Abstract
Background
Antithrombotic treatment represents a dilemma in elderly patients with atrial fibrillation since both risk of thromboembolism and bleeding are age-dependent complications. A paradigm shift occurred over the past 10 years when aspirin was overcome by warfarin and further by the direct oral anticoagulants. Here we present a clinical practice-based analysis of a cohort of elderly inpatient atrial fibrillation patients and investigate the influence of clinical factors in the choice of antithrombotic strategy.
Methods
Study participants (n = 2943) are consecutive patients aged 75–104 years discharged from a Swedish university hospital with atrial fibrillation or atrial flutter as main diagnosis between November 1st 2010 and December 31st 2017. Cardiovascular risk factors, comorbidities and antithrombotic treatment at discharge were manually extracted from medical charts. A logistic regression analysis was performed to estimate predictors of the probability to receive direct oral anticoagulants (DOACs) compared to warfarin.
Results
Patients aged ≥90 y (n = 446, women 73%) showed the highest prevalence of cardiovascular comorbidities and the highest bleeding and thromboembolic risk. DOACs became more commonly prescribed than warfarin in 2016/2017 across all ages. However, the probability to receive DOAC as compared to warfarin was lower in the presence of high bleeding risk (OR 0,55; 95% CI 0,40–0,77; p = 0,00) and high thromboembolic risk (OR 0,74; 95% CI 0,59–0,94; p = 0,01).
Conclusion
Elderly atrial fibrillation patients represent a heterogenous group where the oldest (≥90 years) show both a very high thromboembolic and bleeding risk profile. In the presence of high thromboembolic and bleeding risk, warfarin was still preferred over DOAC.
1. Introduction
Thromboembolism (TE), including ischemic stroke (IS) and systemic embolism (SE), prevention in atrial fibrillation (AF) patients relies on treatment with oral anticoagulants (OAC). While on OAC treatment, AF patients are exposed to an increased bleeding risk, the most feared being intracranial hemorrhage (ICH). Both thromboembolic and bleeding risk increase markedly with age [1], complicating clinical decisions of OAC treatment in elderly.
Warfarin was, during many years, the cornerstone of OAC treatment after it was proven to be superior to antiplatelet therapy (APT) with aspirin in thrombosis prevention [2]. The introduction of the direct oral anticoagulants (DOACs) with dabigatran, rivaroxaban and apixaban in 2011–2013 and edoxaban in 2016, has changed treatment guidelines and clinical practice of thrombosis prevention in AF patients [3], [4], [5]. The four pivotal randomized clinical trials (RCT) designed to assess efficacy of DOAC in comparison to warfarin, showed equivalence [6], [7] or superiority [8], [9] in prevention of thromboembolic episodes and a favorable safety profile with a reduced risk of ICH [6], [7], [8], [9].
Elderly AF patients represented a minority of the DOAC trial populations [10] and notably elderly with multimorbidity often met in clinical practice were lacking in the trials, reducing the external validity of the results. Nonetheless, observational studies have shown that elderly, in particular, seem to benefit from IS preventive treatment with OAC [11], [12], [13], contributing to the conviction of a great need for inclusion of elderly in RCTs [14]. An emerging number of registry-based studies include elderly. However, these studies often lack an extensive characterization of the heterogenous patient group that elderly constitute [15] and bleeding events can be missed when registered by administrative coding [16]. These findings along with variability in bleeding definitions may contribute to differences in bleeding incidence among observational studies, even though higher bleeding rates than in clinical trials are generally noted [17].
Observational studies with clinical practice-based data may contribute to fill the knowledge gap on the optimal OAC treatment strategy in elderly AF patients. To this extent, we have established a large cohort, the Carebbean-elderly (Atrial fibrillation: Risks and Benefits of ANticoagulation in elderly), to analyse clinical risk profiles for TE and bleeding in elderly patients amenable to OAC treatment. Here we present a cross-sectional analysis of this population along with the analysis of how clinical characteristics have influenced the choice of OAC regimen in this patient group.
