Abstract
The purpose of the current systematic review was to identify the prevalence of hypovitaminosis-D in LE-TJA patients; and outline the association between pre-operative hypovitaminosis and post-operative outcomes. A search of PubMed-Medline and the Cochrane-Library databases was performed for literature published before November 27th, 2019. The eighteen studies analyzed had a pooled prevalence for vitamin D insufficiency (20 - <30 ng/mL) and deficiency (<20 ng/mL) of 53.4% and 39.4%, respectively. Hypovitaminosis-D was associated with higher complication rates (p = 0.043), and a greater prevalence among septic versus aseptic revisions (p = 0.016). Therefore, pre-operative screening for hypovitaminosis-D can be beneficial in patients undergoing LE-TJA.
Level of evidence
Systematic Review (Level III)
Keywords: Vitamin-D, Total hip arthroplasty, Total knee arthroplasty, Vitamin-D deficiency, Hypovitaminosis-D
1. Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) have been established as effective surgeries in terms of achieving high 10-year implant survival (91.3% and 93.8%, respectively) and overall patient satisfaction (87.5% and 88%, respectively) rates.1, 2, 3, 4 Over 7.2 million people In the U.S. are estimated to be living with a TKA or THA.5 Furthermore, the annual number of lower extremity arthroplasty (LE-TJA) surgeries is expected to grow between 2015 and 2020 by 35.4% and 48.5% for THA and TKA, respectively.6,7 Nevertheless, there is a small percentage of patients who suffer complications, including: persistent pain,8 infection,9, 10, 11 and functional limitation.12, 13, 14, 15, 16 An anticipated increase to an already large volume of cases makes it paramount that all major risk factors of LE-TJA are thoroughly explored in order to ensure maximum patient satisfaction and cost-effectiveness.
Vitamin D is known to affect bone and muscle health, as well as immunological functions through a variety of mechanisms.17 Hypovitaminosis-D is a widespread nutritional deficiency, even more among patients scheduled to undergo orthopedic surgery.18,19 The predominant action of vitamin D is on the skeletal system due to its role in maintaining calcium homeostasis.20 Vitamin D deficiency places patients at increased risk of hip and non-vertebral fracture, muscle weakness in addition to being a significant independent risk of falls.21, 22, 23 In addition to these skeletal effects, low levels of vitamin D have been associated with an increased risk of cancers, cardiovascular disease, autoimmune diseases, and psychiatric illness.24, 25, 26, 27, 28 There is no clear consensus regarding the prevalence of pre-operative Hypovitaminosis-D in patients undergoing LE-TJA.29 Furthermore, reports on the potential association between pre-operative hypovitaminosis-D and post-operative adverse outcomes have yielded variable results.29
Therefore, we performed a systematic review to determine: 1) the prevalence of hypovitaminosis-D in patients undergoing LE-TJA (specifically total hip (THA) and knee (TKA) arthroplasty); 2) the association between pre-operative vitamin D levels and post-operative patient-reported outcomes; and 3) the association between pre-operative vitamin D levels and the development of post-operative complications.
2. Methods
2.1. Search strategy
This study was conducted in accordance with the 2009 Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement.30 A systematic review of the literature was performed using PubMed-Medline and the Cochrane Database of Systematic Reviews. The query was performed on November 17th, 2019 using the following keywords in combination with Boolean operators AND or OR for the literature search: “vitamin D,” “arthroplasty,” “THA,” “LE-TJA,” “TKA,” Total hip arthroplasty,” “Total knee arthroplasty,” “deficiency,” and “insufficiency”.
2.2. Inclusion and exclusion criteria
Inclusion criteria were: human clinical trials (prospective and retrospective) on LE-TJA (specifically TKA and THA), presented in the English language that reported serum vitamin D levels were included. On the other hand, basic science articles, editorials, surveys, special topics, letters to the editor, personal correspondence and review articles, and studies reporting the effect of post-operative levels of vitamin D without pre-operative vitamin D levels were excluded from the present study.
2.3. Data extraction
Three investigators (AE, EN, and NSP) independently reviewed the abstracts from all articles identified in these searches. Full-text articles were reviewed when necessary to confirm inclusion/exclusion criteria. Reference lists were also reviewed to minimize the risk of missing relevant articles. Data was recorded into a custom information extraction table.31 We collected data on the prevalence of hypovitaminosis-D among LE-TJA patients, the correlation between low levels of serum vitamin D and clinical/functional outcomes, and whether hypovitaminosis-D was associated with a higher risk of post-operative complications.
2.4. Data analysis
The effect of Vitamin D on clinical outcomes was compiled when at least three studies reported a common outcome. Heterogeneity was assessed using I2 statistics. If significant heterogeneity (I2 >50%) was present, a random-effects model was used. A Meta-analysis of proportion was conducted to calculate the pooled prevalence of pre-operative vitamin-D insufficiency and deficiency through a random-effects model due to data heterogeneity. This was performed using Comprehensive Meta-Analysis (Comprehensive Meta-Analysis Version 3, H. Biostat, Englewood, NJ 2013). On the other hand, the two arm meta-analysis for comparing WOMAC scores between vitamin-D deficient and sufficient cohorts was conducted using Review Manager (Review Manager. Version 5.3. Copenhagen, the Cochrane Collaboration, 2014) which cannot perform analyses of proportions. The threshold for statistical significance for all analyses was set at p < 0.05.
3. Results
3.1. Article identification and selection
After the exclusion of duplicates, 2861 individual reports were identified. After the application of inclusion/exclusion criteria, 2828 studies were eliminated, leaving 33 articles for full-text review. After a comprehensive review of these articles, a total of 18 articles met complete inclusion criteria for analysis (Fig. 1).
