Summary
Background
Although fomites or contaminated surfaces have been considered as transmission routes, the role of environmental contamination by human parainfluenza virus type 3 (hPIV-3) in healthcare settings is not established.
Aim
To describe an hPIV-3 nosocomial outbreak and the results of environmental sampling to elucidate the source of nosocomial transmission and the role of environmental contamination.
Methods
During an hPIV-3 outbreak between May and June 2016, environmental surfaces in contact with clustered patients were swabbed and respiratory specimens used from infected patients and epidemiologically unlinked controls. The epidemiologic relatedness of hPIV-3 strains was investigated by sequencing of the haemagglutinin–neuraminidase and fusion protein genes.
Findings
Of 19 hPIV-3-infected patients, eight were haematopoietic stem cell recipients and one was a healthcare worker. In addition, four had upper and 12 had lower respiratory tract infections. Of the 19 patients, six (32%) were community-onset infections (symptom onset within <7 days of hospitalization) and 13 (68%) were hospital-onset infections (≥7 days of hospitalization). Phylogenetic analysis identified two major clusters: five patients, and three patients plus one healthcare worker. Therefore, seven (37%) were classified as nosocomial transmissions. hPIV-3 was detected in 21 (43%) of 49 environmental swabs up to 12 days after negative respiratory polymerase chain reaction conversion.
Conclusion
At least one-third of a peak season nosocomial hPIV-3 outbreak originated from nosocomial transmission, with multiple importations of hPIV-3 from the community, providing experimental evidence for extensive environmental hPIV-3 contamination. Direct contact with the contaminated surfaces and fomites or indirect transmission from infected healthcare workers could be responsible for nosocomial transmission.
Keywords: Human parainfluenza virus type 3, Nosocomial outbreak, Haematology unit, Phylogenetic analysis, Environmental contamination
Introduction
Human parainfluenza viruses (hPIV), along with human respiratory syncytial virus (RSV), are well-recognized respiratory pathogens in infants and young children, ranging from mild upper respiratory tract (URI) symptoms to croup and pneumonia [1], [2]. In the immunocompetent adult, URIs are mild and self-limiting in nature, and reinfections may occur due to incomplete immunity to hPIV. However, in the immunocompromised host, especially in haematopoietic stem cell transplant (HSCT) recipients and patients with haematologic malignancies, hPIV causes a significant morbidity and mortality with prolonged virus shedding [3], [4], [5]. Of the five known human serotypes, human parainfluenza virus type 3 (hPIV-3) is the most frequently identified pathogen [4]. Epidemics of hPIV are seasonal, with the peak of activity usually between May and June each year in South Korea, accounting for about 20–35% of detected respiratory virus agents in the community [6].
There are several reports of hPIV-3 nosocomial outbreaks in HSCT recipients or patients with haematologic malignancy [7], [8], [9], [10], [11], [12], [13], [14]. In these reports, whereas some outbreaks were due to a single strain circulating within the unit, others originated from multiple hPIV-3 strains introduced from the community [7], [11], [12], [14]. Sequencing and phylogenetic analysis of hPIV-3 strains allowed for verification of whether a single strain or multiple strains were responsible for the outbreak. In addition, in some of these reports, infection control, including symptomatic surveillance and isolation, were often ineffective in terminating transmission, suggesting asymptomatic viral shedding among patients, staff, or outside visitors, or environmental contamination as a possible explanation [9]. Although fomites or contaminated surfaces have been considered as possible transmission routes of hPIV-3, in addition to droplets or close personal contact, the role of environmental contamination by hPIV-3 in healthcare settings has not been well defined [15], [16]. The present study describes an hPIV-3 nosocomial outbreak in a haematology ward and the results of environmental sampling to elucidate the source of nosocomial transmission and the role of environmental contamination.
Methods
Hospital setting and patient population
This study was conducted at a haematology unit in a 2700-bed tertiary care hospital in Seoul, South Korea. The haematology unit, which serves chemotherapy for patients with haematologic malignancy and haematopoietic stem cell transplantation (HCT), consists of two wards located on different floors. One ward (A) has 42 beds; 12 of these are for patients undergoing HCT. The other ward (B) has 50 beds, including a mixture of single-, two-, five-, and six-bedded rooms, communal showers, and a visiting room for patients on post-HCT care or cytotoxic chemotherapy. These wards routinely allow limited visitation due to the immunocompromised status of admitted patients.
In mid-June 2016, an increased number of hPIV-3-positive cases were identified, clustered in a six-bed room on ward B. By the end of June, one or two more cases were confirmed each day, leading to the notification of an outbreak in the haematology unit by the infection control team. After thorough review, all laboratory-confirmed hPIV-3 patients and symptomatic healthcare workers in the haematology ward during this period were enrolled in this study. Since the incubation period of hPIV-3 infection in adults has been estimated to be two to six days, an infection was considered to be hospital-onset if the patient had been hospitalized for ≥7 days before the onset of respiratory symptoms [17], [18], [19]. All rooms occupied by the original cluster of diagnosed patients, including the six-bedded room, the adjacent room, and the isolation room were selected for the environmental study. This study was approved by the institutional review board of Asan Medical Center.