2. Methods
2.1. Study population
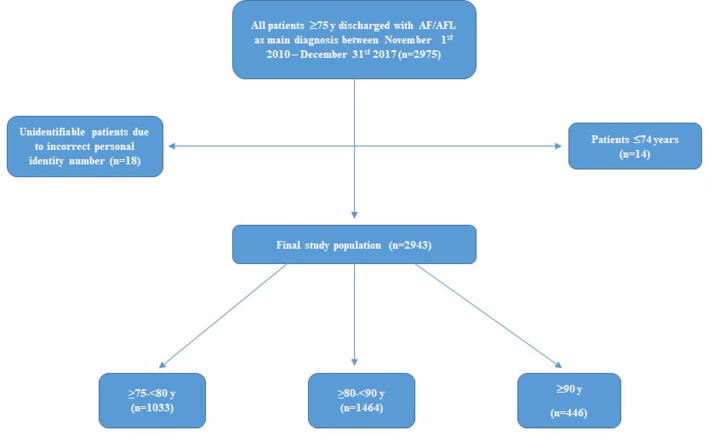
The Carebbean-elderly (Clinicaltrials.gov, NCT03828162) is a prospective cohort of consecutive elderly patients ≥75 years (y) discharged from the Department of Cardiology at Danderyd University Hospital (Stockholm, Sweden), a secondary referral center with a catchment area of approximately 500 000 inhabitants, with AF or atrial flutter (AFL) as main diagnosis between November 1st 2010 and December 31st 2017.
AF and AFL diagnoses were extracted from the hospital database by the QlikView software using the diagnosis codes I48.0–99 (International Classification of Diseases 10th Revision; ICD-10) linked to the patients by their personal identity number.
Supplementary Fig. 1 shows the flowchart of the study along with the inclusion and exclusion criteria. In order to verify complete coverage of eligible study patients and ascertain consecutive sampling, we validated the number of included patients comparing our records with the Stockholm Healthcare Analyses Database (Vårdanalysdatabasen) that covers all recorded diagnosis codes and reasons for hospitalizations and consultations in both primary and specialist care in Stockholm County [18].
2.2. Clinical data collection and definition of variables
Medical charts relative to the first hospital stay when discharged with AF/AFL as main diagnosis, were reviewed manually in TakeCare (electronic health system by CompuGroup Medical) for each single patient according to a prespecified protocol. We recorded date of birth, age at admission, sex, whether AF/AFL was recurrent or diagnosed for the first time and symptom at admission. A detailed description of the definitions used to define cardiovascular (CV) risk factors and comorbidities collected from the medical charts, is reported in the Supplementary Material.
Briefly, anamnestic records of previous CV disease, CV risk factors, history of bleeding, cancer, venous thromboembolism, chronic inflammatory disease, dementia and cognitive impairment were retrieved from medical charts if self-reported at admission, and/or previously stated in chart notes and/or diagnosed according to ICD-10. Anthropometric measures, systolic and diastolic blood pressure and biochemical parameters as well as echocardiographic data were recorded at discharge. Renal function was estimated by glomerular filtration rate (eGFR) in milliliters (ml)/minute (min) according to the Cockcroft-Gault (C-G) formula and categorized as described in the Supplementary Material.
Antithrombotic (AT) treatment was recorded at admission for patients with known AF/AFL. Time in Therapeutic Range (TTR) for warfarin was calculated by the number of therapeutic international normalized ratio (INR) values (2.0–3.0) divided by the total number of values registered, i.e. the fraction of INR values in the target range, during the year prior to admission for all patients on warfarin at admission [19]. Similarly, AT treatment was recorded at discharge for all patients. Type and dosing of DOACs were annotated. We also recorded treatment with APT, low molecular weight heparin (LMWH) and no AT treatment and the underlying reason for being withheld treatment.
Risk of TE was evaluated for each participant by calculation of CHA2DS2 VASc [20]. Bleeding risk was estimated using multiple scores; HAS-BLED [21], ORBIT [22] and ATRIA [23]. Definitions and categorisations of the risk scores are available in the Supplementary Material.
2.3. Ethics
All included patients have given their informed consent to use of medical data for research purposes and for linkage to national mandatory registries. On these premises, the study was approved in 2016 by the Regional Ethics Review Board, Stockholm, Sweden (Dnr 2016/63–31/1, 2017/1520–32 and 2019–01850).
2.4. Statistical analysis
Continuous variables are reported as median (interquartile range) and categorical as percentages. Patient characteristics, comorbidities, AT treatment and risk of TE and bleeding are reported for the population in total and according to three age groups: ≥75–<80 y (septuagenarians), ≥80–<90 y (octogenarians) and ≥90 y (nonagenarians/centenarians) (Supplementary Fig. I).
Clinical practice and guidelines for stroke prevention in patients with AF/AFL have changed throughout the years of 2010–2017 [3], [4], [5]. With this in mind, we analysed how prescriptions of the three most commonly used AT regimens (ASA/warfarin/DOAC) changed over the years in relation to patients' clinical characteristics. Basic descriptives were used to report the distribution of patient characteristics within four time periods (2010/2011, 2012/2013, 2014/2015 and 2016/2017).