Fig. 1.
Flow Diagram (PRISMA Chart) Presenting the Systematic Review Process Used in this Study.
3.2. level of evidence
There were three Level I studies,32, 33, 34 10 Level II studies,35, 36, 37, 38, 39, 40, 41, 42, 43, 44 and 5 Level III study.45, 46, 47, 48, 49 Level of evidence was determined or verified using the defined criteria by Wright J et al.50 (Table 1).
Table 1.
Demographic characteristics of patients in the analyzed studies.
| Author | Year | LOE | Patients (n) | THA | TKA | Both | Primary/Revision/both | Diagnosis | Mean Age | F/M |
|---|---|---|---|---|---|---|---|---|---|---|
| Unnanuntana et al. | 2014 | 1 | 219 | 219 | 0 | No | Primary | OA(122) Dysplastic hip (63) Other (34) | 67 | 126/93 |
| Nawabi et al. | 2010 | 1 | 62 | 62 | 0 | No | Primary | OA | 71 | 39/23 |
| Allain et al. | 2008 | 1 | 92 | 0 | 92 | No | 79/9/4(Unicompartmental JR) | OA(86), 6(RA) | 74 | 54/38 |
| Traven et al. | 2017 | 2 | 126 | 62 | 64 | Yes | Revision | All Revision THA or TKA | 65 | 70/56 |
| Shin et al. | 2016 | 2 | 87 | 0 | 87 | No | Primary | OA | 72 | 78/9 |
| Maniar et al. | 2016 | 2 | 120 | 0 | 120 | No | Primary | OA | 68 | 97/23 |
| Cunha et al. | 2016 | 2 | 93 | 93 | 0 | No | Primary | OA | 60 | NA |
| Lee et al. | 2015 | 2 | 214 | 0 | 214 | No | Both, #NA | All major primary or revision TKA | NA | 153/61 |
| Maier et al. | 2014 | 2 | 190 | NA | NA | yes + Shoulder | 109/81 | Primary (109), PJI (50), Aseptic Loosening (31) | 65 | 62/47 |
| Lavernia et al. | 2014 | 2 | 60 | 60 | 0 | No | Primary | All Primary | 70 | 48/12 |
| Unnanuntana et al. | 2012 | 2 | 200 | 200 | 0 | No | Primary | OA(187) ON(6) Developmental Dysplasia(3), PTOA(4) | 67 | 112/88 |
| Nixon et al. | 2007 | 2 | 80 | 80 | 0 | No | NA | OA | 68 | 81/46 |
| Visser et al. | 2018 | 2 | 87 | 87 | 0 | No | Primary | Elective Arthroplasty excluding fractures | 74 | 66/21 |
| Gao et al. | 2019 | 3 | 128 | 129 | 257 | yes | Primary | Elective, OA | 72.35 | 149/108 |
| Hegde et al. | 2018 | 3 | 6593 | 0 | 6593 | No | Primary | Elective Arthroplasty excluding fractures | NA | 4944/1599 |
| Piuzzi et al. | 2018 | 3 | 297 | 148 | 149 | Yes | Primary | Elective Arthroplasty excluding fractures | 66.4 | 198/99 |
| Maier et al. | 2016 | 3 | 1083 | 606 | 477 | yes | NA | Elective Arthroplasty excluding fractures | 76 | 567/515 |
| Kelly et al. | 2017 | 3 | 164 | 0 | 79 | No+ 85 non-operative control patients. | Primary | Elective arthroplasty excluding fractures | 68.2 | 89/75 |
OA: Osteoarthritis, THA: total hip arthroplasty, TKA: total knee arthroplasty, RA: rheumatoid arthritis, PJI: periprosthetic joint infection, PTOA: post-traumatic osteoarthritis, ON: osteonecrosis.
3.3. Patient demographics
The eighteen studies included 9,895 patients, of which 1,746 had a THA, and 8132 had a TKA. One study included a small and undisclosed number of shoulder arthroplasty patients45 while another included 85 patients as a non-operative control group.49 Thirteen studies included only primary surgeries, three looked at either primary or revision surgeries, and one looked only at revision surgeries. Sixteen studies reported mean ages, with the mean overall age these studies being 70.2 years. The seventeen studies which reported gender were comprised of 6,933 (70.4%) females and 2,913 (29.6%) males. The most common reason for surgery among primary arthroplasty patients was osteoarthritis.
