Abstract
Background
Meticillin-resistant Staphylococcus aureus (MRSA) has been entrenched in Singapore hospitals since the 1980s, with an excess of 600 non-duplicate cases of infections (120 bacteraemia episodes) each year in our 995-bed university hospital. Approximately 5% of our hospital beds are used as isolation facilities.
Aim
To study the impact of an MRSA control bundle that was implemented via gradual geographic extension across hospital wards.
Methods
The bundle included active surveillance on admission and transfer/discharge to identify ward-based acquisition of MRSA, isolation and cohorting of MRSA-infected patients, enhanced hand hygiene initiatives, and publicly displayed feedback of MRSA acquisition and hand hygiene compliance rates. Implementation was between October 2006 and June 2010 in order to provide lead-time for the incremental development of infrastructural capacity, and to develop an ethic of infection prevention among staff. Results were analysed via interrupted time-series analysis.
Findings
MRSA infections fell midway through the implementation, with MRSA bacteraemia declining from 0.26 [95% confidence interval (CI): 0.18–0.34] cases per 1000 inpatient-days in the first quarter of 2004 to 0.11 (95% CI: 0.07–0.19) cases per 1000 inpatient-days in the first quarter of 2012. MRSA acquisition rates fell a year after the programme had been fully implemented, whereas hand hygiene compliance rose significantly from 47% (95% CI: 44–49) in the first quarter of 2009 to 69% (95% CI: 68–71) in the first quarter of 2012.
Conclusion
Successful staged implementation of an MRSA bundle in a hyper-endemic setting is sustainable and represents a model that may be adapted for similar settings.
Keywords: Active surveillance, Cohorting, Hand hygiene, Infection control, Meticillin-resistant Staphylococcus aureus, Time-series analysis
Introduction
Since the worldwide establishment of healthcare-associated meticillin-resistant Staphylococcus aureus (MRSA), sustainable reductions have been demonstrated in several parts of the world. The Netherlands has maintained MRSA levels of <1.0% and managed outbreaks successfully using a search and destroy policy.1 Scandinavian countries and Western Australia cite similar success stories, as has the Veteran Affairs chain of hospitals in the USA after the establishment of an MRSA bundle.2, 3, 4, 5 More reports from across Australia, France, and the UK have also shown significant reductions in MRSA infections in the last few years.6, 7, 8, 9
Effective interventions for controlling MRSA transmission in a hospital setting are well known and include active surveillance, improving hand hygiene compliance, and isolating all MRSA cases, whereas general strategies such as obtaining focused and committed hospital leadership are critical towards lowering implementation barriers and improving sustainability.1, 4, 5, 10 Such successes are rare in Asia and parts of the world where MRSA is hyper-endemic, and where high antimicrobial resistance rates in other nosocomial bacteria result in competition for limited resources and isolation facilities – infrastructural hurdles that have been difficult to overcome.11, 12
Estimates of the burden of MRSA in Singapore and at the National University Hospital of Singapore (NUH) have been published in separate reports which describe the issue microbiologically, and clinically.13, 14 More than 600 MRSA infections including at least 120 bloodstream infections were previously seen per year at NUH. The sheer volume of cases coupled with infrastructural and societal constraints traditionally has hindered MRSA control efforts here. Few beds are available for contact isolation despite 42 negative pressure isolation rooms being built after the severe acute respiratory syndrome (SARS) outbreak in 2003. There is insufficient capacity to isolate all MRSA patients, as they constitute up to 15% of all inpatients, and prioritizing MRSA would impair the ability to isolate or cohort patients with other transmissible diseases.15 Adding to the problem is the average daily bed occupancy rate exceeding 85% – the result of a recent, rapid population boom in Singapore.
In 2006, the senior hospital management at NUH, in response to endemic rates of MRSA infections of 40% and large numbers of clinical infections, called for a more aggressive response. We describe our strategy to contain MRSA in a hyper-endemic hospital with infrastructural constraints and its implementation, as well as analyse its impact via time-series analysis.
Methods
Study design and setting
We conducted a prospective interrupted time-series study at NUH – a 995-bed acute care tertiary university in Singapore. The intervention (described further below) was implemented via gradual geographic extension between October 2006 and June 2010.