Sample collection
Respiratory samples, including nasopharyngeal swabs/aspirates and bronchoalveolar lavage (BAL) fluid, were taken as part of routine clinical practice. After learning of the hPIV-3 outbreak, respiratory samples were tested for respiratory viruses including hPIV types 1–4, adenovirus, influenza A and B virus, rhinovirus, human metapneumovirus, bocavirus, coronavirus 229E, NL63, and OC43, enterovirus, and RSV types A and B by real-time multiplex reverse transcription–polymerase chain reaction (PCR) as a routine screening of any patient or healthcare worker presenting with respiratory symptoms and/or fever. Additional microbiological investigations performed on sputum or BAL fluid included Gram stain, acid-fast stain, and cultures for conventional bacteria, mycobacteria, and fungi, as needed. Samples were also tested for galactomannan and CMV DNA load (CMV qPCR) on blood and BAL fluid, if invasive aspergillosis or CMV disease was suspected. Once the hPIV-3 infection was diagnosed, respiratory samples for hPIV-3 testing were taken weekly until negative or patient discharge. Thus, no further respiratory samples were taken at the time of environmental sampling.
Dacron swabs pre-moistened with viral transport medium were used to aseptically swab surfaces that were frequently touched by patients or healthcare workers. The following types of surface were swabbed: (i) fomites (stethoscopes, infusion regulator, hand sanitizer tip, nasal prongs or masks, pillows, curtains, and television remote control); (ii) fixed structures in the rooms and their associated restrooms (doorknobs, bedside rails, toilet seats, call button, telephone buttons, hand sanitizing dispensor, and light switch). All environmental samples were collected after routine cleaning or periodic linen change. The standard cleaning procedures of the rooms included routine bed cleaning (e.g. bed rails, control panel, call bell, bedside locker, switches, telephone, and main door knob) and toilet cleaning at least daily; and discharge bed cleaning after the patients moved or were discharged.
Eleven isolates that were concurrently circulating in the community were obtained from respiratory specimens of epidemiologically unlinked patients from different wards of our institution during the same period to be used as controls.
Real-time RT–PCR and hPIV-3 strain sequencing
Viral RNA was extracted from all respiratory or environmental specimens using QIAmp® Viral RNA Mini Kit (Qiagen, Germany). The respiratory specimens from 19 patients with confirmed hPIV-3 and environmental samples were included in the subsequent epidemiological investigation, involving partial sequence analysis of the haemagglutinin–neuraminidase (HN) gene. In addition, 11 hPIV-3 isolates that were obtained from respiratory specimens of epidemiologically unlinked patients on different wards of our institution during the same period were also sequenced as controls. RT–PCR was performed on the extracts using primers F (5′-ATTACTCGAGGTTGCCAGGA-3′) and R (5′-CCGCGACACCCAGTTGTG-3′) covering 450 bp of the HN gene. The OneStep RT–PCR Kit (Qiagen, Hilden, Germany) was used according to the manufacturer's instructions, with 5 μL of extract being amplified in a final volume of 25 μL, containing 5× one-step RT–PCT buffer, 0.25 mM deoxynucleotide triphosphate, 25 pmol of each primer, and one unit of one-step RT–PCR Enzyme Mix. Thermal cycling conditions were as follows: 30 min at 50°C, followed by 15 min at 95°C and 50 cycles of 30 s at 95°C, 30 s at 50°C, and 30 s at 72°C, and a final elongation step at 72°C for 10 min. Sequences were aligned with MEGA 7.0 software using the Clustal W method. Phylogenetic analysis was carried out using the MEGA 7.0 software maximum likelihood method; the kimura 2-parameter was selected as an evolution model, and the bootstrap value was 1000. The sequence of fusion protein (F) gene was further analysed for clarifying the epidemiologic links within an equivocal cluster. The OneStep RT-PCR Kit was also used with primers F (5′-CTTTGGAGGGGTAATTGGAACTA-3′) and R (5′-ATGATGTGGCTGGGAAGAGG-3′) covering 621 bp of F gene. Thermal cycling conditions were followed as previously described. A positive result was confirmed by agarose gel electrophoresis and visualization by UV transilluminator.
Statistical analysis
The Kaplan–Meier method was used to construct survival curves for the period during which patients had positive RT–PCR results. As flock swab samples were not obtained at discharge from patients who recovered, we defined these patients who did not undergo follow-up flock swab sampling as ‘censored’ patients. All statistical analyses were performed using SPSS version 21.0 (IBM Co., Armonk, NY, USA).
Results
hPIV-3 outbreak
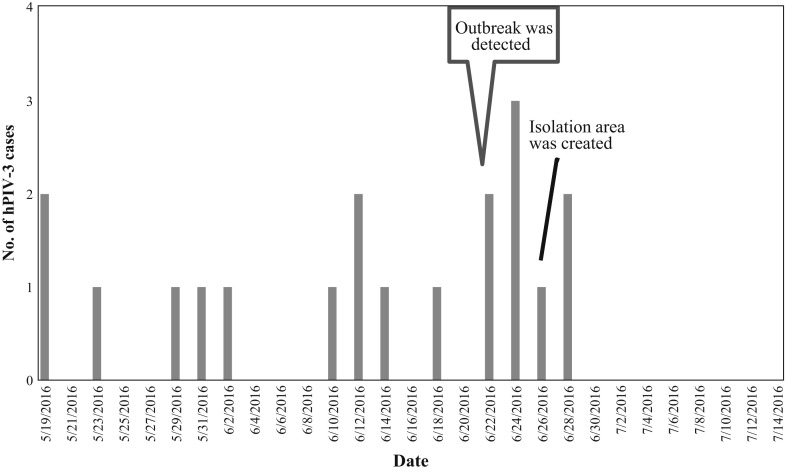
From May 19, 2016 through June 30, 2016, 19 patients with hPIV-3, including eight HCT recipients and one healthcare worker, were identified in haematology ward B (Figure 1 ). Table I shows the clinical characteristics of these patients. The mean age of the patients was 44 years. The majority of the patients were lymphopenic (12; 64%). Six of the eight HCT recipients had undergone transplantation <12 months before hPIV-3 infection developed. Of the 19 infected patients, four had upper and 12 had lower respiratory tract infections, and five (26%) died of hPIV-3 infection. Of the 19 patients, six (32%) were community-onset infection (symptom onset within <7 days of hospitalization) and 13 (68%) were hospital-onset infection (symptom onset within ≥7 days of hospitalization). Fourteen patients (74%) had other serious concurrent infections, including invasive fungal infections (eight patients). Median length (interquartile range) of viral shedding from respiratory specimen was 18 days (14–19 days).
