Abstract
Infectious micro-organisms may be transmitted by a variety of routes, and some may be spread by more than one route. Respiratory and facial protection is required for those organisms that are usually transmitted via the droplet/airborne route, or when airborne particles have been artificially created, such as during ‘aerosol-generating procedures’. A range of personal protective equipment that provides different degrees of facial and respiratory protection is available. It is apparent from the recent experiences with severe acute respiratory syndrome and pandemic (H1N1) 2009 influenza that healthcare workers may have difficulty in choosing the correct type of facial and respiratory protection in any given clinical situation. To address this issue, the Scientific Development Committee of the Healthcare Infection Society established a short-life working group to develop guidance. The guidance is based upon a review of the literature, which is published separately, and expert consensus.
Keywords: Respiratory infection, Facial protection equipment, Respiratory protection equipment, Personal protective equipment, Filtering face piece, Aerosol-generating procedure, Droplet transmission, Airborne transmission
Key points
-
–
Healthcare workers (HCWs) may have difficulty in deciding whether facial and respiratory protection is required, and in choosing which combination is appropriate in any given clinical situation. This document provides guidance to support HCWs to select appropriate respiratory and facial protection.
-
–
Respiratory and facial protection as considered in this guidance is required to deal with the presence of potentially infectious particles in the air.
-
–
A range of personal protective equipment (PPE) that provides facial and respiratory protection is available. In most clinical scenarios where this is required, it will comprise either a surgical mask or a respirator, with or without eye protection.
-
–
The requirement to wear respiratory and facial protection will be determined by a range of factors that involve a risk-assessment-based approach related to: the procedure/task to be undertaken; known/suspected infection; and presenting patient symptoms.
-
–
The selection and use of respiratory and facial protection equipment must be underpinned by appropriate staff education and training.
-
–
Specific recommendations on selection of equipment based upon this approach are presented in detail within the main body of the document.
-
–
In the majority of situations where respiratory and facial protection is required, a surgical mask will be adequate. For a very small number of pathogens that are truly transmissible via the airborne route, or where aerosol-generating procedures (AGPs) involving infectious body fluids are being undertaken, a respirator will be required. The requirement for eye protection will largely be determined by the risk of splashing/spraying of blood and/or body fluids to the eyes/face.
-
–
Recommendations for future research to address outstanding evidence gaps are provided.
Introduction
A range of PPE that provides different degrees of facial and respiratory protection is available. This includes surgical face masks, respiratory protection equipment (RPE), protective spectacles, goggles and visors. It is apparent from recent experiences with severe acute respiratory syndrome and pandemic (H1N1) 2009 influenza that HCWs may have difficulty in choosing the correct type of facial and respiratory protection in any given clinical situation.1, 2
To address this issue, the Scientific Development Committee of the Healthcare Infection Society established a short-life working group in May 2011 to develop appropriate guidance. The working group included representation from the Healthcare Infection Society, Public Health England, Health and Safety Executive (HSE), Association of National Health Occupational Physicians, Health Protection Scotland, Infection Prevention Society, Intensive Care Society, Clinical Virology Network and British Infection Association. The guidance is based upon a review of the literature (which can be accessed separately) and expert consensus.3 Although the guidance also takes account of relevant current UK health and safety legislation, the majority of the material will be more widely relevant.
Implementation of this guidance is a matter for local determination based on risk assessment and the need to adhere to any relevant health and safety legislation. Employers in the UK have a duty of care to their employees to provide a safe working environment, which may include the provision and use of PPE (see Appendix 3).
Aim
This article provides guidance (best practice guidelines) to support HCWs in hospital or community settings to select and wear the appropriate respiratory and facial protection to minimize the risk of acquisition of infection in the workplace.
Exclusions
This guidance does not cover the use of powered respirators, chemical exposures and laser plumes, or Category 4 pathogens. Recommendations on the latter are available from the Advisory Committee on Dangerous Pathogens4 and the HSE document ‘Respiratory protective equipment at work: a practical guide’.5
Advice on the wearing of respiratory and facial protection by patients and visitors is also out with the scope of the current guidance.
Risks associated with infectious particles and routes of transmission
The respiratory and facial protection considered in this guidance is required to deal with the presence of infectious particles in the air. These particles form a continuous spectrum of sizes and resultant properties. The size of the particle determines the microbial numbers it can carry, the distance it can travel, how deeply it can penetrate the host's respiratory tract, and what form of protection will be necessary.
For convenience, particles can be grouped into functional units categorized by their routes of transmission.
-
–
Splashes: large particles (>100 μm in diameter) that fall out of airborne suspension within a few seconds.
-
–
Droplets: smaller particles but larger than aerosols (approximately 5–100 μm in diameter). While the lower range of these particle sizes (<20 μm) will remain airborne for many minutes, particles >20 μm fall out of airborne suspension within seconds. Droplet particles penetrate the respiratory tract to above the alveolar level. However, if a liquid (aqueous) droplet evaporates before falling to the ground, it can shrink to become an aerosol particle known as a ‘droplet nucleus’.
