Highlights
-
•
Sambucus FormosanaNakai extract reduced cytopathicity and virus yield in HCoV-NL63-infected cells.
-
•
Among phenolic acid constituents, caffeic acid, chlorogenic acid and gallic acid sustained the anti-HCoV-NL63 activity.
-
•
Sambucus FormosanaNakai extract and caffeic acid concentration-dependently inhibited HCoV-NL63 attachment onto cells.
Keywords: Human coronavirus NL63, Sambucus FormosanaNakai, Caffeic acid, Antiviral, Virus yield, Attachment inhibition
Abstract
Human coronavirus NL63 (HCoV-NL63), one of the main circulating HCoVs worldwide, causes respiratory tract illnesses like runny nose, cough, bronchiolitis and pneumonia. Recently, a severe respiratory illness outbreak of HCoV-NL63 has been reported in a long-term care facility. Sambucus FormosanaNakai, a species of elderberry, is a traditional medicinal herb with anti-inflammatory and antiviral potential. The study investigated the antiviral activity of Sambucus FormosanaNakai stem ethanol extract and some phenolic acid constituents against HCoV-NL63. The extract was less cytotoxic and concentration-dependently increased anti-HCoV-NL63 activities, including cytopathicity, sub-G1 fraction, virus yield (IC50 = 1.17 μg/ml), plaque formation (IC50 = 4.67 μg/ml) and virus attachment (IC50 = 15.75 μg/ml). Among the phenolic acid constituents in Sambucus FormosanaNakai extract, caffeic acid, chlorogenic acid and gallic acid sustained the anti-HCoV-NL63 activity that was ranked in the following order of virus yield reduction: caffeic acid (IC50 = 3.54 μM) > chlorogenic acid (IC50 = 43.45 μM) > coumaric acid (IC50 = 71.48 μM). Caffeic acid significantly inhibited the replication of HCoV-NL63 in a cell-type independent manner, and specifically blocked virus attachment (IC50 = 8.1 μM). Therefore, the results revealed that Sambucus Formosana Nakai stem ethanol extract displayed the strong anti-HCoV-NL63 potential; caffeic acid could be the vital component with anti-HCoV-NL63 activity. The finding could be helpful for developing antivirals against HCoV-NL63.
1. Introduction
Human coronavirus (HCoV) NL63, the member of the genus alphacoronavirus in the Coronaviridae family, is one of common human coronaviruses (Li and Lin, 2013; Huang et al., 2017). HCoV-NL63 genome is a positive-strand RNA with near 27.5 kb nucleotides containing 5′ untranslated regions (UTR), ORF1a/b, spike(S), ORF3, envelope(E), membrane(M), nucleoprotein (N), and 3′UTR (Geng et al., 2012). ORF1a/b encodes overlapping replicase 1a and 1ab via the −1 ribosomal frameshift at the nucleotide 12,424. The papain-like (PLpro) and 3C-like (3CLpro) proteases embedded within replicase 1a and 1ab process cis- and trans-cleavage activity to divide replicase 1a and 1ab into nonstructural proteins (nsps) that modulate in viral RNA replication. Among common human coronaviruses like HCoV-229E, HCoV-HKU1, and HCoV-OC43, HCoV-NL63 is one of main circulating HCoVs in the fall and winter worldwide, causing mild upper respiratory tract illnesses like runny nose, cough and sore throat in young children, young adults and elderly (Cui et al., 2011; Dijkman et al., 2012). Interestingly, HCoV-NL63 has been frequently detected than other HCoVs, influenza viruses, and rhinovirus in the specimens from the young adults with acute respiratory infection (cough and body aches or chills or fever/feverishness) (Davis et al., 2018). Importantly, HCoV-NL63 is also associated with lower respiratory tract illnesses, such as pneumonia and bronchiolitis in young children and elderly (Huang et al., 2017). HCoV-NL63 infection is the high prevalence (8.4%) in hospitalized patients with pneumonia in winter. Recently, a severe respiratory illness outbreak in a long-term care facility in Louisiana has been reported to be associated with the HCoV-NL63 infection in winter 2017 (Hand et al., 2018). Among 20 cases aged from 66 to 96, 6 patients with pneumonia have to be hospitalized and 3 patients are dead. Moreover, screening children with acute undifferentiated febrile illness in rural Haiti indicates that HCoV-NL63 is identified in the blood samples from four patients aged from 3 to 10 years who have no respiratory symptom, but two cases have headache and the others exhibit influenza virus causing abdominal symptoms (Beau De Rochars et al., 2017). Therefore, HCoV-NL63 is the notable pathogen as the etiology of mild and severe respiratory diseases, even acute undifferentiated febrile illness.
Sambucus FormosanaNakai, also known by Sambucus chinensis Lindl and Sambucus javanica, is a species of elderberry, belongs to family Adoxaceae, and grows in subtropical and tropical Asian areas, including Taiwan, China, Japan, Cambodia, India etc. (Lin and Tome, 1988; Yang and Chiu, 1998; Hong et al., 2013). Sambucus FormosanaNakai is a traditional medicinal herb in Taiwan for reducing inflammation, enhancing circulation, and treating rheumatoid and low back pain, neuritis, dermatitis, and infection diseases (Yang and Chiu, 1998). The chemical components of S.ambucus FormosanaNakai include sambuculin A, oleanolic acid, α-amyrin and β-amyrin, β-sitosterol, ursolic acid, pomolic acid, lupeol palmitate, glycyrrhetic acid, phenolic acid constituents, and flavonoids (Chen et al., 2001, 2019; Liao et al., 2006; Lin and Tome, 1988; Yang and Lin, 2004; Zhang et al., 2010). In addition, phenolic acid constituents of S.ambucus FormosanaNakai, including caffeic acid, caffeotannic acid, chlorogenic acid, coumaric acid, ferulic acid, and gallic acid, have been identified as the members of the most important active components with anti-inflammatory, anti-tumor and anti-hepatotoxic activities (Chen et al., 2001; Liao et al., 2006; Yang and Lin, 2004; Zhang et al., 2010). Active components of Sambucus FormosanaNakai are similar to those of other Sambucus species, including Sambucus nigra L. and Sambucus lanceolata, which process antioxidant, antiradical, antiviral, antimicrobial, and anti-inflammatory activities (Barak et al., 2001; Krawitz et al., 2011; Mandrone et al., 2014; Pinto et al., 2017; Pliszka, 2017; Porter and Bode, 2017; Turek and Cisowski, 2007). Especially, the extract of Sambucus nigra L. exerts the antiviral activity against influenza A and B viruses, human immunodeficiency virus, and the herpes simplex virus type 1 (Krawitz et al., 2011; Serkedjieva et al., 1990; Zakay-Rones et al., 1995; Amoros et al., 1992; Mahmood et al., 1993; Roschek et al., 2009). Sambucus nigraphenolic acids like caffeic acid show the highly inhibitory effect on the in vitro replication of influenza A virus (Porter and Bode, 2017; Utsunomiya et al., 2014). Since Sambucus FormosanaNakai contains such antiviral active components in the extract of Sambucus nigra L., the antiviral activity of Sambucus FormosanaNakai is worthy to further investigate.
