Summary
Aerosols containing microbes from the oral cavity of the patient are created when using modern high-speed rotating instruments in restorative dentistry. How far these aerosols spread and what level of contamination they cause in the dental surgery has become a growing concern as the number of patients with oro-nasal meticillin-resistant Staphylococcus aureus colonization has increased. The present study aimed to determine how far airborne bacteria spread during dental treatment, and the level of contamination. Fall out samples were collected on blood agar plates placed in six different sectors, 0.5–2 m from the patient. Restorative dentistry fallout samples (N = 72) were collected from rooms (N = 6) where high-speed rotating instruments were used, and control samples (N = 24) were collected from rooms (N = 4) used for periodontal and orthodontic treatment where rotating and ultrasonic instruments were not used. The collection times were 1.5 and 3 h. In addition, samples were taken from facial masks of personnel and from surfaces in the rooms before and after disinfection. After 48 h of incubation at 37 °C, colonies were counted and classified by Gram stain. The results showed significant contamination of the room at all distances sampled when high-speed instruments were used (mean 970 colony-forming units/m2/h). The bacterial density was found to be higher in the more remote sampling points. Gram-positive cocci, namely viridans streptococci and staphylococci, were the most common findings. The area that becomes contaminated during dental procedures is far larger than previously thought and practically encompasses the whole room. These results emphasize the need for developing new means for preventing microbial aerosols in dentistry and protection of all items stored temporarily on work surfaces. This is especially important when treating generally ill or immunocompromised patients at dental surgeries in hospital environments.
Keywords: Bacterial aerosol, Dental surgery, Hygiene, Hospital bacteria
Introduction
Patients and oral health personnel are surrounded by microbial aerosols during dental treatment with turbine burs, water–air sprays and other aerosol-forming instruments.1, 2, 3, 4, 5 Nevertheless, only after identification of the human immunodeficiency virus in the 1980s and the subsequent media alert was attention truly focused on hygiene in dental surgeries.6 Subsequently, disposable gloves, face masks and eye glasses have come into widespread use among dental staff, and questions have been raised regarding how and where patients with communicable diseases should be treated.7 Dental schools have been alerted and infection control has become an essential part of their curricula.8
Micro-organisms may also colonize dental equipment and water pipes, and form biofilms on the surfaces.9 Sterilization processes have often been shown to be inadequate in dental primary care.10 Bacteria and yeasts from the biofilms may produce aerosols in the dental surgery.11 Bacterial species such as Pseudomonas aeruginosa, Pseudomonas cepacia, Legionella pneumophila and Mycobacterium chelonae have been identified in biofilms.12 The concentration of total bacterial aerosols has been shown to be clearly associated with clinical working hours in dental surgeries.4
However, there are still uncertainties in aspects of cross-infection and infection control in dentistry, as shown, for example, in a questionnaire study from Germany. In this, 1100 patients were asked about their opinions of contracting an infection and the resulting problems such as the situation for patients in the waiting room, hygiene practices in the dental surgery, the course of dental treatments and the trust placed in the dentist.13 The results revealed a number of uncertainties that indicate a lack of information about infectious diseases, as well as in the confidence placed in the competence of dentists. In the present era of severe acute respiratory syndrome, avian flu and global problems with multi-resistant bacteria, reliable information about these aspects would be highly relevant and the importance of infection control in dentistry cannot be underestimated.14
It is not known how far airborne micro-organisms spread in a dental surgery when modern high-speed rotating instruments are used. Therefore, the present study aimed to determine this by placing agar plates systematically around dental units in order to collect airborne bacteria during routine treatment of patients. The study hypothesis was that different treatment modes cause different amounts of aerosol with potential spread of bacteria. It was anticipated that the facial masks of personnel and various surfaces in the rooms would become differentially contaminated during working sessions. The aim was to obtain data for updated guidelines of infection control in dentistry.
Materials and methods
This study was performed at the specialist care unit of the Helsinki City Health Department in 2004–2005. Restorative dentistry fallout samples (N = 72) were collected from rooms (N = 6) where high-speed rotating instruments were used, and control samples (N = 24) were collected from rooms (N = 4) used for periodontal and orthodontic treatment where rotating and ultrasonic instruments were not used. Rooms at rest (N = 3) were also sampled for background contamination. The rooms have two-directional forced ventilation with class F7 filters (installed in 2001). The air flow is adjusted to 84 L/s in four of the rooms (high-speed instruments, control, rest) and to 440 L/s in the two larger rooms (high-speed instruments, control). This results in one air exchange every 10 min in the slightly smaller rooms and one air exchange every 5 min in the larger rooms. The ventilation was not altered during the sampling period. The ventilation system is checked and serviced annually. The rooms do not have air-conditioning units as they are not necessary in the Finnish climate.