Splash and droplet transmission occurs as a result of droplets being expelled from the respiratory tract of an infected individual (e.g. during coughing and sneezing). These may impact directly on to a mucosal surface or conjunctiva of a susceptible individual. Such transmission tends to be relatively efficient (large particles can contain high numbers of microbes), but is only effective over the short distance before these particles fall out of the air.
Traditionally, a distance of 1 m6, 7, 8 has been used to define the need for droplet precautions; however, this distance is recommended as the minimum rather than an absolute distance.
Protection against splashes and droplets requires barriers to protect the eyes, nose, mouth and upper respiratory tract of those exposed.
-
–
Aerosols: very small, lightweight particles with neutral buoyant density that can remain in suspension in the air for long time periods and travel long distances. These particles can penetrate the respiratory system to the alveolar level and are generally <5 μm in diameter. Aerosol particles can be formed from the evaporation of a larger droplet particle. Aerosols formed in this way are termed ‘droplet nuclei’ but behave as all other aerosols.
Airborne transmission (literally ‘via the air’) occurs as a result of aerosol particles being generated from the respiratory tract of an infected individual during, for example, coughing and sneezing and during procedures that put sufficient energy into infectious body fluids to break them up into small enough particles to form aerosols directly or via droplet nuclei (AGPs). Such transmission tends to be relatively inefficient and requires close proximity, prolonged proximity, high levels of dispersion, high susceptibility or a combination.
Protection against aerosols requires filtration of inhaled contaminated air (i.e. the use of respirators).
-
–AGPs can generate an aerosol hazard from an infection that may otherwise only be transmissible via splashes or droplets. The following procedures are considered to be AGPs (this is currently an area of active research and this list is likely to be subject to change to reflect this):7
-
–intubation, extubation and related procedures (e.g. manual ventilation and open suctioning);
-
–cardiopulmonary resuscitation;
-
–bronchoscopy;
-
–surgery and post-mortem procedures involving high-speed devices;
-
–some dental procedures (e.g. drilling);
-
–non-invasive ventilation (e.g. bi-level positive airway pressure and continuous positive airway pressure ventilation);
-
–high-frequency oscillating ventilation; and
-
–induction of sputum.
-
–
Whilst other procedures/equipment may generate an aerosol from material other than patient secretions, they are not considered to represent a significant infectious risk. Procedures in this category include:
-
–
pressurized humidified oxygen (unless other risks exist); and
-
–
medication administered via nebulization.
Protection against aerosols requires RPE to remove (filter) aerosols from inhaled air. As aerosols are frequently generated along with droplets and splashes, barrier protection of eyes is also usually required if close to a dispersion source.
The rate of clearance of aerosols in an enclosed space (room) is dependent on the extent of ventilation: the greater the number of air changes per hour (ventilation rate), the faster any aerosols will be diluted. The time required for dilution of aerosols, and thus the time after which the room can be entered without respiratory protection, can be determined following a risk assessment. The risk assessment should take into account the number of air changes per hour (assuming perfect mixing, a single air change removes 63% of airborne contamination, and each subsequent air change removes 63% of what remains; therefore, five air changes reduces contamination to <1% of its former level, assuming dispersion has ceased).9
A list of the more commonly encountered infections and their routes of transmission is given in Table I .
Table I.
Commonly encountered infections and their routes of transmission
| Pathogen | Disease | Immunization (refer to appropriate immunization guidance, e.g. ‘Green Book’ in UK20) | Main route of transmission | Respiratory personal protective equipment for healthcare workers |
||
|---|---|---|---|---|---|---|
| Surgical face mask required | FFP3 required | FFP3 for AGP requireda | ||||
| Bordetella pertussis | Pertussis/whooping cough | Should be vaccinated as part of childhood programme. Protective response in approximately 80% | Droplet | ✓ Until patient has received five days of appropriate antibiotics |
✓ | |
| Chlamydia pneumoniae | Pneumonia | Not available | Droplet | ✓ Duration of acute symptoms until patient is no longer considered infectious |
✓ | |
| Haemophilus influenzae | Epiglottitis Meningitis | Hib vaccine given as part of childhood immunization in UK20 | Droplet | ✓ Until patient has received 24 h of appropriate antibiotics |
✓ | |
