Abstract
Macrophages, circulating in the blood or concatenated into different organs and tissues constitute the first barrier against any disease. They are foremost controllers of both innate and acquired immunity, healthy tissue homeostasis, vasculogenesis and congenital metabolism. Two hallmarks of macrophages are diversity and plasticity due to which they acquire a wobbling array of phenotypes. These phenotypes are appropriately synchronized responses to a variety of different stimuli from either the tissue microenvironment or – microbes or their products. Based on the phenotype, macrophages are classified into classically activated/(M1) and alternatively activated/(M2) which are further sub-categorized into M2a, M2b, M2c and M2d based upon gene expression profiles. Macrophage phenotype metamorphosis is the regulating factor in initiation, progression, and termination of numerous inflammatory diseases. Several transcriptional factors and other factors controlling gene expression such as miRNAs contribute to the transformation of macrophages at different points in different diseases. Understanding the mechanisms of macrophage polarization and modulation of their phenotypes to adjust to the micro environmental conditions might provide us a great prospective for designing novel therapeutic strategy. In view of the above, this review summarises the activation of macrophages, the factors intricated in activation along with benefaction of macrophage polarization in response to microbial infections, pulmonary toxicity, lung injury and other inflammatory diseases such as chronic obstructive pulmonary dysplasia (COPD), bronchopulmonary dysplasia (BPD), asthma and sepsis, along with the existing efforts to develop therapies targeting this facet of macrophage biology.
Keywords: M1/M2 macrophages, Classical activation, Alternative activation, Lung inflammation, COPD, BPD, Asthma
1. Background
Macrophages are the ultimate cells of differentiation of the mononuclear phagocyte system. The system further embraces dendritic cells, blood monocytes in circulation and committed myeloid lineage cells in the bone marrow. A marked heterogeneity is displayed by monocytes and dendritic cells in response to environmental stimuli. However, in case of human macrophages, some apprehensions with respect to marked expression motifs of surface markers that delineate different macrophage subsets still persist (Cassetta et al., 2011). They form an important component of immunity, playing indispensable characters in innate as well as acquired immunity. Macrophages residing in the tissues are the first barriers of defense against extrinsic invaders and coordinate leukocyte penetration in innate immunity. They subscribe equilibrium between antigen removal by phagocytosis and degradation of microbes, apoptotic cells, and neoplastic cells (Gordon, 2003). They are able to acquire well defined phenotypes via phenotypic polarization in response to variegated environmental signals (which could be pathogenic parts or products, injured cells, cellular debris or initiated lymphocytes);and during divergent states of disease (O'Shea and Paul, 2010).
Macrophages amalgamate with B and T cells through systems grounded on the secretion of enzymes, reactive radicals, cytokines, chemokines and metabolites of arachidonic acid (fluid phase mediated mechanism); and through cell to cell communication (Gordon, 2003, Stout and Suttles, 2005, Duffield, 2003). Their activation is dependent upon the signals they receive from their microenvironment which can potentially promote or suppress inflammation leading to smashing of tissue or resuscitation and wound repair, respectively. Thus, the functional phenotype of macrophages acquired in sequel to signals derived from tissues finally trigger, instruct and adjourn the adaptive immune response (Mantovani et al., 2005). Moreover, these cells also bestow themselves in homoeostatic functions such as reconstruction of tissues in ontogenesis and metabolic function symphonization (Gordon, 2003, Biswas and Mantovani, 2010, Sica and Bronte, 2007).
Monocytes migrating from blood stream to the peripheral tissues are the progenitor cells of macrophages. The demarcation (differentiation) of these monocytes into macrophages and dendritic cells occurs on exposition to microbial compounds, cytokines favouring inflammation and local growth factors (Tacke et al., 2006). Different regulatory levels and attainment of enhanced resistance to microbes has been achieved by intercellular communication between activated T and B lymphocytes and macrophages. A broader range of trophic functions such as the job of Th2- derived interleukin-4 (IL-4) against extracellular parasitic infection and Th1-derived interferon-gamma (IFN-g) against intracellular infection in acquired immunity and several immunomodulatory agents are responsible for the emergence of the concept of the two correspondents: M1 and M2 macrophages (Martinez and Gordon, 2014). The multiple properties of different phenotypic variants of macrophages possess substantial effects on the residing tissues under different diseased conditions. A specific subset can be either defensive during disease or homeostasis or can be modified in order to complement disease pathogenesis and progression.
2. Heterogeneity in macrophage activation and polarization
Two hallmarks of macrophages are diversity and plasticity. Classically activated M1 and alternatively activated M2 phenotypes of macrophages portray two zestful changing states of macrophage activation. M1/M2 macrophage polarization is a tightly coordinated process involving numerous pathways of signal transduction, transcriptional and post-transcriptional networks of regulation. M1 macrophages release pro-inflammatory cytokines which retard cellular proliferation surrounding the tissue leading to tissue damage. In contrast, M2 macrophages release anti-inflammatory cytokines which aids in cellular proliferation and promotes wound healing and tissue repair. Any imbalance in macrophage M1/M2 polarization may have detrimental effects resulting in varied diseases or states of inflammation (Wang et al., 2014).
2.1. Macrophage polarization: classical and alternate activation
IFN-γ or a combination of IFN-γ with microbial stimuli like lipopolysaccharide (LPS present in the exterior membrane of Gram-negative bacteria) or other cytokines such as GM-CSF (granulocyte-macrophage colony stimulating factor) and TNF-α is responsible for the differentiation of classically activated M1 macrophage (Martinez and Gordon, 2014, Murray et al., 2014). Lipopolysaccharide (LPS), is carried to the cell surface receptor complex via an LPS-binding protein (Guha and Mackman, 2001). Th1, CD8+ cytotoxic lymphocytes, natural killer (NK) cells, antigen-presenting cells (APC) and B cells, all secrete IFN-γ. IFN-γ is then recognized by IFN-γ receptor (IFNGR), and binding of IFN-γ to IFNGR triggers a series of signal cascades leading to activation and differentiation of M1 macrophages. M1 macrophages possess a specific set of characteristics determined on the basis of tissue location. These characteristics are: a. enhanced secretion of pro-inflammatory cytokines like TNF-α, IL-15, IL-23, IL-1β; b. amplified antigen presenting capacity; c. increased production of iNOS-dependent reactive nitrogen intermediates (RNI) and reactive oxygen intermediates (ROI) and d. aggravated release of IL-12. M1 macrophages, being the first shield to safeguard against intracellular pathogens, display enhanced endocytic functions. Moreover, they secrete high quantities of IL-12 which aids in amplifying Th1 polarization of CD4+ lymphocytes. Thus, M1 macrophages are compelling effector cells, highly efficient of producing abundant pro-inflammatory cytokines and exert microbicidal and cytotoxic activities (Martinez and Gordon, 2014, Arango Duque and Descoteaux, 2014) (Fig. 1 ).
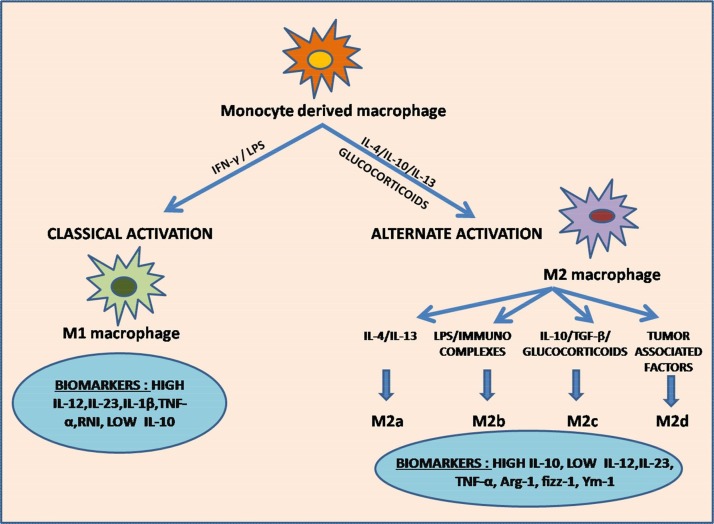
Fig. 1.
Classical and Alternate pathway of Macrophage polarization.
The figure depicts the pathways involved in macrophage polarization in response to signals received from micro-environment. Classical activation of M1 macrophages is induced by LPS/IFN-γ exposure. Activated M1 macrophages promote enhanced secretion of M1 chemokines, Th1 response elements, i-NOS (inducible nitric oxide synthase) dependent reactive nitrogen intermediates (RNI), high levels of IL-12, IL-23 IL-1β and TNF-α, and low levels of IL-10, which exert pro-inflammatory and cytotoxic effects. They are also involved in tumor suppression and immunostimulation. Alternately activated M2 macrophages are stimulated by IL-4, IL-10, IL-13 and glucocorticoids. IL-4 and IL-13 activates M2a subtype. Presence of immunocomplexes and LPS activates M2b subtype. M2c subtype is induced by IL-10, TGF-β and glucocorticoids. Presence of tumor associated factors triggers the activation of M2d subtype. Activated M2 macrophages enhance the secretion of IL-10 and reduces the secretion of IL-12 and IL-23 due to which they exert anti-inflammatory effects and roles in tissue repair and wound healing. M2d subtype is the prime constituent of TAMs (tumor associated macrophages) and hence promote tumor growth.
The type of activation in which LPS acts as a stimulus for in vitro macrophage activation is known as microbial pathogen associated molecular patterns (PAMPs) based activation (Janeway and Medzhitov, 2002, Mukhopadhyay et al., 2004). It is also known as innate activation. It resembles the M1 activation but is actually different from M1 macrophages in the way that innate activated macrophages are unable to secrete biologically-active IL-12 as M1 do. They do not possess increased phagocytic activity too (Trinchieri, 2003). Therefore, basing on their activation property, Martinez et al. further categorized these macrophages into M1a (classical) and M1b (innate) type. (Martinez et al., 2008).