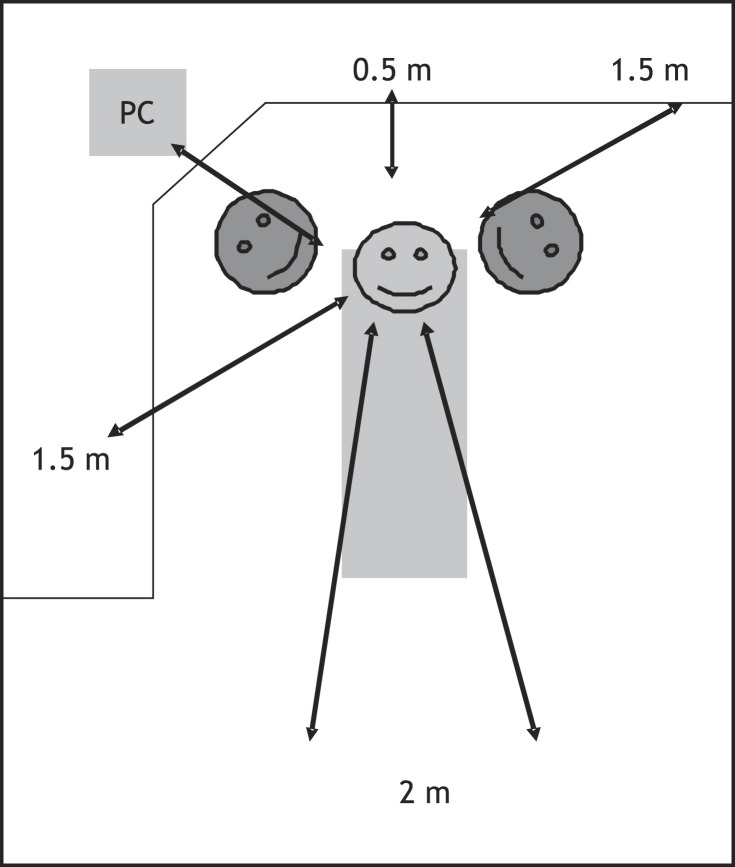
The experimental setting is outlined in Figure 1. Two adjacent horse blood chocolate agar plates (HUSLAB, Helsinki, Finland) supplemented with Isovitalex® 1 and 2 (BBL Microbiology systems, Cockeysville, MD, USA) were placed in six different sectors, 0.5–2 m from the patient as shown in Figure 1. The plates were opened when treatment started, one of each pair of plates was closed after 1.5 h, and the other plates were closed after 3 h. Sampling was repeated once. In addition, surface samples were collected with sterile cotton swabs from the facial masks of the dentists and dental nurses, and from different surfaces of the dental chair and cabinets, keyboards and door knobs. Samples were immediately plated on to the chocolate agar plates. Sampling was undertaken before and after disinfecting the working area in preparation for the next patient. The plates were incubated at 37 °C for 48 h. Colonies were counted and bacteria were classified by Gram stain using a light microscope with 1000 × magnification. All patients treated during sampling were otherwise healthy and scheduled for routine dental treatments. The results are given as means of colony-forming units (CFU)/m2/h with standard deviations. Differences between the sample collection times, sites of collection, treatment modes and bacterial counts were analysed by the Mann-Whitney test.
Figure 1.
Schematic representation of the placement of agar plates for collection of airborne bacteria. The dental unit with the patient is in the middle, and the dentist and dental nurse are at 11 and 1 o'clock positions, respectively. PC, computer with patients' records etc.