| Influenza virus | Upper +/− lower respiratory tract infection | Healthcare workers should be vaccinated. Protective response in approximately 70% | Droplet, aerosol if AGP | ✓ Duration of respiratory symptoms, particularly cough. Prolonged shedding and detection of virus in nasopharynx of immunocompromised patients |
✓ Duration of respiratory symptoms, particularly cough. Prolonged shedding and detection of virus in nasopharynx of immunocompromised patients |
|
| Legionella spp. | Lower respiratory tract infection | Not available | Person-to-person transmission not considered a significant transmission route | Standard infection control precautions apply | ||
| Measles virus | Measles | Live-attenuated vaccine. Protective response in approximately 90% | Droplet/aerosol | ✓b (recommended to be worn until patient is no longer considered infectious) |
✓ | |
| Mumps virus | Mumps | Live-attenuated vaccine (effectiveness 64%) | Droplet | ✓b (recommended to be worn until patient is no longer considered infectious) |
✓ | |
| Mycobacterium tuberculosis | Smear-positive pulmonary or laryngeal disease | Healthcare workers should be vaccinated. Response rate/protection is 70–80% and <70% protection against respiratory disease | Aerosol | ✓ Until multi-drug-resistant or extensively-drug-resistant tuberculosis excluded by laboratory testing |
✓ | |
| Mycoplasma pneumoniae | Pneumonia | Not available | Droplet | ✓ Duration of acute symptoms |
✓ | |
| Neisseria meningitidis | Meningitis | Healthcare workers are not routinely vaccinated but may require risk assessment for prophylaxis | Droplet | ✓ Until patient has received 24 h of appropriate antibiotics |
✓ | |
| Norovirus | Winter vomiting disease | Not available | Droplet | ✓ Only if spillage/risk of splashing |
||
| Rubella virus | Rubella | Live-attenuated vaccine (95–100% protection) | Droplet | ✓b (recommended to be worn until patient is no longer considered infectious) |
✓ | |
| SARS coronavirus | Pneumonia | Not available | Droplet/aerosol | ✓ (recommended to be worn until patient is no longer considered infectious) |
✓ | |
| Streptococcus pneumoniae | Pneumonia, meningitis | Pneumococcal polysaccharide vaccines available for various at-risk groups, but are not routinely indicated for healthcare workers in the UK20 | Droplet | ✓c Only required if evidence of ongoing transmission within a healthcare facility; if so, continue until patient has received 24 h of appropriate antibiotics |
✓ | |
| Varicella zoster virus | Chickenpox | Live-attenuated vaccine. Protection is 75% (for adolescents and adults) | Droplet/aerosol | ✓b (recommended to be worn until patient is no longer considered infectious) |
✓ | |
| Other respiratory virusesc Adenovirus Rhinovirus Coronavirus (non- SARS) Parainfluenza virus Respiratory syncytial virus |
Upper +/−lower respiratory tract infection | Not available (although adenovirus vaccine in production for military services) | Droplet | ✓c Duration of respiratory symptoms, particularly cough. Prolonged shedding and detection of virus in nasopharynx of immunocompromised patients |
✓ | |
FFP, filtering face piece; AGP, aerosol-generating procedure; SARS, severe acute respiratory syndrome.
AGPs are defined as: intubation, extubation and related procedures (e.g. manual ventilation and open suctioning); cardiopulmonary resuscitation; bronchoscopy; surgery and post-mortem procedures in which high-speed devices are used; dental procedures; non-invasive ventilation (e.g. bi-level positive airway pressure ventilation and continuous positive airway pressure ventilation); high-frequency oscillatory ventilation; and induction of sputum. NB. These procedures are likely to change as new evidence emerges.
Maintaining immunity in the healthcare worker population helps to prevent transmission of vaccine-preventable diseases to and from healthcare workers and patients. Staff who have regular clinical contact with patients and who are directly involved in patient care should have a documented history of all immunization. NB. In relation to childhood illnesses and use of masks, no vaccine offers 100% protection and a small proportion of individuals get infected despite vaccination.20 Personal protective equipment should be used as a means of protecting from the risks that remain.4 For further information regarding selected vaccinations for healthcare workers, please refer to the Green Book.20
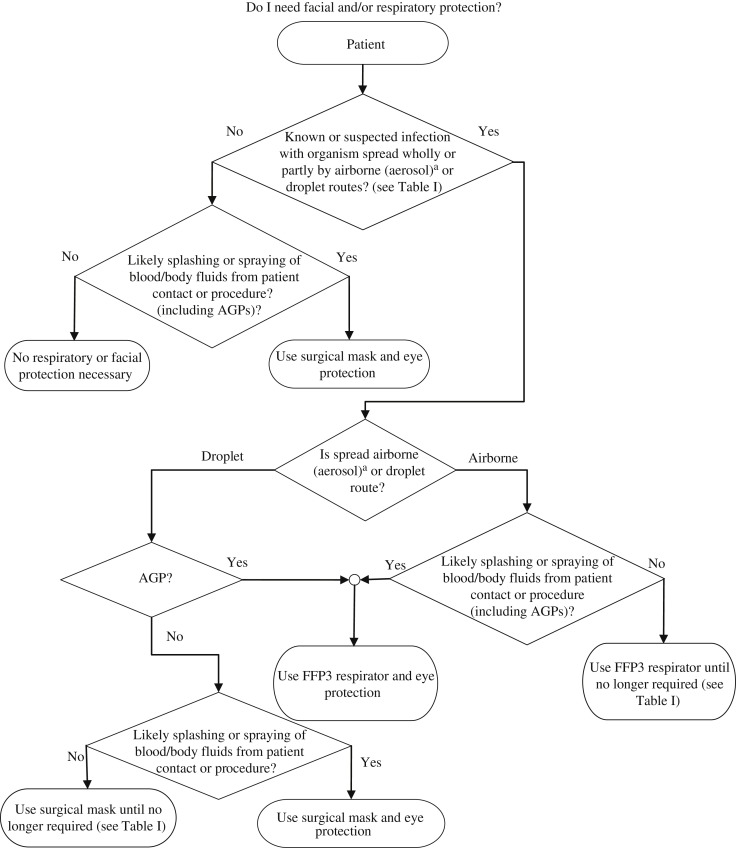
In routine clinical practice, healthcare workers do not commonly wear masks when dealing with patients presenting with symptoms of a ‘common cold’ or ‘influenza-like illness’. However, in a patient with undiagnosed respiratory illness where coughing or sneezing are significant features, or in the context of known widespread respiratory virus activity in the community or a suspected or confirmed outbreak of a respiratory illness in a closed or semi-closed setting, the need for appropriate respiratory and facial protection to be worn should be considered as per Figure 1.
