Abstract
This paper briefly reviews the physiological components of the microcirculation, focusing on its function in homeostasis and its central function in the realization of oxygen transport to tissue cells. Its pivotal role in the understanding of circulatory compromise in states of shock and renal compromise is discussed. Our introduction of hand-held vital microscopes (HVM) to clinical medicine has revealed the importance of the microcirculation as a central target organ in states of critical illness and inadequate response to therapy. Technical and methodological developments have been made in hardware and in software including our recent introduction and validation of automatic analysis software called MicroTools, which now allows point-of-care use of HVM imaging at the bedside for instant availability of functional microcirculatory parameters needed for microcirculatory targeted resuscitation procedures to be a reality.
Keywords: Microcirculation, Sepsis, Shock, Sidestream dark field imaging, Incident dark field imaging, Tissue red blood cell perfusion
Introduction
Resuscitation from states of shock is conventionally achieved by the restoration of systemic hemodynamic variables using fluid and vasoactive compounds with the aim of promoting tissue perfusion and oxygen transport to tissue. However, whether this aim is actually achieved is uncertain. This condition leads to inappropriate use of drugs, which in turn can cause an increase in organ injury and adverse outcome. The physiological basis of this clinical dilemma has been exposed by our clinical introduction of hand-held vital microscopes (HVM) for bedside monitoring of the microcirculation. To this end, a deeper insight into the functional anatomy and (patho)physiology of microcirculatory alterations associated with disease and therapy is needed.
The Microcirculation
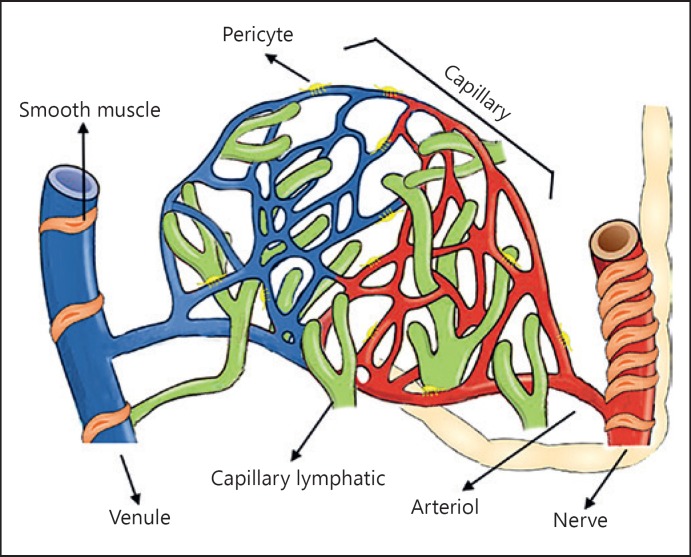
The microcirculation is the terminal vascular network of the systemic circulation consisting of microvessels with diameters <20 µm. These microvessels consist of arterioles, post-capillary venules, capillaries, and their (sub) cellular constituents (Fig. 1). The microcirculation is the final destination of the cardiovascular system and is ultimately responsible for oxygen transfer from the red blood cells (RBC) in the capillaries to the parenchymal cells where oxygen is delivered to meet the energy requirements of the tissue cells in support of their functional activity. Other functions of the microcirculation include the regulation of solute exchange between the intravascular and tissular space and is responsible for the transport of all blood-borne hormones and nutrients to the tissue cells including mediating the functional activity of the immune system and hemostasis. It is arguably the most important compartment of the cardiovascular system, since it is in direct contact with the parenchymal cells, which rely on its proper function to maintain their viability to support organ function.
Fig. 1.
Microvascular anatomy. The microcirculation is the part of the vascular system and consists of the small vessels so-called arterioles, capillaries, and venules. The lymphatic capillaries carry the extravascular fluid into the venous system. The arterioles are surrounded by vascular smooth muscle cells responsible for the regulation of arteriole tone.
Oxygen transport by RBC flow in the microcirculation to the tissues is accomplished by 2 primary mechanisms. These are convection of the oxygen-carrying RBCs and diffusion of the oxygen from the RBCs to the respiring mitochondria of the tissue cells. The former component of oxygen transport to the tissues is described by RBC flux or flow, and the diffusional component of oxygen transport can be quantified by the functional capillary density (FCD) of the microcirculation [1].
