Summary
Aim
To describe a hospital outbreak of influenza B virus (InfB) infection during season 2015/2016 by combining clinical and epidemiological data with molecular methods.
Methods
Twenty patients diagnosed with InfB from a hospital outbreak over a four-week-period were included. Nasopharyngeal samples (NPS) positive for InfB by multiplex real-time polymerase chain reaction were sent for lineage typing and whole genome sequencing (WGS). Medical records were reviewed retrospectively for data regarding patient characteristics, localization, exposure and outcome, and assembled into a timeline. In order to find possible connections to the hospital outbreak, all patients with a positive NPS for influenza from the region over an extended time period were also reviewed.
Findings
All 20 cases of InfB were of subtype B/Yamagata, and 17 of 20 patients could be linked to each other by either shared room or shared ward. WGS was successful or partially successful for 15 of the 17 viral isolates, and corroborated the epidemiological link supporting a close relationship. In the main affected ward, 19 of 75 inpatients were infected with InfB during the outbreak period, resulting in an attack rate of 25%. One probable case of influenza-related death was identified.
Conclusion
InfB may spread within an acute care hospital, and advanced molecular methods may facilitate assessment of the source and extent of the outbreak. A multi-faceted approach, including rapid diagnosis, early recognition of outbreak situations, simple rules for patient management and the use of regular infection control measures, may prevent nosocomial transmission of influenza virus.
Keywords: Nosocomial transmission, Influenza B virus infection, Whole genome sequencing, Hospital outbreak, Infection control
Introduction
Influenza virus causes respiratory tract infections, and spreads by inhalation of virus-loaded aerosols or droplets, or via direct or indirect contact with infected individuals. Hospital outbreaks of influenza A have been described [1], [2], [3]. Patients, visitors and healthcare workers (HCWs) might be part of the transmission chain, and outbreaks may be facilitated within closed settings [4], possibly by contamination of hospital surfaces [5]. The relative importance of different modes of transmission is contentious; however, aerosol is suggested to be an important factor [6], [7], [8]. The epidemiological understanding of influenza transmission in healthcare settings is incomplete due to variations in case identification, source and route of transmission, patient-related factors, and the use of infection control measures. Outbreak reports of influenza from long-term care facilities have been of particular concern due to the high-risk population, but reports using laboratory confirmation combined with accurate clinical definitions have been rare [9]. Molecular characterization has commonly shown multiple viral strains during hospital outbreaks, reflecting simultaneous circulation of various strains in the community rather than hospital clustering [10], [11]. Prevention and control strategies are essential in controlling hospital outbreaks, as well as defining the onset and end of influenza seasons in the community [12]. Properly designed studies are needed to optimize preventive measures [13].
Human influenza virus is classified into types A, B and C, with type A as the dominant cause of seasonal epidemics. Influenza B virus (InfB) is divided into two lineages, Victoria and Yamagata, and is mainly associated with smaller epidemics. Few reports have described InfB outbreaks in a hospital setting [14], [15], [16]. Whole genome sequencing has yielded insights into transmission dynamics in nosocomial outbreaks for other agents (e.g. carbapenem-resistant Klebsiella pneumoniae [17]) and community outbreaks of influenza A [18]. To the authors' knowledge, there have been no reports of hospital outbreaks including a detailed molecular characterization of InfB. This study identified one virus strain as the probable cause of multiple secondary cases, causing negative effects for both patients and hospital functions.
Methods
Setting
The outbreak occurred in a 200-bed acute care hospital, serving a population of approximately 125,000 inhabitants in Western Sweden, and lasted for a period of four weeks in May 2016. The period of increased seasonal influenza activity in this area peaks from December to March, and this particular season lasted from 5th December 2015 to 23rd April 2016. Diagnostic services were provided by the virology laboratory at a larger teaching hospital, serving a population of approximately 700,000 inhabitants. The time between nasopharyngeal samples (NPS) being taken and laboratory confirmation was 12–48 h, depending on time of day and transportation logistics. The ward most affected by the outbreak (Ward A) has 25 beds but no isolation facilities (Figure A, see online supplementary material). In situations when the hospital is filled to capacity, inpatients might be placed in rooms not fully equipped for patient care. The mean bed occupancy rate for Ward A during 2016 was 106%, and the mean length of hospital stay was 5.4 days.
Study design
Stored NPS from patients involved in the outbreak were used for InfB lineage typing and sequence analysis [19]; a detailed description is given in the supplementary material. Retrospective review was undertaken of the medical records for all patients with an NPS positive for InfB by a real-time in-house polymerase chain reaction (PCR) described previously [20] during an extended time period to find possible connections with the hospital cases. This period precedes the admission of the index case of the study by one week, and terminates one week after confirmation of the final case. In addition, medical records for all patients admitted to Ward A were reviewed in order to estimate the attack rate.