The study explored the anti-HCoV-NL63 activity of Sambucus FormosanaNakai stem ethanol extract and some markers of its phenolic acid constituents, like caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, gallic acid. The study indicated the inhibitory activity of Sambucus FormosanaNakai extract and its phenolic acid constituents on HCoV-NL63 induced cytopathic effect, virus yield, and the early stage of HCoV-NL63 replication in concentration-dependent and cell-type independent manners.
2. Materials and methods
2.1. Viruses and cells
HCoV-NL63 provided by Dr. Lia van der Hoek (Academic Medical Center, The Netherlands) was amplified in LLC-MK2 cells, as described in our prior study (Huang et al., 2017). LLC-MK2 cells were cultured in the Minimum Essential Medium (MEM) containing 2 mM L-Glutamine, 50 μg/ml penicillin, 50 μg/ml streptomycin, and 5% fetal bovine serum (FBS) at 37 ℃ in a 5% CO2 incubator. LLC-MK2 cells were further used to perform antiviral assays and examine antiviral mechanism. Human airway Calu-3 cells were cultured in MEM supplemented 10% FBS and antibiotics mentioned above, maintained at the 80–90% confluent, and then also used to confirm the antiviral activity of indicated phenolic acid constituents.
2.2. Preparation of Sambucusformosana Nakai stem ethanol extract
Dried stems of Sambucus Formosana Nakai (Supplemental Fig. 1A) were purchased from the medicinal herb pharmacy in Taichung, and identified as described in Flora of Taiwan (Yang and Chiu, 1998). Dried stem slices (Supplemental Fig. 1B) were soaked in 95% ethanol with the sonication for 2 h; the stem ethanol extract was filtered by Whatman No. 1 paper, then lyophilized in an IWAKI FDR-50 P freeze dryer, as described in our previous study (Wang et al., 2012). The powder of Sambucus Formosana Nakai stem ethanol extract was kept in the sterile bottle; the stock solution of 10 mg/ml in dimethyl sulfoxide was prepared and stored in −20 °C freezer and the test solutions of the stem extract were diluted by the media. The phenolic acid constituents such caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, and gallic acid, were purchased from Sigma-Aldrich company.
2.3. Cell viability assay
LLC-MK2 cells (3 × 104 cells/well) were cultured in the 96-well plates overnight, quintuplicate treated with Sambucus Formosana Nakai stem ethanol extract or its phenolic acid constituents (caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, and gallic acid) for 2 days, and then incubated with 0.5 mg/ml 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for additional 4 h. After the cell lysis by isopropanol, yielding absorbance (OD570-630) at the wavelength of 570 nm and 630 nm was assessed using a micro-ELISA reader. Cell viability (%) and cytotoxic concentration showing 50% toxic effect (CC50) were subsequently determined, as previously described (Wang et al., 2012).
2.4. Cytopathic effect (CPE) reduction, cell cycle, and virus yield
LLC-MK2 cells (3 × 105 cells/well) were cultured in the 6-well plates overnight, and then infected by 3 × 104 pfu (plaque formation unit) HCoV-NL63, representing as a MOI (multiplicity of infection) of 0.1 to significantly induce viral cytopathic effect (CPE) in LLC-MK2. The cells were simultaneously added with HCoV NL63, and treated with Sambucus Formosana Nakai stem ethanol extract at the concentrations of 0, 1, 10, and 50 μg/ml or the phenolic acid constituents at the concentrations of 0, 10, 50, and 100 μM, respectively. After the 36-h incubation at 37 ℃ and 5% CO2, HCoV NL63-induced cytopathic effect (CPE) with cell swelling, rounding, vacuoles, and eventual detachment was photographed using microscope, in which vacuoles in CPE from HCoV NL63-infected LLC-MK2 cells were more predominant at 37 °C (Lednicky et al., 2013). In the cell cycle assay, the cells were harvested 36 h post infection, stained with propidium iodide, and then examined using flow cytometry, as described in our prior study (Wang et al., 2018). To determine the production of progeny virions (virus yield assay), the cultured media were collected for determined the virus titers using plaque assay. LLC-MK2 cell monolayer in the well of 6-well plates was added with 100 μl of serial 10-fold dilutions of above cultured media. After a 1-h incubation, the cell monolayer was overlaid by the medium containing 0.75% agarose (3 ml per well) for 2-day incubation at 37 °C in a CO2 incubator to allow the plaque formation. Finally, the cell monolayer was stained with the solution of naphthol blue-black dye and plaque number calculated. Fifty percent (50%) inhibitory concentration (IC50) for virus yield reduction by the stem ethanol extract and the phenolic acid constituents was determined.