Vessels of the microcirculation are almost entirely lined by endothelial cells (EC). These cells contain fenestrations and pores and are held together by various molecules, including cadherins as well as current-carrying gap junctions, which allow upstream electrical communication between EC. These endothelial structures can vary in density and morphology between the different organs and vessels. EC in symbiosis with smooth muscle cells regulate the microvascular blood flow predominantly by regulation of the vasotone of arterioles. There are 3 main mechanisms that cause this regulation: myogenic, metabolic, and neurohumoral control mechanisms. One of the most important subcellular structures of the endothelium mediating its function is the glycocalyx present on the luminal side of the endothelium [2, 3, 4]. It is a 0.2–0.5 µm gel-like layer synthesized by EC. It is composed of 3 major components, proteoglycans, glycosaminoglycans, and plasma proteins, and harbors various substances such as antithrombin and superoxide dismutase. The glycocalyx is responsible for several critical physiologic processes including homeostasis, solute transport, hemostasis, and immunological functions. Although it is generally thought that the glycocalyx integrity is the main determinant of the vascular barrier, we showed in a recent study that this is not the case and that the glycocalyx can be shed in conditions of shock without compromising the vascular barrier function [5]. It is generally considered that endothelium dysfunction can be considered one of the main cellular events responsible for hemodynamic collapse seen in states of shock and responsible for the ineffectiveness of routine resuscitation procedures [4]. The microcirculation is of key importance for the functioning of the kidney due to its central role in delivering oxygen to the renal microcirculation [6, 7]. The majority (>80%) of oxygen delivered to the kidney is utilized for production of ATP needed by the Na+/K+ pump whose activity is essential for tubular sodium reabsorption [8]. Injury of the renal microcirculation resulting in acute kidney injury (AKI) can be caused by hypoxia, oxidative/nitro stress, and/or inflammatory mediators, and is thought to be central in the sequelae leading to AKI [9]. Experimental models have shown that targeting inflammation [10] and microcirculatory oxygen delivery [11] can be successful in resolving AKI in such models.
Clinical Measurement of the Microcirculation Using HVM
Previously the measurement of the microcirculation in vivo was limited to experimental studies where intravital microscopes were used to observe the microcirculation in mainly muscle tissues (cremaster and hind limb muscle). In clinical studies, such microscopes were used to asses the function of the nail fold capillary bed in patients with peripheral vascular disease. In the 1990s, however, our group introduced HVM to the clinics, which allowed the first time observation of the microcirculation of the brain during surgery [12]. These first-generation HVM devices were based on orthogonal polarized spectral imaging and made use of cross-polarized green light to image the microcirculation without the need to transilluminate the organ surface from below as was needed before [13]. This methodology allowed clear video observation of the flowing RBCs of the microcirculation. Due to the limited applicability of the bulky apparatus and the need for high-powered light sources of orthogonal polarized spectral imaging, we developed a battery-based device based on sidestream dark field imaging [14]. Later a third-generation device with improved image quality was introduced based on incident dark field imaging [15]. In clinical conditions, these devices were mostly used to observe the sublingual microcirculation. In a large number of studies, sublingual microcirculation proved to be a highly clinically relevant location, where alterations were found to be highly sensitive and specific, much more than alterations in systemic hemodynamic variables, in predicting morbidity and mortality in various clinical patient groups [16, 17, 18, 19, 20, 21, 22].
To identify the sublingual microcirculation as a clinically relevant location representative of microcirculatory dysfunction in other organ beds, studies were carried out showing that sublingual microcirculation alterations paralleled microcirculatory alterations in other organs such as the intestines and kidneys [23, 24, 25]. Addressing this question in a clinical study, Boerma et al. [26] looked out the correlation between sublingual and intestinal microcirculation in patients having developed sepsis as a result of stoma surgery. Although they found no correlation early on in sepsis, later there was a correlation between the intestinal and sublingual microcirculation in these septic patients showing how regional microcirculatory alterations can develop into a systemic microcirculatory alteration in the course of time [26].
HVM studies were carried out in a range of different clinical applications including on organ surface during surgery [12, 27, 28, 29]. Furthermore, methodologies were developed to identify other aspects of the microcirculation such as methodologies to identify leucocyte kinetics [30] and the presence of the glycocalyx [31] and methodologies to identify microcirculatory reserve by topical application on the sublingual area of nitroglycerine [32].
The prevalence of the HVM devices and the growth in the number of studies showing adverse outcome to be linked to the persistence of microcirculatory alterations independent of alterations in systemic hemodynamic variables led the publication of an international consensus paper under the auspices of a task force of the European Society for Intensive Care Medicine on the measurement of sublingual microcirculation in critically ill patients using HVM [33]. One of the most important recommendations of their consensus guidelines was the need for the development of a validated automatic software analysis platform. At the time, the only validated software analysis platform was the semi-automatic AVA software developed by us [34] requiring time-consuming offline analyzing of images to produce functional microcirculatory parameters. Several automatic software platforms have been attempted, but these were either inadequate or not validated with sufficient rigor to allow these to be used in a reliable point-of-care application. Especially the quantitative measurement of capillary flow was found to be a major challenge in the automatic analysis of microcirculatory images. This changed with our recent development of an experimentally and clinically validated automatic software platform, called MicroTools, which allowed an almost 500 × faster automatic analysis of HVM generated microcirculatory than the previous AVA software [35] (website: [36]). This software platform calculates all the relevant parameters identified by the consensus paper as necessary for describing the functional state of the microcirculation, including quantitative velocity measurements of each vessel in the field of view. MicroTools thereby allowed a quantitative measure of the convective (RBC velocity and flow) and diffusive capacity (FCD) of the microcirculation instantaneously at the bedside. This important development has now made integrating the monitoring of the microcirculation using HVM into conventional systemic hemodynamic monitoring at the bedside as a point-of-care modality a reality.