The intervention
The intervention comprised a series of MRSA control measures put together as a bundle, and included:
-
−
Active surveillance for MRSA carriage. This consisted of swabs of nares, axillae and groin (plus any wounds) cultured on chromogenic agar MRSA Select (Bio-Rad Laboratories, Marnes-la-Coquette, France).16 Patients not known to have MRSA had entry swabs, while those who had been in a hospital ward >48 h, had no prior positive MRSA cultures (screening or clinical) and had not died, also had exit swabs upon discharge or transfer from the ward.
-
−
Promotion of hand hygiene. This was done primarily via educational and socio-behavioural measures. Hand hygiene education focused on the World Health Organization (WHO) ‘five moments for hand hygiene’, which was audited. Continuous reminders were developed, including via posters in wards, computer screensavers, and audio on ward entry. Hospital-wide training programmes ran in 2009 and annually to coincide with the WHO World Hand Hygiene Day. All frontline clinical staff had to participate in a compulsory 20 min training programme and assessment over one week on both years. A sticker placed on the staff identification tag confirmed certification. All new staff received the same programme at orientation. To further reinforce the message, the clinical examination template in the National University of Singapore final MBBS degree stipulated a deduction in marks for failed hand hygiene.17
-
−
Auditing of hand hygiene compliance. This was performed primarily by appointed ward liaison nurses that had been trained by infection control nurses, supplemented and cross-checked by medical and nursing students who were engaged via innovative educational programmes in which they were trained as auditors and observed staff in their work covertly.18
-
−
Isolation or cohorting of MRSA-positive patients. Such patients were placed in isolation or, more usually, in a designated cohort cubicle, established on each ward. Periodic snapshots of cohorting compliance were undertaken. A close relationship between the bed management unit and the project stakeholders was developed to facilitate the siting of MRSA-positive patients.
-
−
Public and regular feedback of results. Data on hand hygiene compliance and MRSA acquisition were posted on ward-based ‘dashboards’ that had been requested by hospital administration. The signage was publicly displayed, ward-specific and updated monthly.
-
−
Other measures included a bare-below-the-elbows policy for all clinical staff, coloured bracelets to identify all colonized and infected patients, and cash rewards (of around US$250) for exemplary performances by wards or departments with regards to hand hygiene compliance and MRSA transmission rates to enjoy a celebratory lunch or similar.
-
−
To ensure that any progress made was primarily due to the MRSA bundle and not changes in prescribing habits, surveillance of major classes of antibiotics (fluoroquinolones, carbapenems, third or fourth generation cephalosporins and vancomycin) was undertaken from 2006 using pharmacy-based electronic data. This was expressed as defined daily doses (DDD) per 1000 inpatient-days.
In order to fully implement this in an MRSA-hyper-endemic hospital with infrastructural and resource constraints, the MRSA bundle was implemented via planned gradual geographic extension over 4 years – a few wards at a time. This would allow lead-time for the incremental development of infrastructural capacity in a number of areas including the number of microbiology laboratory technical staff and an infection control data management system. It was also believed that a gradual introduction would help the growth of an ethic of infection prevention among staff.
To improve the odds of sustainability, two actions were undertaken simultaneously. First, an overall MRSA taskforce was formed that included members of the senior hospital management, comprising the chair of the medical board, chief operations officer, director of nursing, the infection control chair and a full-time MRSA data manager among others. Second, an assessment of the local clinical and economic impact of MRSA infections was undertaken.19
Outcome measures
The key indicators for improvement included:
-
−
The incidence-density of all MRSA clinical isolates (measured as the number of MRSA clinical specimens cultured per 1000 inpatient-days, with duplicates within 2 weeks and same admission excluded). Uniform data have been kept since 1999.
-
−
The incidence-density of severe MRSA infections (MRSA bacteraemia episodes per 1000 inpatient-days, excluding duplicates within 2 weeks). Consistent data capture dated back to 2004.
-
−
The rates of MRSA acquisition during the inpatient stay (the percentage of patients with positive MRSA clinical specimen or exit swab on discharge in patients who tested MRSA-negative on hospital admission). Such active surveillance began in 2006.
-
−
Hand hygiene compliance (the percentage of compliance in all assessed hand hygiene opportunities). Standardized audits began in 2008.
Collectively, these indicators would represent the overall burden of MRSA infections in the institution, the likelihood of MRSA acquisition in MRSA-naïve patients during their hospitalization, and the overall infection control adherence among healthcare staff. Process indicators included cohorting compliance rates and active surveillance testing rates as well as the results of hand hygiene compliance audits.