Figure 1.
Number of hPIV-3 cases detected during outbreak in ward B.
Table I.
Characteristic of 19 patients infected with parainfluenza virus 3 (PIV3)
| Patient characteristics | Total (N = 19) |
|---|---|
| Mean age (years) (± SD) | 44 ± 19 |
| Male gender (%) | 7 (37%) |
| Underlying malignancy a (%) | 18 (95%) |
| Leukaemia b | 12 |
| Lymphoma c | 1 |
| Multiple myeloma | 2 |
| Myelodysplasia | 2 |
| Haemophagocytic lymphohistiocytosis | 1 |
| Neutropenia (<0.5×109 cells/L) (%) | 6 (32%) |
| Lymphopenia (<1.5×109 cells/L) (%) | 12 (64%) |
| Haematopoietic stem cell transplant (%) | 8 (42%) |
| Allogeneic | 7 |
| Within 12 months (allogeneic) | 6 (5) |
| More than 12 months ago | 2 |
| Admission with PIV3 infection (%) | 2 (11%) |
| Associated symptoms/signs | |
| Asymptomatic | 1 |
| Upper respiratory tract infection | 4 |
| Lower respiratory tract infection | 12 |
| Fever only | 3 |
| Neutropenic sepsis | 3 |
| Co-infection (%) | 14 (74%) |
| Bacterial d | 5 |
| Viral e | 2 |
| Fungal f | 8 |
| Length of PIV3 excretion (days ± SD) g | 18 ± 6 |
| Mortality (%) | 5 (26%) |
One patient was a nurse working in ward B, who was previously healthy.
Four acute lymphoblastic, seven acute myeloid, and one aggressive natural killer cell leukaemias.
One diffuse large B-cell lymphoma.
Bacterial co-infection included vancomycin-resistant enterococcal bacteraemia, meticillin-susceptible Staphylococcus aureus, Escherichia coli and Aeromonas hydrophila bacteraemia, and Clostridium difficile infection.
Virus co-infection included cytomegalovirus (CMV) pneumonia and CMV antigenaemia.
Fungal co-infection included five invasive pulmonary aspergilloses, two Pneumocystis jirovecii pneumonia, and one chronic disseminated candidiasis.
The Kaplan–Meier method was used to construct survival curves for the period during which patients had positive reverse transcription–polymerase chain reaction results. Once the hPIV-3 infection was diagnosed, respiratory samples for hPIV-3 testing were taken weekly until negative conversion or patient discharge.
Molecular analysis
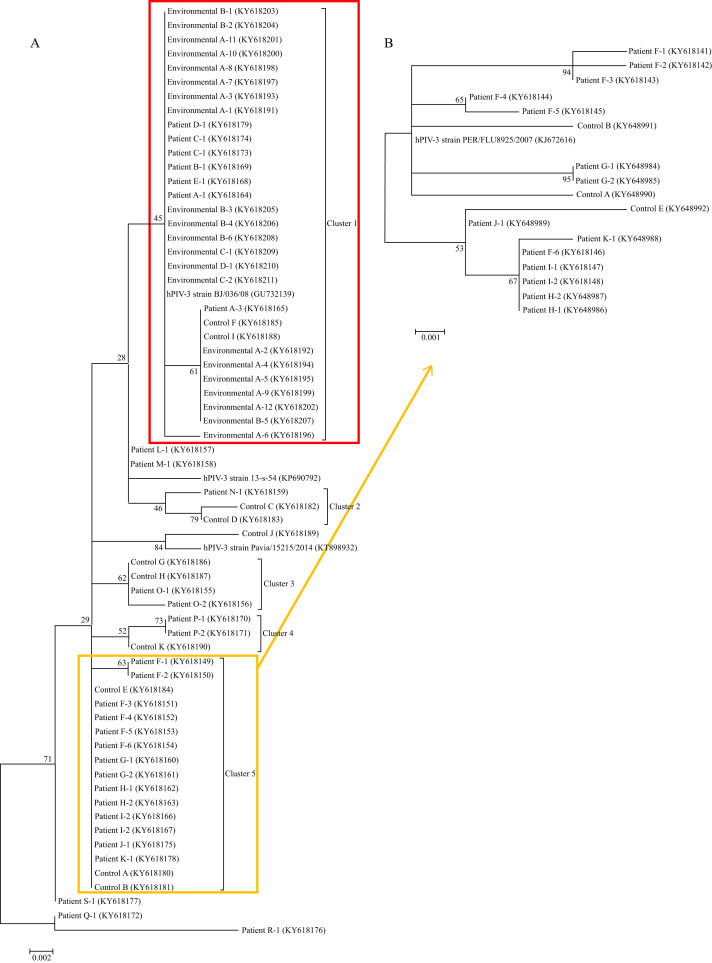
Phylogenetic analysis of the HN gene of the 42 hPIV-3 strains relevant to the 19 infected patients, 11 control patients unrelated to this outbreak, and 21 environmental samples of 49 swab specimens are shown in Figure 2 . Two major clusters were identified: cluster 1, including 30 identical hPIV-3 strains (relevant to patient A, E, B, C, D, and two control patients), and cluster 5, including 17 identical hPIV-3 strains (relevant to patients F, G, H, I, J, K, and three control patients). Further analysis of the F gene within cluster 5 (17 hPIV-3 strains relevant for the six patients and three control patients) was performed because the HN gene had low discriminate power within cluster 5. The F gene analysis revealed the subcluster of eight identical hPIV-3 strains (relevant to patients H, I, J, K, the last hPIV-3 strains from patient F, and one control patient). Other clusters consisted of three or four identical hPIV-3 strains isolated from one patient and one or two control patients.