There are several stimulatory factors of M2 macrophages some of which are cytokines (IL-4, IL-10 and IL-13); glucocorticoids and immunoglobulin complexes or Toll-like receptor (TLR) ligands. M2 macrophages are known to control inflammation and adaptive Th2 immunity, support angiogenesis, refurnishing and repair of damaged tissue, scavenge debris, and aid tumor progression (Mantovani et al., 2004). At the same time, they mediate clearance by expressing the receptor Stabilin-1 which binds the matricellular protein SPARC. Thus, they play a paramount role in harmonized reconstruction of extracellular matrix and repair of damage in normal and tumorous tissues (Kzhyshkowska et al., 2006). High IL-10, low IL-12 and IL-23 are the specific characteristics of M2 macrophages (Fig. 1). Depending upon the gene expression profiles, M2 macrophages are further subdivided into 3 subgroups: M2a, M2b, and M2c (Martinez and Gordon, 2014, Martinez et al., 2008, Mantovani et al., 2004). IL-4 and IL-13, chiefly secreted by mast cells, Th2 cells, and basophils initiate M2a subtype. These two cytokines negatively regulate pro-inflammatory intermediators like TNF-α, IFN-γ, IL-6, IL-12, IL-1β and production of superoxide anions. It has also been found that both IL-4 and IL-13 can also regulate beta2 integrins, MHC-II molecules, metalloproteinase (MMP)-1 and tissue-type plasminogen activator. Furthermore, proteins involved in tissue proliferation and repair; and fibrogenesis are also expressed by M2a cells (Hart et al., 1989, Chizzolini et al., 2000, Gratchev et al., 2001, Torocsik et al., 2005). Exposure to immune complexes in combination with LPS or IL-1R ligand stimulates the subset M2b which is outlined by decreased production of IL-12 and increased production of IL-10 (Anderson and Mosser, 2002). M2b is also favoured by Th2 immune response. The third subtype i.e. M2c is prompted by glucocorticoids, TGF-β or IL-10. It negatively regulates the production of pro-inflammatory cytokines and enhances scavenging activity of debris by M1 macrophages. Wang et al. also identified an additional M2 subset, the M2d macrophage, designated by reduced secretion of IL-12 and enhanced release of IL-10.They exhibit a few attributes of tumor-associated macrophages (TAMs) (Wang et al., 2010). There are evidences to show that TAMs play a role of key effectors in provoking tumor angiogenesis and progression (Guo et al., 2013a).
2.2. Molecular basis of macrophage polarization
M1 macrophages secrete inflammation promoting mediators such as IL-1, IL-6 and tumor necrosis factor (TNF)-α; reactive nitrogen and oxygen intermediates, thereby exerting strong microbicidal and tumoricidal actions. M2 phenotype express cell surface markers such as Arginase 1(Arg1), resistin-like-α (Fizz1), Mrc1 (CD206), chitinase 3-like 3 (Ym1) and anti-inflammatory cytokines such as IL-10. These markers are known to be involved in immunoregulatory activities such as parasite affliction, tissue remodelling and tumor progression (Gordon and Martinez, 2010). Switch over of M1-M2 macrophage phenotype can take place in their specialised tissue microenvironment but this transformation is quite different from that of Th1 and Th2. The leading transcription factors involved in macrophage polarization are STATs (signal transducer and activator of transcription) (Lawrence and Natoli, 2011, Ohmori and Hamilton, 1997), NF (nuclear factor)-κβ (Oeckinghaus et al., 2011), interferon-regulatory factor (IRFs) (Satoh et al., 2010, Krausgruber et al., 2011), AP 1 (activator protein) (Schonthaler et al., 2011), PPAR-γ (peroxisome proliferator-activated receptor) (Odegaard et al., 2007, Bouhlel et al., 2007) and CREB (cAMP-responsive element-binding protein) (Ruffell et al., 2009), which forms a highly coordinated interface with each other to drive macrophage polarization to a particular phenotype in different diseases of inflammation (Fig. 2 ).
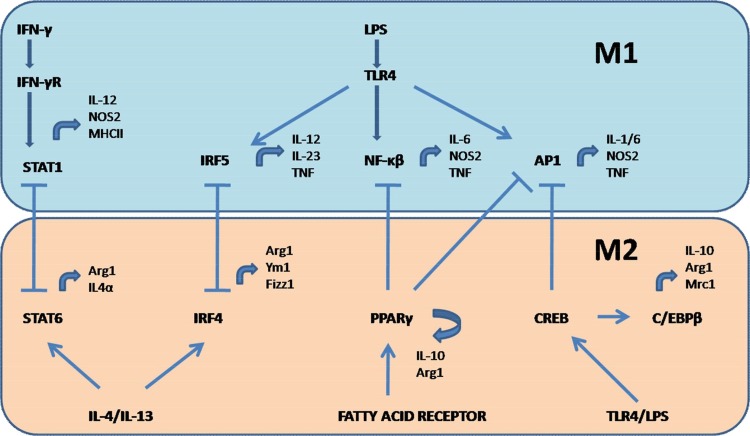
Fig. 2.
Transcription factors involved in macrophage polarization.
The figure represents the role of transcription factors involved in M1/M2 polarization and their feedback control. M1 polarization: Binding of IFN-γ to its receptor activates STAT1, involved in transcription of IL-12, NOS-2 and MHC-II genes. Binding of LPS to TLR4 causes activation of IRF5, NF-κβ and AP1, all of which are involved in increased production of proinflammatory cytokines – IL-1, IL-6,IL-12,IL-23 and TNF. M2 polarization: Binding of free fatty acids to their receptors induces activation of PPARγ, involved in transcription of IL-10 and Arg-1 genes. IL-4/IL-13 triggers the activation of STAT6 and IRF4, responsible for transcription of Arg-1, IL-4α, Ym-1 and fizz-1 genes. TLR4/LPS binding activates CREB, which induces enhanced production of IL-10. M1/M2 polarization also exert feedback regulation mediated by STAT1-STAT6, IRF5-IRF4, NF-κβ-PPARγ, AP1-PPARγ and AP1-CREB. Blocked arrows represent feedback control.
A number of signalling pathways are involved in the process of macrophage polarization. These pathways are well organised in the form of a network co-ordinating appropriate responses to different stimuli. One of the foremost pathway is JAK/STAT pathway which crops up by binding of IFNγ to its cell surface receptor. This causes activation of JAKs leading to dimerization and translocation of STAT1 to the nucleus. STAT1, then initiates transcription of M1-associated genes resulting in production of pro-inflammatory cytokines. On the other hand, STAT6 gets activated by IL-4 and IL-13 to induce M2 polarization. C-Jun-N-Terminal Kinase and MAPK are involved in this pathway. STAT6 activation further activates PPARγ and PPARδ important for M2 polarization. KLF4 cooperates with STAT6 to transform polarization towards M2 phenotype by impairing co activators of NF-κβ. KLF-2 and KLF-4 are the two known factors associated with macrophage activation. Both the factors inhibit NF-κβ activity by impaired recruitment of a NF-κβ transcriptional co activator complex, PCAF/p300 to the target gene promoter (Mahabeleshwar et al., 2011). Deficiency of KLF4 promotes M1 polarization and inhibits M2 polarization. Thus, it acts as a tipping point in M1/M2 polarization (Tugal et al., 2013). Furthermore, activation of Akt2 via PI3K signalling pathway favours M1 polarization and Akt-1 activation favours M2 polarization. In addition, IRF5 (interferon regulatory factor) enhances the expression of genes encoding IL-12 and represses the genes encoding IL-10, hence promoting M1 polarization. In contrast, IRF-4 facilitates M2 polarization (Labonte et al., 2014) (Fig. 3 ).
Fig. 3.
Macrophage polarization – JAK/STAT pathway.
Binding of IFNγ, LPS or IL-4/13 to their corresponding surface receptors triggers activation of JAKs (Janus Kinases) which induces activation of STATs (Signal transducer and activators of transcription) and transcription of M1and M2 genes. These genes are also transcribed by the differential activation of Akt1/2 via PI3K or PIP3. Binding of IFNγ to IFNγR1/2 activates JAK1/2, which in turn, activates STAT1/3. STAT1/3 induces activation of NF-κβ. Binding of LPS to TLR4 also activates NF-κβ via adapter proteins MyD88 (Myeloid differentiation primary response 88)/TRIF (TIR-domain containing adapter-inducing interferon-β). KLF4 inhibits the activity of NF-κβ. SOCS3 (suppressor of cytokine signalling 3) negatively regulates the cytokine signalling by binding to JAK2 kinase and inhibiting its activity. Binding of IL-4 to its receptor activates JAK1/2/3 kinases or PI3 kinases. Activated JAKs trigger activation of PPARγ/δ (Peroxisome proliferator-activated receptor gamma) via STAT6, which in turn, activates M2 genes. Akt1 activation promotes activation of M1 genes and Akt2 activation promotes activation of M2 genes.
Additionally, macrophages selectively express certain receptors of the super immunoglobulin family known as Triggering Receptor expressed on myeloid cells (TREM). This family contains two activating receptors – TREM1 and TREM2. These receptors are known to harmonize inflammatory responses by aggravating the TLR-initiated responses to pathogenic attack, enabling the release of pro inflammatory cytokines and chemokines. Their ligands have not been identified but some of our previous studies have shown that exposure to bacteria/microbial products induce TREM-1 expression. We have also scrutinized the role of lipid mediators in TREM-1 expression. It has been established that on exposure to bacterial and LPS, prostaglandins PGD2, PGJ2 and 15-dPGJ2 (naturally occurring derivatives of prostaglandins) inhibit the expression of TREM-1. This inhibition occurs due to NF-κβ inhibition and Nrf-2 activation i.e. transcriptional factor dependent but is receptor (PGD2 receptors and PPARγ) independent. This prostaglandin mediated inhibition of TREM-1 receptors results in anti-inflammatory effects (Syed et al., 2010). Previous work carried in our laboratory have also shown that TREM-1 inhibition in macrophages leads to alterations in prime signalling (IKβα, CD14 and MyD88) and effector molecules (MCP-1,IL-6 and IL-1β) downstream of TLR activation. In addition, TREM-1 expression requires TLR activation by specific TLR ligands (Zeng et al., 2007, Yuan et al., 2014).
2.3. Metabolic determinants of macrophage polarization
Differential metabolism is a peculiar characteristic of different phenotypic variants of macrophages under different conditions of tissue microenvironment and pathophysiological conditions. Differences in sugar, lipid and protein profiles of different phenotypes are observed. Classically activated M1 macrophages persuade an aerobic glycolytic program leading to lactate production. Moreover, PPP (pentose phosphate pathway) is also instigated by IFN-γ or LPS mediated activation. NADPH needed for ROS production is generated by this pathway. The generation of M1 phenotype also requires carbohydrate kinase-like protein (CARKL) involved in catalysis of sedoheptulose-7-phosphate. CARKL is considered to be sedoheptulose kinase choreographing the metabolic controls of pro and anti-inflammatory immune responses in the following manner: the development of M1 phenotype requires down-regulation of CARKL which leads to a reduction in NADH levels in cells over expressing CARKL resulting in a redox shift. PPP (Pentose phosphate pathway) also bestows itself in the reduction of redox couples via NADPH. Thus, M1 activation requires increased GSH and NADH generation whereas M2 activation leads to positive regulation of CARKL which is not a result of elevated GSH or NADH. Further, we can say that differences in metabolic states of macrophage polarization are CARKL dependent (Haschemi et al., 2012). In the case of fatty acid oxidation and oxidative metabolism, IL-4 activates STAT-6 which induces mitochondrial respiration. Therefore, M2 activation can direct the pyruvate into Krebs cycle and initiate Electron Transport Chain (ETC) thereby providing energy for tissue remodelling and repair. M1 activation leads to increased glycolysis which aids in microbicidal activity of M1 macrophages and coping up with hypoxia generated in the tissue microenvironment. In lipid metabolism, the pathways activated in M1 macrophages are suppressed in M2 macrophages. Eg: COX-2 is up-regulated in IFN-γ or LPS activated M1 macrophages (Martinez et al., 2006) while COX-1 is down-regulated. On the contrary, COX-1 is up-regulated in IL-4activated M2 macrophages.
