016) and in part from the Wellcome Trust DBT India Alliance Senior Fellowship (IA/S/16/1/502355), awarded to G.R.J. N.K. and S.M. were supported by a PhD fellowship (MHRD grant to IIT-Kanpur). S.B. is supported by an IIT-Kanpur Institute postdoctoral fellowship.
References
- 1.Rolling F., Samulski R.J. AAV as a viral vector for human gene therapy. Generation of recombinant virus. Mol. Biotechnol. 1995;3:9–15. doi: 10.1007/BF02821330. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 5.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J., Jacobson S.G. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Z., Qiao C., Hu P., Li J., Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011;22:613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark K.R. Recent advances in recombinant adeno-associated virus vector production. Kidney Int. 2002;61(1, Suppl):S9–S15. doi: 10.1046/j.1523-1755.2002.0610s1009.x. [DOI] [PubMed] [Google Scholar]
- 10.Mary B., Maurya S., Arumugam S., Kumar V., Jayandharan G.R. Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. 2019;286:4964–4981. doi: 10.1111/febs.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potter M., Lins B., Mietzsch M., Heilbronn R., Van Vliet K., Chipman P., Agbandje-McKenna M., Cleaver B.D., Clément N., Byrne B.J., Zolotukhin S. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. Methods Clin. Dev. 2014;1:14034. doi: 10.1038/mtm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao G., Qu G., Burnham M.S., Huang J., Chirmule N., Joshi B., Yu Q.C., Marsh J.A., Conceicao C.M., Wilson J.M. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 13.Vandenberghe L.H., Xiao R., Lock M., Lin J., Korn M., Wilson J.M. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum. Gene Ther. 2010;21:1251–1257. doi: 10.1089/hum.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire C.A., Balaj L., Sivaraman S., Crommentuijn M.H., Ericsson M., Mincheva-Nilsson L., Baranov V., Gianni D., Tannous B.A., Sena-Esteves M. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudry E., Martin C., Gandhi S., György B., Scheffer D.I., Mu D., Merkel S.F., Mingozzi F., Fitzpatrick Z., Dimant H. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23:380–392. doi: 10.1038/gt.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.György B., Fitzpatrick Z., Crommentuijn M.H., Mu D., Maguire C.A. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials. 2014;35:7598–7609. doi: 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meliani A., Boisgerault F., Fitzpatrick Z., Marmier S., Leborgne C., Collaud F., Simon Sola M., Charles S., Ronzitti G., Vignaud A. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017;1:2019–2031. doi: 10.1182/bloodadvances.2017010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.György B., Sage C., Indzhykulian A.A., Scheffer D.I., Brisson A.R., Tan S., Wu X., Volak A., Mu D., Tamvakologos P.I. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017;25:379–391. doi: 10.1016/j.ymthe.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassmer S.J., Carvalho L.S., György B., Vandenberghe L.H., Maguire C.A. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep. 2017;7:45329. doi: 10.1038/srep45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan N., Bammidi S., Chattopadhyay S., Jayandharan G.R. Combination Suicide Gene Delivery with an Adeno-Associated Virus Vector Encoding Inducible Caspase-9 and a Chemical Inducer of Dimerization Is Effective in a Xenotransplantation Model of Hepatocellular Carcinoma. Bioconjug. Chem. 2019;30:1754–1762. doi: 10.1021/acs.bioconjchem.9b00291. [DOI] [PubMed] [Google Scholar]
- 21.Patel G.K., Khan M.A., Zubair H., Srivastava S.K., Khushman M., Singh S., Singh A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019;9:5335. doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreu Z., Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller L.T., Lemus-Diaz N., Rinaldi Ferreira R., Böker K.O., Gruber J. Enhanced Production of Exosome-Associated AAV by Overexpression of the Tetraspanin CD9. Mol. Ther. Methods Clin. Dev. 2018;9:278–287. doi: 10.1016/j.omtm.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada T., Nonaka-Sarukawa M., Uchibori R., Kinoshita K., Hayashita-Kinoh H., Nitahara-Kasahara Y., Takeda S., Ozawa K. Scalable purification of adeno-associated virus serotype 1 (AAV1) and AAV8 vectors, using dual ion-exchange adsorptive membranes. Hum. Gene Ther. 2009;20:1013–1021. doi: 10.1089/hum.2009.006. [DOI] [PubMed] [Google Scholar]
