Abstract
A high number of infectious diseases affecting livestock and companion animals are caused by pathogens of viral etiology. Ensuring the maximum standards of quality and welfare in animal production requires developing effective tools to halt and prevent the spread of those infectious diseases affecting animal husbandry. To date, one of the best strategies is to implement vaccination policies whenever possible. However many of the currently manufactured vaccines relies in classical vaccine technologies (killed or attenuated vaccines) which, under some circumstances, may not be optimal in terms of safety or adequate for widespread application in disease-free countries at risk of disease introduction. One step ahead is needed to improve and adapt vaccine manufacturing to the use of new generation vaccine technologies already tested in experimental settings. Here we present in the context of animal viral diseases of veterinary interest, an overview of some current vaccine technologies that can be approached for virus pathogens with a brief insight in the type of immunity elicited.
Key words: Virus vaccines, Attenuated vaccines, Viral vectors, DNA vaccines, Subunit vaccines, Innate immunity, Adaptive immunity, Vaccine technologies
Viral Diseases of Animals and the Need for Vaccination
One of the biggest transformations in history occurred when mankind shifted from a hunter-gatherer to an agricultural lifestyle. During millenniums livestock and companion species (ruminants, swine, poultry, cats and dogs) were domesticated and raised first for survival (in this sense the word “livestock” is meaningful) then for profit and commerce. Since then, animal husbandry evolved as one of the most important activities for civilization and development. The importance of such activity is obvious since a proper management of land use and animal resources is always required to avoid malnutrition and famine in developing countries or in countries where intensive farming is essential for subsistence. The explosive growth rate of the world’s human population complicates this picture (particularly in developing countries) so other sources of dietary consumption, such as farmed fish, will be more demanded in the near future. Inevitably, the intensive farming of animal species leads to the onset of diseases mainly caused by propagation of infectious pathogens, affecting animal welfare, reducing productivity, and in the worst cases, seriously undermining the economy of nations. In some cases, livestock or animal pathogens can also cause disease in humans, so means to control and eradicate them have to be implemented.
Among the plethora of infectious diseases in animals, those of viral etiology account for a high burden of cases and are among the most relevant from a veterinary perspective. In fact, approximately half of the most important animal diseases are caused by viruses, according to the OIE’s classification for terrestrial and aquatic notifiable animal diseases (see Table 1). The listed viral diseases comprise virus from 22 different viral families and four families of virus (herpesvirus, rhabdovirus, poxvirus and paramyxovirus) concentrate a high number of diseases (Fig. 1). Several of the listed animal virus diseases can be also transmitted to humans (zoonotic diseases) either by direct contact with infected animals, infected animal tissues and fluids or by means of arthropod vectors, impacting both public health and food security. Thus, preventing transmission of infectious diseases at the animal–human interface is important for protecting the world population from both epizootics and pandemics, constituting the basis for the “One Health” concept [1, 2]. Prevention by vaccination remains as one of the most cost-effective intervention strategies against infectious diseases. For most of the listed diseases there are “licensed” or available vaccines, eventually obtained by “classical” production methodologies. An important exception is that of diseases caused by retroviruses, for which classical vaccine technologies have not been successful, and that of aquatic diseases, for which only vaccines for fish have been so far developed. In some cases the efficacy of vaccination against viral diseases of animals has been very successful, as it can be illustrated by the eradication of rinderpest [3] (probably the most deadly disease of cattle and ruminants, caused by a morbillivirus) by the use of an attenuated/avirulent strain of the causative virus. Recent evidences advice to support efforts to control emerging viral pathogens where they primarily occur, in order to avoid uncontrolled spread of deadly viruses [4]. Within this perspective, some technologies for vaccine design may constitute powerful platforms to rapidly generate new experimental vaccines based on previous knowledge about the immune responses generated in the host.
Table 1.