The most studied metabolic difference is presented by catabolism of arginine. L-arginine is a substrate for both i-NOS and arginase-1 (Arg-1). i-NOS produces nitric oxide (NO) and L-citrulline. NO is the major effector molecule in cytotoxicity mediated by M1 macrophages. Arg-1 produces L-ornithine, polyamines and urea which is responsible for wound healing actions of M2 macrophages (El-Gayar et al., 2003, Modolell et al., 2009) (Fig. 4 ).
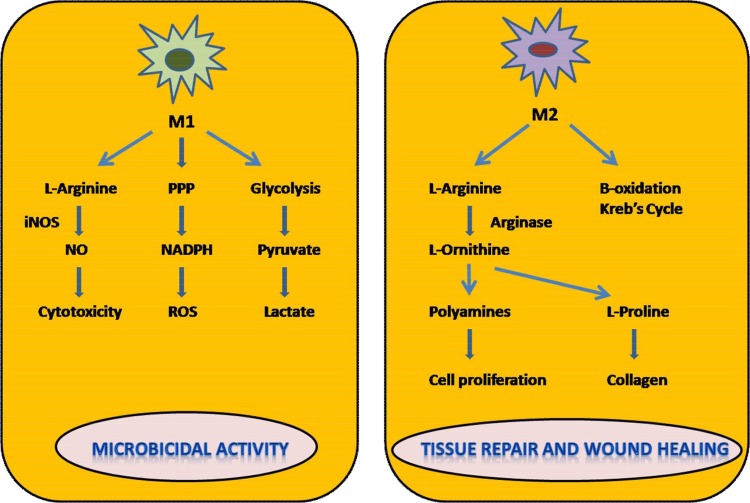
Fig. 4.
Differential metabolism in macrophage polarization.
This figure represents the metabolic differences in macrophage polarization. There is increased glycolytic activity induced by M1 macrophages which leads to higher production of lactate. Increase in NADPH is observed due to PPP (Pentose Phosphate Pathway) which contributes to the generation of ROS (reactive oxygen species) responsible for killing activity exerted by M1 cells. The cytotoxic activity of M1 cells is due to the production of NO (Nitric oxide) which is produced by catabolism of L-Arginine via i-NOS (Inducible Nitric oxide synthase) dependent pathway. M2 cells are involved in the catabolism of L-ornithine and polyamines mediated by Arg-1 expression. They also induce β oxidation, Krebs cycle and oxidative phosphorylation. Polyamines and L-Ornithine generated are involved in tissue repair and regeneration activity of M2 macrophages.
Recently, two additional metabolic pathways involving M2 polarization were also identified. They are glutamine linked metabolic pathway and the uridine diphosphate N-acetyl glucosamine (UDP-GlcNAc) pathway. Aspartate-arginine succinate shunt has also been identified in co-ordinating NO and TCA cycles for M1 activation (Jha et al., 2015).
2.4. Switch over of M1–M2 phenotype
Dynamic differentiation of macrophages in response to micro environmental signals enables them to swiftly transform their phenotypes. Under several pathological conditions, stimulation of members of IRF or NF-κβ family in macrophages scan TLR4 or other TLRs and direct macrophages to each of two M1 or M2 phenotype which can then be switched over depending upon the progression states of diseased conditions (Doyle et al., 2005, Doyle et al., 2007, Horwood et al., 2003, Horwood et al., 2006, Ni Gabhann, 2014, Lee et al., 2012, Schlaepfer et al., 2014). The spatio-temporal provocation of NF-κβ is a prime regulator of macrophage heterogeneity seen in progression of numerous inflammatory diseases. It can be explained with an example i.e., M1 macrophage stimulation of NF-κβ discerns inflammation linked to cancer during the early stage of tumorigenesis. In contrast, remodelling of macrophages to TAM or M2 type is observed in the late phase of tumorigenesis. These macrophages exhibit decreased capacity of NF-κβ stimulation and increased level of immunosuppression (Biswas et al., 2006). Congruently, in different stages of sepsis, a similar type of situation is displayed wherein M1 mediated NF-κβ activation is convoluted in the early phase of inflammation, while there is preponderance of tumor promoting and inflammation suppressing M2 phenotype in the later phases of inflammation and endotoxin clearance. NF-κβ activation is debilitated in the later phases (Porta et al., 2009). Another example is RSV infection, the utmost convincing cause of infection in lower respiratory tract occurring in infants and young children. It couples a mixed community of “Th1”and“Th2” cytokines. RSV stimulates the expression of several anti-viral genes such as IFN-b in airway epithelial cells during the initial stages of RSV infection, while during the later stages, it fosters the assertion of numerous pro-inflammatory genes activated by NF-κβ in macrophages by prompting TLR2, TLR3, TLR4, and retinoic acid-inducible gene I (RIG-I). Such expression directs macrophages towards M1 phenotype exhibiting anti-viral and pro-inflammatory activities. Nonetheless, RSV also persuades disease resolution via activation of IL-4 and IL- 13 mediated TLR-4, IL-4Ra/STAT-6, and IFN-b-dependent signalling pathways leading to M2 polarization of alveolar macrophages (Shirey et al., 2010). Besides several other factors, a metabolic determinant phospholipase Cβ2, involved in catalysis of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate, has also been involved in switching over of phenotypic determinants of macrophages (Grinberg et al., 2009).
3. Macrophages in acute lung injury
Acute lung injury or ALI is a traumatic disease which maybe caused by several direct (shock, severe sepsis, pulmonary contusion, gastroesophageal reflux, pneumonia, drug toxicity, transfusion, and acute pancreatitis) or indirect factors like ventilator induced or mechanical injury etc (Cao et al., 2016). The peculiar characteristics of ALI are enhanced permeability of capillary endothelial and alveolar epithelial cells, scattered pulmonary alveolar and interstitial edema, progressive and refractory hypoxemia and impaired gas exchange all of which expedite arterial hypoxemia,ultimately leading to respiratory failure (Zeng et al., 2013, Tamarapu Parthasarathy et al., 2012, Xie et al., 2012, Cai et al., 2012a, Agarwal et al., 2006). The disease pathogenesis is initiated by alveolar septa burst, destruction of the epithelium‐capillary interface, the disgorging of high protein containing fluid, acquittal of inflammation causing chemokines and cytokines alongwith the intrusion of monocytes, neutrophils and other cells causing inflammation (Oh and Lee, 2014, Li et al., 2014). As a result, the pathophysiological conditions such as reduced lung volume, uneven ventilation or perfusion and decreased lung compliance prevails. Acute Respiratory Distress Syndrome (ARDS) is the more relentless form of ALI and can lead to tenacious respiratory failure and high susceptibility to multi-organ functional imbalances or mortality (Cai et al., 2012a, Guo et al., 2013b). Several evidences have indicated that macrophages also contributes unequivocally to lung injury by modulating inflammatory responses (Kooguchi et al., 1998, Marriott and Dockrell, 2007, Herold et al., 2008). Macrophage polarization is critical in terms of the meticulous benefaction of their different phenotypes to disease pathogenesis. This can be seen in case of ALI which involves an extended or magnified M1 response and faulty M2 mediated repair, whereas in case of chronic diseases like fibrosis and cancer, hyper-responsive, alternatively activated M2 cells dominate.
Two macrophage populations persist in lungs. Alveolar macrophages residing in the tissue acquire lung tissue in the course of early stages of embryogenesis. They endure to be expedient for longer periods and are not restored by monocytes. On the other hand, interstitial macrophages which upon stimulation by various environmental stimuli.are recruited from circulation, have a short life span. Both of them play divergent roles in lung injury. Alveolar macrophages limit neutrophil influx while interstitial macrophages promote neutrophil expulsion (Misharin et al., 2013). The incursion of macrophages inside the lung crop up all along the induction and resolution phase of inflammation. Some of the earlier studies have illustrated that the elevated recruitment of macrophages to the lungs using monocyte chemotactic protein-1 was correlated with mitigated lung injury (Amano et al., 2004). Nonetheless, it has also been shown by studies based on macrophage depletion techniques, that macrophages contribute equally well in the pathogenesis of lung injury (Sone et al., 1999). A reason for these unlike effects could be the plasticity or heterogeneity of macrophage polarization, and the scrupulous acquisition or depletion of leukocytes such as monocytes and macrophages in order to promote or retard inflammation. (Johnston et al., 2012). Thus, exploration of the attributes of these subsets is crucial to modify disease −treatment and design therapeutic interventions.
Pathogen recognition, protective host defense by initiation of inflammation, and airway pathogen clearance are the dominant roles of lung macrophages. During lung injury, they recognise microbial impressions through TLRs (toll-like receptors), NODs (NOD-like receptors), intracellular helicases like retinoic acid inducible gene I (RIG-I) as well as various other pattern recognition receptors, and scavenge and phagocytose them. Phagocytosis is mediated by several receptors such as CD13 and receptors for IgG antibodies (FcγRs). When IgG opsonised particles bind to FcγRs present on the surface of a phagocyte, a series of events such as release of pro-inflammatory mediators, production of cytokines and ROS; and antibody-dependent cell mediated cytotoxicity pursues. It has been found that macrophages highly express CD13 which induce ROS production and modulate FcγRs mediated phagocytosis. Usually, it was presumed that changes in expression levels of these receptors determines the extent of phagocytosis. However, it has now been confirmed that macrophage polarization state determines the extent of phagocytosis and ROS production rather than the changes in receptor expression (Mendoza-Coronel and Ortega, 2017). Further, pattern recognition leads to release of cytokines involved in initial response. These cytokines are TNF-α, type I IFN, and IL-1β which upon release, consequently leads to the activation of lung macrophages in an NF-κβ- or IRF-dependent way. These cytokines exhilarate neighbouring AECs (alveolar epithelial cells) and tissue inhabiting macrophages to produce an array of chemokines in either autocrine or paracrine manner. The chemokines released, in turn, arbitrates the avocation of neutrophils, macrophages and lymphocytes to the infection site, eventually leading to pathogen clearance, Reversal of all of the inflammatory initiating steps and ordination of mechanisms of counter regulation leads to termination of inflammation. Repair and settlement of lung inflammation and recurrence of healthy tissue homeostasis is a highly organized process. This process involves interruption of blood vessel displacement of granulocytes, eviction of extravasated fluids and refurbishment of normal permeability of blood vessels, removal of apoptotic neutrophils, cessation of monocyte displacement and their maturation initiation in tissue residing alveolar macrophages, and ultimately, rehabilitation of functional endothelial and epithelial monolayers by “passerby” injury repair. Functional plasticity of alveolar macrophages enables them to acquire different phenotypes during the course of inflammation. The pro- inflammatory or anti-inflammatory or tissue- reparative role of these phenotypes is determined by the signals received from encompassing cells or from the pathogen itself. Thus, the major role of alveolar macrophages is to assimilate these cues during inflammatory progression, to stage a ratchet feedback in order to abort and reconcile alveolar inflammation in the subsequential stages of ALI and to firmly correlate processes of parenchymal repair which is indispensable for restoration of homeostasis (Fig. 5 ) (Herold et al., 2011).