From the year 2014, both warfarin and DOAC were fully adopted in the management of elderly AF patients. From that time until the end of the study period (2014–2017), we analysed whether the probability to receive DOAC compared to warfarin could be predicted by age, gender, BMI, eGFR and thromboembolic/bleeding risk. To estimate the predictors of the probability to receive a specific OAC treatment at discharge, we performed logistic regression presented as odds ratio (OR) and a 95% confidence interval (CI). In the analysis, all patients discharged with a DOAC (n = 810) including those on combination therapy with DOAC and APT were compared to all patients discharged with warfarin (n = 1377) including those on treatment with both warfarin and APT. Here, we did not consider patients discharged with APT, LMWH or no treatment (n = 714) since they represent a selected group of patients where the choice of AT treatment was driven by other concomitant medical conditions (e.g. malignancy or palliative treatment).
In the analysis of age categories as possible predictors, the lowest age group (≥75–<80 y) served as reference group. Further, mildly decreased-normal eGFR (≥60 ml/min) served as reference group for eGFR categories. The low risk groups of CHA2DS2 VASc and HAS-BLED served as references in the analyses of risk scores.
The regression models were performed with complete-case analysis due to few missing values (<2%) of the studied variables.
All analyses were performed with STATA version 14.2.
3. Results
The validation of our sampling with the Stockholm Healthcare Analyses Database, showed congruence in numbers of patients discharged with AF/AFL as main diagnosis during the years 2010–2017 after exclusion of the patients who were readmitted to our hospital during the study period.
3.1. Clinical characteristics and prescription of AT regimens in the study population
Table 1 and Supplementary Table 1 summarize the characteristics of the study population as a whole and after stratification in three age categories. Women represented 58.4% of the study population (73.8% of ≥90 y). Almost half of the admitted patients (47.6%) had a newly diagnosed AF/AFL. Patients most commonly presented with dyspnea (30.5%) and palpitations (20.9%). With age, dyspnea became more, and palpitations less, prevalent (Supplementary Table 1).
Table 1.
Clinical characteristics of the studied population in total and stratified by age groups.
| All patients | ≥75–<80 | ≥80–<90 | ≥90 | |
|---|---|---|---|---|
| n | 2943 | 1033 | 1464 | 446 |
| Age (y) | 82 (78–87) | 77 (75–78) | 84 (82–87) | 92 (91–94) |
| Female gender | 1718 (58.4) | 524 (50.7) | 865 (59.1) | 329 (73.8) |
| BMI | 24.5 (21.9–27.6) | 25.7 (23.0–29.0) | 24.2 (21.8–27.1) | 22.8 (20.3–25.1) |
| Underweight | 137 (4.7) | 26 (2.6) | 66 (4.6) | 45 (10.3) |
| Normal weight | 1460 (50.6) | 425 (41.9) | 762 (53.0) | 273 (62.8) |
| Overweight | 895 (31.0) | 367 (36.2) | 439 (30.5) | 89 (20.5) |
| Obese | 396 (13.7) | 196 (19.3) | 172 (12.0) | 28 (6.4) |
| New onset AF/AFL | 1402 (47.6) | 476 (46.1) | 711 (48.6) | 215 (48.2) |
| Prevalent CV disease | ||||
| IS/TIA/SE | 563 (19.1) | 157 (15.2) | 297 (20.3) | 109 (24.4) |
| Myocardial infarction | 414 (14.1) | 109 (10.6) | 220 (15.0) | 85 (19.1) |
| Other vascular disease | 484 (16.5) | 153 (14.8) | 257 (17.6) | 74 (16.6) |
| Heart failure | 962 (32.7) | 248 (24.0) | 506 (34.6) | 208 (46.6) |
| Previous bleeding | 430 (14.6) | 124 (12.0) | 216 (14.8) | 90 (20.2) |
| Prevalent CV risk factors | ||||
| Diabetes mellitus | 453 (15.4) | 173 (16.8) | 239 (16.3) | 41 (9.2) |
| Hypertension | 1962 (66.7) | 694 (67.2) | 969 (66.2) | 299 (67.0) |
| Lipidlowering treatment | 712 (24.2) | 292 (28.3) | 352 (24.0) | 68 (15.3) |