2.5. Plaque formation inhibition, virucidal activity, and attachment reduction assays
In the plaque formation inhibition assays, LLC-MK2 cell monolayer cultured in 6-well plates were infected with 200 pfu of HCoV-NL63 that were the maximum number of plaques counted in the well of 6-well plates. After the addition with HCoV NL63, the cells were immediately treated with the ethanol extract at the concentrations of 0, 1, 5, and 10 μg/ml, caffeic acid and chlorogenic acid at the concentrations of 0, 10, 50, and 100 μM, respectively. After a 1-h incubation at 37 °C in a 5% CO2 incubator, the mixtures were removed from the wells; the cell monolayer was cultured with the medium containing 0.75% agarose and performed by the plaque assay mentioned above. In the virucidal assay, HCoV-NL63 (2 × 106 pfu) was added into in the Eppendorf tube and directly treated with the ethanol extract, caffeic acid, or chlorogenic acid for 1 h at 37 °C. For minimizing the antiviral effect of indicated agents in the cells, 100 μl (near 200 pfu HCoV-NL63) of the 10000-fold dilution from the mixtures of virus and the extract or phenolic acids was added to the MK2 cell monolayer in the 6-well plate to determining the residual viral infectivity using the plaque assay described above. Virucidal activity was calculated based on the comparison of the residual infectivity of HCoV-NL63 in the treated groups with the un-treated group. In the HCoV-NL63 attachment assay, LLC-MK2 cell monolayer in 6-well plate was placed at 4 °C for 1 h, infected with HCoV-NL63 (200 pfu), and then immediately added with the ethanol extract, caffeic acid or chlorogenic acid. After an additional 1-h incubation at 4 °C, the mixture of virus and the extract or phenolic acids was removed from the well; the cell monolayer was overlaid with MEM medium containing0.75% agarose at 37 °C in a 5% CO2 incubator, and then executed by plaque assay, as mentioned above. The attachment inhibition was analyzed according to the ratio of the plaque number in treated groups to the un-treated group.
2.6. Inhibitory effect of caffeic acid on viral infectivity in human airway epithelial cells using immunofluorescent staining
To measure the inhibitory activity of caffeic acid on the replication of HCoV-NL63 in human airway epithelial cells, Calu-3 cells (1 × 105 cells/well) were cultured in the 6-well plates overnight, and then infected by 5 × 103 pfu HCoV-NL63 (MOI = 0.05). After the addition of HCoV NL63, the cells were simultaneously treated with caffeic acid at the concentrations of 0, 10, and 50 μM. After the 36-h incubation at 32 ℃ in a 5% CO2 incubator, swelling, rounding, and eventual de-attachment in HCoV-NL63-induced CPE were more predominant at 32 °C (Lednicky et al., 2013), and then the images were recorded by microscope. Subsequently, the cells were fixed with 4% paraformaldehyde solution in PBS for 30 min, incubated with the quench buffer (50 mM NH4CI) for 15 min, permeabilized and blocked using the cell perforation and blocking solution containing 1% albumin bovine (Affymetrix) plus triton X-100 (ThermoFisher) for 4 h at 4 °C, and then reacted with HCoV-NL63-immunized sera in 1% BSA (1:2000) overnight at 4 °C and secondary antibody Alexa Fluor anti-mouse IgG in 1% BSA (1:3000) for 1 h at 4 °C (ThermoFisher). After staining with 4′,6-diamidino-2-phenylindole (DAPI, ThermoFisher) 20 min at room temperature, the images of mock, infected, and infected/treated cells were photographed using fluorescent microscopy. The infectivity was represented as the ratio of red fluorescent signals (HCoV-NL63-positive cells) to blue fluorescent signals (total nucleuses) was calculated by Image J.
2.7. Statistical analysis
The p value was calculated based ANOVA using SPSS program and Student t-test with the data from three independent experiments. When the p value was less than 0.05, the result of the assay was statistically significant.
3. Results
3.1. Antiviral activity of Sambucusformosana Nakai stem ethanol extract against HCoV-NL63
Sambucus Formosana Nakai stem ethanol extract had a low cytotoxicity with a CC50 value of 180.62 μg/ml to LLC-MK2 cells (Supplemental Fig. 2, Table 1 ). Subsequently, anti-HCoV-NL63 ability of the stem ethanol extract was assessed with cytopathicity, cell cycle and virus yield assays (Fig. 1, Fig. 2 ). Sambucus Formosana Nakai extract concentration-dependently reduced cytopathicity in HCoV-NL63 infected cells (Fig. 1A), in which vacuolation in infected cells at 37 °C appeared more predominantly, as described in the prior report (Lednicky et al., 2013). The extract also significantly decreased the sub-G1 fraction in infected cells (Fig. 1B). In addition, Sambucus FormosanaNakai extract inhibited the in vitro production of progeny HCoV-NL63 by concentration-dependent manners. The plaque assay indicated that the IC50 value of Sambucus FormosanaNakai extract on the virus yield was 1.17 μg/ml (Fig. 2). Remarkably, the results demonstrated that Sambucus FormosanaNakai stem ethanol extract served the significantly antiviral activity against HCoV-NL63.
Table 1.
Cytotoxicity and anti-HCoV-NL63 activity of Sambucus Formosana Nakai stem ethanol extract and the phenolic acid constituents.
| Cytotoxicity (CC50) | Virus yield (IC50) | Plaque formation (IC50) | Virus attachment (IC50) | |
|---|---|---|---|---|
| Stem ethanol extract | 180.62 ± 63.04 μg/mL | 1.17 ± 0.75 μg/mL | 4.67 ± 1.21 μg/mL | 15.75 ± 6.65 μg/mL |
| Caffeic acid | >500 μM | 3.54 ± 0.77 μM | 5.40 ± 1.20 μM | 8.10 ± 0.05 μM |
| Chlorogenic acid | >500 μM | 43.45 ± 6.01 μM | 44.38 ± 3.64 μM | – |
| Gallic acid | >500 μM | 71.48 ± 18.40 μM | – | – |
Fig. 1.
Inhibitory effects of Sambucus Formosana Nakai extract on viral cytopathicity and Sub-G1 fraction in HCoV-NL63 infected cells. HCoV-NL63 at MOI of 0.1 was mixed with the extract, and immediately added to LLC-MK-2 cell culture. Virus-induced cytopathic effect was photographed 36 h post-infection by microscopy (A). Infected cells in the presence or absence of the extract were harvested 36 hpi, stained using propidium iodide, and then examined using flow cytometry. The flow cytometry histograms (top) and the percentage of sub-G1 phase (bottom) in HCoV-NL63 infected cells was displayed (B). **, p value < 0.01 compared with mock-treated infected cells.
Fig. 2.
Reduction of HCoV-NL63 yield in MK-2 cells by Sambucus Formosana Nakai extract. Supernatant of HCoV-NL63-infected cells in the presence or absence of the extract was harvested 36 hpi and HCoV-NL63 yield in the supernatant was determined by plaque assay (A). The rate of virus yield reduction was calculated based on the ratio of loss particle number to mock-treated group (B). **, p value < 0.01. compared with mock-treated infected cells.