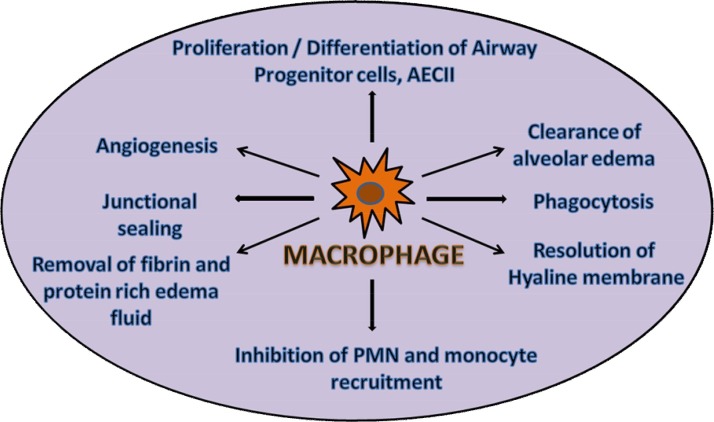
Fig. 5.
Macrophages in homeostasis.
This figure portrays all the processes involved in cessation and repair of alveolar inflammation after acute inflammatory lung injury Homeostasis is a timely coordinated, active process in which alveolar macrophages are directly or indirectly involved. These processes include blockage of granulocyte (PMN) and monocyte influx from the circulation, phagocytosis of apoptotic polymorphonuclear neutrophils or parenchymal cells, initiation of angiogenesis, repair of the endo-and epithelial barrier by junctional sealing, clearance of alveolar edema, proliferation/differentiation of epithelial progenitor cells including type II alveolar epithelial cells (AEC), removal of fibrin and protein rich edema fluid. These processes are well synchronized and are critical for healthy tissue homeostasis.
One of the important factors necessary for maintaining homeostasis is apoptosis (programmed cell death), which is a highly co-ordinated process carried out to balance cellular proliferation and death. The pro inflammatory macrophages are evacuated by the process of apoptosis and efferocytosis. Delayed apoptosis of these cells result in inflammation and continues with enhanced secretion of pro inflammatory intermediates. In one of our study, we have found that TREM-1 receptors, a member of super immunoglobulin family selectively expressed on macrophages and neutrophils, play an important role as an anti-apoptotic molecule responsible for prolonged survival of inflammatory macrophages. They induce major anti-apoptotic proteins Bcl-2 and Bcl-XL along with reduced activation of Caspase-3 (apoptosis executioner). This induction is Egr-2 mediated which is ERK dependent. Egr proteins are a family of zinc finger transcriptional regulators (Egr 1–4) involved in gene expression during growth, differentiation and survival of a number of cell types (Yuan et al., 2014).
Recently, miRNAs have also emerged to be involved in the establishment of pathogenesis of ALI/ARDS. miR-21 and miR-16 alleviate pulmonary edema associated with ALI. Let-7, miR-155 and miR-146 are associated with inflammation. miR-127 is found to be down modulated in macrophages of mice with bleomycin-induced lung injury in one study (Xie et al., 2012, Vaporidi et al., 2012, Yehya et al., 2012, Xie et al., 2011). In one of the studies carried out on differential miRNA analysis of patients with acute lung injury, it was found that miR-181b inhibits NF-κβ nuclear localization and signalling and hence reducing leukocyte influx and consequently reducing lung injury (Song et al., 2012).
4. Polarization of macrophages in inflammatory diseases
It has been suggested by several studies that during inflammation, monocytes and macrophages express myeloid related proteins (belonging to S100 protein family), at a higher level. Two of them, S100A8 and S100A9 exist as calcium dependent noncovalent homodimers or heterodimers and exhibit pro inflammatory functions. It has been demonstrated that murine S100A8, also called CP-10 is a strong chemotactic factor and promotes continued leukocyte employment. S100A9 and S100A8/9 increase attachment of monocytes to endothelial cells via Mac-1/ICAM-1interactions (Ryckman et al., 2003). S100A8 proteins have been reported to stimulate chemotactic migration and persuading macrophages to induce pro-inflammatory mediators such as TNF-α (Sekimoto et al., 2015). Some of the exemplary inflammatory diseases are discussed here.
5. Infection by various pathogens
A pathogenic attack on a tissue calls for the recruitment of monocytes in circulation, which then – differentiate into macrophages depending upon the stimulus they receive from the pathogen of the environment. In the early stage of bacterial infection, macrophages express M1 phenotype (Liu et al., 2014). The macrophage populations scan their resident tissue of PAMPs (pathogen-associated molecular patterns) and DAMPs (danger associated molecular patterns). They respond to these stimuli by initiating an acute inflammatory cascade (Wynn et al., 2013). When the PAMPs present in bacteria are identified by pattern recognition receptors like TLRs (Toll-like receptors), macrophages are stimulated and polarized towards M1 phenotype thereby leading to high production of pro-inflammatory intermediators such as IL-1, TNF-α, and NO (nitric oxide), which kill the conquering pathogens and trigger adaptive immunity (Kapellos and Iqbal, 2016). This scheme of inflammation is also involved in infection with Salmonella typhi, the initial infection stages of Mycobacterium ulcerans, Mycobacterium avium and Mycobacterium tuberculosis (Sica and Mantovani, 2012). Any delay in control of macrophage-mediated inflammation leads to the discharge of a cytokine blast which plays an important role in the pathogenesis of severe sepsis (Stearns-Kurosawa et al., 2011). To counterbalance the exorbitant inflammation, macrophages go through apoptosis or transform their phenotype towards M2 type to defend the host from extreme injury and trigger wound healing (Murray and Wynn, 2011).
In case of Bronchiectasis (a chronic devitalizing airways disease designated by a domino effect of inflammation and bacterial squatting), it was found that inflammatory effects are enhanced by the presence of certain commensal bacteria such as Stromatosus mucilaginosus which could provide a cordial environment for the growth of other microbes such as Pseudomonas aeruginosa contributing to lung infection. S.mucilaginosus activates TLR2 to induce host immune response by releasing pro inflammatory cytokines from M1 macrophages (Yuan et al., 2013).
On the other hand, M2 macrophages are involved in repressing inflammation and promote tissue healing in a viral infection. In acute viral infection, stimulation by chemokines directs the recruitment of macrophages from circulation and polarizes them to a particular phenotype depending upon the type of chemokines released. These macrophages act as a robust exterminator of conquering pathogens by secreting inflammatory intermediators, such as i-NOS (inducible nitric oxide synthase) and TNF-α (Sitia et al., 2011) though, the tissue-inhabiting macrophages behave differently in viral infections. As for e.g in case of influenza virus infection, M2 phenotype accelerates the resolution of inflammation by elevating the process of phagocytosis (Hoeve et al., 2012). In severe acute respiratory syndrome (SARS)-Cov infection, STAT dependent pathway of macrophage polarization insulates the host from development of chronic fibrotic lung disease. M2 macrophages are critical for this process (Page et al., 2012). In addition, mixed M1 and M2 macrophages are associated with severe respiratory syncytial virus (RSV) induced bronchiolitis (Shirey et al., 2010). Moreover, it has been found that the activity of cyclooxygenase-2 inhibitor is impaired by IL-4-STAT6 dependent M2 macrophage polarization. Cyclooxygenase -2 inhibitor is known to exaggerate inflammation, epithelial damage and M2 macrophage expression in lungs. For this reason, it has also been proposed as a treatment strategy (Liddiard et al., 2011).
5.1. Pulmonary toxicity
The continuous exposure of lungs to extrinsic environment makes it notably susceptible to the injurious effects of acid aerosols, inhaled gases, radiation, particles and systemically administered drugs such as bleomycin and nitrogen mustard (Laskin et al., 2011). These agents may instigate acute injury and pneumonitis succeeded by pulmonary fibrosis. The severity of the pathologic response is the outcome of the dose, duration and the nature of the toxic agent. Alveolar and interstitial macrophages most prominently behave as the first line of defense against xenobiotics, although macrophages are located all over the lungs. Several receptors such as TLRs Fc portion of IgG, IgA, and IgE; and other pattern-recognition receptors are present on lung macrophages. These receptors, upon binding of the suitable ligand cause their activation and dispense proteases, ROS and RNS, pro-inflammatory lipids, and cytokines steered to safeguard the host. Moreover, they can phagocytose a number of remote substances (Laskin et al., 2001).
Ingestion of mineral dusts like silica and asbestos also leads to acute pulmonary injury succeeded by fibrosis. In case of such pulmonary injury, lung macrophages produce mediators which initiate and perpetuate inflammation along with transformation of mesenchymal cells (Gordon, 2003, Martinez et al., 2008, Mosser and Edwards, 2008). These inhaled particles bind with the MARCO (macrophage receptor with a collagenous structure) receptors present on the surface of macrophages and are phagocytosed by alveolar macrophages (Hamilton et al., 2006). However, these inert particles are indigestible; hence triggering of macrophage breach leads to a burst of proteases and chemokines which intensifies inflammation, subsequently leading to tissue injury. Latest evidences have demonstrated that it is an NLRP3 inflammasome dependent response. Inflammasome is a multiprotein scaffold present in cytosol, and is critical for inflammatory response activation (Cassel et al., 2008). Silica activated alveolar macrophages also release mitogens, TGF-β and chemotactic factors for lung fibroblasts which aid in promoting lung fibrosis (Kuhlmann et al., 2009). When animals are subjected to bleomycin and radiation, macrophages and intermediators of inflammation to lung fibrosis contribute in a similar fashion (Murray et al., 2010, Buttner et al., 1997).