Microvasculatory Shock and Renal Compromise
Sepsis is associated with profound changes in microcirculation due to several mechanisms including endothelial dysfunction, glycocalyx degradation, altered blood cell rheology (reduced RBC deformability), and dysbalance between the levels of vasodilating and vasoconstricting substances [37] (Fig. 2). An oxygen extraction deficit by the tissues is considered a main characteristic hemodynamic defect in sepsis. This defect was found to be unresolved by therapeutic increases in systemic oxygen delivery [38]. This property in sepsis has made it difficult to choose an effective resuscitation target for its hemodynamic resolution. The underlying mechanism of the reduced capacity of the tissues to extract oxygen from the circulation was identified in a series of experimental studies to be caused by microcirculatory dysfunction resulting in functional oxygen shunting of the microcirculation [39]. This condition manifests itself clinically as a reduction in the oxygen extraction capacity of the tissues, a condition that can occur in the presence of normalized systemic hemodynamic variables following resuscitation. The clinical introduction of HVM to the study of critically ill patients verified this mechanism by the observation of persistent RBC plugging of capillaries next to capillaries with normal RBC flow despite apparent adequate resuscitation based on the normalization of systemic hemodynamic variables. Studies using HVM in states of sepsis and shock showed these microcirculatory alterations to be related to adverse outcome and organ failure independent of systemic hemodynamic conditions [17, 18, 40, 41].
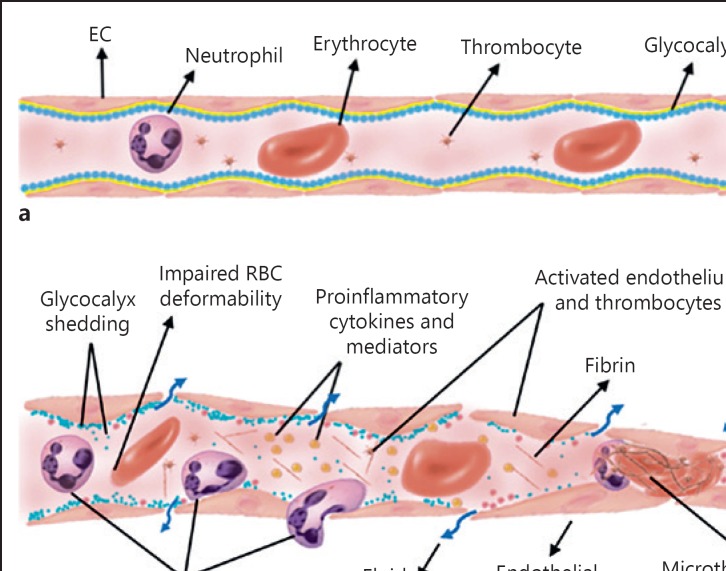
Fig. 2.
Microvascular dysfunction and vascular endothelial damage. a The structure of a healthy microvessel is shown. EC and glycocalyx cover the lumen of the microvessel. The blood cells (leukocytes, RBC, thrombocytes) flow together with plasma inside the microvessels. b Microcirculatory damage can be caused by ischemia, reperfusion, inflammation, and hypoxia, resulting in endothelial and glycocalyx and RBC damage. Activation of leukocytes induces rolling, adhesion, and ultimately extravasation to the tissue, which further accelerates the inflammation. Decreased vascular permeability causes vascular leakage and edema formation. RBC, red blood cell; EC, endothelial cells.
During states of cardiovascular compromise, resuscitation-induced improvement in systemic hemodynamic parameters does not necessarily result in a parallel improvement in the microcirculation. Such a condition, which is expected to occur under normal physiology, we termed as there being a “loss of hemodynamic coherence” between the macrocirculation and microcirculation. If persistent it has been shown to be an independent predictor of adverse patient outcome despite macrocirculatory normalization [36].