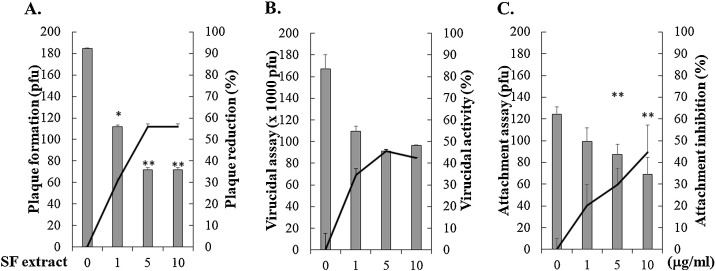
3.2. Inhibitory effect of Sambucusformosana Nakai extract on the viral plaque formation and attachment
To discover the inhibitory action of Sambucus FormosanaNakai extract on stages of HCoV-NL63 replication, plaque formation, virucidal activity, and virus attachment assays were performed using the plaque assays (Fig. 3 , Table 1). Sambucus FormosanaNakai extract meaningfully inhibited the plaque formation with an IC50 value of 4.67 μg/ml (Fig. 3A). In the virucidal assay, Sambucus FormosanaNakai extract at 1, 5, and 10 μg/ml had a slight virucidal activity with lower than 50% inhibition to interfere with the HCoV-NL63 particle infectivity compared to the mock control (Fig. 3B). In the virus attachment assay, Sambucus FormosanaNakai extract concentration-dependently reduced the HCoV-NL63 attachment onto LLC-MK2 cell monolayer in 6-well plates incubated at 4 °C, which result demonstrated that the ethanol extract had a influentially inhibitory effect on HCoV-NL63 attachment with an IC50 value of 15.75 μg/ml (Fig. 3C). The results demonstrated that Sambucus FormosanaNakai extract specifically suppressed the viral plaque formation and virus attachment during HCoV-NL63 replication.
Fig. 3.
Effects of Sambucus Formosana Nakai extract on plaque formation, virucidal activity and virus attachment. MK-2 cell monolayer was infected with HCoV-NL63, simultaneously treated with the extract for 1 h, and then covered with the agarose overlay medium. After 3-day incubation at 37 °C in a CO2 incubator, plaques were determined after crystal violet staining. The inhibitory activity of the extract on the plaque formation was according to on the ratio of loss plaque number to mock-treated group (A). In the virucidal assay, the extract was mixed with HCoV-NL63 (106 pfu), then incubated at 37 °C for 1 h. The extract/virus mixture was diluted by 1000-fold dilution and examined for the residual infectivity by plaque assay (B). In the attachment assay, HCoV-NL63 was mixed with the extract, then immediately added onto MK2 cell monolayer for 1 h at 4 °C. After washing, the cell monolayer was overlaid with 0.75% agarose medium for 3 days at 37 °C in CO2 incubator. Attachment inhibition was determined based on the residual plaques (C). *, p value < 0.05; **, p value < 0.01 compared with mock-treated cells.
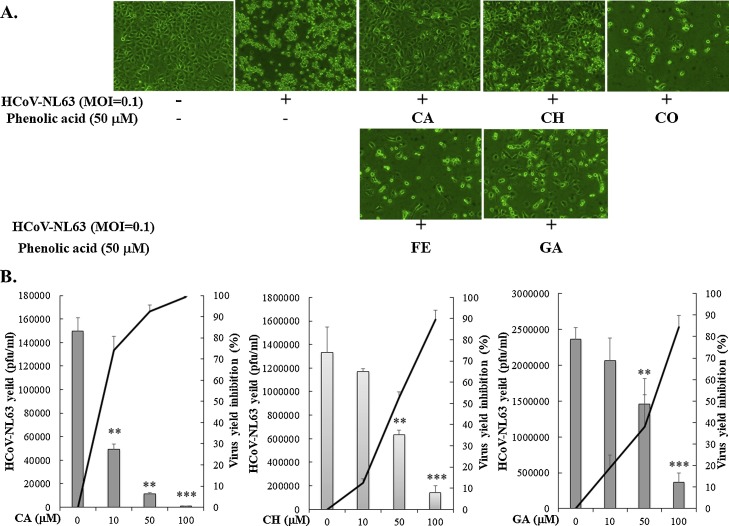
3.3. Anti-HCoV-NL63 activity of the phenolic acid constituents
The phenolic acid constituents were rich in the extract of Sambucus FormosanaNakai and Sambucus australis, and the presence of chlorogenic acid, caffeic acid, coumaric acid and ferulaic acid was identified as the major compounds using LC-MS/MS analyses (Benevides Bahiense et al., 2017; Zhang et al., 2010). Subsequently, caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, and gallic acid represented the key phenolic acid constituents in the ethanol stem extract of Sambucus FormosanaNakai, as further assessed the cytotoxicity to LLC-MK2 cells and the antiviral activity against HCoV-NL63 (Supplemental Figs. 2 and 4). The markers of phenolic acid constituents in Sambucus Formosana Nakai extract were less cytotoxic to LLC-MK2 cells, in which CC50 values of caffeic acid, chlorogenic acid, and gallic acid were greater than 500 μM (Supplemental Fig. 2, Table 1). In the inhibitory assay of HCoV-NL63 induced cytopathic effect, caffeic acid, chlorogenic acid, and gallic acid, but not coumaric acid and ferulic acid, diminished the cytopathicity in the infected cells (Fig. 4 A). The antiviral activity of the phenolic acid constituents in the in vitro production of HCoV-NL63 was ranked in the following order of virus yield reduction: caffeic acid (IC50 = 3.54 μM) > chlorogenic acid (IC50 = 43.45 μM) > coumaric acid (IC50 = 71.48 μM) (Fig. 4B, Table 1). The results verified that the phenolic acid constituents, like caffeic acid, chlorogenic acid, and gallic acid exhibited the prominent antiviral activity against HCoV-NL63, as potential anti-HCoV-NL63 components in the Sambucus FormosanaNakai extract.
Fig. 4.