3.4. Prevalence of Hypovitaminosis-D
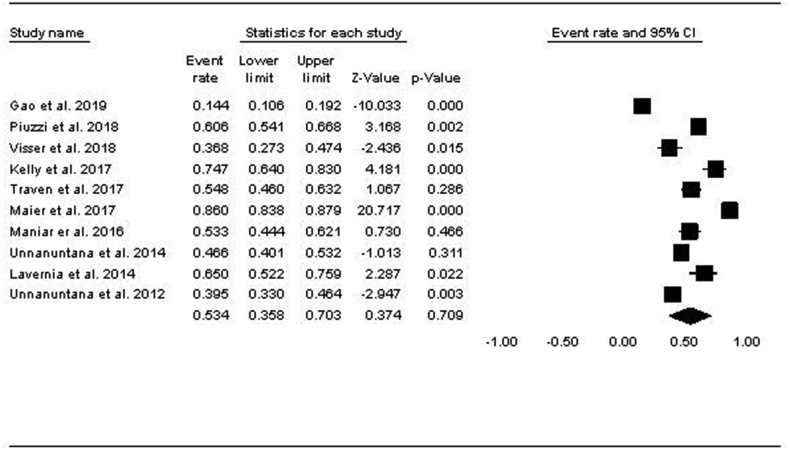
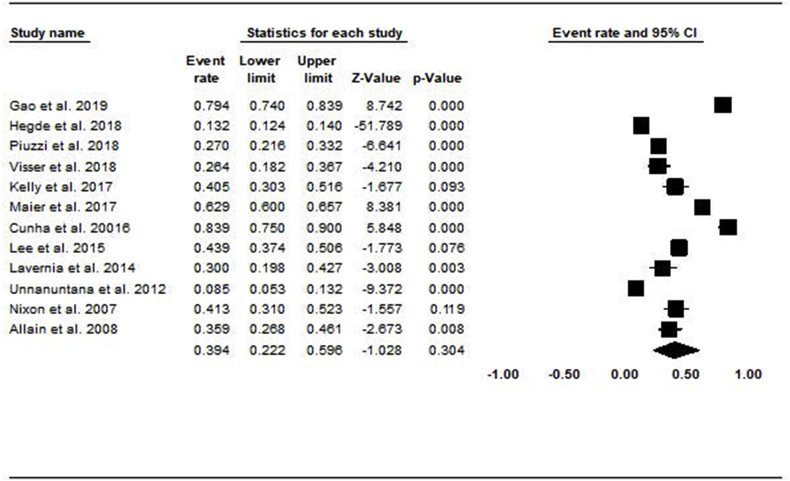
Among the 10 studies that looked into vitamin D insufficiency (serum levels 20 - <30 ng/mL), 63% of subjects (1549/2457) were in the vitamin D insufficiency range. The pooled prevalence of vitamin D insufficiency using the random-effects model was 53.4% (CI: 35.8–70.3) (Fig. 2). Among the 12 studies that reported vitamin D deficiency serum levels (<20 ng/mL), 52% of patients (2142/9064) met the criteria. The pooled prevalence of vitamin D deficiency using the random-effects model was 39.4% [CI: 22.2–56.6] (Fig. 3).
Fig. 2.
Forest Plot showing the Pooled Prevalence of Vitamin D Insufficiency.
Fig. 3.
Forest plot showing the pooled prevalence of vitamin d deficiency.
Nawabi et al.33 defined Vitamin D deficiency as <16 ng/mL and found that only 24% of patients (15/62) in their study met the criteria while Shin et al. used <12 ng/mL as the threshold and found that 51% of patients (44/87) in their study were severely vitamin D deficient. Traven et al.35 found that the prevalence of vitamin D deficiency among revision patients was higher than the prevalence of vitamin D deficiency among all primary LE-TJA patients (55% vs. 42%, respectively). Finally, in a cohort of 25 patients undergoing TKA, Jensen et al.51 found a 16% prevalence of vitamin D levels under 25 ng/mL.
3.5. Patient reported outcomes
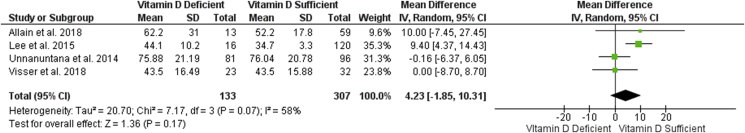
Nine articles examined post-operative patient-reported outcomes (PROs), of these, five found that vitamin D sufficient patients performed better than vitamin D deficient patients in at least one of the score assessed,33,34,41,52,53 while four studies found no differences32,37,39,44 between the two groups. There was no correlation between subsets of LE-TJA (TKA and THA) and outcomes for vitamin D deficient patients. Of the eight studies which demonstrated worse PROs in patients with hypovitaminosis: four were composed of THA patients, and four were composed of TKA patients. Outcomes for which a significant difference in favor of the non-vitamin D deficient groups was found include: both pre and post-op Harris Hip scores,33,41 Merle d’Aubigne'-Postel scores,41 post-operative Knee Society Scores (KSS),36 the functional subdomain of the KSS,53 the total WOMAC score52 and the stiffness subset of WOMAC scores among TKA patients34 (Table 2). Pooled analysis of the four studies reporting post-operative WOMAC32,34,39,44 was conducted through a random-effects model (I2 = 58%). Results demonstrated a trend towards poorer post-operative outcomes in patients with pre-operative vitamin D deficiency compared to vitamin D sufficient patients (4.32, 95% CI [-1.85, 8.70]). However, this trend failed to reach statistical significance (p = 0.07) (Fig. 4).
Table 2.
Summary of the effect of vitamin D on the clinical outcomes.