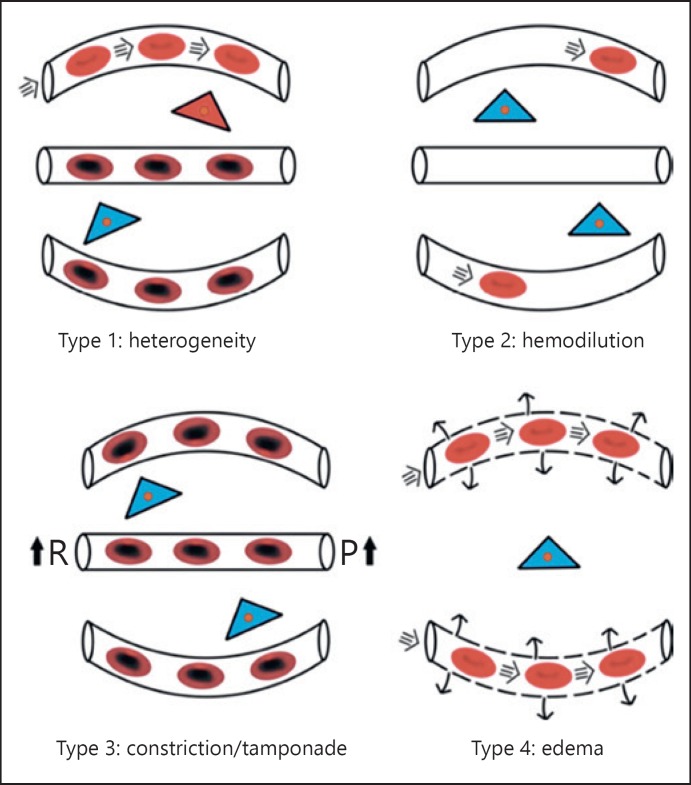
The loss of hemodynamic coherence has been found to be associated with 4 main types of hemodynamic microcirculatory alterations, all associated with a loss of oxygen extraction capacity of the tissues (Fig. 3). Type 1 alteration is characterized by heterogeneity in capillary density and blood flow and shunts in microvascular blood flow (as seen in sepsis). Type 2 alteration is associated with inadequate transport of oxygen to the microcirculation due to dilutional anemia caused, for example by hemodilution. Type 3 alteration is seen as a stasis of microcirculatory blood flow, for example, by the use of too much vasopressors [42] or tamponade caused by an increase in venous pressure [43]. Type 4 alteration is typically seen in states of edema where FCD is low.
Fig. 3.
Condition of microcirculatory alterations associated with loss of hemodynamic coherence and reduced oxygen capacity of the tissues. Type 1: Heterogenous RBC flow caused by RBC and endothelial cell injury induced for example by sepsis results in RBC stagnant capillaries next to perfused capillaries resulting in microcirculatory shunts and a reduction of tissue oxygen extraction capacity. Type 2: A decrease in the oxygen-carrying potential of the microcirculation due to hemodilution induced anemia resulting from a low FCD. Type 3: A stasis in the RBC flow due to increased vascular resistance (R) [37] and/or elevated venous pressure (P) [38]. Type 4: Increased oxygen diffusion distances due to edema caused by capillary leak syndrome. Adapted from Ince [67].
The observations of states of microcirculatory shock using HVM despite normalized systemic hemodynamics achieved by resuscitation have led to many studies being carried out to investigate the efficacy of various therapeutic interventions to resuscitate the microcirculation. The response of the microcirculation to vasoactive compounds have generally been shown that vasopressors targeting increases in blood pressure to have a limited effect on improving microcirculation unless there was initial microcirculatory hypoperfusion; otherwise, vasopressors could actually decrease microcirculatory flow [42, 44, 45]. Vasoactive compounds having dilatory effects such as dobutamine, enoximone, and nitroglycerin, on the other hand, were much more effective in recruiting the microcirculation [40, 46, 47].
Fluids are a mainstay therapeutic option for states of hypovolemia and shock, and many studies have been conducted investigating various aspects of the response of the microcirculation to fluid administration. Studies in sepsis have shown fluid resuscitation to be effective in promoting microcirculatory flow only when such microcirculatory flow was initially low in value [48]. Such a condition was shown to occur independently of normally used surrogates of hypovolemia as indicative of fluid need such as oliguria, stroke volume, tachycardia, and low lactate [49]. In abdominal surgical patients, Bouattour et al. [50] found pulse pressure variation to identify preload dependence to be associated with reduced sublingual microcirculation, which was successfully improved by fluid administration. A consistent finding especially in cardiac surgery patients is that fluid administration leads to a Type 2 loss of hemodynamic coherence (Fig. 3) where an RBC dilution quantified by a decrease in FCD indicated a reduction in oxygen extraction capacity of the microcirculation [51], whereas a de-escalation by use of diuretic therapy leads to an increase in FCD [52]. These consistent findings of the presence of dilution anemia being caused by excessive use of fluid therapy, identified in the microcirculation as a reduction in FCD, let us to identify anemic shock as a possible fifth category of shock, which should be added to the classic four states of circulatory shock (cardiogenic, hypovolemic, obstructive, and distributive) described by Weil [53]. Blood transfusions have been shown to be an effective option for recruiting the microcirculation [54], especially in improving the diffusional component of microcirculatory oxygen transport by an increase in FCD [55].
Heart failure and cardiogenic shock have been found in a number of studies to be associated with a decrease in the microcirculatory convective flow [41, 47]. Mechanical support of the circulation by use of VA-ECMO in adult and pediatric patients has shown that the inability of VA-ECMO to improve the microcirculation was associated with adverse outcome [16, 21, 56, 57]. It is clear from the above studies that the next phase in the study of the microcirculation must be to investigate the efficacy of microcirculatory-guided resuscitation strategies. For such studies to be effective, however, a point-of-care analysis of methodology for instant bedside evaluation of microcirculatory alterations is needed.