Types of respiratory and facial protection
The range of different types of respiratory protection and other components of facial protection that are available for routine use by HCWs are described below (the key attributes of surgical masks compared with RPE are summarized in Table II ).
Table II.
Comparison of attributes of surgical masks compared with respiratory protection equipment
| Surgical mask | Respirator (e.g. FFP3) | Powered respirator | |
|---|---|---|---|
| Cost | Inexpensive (depends on type) | Moderately expensive (depends on type) | Expensive |
| Level of protection | Protects mouth, nose and respiratory tract against splashes and droplets (but not aerosols) | Protects mouth, nose and lower respiratory tract against splashes, droplets and aerosols | Protects mouth, nose and lower respiratory tract against splashes, droplets and aerosols |
| Fit testing requirement | Not required | Required | Not required |
| Eye protection | May be included | Not included | Included |
| Relative comfort | Reasonable comfort for wearer | Can be uncomfortable if used for long durations | Reasonable comfort for wearer |
| Decontamination requirement | Single use: no decontamination required | Single-use type: no decontamination required Re-usable type: must consider decontamination |
Re-usable: must consider decontamination |
| Power requirement | No power required | No power required | Rechargeable battery pack required |
| Duration of use | Can keep same mask on for duration of the specified activity. Should be changed:
|
Can keep same respirator in use for the duration of the specified activity. Should be changed:
|
Useable for as long as battery has sufficient power |
Surgical face mask
Surgical face masks provide a barrier to splashes and droplets impacting on the wearer's nose, mouth and respiratory tract. They do not provide protection against airborne (aerosol) particles and are not classed as RPE. Whilst some surgical masks claim to have particulate filtration properties, they do not have the filtering efficiencies required for adequate respiratory protection. Moreover, most are not designed to fit closely to the wearer's face, and the poor fit means that aerosols can be inhaled having passed through the gap between the mask and the wearer's face, escaping any filtration. Although not classified as RPE, surgical face masks used for protection against infection must be fluid repellent, compliant with the Medical Devices Directive (MDD 93/42/EEC),10 and be ‘CE’ marked.11 Some surgical masks have integral eye protection.
Surgical masks should be worn for the duration of the relevant exposure, task or procedure. They should be changed if they become damaged or contaminated with respiratory secretions, only worn once, and discarded as healthcare waste following use.
Respirator
A respirator is used by an individual to provide respiratory protection. In the healthcare setting, this most commonly relates to the filtering half face mask. The European standard for filtering face masks [EN 149 (currently 2009)] lists three classes of filtering face piece (FFP): FFP1, FFP2 (approximately equivalent to N95) and FFP3.5 These are classified by inward leakage in laboratory tests and simulated real-life use. Inward leakage can result from penetration through the material matrix of the face piece or through any gap between the face piece and the wearer's face (see Appendix 1 ‘fit testing’). FFP3 offers the highest level of protection. Although most of the evidence base supporting the use of FFP respirators in the prevention of airborne transmission of infection is based upon N95/FFP2 devices, FFP3 is the only FFP class acceptable to HSE for use against infectious aerosols in health care in the UK (Appendix 3). In the USA, N95 (approximately equivalent to FFP2) is acceptable, as is the case in a number of other countries.
FFP respirators are available with or without an exhalation valve. Valved FFPs, although slightly more expensive, are more comfortable to wear than non-valved FFPs as the valve reduces overall breathing resistance, and heat and humidity build up in the face piece.
FFP3 respirators should be changed after each use, if breathing becomes difficult, if it is damaged, or if it becomes obviously contaminated with respiratory secretions or other body fluids. Single-use respirators should be worn once and disposed of as healthcare waste. Re-usable devices will require appropriate decontamination. The manufacturer of the respirator should provide clear instructions on the method of decontamination to be used between uses.
Eye protection
An element of facial protection that is often forgotten, eye protection provides a barrier to droplets and splashes impacting on the wearer's conjunctivae. The most commonly available items are safety spectacles, full-face visors or an integral transparent panel on the top of a surgical face mask. Eye protection should be used when there is a risk of contamination of the eyes from splashing [e.g. by secretions (including respiratory secretions), blood, body fluids or excretions]. Eye protection should always be worn by all those present in the room during potentially infectious AGPs. Disposable, single-use eye protection is recommended; however, if this is re-usable, appropriate decontamination between uses is required.