Case and outbreak definitions
All cases required laboratory confirmation by multiplex real-time PCR of InfB in combination with symptoms of influenza-like-illness (ILI) as stated by the US Centers for Disease Control and Prevention (fever >37.8 °C and cough or sore throat) or acute respiratory infection (ARI), defined here as sudden onset of cough, sore throat or shortness of breath regardless of fever with no other plausible cause. A probable case referred to symptoms of ILI/ARI in addition to an epidemiological link [21]. Healthcare-associated infection (HCAI) was defined as onset of ILI/ARI symptoms ≥48 h after hospital admission or ≤48 h after discharge [22].
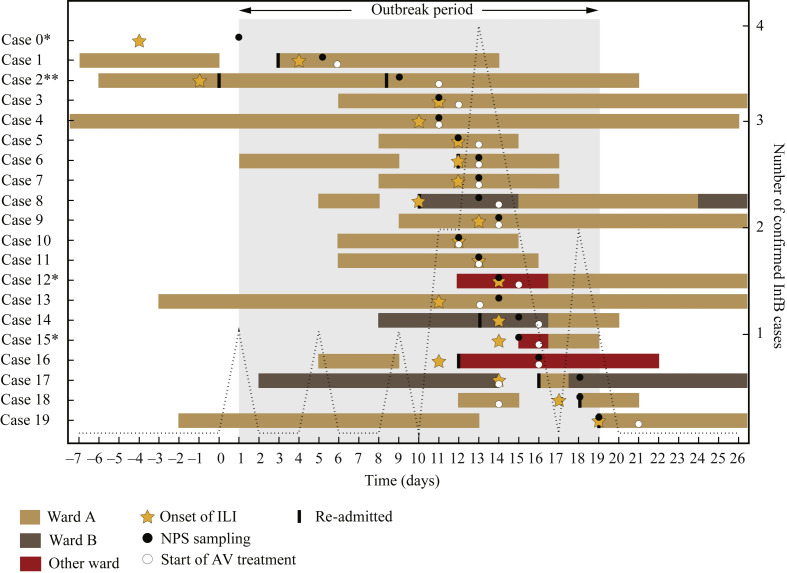
The outbreak period was defined as the period between the NPS sampling day of Case 0 and the NPS sampling day of Case 20 (hereafter referred to as Day 1–Day 19).
Infection control measures
Current recommendations from the infection control unit and hospital management for any patient with suspected influenza include inpatient care in a closed single room. Patients are instructed to cover their mouth and nose while coughing or sneezing (not specified in what way), and HCWs are instructed to observe droplet precautions (visor or surgical mask/protective glasses) in addition to standard precautions [23] when in close contact (distance <1 m) with patients. The incubation time was considered to be within four days of exposure.
Antiviral treatment and chemoprophylaxis
Antiviral treatment of InfB infection (75 mg of oseltamivir twice daily for five days) was recommended for symptomatic patients with signs of severe illness (by clinical judgement; for example, respiratory rate >30/min, systolic blood pressure <90 mmHg and/or diastolic blood pressure <60 mmHg, signs of disorientation or dehydration) or with estimated high risk of complications [24]. All hospitalized patients were generally considered to be at high risk. Chemoprophylaxis (75 mg of oseltamivir once daily for 10 days) was considered for patients exposed by sharing a room with a suspected or verified case of influenza, regardless of vaccination status.
Results
Case 0
In order to find InfB-positive cases with a possible link to the hospital, the outbreak period was extended as described above. One patient, a 20-year-old male (Case 0), was diagnosed during a visit to the emergency room (ER) two days prior to admission of the index patient. He was not admitted to the hospital and no epidemiological link to the other cases could be established.
Index case
The index case (Case 1) was a 66-year-old male who was re-admitted three days after discharge from Ward A (Figure 1 ). The ER nurse noted that the patient's wife had ILI. No infection control measures for influenza were initiated, and he went home for short periods during the hospital stay. On Day 4, the patient developed symptoms of ILI. InfB infection was confirmed on Day 6, and he was moved to a single room and received oseltamivir treatment.
Figure 1.
Overview of all confirmed cases of influenza B (InfB) from the hospital over an extended time period. Location, onset of influenza-like illness (ILI)/acute respiratory infection (ARI) in relation to nasopharyngeal samples (NPS) and initiation of antiviral (AV) treatment are shown. The defined outbreak period ranges from the NPS sampling day of Case 0 to the NPS sampling day of Case 20. ∗Cases 0, 12 and 15 could not be linked to the ‘true’ outbreak, starting with the index patient on Ward A. ∗∗Case 2 developed diffuse respiratory symptoms meeting the criteria for ARI 10 days before NPS sampling, and in addition also had a high cycle threshold value (30.3). Clinical picture and time of InfB infection are unclear in this case.