Microcirculatory alterations and hypoxemia have been reported in patients with chronic kidney disease and patients on hemodialysis. Studies using the BOLD technique for measuring renal tissue oxygenation showed that chronic kidney disease patients with renal hypoxemia had a 3 times more likely chance to develop the need for renal replacement therapy or show a 30% or more increase in serum creatinine [58]. During the course of hemodialysis and fluid withdrawal, microcirculatory flow is reduced as shown in several HVM studies [59, 60]. This effect could be reversed following renal transplantation [59]. In a large cohort of hemodialysis patients, Meyring-Wosten et al. [61] identified, by measurement of arterial and venous oxygen saturation, states of “prolonged intradialysis hypoxemia,” a condition they found to be associated with all-cause hospitalization and mortality. De-escalation following volume overload can be accomplished by hemodialysis or by diuretic therapy. Campos et al. [62] showed how arterial saturation can improve upon volume removal during hemodialysis. In an HVM study volume overloaded post-cardiac surgery patients receiving diuretic therapy were shown to be successful in increasing sublingual FCD, thereby improving the diffusive capacity of the microcirculation [52].
In states of inflammation and infection, such as in sepsis, blood purification of inflammatory mediators can be accomplished by the use of specialized cytokine removal filters, such as Cytosorb, in line with continuous replacement therapy. In a propensity score weighted retrospective clinical trial, we showed that such an approach was successful in improving 28 day mortality in severely ill septic ICU patients [63]. In an HVM study, Zuccari et al. [64] showed that the use of such Cytosorb filters were associated with a reduction in cytokine levels in parallel with a recovery of microcirculatory alterations associated with sepsis.
New Directions in the Clinical Monitoring of the Microcirculation
There is general agreement in the literature that the ultimate expectation of achieving an adequate hemodynamic resuscitation target is when there is a normalization of tissue perfusion (e.g., [65]). However, there is no clarity about what is precisely meant by “tissue perfusion.” It is presumed that tissue perfusion must be equivalent to the promotion of the flow of RBCs in the microcirculation with the aim of promoting tissue oxygenation to sustain cell viability needed to support organ function. However, the most common resuscitation procedure being fluid resuscitation may increase convection but at the expense of diffusion of oxygen due to the increase in diffusion distance between the RBCs reducing the oxygen extraction capacity of the tissues [66, 67]. Thus, a technique such as a laser Doppler is inadequate in measuring these variables because it only measured the flux. Near-infrared spectroscopy is equally inadequate because it only measures approximate hemoglobin oxygen saturation instead of actual delivery of oxygen availability. Thus, what is needed is a metric combining cellular RBC transport as well as RBC availability which represent the 2 primary determinants of microcirculatory oxygen transport: RBC convection and diffusion capacity, the latter consisting of the density of RBC-filled capillaries (FCD) and the capillary hematocrit [1]. To measure both microcirculatory convection and diffusion capacity, direct visualization of the microcirculation is mandatory (for identification of single RBC). In addition, these functional microcirculatory parameter values have to be directly calculated at the bedside in a point-of-care manner. Only then can clinicians titrate resuscitation compounds to optimize microcirculatory perfusion and oxygen transport values. HVM meets the requirements to allow clear visualization of flowing RBCs in the microcirculation and our recently introduced clinically validated automatic analysis software called MicroTools [35] allows instant calculation of all of the required parameters to quantify microcirculatory oxygen transport values thus fusing capillary hematocrit, FCD, and the flow of RBC into one parameter defining the determinants of microcirculatory oxygen transport variables has led us to introduce a new resuscitation target variable we call tissue “RBC perfusion”. We expect this parameter, which can be instantaneously measured using HVM in conjunction with MicroTools, to provide a gold standard as a microcirculatory resuscitation target. A current research in our group in a large international multicenter database has gathered microcirculatory measurements in various patient categories, and therapeutic interventions will provide the validation of the use of tissue RBC perfusion as this new resuscitation target.
Conclusion
Over the last several decades, much progress has been made in our understanding of microcirculatory (dys-) function in various clinical conditions due to the introduction of HVM for bedside observations of the microcirculation. Over the years, technological advances have led to improvements in the development of hardware related to HVM as well as development of fully automated software (MicroTools) for analysis of images to provide functional microcirculatory parameters. It is expected that direct visualization of the microcirculation and performing point-of-care analysis of functional parameters will identify patients at risk where apparent resuscitation targets have been met based on the normalization of systemic hemodynamic variables and possibly provide new microcirculatory-based resuscitation targets in conjunction with systemic hemodynamic targets [68].
Disclosure Statement
C.I. has received grants and speaker fees from Fresenius Medical, Fresenius-Kabi, Cytosorbents, La Jolla Pharmaceutical Company, AM Pharma, Covidean, Baxter Health Care. Dr. Ince and his team provided services and training with regard to clinical microcirculation. To this purpose, he runs an internet site called https://www.microcirculationacademy.org. The internet site and its activities are run by a company called Active Medical BV of which CI is shareholder and MPH has received financial support.