Selecting and wearing respiratory and facial protection
The requirement for and selection of appropriate respiratory and facial protection is determined by a number of factors that involve a risk-assessment-based approach considering:
-
–
the task or procedure to be undertaken;
-
–
the suspected or known infectious status of the patient; and
-
–
the presenting symptoms of the patient.
These factors are represented diagrammatically in the flow diagram in Figure 1 . This, used in conjunction with Table I, may be helpful in guiding HCWs in assessing the need for, and selection of, appropriate respiratory and facial protection. Worked examples are provided in Appendix 2.
Figure 1.
Flow diagram for the selection of respiratory and facial protection. FFP, filtering face piece; AGP, aerosol-generating procedure. a Airborne (aerosol) spread refers to inhaled infectious particles small enough to penetrate down to, and be retained in, the deepest part of the lungs (alveoli). These particles are <5 μm in size, too small to be seen by eye and can remain suspended in the air for prolonged periods of time. This is in contrast to droplet spread, which is associated with larger particles (>5 μm) that do not readily penetrate the lower respiratory tract, but may cause infection by impacting directly upon a mucosal surface or conjunctiva. Table I describes the transmission route for a range of pathogenic micro-organisms spread wholly or partly by airborne (aerosol) or droplet routes.
It should be noted that Table I also provides information on the availability of immunization for the range of pathogens considered. Where such an option is available, or where immunity arising from prior infection can be established reliably, this is a powerful tool in managing the susceptibility of HCWs and a potentially important component of the hierarchy of controls. However, the degree of protection offered by immunization against different agents is variable, and the durability of protection may wane over time. In this context, the need for use of PPE still exists irrespective of immune status as it provides the last line of protection to manage any residual risk that has not been mitigated by other control measures (Appendix 3).
In summary, it should be emphasized from the foregoing that in the majority of situations where respiratory and facial protection is required, a surgical mask will be adequate. For a very small number of pathogens truly transmissible via the airborne route, or where AGPs involving infectious body fluids are being undertaken, a respirator will be required. The requirement for eye protection will largely be determined by the risk of splashing/spraying of blood and/or body fluids to the eyes/face.
With specific reference to tuberculosis, it should be noted that this guidance is essentially in agreement with the recommendations of the National Institute for Clinical Excellence12 and the British Thoracic Society.13 However, it is the authors' opinion that with the widespread availability of rapid susceptibility testing, increasingly by molecular methods, appropriate respiratory protection should be employed until multi-drug-resistant/extensively-drug-resistant disease has been excluded, rather than relying upon risk assessment (unless an AGP is being performed, in which case respiratory protection is required regardless of antimicrobial sensitivity). This is increasingly relevant in light of concerns over the rising prevalence of antibiotic-resistant tuberculosis.14
The appropriate selection and use of all PPE, including respiratory and facial protection, must be underpinned by staff education and training.
A summary of current UK legislation pertaining to respiratory and facial protection is provided in Appendix 3.
Dos and don'ts of respiratory and facial protection
Do
-
–
Ensure the eye protection, surgical face mask or FFP respirator is worn correctly, completely covering the eyes, nose and mouth and secured on to the face according to the manufacturer's instructions.
-
–
Ensure the FFP respirator is ‘fit tested’ as it will be worn in practice (i.e. with eye protection if required) (see Appendix 1).
-
–
Ensure the FFP respirator is ‘fit checked’. This should be undertaken every time a respirator is worn (see Appendix 1).
-
–
Change the surgical face mask or FFP respirator if it becomes moist, wet or damaged in any way.
-
–
Ensure that the eye protection, surgical face mask or FFP respirator is removed at the end of a clinical procedure or task in the correct order to minimize the risk of self-contamination (see Appendix 1).
-
–
Dispose of single-use eye protection, surgical face mask or FFP respirator immediately after removal and in accordance with local policy.
-
–
Perform hand hygiene after removing PPE.
Don't
-
–
Pull surgical face mask or respirator down to hang around the neck.
-
–
Re-use eye protection, surgical face masks or RPE that are designated for single use.
-
–
Assume corrective spectacles will protect the eyes.
-
–
Fiddle with eye protection, surgical face mask or respirator while in position on the face.
-
–
Continue to wear eye protection if it is visibly soiled and/or vision is impaired.
Appendix 1 gives instructions regarding how to don and remove respiratory and facial protection.
Future research and evidence gaps
A survey of current UK infection prevention and control policies should be undertaken to establish the degree to which this guidance on the use of respiratory and facial protection differs from what is currently performed, and if possible to establish the source of any variations.
The uptake of use of facial and respiratory protection in areas where it is recommended, and also where it is not, should be established. This should focus on looking at areas of highest potential usage (e.g. accident and emergency departments).