Outbreak
On Day 9, an 88-year-old woman (Case 2), who had reported mild respiratory symptoms for nine days and had been discharged from Ward A less than 24 h previously, presented at the ER with an acute onset of fever (Figure 1). She was not defined as exposed to the index patient. Oseltamivir treatment was initiated on Day 11 when InfB infection was confirmed. Over the following 12 days, another 17 cases of InfB infection were confirmed at the hospital, with a cluster of 14 patients between Days 11 and 16. Oseltamivir treatment was initiated based on clinical presentation in eight cases, but treatment was initiated upon confirmation of InfB infection in 10 cases. One patient (Case 18) was defined as exposed on the ward and received oseltamivir prophylaxis within 48 h, but had a breakthrough infection.
On Day 10 of the outbreak, a 90-year-old man (Case 8) returned to the hospital with fever, three days after being discharged from Ward A. Like Case 2, he had not been defined as being exposed on the ward. Influenza was not suspected, and he was admitted to Ward B. InfB was confirmed on Day 14, which was the same day as two other patients from Ward B (Cases 14 and 17) developed ILI symptoms. Furthermore, two patients (Cases 12 and 15) were reported on Days 15 and 16 from other wards in the hospital. No epidemiological link to the other hospital cases could be established, and as they presented with ILI after less than 48 h of hospital care or with symptoms on admission, they were not classified as HCAI.
When medical records of all patients admitted to Ward A were reviewed, one additional patient (ILI onset on Day 13 without laboratory confirmation) met the criteria for a probable case. Furthermore, 15 HCWs on Ward A reported sick leave due to fever and/or respiratory symptoms between Days 8 and 19, five of them on Day 13 when the outbreak peaked. Ward A was closed to admission of new inpatients due to staff shortages on Days 13–14. The infection control unit was contacted on Day 13. Recommended additional infection control measures included distancing of symptomatic and asymptomatic patients on the ward, and ensuring easy access to personal protective equipment for HCWs. High vigilance for ILI/ARI was initiated from Day 16, including individual assessment of infectivity, criteria for re-admissions and continuous staff information.
Patient characteristics and outcome
In total, 20 cases of InfB infection from the hospital were included in the study. Demographic data, Charlson morbidity score [25] and clinical data for all 20 patients are listed in Table I . Data regarding vaccination status were not available from medical records. The multiplex real-time PCR indicated a high InfB viral load in most cases, and detected no co-infections with other respiratory pathogens. The mean length of hospital stay was 11.3 days and the all-cause 30-day mortality rate of the confirmed cases was zero. One case of potential influenza-related death was found. The patient defined above as a probable case had a medical history of a malignant disorder, developed ILI on Day 13 and died within 24 h. An NPS was not obtained. Overall during the outbreak period, 19 of 75 patients who were admitted to Ward A were diagnosed with InfB, resulting in an attack rate of 25%. Furthermore, during the outbreak period, nine of 75 (12%) patients on Ward A received oseltamivir prophylaxis, and a total of 30 NPS were found positive to be positive for InfB in the laboratory, of which 20 (67%) were taken from patients involved in this outbreak.
Table I.