Figure 2.
Phylogenetic analysis of the 65 identified hPIV-3 strains. (A) Sequence comparison of PIV3 sequenced in the haemagglutinin–neuraminidase (HN) gene of 450 bp. (B) Sequence comparison of PIV3 sequenced in the fusion protein (F) gene from cluster 5. GenBank accession numbers are KP690792 for hPIV-3 strain 11-s-83, GU732139 for hPIV-3 strain BJ/036/08, KT898932 for hPIV-3 strain Pavia/15215/2014, and KJ672616 for hPIV-3 strain PER/FLU8925/2007.
Epidemiological analysis
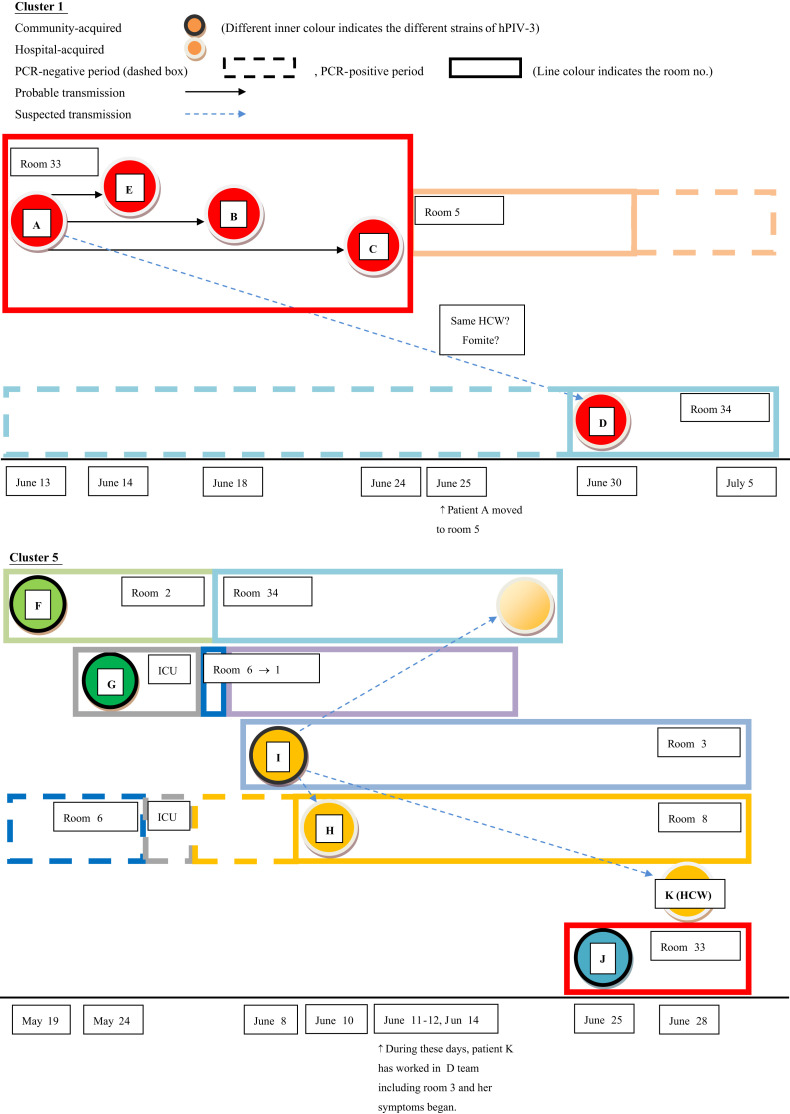
The temporal and spatial relationships among the 11 outbreak cases of hPIV-3 belonging to the two identified major clusters (i.e. clusters 1 and 5) are shown in Figure 3 . These analyses revealed that seven (37%) cases including one reinfection case were classified as nosocomial transmissions. The six cases except one reinfection case were initially classified as hospital-onset infection (symptom onset within ≥7 days of hospitalization). Genetically identical strains are identified by the same inner circle colours; likewise different rooms are indicated by different box colours. Within cluster 1, patient A, the index case, had positive hPIV-3 PCR results over 10 days and infected four patients. Of these four patients, three were hospitalized in the same room with patient A at the time of hPIV-3 diagnosis. Finally, patient A moved to a single room (room 5) for effective isolation. After isolation of patient A, the same nursing team cared for the patients in room 5 (patient A) and room 34 (patient D).