| Renal function | ||||
| absolute eGFR (ml/min) | 52.1 (36.6–70.9) | 67.8 (53.4–86.7) | 48.3 (36.2–63.6) | 31.2 (24.3–42.2) |
| ≥60 | 1087 (37.9) | 643 (64.4) | 419 (29.3) | 25 (5.8) |
| 45–59 | 661 (23.1) | 214 (21.4) | 387 (27.0) | 60 (13.8) |
| 30–44 | 687 (24.0) | 108 (10.8) | 430 (30.1) | 149 (34.3) |
| 15–29 | 379 (13.2) | 26 (2.6) | 175 (12.2) | 178 (40.9) |
| <15 | 51 (1.8) | 8 (0.8) | 20 (1.4) | 23 (5.3) |
| Antithrombotic treatment at discharge | ||||
| Warfarin | 1377 (46.8) | 553 (53.5) | 695 (47.5) | 129 (28.9) |
| DOAC | 810 (27.5) | 313 (30.3) | 397 (27.1) | 100 (22.4) |
| ASA | 461 (15.7) | 93 (9.0) | 236 (16.1) | 132 (29.6) |
| None | 157 (5.3) | 37 (3.6) | 70 (4.8) | 50 (11.2) |
| TTR* | 0.67 (0.53–0.78) | 0.70 (0.57–0.80) | 0.65 (0.50–0.75) | 0.62 (0.50–0.71) |
| Risk scores | ||||
| CHA2DS2 VASc | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (4–5) |
| 2–4 | 1720 (58.4) | 724 (70.1) | 928 (63.4) | 273 (61.2) |
| ≥5 | 1018 (34.6) | 309 (29.9) | 536 (36.6) | 173 (38.8) |
| HAS-BLED | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| <2 | 571 (19.4) | 203 (19.7) | 280 (19.1) | 88 (19.7) |
| 2 | 1359 (46.2) | 495 (47.9) | 679 (46.4) | 185 (41.5) |
| ≥3 | 1013 (34.4) | 335 (32.4) | 505 (34.5) | 173 (38.8) |
Continuous variables are reported as median (interquartile range), categorical variables as number (percentage).
Abbreviations: Y: years; BMI: body mass index; AF: atrial fibrillation; AFL: atrial flutter; CV: cardiovascular; IS: ischemic stroke; TIA: transient ischemic attack; SE: systemic embolism; eGFR: estimated glomerular filtration rate; DOAC: direct oral anticoagulants; ASA: acetylsalicylic acid; TTR: time in therapeutic range; Missing values for BMI (1,9%) and eGFR (2,0%).
Definition of BMI and eGFR categories as well as risk score categories of CHA2DS2 VASc and HAS-BLED are reported in the Supplementary Material.
TTR was calculated for all patients (n = 791) already on warfarin treatment at admission.
Prevalent CV comorbidities, thromboembolic and bleeding risk increased markedly with age. Moreover, a higher prevalence of underweight (BMI < 18.5) and severely decreased renal function (eGFR < 30 ml/min) were noted in the ≥90 y group. A history of bleeding was recorded in 14.6% of the total population and in 20.2% of the ≥90 y. The proportion of patients with a very high thromboembolic risk, constituted 38.8% of the ≥90 y olds. Risk of major bleed, assessed by the HAS-BLED score, was intermediate for half of the patients (46.2%). However, when bleeding risk was assessed by the ORBIT and ATRIA scores (Supplementary Table 1), the majority of the ≥90 y were classified as high bleeding risk.
Overall, during the years 2010–2017, warfarin was the most prescribed OAC (46,8%) followed by DOAC (27,5%) and ASA (15,7%). In both the ≥75–<80 y and the ≥80–<90 y groups, warfarin outnumbered the DOACs while ASA prescriptions were more common than both warfarin and DOAC in the ≥90 y group. The number of patients not receiving any AT treatment also increased with age constituting 11,2% of the ≥90 y (Supplementary Table 1).
3.2. Prescription of antithrombotic regimens over time in relation to clinical characteristics
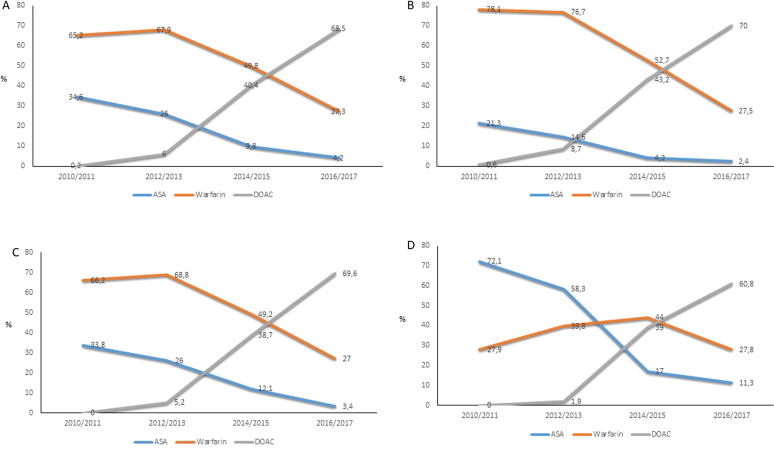
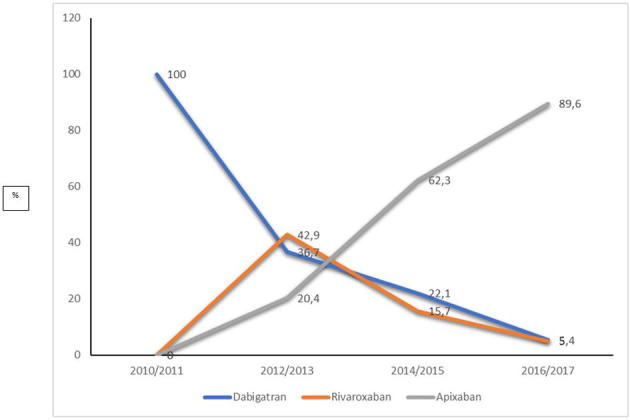
As reported in Fig. 1, from 2014, DOACs were actively prescribed to elderly patients. However, first in 2016/2017 was DOAC the most commonly prescribed AT treatment in all age groups of the study population. At this time, apixaban contributed to nearly 90% of the DOAC prescriptions (Supplementary Fig. 2). Among the age groups, the ≥90 y patients differed in primarily being prescribed ASA until 2014/2015 when instead warfarin started to dominate followed by DOAC in 2016/2017 (Fig. 1, Panel D).