Case number, age, sex, co-morbidity estimated by Charlson scoring system, probable mode of exposure, cycle threshold (CT) for nasopharyngeal samples, total length of hospital stay (LOS), number of re-admissions during the outbreak period, healthcare-associated infection (HCAI) and antibiotic treatment (AB)
| Case no. | Age | Sex | Charlson score | Exposure | CT | LOS (days) | Re-admissions (N) | HCAI (Y/N) | AB (Y/N) |
|---|---|---|---|---|---|---|---|---|---|
| 0a | 20 | M | 0 | – | 22.2 | 0 | 0 | N | N |
| 1 | 66 | M | 6 | Index | 22.3 | 18 | 1 | Y | Y |
| 2 | 88 | F | 4 | Shared ward | 30.3 | 26 | 2 | Y | Y |
| 3 | 68 | F | 4 | Shared room | 27.1 | 24 | 0 | Y | Y |
| 4b | 75 | F | 5 | Shared room | 23.6 | 47 | 0 | Y | Y |
| 5 | 67 | F | 0 | Shared room | 18.4 | 6 | 0 | Y | N |
| 6 | 45 | F | 4 | Shared room | 26.2 | 12 | 1 | Y | Y |
| 7 | 65 | M | 4 | Shared room | 24.6 | 8 | 0 | Y | Y |
| 8 | 90 | M | 9 | Shared ward | 18.8 | 21 | 1 | N | Y |
| 9 | 87 | F | 0 | Shared ward | 20.4 | 25 | 0 | Y | Y |
| 10b | 90 | M | 5 | Shared room | 30.1 | 9 | 0 | Y | N |
| 11 | 57 | M | 3 | Shared ward | 21.6 | 10 | 0 | Y | N |
| 12a | 82 | F | 1 | - | 17.5 | 23 | 0 | N | Y |
| 13 | 68 | F | 2 | Shared room | 21.9 | 37 | 0 | Y | Y |
| 14 | 66 | F | 8 | Shared ward | 25.7 | 10 | 1 | Y | Y |
| 15a | 85 | M | 3 | – | 30.6 | 3 | 0 | N | Y |
| 16 | 78 | M | 4 | Shared ward | 19.9 | 12 | 1 | Y | Y |
| 17 | 87 | F | 7 | Shared room | 19.7 | 27 | 1 | Y | N |
| 18 | 77 | F | 2 | Shared room | 28.0 | 4 | 1 | Y | Y |
| 19 | 77 | F | 2 | – | 23.4 | 21 | 1 | N | Y |
| Median | 77 | 4 | 23.4 |
Cases 0, 12 and 15 could not be linked to Ward A.
Cases 4 and 10, sequence analysis was not possible.
Molecular characterization of viral isolates
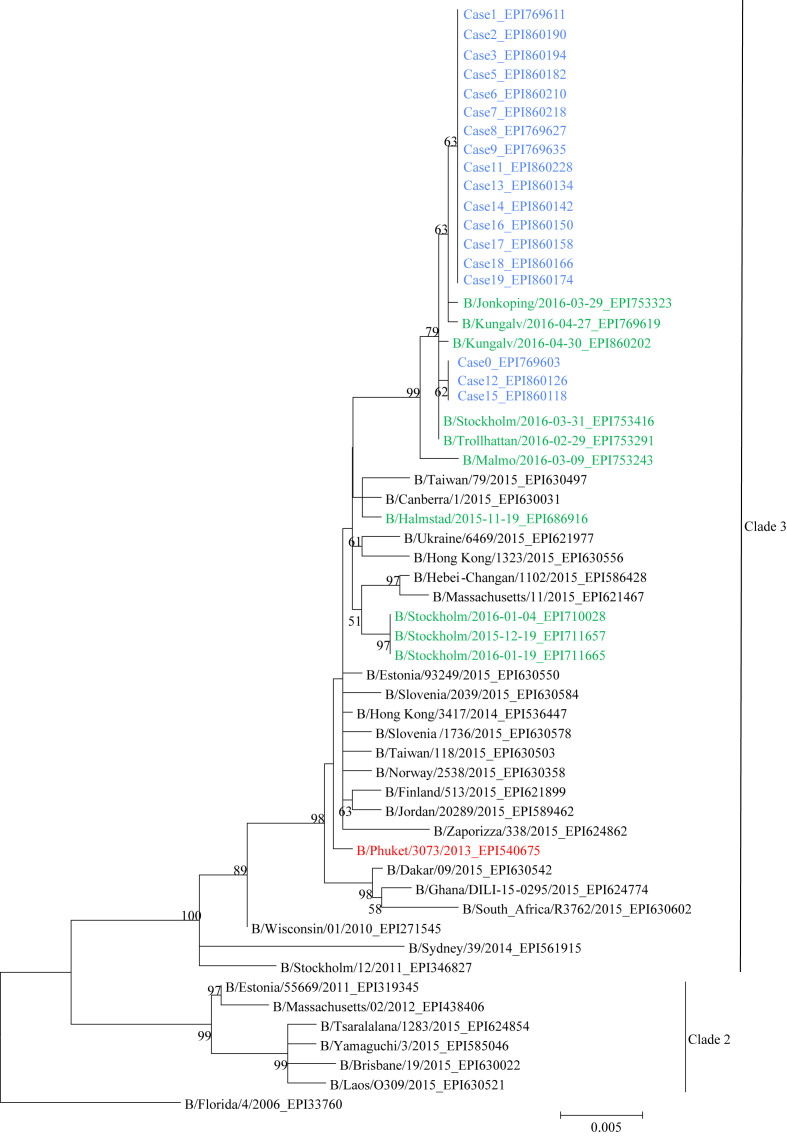
The phylogenetic analysis of 18 full-length (1755 nucleotides) haemagglutinin (HA) sequences from the hospital outbreak, along with all collected and sequenced Swedish B/Yamagata viruses from season 2015/2016, the seasonal vaccine strain for the northern hemisphere and reference viruses are shown in Figure 2 . A high CT value (cycle threshold >36) prevented sequencing of InfB from Case 10, and no HA sequence could be obtained from Case 4. Fifteen of the 18 sequences had identical HA sequences, although Case 9 did contain a mix of two nucleotides in one position. The remaining three cases (Cases 0, 12 and 15) had identical HA sequences but differed in three nucleotide positions from the other 15 cases. The HA nucleotide sequences of the 18 cases were not identical to any other Swedish B/Yamagata viruses collected and sequenced during season 2015/2016. All 18 cases were identical at amino acid level (HA) and belonged to genetic clade 3. Information regarding the identity of the respective nucleotide alterations and the accession numbers for the viruses in GISAID EpiFlu (www.GISAID.org/) are shown in Table A (see online supplementary material).