| Author | Patient (n) | Follow-up Period | Groups Compared | Post-Operative Outcomes | Findings |
|---|---|---|---|---|---|
| Allain et al., 2008 | 92 | 6 Months | (<12 ng/mL) vs (12–19 ng/mL) vs (>20 ng/mL) | WOMAC Stiffness WOMAC Physical WOMAC Total Pain | (12–19 ng/mL) group had significantly lower WOMAC stiffness scores than (<12 ng/mL) group. There was no difference in Pain scores, Total WOMAC scores, or Physical WOMAC scores. |
| Joris et al., 2017 | 138 | 8 Years | vitamin D deficient (<40 nmol/L) and vitamin D sufficient (>40 nmol/L) | Difference in WOMAC scores at 8 years postoperatively. | On average, Vitamin D deficient patients had +5.0 points (range: +0.2 to 9.8) compared to vitamin D sufficient patients (p = 0.04). |
| Lavernia et al., 2014 | 60 | 11 Months | (>30 ng/mL) vs (<30 ng/mL) And (>20 ng/mL) vs (<20 ng/mL) | HHS Merle d’Aubigne'-Postel score WOMAC Total | >30 ng/mL group had significantly better HHS and Merle d’Aubigne'-Postel scores compared to <30 ng/mL group (92 vs 83 and 17 vs 14, respectively). There was no difference in HHS or Merle d’Aubigne'-Postel score when deficiency was defined as <20 ng/mL. There was no difference in total WOMAC score at any threshold. |
| Lee et al., 2015 | 214 | 3 Months | (>20 ng/mL) vs (<20 ng/mL) | WOMAC | There was no difference in any other WOMAC sub-section or total. |
| Maniar et al., 2016 | 120 | 3 Months | (>30 ng/mL) vs (<30 ng/mL) | WOMAC Functional KSS Total KSS SF-PCS SF-MCS | WOMAC, KSS, SF-PCS, and SF-MCS scores were not statistically different between groups. |
| Nawabi et al., 2010 | 62 | 6 Months | (>16 ng/mL) vs (<16 ng/mL) | HHS | Mean HHS at 6 months were not significantly different between the two groups. The >16 ng/mL group had a significantly higher number of patients attaining excellent HHS (P = 0.003). Plasma 25(OH) D3 levels correlated positively with post-operative HHS's yielding (Spearman's correlation coefficients of +0.332, p = 0.008). |
| Shin et al., 2017 | 87 | 3 Months | (<16 ng/mL) vs (>16 ng/mL) | Clinical and Functional KSS | There was no difference between vitamin D deficient and non-deficient cohorts in terms of the Clinical KSS (56.2 ± 10.1 vs 58.9 ± 13.6; p > 0.05). On the other hand, functional KSS demonstrated significant superiority in the non-deficient group (67.8 ± 12.0 vs 73.6 ± 14.4; p = 0.045). |
| Unnanuntana et al., 2014 | 219 | 6 Weeks | (>30 ng/mL) vs (<30 ng/mL) | WOMAC SF-36 Physical Function | There were no differences in WOMAC or SF-36 scores between the two groups. |
| Visser et al., 2018 | 87 | 6 Weeks | Deficient (<50 nmol/L), insufficient (50–75 nmol/L) and sufficient (>75 nmol/L) | LASA Physical Activity Questionnaire (LAPAQ) and total WOMAC scores. | Vitamin D status did not affect physical recovery after THA. |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index, HHS: Harris hip score, KSS: American Knee Society Scores, SF-36: short form-36, LASA: Longitudinal Aging Study Amsterdam.
Fig. 4.
Forest plot of total WOMAC scores in vitamin d deficient versus sufficient groups.
Lee et al.39 found similar pain scores in vitamin D deficient and non-deficient TKA patients pre-operatively. However, a significantly higher prevalence of moderate-to-severe pain was detected post-operatively in patients with hypovitaminosis-D compared to vitamin D sufficient patients (13.8% vs. 5.9% respectively, p = 0.05).
3.6. Post-operative complications
Three studies evaluated the effect of pre-operative hypovitaminosis-D on the post-operative risk of infection. Traven et al.35 found that patients with serum vitamin D levels <30 ng/mL had an increased risk of periprosthetic joint infection (PJI), as well as an increased risk of having a complication within 90 days post-operatively when controlling for PJI (37.7% vs. 21.1%, p-value = 0.043). Maier et al.40 studied patients undergoing revision LE-TJA and found a significant difference in Vitamin D levels between patients who were revised for PJI (13.29 ng/ml ± 6.54) compared to patients who were undergoing primary LE-TJA (19.46 ng/m l± 9.49; p < 0.001) or who were revised for any aseptic reason(13.29 ng/ml ± 6.54; p < 0.001). Furthermore, Hegde et al.47 queried the humana administrative claims registry for the incidence of pre-operative vitamin D deficiency and its association with the development of early post-operative complications. They found that pre-operative vitamin D levels under 20 ng/mL were more likely to develop post-operative surgical site infection requiring irrigation and debridement (OR = 1.76, 95% confidence interval (CI) [1.25–2.48]; p = 0.001) after TKA.
Traven et al.35 also found that 13% of patients with hypovitaminosis-D needed an unplanned reoperation compared to 5.3% of patients with normal vitamin D levels (P<.001). Patients with low vitamin levels had more cases of multiple post-operative complications compared to patients with normal vitamin D levels (20.3% vs. 8.8%, p<.001), independent of pre-operative infection and poor nutrition.35
In the study by Hegde et al.,47 pre-operative vitamin D deficiency (>20 ng/mL) in TKA patients was associated with a greater likelihood of development of stiffness requiring manipulation under anesthesia (OR = 1.69, 95% CI [1.36–2.04]; p < 0.001), need for implant explantation (OR = 2.97, 95% CI [2.04–4.3]; p < 0.001). Furthermore, medical complications including post-operative deep venous thrombosis (OR = 1.8, 95% CI [1.3–2.38]; p < 0.001), myocardial infarction (OR = 2.11, 95% CI [1.41–3.15]; p < 0.001) and cerebrovascular accidents (OR = 1.73, 95% CI [1.17–2.57]; p = 0.006) were more likely to occur in pre-operatively vitamin D deficient patients.