Evidences have clearly stated the part of M2 macrophages in the augmentation of chronic lung disease from studies that states pulmonary fibrosis worsens in animals over expressing IL-10 or IL-13 (Barbarin et al., 2005, Lee et al., 2001) but decreases in animals that (a) are treated, with serum amyloid P (an inhibitor of M2 macrophage development in the lung) (Murray et al., 2010); (b) lack M2 chemokine CCR2 (Okuma et al., 2004) or are IL-4 deficient (Migliaccio et al., 2008); or (c) have been administered with anti-CCL2/MCP-1 gene therapy (Inoshima et al., 2004). Impressively, it has been shown that alternatively activated M2 macrophages are cynical in retarding actions of M1 phenotype and an equilibrium in their action determines the output of the harmful response. These results are in concord with reports stating that development of fibrosis in a granulomatous lung disease model is analogous with up-regulation of arginase, the M2 marker, and that over expression of NOS-2 and reduced fibrosis is due to administration of IL-12 (Hesse et al., 2000).
5.2. Allergy/asthma
Allergy is associated with M2 polarization of macrophages driven by Th2 cells and products (Kim et al., 2010, Melgert et al., 2011). It represents an instance of type 2 inflammation driven by IL-4/IL-13. IL-4 inducible chemokines acting on CCR4 (e.g., CCL22) has been noted to crook macrophage function (Trujillo et al., 2008). It has been evidenced that pathogenesis of allergy involves effective role of chitin and arginase-dependent M2 pathways (Zhu et al., 2004, Reese et al., 2007). However, there are certain evidences stating that allergic inflammation can also be driven by the inflammasome/IL-1/Th17 pathway (Ather et al., 2011). In addition, allergic inflammation also incriminates IL-18, (a Th1-associated cytokine) (Tsutsui et al., 2004). Therefore, it is possibly unsurprising that mixed phenotype of macrophages are involved in allergic inflammation.
Asthma is a chronic inflammatory disease prevalent in both developing and developed countries. Disturbances in the reconciliation of pulmonary macrophage phenotypes contributes to the pathogenesis of asthma (Moreira and Hogaboam, 2011). M2 phenotype of macrophages, appreciative of tissue repair and refurbishment of homeostasis in the lung tissue microenvironment, play a leading role in asthma. However, airway hyper-responsiveness may result due to imprudent M2 macrophages which increase cell recruitment and mucus secretion. It has been found that the inflammatory response, collagen deposition and pathophysiological process of asthma is accelerated on transferring M2 macrophages into the lungs of fungus-induced asthmatic model of mice (Moreira et al., 2010). With increasing research in macrophage polarization, several evidences have been found to reassure that M1 macrophages subscribe to the progression of asthma. In cases of more complicated asthmatic forms, particularly glucocorticoid therapy resistant patients, macrophages adopt M1 phenotype resulting in high production of pro-inflammatory mediators, including TNF-α, IL-1β, NO, intensification of the lung injury and acceleration of the airway remodelling (Kim et al., 2007). This can be explained as NO released by M1 macrophages mediates oxidative DNA damage, inflammation and aggravated mucus secretion which intensifies lung injury in murine model of allergen induced airway disease (Naura et al., 2010). Thus, exploring the ways to maintain the order of regulation of pulmonary macrophage phenotypes would provide us the way for development of improved therapeutic strategies.
It has been recently found that some of the prospects of asthma are also associated with miRNA deregulation. miR-145 reduces pro-inflammatory cytokines IL-13, IL-5 and hence diminishes asthmatic symptoms. miR-146a decreases the secretion of several pro-inflammatory cytokines and chemokines. miR-21 targets IL-21, the prime cytokine implicated in Th1 cell polarization. Similarly, negative regulation of let-7 reduces the level of IL-13, an important molecule of allergic events exhibiting a role in Th2 response. (Kumar et al., 2011, Nahid et al., 2009)
5.3. Sepsis
Sepsis is an immoderate inflammatory response and an immunosuppressive disorder that arises due to infection that gets into the blood or acute insults. (Hotchkiss et al., 2013). Secondary infection is the most prominent cause of severe sepsis or septic shock related mortality. It is a result of debilitation of the host immune response due to apoptosis of immune cells and high production of regulatory T cells (Jiang et al., 2012) and myeloid derived suppressor cells (Delano et al., 2007). M2 phenotype is commonly involved in the development of sepsis related immunosuppressive state. It has been evidenced by Porta and his co researchers that p50 subunit of the NF-κβ is the main controller of this process. They have also stated that LPS-resistant macrophages and M2 macrophages exhibit similar characteristics (Porta et al., 2009). Therefore, revealing the mechanisms of macrophage polarization in differential progression of sepsis and maintaining its equilibrium may cater a probable therapeutic target for septic complications.
For the treatment of sepsis related ARDS, strategies based upon deletion of Akt2 kinase have been used in mice. These strategies work on enhancing M2 polarization of alveolar macrophages while totally negating M1 polarization. However, innate immune response required for effective bacterial clearance by alveolar macrophages is impeded on complete arrest of classical macrophage activation. Hence, its use is limited to only aseptic conditions and not live bacteria. Therefore, therapeutic strategies based on augmenting M2 activation while retaining M1 plasticity would be more favourable for the treatment. Immunoproteasome-mediated inhibition seems to be a more appreciative approach according to some authors (Moreira-Teixeira et al., 2016). The proteasome is involved in regulating intracellular signalling by degradation of a number of signalling mediators. Immunoproteasome activity increases on stimulation of all three subunits. The three subunits, LMP, LMP7 and multicatalytic endopeptidase complex-like 1 are induced by bacterial LPS or IFNγ which drives alveolar macrophage polarization towards M1 phenotype. Thus, M1phenotype prompts all the three subunits of immunoproteasome. On the other hand, alternatively activated M2 macrophages increases immunoproteasome activity by aggravating expression of LMP2 and LMP7 subunits only (Chen et al., 2016).
5.4. Chronic obstructive pulmonary disorder (COPD)
Chronic exposure of the airways to irritants such as cigarette smoke leads to epithelial cell injury, destruction of pulmonary capillary vasculature, acceleration of epithelial cell senescence and airway remodelling resulting in the loss of lung compliance which ultimately leads to COPD (MacNee and Donaldson, 2003, Di Stefano et al., 2004). The prolonged persistence of inflammation is an important specifying feature of COPD (Pearson, 2013). It has been found that a group of endothelial-derived microparticles (EMPs) increase in patients with stable COPD and during exacerbation. These EMPs contain E-selectin, platelet endothelial cell adhesion molecule and vascular endothelial cadherin. Lung destruction and air flow limitation can be correlated with levels of EMPS in stable COPD (Kratzer et al., 2013, Takahashi and Kubo, 2014). Most of the alveolar macrophages are normally non-polarized but in COPD, there is a significant increase in macrophage polarization and co-expression of M1 and M2 macrophages (Bazzan et al., 2017). An important feature of COPD pathogenesis is the release of a number of inflammatory mediators such as TNF-α, IL-1β, GM-CSF, TGFβ, and CXCL-8, by lung epithelial cells. These mediators act in both autocrine and paracrine manner. The main cause of fibrosis development is airway remodelling which is induced by TGFβ mediated myofibroblast differentiation.The severity of airway obstruction can be correlated with the level of TGFβ in the small airway epithelium of COPD patients (Tufekci et al., 2014).
Zaynagetdinov et al. (Zaynagetdinov et al., 2016) demonstrated that chronic airway inflammation is a NF-κβ driven process which leads to the alternative activation of alveolar macrophages, resulting in TGFβ/IL-10/retinoic acid-dependent generation and maintenance of Foxp3+ Tregs. In addition, they also demonstrated that despite the presence of chronic airway inflammation, the development and maintenance of an immunosuppressive microenvironment in the lungs is induced by the amplified expression of TGFβ and IL-10 by alveolar macrophages. Thus, these molecules and pathways could be potential targets to reduce the excessive risk of lung cancer in individuals accompanied by chronic lung inflammation related to COPD.
In a recent study based on miRNA/mRNA microarray expression analysis on human alveolar macrophages from smokers and non-smokers it was found that – reduced expression of miR-152 up regulates the expression of MMP12, responsible for development of emphysema. This study demonstrated a correlation between macrophage polarization and miRNA deregulation (Graff et al., 2012). Another study demonstrated that miR-146a regulates the duration and intensity of inflammation by down regulating COX2 and PGE2 which are the important mediators of tissue inflammation (Sato et al., 2010).
5.5. Tuberculosis
Tuberculosis (TB) is a worldwide spread infectious disease, caused by Mycobacterium tuberculosis (Mtb). The precise mechanisms of its pathogenesis are unspecified. Macrophages play an important role in eliminating Mtb in the way as Mtb are inhaled as droplets from air are phagocytosed by alveolar macrophages in the lung and killed by ROS in the phagolysosome resulting in the recruitment of mononuclear cells (Behar et al., 2011). They are the cells of first line of immune defense to confront and eliminate mycobacteria (Behar et al., 2010). Resistance against intracellular parasites and acceleration of inflammatory responses is mediated by classically activated (M1) macrophages (Behar et al., 2010). On the other hand, alternatively activated M2 macrophages participate in tissue repair, tumour progression, restraint of inflammation, and promotion of Th2 responses (Liu et al., 2014). Macrophage polarization is a very important aspect of disease progression and resolution of inflammatory or infectious processes (Labonte et al., 2014, Sica et al., 2014, Benoit et al., 2008). In fact, it has also been shown that anti-microbial activity and cytokine production during TB granuloma formation is modulated by macrophage polarization (Marino et al., 2015, Huang et al., 2015). Thus, it might also play an important role in Mtb infection (Saiga et al., 2011, Sakamoto, 2012). Host protection is impeded by type I IFN which is being evidenced to play an undesirable role in pathogenesis of tuberculosis by interfering IFN-γ dependent immunity (Moreira-Teixeira et al., 2016).
It is well evidenced that fusion of lysosomes with Mtb-containing phagosomes and iNOS up-regulation is triggered by M1 polarized macrophages (Sica et al., 2014). Up-regulation of microbicidal enzymes and iNOS generation is also associated with M1 macrophages. Importantly, utilization of TLR2 signalling pathway is also ensured by M1 polarization. It has also been suggested that MyD88-dependent TLR2 signalling is inhibited by Mtb (Pathak et al., 2007). Lim et al. suggested that during Mtb infection, the TLR2/MyD88 signalling pathway is relatively more associated with M1 macrophage activation rather than M2 polarization (Lim et al., 2016). Therefore, inhibition of the intracellular survival of Mtb would be furnished by M1 macrophage activation via the TLR2 signalling pathway which would be beneficial for the host. Cell death by apoptosis is also an important mechanism of host defense against Mtb infection, but the underlying molecular mechanisms are not clear. Intrinsic and extrinsic signals can trigger apoptosis, and such signals can also activate macrophages.
5.6. Bronchopulmonary dysplasia (BPD)
It is a chronic lung disease prevalent in premature babies. The causative factors of BPD are life saving interventions including high airway pressures and oxygen tension in the course of mechanical ventilation. The pathogenesis of BPD consist of postnatal suspension of alveolarisation along with noticeable decrease in type I alveolar epithelial cells (AEC1); and reduction in septal crust density (Lim et al., 2015).