Figure 3.
Chronological appearance of the hPIV-3 strains of the 11 infected patients in cluster 1 and cluster 5 during the outbreak.
Within cluster 5, patients F, G, I, and J presented symptoms associated with hPIV-3 infection or positive hPIV-3 PCR results on admission. According to the additional F-gene sequence analysis, initial hPIV-3 strains isolated from these four patients were distinctive community-acquired strains. Patient I, who had acquired hPIV-3 in the community, was admitted through the emergency department and transmitted hPIV-3 to patients H, F (reinfection), and K (healthcare worker). Even though the healthcare worker (patient K) was positive for hPIV-3 by PCR on 28 June, she developed symptoms two weeks prior. During her symptomatic period, she cared for the patients in room 34, including patient F. The last (sixth) hPIV-3 isolate from patient F was a different strain from the other isolates from patient F, and was the same strain as that of the subcluster. Patient F succumbed to reinfection by the last hPIV-3 strain in a hospital ward, either from other patients (i.e. patient I or H) or the healthcare worker (patient K). The pairwise comparison among all six strains from patients F and I also support our explanation of reinfection in patient F (Supplementary Figure 1, Appendix A).
Environmental contamination
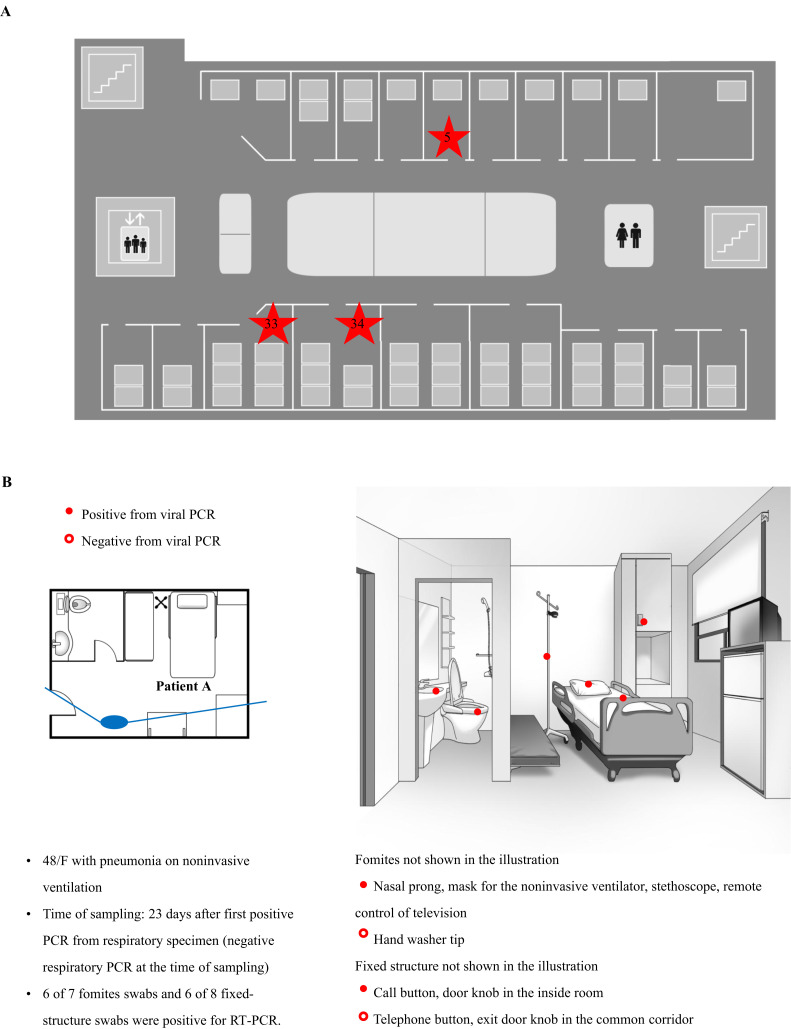
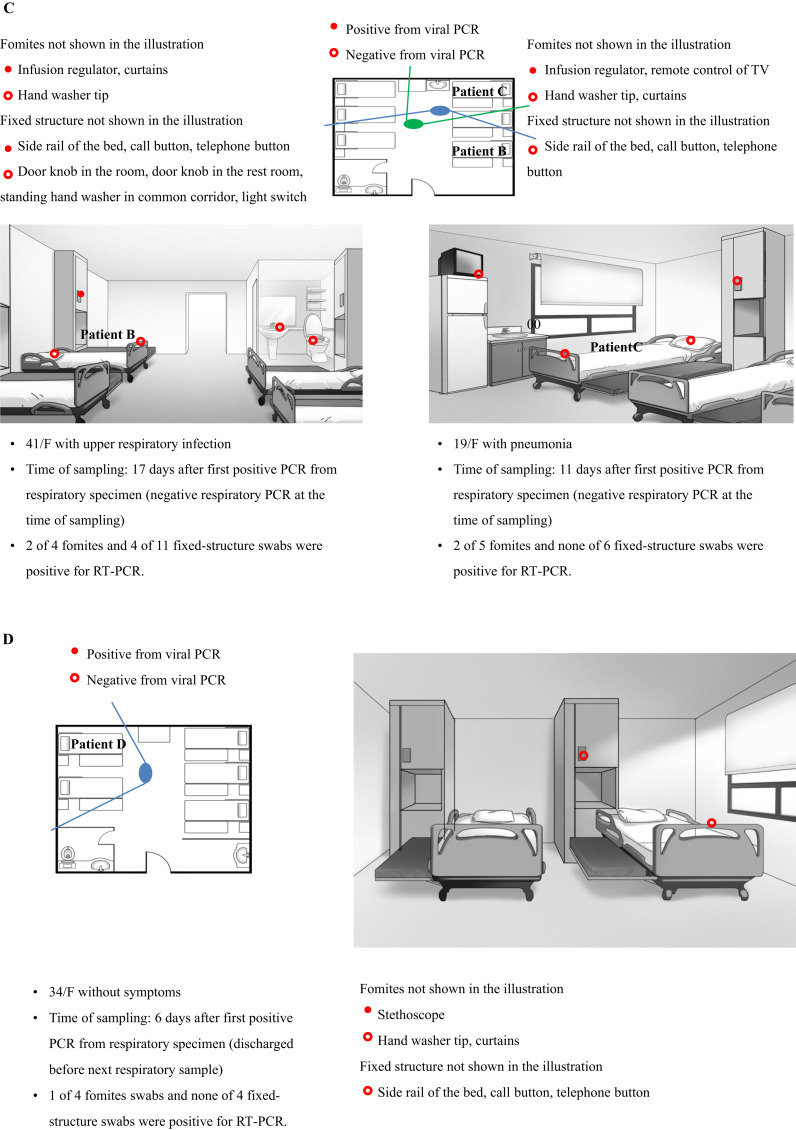
On July 5th, environmental samples were collected in three rooms of four patients (patients A, B, C, and D) within cluster 1. By this day, all but one patient, who was discharged just before environmental sampling, had negative PCR results from their respiratory specimens. Of the 49 swab samples collected, 21 samples tested positive for hPIV-3 by RT–PCR and HN sequencing (Supplementary Table I, Appendix A). In particular, 11 out of 20 fomite swabs and 10 out of 29 fixed-structure swabs were positive for hPIV-3. Room 5 (Figure 4 B) was the single-bed room used for the isolation of patient A, the index case of cluster 1. Out of 15 swab samples, 12 tested positive for viral RNA despite being swabbed, 12 days after the patient's last positive PCR and five days after negative PCR for hPIV-3.
Figure 4.
(A) Floor plan of the haematology unit. Room numbers are indicated by red stars. (B) Results of viral reverse transcription–polymerase chain reaction (RT–PCR) of swabs from patient room 5. Patient A was a woman aged 48 years with pneumonia on non-invasive ventilation. She moved from room 33 to room 5 on day 12 from after the first positive hPIV-3 PCR. (C) Results of viral PCR of swabs from patient room 33. Patient B was a woman aged 41 years with upper respiratory infection, and patient C was a woman aged 19 years with pneumonia. (D) Results of viral PCR of swabs from patient room 34. Patient D was a woman aged 34 years without definite symptoms. The solid lines radiating from the large ovals indicate the angles of observation used for drawing the illustrations of the patients' rooms. HCW, healthcare worker; ICU, intensive care worker.