Finally, Gao et al.46 prospectively analyzed the incidence of post-operative cognitive dysfunction (POCD) in a cohort of 257 elderly patients (mean age: 72.35) receiving elective LE-TJA (THA: n = 128; TKA n = 129). POCD was determined based on cognitive function decline at post-operative day seven compared to the baseline evaluated at one day pre-operatively. They found that the patients who experienced POCD were associated with significantly lower vitamin D levels (12.2 ± 4.7 vs. 15.4 ± 5.8; p = 0.001). Furthermore, multivariate analysis demonstrated that vitamin D deficiency (<20 ng/mL) posed a significant risk of POCD development (OR = 1.77, 95% CI [1.13–2.78], p = 0.016).
3.7. Miscellaneous
Four studies compared length of stay. Maier et al.45 (n = 1,083) found that primary LE-TJA patients with hypovitaminosis-D (<20 ng/mL) had a significantly longer mean length of stay compared to those with sufficient serum vitamin D levels (15.6 ± 7.2 vs 11.3 ± 7.9 days, respectively; p = 0.014). Unnanuntana et al. (n = 217) found no significant difference in length of stay among primary arthroplasty patients who were vitamin D sufficient, vitamin D insufficient, and vitamin D deficient.42 Traven et al.35 reviewed 126 revision patients and did not find a statistically significant difference in length of stay between vitamin D insufficient (<30 ng/mL) and vitamin D sufficient (>30 ng/mL) revision patients. Finally, Joris et al.52 found that TKA patients with low pre-operative levels of vitamin D (<30 ng/mL) demonstrated a longer length of stay (mean + 1 days; range +0.2- +1.6 days) compared to patients with sufficient pre-operative vitamin D levels (>30 ng/mL).
In the gait analysis study by Cunha et al., they demonstrated that serum vitamin D levels correlated with greater post-operative peak hip extension (R = 0.25, p = 0.017) and peak power generation (R = 0.25, p = 0.04) in the gait cycle of patients undergoing THA.38 Unnanuntana et al. found that patients who had hypovitaminosis-D achieved in-hospital functional milestones at the same pace as those with sufficient levels of vitamin D.42 The percentage of patients who had ASA class 3 or 4 was significantly higher in the vitamin D deficiency group (52.9%, 22.6%, and 23.9% for vitamin D deficiency, insufficiency, and normal groups, respectively; p = 0.032)42.
Shin et al.53 compared post-operative outcomes in terms of the alternate-step test (AST), 6 m walk test (SMW), sit to stand test (STS) and timed up and go test (TUGT) between pre-operatively vitamin D deficient (<20 ng/mL) and non-deficient cohorts. All patients underwent TKA and were evaluated at three months postoperatively. Vitamin D deficient patients demonstrated worse AST (16.7 ± 12.0 vs 14.6 ± 3.7; p = 0.032) and SMW (8.8 ± 2.3 vs 7.7 ± 1.6; p = 0.012) compared to non-deficient patients. On the other hand, there was no significant difference in the results of STS and TUGT (p > 0.05 for both) between the deficient and non-deficient cohorts.
4. Discussion
The prevalence and role hypovitaminosis-D plays in LE-TJA must be understood so medical teams and their patients can make educated and prudent treatment choices. Our current understanding of vitamin D with regards to LE-TJA remains low, and even the threshold by which hypovitaminosis-D itself is defined remains disputed.54 When reviewing LE-TJA studies that measured serum vitamin D, we found that the two most common thresholds used to define hypovitaminosis-D were vitamin D insufficiency (<30 ng/mL) and vitamin D deficiency (<20 ng/mL). Our findings show that hypovitaminosis-D was prevalent using both values.
The pooled prevalence of vitamin D insufficiency (20 - <30 ng/mL) was 53.4%, and that of deficiency (<20 ng/mL) was 39.4%. The prevalence of vitamin D deficiency was even higher among patients undergoing revision surgeries (55%).35 One study reported that among patients scheduled to undergo orthopedic surgery, the prevalence of vitamin D insufficiency (<32 ng/mL) was 43%, and that of deficiency (<20 ng/mL) was 17%.19 In the U.S, the prevalence of vitamin D deficiency (<20 ng/mL) among the general adult population has been reported to be as high as 41.6%, a similar prevalence to the one found in this review.55 A similar prevalence of vitamin D deficiency in the LE-TJA population and the general population is not surprising, as reported in other previous studies.56,57
A sufficient vitamin D level was associated with superior patient-reported outcomes in five of the 9ninearticles that reported on this variable. These studies utilized variable patient-reported outcome scores, which prevented conducting a pooled analysis on many of the reported outcomes. However, it is clear that as of date, no consensus exists on the effect of pre-operative hypovitaminosis-D on post-operative patient-reported outcomes (Table 2). Furthermore, pooled analysis failed to demonstrate a statistically significant difference (p = 0.07) in the most frequently described patient-reported outcome (WOMAC) among the included studies (Fig. 4). Further research is required in order to assess the effect vitamin D deficiency has on accepted patient-reported outcomes.