Statistics
Preliminary analysis of pre-intervention time-series yielded non-significant autoregressive coefficients, suggesting that the observed autocorrelations at lag 1 across the entire time-series resulted from gradual changes in disease incidence rather than inherent correlations between time-windows. Subsequent analysis accounted for autocorrelation by modelling changes in the mean incidence or proportion as a function of time. The numbers of MRSA clinical cases and MRSA bacteraemia cases per quarter were modelled as Poisson with mean μ(t) per hospital inpatient-day. Extending the model to negative binomial to account for super-Poisson heterogeneity did not improve the fit. The quarterly number of positive exit swabs among initially non-colonized patients was assumed to be binomial with proportion p(t). Both models used linear (on a log or logit scale) changes in location with time, with a single change in gradient allowed. For the number of MRSA or bacteraemia cases, the models have the form:
where α quantifies average incidence at the temporal change point, τ, while β(γ) characterizes per-quarter increase (decrease) in incidence after (before) τ, and 1(A) = 1 if A is true and 0 otherwise. A similar model, replacing log μ(t) by logit p(t), was used for within-hospital colonization and handwashing performance.
The parameters α, β, γ and τ, were inferred independently of the time-course of interventions, thereby to assess the plausibility that interventions coincided with the inferred change-point, τ. Because the likelihood surface suggested that the maximum likelihood estimate of τ, in particular, may not be normally distributed, Bayesian methods were used to derive 95% confidence intervals (CIs), taking uniform prior distributions over the parameter support, using Markov chain Monte Carlo samplers with 100,000 samples and every 10th retained, with multivariate normal proposals with covariance tuned on trial runs. Approximate P-values were obtained by extending credible intervals by analogy to the relationship between P-values and the end-points of confidence intervals. Point estimates reported were maximum likelihood estimates or posterior modes. All analysis was performed in the R statistical environment.20
Results
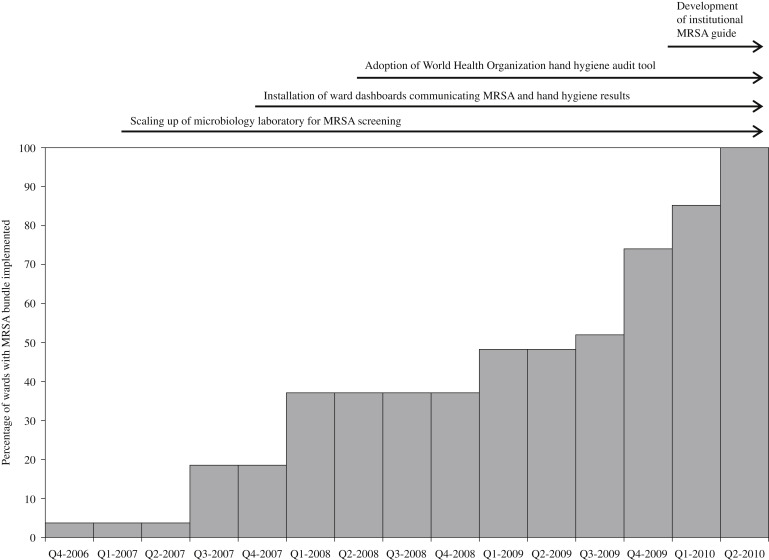
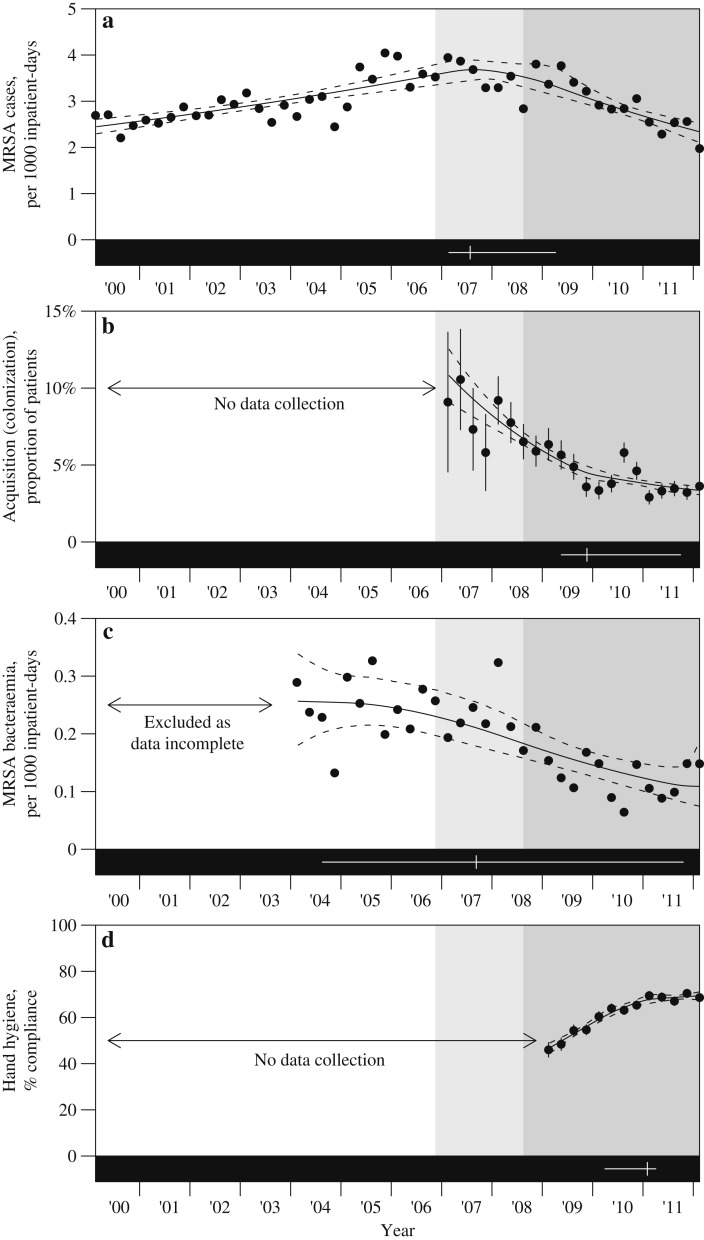