Fig. 1.
Pattern of prescription of ASA, warfarin and DOAC in elderly patients ≥75 y with AF/AFL during 2010–2017 in the total population (Panel A) and in the three different age groups (Panel B-D). Time trend in prescription of ASA (blue), warfarin (orange) and DOAC (grey) reported in percentage in the total study population in time periods between 2010 and 2017 (A), and in the three age groups (≥75–<80 y (B), ≥80–<90 y (C) and ≥90 y (D)). Abbreviations: ASA; acetylsalicylic acid, DOAC; direct oral anticoagulants, y; years, AF; atrial fibrillation, AFL; atrial flutter. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The other investigated clinical characteristics (sex, BMI and eGFR) were distributed similarly among the patients in every treatment group across all time periods (Supplementary Table 2).
3.3. Influencing factors in decision-making of OAC treatment
As shown in Table 2, and as expected, the probability of receiving DOAC was lower in patients with impaired renal function. We also observed that the probability of receiving DOAC instead of warfarin during 2014–2017 was lower in the presence of a high thromboembolic (OR 0.74; 95% CI 0.59–0.94; p = 0.01) and high bleeding risk (OR 0.55; 95% CI 0.40–0.77; p < 0.001).
Table 2.
Crude univariate analysis of clinical characteristics as predictors of the probability of receiving DOAC in comparison to warfarin during 2014–2017.
| DOAC OR (95% CI) | p-value | |
|---|---|---|
| Age (y) | 0.99 (0.98–1.01) | 0.55 |
| ≥75–<80 | ref | |
| ≥80–<90 | 1.04 (0.82–1.33) | 0.72 |
| ≥90 | 1.00 (0.70–1.42) | 0.98 |
| Female gender | 1.07 (0.86–1.34) | 0.53 |
| BMI | 0.98 (0.96–1.01) | 0.13 |
| eGFR, absolute C-G | 1.01 (1.00–1.01) | 0.01 |
| ≥60 | ref | |
| ≥45-<60 | 0.90 (0.67–1.20) | 0.47 |
| ≥30-<45 | 0.74 (0.55–0.98) | 0.04 |
| ≥15-<30 | 0.70 (0.47–1.02) | 0.06 |
| <15 | 0.12 (0.03–0.56) | 0.01 |
| CHA2DS2 VASc | 0.91 (0.84–1,00) | 0.05 |
| 2–4 | ref | |
| ≥5 | 0.74 (0.59–0.94) | 0.01 |
| HAS-BLED | 0.71 (0.63–0.81) | <0.001 |
| <2 | ref | |
| 2 | 1.01 (0.74–1.38) | 0.95 |
| ≥3 | 0.55 (0.40–0.77) | <0.001 |
The probability of receiving DOAC (n = 810) as compared to warfarin (n = 1377) is expressed as odds ratio (OR) and a 95% confidence interval (CI).
Abbreviations: DOAC: direct oral anticoagulants; OR: odds ratio; CI: confidence interval; BMI: body mass index; eGFR: estimated glomerular filtration rate; C-G: Cockcroft-Gault.
Definition of eGFR categories as well as risk score categories of CHA2DS2 VASc and HAS-BLED are reported in the Supplementary Material.
The regression analysis was performed with complete-case analysis due to few missing values for BMI (1,9%) and eGFR (2,0%).