Macrophages are significant components of both neonatal and adult lung immunity. Neonates are vulnerable to several pathogenic infections such as pneumonia leading to localized lung inflammation which is considered very important in the pathogenesis of BPD (Sierra-Filardi et al., 2011) (Kollmann et al., 2012). Neonatal monocytes respond normally to such pathogenic attacks leading to release of inflammatory cytokines such as TNFα and IL-6 but preterm infants have decreased IL-10 release (Huber et al., 2009). Aggravated release of these pro-inflammatory cytokines may have a deleterious effect on host’s health (Syed and Bhandari, 2013).
It has been shown by our group that hyperoxia or hyperoxia plus LPS exposure leads to aggravation of inflammation induced-M1phenotype and suppression of M2 phenotype in macrophages. The suggested causal factor behind these modifications in macrophage polarization state from M2 to M1 is the lack of BRP-39 in the developing lung (Syed and Bhandari, 2013). BRP-39 (Breast Regression Protein-39) is a chitinase-like protein present in a number of cells such as monocytes and macrophages (Gwyer Findlay and Hussell, 2012). There are several other factors which have potential clinical relevance with neonatal inflammatory imbalances during pulmonary development. One of the factors is Activin A which is a member of TGFβ family and is implicated in repair and survival of type-II AEC (Buckley et al., 2008). Inhibition of Activin A signalling provides protection against hyperoxia induced pathological lung remodelling, growth retardation and leukocyte infiltration. Moreover, it also inhibits anti-inflammatory M2 phenotype by down regulating IL-10 production in-vitro, suggesting their role in progressive BPD (Sierra-Filardi et al., 2011). Thus, addressing the inter-link between inflammatory imbalances in lung development of neonates and macrophage modulation may provide us a potential interposition to cure BPD.
6. Repolarization of macrophages – potential therapy
Reprogramming of macrophages to switch their phenotype could be a potential therapeutic strategy (Das et al., 2015). This can be explained with the help of few examples such as (a) Activin A, a pluoripotent TGFβ family growth and differentiation factor, regulates the cellular growth of a number of cell types and attunes myeloid cell release of cytokines and chemokines. It is also inculcated in macrophage polarization by stimulating Arg-1 expression and hindering expression of IFN-γ-induced NO synthase 1 suggesting its role as a Th2 cytokine and thereby promoting alternate activation of M2 macrophages in mouse. Contrarily, in humans, macrophage polarization is directed towards M1 phenotype by high levels of Activin A (Sierra-Filardi et al., 2011). (b) Schistosoma mansoni – derived soluble egg antigens (SEAs) initiates immune responses involving M2 macrophages and can forestall inflammatory responses. Therefore, its use may be advantageous in treatment of chronic inflammatory diseases (Wolfs et al., 2014). Some of the phenotype switch strategies are based upon inhibition of mitochondrial respiration thereby blocking the M2 phenotype and initiating M1 phenotypic state. Propelling oxidative metabolism towards M1 macrophage enhances M2 macrophage activation. Haschemi etal measured the effects on macrophage polarization by changing CARKL expression. It was found that upon LPS stimulation, constitutional CARKL expression decreased glycolysis in macrophage cell lines resulting in activation of M2 macrophages. On the contrary, RNAi mediated abscission of CARKL expression drive macrophages towards M1 phenotype before LPS stimulation (Haschemi et al., 2012).
As suggested by above illustrations, it can be stated that manipulation of macrophage polarization is clinically successful to some extent. At the same time, it is also well established that many pathological conditions express a mixed population of macrophages. Thus, the influence of disease evolutionary changes and progression should be taken into account very cautiously on a case-to-case basis.
6.1. Glutathione: role in macrophage repolarization
In the last few years, it has been found that reduced glutathione (GSH) is also cardinally involved in adaptive immunity by affecting the activities of macrophages and lymphocytes. It is a ubiquitous non-protein thiol present in animal cells and is involved in numerous cellular functions such as synthesis and degradation of DNA and proteins; and toxin and carcinogen detoxification. Depletion of GSH in APC (antigen presenting cells like macrophages) is related to defects in antigen processing and also inhibits Th-1 associated cytokine release. In mouse, depletion of GSH in APC reduces secretion of IL-12 (responsible for synchronization of IFN-γ production) resulting in switching over of Th1 cytokine profile towards Th2 cytokine profile (Short et al., 1996, Peterson et al., 1998, Murata et al., 2002a, Murata et al., 2002b). It also plays a key role in determining the type i.e. Th1 or Th2 response in human alveolar macrophages (Dobashi et al., 2001). Hence, we can say that M1 cells have an elevated ratio of GSH/GSSG or reduced −to-oxidized glutathione wherein IFN-γ and IL-4 exert antagonistic effects on reductive status. Further, by modifying the ratio of intracellular GSH/GSSG, M2 macrophages can be reprogrammed to M1 phenotype. It has been accomplished by using γ-Glutamylcysteinylethyl ester (γ-GCE) which could strengthen Th1 response via high production of IL-12 (Koike et al., 2007). It is also observed that low GSH/GSSG ratio favours M2 macrophages. As a consequence, measurement of GSH level of human and murine macrophages can provide us a steady biomarker. Mycobacterium tuberculosis infection is a compelling example of modification of intra-cellular redox state by intra-macrophage pathogen leading to alteration of T-cell responses. Alam et al. have illustrated the manipulation of redox state of macrophages using N-acetyl cysteine (NAC, a glutathione precursor) (Alam et al., 2010).
Other molecules that can also be used to fortify Th1 response are I-152 (a cysteine precursor involved in synthesis of glutathione) (Utsugi et al., 2003); n-butanoyl GSH derivative (GSH-C4); GSH-monoethyl ester (GSH-OEt) and LPS- induced IL-12 p40 protein production in human macrophages (Fraternale et al., 2010). Therefore, these molecules can be considered as new immunomodulators (Fraternale et al., 2011).
7. mi-RNA (micro RNA) and macrophage plasticity
Micro RNAs or miRNAs are highly conserved and regulatory small non coding RNAs ~ 22nts in length. They are indistinguishable to intermediators of RNA interference and attune various physiological processes like immune cell activation, differentiation and apoptosis. They bind to complementary base pairs on target mRNAs leading to inhibition of translation. Three miRs, miR-155, miR-124 and miR-223 have been identified to be intricated in macrophage polarization (Zhuang et al., 2012). miR-124 is known to promote latency of macrophages and clampdown of experimental autoimmune encephalomyelitis. It binds three presumptive binding sites present in the 3′ UTR (untranslated region) of CCAAT/enhancer binding protein-α (C/EBPα) (a monocytic transcription factor) leading to inhibition of mRNA translation. High expression of miR-124 up regulates M2 associated cytokines such as Arg-1 and TGF-β1, and decreases activation of myelin-specific T cells and notable disease annihilation (Tugal et al., 2013). Expression of miR-155 is initiated by bacterial LPS. It limits expression of C/EBPβ and assists M1 macrophage polarization (He et al., 2009), miR-223, also favours M1 polarization and adjourns M2 polarization on LPS/IL-4 stimulation. Pknox1, gene has been known to be implicated in miR-223 mediated macrophage polarization (Zhuang et al., 2012). miRNAs such as miR-146, miR-9, miR-125b and miR-155 ameliorate TLR4/IL-1R signalling pathways in macrophages and monocytes suggesting macrophage phenotype transformation from pro-inflammatory to anti-inflammatory types (Baltimore et al., 2008). miR-17, miR-20a and miR-106a down regulates the expression of signal regulatory protein α (SIRPα), a macrophage differentiation related marker (Zhu et al., 2013). miR-21 and miR-98 down regulates the expression of inflammatory genes in monocytes and macrophages (Liu et al., 2011). Let-7c has been found to be associated with macrophage modulation. It is highly expressed in M2 macrophages (Banerjee et al., 2013). miR-155 has also been established as a key molecule in shifting macrophage polarization towards M1-type (Cai et al., 2012b). One of our study has shown that up-regulation of miR-155 is responsible for the pro inflammatory effects of TREM-1 receptors selectively expressed on macrophages. These changes are mediated by down-regulation of SOCS-1(suppressor of cytokine signalling), target of miR-155. Our data shows thatTREM-1gene silencing leads to survival improval in LPS-induced neutrophillic lung inflammation. It could also improve mitochondrial bioenergetics which would be immunometabolically advantageous to mitochondrial respiration with low rate of O2 consumption. We have also demonstrated a novel nanomicellar (LP17 nanomedicine) approach to inhibit TREM-1 effects. Thus, our study suggests TREM-1 to be a potential therapeutic target for treatment of ARDS (Yuan et al., 2016).
8. Compounds affecting macrophage polarization
Some natural compounds affect macrophage polarization to exert their anti inflammatory effect. Shikonin, the major component of root extracts of Lithospermum erythrorhizon, inhibits the transcriptional activity of the TNF-α promoter suggesting its potential as an anti-inflammatory therapeutic (Cheng et al., 2008). Chlorogenic acid is a phenolic compound found in coffee, apples, pears and green tea which exerts anti-inflammatory effects through NF-κB inhibition of PGE synthesis and COX-2 and by suppressing JNK/AP-1 activation (Xue et al., 2017). It has been reported that ACA, a glycoprotein obtained from the mycelium extract of A. camphorata is capable of macrophage activation via TLR2/MyD88-dependent pathway. It is thus able to exhibit a pro inflammatory response favouring M1 phenotype of macrophages (Sheu et al., 2009). A new, sulfur-containing compound, Onionin A isolated from acetone extracts of bulbs of onion (Allium cepa), possess the ability to suppress tumor-cell proliferation by inhibiting the polarization of M2 alternatively activated macrophages (El-Aasr et al., 2010). Curcumin (diferuloylmethane), the yellow pigment of turmeric, a prime ingredient of curry spice is also known to exhibit anti-inflammatory effects induced by inhibition of TREM-1 receptors on LPS exposure. It inhibits the p300 activity in TREM promoter resulting in epigenetic changes involving hypoacetylation of histone 3 and 4 in the lysine residues of chromatin. This leads to reduced binding of p65 component of NF-κβ to TREM-1 promoter resulting in dampening of TREM-1 expression (Yuan et al., 2012).