Figure 2.
Phylogenetic analysis of full-length (1755 nucleotides) haemagglutinin sequences. Included are 18 viruses from the hospital outbreak (blue), all Swedish B/Yamagata viruses collected and sequenced during season 2015/2016 (N = 10, black), date and geographical location shown, and reference viruses (grey) and the vaccine strain for northern hemisphere season 2015/2016: B/Phuket/3073/2013 (red). Sequencing data are missing for Cases 10 and 4. The tree was constructed using the maximum likelihood method in Mega Version 5.1. Bootstrap values were obtained from 1000 replicates and values >70% are displayed on nodes. The EPI numbers shown in the tree correspond to haemagglutinin sequences in GISAID's EpiFlu Database (www.GISAID.org).
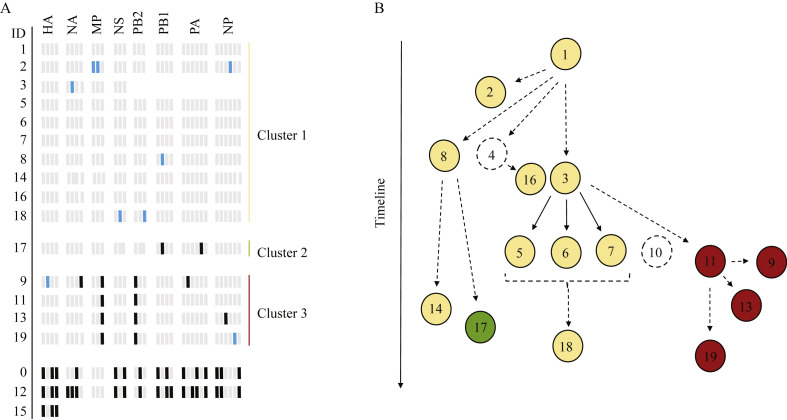
Further analysis of nucleotide differences within the entire InfB genome could arrange the isolates in three groups, distinctly separated from Cases 0, 12 and 15 (Figure 3 A). Within Group 1, four viruses displayed a mixed population of nucleic acids in one to two positions. For Case 3, sequencing results were lacking for PB2, PB1, PA and NP. Within Group 2, which consisted of a single isolate, two positions exhibited altered nucleotide sequences compared with the viruses in Group 1. Group 3 consisted of four viruses with two positions where the same nucleotide variations were observed for all isolates. Additional variations were also found in three of the isolates.
Figure 3.
(A) Single nucleotide variants (SNVs) identified in the eight segments of the sequenced influenza B virus (InfB) genomes. Nucleotide positions that had identified variations compared with the index case are shown, with variable positions on the x-axis and case ID on the y-axis. Light grey, nucleotide variant based on sequence of index case (ID 1); black, altered nucleotide (SNV) compared with index case; blue, mixed nucleotide population; missing values, no sequence data obtained. Variants present at a frequency of at least 20% (in positions with coverage ≥20X) were included. In some cases, the coverage was <20X for some nucleotide positions, which were therefore not analysed for variants. Information regarding the identity of respective nucleotide alteration can be found in Table A (see online supplementary material). Variant detection and alignment of the sequences were performed with CLC Genomics Workbench (Qiagen). (B) Putative map for InfB transmission based on SNV analysis of the whole InfB genome and patient overlap within a ward. Nodes represent cases and arrows indicate transmission events, directly or indirectly, from one patient to another. Dashed open nodes represent cases where sequence data are lacking. Solid arrows indicate highly likely transmissions and dashed arrows represent possible transmission events. Yellow nodes, Group 1; green node, Group 2; red nodes, Group 3.