Inhibitory effects of the phenolic acid constituents on viral cytopathicity and virus yield in HCoV-NL63 infected cells. HCoV-NL63 at MOI of 0.1 was added to LLC-MK-2 cell culture and then immediately treated with the phenolic acid constituents. Virus-induced cytopathic effect was photographed 36 h post-infection by microscopy (A). Supernatant of HCoV-NL63-infected/treated cells was harvested 36 hpi; virus yield in supernatant was determined plaque assay. The rate of virus yield reduction was calculated based on the ratio of loss particle number to mock-treated group (B). CA, caffeic acid; CH, chlorogenic acid; CO, coumaric acid; FE, ferulic acid; GA, gallic acid. **, p value < 0.01, ***, p value < 0.001 compared with mock-treated infected cells.
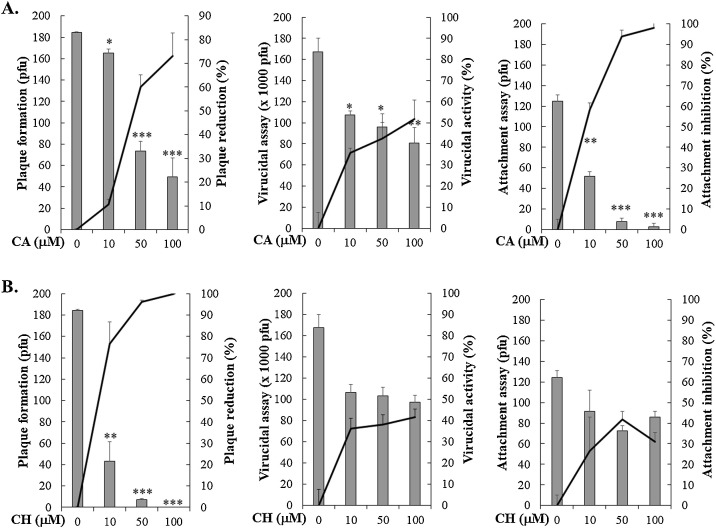
3.4. Caffeic acid, a phenolic acid constituent in Sambucus spp., potently inhibits HCoV-NL63 attachment onto cells
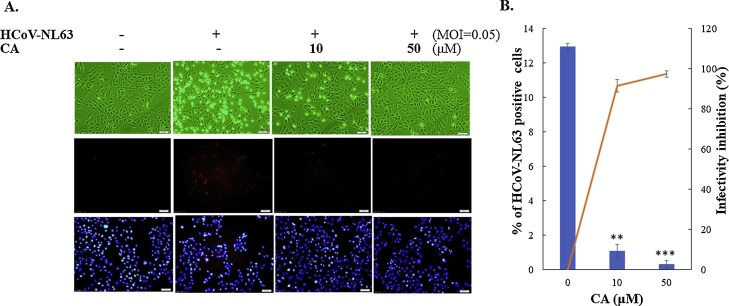
To examine the antiviral mechanism of caffeic acid and chlorogenic acid against HCoV-NL63, the assays of plaque formation, virucidal activity and virus attachment were subsequently performed (Fig. 5 , Table 1). Caffeic acid had a stronger inhibitory activity on the plaque formation than chlorogenic acid, in which the IC50 values on the plaque formation were 5.4 μM for caffeic acid and 44.38 μM for chlorogenic acid, respectively (Fig. 5). Caffeic acid, but not chlorogenic acid, concentration-dependently served the virucidal activity (IC50 = 91.3 μM) and powerfully reduced HCoV-NL63 attachment to the cell surface (IC50 of 8.1 μM), respectively (Fig. 5). In addition, infectivity inhibition assay with human airway epithelia Calu-3 cells was performed when HCoV-NL63-infected cells with a MOI of 0.05 were treated with 10 and 50 μM caffeic acid after a 36-h incubation at 32 °C. Microscopic photography indicated that swelling and rounding were observed in HCoV-NL63-infected Calu-3 cells (Fig. 6 A, top), as described in the previous study (Lednicky et al., 2013). Caffeic acid at 10 and 50 μM significantly lessening viral CPE (Fig. 6A, top). Immunofluorescent staining demonstrated that caffeic acid concentration-dependently reduced HCoV-NL63 infectivity determined according to the ratio of HCoV-NL63-positive cells to total cells stained with DAPI (Fig. 6A, middle and bottom). The inhibitory assay with immunofluorescent staining on HCoV-NL63 infectivity indicated that caffeic acid had a potent antiviral activity against the replication of HCoV-NL63 in Calu-3 cells (IC50 = 0.2 ± 0.1 μM) (Fig. 6B). The results revealed that caffeic acid displayed the potent anti-HCoV-NL63 activity in a cell-type independent manner, specifically inhibiting on the attachment stage of HCoV-NL63 replication.
Fig. 5.
Effects of caffeic acid and chlorogenic acid on plaque formation, virucidal activity and virus attachment. MK-2 cell monolayer was infected with HCoV-NL63, simultaneously treated with the caffeic acid or chlorogenic acid for 1 h, and then covered with the agarose overlay medium for 3-day at 37 °C in a CO2 incubator. After crystal violet staining, the inhibitory activity of caffeic acid and chlorogenic acid on the plaque formation was according to on the ratio of loss plaque number to mock-treated group (A). In the virucidal assay, the caffeic acid or chlorogenic acid was mixed with HCoV-NL63 (106 pfu), then incubated at 37 °C for 1 h. The 1000-fold dilution of the compound/virus mixture was examined for the residual infectivity by plaque assay (B). In the attachment assay, MK2 cell monolayer was infected with HCoV-NL63 (100 pfu), immediately treated with the caffeic acid or chlorogenic acid for 1 h at 4 °C, washed, and overlaid with 0.75% agarose medium for 3 days at 37 °C in CO2 incubator. Attachment inhibition was determined based on the residual plaques (C). CA. caffeic acid; CH, chlorogenic acid. *, p value < 0.05;**, p value < 0.01; ***, p value < 0.001 compared with un-treated infected cells.
Fig. 6.