4. Discussion
We report a cross-sectional analysis of a contemporary elderly inpatient AF/AFL population. Across this group of patients we have observed that the oldest patients (≥90 y), who are largely underrepresented in clinical trials yet constitute a growing patient group with high risk of vascular complications, have clinical characteristics that differ from the general elderly AF/AFL population. A shift in OAC treatment from warfarin to DOAC occured in 2016/2017 in this elderly group of AF/AFL patients, however, a high thromboembolic and bleeding risk along with decreased renal function were factors still predisposing to warfarin use instead of DOAC.
AF/AFL patients ≥90 y differentiate in terms of clinical characteristics compared to general elderly AF/AFL patients, potentially affecting the effectiveness and safety of OAC treatment. Thus, our study population differs from the DOAC trial populations as shown by a higher median age (82 vs. 70–73), a higher proportion of women (58,4% vs. 35,3–39,7%), a lower median BMI (24 vs. 28) and more impaired renal function (moderately decreased renal function or worse in 39% vs. 17–19%). With regard to thromboembolic risk, study participants in the ARISTOTLE and RE-LY trials [8], [9] and particularly study participants enrolled in the ROCKET-AF and ENGAGE AF-TIMI 48 trials [7], [8], were at increased risk of IS due to inclusion criteria and the prevalence of CV comorbidities were similar or even higher than in the present study. On the other side, participants with increased bleeding risk including severely decreased renal function (i.e. eGFR < 30 ml/min) were excluded from the DOAC trials, partly explaining why so few very elderly patients were included. This was also confirmed by the ETNA-AF-Europe registry [24]. When baseline characteristics of an unselected European AF population prescribed edoxaban treatment were compared to the trial population of ENGAGE AF-TIMI 48, a higher bleeding risk among the clinic-based registry patients was observed. Our data show that the population of elderly AF/AFL patients is highly heterogenous and therefore, data from the DOAC trials must be translated to clinical practice with caution, in particular that concerning elderly patients above 90 y of age. In this group, the burden of comorbidities and the associated bleeding risk clearly depart from what is observed in younger AF/AFL patients.
Indeed, bleeding and bleeding risk will be of even greater importance in coming years, considering the growing population of elderly and frail patients, a group with a high prevalence of AF/AFL and high risk of TE. Thus, while clinical trials have excluded very elderly, several observational studies have focused on investigating OAC treatment in elderly AF/AFL patients. As compared to existing studies, however, our investigation shed lights on the heterogeneity of elderly patients which is often not addressed by registry-based studies. In the present study, the highest proportion of patients not receiving any AT treatment was seen in the ≥90 y patient group (11,2%) which is defined by a particularly high proportion of patients with high (61,2%) and very high (38,8%) thromboembolic risk as well as high bleeding risk (38,8%). The FRAIL-AF Study concluded that in octogenarians, OAC therapy was more often prescribed when higher risk of TE, lower risk of bleeding and lower frailty score were present [25]. Although consisting of younger populations, the GARFIELD-AF and the ORBIT-AF I and II, two large international prospective registries of patients with AF, have shown that OAC is more consistently used in patients with a low risk of IS than in those with a high risk [26]. Taken together, these observations indicate a “treatment paradox” and confirm the lack of solid evidence to reassure safe OAC use in the oldest patients with the highest risk of TE.
DOACs are recommended in preference to warfarin as IS prevention in Europe and in the US [3] and APT is no longer recommended [27]. In 2015, the National Board of Health and Welfare in Sweden recommended warfarin and DOAC with equal weight which, the same year, was followed by the Regional Drug and Therapeutic committee (DTC) in Stockholm county to equally recommend warfarin or a DOAC (apixaban) [28]. In our population, DOAC prescription started to increase in 2014/2015 and dominated warfarin prescription in 2016/2017 in all age groups. However, nearly 20% of the OAC initiations were on a DOAC in AF patients in the Region of Stockholm in both primary and secondary care, after the publication of the 2012 ESC guidelines [28], while the corresponding proportion in our elderly population was 6%. In spite of European guidelines recommendations, warfarin was generally preferred over DOACs in patients with a high thromboembolic and high bleeding risk and, as expected, in patients with a decreased renal function, in our single-center cohort. This finding, i.e. a more conservative treatment approach in the most vulnerable patients, is consistent with previous studies [29]. In the abscence of RCTs including elderly, multimorbid patients, clinicians likely lean on observational studies having included elderly patients of high-risk met in clinical practice. However, by the time of our study period (2010–2017), few observational studies of this particular patient group had been conducted showing a convincing net clinical benefit of the DOACs compared to warfarin. Our present data, showing a somewhat slower implementation of DOACs in elderly patients, suggest that regional recommendations, mainly based on observational studies from clinical practice, might have been more influential than European guidelines, based on results from RCTs, for the adoption of DOACs in these elderly patients with multimorbidity.