Some studies reported higher rates of complications in patients with hypovitaminosis-D. But, the nature of the complications assessed showed wide heterogeneity among the different studies, making it difficult to make definite conclusions about the role of vitamin D on complications after LE-TJA. One study found an increased risk of infection,35 while one study found a higher incidence of vitamin D deficiency in infected patients.40 The possible association between infection and vitamin D levels is substantiated by the role vitamin D is known to play in immune function.24 Since the discovery of the expression of vitamin D receptors and vitamin D metabolizing enzymes in cells of the immune system, an increasing number of studies have been performed to elucidate the mechanism by which vitamin D affects the immune system.58,59 Some of the possible mechanisms include upregulation of antibacterial peptides such as cathelicidin and defensins and stimulation of toll-like receptors (TLRs), which are involved in the activation of macrophages.58, 59, 60, 61 Although the effect of preoperative vitamin D supplementation on the development of PJI remains controversial, Hegde et al. demonstrated the efficacy of a single preoperative supplement dose of vitamin-D in decreasing the incidence of post-operative PJI (p < 0.05) in a mouse model.62
The current study was limited by the quality of the studies that were included. All studies that were included; however, followed reasonable protocols and methods, and the level of evidence of each study is shown in Table 1. The variation in the utilized cutoffs for stratification of vitamin D levels contributed to the exclusion of some data from the pooled analysis despite reporting on pre-operative vitamin D levels. Furthermore, it is unclear whether all studies measured vitamin D levels in a consecutive number of patients; thus, there is a possibility that the prevalence reported in this review was affected by sample bias. The heterogeneity of variables collected from the included studies made rigorous statistical analysis difficult, and the wide variety of places in which the studies were conducted may make generalizing their findings difficult (Table 1). Another limitation is the methods by which each study collected serum vitamin D levels. It has been shown that the most commonly accepted methods of measurement can give drastically different results.63 The included studies were comprised mainly of primary surgeries with the exception of one Traven et al.35 This limits how confident we can be in generalizing our findings outside the primary arthroplasty population. The included studies had various diagnoses, which limits our ability to determine the effect of hypovitaminosis-D on a specific population but is representative of the LE-TJA population as a whole. Finally, the variation of the included implant designs, follow-up intervals, and the subset of surgery performed (primary versus revision; THA versus TKA) are all limiting factors inherent in our attempt to provide a comprehensive analysis of the prevalence of pre-operative vitamin D deficiency in patients undergoing LE-TJA. Despite these shortcomings, the methods used in screening and comparing the included papers ensure confidence in our findings and main conclusions.
In summary, our study found that more than half of the patients undergoing LE-TJA had low levels of vitamin D. Although some studies suggest that hypovitaminosis-D might lead to unfavorable outcomes, there is insufficient literature to conclusively establish an association between vitamin D and adverse outcomes after LE-TJA. Additionally, it is still to be determined whether pre-operative correction of vitamin D levels in patients with deficiency or insufficiency status can improve the outcomes. This can only be answered by large randomized controlled trials, the feasibility of which is a concern. Given the paucity on the effects of vitamin D, further studies are required to fully understand the effects of hypovitaminosis-D and its correction on the outcomes after LE-TJA. Nevertheless, due to the high prevalence and plausible association with poor outcomes, routine pre-operative screening of vitamin D levels should be considered in patients scheduled for LE-TJA. Routine screening is further supported by the potential association between pre-operative vitamin D deficiency and a higher risk of adverse outcomes. Furthermore, additional attention should be directed towards pre-operatively vitamin D deficient patients in anticipation of potential complications.
Authors' contributions
A.K.E. and E.N. collected the data and drafted the manuscript, J.G. and N.S.P. wrote the manuscript and reviewed the data, M.A.B. participated in data collection and reviewed the manuscript and C.H. supervised the project, reviewed the collected data and the manuscript.
Funding
No funding was required for the current study.
Declaration of competing interest
-
•
Carlos Higuera has the following disclosures to declare, all of which being unrelated to the topic of this study: Research Support: CD Diagnostics, Cymedica, Ferring Pharmaceuticals, Orthofix, Inc, Orthogenics, Stryker, Zimmer, OREF, and KCI. Editorial or governing board: American Journal of Orthopedics, Journal of Arthroplasty, Journal of Hip Surgery, Journal of Knee Surgery. Paid consultant/presenter: KCI. Stock or Stock Options: PSI.
-
•
Nicolas S. Piuzzi has the following disclosures to declare, all of which being unrelated to the topic of this study: ISCT: Board or committee member; Orthopaedic Research Society: Board or committee member; Zimmer: Research support.
-
•
Ahmed K. Emara, Emmanuel Nageeb, Jaiben George, Martin A. Buttaro, and Nicolas S. Piuzzi declare that they have no conflict of interest.