Results
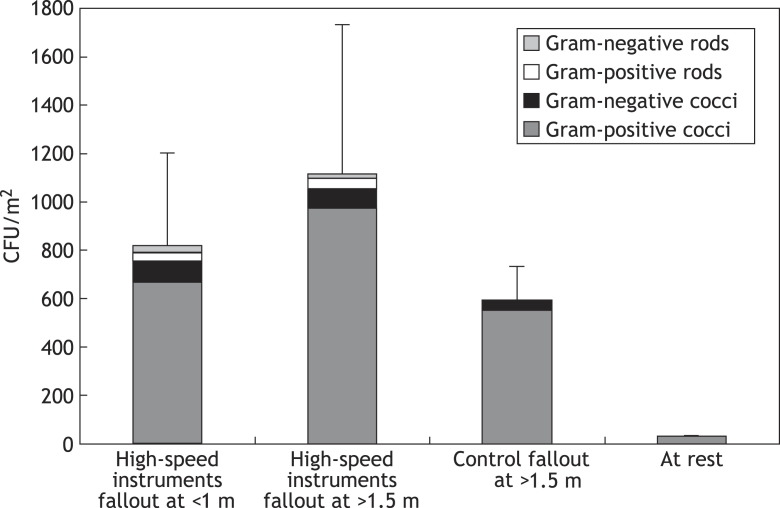
The results showed significant contamination at all distances sampled when high-speed instruments were used. The mean density of aerobic oral bacteria was 823 CFU/m2/h at <1 m distance from the patient. At distances > 1.5 m, the density was 1120 CFU/m2/h. The increase in the contamination density with respect to distance from the patient was not statistically significant (P > 0.5). Figure 2 shows the results of the bacterial fallout collection during restorative dental treatment. Gram-positive cocci, namely viridans streptococci and staphylococci, were the most common findings. Contamination was less intense during periodontal and orthodontic treatment (mean 598 CFU/m2/h) where high-speed rotating and ultrasonic instruments were not used, as shown in Figure 3. Gram-positive cocci were also predominant in these samples. The difference in the contamination level between the rooms where high-speed instruments were used and the rooms where they were not used was statistically significant (P = 0.001). Contamination was practically non-existent (mean 35 CFU/m2/h) in rooms at rest.
Figure 2.
Colony-forming units (CFU) of different types of bacteria at various distances from the treatment units after 1.5 and 3-h collection times. Significant contamination was detected at all distances sampled when high-speed instruments were used. Contamination was less intense during periodontal and orthodontic treatment (control) where high-speed rotating and ultrasonic instruments were not used, and was practically non-existent in rooms at rest. Means with standard deviations of total counts are shown.
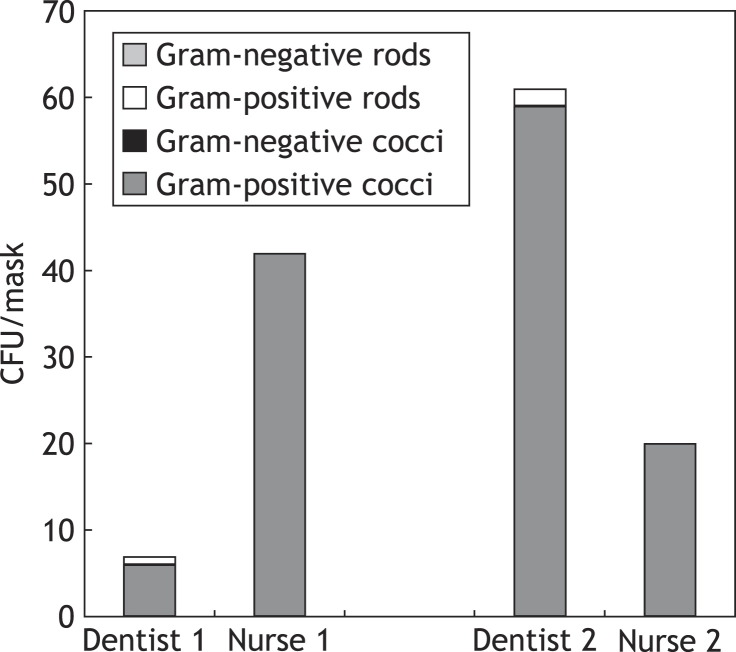
Figure 3.
Colony-forming units (CFU) of different bacteria cultivated from facial masks of two dentists and two dental nurses after a 40-min treatment session where high-speed rotating instruments were used. A notable difference can be seen in the facial contamination of the two teams. The operating area, working positions and suction technique may be responsible for the differences.
The facial masks became equally contaminated during the use of high-speed rotating instruments. Figure 3 gives the results of bacterial counts from the facial masks of two dentists and two dental nurses after a 40-min treatment session where high-speed rotating instruments were used. A notable difference was found in the facial contamination between the two teams. Using surface sampling, high counts of Gram-positive cocci were detected on various surfaces before disinfection, but the samples were predominantly negative after disinfecting the working area in preparation for the next patient.