The programme was initiated in October 2006 in a single orthopaedic ward, followed by a subsequent roll-out to all targeted wards within the hospital that was completed by June 2010 (Figure 1 ). By the end of June 2008, all adult intensive care units, and up to 30% of general wards including the majority of surgical general wards, had been included in the programme. Figure 2 shows the incidence-density of all MRSA clinical isolates since 1999, all MRSA blood isolates since 2004, and MRSA acquisition rates since 2006.
Figure 1.
Time-scale of the roll-out of the meticillin-resistant Staphylococcus aureus (MRSA) bundle, showing the percentage of wards covered, as well as other interventions.
Figure 2.
Outcome measures over time. (a) Incidence-density of meticillin-resistant Staphylococcus aureus (MRSA) cases per 1000 inpatient-days. (b) Proportion of patients with inpatient acquisition of MRSA. (c) Incidence-density of MRSA bacteraemia per 1000 inpatient-days. (d) Percentage of hand hygiene compliance according to the World Health Organization audit tool.
There is strong evidence of a rising incidence of all MRSA clinical cases until a peak in the first quarter of 2008 (95% CI: Q1-2007 to Q2-2009) with a notable decline thereafter (Figure 2a); this peak is consistent with the completion of the roll-out by the end of the second quarter of 2008 (P = 0.57) but not with the first roll-out of the programme in the fourth quarter of 2006 (P = 0.005). The decline after the change-point was 2.3 times (95% CI: 1.3–4.7) as rapid as the preceding rise. The MRSA acquisition rate is presented in Figure 2b alongside the modelled fit. Here, too, there is evidence of a change in the acquisition rate over time, with a decelerating fall around the first quarter of 2010 (95% CI: Q2-2009 to Q3-2011), i.e. around a year after the control programme was fully rolled out. The empirical and modelled MRSA bacteraemia rates are presented in Figure 2c. Here, there was a significant fall from 0.26 cases (95% CI: 0.18–0.34) per 1000 inpatient-days in the first quarter of 2004 to 0.11 cases (95% CI: 0.07–0.19) in the first quarter of 2012, but no evidence of the fall levelling off, with the 95% CI for a change-point spanning almost the entire period of recorded data.
The evolution of hand hygiene compliance is displayed in Figure 2d. Compliance rose significantly since audits started, from 47% (95% CI: 44–49%) in the first quarter of 2009 to 69% (95% CI: 68–71%) in the first quarter of 2012. There was, however, evidence that compliance stalled around the last quarter of 2010 (95% CI: Q1-2010 to Q2-2011) with a rate of change after that time that was not statistically distinguishable from 0 (P = 0.5).