References
- 1.Bourne R.B. Measuring tools for functional outcomes in total knee arthroplasty. Clin Orthop Relat Res. 2008;466(11):2634–2638. doi: 10.1007/s11999-008-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae D.K., Song S.J., Park M.J., Eoh J.H., Song J.H., Park C.H. Twenty-year survival analysis in total knee arthroplasty by a single surgeon. J Arthroplasty. 2012;27(7):1297–1304. doi: 10.1016/j.arth.2011.10.027. e1. [DOI] [PubMed] [Google Scholar]
- 3.Mäkelä K.T., Matilainen M., Pulkkinen P. Failure rate of cemented and uncemented total hip replacements: register study of combined Nordic database of four nations. BMJ. 2014;348:f7592. doi: 10.1136/bmj.f7592. [DOI] [PubMed] [Google Scholar]
- 4.Dailiana Z.H., Papakostidou I., Varitimidis S. Patient-reported quality of life after primary major joint arthroplasty: a prospective comparison of hip and knee arthroplasty. BMC Muscoskel Disord. 2015;16:366. doi: 10.1186/s12891-015-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maradit Kremers H., Larson D.R., Crowson C.S. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer B.D., Parvizi J., Austin M. 2013. Obesity and Total Joint Arthroplasty A Literature Based Review. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz S.M., Ong K.L., Lau E., Bozic K.J. Impact of the economic downturn on total joint replacement demand in the United States. J Bone Jt Surgery-American. 2014;96(8):624–630. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 8.Wylde V., Hewlett S., Learmonth I.D., Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Peersman G., Laskin R., Davis J., Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. [PubMed] [Google Scholar]
- 10.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Voigt J., Mosier M., Darouiche R. Systematic review and meta-analysis of randomized controlled trials of antibiotics and antiseptics for preventing infection in people receiving primary total hip and knee prostheses. Antimicrob Agents Chemother. 2015;59(11):6696–6707. doi: 10.1128/AAC.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirwan J.R., Currey H.L., Freeman M.A., Snow S., Young P.J. Overall long-term impact of total hip and knee joint replacement surgery on patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1994;33(4):357–360. doi: 10.1093/rheumatology/33.4.357. [DOI] [PubMed] [Google Scholar]
- 13.Walsh M., Woodhouse L.J., Thomas S.G., Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther. 1998;78(3):248–258. doi: 10.1093/ptj/78.3.248. [DOI] [PubMed] [Google Scholar]
- 14.Ng M., Song S., George J. Associations between seasonal variation and post-operative complications after total hip arthroplasty. Ann Transl Med. 2017;5 doi: 10.21037/atm.2017.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry J.B., Gwam C.U., Chughtai M. Factors influencing patients' hospital rating after total joint arthroplasty. Orthopedics. 2017;40(6) doi: 10.3928/01477447-20171019-03. [DOI] [PubMed] [Google Scholar]
- 16.Piuzzi N.S., Muschler G.F. CORR Insights®: which clinical and patient factors influence the national economic burden of hospital readmissions after total joint arthroplasty. Clin Orthop Relat Res. 2017:1–3. doi: 10.1007/s11999-017-5324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laird E., Ward M., McSorley E., Strain J.J., Wallace J. Vitamin D and bone health; Potential mechanisms. Nutrients. 2010;2(7):693–724. doi: 10.3390/nu2070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mithal A., Wahl D.A., Bonjour J.-P. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 19.Bogunovic L., Kim A.D., Beamer B.S., Nguyen J., Lane M.J., Hypovitaminosis D. In patients scheduled to undergo orthopaedic surgery. A single-center analysis. J Bone Jt Surgery, Am. 2010;92-A(13):2300–2304. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff-ferrari H.A., Giovannucci E., Willett W.C., Dietrich T., Dawson-hughes B. vol. 25. 2006. pp. 18–28. (Estimation of Optimal Serum Concentrations of 25-hydroxyvitamin D for Multiple Health Outcomes 1 – 3). [DOI] [PubMed] [Google Scholar]
- 22.Holick M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Broe K.E., Chen T.C., Weinberg J., Bischoff-Ferrari H.A., Holick M.F., Kiel D.P. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 24.Cantorna M.T., Zhu Y., Froicu M., Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 suppl l) doi: 10.1093/ajcn/80.6.1717S. 1717S-20S. [DOI] [PubMed] [Google Scholar]
- 25.McGrath J., Selten J.-P., Chant D. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration--data from Australia and The Netherlands. Schizophr Res. 2002;54(3):199–212. doi: 10.1016/s0920-9964(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 26.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Ahonen M.H., Tenkanen L., Teppo L., Hakama M., Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11(9):847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 28.Gorham E.D., Garland C.F., Garland F.C. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1-2):179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Iglar P.J., Hogan K.J. Vitamin D status and surgical outcomes: a systematic review. Patient Saf Surg. 2015;9(1) doi: 10.1186/s13037-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 31.Harris J.D., Quatman C.E., Manring M.M., Siston R.A., Flanigan D.C. How to write a systematic review. Am J Sports Med. 2014;42(11):2761–2768. doi: 10.1177/0363546513497567. [DOI] [PubMed] [Google Scholar]
- 32.Unnanuntana A., Saleh A., Nguyen J.T. Low vitamin D status does not adversely affect short-term functional outcome after total hip arthroplasty. J Arthroplasty. 2013;28(2):315–322. doi: 10.1016/j.arth.2012.04.027. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nawabi D.H., Chin K.F., Keen R.W., Haddad F.S. Vitamin D deficiency in patients with osteoarthritis undergoing total hip replacement: a cause for concern? J Bone Joint Surg Br. 2010;92(4):496–499. doi: 10.1302/0301-620X.92B3.23535. [DOI] [PubMed] [Google Scholar]
- 34.Allain T.J., Beresford P.A., Newman J.H., Swinkels A. Vitamin D levels in patients undergoing knee arthroplasty: does vitamin D status effect postoperative outcomes? e-SPEN. 2008;3(1):e17–e21. [Google Scholar]
- 35.Traven S.A., Chiaramonti A.M., Barfield W.R. Fewer complications following revision hip and knee arthroplasty in patients with normal vitamin D levels. J Arthroplasty. 2017:1–4. doi: 10.1016/j.arth.2017.02.038. Mar 8 [Epu. [DOI] [PubMed] [Google Scholar]