A putative transmission map was created using nucleotide and patient data in relation to time and location within the hospital (Figure 3B). For Case 4, Case 10 and HCWs, no transmission could be supported by sequence analysis due to lack of data. The transmission map generated by integrating nucleotide analysis and epidemiological data highlights the complexity of the outbreak progression. Three putative transmission events stemmed from the index patient, and two secondary transmission events subsequently stemmed from Cases 8 and 3, which likely resulted in six additional infections. Transmission from Case 3 to Case 11 eventually resulted in transmission to another three patients, according to the generated map, before the outbreak came to a halt. No mutations associated with resistance to oseltamivir or zanamivir were identified in the neuraminidase gene in any of the 17 cases where sequence analysis was complete.
Discussion
In this study, 17 of 20 patients with InfB infection could be epidemiologically linked to each other. The connections in time and space were supported by molecular data, adding more weight to the hypothesis that transmission occurred within the hospital. Furthermore, detailed analysis of all gene segments identified three separate groups within the outbreak, where mutations of single nucleotides had occurred. The mutation rate for influenza B is estimated to be two or three times lower than for influenza A (which is predicted to be two to three mutations per replicated genome) or one mutation per replication cycle [26], [27]. This is in line with the single nucleotide variant analysis, indicating that rapid changes occurred within the influenza genome during the outbreak period.
Delayed initiation of antiviral therapy and infection control measures at the beginning of the outbreak may have enabled the first cases to spread the virus efficiently. The median incubation time for InfB is 0.6 days [28], explaining why the impact of Case 3 or 4 might have been of greater importance. A few individuals appear to be more important for spreading the virus, as determined by the putative transmission map. The gap between Days 4 and 10, when no new patients reported symptoms, suggest that during these days, HCWs or asymptomatic patients might have been part of the transmission chain. At the beginning of the outbreak, no preventive measures were taken before laboratory confirmation. During weekends, access to the on-call physician is limited and no regular ward rounds are performed, which may have added to the delay.
None of the 15 HCWs who reported sick leave were vaccinated. Five of them reported sick at the peak of the outbreak, suggesting a common source of infection. HCWs tend to work despite mild respiratory symptoms, increasing the exposure of co-workers and patients to influenza [29]. It has been shown that the highest number of contacts in hospital care occurs between nurses, or between nurses and patients, which highlights the role of HCWs in nosocomial influenza transmission [30]. Analysis of the severe acute respiratory syndrome outbreak in Beijing identified a high number of close contacts, misdiagnosis and overcrowding as risk factors for ‘super-spreading’ in a hospital setting [31]. Intrahospital transfer of patients with suspected influenza within or between wards may be counterproductive, and may increase the number of exposed individuals. This is illustrated in this study by Case 8, who was re-admitted to another ward despite recent exposure, resulting in Cases 14 and 17.
Defining a patient as exposed to influenza may be difficult, especially when HCWs may be involved. The total number of infectious individuals in a hospital unit might increase the overall risk of exposure. In the present study, seven cases of influenza were diagnosed in patients who did not share a room with a confirmed case. To estimate the number of exposed patients demands knowledge of local conditions, patient transfer patterns within a facility, and individual assessment of infectivity. Access to isolation facilities is, as in the present study, often limited. Patients and HCWs might also be exposed to influenza virus in public areas outside patient rooms, such as corridors, elevators and waiting rooms.
The attack rate for confirmed InfB among inpatients in Ward A was 25%. Only one patient was defined as a probable case. This indicates a low threshold for NPS sampling in patients with ILI/ARI. In outbreak reports from long-term care facilities, attack rates for influenza (A and B) ranges between 1% and 65%, with an adjusted mean of 28%; however, the attack rate of InfB is commonly lower than for influenza A [9]. It has also been hypothesized that elderly people are particularly vulnerable to the Yamagata lineage [32]. The short incubation period might also lead to underestimation of nosocomial transmission rates. In this study, five patients did not meet the defined criteria for an HCAI (Cases 0, 8, 12, 15 and 19), although the results strongly suggest that Cases 8 and 19 had a hospital-acquired influenza infection.
Chemoprophylaxis for exposed individuals may reduce attack rates and shorten outbreak durations [33], [34], and oseltamivir used for both treatment and prophylaxis has been shown to be more effective than treatment alone [35]. When nosocomial transmission is suspected, it might be useful to provide extended and prolonged prophylaxis, which is probably easier to implement than coherent social distancing. However, oseltamivir seems to be less effective for InfB than for influenza A regarding fever duration and virus persistence [36], [37], which may have influenced the course of the outbreak reported here.