Moreover, the high prevalence of prior bleedings among the study participants (20,2% in the ≥90 y patient group), may have concerned the clinicians and affected their choice of OAC regimen when favoring warfarin to the patients with high risk of thromboembolism and bleeding. This is likely explained by the lack of available antidotes for the Factor Xa inhibitors rivaroxaban and apixaban during the study period, while well-established routines existed for reversal of anticoagulation by warfarin.
Finally, the limited knowledge of the DOACs' pharmacological interactions might have prevented their use in elderly patients where the higher burden of comorbidities requires treatment with multiple drugs.
In a broader perspective, it strenghens the need for efficient implementation of both national and regional recommendations concerning elderly where the regional DTC might have used a more active approach in its implementation strategy, to reach prescribers. It also highlights the need for a more clear definition of indication for OAC treatment and different OAC regimens specified to different age categories of elderly patients.
This study has both strengths and limitations. We conducted an analysis of a contemporary elderly AF/AFL population often excluded from clinical trials making the results generalizable to elderly patients often met in the clinic. We collected clinical data by detailed chart review allowing a consistent and qualitative evaluation of patient data beyond what might be obtainable from registry data based solely on diagnosis codes. Consecutive enrollment of the study population and no explicit exclusion criteria were used to avoid selection bias. Overall, there were few missing data for all variables.
As for limitations, we cannot rule out that we may have underestimated the correct number of eligible study patients when relying on registered diagnosis ICD-10 codes for AF/AFL in our inclusion. The sensitivity and specificity of the ICD-10 codes of AF and AFL have, however, been proven to be high [25]. The medical record system implemented for healthcare in the Stockholm Region (TakeCare) and herein used for data extraction, has only regional coverage. Patient information from outside the Region of Stockholm were therefore not obtainable in this study based on medical chart review. However, very few elderly move at all after the age of 75 and even fewer outside of a larger city. Since this is a single-center study, our data may differ from other populations or regions. Differences in treatment traditions and/or geographical differences in socioeconomics and ethnicity may influence data and prescription patterns. Future studies, especially in elderly, performed in other regions and populations are therefore awaited.
Our analysis is cross-sectional, hence, does not include outcome data. The main focus of this study was to provide a “snapshot” of the clinical complexity of the population of elderly AF/AFL patients and thereby highlighting its heterogeneity in clinical characteristics. For this purpose, we believe a descriptive study design was the most appropriate choice for guidance of understanding the entity of the presented problem. On the other side, cross-sectional studies do not allow analyses of temporality and causality. A prospective study is warranted to analyse if the chosen OAC treatment in this group of patients affects the incidence rate of adverse events.
In conclusion, elderly hospitalized AF/AFL patients constitute a highly heterogenous patient group. CV comorbidities and bleeding risk increase with age making the oldest patients particularly vulnerable to anticoagulation. After the introduction of the DOACs, clinicians at our center did not adopt DOAC prescription as rapidly in the elderly as seen in more general AF/AFL populations. These findings suggest that the underrepresentation of elderly with multimorbidity in the DOAC trials, had major influence on the implementation of DOAC use in elderly. Our data underscore the need for studies, in particular randomized controlled trials, including elderly patients.