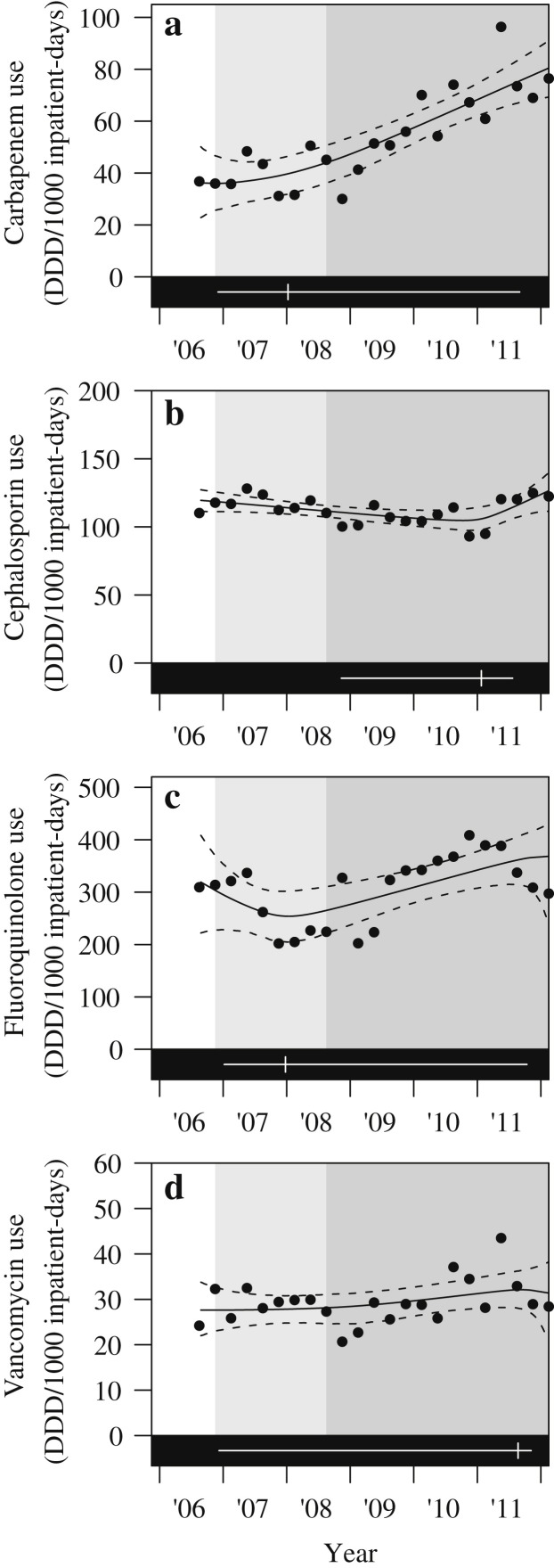
Throughout the period of the study, the changes in consumption of the antibiotics measured did not correlate with the improvement in MRSA rates (Figure 3 ), and therefore antibiotic prescription per se did not affect MRSA rates at our institute.
Figure 3.
Trends of defined daily doses (DDD) per 1000 inpatient-days of major broad-spectrum antibiotics prescribed at our institution.
Discussion
Our results reinforce the concept that an ‘MRSA bundle’ comprising standard well-known MRSA control measures is able to bring down MRSA transmission and infection rates substantially, even in a setting where MRSA is hyper-endemic.1, 4, 5
This study has shown significant declines in MRSA acquisitions, all clinical specimens and also bacteraemias. Concurrently there was no decline in antibiotic prescribing habits. Adding to the likelihood that the interventions caused the MRSA improvements is the fact that vancomycin-resistant enterococci and Clostridium difficile rates have increased at our hospital (unpublished data) while multidrug-resistant Gram-negative infection rates have remained stable.
Our approach can perhaps be extrapolated to other similar settings, as it shows how implementation barriers in terms of manpower, cultural and infrastructural constraints can be overcome using a gradual and staged roll-out of the MRSA bundle, with active involvement of senior hospital management. Kotter broadly mapped out the process of initiating and sustaining change efforts more than a decade ago.21
Sustainability is critical in such efforts, and requires cultural change. Data management and communication was key to sustaining the change. One full-time member of staff was appointed to collate and feed performance metrics back to ward-based healthcare workers, nurse managers, the infection control team and senior management. Ward-specific hand hygiene compliance data from trained auditors and MRSA acquisition rates were posted graphically each quarter in publicly displayed dashboards on each ward.