It was speculated that the hPIV-3 outbreak began in room 33 (Figure 4C), the six-bedded room. Six out of 15 swab samples were positive for hPIV-3, 17 days after patient B's last positive PCR and 12 days after negative PCR. Her symptoms had resolved by the time of environmental sampling. Patient C had only mild rhinorrhoea at the time of environmental swab. Of 11 swab samples, two were positive for viral RNA, eight days after patient C's last positive PCR and one day after negative PCR. Patient E was discharged five days prior to environmental sampling. In room 34 (Figure 4D), the five-bedded room, only the stethoscope tested positive for viral RNA. Environmental sampling of this room was performed immediately after clean-up following patient D's discharge. The RT–PCR results of the environmental specimens revealed that the fluid infusion regulator (three out of three specimens), television remote control (two out of two specimens), and stethoscope (two out of two specimens) were frequently positive for hPIV-3. Detailed information of all tested environmental samples is shown in Figure 4. The virus strains isolated from the environmental samples were consistently found to be identical to their source patients (Figure 2).
Discussion
The data demonstrated that an hPIV-3 outbreak in a haematology unit caused considerable morbidity and mortality in a substantial portion of patients with haematologic malignancies, and that at least one-third of an hPIV-3 outbreak resulted from nosocomial transmission, originating from multiple importations of hPIV-3 strains from the community during its peak epidemic season. In addition, the extensive environmental contamination of hPIV-3 occurred in patients with negative PCR conversion from his/her respiratory specimen despite the routine disinfection procedures. Therefore, these findings provide evidence for the probable routes for nosocomial transmission and emphasize the importance of strict adherence to infection control precautions and the limited visitation during periods of high hPIV-3 activity in the community.
This study clearly showed that both multiple importations from the community and subsequent nosocomial transmission equally contributed to this hPIV-3 outbreak. As the vulnerability of our healthcare system – such as patients occupying rooms with many beds, communal showers or a visiting room, and the visitation to hospitalized patients by friends and family members – was revealed in the large outbreak of Middle East respiratory syndrome in South Korea, we would like to elucidate which transmission route is important in our complex situation during an hPIV-3 outbreak. Previous studies have shown that molecular investigations are useful in discerning routes of transmission in hPIV-3 outbreaks [7], [8], [9], [10], [11], [14], [20], [21]. One study demonstrated that both community-acquired and nosocomial transmitted infections occurred during one haematology unit outbreak, whereas others reported that nosocomial infections play a more significant role than multiple importations from the community [7], [8], [14], [21]. This discrepancy among studies arises from the differences in terms of hPIV-3 activity in the community. It has been accepted that the epidemiology of community respiratory viral infections, including hPIV-3, in inpatients often mirrors the epidemiology in the outpatient population [22]. In addition, differences in infection control procedure, the presence of multi-patient rooms, and visits to hospitalized patients by friends and family members might contribute to the conflicting results of previous studies.