Discussion
Aerosols containing microbes from the oral cavity are created when modern high-speed rotating instruments are used in restorative dentistry.1, 2, 3, 4, 5 How far these aerosols spread and what level of contamination they cause in the dental surgery has become a growing concern as the number of patients with oro-nasal meticillin-resistant Staphylococcus aureus colonization has increased.
The role and challenges of hygiene in dentistry have changed radically over the past decades. New pathogens have emerged and old pathogens have developed resistance against antibiotics. More patients are receiving immunomodulatory medication or are for other reasons at increased risk for infections. Concomitantly, faster rotating instruments and new devices, equipment and treatment modes have been introduced in dentistry, often into old facilities and settings. In many surgeries, there is a clear lack of appropriate storage space, and many items coming into contact with patients are temporarily or semi-permanently stored on work surfaces. The contaminated area was thought to extend 1–1.5 m from the patient's mouth, and the risk for contamination and cross-infection beyond that distance was assumed to be minimal.1
There is no current consensus regarding the acceptable level of contamination in dental practice. Suggested guidelines for hospitals have been published but how useful they are in dentistry, how they should be construed and how up to date they are in the infection situation of today is another matter.15, 16 A suggested guideline by Fischer et al. employing the IMA-index for microbial air contamination17 propose that counts of 45 100–75 000 CFU/m2/h in medical wards, and counts of 25 100–45 000 CFU/m2/h in surgeries are acceptable by Fischer et al. 15 However, for operating theatres at rest, equivalent to background contamination, counts of 0–400 CFU/m2/h have been suggested. Dental surgeries are often compared with doctors' surgeries or hospital wards although procedures are not normally performed there. Most dental procedures cause some mucosal damage and significant wounds are created in periodontal treatment and dento-alveolar surgery. Thus, a dental surgery is more of an operating theatre than an office or a ward. On the other hand, the hygiene requirements for operating theatres seem exaggerated for normal dental surgeries but may be applicable to hospital environments where dental treatment is given to generally ill, hospitalized patients.
When high-speed rotating instruments are used, the air is momentarily contaminated; as the bacteria settle, the air quality increases. It has been claimed that settle plates have no role in monitoring operating theatre counts, and that air samplers collecting, for example, 1 m3 of air over a 15-min sampling period would be more appropriate.17 This thinking may not, however, apply for dental surgeries because of the evident fluctuation of contamination and problems with defining the most relevant sampling moment and interpretation of the results. Passive air sampling, as used in the present study, gives a better description of the end result of a treatment session. It reflects the extent of contamination on the critical surfaces, and highlights areas that should be disinfected between patients and where items are at risk for becoming contaminated.
These results show that a dental procedure is a potential hospital infection risk if the extent and nature of microbial aerosols created by high-speed rotating instruments is underestimated. The study showed significant contamination of the room at all distances sampled when high-speed instruments were used (mean 1119 CFU/m2/h at >1.5 m from the patient). Contamination was less intense during periodontal and orthodontic treatment (598 CFU/m2/h at >1.5 m from the patient) where high-speed rotating and ultrasonic instruments were not used, and was practically non-existent in rooms at rest. The present findings of the density of bacteria detected on various surfaces in the vicinity of the treatment unit are in line with previous studies.4, 18 However, the present finding that bacterial counts were generally higher in the more remote sampling points has not been reported previously. This phenomenon may be due to the increased rotating speeds of the instruments giving a higher angular velocity and longer trajectory of the bacteria.
Various means have been investigated to prevent or reduce bacterial aerosols during dental treatment. These include use of a rubber dam, which has been shown to be highly significant in reducing contamination of the atmosphere, and giving the patient antiseptic mouth rinse before treatment.19, 20 In Japan, Noro et al. found that an extra oral vacuum aspirator was effective in reducing the spread of oral streptococci, and recommended this for treating patients with infectious diseases.21 However, in practice, it is impossible to totally eliminate bacterial aerosols during dental treatment. Furthermore, new monitoring techniques have revealed infectious agents such as Legionella spp. that may contaminate the atmosphere during dental treatment.22
These results show that the area becoming contaminated during dental procedures is far larger than previously thought and practically encompasses the whole room. Only items necessary for ongoing treatment should be out on work surfaces, and other items should be stored in closed cupboards. Disinfection between patients should be made as easy as possible and should extend to all contaminated areas. In general, dental surgeries should be seen more as operating theatres than offices in order to minimize the risks of cross-infection. Protection of the face and hair and personal hygiene of the personnel after work should also be emphasized to control colonization and spread of hospital bacteria in the community.