- 25.Lock M., Alvira M., Vandenberghe L.H., Samanta A., Toelen J., Debyser Z., Wilson J.M. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piras B.A., Drury J.E., Morton C.L., Spence Y., Lockey T.D., Nathwani A.C., Davidoff A.M., Meagher M.M. Distribution of AAV8 particles in cell lysates and culture media changes with time and is dependent on the recombinant vector. Mol. Ther. Methods Clin. Dev. 2016;3:16015. doi: 10.1038/mtm.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagyu S., Hoyos V., Del Bufalo F., Brenner M.K. An Inducible Caspase-9 Suicide Gene to Improve the Safety of Therapy Using Human Induced Pluripotent Stem Cells. Mol. Ther. 2015;23:1475–1485. doi: 10.1038/mt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sápi J., Kovács L., Drexler D.A., Kocsis P., Gajári D., Sápi Z. Tumor Volume Estimation and Quasi-Continuous Administration for Most Effective Bevacizumab Therapy. PLoS ONE. 2015;10:e0142190. doi: 10.1371/journal.pone.0142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S.S., Mehedint D.C., Ford O.H., 3rd, Jeyaraj D.A., Pop E.A., Maygarden S.J., Ivanova A., Chandrasekhar R., Wilding G.E., Mohler J.L. Comparison of ACINUS, caspase-3, and TUNEL as apoptotic markers in determination of tumor growth rates of clinically localized prostate cancer using image analysis. Prostate. 2009;69:1603–1610. doi: 10.1002/pros.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan W.R., Garner D.S., Williams S.D., Funckes-Shippy C.L., Spath I.S., Blomme E.A. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J. Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- 31.Wieder R. TUNEL assay as a measure of chemotherapy-induced apoptosis. Methods Mol. Med. 2005;111:43–54. doi: 10.1385/1-59259-889-7:043. [DOI] [PubMed] [Google Scholar]
- 32.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baixauli F., López-Otín C., Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessvik N.P., Øverbye A., Brech A., Torgersen M.L., Jakobsen I.S., Sandvig K., Llorente A. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell. Mol. Life Sci. 2016;73:4717–4737. doi: 10.1007/s00018-016-2309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.A., Bernad A., Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolte-’t Hoen E.N.M., Buermans H.P.J., Waasdorp M., Stoorvogel W., Wauben M.H.M., ’t Hoen P.A. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pigati L., Yaddanapudi S.C., Iyengar R., Kim D.J., Hearn S.A., Danforth D., Hastings M.L., Duelli D.M. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouwaki T., Okamoto M., Tsukamoto H., Fukushima Y., Oshiumi H. Extracellular Vesicles Deliver Host and Virus RNA and Regulate Innate Immune Response. Int. J. Mol. Sci. 2017;18:666. doi: 10.3390/ijms18030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Z., Hensley L., McKnight K.L., Hu F., Madden V., Ping L., Jeong S.H., Walker C., Lanford R.E., Lemon S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y., Han Q., Hou Z., Zhang C., Tian Z., Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell. Mol. Immunol. 2017;14:465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiley R.D., Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masciopinto F., Giovani C., Campagnoli S., Galli-Stampino L., Colombatto P., Brunetto M., Yen T.S., Houghton M., Pileri P., Abrignani S. Association of hepatitis C virus envelope proteins with exosomes. Eur. J. Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X.C., Gao J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017;521:167–175. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 47.Orefice N.S., Souchet B., Braudeau J., Alves S., Piguet F., Collaud F., Ronzitti G., Tada S., Hantraye P., Mingozzi F. Real-Time Monitoring of Exosome Enveloped-AAV Spreading by Endomicroscopy Approach: A New Tool for Gene Delivery in the Brain. Mol. Ther. Methods Clin. Dev. 2019;14:237–251. doi: 10.1016/j.omtm.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 49.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 50.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayroo R., Nolasco D., Yin Z., Colon-Cortes Y., Pandya M., Ling C., Aslanidi G. Development of novel AAV serotype 6 based vectors with selective tropism for human cancer cells. Gene Ther. 2016;23:18–25. doi: 10.1038/gt.2015.89. [DOI] [PubMed] [Google Scholar]
- 52.Rezvani M., Español-Suñer R., Malato Y., Dumont L., Grimm A.A., Kienle E., Bindman J.G., Wiedtke E., Hsu B.Y., Naqvi S.J. In Vivo Hepatic Reprogramming of Myofibroblasts with AAV Vectors as a Therapeutic Strategy for Liver Fibrosis. Cell Stem Cell. 2016;18:809–816. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 54.Aurnhammer C., Haase M., Muether N., Hausl M., Rauschhuber C., Huber I., Nitschko H., Busch U., Sing A., Ehrhardt A., Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods. 2012;23:18–28. doi: 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
- 55.Tomayko M.M., Reynolds C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]