The current scientific evidence suggests that fomites and contaminated surfaces are more concerning routes of hPIV transmission compared to aerosol [2], [23]. By air sampling, hPIV-1 was isolated from air obtained in only one of 150 infected children at a distance of 60 cm [24]. However, nasal secretions can travel a distance greater than 10 feet to contaminate surrounding fomites [23]. An experimental study demonstrated that hPIV can survive in stainless steel for 10 h [16]. Although the transfer of hPIV-3 from finger to finger or from finger to metal disc did not occur, hPIV-3 could be transferred from contaminated disks to clean hands [25]. Therefore, person-to-person spread by direct hand contact appears to be an unlikely mode of transmission, whereas contaminated surfaces may lead to direct self-inoculation. To date, only these pieces of indirect experimental evidence have supported contaminated surfaces as a mode of hPIV-3 transmission. In this context, the extensive environmental contaminations and prolonged environmental presence of hPIV-3 were clearly demonstrated, and these findings may partially explain why hPIV-3 is easily spread in the haematology ward. In addition, this emphasizes the importance of disinfection of environmental surfaces. It is noteworthy that hPIV-3 infection was documented in one minimally symptomatic healthcare worker who obtained this virus from the hPIV-3-infected patients. Although it is not exactly known whether asymptomatic or mildly symptomatic healthcare workers can transmit hPIV-3, asymptomatic hPIV-3 viral shedding in the nasopharynx among immunocompetent hosts and the previous outbreak reports suggest the potential role of asymptomatic healthy carriers in nosocomial transmission [9], [17], [26]. In our data, we could not carry out universal screening for healthcare workers during the outbreak, thus precluding our ability to define the precise role of none-to-minimal symptomatic healthcare workers in nosocomial transmission. However, since the same nursing team cared for the index patients and the latterly infected cases of cluster 1, and one of the nursing team members (patient K in cluster 5) had documented hPIV-3 infection, healthcare workers may have a role in nosocomial transmission. Further studies are needed on this issue.
In the immunocompromised host, hPIV is associated with significant morbidity and mortality. We observed a mortality rate of 26% during a single hPIV-3 outbreak episode. In previous reports, the mortality rate of hPIV-3 infection varied, ranging from 3% to 47% according to the type of transplant, conditioning and immunosuppression therapy, and post-transplant complications [4], [8], [10], [11]. However, in the majority of cases, it is difficult to verify the cause of death as hPIV-3 infection, and thus only an association of hPIV-3 infection and death can be documented. Particularly in HSCT recipients, LRTI with hPIV significantly increased the risk of airflow decline (odds ratio: 17.9; 95% confidence interval: 2.0–160), and this complication is associated with increased mortality risk. In our study, one out of five patient deaths had a post-HSCT pulmonary complication and died due to pulmonary function deterioration following hPIV-3 pneumonia [27]. Considering the morbidity and mortality associated with hPIV-3 infection in the immunocompromised hosts and the absence of effective antiviral agents or vaccination, prevention via more thorough infection control is essential. Interestingly, we clearly demonstrated that hPIV-3 reinfection occurred during the hospital stay (patient F, Figure 3, and Supplementary Figure 1, Appendix A). There is a need for more careful and comprehensive prevention guidelines to be applied during hPIV-3 outbreak in the haematology unit.
This study has some limitations. First, samples positive for viral RNA by RT–PCR may not contain live virus with infectivity. However, the molecular analysis showed extensive and prolonged environmental viral contamination surrounding clustered hPIV-3-infected patients by a nearly identical strain. In particular, the fomites, easily overlooked during routine daily cleaning (i.e. television remote control) or frequently manipulated by healthcare workers (i.e. the fluid infusion regulator and stethoscope), were consistently positive during environmental sampling and might have served as the episource during this outbreak. Therefore, these data provide more direct insight into the possible routes of nosocomial transmission of hPIV-3. Second, we did not perform air sampling. A previous study showed the limited role of aerosol as a mode of hPIV transmission [23]. Finally, in the phylogenetic analysis of the HN gene, differences in a small number of nucleotides (nt) (1–3 bp) within the given 450 bp were grouped together. Although one might postulate that even more than three nucleotide differences may not represent different strains of viruses but rather reflect errors introduced during RT–PCR, the clear epidemiologic link supports the grouping in cluster 1. On the contrary, in cluster 5, due to the low discriminative ability of the HN gene analysis and uncertain epidemiologic link, we further analysed the F gene, another variable region, and carried out sequence alignments for hPIV-3 amplicons in some strains.
In conclusion, this study suggested that an apparently single nosocomial outbreak was equally attributed to multiple importations of hPIV-3 strains from the community and subsequent nosocomial transmission. Furthermore, these data provide further direct experimental evidence for extensive and prolonged viral contamination of materials surrounding hPIV-3 patients and/or from minimally symptomatic healthcare workers as probable nosocomial transmission routes. The findings of the present study indicate and reinforce the idea that preventive measures should consist of the isolation of infected patients, routine handwashing with ethanol-based disinfectants by healthcare workers, patients, and visitors, meticulous environmental cleaning, and limited patient visitation.