It is difficult to make an aetiologic diagnosis of ARI based on the clinical presentation alone [38]. Also, low suspicion of influenza outside the ordinary season may have delayed the recognition of InfB cases in this study. To what extent HCWs and patients followed the recommended infection control measures is unknown. An outbreak was not suspected until Day 13, when seven cases had already been confirmed. Risk perception may promote protective behaviour [39], and no onward transmission could be demonstrated for Cases 12 and 15 (diagnosed on two different wards on Days 15 and 16) when the vigilance of influenza was high throughout the hospital. Additional infection control measures were introduced on Days 13 and 16, but most likely had limited impact as the number of susceptible individuals decreased continuously.
This report has several limitations. Unknown factors may have affected the course of the outbreak. Data were collected retrospectively and this may have generated bias. Detailed contact patterns for the infected patients (i.e. shared room for short time periods) could not be established. The HCWs' part of the transmission chain is unclear as information regarding symptoms, laboratory confirmation and adherence to control measures was lacking. Sequence data were only partially complete for Case 3, and were lacking for Cases 4 and 10, making the putative transmission map incomplete. The total number of InfB cases may have been underestimated as asymptomatic patients not meeting the definition of a probable case were not included. There may also be unknown patients, discharged during the outbreak, who contracted InfB on the ward but did not seek further medical care. Patient data regarding vaccination are also lacking. Vaccination coverage for influenza in age group >65 years in Sweden was estimated to be 50% in season 2015/16. However, the protective effect of the vaccine in this study is probably of limited importance due to late seasonal onset and waning immunity.
In conclusion, InfB may spread within an acute care hospital, and advanced molecular methods may facilitate assessment of the source and extent of the outbreak. A multi-faceted approach including rapid diagnosis, early recognition of outbreak situations, simple rules for patient management and the use of regular infection control measures may prevent nosocomial transmission of influenza virus.
Acknowledgements
The authors wish to thank the staff at the virology laboratory at Sahlgrenska University Hospital, the staff at the Public Health Agency of Sweden, and the originating and submitting laboratories of sequences from GISAID's EpiFlu Database used in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jhin.2018.06.004.
Conflict of interest statement
None declared.
Funding source
This work was supported by a grant from the Region Vastra Gotaland research fund (Grant No. ALFGBG-672131).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig S1.
References
- 1.Carnicer-Pont D., White D., Pike C., Lyons M. Influenza A outbreak in a community hospital in south east Wales, February 2005. Euro Surveill. 2005;10 doi: 10.2807/esw.10.07.02645-en. [DOI] [PubMed] [Google Scholar]
- 2.Andrieu A.G., Paute J., Glomot L., Jarlier V., Belmin J. Nosocomial influenza outbreak in a geriatrics department: effectiveness of preventive measures. Presse Med. 2006;35:1419–1426. doi: 10.1016/s0755-4982(06)74830-8. [DOI] [PubMed] [Google Scholar]
- 3.Vanhems P., Benet T., Munier-Marion E. Nosocomial influenza: encouraging insights and future challenges. Curr Opin Infect Dis. 2016;29:366–372. doi: 10.1097/QCO.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 4.Wong B.C., Lee N., Li Y., Chan P.K., Qiu H., Lou Z. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin Infect Dis. 2010;51:1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges C.B., Kuehnert M.J., Hall C.B. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 7.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl. 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rainwater-Lovett K., Chun K., Lessler J. Influenza outbreak control practices and the effectiveness of interventions in long-term care facilities: a systematic review. Influenza Other Respir Viruses. 2014;8:74–82. doi: 10.1111/irv.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagani L., Thomas Y., Huttner B., Sauvan B., Notaridis G., Kaiser L. Transmission and effect of multiple clusters of seasonal influenza in a Swiss geriatric hospital. J Am Geriatr Soc. 2015;63:739–744. doi: 10.1111/jgs.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eibach D., Casalegno J.S., Bouscambert M., Bénet T., Regis C., Comte B. Routes of transmission during a nosocomial influenza A(H3N2) outbreak among geriatric patients and healthcare workers. J Hosp Infect. 2014;86:188–193. doi: 10.1016/j.jhin.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Maltezou H.C., Drancourt M. Nosocomial influenza in children. J Hosp Infect. 2003;55:83–91. doi: 10.1016/s0195-6701(03)00262-7. [DOI] [PubMed] [Google Scholar]