Acknowledgement
The authors thank Mr. Jussi Furuholm for help with statistics.
References
- 1.Micik R.E., Miller R.L., Mazzarella M.A., Ryge G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J Dent Res. 1969;48:49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 2.Shpuntoff H., Shpuntoff R.L. High-speed dental handpieces and spread of airborne infections. N Y State Dent J. 1993;59:21–23. [PubMed] [Google Scholar]
- 3.Bentley C.D., Burkhart N.W., Crawford J.J. Evaluating splatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125:579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 4.Al Maghlouth A., Al Yousef Y., Al Bagieh N. Qualitative and quantitative analysis of bacterial aerosols. J Contemp Dent Pract. 2004;5:91–100. [PubMed] [Google Scholar]
- 5.Timmerman M.F., Menso L., Steinfort J., van Winkelhoff A.J., van der Weijden G.A. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31:458–462. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson E.O. AIDS: today's vital challenge to dentistry. J Dent Pract Adm. 1985;2:2–8. [PubMed] [Google Scholar]
- 7.de Graaff J., van Amerongen W.E., Mulder G.R. Hygiene in dental practice. Part II: Measures to reduce the risk of contamination. ASDC J Dent Child. 1988;55:56–63. [PubMed] [Google Scholar]
- 8.Sampson E., Dhuru V.B. Infection control in North American dental schools. J Dent Educ. 1989;53:532–537. [PubMed] [Google Scholar]
- 9.Barbeau J. Waterborne biofilms and dentistry: the changing face of infection control. J Can Dent Assoc. 2000;66:539–541. [PubMed] [Google Scholar]
- 10.Smith A., Letters S., Lange P., Perrett D., McHugh S., Bagg J. Residual protein levels on reprocessed dental instruments. J Hosp Infect. 2005;61:237–241. doi: 10.1016/j.jhin.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Harrel S.K. Airborne spread of disease – the implications for dentistry. J Calif Dent Assoc. 2004;32:901–906. [PubMed] [Google Scholar]
- 12.Miller C.H., Palenic C.J. Aseptic techniques. In: Miller C.H., Palenic C.J., editors. Infection control and management of hazardous materials for the dental team. Mosby; St. Louis: 1998. pp. 205–209. [Google Scholar]
- 13.Pistorius A., Willershausen B., Heffner N. Treatment aspects for patients with infectious diseases in dental practices – results of a survey. Eur J Med Res. 2002;7:457–462. [PubMed] [Google Scholar]
- 14.Harrel S.K., Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer G., Fodre S., Nehez M. Results of the study to determine marginal pathogen count values in the air of operating rooms. Z Gesamte Hyg. 1972;18:729–733. [PubMed] [Google Scholar]
- 16.Pitzurra M., Savino A., Pasquarella C. Microbiological environment monitoring (MEM) Ann Ig. 1997;9:439–454. [PubMed] [Google Scholar]
- 17.Pasquarella C., Pitzurra O., Savino A. The index of microbial air contamination. J Hosp Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 18.Kedjarune U., Kukiattrakoon B., Yapong B., Chowanadisai S., Leggat P. Bacterial aerosols in the dental clinic: effect of time, position and type of treatment. Int Dent J. 2000;50:103–107. doi: 10.1002/j.1875-595x.2000.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 19.Samaranayake L.P., Reid J., Evans D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC J Dent Child. 1989;56:442–444. [PubMed] [Google Scholar]
- 20.Logothetis D.D., Martinez-Welles J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126:1634–1639. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 21.Noro A., Yanaka N., Takahashi K. A study on prevention of hospital infection control caused by tooth preparation dust in the dental clinic. Part 1. Preventive measures against environmental pollution in the dental clinic caused by microbial particles. Bull Tokyo Dent Coll. 1995;36:201–206. [PubMed] [Google Scholar]
- 22.Williams H.N., Paszko-Kolva C., Shahamat M., Palmer C., Pettis C., Kelley J. Molecular techniques reveal high prevalence of Legionella in dental units. J Am Dent Assoc. 1996;127:1188–1193. doi: 10.14219/jada.archive.1996.0410. [DOI] [PubMed] [Google Scholar]