Funding Sources
There is no funding source to declare.
Author Contributions
G.G., M.H., and C.I.: design of the work, revising the manuscript critically for important intellectual content.
References
- 1.Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review microvascular dysfunction in sepsis—hemodynamics, oxygen transport, and nitric oxide. Crit Care. 2003 Oct;7((5)):359–73. doi: 10.1186/cc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019 Jan;23((1)):16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9((1)):121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 4.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, et al. ADQI XIV Workgroup. The Endothelium in Sepsis. Shock. 2016 Mar;45((3)):259–70. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerci P, Ergin B, Uz Z, Ince Y, Westphal M, Heger M, et al. Glycocalyx Degradation Is Independent of Vascular Barrier Permeability Increase in Nontraumatic Hemorrhagic Shock in Rats. Anesth Analg. 2019 Aug;129((2)):598–607. doi: 10.1213/ANE.0000000000003918. [DOI] [PubMed] [Google Scholar]
- 6.Zafrani L, Ince C. Microcirculation in Acute and Chronic Kidney Diseases. Am J Kidney Dis. 2015 Dec;66((6)):1083–94. doi: 10.1053/j.ajkd.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Post EH, Kellum JA, Bellomo R, Vincent JL. Renal perfusion in sepsis from macro- to microcirculation. Kidney Int. 2017 Jan;91((1)):45–60. doi: 10.1016/j.kint.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Welch WJ. Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol. 2006 Oct;33((10)):1002–5. doi: 10.1111/j.1440-1681.2006.04478.x. [DOI] [PubMed] [Google Scholar]
- 9.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol. 2011;174:119–28. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 10.Ergin B, Heger M, Kandil A, Demirci-Tansel C, Ince C. Mycophenolate mofetil improves renal haemodynamics microvascular, oxygenation, and inflammation in a rat model of supra-renal aortic clamping-mediated renal ischaemia reperfusion injury. Clin Exp Pharmacol Physiol. 2017 Feb;44((2)):294–304. doi: 10.1111/1440-1681.12687. [DOI] [PubMed] [Google Scholar]
- 11.Zafrani L, Ergin B, Kapucu A, Ince C. Blood transfusion improves renal oxygenation and renal function in sepsis-induced acute kidney injury in rats. Crit Care. 2016 Dec;20((1)):406. doi: 10.1186/s13054-016-1581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathura KR, Bouma GJ, Ince C. Abnormal microcirculation in brain tumours during surgery. Lancet. 2001 Nov;358((9294)):1698–9. doi: 10.1016/S0140-6736(01)06722-8. [DOI] [PubMed] [Google Scholar]
- 13.Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, et al. Orthogonal polarization spectral imaging a new method for study of the microcirculation. Nat Med. 1999 Oct;5((10)):1209–12. doi: 10.1038/13529. [DOI] [PubMed] [Google Scholar]
- 14.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007 Nov;15((23)):15101–14. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 15.Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. 2015 Dec;3((1)):40. doi: 10.1186/s40635-015-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Top AP, Ince C, van Dijk M, Tibboel D. Changes in buccal microcirculation following extracorporeal membrane oxygenation in term neonates with severe respiratory failure. Crit Care Med. 2009 Mar;37((3)):1121–4. doi: 10.1097/CCM.0b013e3181962a5f. [DOI] [PubMed] [Google Scholar]
- 17.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012 May;40((5)):1443–8. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 18.Massey MJ, Hou PC, Filbin M, Wang H, Ngo L, Huang DT, et al. ProCESS investigators. Microcirculatory perfusion disturbances in septic shock results from the ProCESS trial. Crit Care. 2018 Nov;22((1)):308. doi: 10.1186/s13054-018-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002 Jul;166((1)):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 20.Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014 Jun;42((6)):1433–41. doi: 10.1097/CCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 21.Kara A, Akin S, Dos Reis Miranda D, Struijs A, Caliskan K, van Thiel RJ, et al. Microcirculatory assessment of patients under VA-ECMO. Crit Care. 2016 Oct;20((1)):344. doi: 10.1186/s13054-016-1519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorcella C, Damiani E, Domizi R, Pierantozzi S, Tondi S, Carsetti A, et al. MicroDAIMON study Microcirculatory DAIly MONitoring in critically ill patients: a prospective observational study. Ann Intensive Care. 2018 May;8((1)):64. doi: 10.1186/s13613-018-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrara G, Edul VS, Canales HS, Martins E, Canullán C, Murias G, et al. Systemic and microcirculatory effects of blood transfusion in experimental hemorrhagic shock. Intensive Care Med Exp. 2017 Dec;5((1)):24. doi: 10.1186/s40635-017-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis a quantitative analysis. Crit Care Med. 2009 Nov;37((11)):2875–81. doi: 10.1097/CCM.0b013e3181b029c1. [DOI] [PubMed] [Google Scholar]