Inhibition of HCoV-NL63 infectivity in human airway epithelial cells by caffeic acid. Calu-3 cells were infected with HCoV-NL63 at a MOI of 0.05 and immediately treated with caffeic acid for 36 h at 32 °C. Images of virus-induced CPE effect were photographed by a light microscope (A, top). In addition, mock, infected, and infected/treated cells were performed using immunofluorescence staining anti-HCoV-NL63 immunized sera and secondary antibody Alexa Fluor anti-mouse IgG (A, middle); total cells were stained with DAPI (A, bottom). Infectivity was determined according to the ratio of HCoV-NL63-positive cells to total cells (B). **, p value < 0.01; ***, p value < 0.001 compared with un-treated infected cells.
4. Discussion
This study was the first report on the antiviral efficacy of Sambucus FormosanaNakai extract and its related phenolic acid constituents against HCoV-NL63. Sambucus FormosanaNakai extract exhibited the low cytotoxicity and markedly decreased cytopathic effect and sub-G1 arrest in HCoV-NL63-infected cells, in which was associated with the virus yield reduction in a concentration-dependent manner (Fig. 1, Fig. 2, Table 1). Moreover, Sambucus FormosanaNakai extract showed the potent antiviral activity against HCoV-NL63 with the IC50 values in low microg/ml ranges, such IC50 of 1.17 μg/ml for virus yield, IC50 of 4.67 μg/ml for plaque formation, and IC50 of 15.75 μg/ml for virus attachment. The results were consistent with the previous reports in that Sambucus spp., such Sambucus nigra L. served the antiviral properties against influenza A and B viruses, and the herpes simplex virus type1 (Krawitz et al., 2011; Serkedjieva et al., 1990; Zakay-Rones et al., 1995). In addition, Sambucus juice was the key recipe for the "Virus Blocking Factor" that processed the in-vitro antiviral activity against many respiratory viral infections, including influenza A H1N1, rhinovirus B subtype 14, respiratory syncytial virus, parainfluenzavirus subtype 3, and adenovirus C subtype 5 (Fal et al., 2016). Sambucus nigra L. has been recognized as was a potentially safer alternative to treat upper respiratory symptoms, such common cold and influenza (Hawkins et al., 2019). Thus, Sambucus FormosanaNakai might be the alternative medicinal herb for caring the respiratory viral infections, especially coronavirus-associated common cold.
Among six phenolic acid constituents in the Sambucus FormosanaNakai extract, caffeic acid had the highest anti-HCoV-NL63 potency with the inhibitory effect on the virus yields (IC50 = 3.54 μM), plaque formation (IC50 = 5.40 μM), and virus attachment (IC50 = 8.10 μM) (Fig. 4, Fig. 5, Table 1). Caffeic acid has also been demonstrated the antiviral activity against HCoV-NL63 in a cell-type independent manner (Fig. 6). In addition, chlorogenic acid and gallic acid exhibited antiviral activity against HCoV-NL63 (Fig. 4, Fig. 5, Table 1). The IC50 value on the inhibition of virus yield was 43.45 μM for chlorogenic acid, and 71.48 μM for gallic acid, respectively. The phenolic acid constituents have been identified as the major components in the Sambucus FormosanaNakai extract and Sambucus australis by LC-MS/MS analyses (Benevides Bahiense et al., 2017; Zhang et al., 2010), therefore caffeic acid, chlorogenic acid and gallic acid might be the key components with anti-HCoV-NL63 activity in the Sambucus FormosanaNakai extract.
Caffeic acid has been reported to process the antiviral activity against hepatitis B and C viruses, influenza A virus, and herpes simplex virus (Wang et al., 2019; Shen et al., 2018; Tanida et al., 2015; Utsunomiya et al., 2014; Ikeda et al., 2011; Langland et al., 2018). Caffeic acid reduced the production of hepatitis C virus in vitro via the initial stage of viral replication (Tanida et al., 2015). The antiviral mechanism of caffeic acid against hepatitis C virus was also associated with the activation of p62-mediated Keap1/Nrf2 signaling pathway for the HO-1-dependent production of interferon α, which significantly suppressed the replication of hepatitis C virus (Shen et al., 2018). Caffeic acid markedly decreased the virus yield and cytopathic effect in influenza A virus-infected cells, particular during the period within 3 h post-infection, suggesting that caffeic acid works at the early stages of influenza A virus infection (Utsunomiya et al., 2014). In addition, caffeic acid had no virucidal effect, but significantly affected the production of progeny herpes simplex virus and the binding attachment to the heparan sulfate proteoglycans on cell surface (Ikeda et al., 2011; Langland et al., 2018). Interestingly, HCoV-NL63 has been demonstrated to utilize the heparan sulfate proteoglycans as the co-receptor for attachment to target cells (Milewska et al., 2014). Caffeic acid has also been identified to exhibit a high inhibitory effect on the enzymatic activity of angiotensin converting enzyme (ACE) (Chiou et al., 2017); docking studies revealed that caffeic acid also presented the good docking score with the hydrogen bond interactions with the residues in the activity site of ACE2 (Zozimus Divya Lobo et al., 2017). Thus, the inhibitory mechanism of caffeic acid on HCoV-NL63 attachment and infection to cells might be due to that caffeic acid directly target and interfere the binding interaction of HCoV-NL63 with heparan sulfate proteoglycans (co-receptor) and ACE2 (receptor) on cell surface.
Chlorogenic acid and gallic acid have also been demonstrated to suppress the in vitro and in vivo replication of influenza A virus, enterovirus 71 and hepatitis B and C viruses (Ding et al., 2017; Li et al., 2013; Wang et al., 2009; You et al., 2018; Govea-Salas et al., 2016). Chlorogenic acid specifically inhibited the neuraminidase activity of influenza A viruses H1N1 and H3N2 that was the crucial mechanism of chlorogenic acid for blocking the release of progeny virions from infected cells (Ding et al., 2017). Gallic acid served the antioxidant capacity that linked with down-regulating the genomic RNA and proteins expression of hepatitis C virus (Govea-Salas et al., 2016). Therefore, the phenolic acid constituents of Sambucus Formosana Nakai extract, like caffeic acid, chlorogenic acid and gallic acid, play the key antiviral components against HCoV-NL63 and the other viruses.