Acknowledgements
We thank H.-W. Jung (e-medical contents team, Asan Medical Center, Seoul, South Korea) for the excellent artwork and Vanessa Topping (Scientific Publications Team, Asan Medical Center, Seoul, South Korea) for assistance and thoughtful comments.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jhin.2017.09.003.
Contributor Information
Y. Shin, Email: shinyongno1@gmail.com.
S.-H. Kim, Email: kimsunghanmd@hotmail.com.
Conflict of interest statement
None declared.
Funding sources
This work was supported by a grant of the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI15C2774).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 2.Henrickson K.J. Parainfluenza viruses. Clin Microbiol Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcolini J.A., Malik S., Suki D., Whimbey E., Bodey G.P. Respiratory disease due to parainfluenza virus in adult leukemia patients. Eur J Clin Microbiol Infect Dis. 2003;22:79–84. doi: 10.1007/s10096-002-0864-4. [DOI] [PubMed] [Google Scholar]
- 4.Lewis V.A., Champlin R., Englund J., Couch R., Goodrich J.M., Rolston K. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23:1033–1037. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 5.Lehners N., Tabatabai J., Prifert C., Wedde M., Puthenparambil J., Weissbrich B. Long-term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One. 2016;11:e0148258. doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korean Centre for Disease Control and Prevention . 2016. Acute Infectious Agents Laboratory Surveillance Reports (Weekly)http://cdc.go.kr/CDC/info/CdcKrInfo0502.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0048-MNU0050&fid=477&q_type=&q_value=&cid=69734&pageNum=1 Available at: [last accessed May 2017] [Google Scholar]
- 7.Zambon M., Bull T., Sadler C.J., Goldman J.M., Ward K.N. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol. 1998;36:2289–2293. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez K.J., Erdman D.D., Peret T.C.T., Gill V.J., Childs R., Barrett A.J. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 9.Nichols W.G., Erdman D.D., Han A., Zukerman C., Corey L., Boeckh M. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biol Blood Marrow Transplant. 2004;10:58–64. doi: 10.1016/j.bbmt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Jalal H., Bibby D.F., Bennett J., Sampson R.E., Brink N.S., MacKinnon S. Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. J Clin Microbiol. 2007;45:1690–1696. doi: 10.1128/JCM.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piralla A., Percivalle E., Di Cesare-Merlone A., Locatelli F., Gerna G. Multicluster nosocomial outbreak of parainfluenza virus type 3 infection in a pediatric oncohematology unit: a phylogenetic study. Haematologica. 2009;94:833–839. doi: 10.3324/haematol.2008.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maziarz R.T., Sridharan P., Slater S., Meyers G., Post M., Erdman D.D. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2010;16:192–198. doi: 10.1016/j.bbmt.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodson A., Kasliwal M., Streetly M., MacMahon E., Raj K. A parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortality. Bone Marrow Transplant. 2011;46:1545–1550. doi: 10.1038/bmt.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvala H., Gaunt E., McIntyre C., Roddie H., Labonte S., Curran E. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a haemato-oncology unit. J Infect. 2012;65:246–254. doi: 10.1016/j.jinf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Goldmann D.A. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19:S97–S102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Brady M.T., Evans J., Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18:18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 17.Kapikian A.Z., Chanock R.M., Reichelderfer T.E., Ward T.G., Huebner R.J., Bell J.A. Inoculation of human volunteers with parainfluenza virus type 3. JAMA. 1961;178:537–541. doi: 10.1001/jama.1961.03040450001001. [DOI] [PubMed] [Google Scholar]
- 18.Tyrrell D.A., Bynoe M.L., Petersen K.B., Sutton R.N., Pereira M.S. Inoculation of human volunteers with parainfluenza viruses types 1 and 3 (HA 2 and HA 1) Br Med J. 1959;2:909–911. doi: 10.1136/bmj.2.5157.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karron R.A., O'Brien K.L., Froehlich J.L., Brown V.A. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167:1441–1445. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- 21.Lee A.V., Bibby D.F., Oakervee H., Rohatiner A., Ushiro-Lumb I., Clark D.A. Nosocomial transmission of parainfluenza 3 virus in hematological patients characterized by molecular epidemiology. Transpl Infect Dis. 2011;13:433–437. doi: 10.1111/j.1399-3062.2011.00603.x. [DOI] [PubMed] [Google Scholar]
- 22.Englund J.A., Whimbey E., Atmar R.L. Diagnosis of respiratory viruses in cancer and transplant patients. Curr Clin Top Infect Dis. 1999;19:30–59. [PubMed] [Google Scholar]
- 23.Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean D.M., Bannatyne R.M., Givan K.F. Myxovirus dissemination by air. Can Med Assoc J. 1967;96:1449–1453. [PMC free article] [PubMed] [Google Scholar]
- 25.Ansari S.A., Springthorpe V.S., Sattar S.A., Rivard S., Rahman M. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29:2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muchmore H.G., Parkinson A.J., Humphries J.E., Scott E.N., McIntosh D.A., Scott L.V. Persistent parainfluenza virus shedding during isolation at the South Pole. Nature. 1981;289:187–189. doi: 10.1038/289187a0. [DOI] [PubMed] [Google Scholar]
- 27.Erard V., Chien J.W., Kim H.W., Nichols W.G., Flowers M.E., Martin P.J. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.