- 36.Shin K.-Y., Park K.K., Moon S.-H., Yang I.H., Choi H.-J., Lee W.-S. Vitamin D deficiency adversely affects early post-operative functional outcomes after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016 doi: 10.1007/s00167-016-4209-8. [DOI] [PubMed] [Google Scholar]
- 37.Maniar R.N., Patil A.M., Maniar A.R., Gangaraju B., Singh J. Effect of preoperative vitamin D levels on functional performance after total knee arthroplasty. Clin Orthop Surg. 2016;8(2):153–156. doi: 10.4055/cios.2016.8.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Cunha B.M., Gava A.D., de Oliveira S.B., de David A.C., dos Santos-Neto L.L. Vitamin d is related to gait recovery after total hip arthroplasty: a prospective analysis. Gait Posture. 2016;50:96–101. doi: 10.1016/j.gaitpost.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Lee A., Chan S.K.C., Samy W., Chiu C.H., Gin T. Effect of hypovitaminosis D on postoperative pain outcomes and short-term health-related quality of life after knee arthroplasty: a cohort study. Medicine (Baltim) 2015;94(42) doi: 10.1097/MD.0000000000001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier G.S., Horas K., Seeger J.B., Roth K.E., Kurth A.A., Maus U. Is there an association between periprosthetic joint infection and low vitamin D levels? Int Orthop. 2014;38(7):1499–1504. doi: 10.1007/s00264-014-2338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavernia C.J., Villa J.M., Iacobelli D.A., Rossi M.D. Vitamin D insufficiency in patients with THA: prevalence and effects on outcome. Clin Orthop Relat Res. 2014;472(2):681–686. doi: 10.1007/s11999-013-3172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unnanuntana A., Rebolledo B.J., Gladnick B.P. Does vitamin D status affect the attainment of in-hospital functional milestones after total hip arthroplasty? J Arthroplasty. 2012;27(3):482–489. doi: 10.1016/j.arth.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Mikosch P. Does bone quality predict loosening of cemented total hip replacements? J fur Miner. 2008;15(2):98. doi: 10.1302/0301-620X.89B10.19038. [DOI] [PubMed] [Google Scholar]
- 44.Visser E., De Roos N.M., Oosting E., Endenburg S.C., Dronkers J.J. Association between preoperative vitamin D status and short-term physical performance after total hip arthroplasty: a prospective study. Ann Nutr Metab. 2018;73(3):252–260. doi: 10.1159/000492938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier G.S., Maus U., Lazovic D., Horas K., Roth K.E., Kurth A.A. Is there an association between low serum 25-OH-D levels and the length of hospital stay in orthopaedic patients after arthroplasty? J Orthop Traumatol. 2016;17(4):297–302. doi: 10.1007/s10195-016-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao B., Zhu B., Wu C. Preoperative serum 25-hydroxyvitamin D level, a risk factor for postoperative cognitive dysfunction in elderly subjects undergoing total joint arthroplasty. Am J Med Sci. 2019;357(1):37–42. doi: 10.1016/j.amjms.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Hegde V., Arshi A., Wang C. Preoperative vitamin D deficiency is associated with higher postoperative complication rates in total knee arthroplasty. Orthopedics. 2018;41(4):e489–e495. doi: 10.3928/01477447-20180424-04. [DOI] [PubMed] [Google Scholar]
- 48.Piuzzi N.S., George J., Khlopas A. High prevalence and seasonal variation of hypovitaminosis D in patients scheduled for lower extremity total joint arthroplasty. Ann Transl Med. 2018;6(16) doi: 10.21037/atm.2018.08.21. 321-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly M.A., Campbell J., Sheahan J., Murray P. Vitamin D insufficiency in patients undergoing total knee arthroplasty in Ireland. Ir Med J. 2017;110(10):649. [PubMed] [Google Scholar]
- 50.Wright J.G., Swiontkowski M.F., Heckman J.D. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A(1):1–3. [PubMed] [Google Scholar]
- 51.Blomberg Jensen M., Husted H., Bjerrum P.J., Juul A., Kehlet H. Compromised activation of vitamin D after elective surgery: a prospective pilot study. JBMR Plus. 2018;2(5):281–288. doi: 10.1002/jbm4.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joris J.R., Jansen A., Tahmassebi J., Haddad F.S. Vitamin D deficiency is associated with longer hospital stay and lower functionaloutcome after total knee arthroplasty. Acta Orthop Belg. 2017;83(4):664–670. [PubMed] [Google Scholar]
- 53.Shin K.Y., Park K.K., Moon S.H., Yang I.H., Choi H.J., Lee W.S. Vitamin D deficiency adversely affects early post-operative functional outcomes after total knee arthroplasty. Knee Surgery. Sport Traumatol Arthrosc. 2017;25(11):3424–3430. doi: 10.1007/s00167-016-4209-8. [DOI] [PubMed] [Google Scholar]
- 54.Dawson-Hughes B., Heaney R.P., Holick M.F., Lips P., Meunier P.J., Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 55.Forrest K.Y.Z., Stuhldreher W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 56.McAlindon T., LaValley M., Schneider E. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. J Am Med Assoc. 2013;309(2):155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin X., Jones G., Cicuttini F. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. J Am Med Assoc. 2016;315(10):1005–1013. doi: 10.1001/jama.2016.1961. [DOI] [PubMed] [Google Scholar]
- 58.White J.H. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13(1):21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 59.Gombart A.F. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C., Wang Y., Gao L. Expression of toll-like receptors 2 and 4 and CD14 during differentiation of HL-60 cells induced by phorbol 12-myristate 13-acetate and 1 alpha, 25-dihydroxy-vitamin D(3) Cell Growth Differ. 2002;13(1):27–38. [PubMed] [Google Scholar]
- 61.White J.H. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121(1-2):234–238. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 62.Hegde V., Dworsky E.M., Stavrakis A.I. Single-dose, preoperative vitamin-D supplementation decreases infection in a mouse model of periprosthetic joint infection. J Bone Jt Surg - Am. 2017;99(20):1737–1744. doi: 10.2106/JBJS.16.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lips P., Chapuy M.C., Dawson-Hughes B., Pols H.A.P., Holick M.F. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9(5):394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]