- 13.Smith S.M., Sonego S., Wallen G.R., Waterer G., Cheng A.C., Thompson P. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20:896–903. doi: 10.1111/resp.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschan J., Ringden O., Ljungman P., Andersson J., Lewensohn-Fuchs I., Forsgren M. Influenza B in transplant patients. Scand J Infect Dis. 1989;21:349–350. doi: 10.3109/00365548909035710. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki Y., Goto N., Iwanami N., Hama M., Fujiwara N., Takahashi Y. Outbreaks of influenza B infection and pneumococcal pneumonia at a mental health facility in Japan. J Infect Chemother. 2017;23:837–840. doi: 10.1016/j.jiac.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Seale H., Weston K.M., Dwyer D.E., Zhu M., Allchin L., Booy R. The use of oseltamivir during an influenza B outbreak in a chronic care hospital. Influenza Other Respir Viruses. 2009;3:15–20. doi: 10.1111/j.1750-2659.2008.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snitkin E.S., Zelazny A.M., Thomas P.J., Stock F., Henderson D.K., Palmore T.N. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4:148ra16. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinel D.M., Heinzinger S., Eberle U., Ackermann N., Schonberger K., Sing A. Whole genome sequencing identifies influenza A H3N2 transmission and offers superior resolution to classical typing methods. Infection. 2018;46:69–76. doi: 10.1007/s15010-017-1091-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B., Lin X., Wang W., Halpin R.A., Bera J., Stockwell T.B. Universal influenza B virus genomic amplification facilitates sequencing, diagnostics, and reverse genetics. J Clin Microbiol. 2014;52:1330–1337. doi: 10.1128/JCM.03265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson M.E., Olofsson S., Lindh M. Comparison of the FilmArray assay and in-house real-time PCR for detection of respiratory infection. Scand J Infect Dis. 2014;46:897–901. doi: 10.3109/00365548.2014.951681. [DOI] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control . ECDC; Brussels: 2018. Influenza case definitions. [Google Scholar]
- 22.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Socialstyrelsen . Swedish National Board of Health and Welfare; Stockholm: 2015. Basal hygiene. [Google Scholar]
- 24.Swedish Medical Products Agency; Uppsala: 2011. Recommendations for antiviral treatment and prophylaxis of influenza. [Google Scholar]
- 25.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Pauly M.D., Procario M.C., Lauring A.S. A novel twelve class fluctuation test reveals higher than expected mutation rates for influenza A viruses. Elife. 2017;6:7–8. doi: 10.7554/eLife.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobusawa E., Sato K. Comparison of the mutation rates of human influenza A and B viruses. J Virol. 2006;80:3675–3678. doi: 10.1128/JVI.80.7.3675-3678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilde J.A., McMillan J.A., Serwint J., Butta J., O'Riordan M.A., Steinhoff M.C. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281:908–913. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 30.Bernard H., Fischer R., Mikolajczyk R.T., Kretzschmar M., Wildner M. Nurses' contacts and potential for infectious disease transmission. Emerg Infect Dis. 2009;15:1438–1444. doi: 10.3201/eid1509.081475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Z., Ning F., Zhou W., He X., Lin C., Chin D.P. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharabi S., Drori Y., Micheli M., Friedman N., Orzitzer S., Bassal R. Epidemiological and virological characterization of influenza B virus infections. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M., Jacobs A., Khan M.N., Jaipaul J., Oda J., Johnson M. Evaluation of the use of oseltamivir prophylaxis in the control of influenza outbreaks in long-term care facilities in Alberta, Canada: a retrospective provincial database analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowles S.K., Lee W., Simor A.E., Vearncombe M., Loeb M., Tamblyn S. Use of oseltamivir during influenza outbreaks in Ontario nursing homes, 1999–2000. J Am Geriatr Soc. 2002;50:608–616. doi: 10.1046/j.1532-5415.2002.50153.x. [DOI] [PubMed] [Google Scholar]
- 35.Booy R., Lindley R.I., Dwyer D.E., Yin J.K., Heron L.G., Moffat C.R. Treating and preventing influenza in aged care facilities: a cluster randomised controlled trial. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai N., Ikematsu H., Iwaki N., Maeda T., Satoh I., Hirotsu N. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006;43:439–444. doi: 10.1086/505868. [DOI] [PubMed] [Google Scholar]
- 37.Sugaya N., Mitamura K., Yamazaki M., Tamura D., Ichikawa M., Kimura K. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis. 2007;44:197–202. doi: 10.1086/509925. [DOI] [PubMed] [Google Scholar]
- 38.Olofsson S., Brittain-Long R., Andersson L.M., Westin J., Lindh M. PCR for detection of respiratory viruses: seasonal variations of virus infections. Exp Rev Anti Infect Ther. 2011;9:615–626. doi: 10.1586/eri.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funk S., Gilad E., Watkins C., Jansen V.A. The spread of awareness and its impact on epidemic outbreaks. Proc Natl Acad Sci USA. 2009;106:6872–6877. doi: 10.1073/pnas.0810762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.