Efforts toward prevention of MRSA acquisition and infection have become embedded in the culture of clinical medicine at NUH. The plateau seen in the data after significant gains suggests that the improvements are sustainable but that better results are unlikely in the absence of further intervention. Potential further interventions beyond those already implemented include those related to technological advancement or cutting edge research. Screening of healthcare staff was not implemented because of both operational concerns – a significant proportion of staff are likely to be colonized but it would be impossible to lay all of them off work during the period required for MRSA decolonization as well as the concern that MRSA recolonization would be common in a hyper-endemic setting.22 Nonetheless, this will be considered in the future as MRSA rates fail to decline further. Decolonization (of patients or staff) remains controversial beyond some preoperative indications and further work in this regard is underway at our institution. It is conceivable that reducing the reservoir of MRSA may prevent transmission, and therefore infection rates. The predictable development of mupirocin resistance is of particular concern and evaluating alternative agents is another priority for research in MRSA control globally.
Current efforts are labour-intensive and carry a risk of fatigue. Maintenance of data allows the tracking of progress and may help to prevent history from repeating itself if a trend toward the original baseline appears. Patients who are colonized but do not develop infection are invisible in the absence of active surveillance, so this monitoring of acquisition (without infection) allows a more ‘upstream’ view of the progress of infection control interventions.
Manual hand hygiene audits using trained auditors are inefficient because they are labour intensive, and are made inaccurate by the Hawthorne effect and necessarily small sample sizes, generally only on weekdays and daytime hours. Technological solutions are evolving and could potentially feature in the infection control programme of tomorrow.23 Functionality and evidence of cost-effectiveness remain barriers to such technology.
Active surveillance testing was a cornerstone of our bundle. Whereas we used culture-based techniques, molecular testing would be more timely and sensitive, albeit it carries a risk in the regional setting of potentially missing certain MRSA strains with arginine-catabolic mobile elements (ACME) inserted into orfX, and incurring greater operational costs.24
There are several limitations of this work. First, this was a non-randomized before-and-after study design involving a single institution. However, this is a common issue in infection control studies and not one that is easily overcome because of the nature of such studies. During this period, there was no concomitant decrease in other nosocomial antibiotic-resistant pathogens such as carbapenem-resistant Acinetobacter baumannii or extended-spectrum beta-lactamase-producing Enterobacteriaceae (data not shown) that would suggest an overall decline in antimicrobial resistance rates. Clinical practices also did not change substantially. Second, the nature of the intervention is such that several measures were included in a single ‘bundle’, and the individual effect of each measure cannot be determined. Nonetheless, such ‘care bundles’ are now accepted in clinical practice, and their effects are greater collectively than when introduced as separate measures.25
In conclusion, hospitals confronting hyper-endemic MRSA with high infection rates and infrastructural and/or resource constraints can introduce sustainable improvement, provided that a proactive team including senior management leadership is driving it. Data maintenance of process and outcome measures and good communication are essential.
Conflict of interest statement
D. Fisher has received honoraria from Pfizer; research support from Merck and from Schulke. P. Tambyah has been a consultant to GlaxoSmithKline; has received honoraria from Sanofi, Astra-Zeneca, Merck, Sharpe & Dohme; and research support from: Sanofi, GSK, Inviragen. L.Y. Hsu has received honoraria from Pfizer, from Merck, Sharpe & Dohme, AstraZeneca, Janssen & Cilag; and research support from Merck, Sharpe & Dohme, AstraZeneca.
Funding source
This work was funded internally by the National University Hospital.