The study demonstrated the potent anti-HCoV-NL-63 activity of Sambucus FormosanaNakai extract through the significant reduction of virus yield, plaque formation and virus attachment. Caffeic acid, chlorogenic acid and gallic acid were reported as the phenolic acid constituents in the Sambucus FormosanaNakai extract, exhibiting the antiviral capacity with reducing the production of progeny HCoV-NL63 particles in vitro. Moreover, caffeic acid plays the important component with antiviral activity, as suggested to influence the binding of HCoV-NL63 to the co-receptors (such heparan sulfate proteoglycans) and receptor (ACE2). Like Sambucus nigra L., Sambucus FormosanaNakai might process the antiviral features against the broad spectrum of human respiratory coronaviruses, as useful for developing the antiviral agents in the future.
Declaration of Competing Interest
All authors declare no potential conflict of interest.
Acknowledgements
This study was supported by China Medical University under the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education, Taiwan (CHM106-6-2 and CMRC-CHM-2). This project was also funded by grants from the Ministry of Science and Technology, Taiwan (MOST107-2923-B-039-001-MY3, MOST105-2320-B-039-053-MY3), China Medical University (CMU106-BC-1, CMU106-ASIA-06, CMU107-ASIA-12, and CMU107-S-14).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2019.197767.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Amoros M., Simões C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992;55(12):1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- Barak V., Halperin T., Kalickman I. The effect of sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. inflammatory cytokines. Eur. Cytokine Networks. 2001;12(2):290–296. [PubMed] [Google Scholar]
- Benevides Bahiense J., Marques F.M., Figueira M.M., Vargas T.S., Kondratyuk T.P., Endringer D.C., Scherer R., Fronza M. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharm. Biol. 2017;55(1):991–997. doi: 10.1080/13880209.2017.1285324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Liu D.L., Wang W., Lv X.M., Li W., Shao L.D., Wang W.J. Bioactive triterpenoids from Sambucus javanica Blume. Nat. Prod. Res. 2019;10(2):1–6. doi: 10.1080/14786419.2019.1596092. [DOI] [PubMed] [Google Scholar]
- Chen W., Li K.Q., Xiong X.J., Zhang J.H. Research on the effective chemical constituents of Sumbucus chinensis Lindl. against hepatitis. J Nanchang Univ. 2001;25(2):165–167. [Google Scholar]
- Chiou S.Y., Sung J.M., Huang P.W., Lin S.D. Antioxidant, antidiabetic, and antihypertensive properties of echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food. 2017;20(2):171–179. doi: 10.1089/jmf.2016.3790. [DOI] [PubMed] [Google Scholar]
- Cui L.J., Zhang C., Zhang T., Lu R.J., Xie Z.D., Zhang L.L., Liu C.Y., Zhou W.M., Ruan L., Ma X.J., Tan W.J. Human coronaviruses HCoV-NL63 and HCoV-HKU1 in hospitalized children with acute respiratory infections in Beijing. China Adv. Virol. 2011;2011 doi: 10.1155/2011/129134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B.M., Foxman B., Monto A.S., Baric R.S., Martin E.T., Uzicanin A., Rainey J.J., Aiello A.E. Human coronaviruses and other respiratory infections in young adults on a university campus: prevalence, symptoms, and shedding. Influenza Other Respi. Viruses. 2018;12:582–590. doi: 10.1111/irv.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D., Yang Q., Malécot V., Boufford D.E. Flora of China. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria; Cambridge, MA: 2013. Sambucus javanica. [Google Scholar]
- Dijkman R., Jebbink M.F., Gaunt E., Rossen J.W., Templeton K.E., Kuijpers T.W., van der Hoek L. The dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 2012;53(2):135–139. doi: 10.1016/j.jcv.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Cao Z., Cao L., Ding G., Wang Z., Xiao W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017;7:45723. doi: 10.1038/srep45723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fal A.M., Conrad F., Schönknecht K., Sievers H., Pawińska A. Antiviral activity of the “Virus Blocking Factor” (VBF) derived i.a. From Pelargonium extract and Sambucus juice against different human-pathogenic cold viruses in vitro. Wiad. Lek. 2016;69(3 pt 2):499–511. [PubMed] [Google Scholar]
- Geng H., Cui L., Xie Z., Lu R., Zhao L., Tan W. Characterization and complete genome sequence of human coronavirus NL63 isolated in China. J. Virol. 2012;86(17):9546–9547. doi: 10.1128/JVI.01457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govea-Salas M., Rivas-Estilla A.M., Rodríguez-Herrera R., Lozano-Sepúlveda S.A., Aguilar-Gonzalez C.N., Zugasti-Cruz A., Salas-Villalobos T.B., Morlett-Chávez J.A. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp. Ther. Med. 2016;11(2):619–624. doi: 10.3892/etm.2015.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand J., Rose E.B., Salinas A., Lu X., Sakthivel S.K., Schneider E., Watson J.T. Severe respiratory illness outbreak associated with human coronavirus NL63 in a long-term care facility. Emerg. Infect. Dis. 2018;24(10):1964–1966. doi: 10.3201/eid2410.180862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.H., Su M.C., Tien N., Huang C.J., Lan Y.C., Lin C.S., Chen C.H., Lin C.W. Epidemiology of human coronavirus NL63 infection among hospitalized patients with pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 2017;50(6):763–770. doi: 10.1016/j.jmii.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Tsujimoto K., Uozaki M., Nishide M., Suzuki Y., Koyama A.H., Yamasaki H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011;28(4):595–598. doi: 10.3892/ijmm.2011.739. [DOI] [PubMed] [Google Scholar]