Factors that influence the degree of compliance with recommendations for the use of respiratory and facial protection in comparison with other types of PPE should be examined.
It should be established which procedures are truly aerosol generating and which are not. The outputs of such research have direct practical implications for assessing the requirement for RPE.
Development of transparent respiratory protection that does not obscure the face is required to reduce patient anxiety and aid communication between HCWs and patients (e.g. in paediatric settings).
Conflict of interest statement
J.S. Nguyen-Van-Tam wishes to declare the following potential conflicts. In December 2008, he was paid for attending a GlaxoSmithKline Plc (GSK) Advisory Board to give scientific advice on the development and commercialization of an antiviral respirator. His unit currently receives research funding from GSK in areas unrelated to facial and respiratory protection.
Funding source
This work was supported by the Healthcare Infection Society.
Appendix 1. Correct fit of FFP respirators
Fit testing
Before using FFP respirators, it must be verified that each user has a respirator suitable for their face shape, and that they can put it on such that it leaves no gaps between the mask and their face for air to pass though unfiltered. This process is known as ‘fit testing’. This is commonly done by a user putting a respirator on and then being challenged with a particulate spray of something they can taste, should it pass through the respirator.
It is a legal requirement that those required to use respirators are fit tested by a competent person, the results are satisfactory, and those results are recorded and available for inspection.
The Control of Substances Hazardous to Health (COSHH) regulations15 recommend that fit testing should be repeated in the following circumstances:
-
–
if it is necessary to change to another type of face piece (e.g. if new types of respirator are procured by the organization);
-
–
if the wearer has lost or gained weight;
-
–
if the wearer has undergone substantial dental work; or
-
–
if the wearer has developed facial imperfections such as scars, moles around the face seal area etc.
There is no stipulated frequency for repeat fit tests as it is the responsibility of the employer to consider what is appropriate and reasonable for the staff within their organization. It is good practice to have a policy of repeat fit testing in a local RPE ‘fit testing’ programme to ensure that the face piece is still providing adequate protection to the wearer as faces change shape over time. A period of every two years is a good starting position. Further information on fit testing is provided on the HSE website.16
In addition, a ‘fit check’ should be performed each time a respirator is worn (see below).
A good fit can only be achieved if the area where the respirator seals against the skin is clean shaven. Beards, long moustaches and stubble may cause leaks around the respirator. Other types of RPE (e.g. powered hoods and helmets) are available and should be considered if a good fit cannot be achieved with disposable respirators. A powered respirator might be the only type suitable for some HCWs.
Correct donning and removal of FFP respirators
Incorrect donning and removal of RPE by HCWs has been commonly observed. It is essential that the wearer be appropriately trained in how to correctly put on and remove the face piece to ensure that it provides maximum protection when worn and to prevent contamination of the wearer during removal.
Figure 2 shows how to don and fit check FFP respiratory protection correctly.
Figure 2.
How to don and fit check filtering face piece (FFP) respiratory protection correctly (adapted from UK Department of Health and HSE poster accessible via UK government web archive at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_110787.pdf).
Fit check
Always perform a fit check before entering the work area.
-
–Cover the front of the respirator with both hands, being careful not to disturb the position of the respirator on the face.
-
–For an unvalved product – exhale sharply.
-
–For a valved product – inhale sharply.
-
–
-
–
If air flows around the nose, re-adjust the nosepiece; if air flows around the edges of the respirator, re-adjust the headbands.
-
–
A successful fit check is when there is no air leaking from the edges of the respirator.
-
–
If a successful fit check cannot be achieved, remove and refit the respirator.
-
–
If you still cannot obtain a successful fit check, do not enter the work area.
Figure 3 shows an example of correctly fitted FFP respiratory protection.
Figure 3.
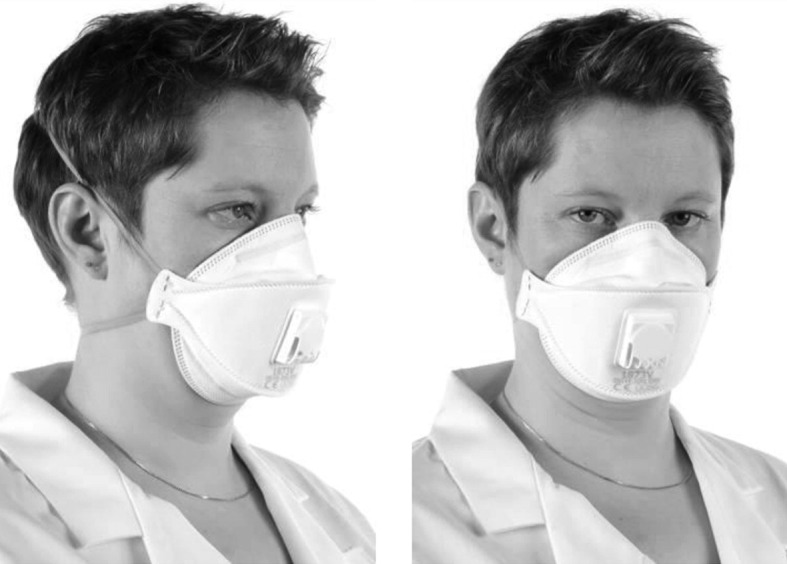
Correctly fitted filtering face piece respiratory protection.