9. Conclusions
Macrophages are important cellular components of both innate and acquired immunity. They are implicated in tissue repair and wound healing activities and also act as a shield against invading pathogens and toxicants. The diverse functions of macrophages are attributable to the heterogeneity in their activation which is a stimulus driven process. Classically activated M1 macrophages are capable of cytotoxicity whereas alternatively activated M2 macrophages are capable of suppressing inflammation and initiation of wound repair. These activities are a result of highly regulated signal pathways involving a number of molecular intermediators such as growth factors, cytokines, oxidants and lipid mediators released by different macrophage subpopulations. These processes are tightly regulated under the conditions of homeostasis. Compromise of control mechanisms, asymmetric activity of macrophages, plethoric release of pro-inflammatory, cytotoxic and fibrogenic products bolster tissue injury and pathogenesis of chronic inflammatory disease. Apart from transient, plastic and irreversible M1-M2 phenotypic transformations, macrophages also have the ability to rapidly switch over to a different phenotype or repolarise in response to the micro environmental stimuli received from encompassing tissues. In addition, several natural compounds possess the ability to modulate macrophage polarisation in order to exhibit their anti-inflammatory, anti-oxidative and anti-carcinogenic actions. Furthermore, a contemporary class of non-coding RNAs, called micro-RNAs or miRNAs are also involved in activation as well as suppression of genes of immune system cells. Thus, miRNA target prediction and comprehensive characterization of differentially expressed miRNAs could help us to elucidate their specific roles in lung injury. Although several studies have clearly indicated the role of resident and recruited alveolar macrophages in process of repair initiation in infectious as well as inflammatory diseases, a lot more remains to be versed about the cellular communication between macrophages and several anti-inflammatory cells. Other questions that need to be answered are the molecular markers involved in macrophage polarization. Thus, finding answers to these questions can pave us a way to manipulate the mechanisms of polarization in order to improve the disease conditions.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Author’s contribution
SA, KD and MAS conceived the idea, SA performed the literature search and wrote the manuscript. MAS, KD, PD and BA provided inputs for the strategy and final edition of the article. All authors read and approved the final manuscript.
Funding
We thank DST (Department of Science and Technology), India for funding under Ramanujan Award (SERB) No: SB/S2IRJN-199/2014.
Ethics approval and consent to participate
Not applicable
Conflict of interest
None.
Acknowledgement
We thank Jamia Millia Islamia for providing internet facility and access to journals.
References
- Agarwal R., et al. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006;130(3):724–729. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- Alam K., et al. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages: possible implications in antituberculosis immunotherapy. J. Immunol. 2010;184(6):2918–2929. doi: 10.4049/jimmunol.0900439. [DOI] [PubMed] [Google Scholar]
- Amano H., et al. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J. Immunol. 2004;172(1):398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- Anderson C.F., Mosser D.M. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 2002;72(1):101–106. [PubMed] [Google Scholar]
- Arango Duque G., Descoteaux G.A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather J.L., et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 2011;187(1):64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., et al. MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 2008;9(8):839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- Banerjee S., et al. MicroRNA let-7c regulates macrophage polarization. J. Immunol. 2013;190(12):6542–6549. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin V., et al. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288(5):L841–8. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- Bazzan E., et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir. Res. 2017;18(1):40. doi: 10.1186/s12931-017-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S.M., Divangahi M., Remold H.G. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 2010;8(9):668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S.M., et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4(3):279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M., Desnues B., Mege J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Biswas S.K., et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Bouhlel M.A., et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Buckley S., et al. TGF-beta signaling promotes survival and repair in rat alveolar epithelial type 2 cells during recovery after hyperoxic injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294(4):L739–48. doi: 10.1152/ajplung.00294.2007. [DOI] [PubMed] [Google Scholar]
- Buttner C., et al. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am. J. Respir. Cell Mol. Biol. 1997;17(3):315–325. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- Cai Z.G., et al. MicroRNAs are dynamically regulated and play an important role in LPS-induced lung injury. Can. J. Physiol. Pharmacol. 2012;90(1):37–43. doi: 10.1139/y11-095. [DOI] [PubMed] [Google Scholar]
- Cai X., et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4(5):341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- Cao Y., et al. MicroRNAs: novel regulatory molecules in acute lung injury/acute respiratory distress syndrome. Biomed. Rep. 2016;4(5):523–527. doi: 10.3892/br.2016.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S.L., et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105(26):9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L., Cassol E., Poli G. Macrophage polarization in health and disease. Sci. World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., et al. Immunoproteasome dysfunction augments alternative polarization of alveolar macrophages. Cell Death Differ. 2016;23(6):1026–1037. doi: 10.1038/cdd.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.W., et al. Shikonin derivatives inhibited LPS-induced NOS in RAW 264. 7 cells via downregulation of MAPK/NF-kappaB signaling. J. Ethnopharmacol. 2008;120(2):264–271. doi: 10.1016/j.jep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chizzolini C., et al. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte-macrophage colony-stimulating factor-differentiated human monocytes. J. Immunol. 2000;164(11):5952–5960. doi: 10.4049/jimmunol.164.11.5952. [DOI] [PubMed] [Google Scholar]
- Das A., et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015;185(10):2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano M.J., et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A., et al. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin. Exp. Allergy. 2004;34(8):1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- Dobashi K., et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin. Exp. Immunol. 2001;124(2):290–296. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S.L., Jefferies C.A., O'Neill L.A. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J. Biol. Chem. 2005;280(25):23496–23501. doi: 10.1074/jbc.C500053200. [DOI] [PubMed] [Google Scholar]
- Doyle S.L., et al. Signaling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. J. Biol. Chem. 2007;282(51):36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- Duffield J.S. The inflammatory macrophage: a story of Jekyll and Hyde. Clin. Sci. (Lond.) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- El-Aasr M., et al. Onionin A from Allium cepa inhibits macrophage activation. J. Nat. Prod. 2010;73(7):1306–1308. doi: 10.1021/np100105u. [DOI] [PubMed] [Google Scholar]
- El-Gayar S., et al. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J. Immunol. 2003;171(9):4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- Fraternale A., et al. The increase in intra-macrophage thiols induced by new pro-GSH molecules directs the Th1 skewing in ovalbumin immunized mice. Vaccine. 2010;28(48):7676–7682. doi: 10.1016/j.vaccine.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Fraternale A., et al. Modulation of Th1/Th2 immune responses to HIV-1 Tat by new pro-GSH molecules. Vaccine. 2011;29(40):6823–6829. doi: 10.1016/j.vaccine.2011.07.101. [DOI] [PubMed] [Google Scholar]
- Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Graff J.W., et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One. 2012;7(8):e44066. doi: 10.1371/journal.pone.0044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratchev A., et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand. J. Immunol. 2001;53(4):386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- Grinberg S., et al. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am. J. Pathol. 2009;175(6):2439–2453. doi: 10.2353/ajpath.2009.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Guo C., et al. The role of tumor-associated macrophages in tumor vascularization. Vasc. Cell. 2013;5(1):20. doi: 10.1186/2045-824X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., et al. Antisense oligonucleotide treatment enhances the recovery of acute lung injury through IL-10-secreting M2-like macrophage-induced expansion of CD4+ regulatory T cells. J. Immunol. 2013;190(8):4337–4348. doi: 10.4049/jimmunol.1203233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyer Findlay E., Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012;2012:140937. doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R.F., Jr., et al. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J. Biol. Chem. 2006;281(45):34218–34226. doi: 10.1074/jbc.M605229200. [DOI] [PubMed] [Google Scholar]
- Hart P.H., et al. Interleukin-4 stimulates human monocytes to produce tissue-type plasminogen activator. Blood. 1989;74(4):1222–1225. [PubMed] [Google Scholar]
- Haschemi A., et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., et al. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol. Immunol. 2009;6(5):343–352. doi: 10.1038/cmi.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S., et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 2008;205(13):3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S., Mayer K., Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., et al. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant: IL-12, in a Th2 model of granulomatous disease. Am. J. Pathol. 2000;157(3):945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeve M.A., et al. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One. 2012;7(1):e29443. doi: 10.1371/journal.pone.0029443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood N.J., et al. Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. J. Exp. Med. 2003;197(12):1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood N.J., et al. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF: but not IL-6, production. J. Immunol. 2006;176(6):3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R.S., Monneret G., Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., et al. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas In vitro. PLoS One. 2015;10(6):e0129744. doi: 10.1371/journal.pone.0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S., et al. Activin a promotes the TGF-beta-induced conversion of CD4+CD25-T cells into Foxp3+ induced regulatory T cells. J. Immunol. 2009;182(8):4633–4640. doi: 10.4049/jimmunol.0803143. [DOI] [PubMed] [Google Scholar]
- Inoshima I., et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286(5):L1038–44. doi: 10.1152/ajplung.00167.2003. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jha A.K., et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Jiang L.N., Yao Y.M., Sheng Z.Y. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. J. Interferon Cytokine Res. 2012;32(8):341–349. doi: 10.1089/jir.2011.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.K., et al. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012;47(4):417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellos T.S., Iqbal A.J. Epigenetic control of macrophage polarisation and soluble mediator gene expression during inflammation. Mediators Inflamm. 2016;2016:6591703. doi: 10.1155/2016/6591703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.K., et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J. Immunol. 2007;178(8):5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., DeKruyff R.H., Umetsu D.T. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010;11(7):577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike Y., et al. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2007;37(3):322–329. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- Kollmann T.R., et al. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooguchi K., et al. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 1998;66(7):3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer A., et al. Endothelial cell adhesion molecule CD146: implications for its role in the pathogenesis of COPD. J. Pathol. 2013;230(4):388–398. doi: 10.1002/path.4197. [DOI] [PubMed] [Google Scholar]
- Krausgruber T., et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- Kuhlmann U.C., et al. Modulation of cytokine production and silica-induced lung fibrosis by inhibitors of aminopeptidase N and of dipeptidyl peptidase-IV-related proteases. Life Sci. 2009;84(1–2):1–11. doi: 10.1016/j.lfs.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Kumar M., et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J. Allergy Clin. Immunol. 2011;128(5) doi: 10.1016/j.jaci.2011.04.034. 1077-85 e1-10. [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J., et al. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J. Immunol. 2006;176(10):5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- Labonte A.C., Tosello-Trampont A.C., Hahn Y.S. The role of macrophage polarization in infectious and inflammatory diseases. Mol. Cells. 2014;37(4):275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin D.L., Weinberger B., Laskin J.D. Functional heterogeneity in liver and lung macrophages. J. Leukoc. Biol. 2001;70(2):163–170. [PubMed] [Google Scholar]