- Krawitz C., Mraheil M.A., Stein M., Imirzalioglu C., Domann E., Pleschka S., Hain T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 2011;11:16. doi: 10.1186/1472-6882-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland J., Jacobs B., Wagner C.E., Ruiz G., Cahill T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antiviral Res. 2018;160:143–150. doi: 10.1016/j.antiviral.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Lednicky J.A., Waltzek T.B., McGeehan E., Loeb J.C., Hamilton S.B., Luetke M.C. Isolation and genetic characterization of human coronavirus NL63 in primary human renal proximal tubular epithelial cells obtained from a commercial supplier, and confirmation of its replication in two different types of human primary kidney cells. Virol. J. 2013;10:213. doi: 10.1186/1743-422X-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu Y., Hou X., Peng H., Zhang L., Jiang Q., Shi M., Ji Y., Wang Y., Shi W. Chlorogenic acid inhibits the replication and viability of enterovirus 71 in vitro. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.W., Lin C.W. Human coronaviruses: clinical features and phylogenetic analysis. Biomed. 2013;3:43–50. doi: 10.1016/j.biomed.2012.12.007. (Taipei) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q.F., Xie S.P., Chen X.H., Bi K.S. Study on the chemical constituents of Sumbucus chinensis Lindl. Zhong Yao Cai. 2006;29(9):916–918. [PubMed] [Google Scholar]
- Lin C.N., Tome W.P. Antihepatotoxic principles of Sambucus formosana. Planta Med. 1988;54(3):223–224. doi: 10.1055/s-2006-962410. [DOI] [PubMed] [Google Scholar]
- Mahmood N., Pizza C., Aquino R., De T., Piacente S., Colman S., Burke A., Hay A.J. Inhibition of HIV infection by flavanoids. Antiviral Res. 1993;22:189–199. doi: 10.1016/0166-3542(93)90095-z. [DOI] [PubMed] [Google Scholar]
- Mandrone M., Lorenzi B., Maggio A., La Mantia T., Scordino M., Bruno M., Poli F. Polyphenols pattern and correlation with antioxidant activities of berries extracts from four different populations of sicilian Sambucus Nigra L. Nat. Prod. Res. 2014;28(16):1246–1253. doi: 10.1080/14786419.2014.898147. [DOI] [PubMed] [Google Scholar]
- Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014;88(22):13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J., Spínola V., Llorent-Martínez E.J., Fernández-de Córdova M.L., L M.-G., Castilho P.C. Polyphenolic profile and antioxidant activities of Madeiran elderberry (Sambucus lanceolata) as affected by simulated in vitro digestion. Food Res. Int. 2017;100(Pt3):404–410. doi: 10.1016/j.foodres.2017.03.044. [DOI] [PubMed] [Google Scholar]
- Pliszka B. Polyphenolic content, antiradical activity, stability and microbiological quality of elderberry (Sambucus Nigra L.) extracts. Acta Sci. Pol. Technol. Aliment. 2017;16(4):393–401. doi: 10.17306/J.AFS.0523. [DOI] [PubMed] [Google Scholar]
- Porter R.S., Bode R.F. A review of the antiviral properties of black elder (Sambucus Nigra L.) products. Phytother. Res. 2017;31(4):533–554. doi: 10.1002/ptr.5782. [DOI] [PubMed] [Google Scholar]
- Roschek B.Jr., Fink R.C., McMichael M.D., Li D., Alberte R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry. 2009;70(10):1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Serkedjieva J., Manolova N., Zgorniak-Nowosielska I. Antiviral activity of the infusion (SHS-174) from flowers of Sambucus Nigra L., aerial parts of Hypericum Perforatum L., and roots of Saponaria Officinalis L. Against influenza and herpes simplex viruses. Phytother. Res. 1990;4:97–100. [Google Scholar]
- Shen J., Wang G., Zuo J. Caffeic acid inhibits HCV replication via induction of IFNα antiviralresponse through p62-mediated Keap1/Nrf2 signaling pathway. Antiviral Res. 2018;154:166–173. doi: 10.1016/j.antiviral.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Tanida I., Shirasago Y., Suzuki R., Abe R., Wakita T., Hanada K., Fukasawa M. Inhibitory effects of caffeic acid, a coffee-related organic acid, on the propagation of hepatitis C virus. Jpn. J. Infect. Dis. 2015;68(4):268–275. doi: 10.7883/yoken.JJID.2014.309. [DOI] [PubMed] [Google Scholar]
- Turek S., Cisowski W. Free and chemically bonded phenolic acids in barks of Viburnum Opulus L. And Sambucus Nigra L. Acta Pol. Pharm. 2007;64(4):377–383. [PubMed] [Google Scholar]
- Utsunomiya H., Ichinose M., Ikeda K., Uozaki M., Morishita J., Kuwahara T., Koyama A.H., Yamasaki H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014;34(4):1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Huang S.C., Zhang Y., Lai Z.R., Kung S.H., Chang Y.S., Lin C.W. Antiviral ability of Kalanchoe gracilis leaf extract against enterovirus 71 and coxsackievirus A16. Evid. Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.Y., Hour M.J., Lai H.C., Chen C.H., Chang P.J., Huang S.H., Lin C.W. Epigallocatechin-3-gallate inhibits the early stages of Japanese encephalitis virus infection. Virus Res. 2018;253:140–146. doi: 10.1016/j.virusres.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Wang G.F., Shi L.P., Ren Y.D., Liu Q.F., Liu H.F., Zhang R.J., Li Z., Zhu F.H., He P.L., Tang W., Tao P.Z., Li C., Zhao W.M., Zuo J.P. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res. 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Yang K.C., Chiu S.T. Flora of Taiwan. 2nd ed. 1998. Caprifoliaceae. [Google Scholar]
- Yang Y.J., Lin J.H. Study on the chemical constituents of Sumbucus Chinensis Lindl. J Chin Med Mater. 2004;27(7):491–492. [Google Scholar]
- You H.L., Huang C.C., Chen C.J., Chang C.C., Liao P.L., Huang S.T. Anti-pandemic influenza A (H1N1) virus potential of catechin and gallic acid. J. Chin. Med. Assoc. 2018;81(5):458–468. doi: 10.1016/j.jcma.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakay-Rones Z., Varsano N., Zlotnik M., Manor O., Regev L., Schlesinger M., Mumcuoglu M. Inhibition of several strains of Influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus Nigra L.) during an outbreak of influenza B panama. J. Altern. Complement. Med. 1995;1(4):361–369. doi: 10.1089/acm.1995.1.361. [DOI] [PubMed] [Google Scholar]
- Zhang T., Yang D., Wang Y., Chen X., Bi K. Simultaneous determination of four components in the herbs of Sumbucus chinensis Lindl. By high performance liquid chromatography. Asian J. Tradit. Med. 2010;5(2):70–74. [Google Scholar]
- Zozimus Divya Lobo C., Syed Mohamed A., Vedhi C., Rajesh S.V., Aroulmoji V., Shanmugam G. Identification of potent angiotensin converting enzyme 2 inhibitors through virtual screening and structure-based pharmacophore design. Int J Adv Sci Eng. 2017;4(1):474–480. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.