Removal of respiratory and facial protection
Respiratory and facial protection is used in conjunction with other types of PPE. All PPE should be put on and removed in an order that minimizes the potential for cross-contamination.
-
–Before leaving the relevant work area
-
–Gloves, gown/apron and eye protection should be removed (in that order, where worn) and disposed of as healthcare waste.
-
–On removal of eye protection, it should be handled by the headband or earpieces only.
-
–Where non-disposable eye protection has been used, appropriate measures for decontamination between uses needs to be in place.
-
–Hand hygiene must be performed after removal and disposal.
-
–
-
–After leaving the area
-
–The respirator or surgical mask can be removed and disposed of as healthcare waste.
-
–Untie or break the bottom ties first, followed by top ties or elastic, and remove by handling ties only.
-
–Hand hygiene must be performed after disposal.
-
–
Appendix 2. Worked examples of use of Figure 1 and Table I
Please note that the examples only consider the selection of appropriate respiratory and facial protection. They do not deal with other infection prevention and control measures and/or other elements of PPE such as gloves and aprons that may also be appropriate in the scenarios described.
Norovirus infection
An elderly patient in a medical ward develops nausea, abdominal pain, vomiting and diarrhoea. He has a fever, headache and myalgia. Another patient has similar symptoms and two members of staff have contacted the ward manager that they will not be coming to work as they have developed diarrhoea and vomiting overnight.
Norovirus infection is the provisional diagnosis and faecal samples are sent to the laboratory for Clostridium difficile as well as norovirus detection.
You are required to clean up a spillage of faeces from one of the symptomatic patients.
-
–
From the clinical history, norovirus infection is likely.
-
–
Table I indicates potential transmission of norovirus via droplet spread from vomit or faeces.
-
–
In Figure 1, the answer to the question ‘Known or suspected infection with organism spread wholly or partly by airborne or droplet routes?’ is yes.
-
–
The answer to the question ‘Likely splashing or spraying of blood/body fluids from patient contact or procedure?’ is yes.
-
–
A surgical mask and eye protection is recommended for this task.
Influenza-like illness
The on-call medical team is contacted by a general practitioner during an influenza outbreak in the community about a patient in the surgery who needs admission to hospital. The patient has a lower respiratory tract infection with fever, cough and myalgia, and is dyspnoeic. Arrangements are made for the patient to be admitted directly to a single side room on the ward. What respiratory and facial protection is appropriate for staff to wear whilst caring for this patient?
-
–
From the clinical history, viral respiratory tract infection with or without secondary bacterial infection is likely.
-
–
Table I indicates potential spread of influenza via the droplet route from respiratory tract secretions.
-
–
In Figure 1, the answer to the question ‘Known or suspected infection with organism spread wholly or partly by airborne or droplet routes?’ is yes.
-
–
The answer to the question ‘Is spread airborne?’ is no.
-
–
Unless an AGP is being undertaken, a surgical mask is recommended.
-
–
If an AGP is being undertaken, an FFP3 mask with eye protection is recommended.
Appendix 3. UK Legislation and requirements pertaining to respiratory and facial protection
In the UK, the Health and Safety at Work Act17 requires a safe working environment and sets the precedence from which all other health and safety regulations follow [e.g. the Management of Health and Safety at Work Regulations,18 COSHH regulations15 and the Personal Protective Equipment at Work Regulations (1992)].11 The use of RPE is contained within the COSHH regulations.
COSHH regulations require that RPE must be:
-
–
adequate and provide the wearer with effective protection;
-
–
suitable for the intended use;
-
–
‘CE’ marked to PPE Directive 89/686/EEC19 which covers PPE requirements;
-
–
selected, used, maintained and tested correctly by properly trained people with appropriate records kept; and
-
–
stored correctly.
It is important to note that when implementing health and safety measures, which include the selection and use of PPE, consultation is required with either a safety representative appointed by recognized trade unions; or employees, either directly or indirectly, through elected representatives. This is of particular importance as one type of RPE or facial protection may not be suitable for all potential wearers.
Hierarchy of controls
In the hierarchy of control measures within the care environment, PPE, including RPE, is often considered the last line of protection because:
-
–
it only protects the wearer (i.e. not all those in the area);
-
–
if PPE is used incorrectly or is badly maintained, the wearer is unlikely to receive adequate protection;
-
–
it can be uncomfortable to wear;
-
–
it may interfere with physical work activities; and
-
–
it may not be compatible with other types of PPE (i.e. face masks and safety goggles).
Under COSHH regulations/guidance,15 where it is not reasonably practicable to prevent exposure to a substance hazardous to health via elimination or substitution (as is the case where HCWs are caring for individuals/patients with suspected or known airborne micro-organisms), the hazard must be adequately controlled by ‘applying protection measures appropriate to the activity and consistent with the risk assessment’.