References
- 1.Wertheim H.F., Vos M.C., Boelens H.A. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in The Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect. 2004;56:321–325. doi: 10.1016/j.jhin.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Benfield T., Espersen F., Frimodt Møller N. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 3.Elstrøm P., Aavitsland P. Methicillin resistant Staphylococcus aureus in Norway. Tidsskr Nor Laegeforen. 2008;128:2730–2733. [PubMed] [Google Scholar]
- 4.Dailey L., Coombes G., O'Brien F. Methicillin-resistant Staphylococcus aureus, Western Australia. Emerg Infect Dis. 2005;10:1584–1589. doi: 10.3201/eid1110.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain R., Kralovic S.M., Evans M.E. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 6.Grayson M.L., Russo P.L., Cruickshank M. Outcomes from the first 2 years of the Australian National Hand Hygiene Initiative. Med J Aust. 2011;195:615–619. doi: 10.5694/mja11.10747. [DOI] [PubMed] [Google Scholar]
- 7.Carlet J., Astagneau P., Brun-Buisson C. French National Program for Prevention of Healthcare-Associated Infections and Antimicrobial Resistance 1992–2008: positive trends, but perseverance needed. Infect Control Hosp Epidemiol. 2009;30:737–745. doi: 10.1086/598682. [DOI] [PubMed] [Google Scholar]
- 8.Wilson J., Guy R., Elgohari S. Trends in sources of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: data from the national mandatory surveillance of MRSA bacteraemia in England, 2006–2009. J Hosp Infect. 2011;79:211–217. doi: 10.1016/j.jhin.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Stone S.P., Fuller C., Savage J. Evaluation of the national Clean your hands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ. 2012;344:e3005. doi: 10.1136/bmj.e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.5 Million Lives Campaign . Institute for Healthcare Improvement; Cambridge, MA: 2008. Getting started kit: reduce methicillin-resistant Staphylococcus aureus (MRSA) infection; how-to guide. [Google Scholar]
- 11.Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:847–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 12.Jean S.S., Hsueh P.R. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37:291–295. doi: 10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Hsu L.Y., Tan T.Y., Jureen R. Antimicrobial drug resistance in Singapore hospitals. Emerg Infect Dis. 2007;13:1944–1947. doi: 10.3201/eid1312.070299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira L., Fisher D. Methicillin-resistant Staphylococcus aureus control in Singapore – moving forward. Ann Acad Med Singapore. 2008;37:891–896. [PubMed] [Google Scholar]
- 15.Clements A., Halton K., Graves N. Overcrowding and understaffing in modern health-care systems: key determinants in meticillin-resistant Staphylococcus aureus transmission. Lancet Infect Dis. 2008;8:427–434. doi: 10.1016/S1473-3099(08)70151-8. [DOI] [PubMed] [Google Scholar]
- 16.Nsira B.S., Dupuis M., Leclercq R. Evaluation of MRSA Select, a new chromogenic medium for the detection of nasal carriage of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2006;27:561–564. doi: 10.1016/j.ijantimicag.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Fisher D., Aw M., Hsu L.Y., Patlovich K., Ho K.Y. The challenge of introducing a hand hygiene standard to clinical examinations. Med Teach. 2011;33:171–172. [PubMed] [Google Scholar]
- 18.Fisher D., Pereira L., Ng T.M., Patlovich K., Teo F., Hsu L.Y. Teaching hand hygiene to medical students using a hands-on approach. J Hosp Infect. 2010;76:86–87. doi: 10.1016/j.jhin.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Pada S., Ding Y., Ling M.L. Economic and clinical impact of nosocomial methicillin-resistant Staphylococcus aureus infections in Singapore: a matched case–control study. J Hosp Infect. 2011;78:36–40. doi: 10.1016/j.jhin.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team . R Foundation for Statistical Computing; Vienna: 2012. R: a language and environment for statistical computing. [Google Scholar]
- 21.Kotter J.P. Harvard Business Press; Boston, MA: 1996. Leading change: why transformational efforts fail. [Google Scholar]
- 22.Chan K.S., Ling M.L., Hsu L.Y. Methicillin-resistant Staphylococcus aureus throat colonization among healthcare workers during an outbreak in Singapore General Hospital. Infect Control Hosp Epidemiol. 2009;30:95–97. doi: 10.1086/593123. [DOI] [PubMed] [Google Scholar]
- 23.Gould D.J., Drey N.S., Creedon S. Routine hand hygiene audit by direct observation: has nemesis arrived? J Hosp Infect. 2011;77:290–293. doi: 10.1016/j.jhin.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Hon P.Y., Chan K.S., Holden M.T. Arginine catabolic mobile element in methicillin-resistant Staphylococcus aureus (MRSA) clonal group ST239-MRSA-III isolates in Singapore: implications for PCR-based screening tests. Antimicrob Agents Chemother. 2013;57:1563–1564. doi: 10.1128/AAC.02518-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marwick C., Davey P. Care bundles; the holy grail of infectious risk management in hospital? Curr Opin Infect Dis. 2009;22:364–369. doi: 10.1097/QCO.0b013e32832e0736. [DOI] [PubMed] [Google Scholar]