- 25.Lima A, van Rooij T, Ergin B, Sorelli M, Ince Y, Specht PA, et al. Dynamic Contrast-Enhanced Ultrasound Identifies Microcirculatory Alterations in Sepsis-Induced Acute Kidney Injury. Crit Care Med. 2018 Aug;46((8)):1284–92. doi: 10.1097/CCM.0000000000003209. [DOI] [PubMed] [Google Scholar]
- 26.Boerma EC, van der Voort PH, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007 Apr;35((4)):1055–60. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 27.Uz Z, Ince C, Rassam F, Ergin B, van Lienden KP, van Gulik TM. Assessment of hepatic microvascular flow and density in patients undergoing preoperative portal vein embolization. HPB (Oxford) 2019 Feb;21((2)):187–94. doi: 10.1016/j.hpb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Uz Z, Kastelein AW, Milstein DM, Liu D, Rassam F, Veelo DP, et al. Intraoperative Incident Dark Field Imaging of the Human Peritoneal Microcirculation. J Vasc Res. 2018;55((3)):136–43. doi: 10.1159/000488392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavy AL, de Bruin AF, van der Sloot K, Boerma EC, Ince C, Noordzij PG, et al. Effects of Thoracic Epidural Anaesthesia on the Serosal Microcirculation of the Human Small Intestine. World J Surg. 2018 Dec;42((12)):3911–7. doi: 10.1007/s00268-018-4746-z. [DOI] [PubMed] [Google Scholar]
- 30.Uz Z, van Gulik TM, Aydemirli MD, Guerci P, Ince Y, Cuppen D, et al. Identification and quantification of human microcirculatory leukocytes using handheld video microscopes at the bedside. J Appl Physiol (1985) 2018 Jun;124((6)):1550–7. doi: 10.1152/japplphysiol.00962.2017. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ, et al. Measuring endothelial glycocalyx dimensions in humans, a potential novel tool to monitor vascular vulnerability. J Appl Physiol (1985) 2008 Mar;104((3)):845–52. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 32.Hilty MP, Merz TM, Hefti U, Ince C, Maggiorini M, Pichler Hefti. J. Recruitment of non-perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m. J Physiol. 2019 May;597((10)):2623–38. doi: 10.1113/JP277590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, et al. Cardiovascular Dynamics Section of the ESICM. Second consensus on the assessment of sublingual microcirculation in critically ill patients results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44((3)):281–99. doi: 10.1007/s00134-018-5070-7. [DOI] [PubMed] [Google Scholar]
- 34.Dobbe JG, Streekstra GJ, Atasever B, van Zijderveld R, Ince C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008 Jul;46((7)):659–70. doi: 10.1007/s11517-008-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilty MP, Guerci P, Ince Y, Toraman F, Ince C. MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun Biol. 2019 Jun;2:217. doi: 10.1038/s42003-019-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. https://www.microcirculationacademy.org. [Google Scholar]
- 37.C Lelubre, JL Vincent. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018 Jul;14((7)):417–27. doi: 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 38.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994 Jun;330((24)):1717–22. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 39.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999 Jul;27((7)):1369–77. doi: 10.1097/00003246-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 40.De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006 Feb;34((2)):403–8. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 41.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004 Jan;147((1)):91–9. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Boerma EC, van der Voort PH, Ince C. Sublingual microcirculatory flow is impaired by the vasopressin-analogue terlipressin in a patient with catecholamine-resistant septic shock. Acta Anaesthesiol Scand. 2005 Oct;49((9)):1387–90. doi: 10.1111/j.1399-6576.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 43.Vellinga NA, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, et al. microSOAP Study Group International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med. 2015 Jan;43((1)):48–56. doi: 10.1097/CCM.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 44.Jhanji S, Stirling S, Patel N, Hinds CJ, Pearse RM. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009 Jun;37((6)):1961–6. doi: 10.1097/CCM.0b013e3181a00a1c. [DOI] [PubMed] [Google Scholar]
- 45.Dubin A, Pozo MO, Casabella CA, Pálizas F, Jr, Murias G, Moseinco MC, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow a prospective study. Crit Care. 2009;13((3)):R92. doi: 10.1186/cc7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002 Nov;360((9343)):1395–6. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 47.den Uil CA, Lagrand WK, van der Ent M, Nieman K, Struijs A, Jewbali LS, et al. Conventional hemodynamic resuscitation may fail to optimize tissue perfusion, an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock. PLoS One. 2014 Aug;9((8)):e103978. doi: 10.1371/journal.pone.0103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010 Jun;36((6)):949–55. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 49.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013 Apr;39((4)):612–9. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouattour K, Teboul JL, Varin L, Vicaut E, Duranteau J. Preload Dependence Is Associated with Reduced Sublingual Microcirculation during Major Abdominal Surgery. Anesthesiology. 2019 Apr;130((4)):541–9. doi: 10.1097/ALN.0000000000002631. [DOI] [PubMed] [Google Scholar]