This includes the following controls listed in order of priority:
-
1.
the design and use of appropriate work processes, systems and engineering controls, and the provision and use of suitable work equipment and materials;
-
2.
the control of exposure at source, including adequate ventilation systems and appropriate organizational measures; and
-
3.
where adequate control of exposure cannot be achieved by other means, the provision of suitable PPE.
The measures referred to above should also include the following:
-
–
consideration of immunizations according to existing guidelines (NB. In relation to childhood illnesses and use of masks, no vaccine offers 100% protection and a small proportion of individuals become infected despite vaccination.20 PPE should be used as a means of protecting from the risks that remain.4);
-
–
arrangements for the safe handling, storage and transport of substances hazardous to health, and of waste containing such substances, at the workplace;
-
–
the adoption of suitable maintenance procedures;
-
–reducing, to the minimum required for work concerned:
-
–the number of employees subject to the exposure,
-
–the level and duration of exposure, and
-
–the quantity of substances hazardous to health present at the workplace;
-
–
-
–
the control of the working environment, including appropriate general ventilation; and
-
–
appropriate hygiene measures including adequate washing facilities.
In the healthcare setting where workers are caring for ill patients who may have infectious diseases, the way of adequately controlling HCW exposure to potentially infectious biological agents that is most reasonably practicable is via the use of PPE. For the control of infectious agents that may be transmissible via the airborne route, and where AGPs are undertaken, the use of PPE would include RPE.
In the USA, all FFPs are given an assigned protection factor (APF) of 10 by the Occupational Safety & Health Administration. In the UK, the APF of FFP2 is 10 and the APF of FFP3 is 20. Therefore, under HSE guidance to reduce exposure to as low as reasonably practicable (ALARP), FFP3 are recommended. The legal requirement is to reduce exposure to safe levels. Where workplace exposure limit exists, this is taken as the level to reduce to or below. For exposures to substances where there is no known safe exposure level, ALARP comes into play, thus the requirement for the use of FFP3.
The only way to move to a lower level of protection is on the basis of having evidence to justify that an APF of 10 is adequate, rather than an APF of 20 or higher.
References
- 1.Loeb M., Dafoe N., Mahony J. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 2.MacIntyre C.R., Wang Q., Cauchemez S. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. 2011;5:170–179. doi: 10.1016/j.jhin.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunyan D., Ritchie L., Jenkins D., Coia J.E. Respiratory and facial protection: a critical review of recent literature. J Hosp Infect. 2013;85:165–169. doi: 10.1016/j.jhin.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advisory Committee on Dangerous Pathogens . UK Health and Safety Executive; London: 2005. Biological agents: managing the risks in laboratories and healthcare premises. [Google Scholar]
- 5.Health and Safety Executive. Respiratory protective equipment at work: a practical guide. UK Health and Safety Executive; London: 2005. [Google Scholar]
- 6.Department of Health. Respirator fit testing leaflet and posters. UK Department of Health; London: 2010. [Google Scholar]
- 7.Health Protection Scotland. General information and infection control precautions to minimise transmission of respiratory tract infections (RTIs) in the healthcare setting. Health Protection Scotland; Glasgow: 2010. V2.0. [Google Scholar]
- 8.Siegel J.D., Rhinehart E., Jackson M., Chiarello L. Centers for Disease Control and Prevention; Atlanta: 2007. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman P. Personal communication. 2012.
- 10.Medicines and Healthcare Products Regulatory Agency. Medical devices directive. Medicines and Healthcare Products Regulatory Agency; London: 2010. [Google Scholar]
- 11.UK Government. Personal Protective Equipment Regulations 2002. HMSO; London: 2002. Statutory Instrument No. 1144. [Google Scholar]
- 12.National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. National Institute for Health and Clinical Excellence; London: 2011. CG 117. [PubMed] [Google Scholar]
- 13.British Thoracic Society. TB guidelines. British Thoracic Society; London: 2012. [Google Scholar]
- 14.Health Protection Agency. Tuberculosis in the UK: 2012 report. Health Protection Agency; London: 2012. [Google Scholar]
- 15.UK Health and Safety Executive. Control of substances hazardous to health (fifth edition) – The Control of Substances Hazardous to Health Regulations 2002 (as amended). Approved code of practice and guidance. UK Health and Safety Executive; London: 2005. [Google Scholar]
- 16.Health and Safety Executive. Fit testing basics. UK Health and Safety Executive; London: 2012. [Google Scholar]
- 17.UK Government. Health and Safety at Work Act 1974. HMSO; London: 1974. [Google Scholar]
- 18.UK Government. The Management of Health and Safety at Work Regulations 1999. HMSO; London: 1999. Statutory Instrument No. 3242. [Google Scholar]
- 19.European Commission. Mechanical engineering – Directive 89/686/EEC on personal protective equipment. European Commission; Brussels: 2012. [Google Scholar]
- 20.Department of Health. Immunisation against infectious disease – ‘The Green Book’. Department of Health; London: 2012. 2006 updated edition. [Google Scholar]