CRediT authorship contribution statement
Hanne Ehrlinder: Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Nicola Orsini: Methodology, Formal analysis, Data curation, Writing - review & editing, Supervision. Karin Modig: Methodology, Writing - review & editing. Claes Hofman-Bang: Conceptualization, Methodology, Writing - review & editing, Supervision. Håkan Wallén: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration. Bruna Gigante: Conceptualization, Methodology, Validation, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgements
This work was supported by a grant from FORTE (2016-00460) to BG. HE was supported by the Stockholm County Council (combined residency and PhD training program).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100505.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
References
- 1.Marinigh R., Lip G.Y., Fiotti N., Giansante C., Lane D.A. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J. Am. Coll. Cardiol. 2010;56(11):827–837. doi: 10.1016/j.jacc.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Mant J., Hobbs F.D.R., Fletcher K., Roalfe A., Fitzmaurice D., Lip G.Y.H. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 3.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019 doi: 10.1161/CIR.0000000000000665. 2019:CIR0000000000000665. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace: Eur. Pacing, Arrhythmias, Cardiac Electrophysiol.: J. Working Groups On Cardiac Pacing, Arrhythmias, Cardiac Cellular Electrophysiol. Eur. Soc. Cardiol. 2016;18(11):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 5.Steffel J., Verhamme P., Potpara T.S., Albaladejo P., Antz M., Desteghe L. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano R.P., Ruff C.T., Braunwald E., Murphy S.A., Wiviott S.D., Halperin J.L. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Granger C.B., Alexander J.H., McMurray J.J., Lopes R.D., Hylek E.M., Hanna M. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 10.Sardar P., Chatterjee S., Chaudhari S., Lip G.Y.H. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J. Am. Geriatr. Soc. 2014;62(5):857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 11.Forslund T., Komen J.J., Andersen M., Wettermark B., von Euler M., Mantel-Teeuwisse A.K. Improved stroke prevention in atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants. Stroke. 2018;49(9):2122–2128. doi: 10.1161/STROKEAHA.118.021990. [DOI] [PubMed] [Google Scholar]
- 12.Chao T.-F., Liu C.-J., Lin Y.-J., Chang S.-L., Lo L.-W., Hu Y.-F. Oral anticoagulation in very elderly patients with atrial fibrillation. Circulation. 2018;138(1):37–47. doi: 10.1161/CIRCULATIONAHA.117.031658. [DOI] [PubMed] [Google Scholar]
- 13.Patti G., Lucerna M., Pecen L., Siller-Matula J.M., Cavallari I., Kirchhof P. Thromboembolic risk, bleeding outcomes and effect of different antithrombotic strategies in very elderly patients with atrial fibrillation: A sub-analysis from the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation) J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.117.005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreotti F., Rocca B., Husted S., Ajjan R.A., ten Berg J., Cattaneo M. Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2015;36(46):3238–3249. doi: 10.1093/eurheartj/ehv304. [DOI] [PubMed] [Google Scholar]
- 15.Poscia A., Collamati A., Milovanovic S., Vetrano D., Liotta G., Petitti T. Methodological issues in the observational studies conducted in older population: a narrative review. Epidemiol. Biostat. Public Health. 2017;14(2) [Google Scholar]
- 16.Li L., Geraghty O.C., Mehta Z., Rothwell P.M. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390(10093):490–499. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes L.C., Spencer F.A., Neumann I., Ventresca M., Ebrahim S., Zhou Q. Systematic review of observational studies assessing bleeding risk in patients with atrial fibrillation not using anticoagulants. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0088131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forslund T., Wettermark B., Wandell P., von Euler M., Hasselstrom J., Hjemdahl P. Risk scoring and thromboprophylactic treatment of patients with atrial fibrillation with and without access to primary healthcare data: experience from the Stockholm health care system. Int. J. Cardiol. 2013;170(2):208–214. doi: 10.1016/j.ijcard.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 19.Reiffel J.A. Time in the therapeutic range (TTR): an overly simplified conundrum. J. Innov. Cardiac Rhythm Manage. 2017;8:2643–2646. doi: 10.19102/icrm.2017.080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 21.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien E.C., Simon D.N., Thomas L.E., Hylek E.M., Gersh B.J., Ansell J.E. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur. Heart J. 2015;36(46):3258–3264. doi: 10.1093/eurheartj/ehv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang M.C., Go A.S., Chang Y., Borowsky L.H., Pomernacki N.K., Udaltsova N. A new risk scheme to predict warfarin-associated hemorrhage. J. Am. Coll. Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Caterina R., Kelly P., Monteiro P., Deharo J.C., de Asmundis C., Lopez-de-Sa E. Characteristics of patients initiated on edoxaban in Europe: baseline data from edoxaban treatment in routine clinical practice for patients with atrial fibrillation (AF) in Europe (ETNA-AF-Europe) BMC Cardiovasc. Disord. 2019;19(1):165. doi: 10.1186/s12872-019-1144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefebvre M.C., St-Onge M., Glazer-Cavanagh M., Bell L., Kha Nguyen J.N., Viet-Quoc Nguyen P. The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF study. Can. J. Cardiol. 2016;32(2):169–176. doi: 10.1016/j.cjca.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg B.A., Gao H., Shrader P., Pieper K., Thomas L., Camm A.J. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am. Heart J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Camm A.J., Lip G.Y., De Caterina R., Savelieva I., Atar D., Hohnloser S.H. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 28.Komen J., Forslund T., Hjemdahl P., Andersen M., Wettermark B. Effects of policy interventions on the introduction of novel oral anticoagulants in Stockholm: an interrupted time series analysis. Br. J. Clin. Pharmacol. 2017;83(3):642–652. doi: 10.1111/bcp.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komen J., Forslund T., Hjemdahl P., Wettermark B. Factors associated with antithrombotic treatment decisions for stroke prevention in atrial fibrillation in the Stockholm region after the introduction of NOACs. Eur. J. Clin. Pharmacol. 2017;73(10):1315–1322. doi: 10.1007/s00228-017-2289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.