- 51.Atasever B, van der Kuil M, Boer C, Vonk A, Schwarte L, Girbes AR, et al. Red blood cell transfusion compared with gelatin solution and no infusion after cardiac surgery effect on microvascular perfusion, vascular density, hemoglobin, and oxygen saturation. Transfusion. 2012 Nov;52((11)):2452–8. doi: 10.1111/j.1537-2995.2012.03802.x. [DOI] [PubMed] [Google Scholar]
- 52.Uz Z, Ince C, Guerci P, Ince Y, P Araujo R, Ergin B, et al. Recruitment of sublingual microcirculation using handheld incident dark field imaging as a routine measurement tool during the postoperative de-escalation phase-a pilot study in post ICU cardiac surgery patients. Perioper Med (Lond) 2018 Aug;(7):18. doi: 10.1186/s13741-018-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent JL, Ince C, Bakker J., Clinical review Circulatory shock—an update: a tribute to Professor Max Harry Weil. Crit Care. 2012 Nov;16((6)):239. doi: 10.1186/cc11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka S, Escudier E, Hamada S, Harrois A, Leblanc PE, Vicaut E, et al. Effect of RBC Transfusion on Sublingual Microcirculation in Hemorrhagic Shock Patients, A Pilot Study. Crit Care Med. 2017 Feb;45((2)):e154–60. doi: 10.1097/CCM.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 55.Yuruk K, Almac E, Bezemer R, Goedhart P, de Mol B, Ince C. Blood transfusions recruit the microcirculation during cardiac surgery. Transfusion. 2011 May;51((5)):961–7. doi: 10.1111/j.1537-2995.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 56.Yeh YC, Lee CT, Wang CH, Tu YK, Lai CH, Wang YC, et al. NTUH Center of Microcirculation Medical Research (NCMMR). Investigation of microcirculation in patients with venoarterial extracorporeal membrane oxygenation life support. Crit Care. 2018 Aug;22((1)):200. doi: 10.1186/s13054-018-2081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akin S, Dos Reis Miranda D, Caliskan K, Soliman OI, Guven G, Struijs A, et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care. 2017 Oct;21((1)):265. doi: 10.1186/s13054-017-1855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018 Apr;93((4)):932–40. doi: 10.1016/j.kint.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Yeh YC, Chao A, Lee CY, Lee CT, Yeh CC, Liu CM, et al. An observational study of microcirculation in dialysis patients and kidney transplant recipients. Eur J Clin Invest. 2017 Sep;47((9)):630–7. doi: 10.1111/eci.12784. [DOI] [PubMed] [Google Scholar]
- 60.Bemelmans RH, Boerma EC, Barendregt J, Ince C, Rommes JH, Spronk PE. Changes in the volume status of haemodialysis patients are reflected in sublingual microvascular perfusion. Nephrol Dial Transplant. 2009 Nov;24((11)):3487–92. doi: 10.1093/ndt/gfp267. [DOI] [PubMed] [Google Scholar]
- 61.Meyring-Wösten A, Zhang H, Ye X, Fuertinger DH, Chan L, Kappel F, et al. Intradialytic Hypoxemia and Clinical Outcomes in Patients on Hemodialysis. Clin J Am Soc Nephrol. 2016 Apr;11((4)):616–25. doi: 10.2215/CJN.08510815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campos I, Chan L, Zhang H, Deziel S, Vaughn C, Meyring-Wösten A, et al. Intradialytic Hypoxemia in Chronic Hemodialysis Patients. Blood Purif. 2016;41((1-3)):177–87. doi: 10.1159/000441271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock a propensity-score-weighted retrospective study. Crit Care. 2019 Sep;23((1)):317. doi: 10.1186/s13054-019-2588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuccari S, Damiani E, Domizi R, Scorcella C, D'Arezzo M, Carsetti A, et al. Changes in Cytokines, Haemodynamics and Microcirculation in Patients with Sepsis/Septic Shock Undergoing Continuous Renal Replacement Therapy and Blood Purification with CytoSorb. Blood Purif. 2019 Aug;:1–7. doi: 10.1159/000502540. [DOI] [PubMed] [Google Scholar]
- 65.Cecconi M, Hernandez G, Dunser M, Antonelli M, Baker T, Bakker J, et al. Fluid administration for acute circulatory dysfunction using basic monitoring narrative review and expert panel recommendations from an ESICM task force. Intensive Care Med. 2019 Jan;45((1)):21–32. doi: 10.1007/s00134-018-5415-2. [DOI] [PubMed] [Google Scholar]
- 66.Perel A, Javidroozi M, Shander A. Blood Transfusion in Sepsis and Iatrogenic Hemodilution. Am J Crit Care. 2018 Nov;27((6)):442–3. doi: 10.4037/ajcc2018450. [DOI] [PubMed] [Google Scholar]
- 67.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legrand M, Ait-Oufella H, Ince C. Could resuscitation be based on microcirculation data? Yes Intensive Care Med. 2018 Jun;44((6)):944–6. doi: 10.1007/s00134-018-5121-0. [DOI] [PubMed] [Google Scholar]