- Laskin D.L., et al. Macrophages and tissue injury: agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lee C.G., et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J. Exp. Med. 2001;194(6):809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.G., et al. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc. Natl. Acad. Sci. U. S. A. 2012;109(15):5791–5796. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., et al. Paracrine factors from mesenchymal stem cells: a proposed therapeutic tool for acute lung injury and acute respiratory distress syndrome. Int. Wound J. 2014;11(2):114–121. doi: 10.1111/iwj.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddiard K., et al. Macrophage heterogeneity and acute inflammation. Eur. J. Immunol. 2011;41(9):2503–2508. doi: 10.1002/eji.201141743. [DOI] [PubMed] [Google Scholar]
- Lim R., et al. Activin A contributes to the development of hyperoxia-induced lung injury in neonatal mice. Pediatr. Res. 2015;77(6):749–756. doi: 10.1038/pr.2015.46. [DOI] [PubMed] [Google Scholar]
- Lim Y.J., et al. Roles of endoplasmic reticulum stress-mediated apoptosis in M1-polarized macrophages during mycobacterial infections. Sci. Rep. 2016;6:37211. doi: 10.1038/srep37211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., et al. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 2011;585(12):1963–1968. doi: 10.1016/j.febslet.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., et al. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014;10(5):520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W., Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur. Respir. J. Suppl. 2003;40:47s–51s. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar G.H., et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34(5):715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sica A., Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Marino S., et al. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect. Immun. 2015;83(1):324–338. doi: 10.1128/IAI.02494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott H.M., Dockrell D.H. The role of the macrophage in lung disease mediated by bacteria. Exp. Lung Res. 2007;33(10):493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.O., et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Martinez F.O., et al. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Melgert B.N., et al. More alternative activation of macrophages in lungs of asthmatic patients. J. Allergy Clin. Immunol. 2011;127(3):831–833. doi: 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- Mendoza-Coronel E., Ortega E. Macrophage polarization modulates fcgammaR- and CD13-mediated phagocytosis and reactive oxygen species production, independently of receptor membrane expression. Front. Immunol. 2017;8:303. doi: 10.3389/fimmu.2017.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio C.T., et al. The IL-4Ralpha pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J. Leukoc. Biol. 2008;83(3):630–639. doi: 10.1189/jlb.0807533. [DOI] [PubMed] [Google Scholar]
- Misharin A.V., et al. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013;49(4):503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell M., et al. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl. Trop. Dis. 2009;3(7):e480. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.P., Hogaboam C.M. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J. Interferon Cytokine Res. 2011;31(6):485–491. doi: 10.1089/jir.2011.0027. [DOI] [PubMed] [Google Scholar]
- Moreira A.P., et al. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J. Allergy Clin. Immunol. 2010;126(4) doi: 10.1016/j.jaci.2010.06.010. 712-721 e7. [DOI] [PubMed] [Google Scholar]
- Moreira-Teixeira L., et al. Type I IFN inhibits alternative macrophage activation during Mycobacterium tuberculosis infection and leads to enhanced protection in the absence of IFN-gamma signaling. J. Immunol. 2016;197(12):4714–4726. doi: 10.4049/jimmunol.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S., Peiser L., Gordon S. Activation of murine macrophages by Neisseria meningitidis and IFN-gamma in vitro: distinct roles of class A scavenger and Toll-like pattern recognition receptors in selective modulation of surface phenotype. J. Leukoc. Biol. 2004;76(3):577–584. doi: 10.1189/jlb.0104014. [DOI] [PubMed] [Google Scholar]
- Murata Y., et al. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol. Immunol. 2002;38(10):747–757. doi: 10.1016/s0161-5890(01)00111-0. [DOI] [PubMed] [Google Scholar]
- Murata Y., Shimamura T., Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.A., et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5(3):e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.J., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid M.A., et al. miR-146a is critical for endotoxin-induced tolerance: Implication in innate immunity. J. Biol. Chem. 2009;284(50):34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naura A.S., et al. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J. Immunol. 2010;185(5):3076–3085. doi: 10.4049/jimmunol.0904214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Gabhann J.et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One. 2014;9(1):e85834. doi: 10.1371/journal.pone.0085834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J.J., Paul W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J.I., et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Oh B., Lee M. Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J. Control. Release. 2014;175:25–35. doi: 10.1016/j.jconrel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T.A. IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J. Immunol. 1997;159(11):5474–5482. [PubMed] [Google Scholar]
- Okuma T., et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J. Pathol. 2004;204(5):594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- Page C., et al. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection. J. Virol. 2012;86(24):13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S.K., et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 2007;8(6):610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- Pearson M. Is the primary mechanism underlying COPD: inflammation or ischaemia? COPD. 2013;10(4):536–541. doi: 10.3109/15412555.2013.763781. [DOI] [PubMed] [Google Scholar]
- Peterson J.D., et al. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. U. S. A. 1998;95(6):3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C., et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl. Acad. Sci. U. S. A. 2009;106(35):14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T.A., et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell D., et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. U. S. A. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman C., et al. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003;170(6):3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- Saiga H., Shimada Y., Takeda K. Innate immune effectors in mycobacterial infection. Clin. Dev. Immunol. 2011;2011:347594. doi: 10.1155/2011/347594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet. Pathol. 2012;49(3):423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- Sato T., et al. Reduced miR-146a increases prostaglandin E(2)in chronic obstructive pulmonary disease fibroblasts. Am. J. Respir. Crit. Care Med. 2010;182(8):1020–1029. doi: 10.1164/rccm.201001-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010;11(10):936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- Schlaepfer E., et al. Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J. Virol. 2014;88(17):9769–9781. doi: 10.1128/JVI.01053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonthaler H.B., Guinea-Viniegra J., Wagner E.F. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann. Rheum. Dis. 2011;70(Suppl. 1):109–112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- Sekimoto R., et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc. Natl. Acad. Sci. U. S. A. 2015;112(16):E2058–66. doi: 10.1073/pnas.1409480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu F., et al. Purification: cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (bitter mushroom) that exhibits TLR2-dependent NF-kappaB activation and M1 polarization within murine macrophages. J. Agric. Food Chem. 2009;57(10):4130–4141. doi: 10.1021/jf900469a. [DOI] [PubMed] [Google Scholar]
- Shirey K.A., et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3(3):291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S., et al. Defective antigen processing correlates with a low level of intracellular glutathione. Eur. J. Immunol. 1996;26(12):3015–3020. doi: 10.1002/eji.1830261229. [DOI] [PubMed] [Google Scholar]
- Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59(5):2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- Sierra-Filardi E., et al. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117(19):5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- Sitia G., et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011;7(6):e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone Y., Serikov V.B., Staub N.C., Sr. Intravascular macrophage depletion attenuates endotoxin lung injury in anesthetized sheep. J. Appl. Physiol. (1985) 1999;87(4):1354–1359. doi: 10.1152/jappl.1999.87.4.1354. [DOI] [PubMed] [Google Scholar]
- Song C., et al. Alveolar macrophage-derived vascular endothelial growth factor contributes to allergic airway inflammation in a mouse asthma model. Scand. J. Immunol. 2012;75(6):599–605. doi: 10.1111/j.1365-3083.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- Stearns-Kurosawa D.J., et al. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R.D., Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol. Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed M.A., Bhandari V. Hyperoxia exacerbates postnatal inflammation-induced lung injury in neonatal BRP-39 null mutant mice promoting the M1 macrophage phenotype. Mediators Inflamm. 2013;2013:457189. doi: 10.1155/2013/457189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed M.A., et al. Expression of TREM-1 is inhibited by PGD2 and PGJ2 in macrophages. Exp. Cell Res. 2010;316(19):3140–3149. doi: 10.1016/j.yexcr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Tacke F., et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. 2006;203(3):583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kubo H. The role of microparticles in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2014;9:303–314. doi: 10.2147/COPD.S38931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarapu Parthasarathy P., et al. MicroRNA 16 modulates epithelial sodium channel in human alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2012;426(2):203–208. doi: 10.1016/j.bbrc.2012.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torocsik D., et al. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell. Mol. Life Sci. 2005;62(18):2132–2139. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Trujillo G., et al. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 2008;172(5):1209–1221. doi: 10.2353/ajpath.2008.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., et al. Induction of allergic inflammation by interleukin-18 in experimental animal models. Immunol. Rev. 2004;202:115–138. doi: 10.1111/j.0105-2896.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Tufekci K.U., et al. The role of microRNAs in human diseases. Methods Mol. Biol. 2014;1107:33–50. doi: 10.1007/978-1-62703-748-8_3. [DOI] [PubMed] [Google Scholar]
- Tugal D., Liao X., Jain M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- Utsugi M., et al. c-Jun N-terminal kinase negatively regulates lipopolysaccharide-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production. J. Immunol. 2003;171(2):628–635. doi: 10.4049/jimmunol.171.2.628. [DOI] [PubMed] [Google Scholar]
- Vaporidi K., et al. Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303(3):L199–207. doi: 10.1152/ajplung.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., et al. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20(6):701–712. doi: 10.1038/cr.2010.52. [DOI] [PubMed] [Google Scholar]
- Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs I.M., et al. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB J. 2014;28(1):288–299. doi: 10.1096/fj.13-235911. [DOI] [PubMed] [Google Scholar]
- Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development: homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., et al. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol. Genomics. 2011;43(9):479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., et al. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcgamma receptor I. J. Immunol. 2012;188(5):2437–2444. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue N., et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017;7:39011. doi: 10.1038/srep39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya N., et al. MicroRNA modulate alveolar epithelial response to cyclic stretch. BMC Genomics. 2012;13:154. doi: 10.1186/1471-2164-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., et al. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int. J. Biochem. Cell Biol. 2012;44(11):2032–2043. doi: 10.1016/j.biocel.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Yuan Z., et al. Induction of cyclooxygenase-2 signaling by Stomatococcus mucilaginosus highlights the pathogenic potential of an oral commensal. J. Immunol. 2013;191(7):3810–3817. doi: 10.4049/jimmunol.1300883. [DOI] [PubMed] [Google Scholar]
- Yuan Z., et al. Triggering receptor expressed on myeloid cells 1 (TREM-1)-mediated Bcl-2 induction prolongs macrophage survival. J. Biol. Chem. 2014;289(21):15118–15129. doi: 10.1074/jbc.M113.536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., et al. TREM-1-accentuated lung injury via miR-155 is inhibited by LP17 nanomedicine. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310(5):L426–38. doi: 10.1152/ajplung.00195.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R., et al. Chronic NF-kappaB activation links COPD and lung cancer through generation of an immunosuppressive microenvironment in the lungs. Oncotarget. 2016;7(5):5470–5482. doi: 10.18632/oncotarget.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., et al. TREM-1 expression in macrophages is regulated at transcriptional level by NF-kappaB and PU.1. Eur. J. Immunol. 2007;37(8):2300–2308. doi: 10.1002/eji.200737270. [DOI] [PubMed] [Google Scholar]
- Zeng Z., et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp. Lung Res. 2013;39(7):275–282. doi: 10.3109/01902148.2013.808285. [DOI] [PubMed] [Google Scholar]
- Zhu Z., et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304(5677):1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- Zhu D., et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. J. Allergy Clin. Immunol. 2013;132(2) doi: 10.1016/j.jaci.2013.02.005. 426-36e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G., et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.