The OIE’s notifiable viral diseases and infections of terrestrial and aquatic animals
| Diseases affecting multiple species | Virus acronym | Virus family | Virus genus | Licensed vaccine type(s) available |
|---|---|---|---|---|
| Bluetongue | BTVa | Reoviridae | Orbivirus | Live attenuated |
| Crimean Congo hemorrhagic feverb | CCHFVa | Bunyaviridae | Nairovirus | Not available |
| Equine encephalomyelitis (Eastern)b | EEEVa | Togaviridae | Alphavirus | Inactivated |
| Foot and mouth diseaseb | FMDV | Picornaviridae | Aphtovirus | Inactivated (BEI) |
| Infection with Aujeszky’s disease virus (Pseudorabies) | SHV-1 | Herpesviridae (α-herpesvirinae) | Suid Herpesvirus | Attenuated (deletion of glycoproteins gE, gC, gG) |
| Infection with rabies virusb | RABV | Rhabdoviridae | Lyssavirus | Inactivated/Attenuated/Recombinant poxvirus; Adenovirus |
| Infection with rinderpest virus | RPV | Paramyxoviridae | Morbillivirus | Attenuated |
| Japanese encephalitisb | JEVb | Flaviviridae | Flavivirus | Inactivated/Attenuated |
| Rift Valley feverb | RVFVb | Bunyaviridae | Phlebovirus | Attenuated |
| Vesicular stomatitisb | VSVb | Rhabdoviridae | Vesiculovirus | Inactivated/Attenuated |
| West Nile feverb | WNVb | Flaviviridae | Flavivirus | Inactivated/Attenuated/Recombinant canarypox/DNA vaccine (USA) |
| Epizootic hemorrhagic disease | EHDVb | Reoviridae | Orbivirus | Inactivated/Attenuated (licensed USA, Japan) |
| Cattle diseases | ||||
| Bovine viral diarrhea | BVDV | Flaviviridae | Pestivirus | Inactivated/Attenuated |
| Enzootic bovine leukosis | BLV | Retroviridae | Lentivirus | Not available |
| Infectious bovine rhinotracheitis/Infectious pustular vulvovaginitis | BoHV-1 | Herpesviridae (α-herpesvirinae) | Varicellovirus | Deleted glycoprotein gE inactivated or attenuated |
| Lumpy skin disease | LSDV | Poxviridae | Orthopoxvirus | Attenuated |
| Sheep and goat diseases | ||||
| Caprine arthritis/encephalitis | CAEV | Retroviridae | Lentivirus | Not available |
| Infection with peste des petits ruminants virus | PPRV | Paramyxoviridae | Morbillivirus | Attenuated/Recombinant capripoxvirus |
| Maedi-visna | MVV | Retroviridae | Lentivirus | Not available |
| Sheep pox and goat pox | SPV | Poxviridae | Orthopoxvirus | Inactivated/Attenuated |
| Equine diseases | ||||
| Equine encephalomyelitis (Western) | WEEVa | Togaviridae | Alphavirus | Inactivated |
| Equine infectious anemia | EIAV | Retroviridae | Lentivirus | Not available |
| Equine influenzab | EIV | Orthomyxoviridae | Influenzavirus | Inactivated/Recombinant canarypox |
| Infection with equid herpesvirus-1 | EHV-1 | Herpesviridae | Inactivated/Attenuated | |
| Infection with equine arteritis virus | EAV | Arteriviridae | Arterivirus | Inactivated/Attenuated |
| Venezuelan equine encephalomyelitisb | VEEVa | Togaviridae | Alphavirus | Inactivated/Attenuated |
| Infection with African horse sickness virus | AHSVa | Reoviridae | Orbivirus | Live attenuated |
| Swine diseases | ||||
| African swine fever | ASFVa | Asfiviridae | Asfivirus | Not available |
| Infection with classical swine fever virus | CSFV | Flaviviridae | Pestivirus | Attenuated/Subunit (E2) |
| Porcine reproductive and respiratory syndrome | PRRSV | Arteriviridae | Arterivirus | Live attenuated |
| Swine vesicular disease | SVDV | Picornaviridae | Enterovirus | Not available |
| Transmissible gastroenteritis | TGEV | Coronaviridae | Alphacoronavirus | Not available |
| Avian diseases | ||||
| Avian infectious bronchitis | IBV | Coronaviridae | Gammacoronaviridae | Inactivated/Attenuated/Inactivated multivalent |
| Avian infectious laryngotracheitis | ILTV | Herpesviridae (α-herpesvirinae) | Gallid herpesvirus-1 | Attenuated/Recombinant herpesvirus/Recombinant fowlpox |
| Duck virus hepatitis | DHV-1 | Picornaviridae | Avihepatovirus | Inactivated/Attenuated |
| Duck virus enteritis | DEV-1 | Herpesviridae (α-herpesvirinae) | Anatid herpesvirus-1 | Attenuated |
| Infection with avian influenza viruses and infection with influenza A viruses of high pathogenicity in birds other than poultry including wild birdsb | AIV | Orthomyxoviridae | Influenzavirus A | LPAI inactivated/Recombinant fowlpox (HPAI vaccination banned or discouraged) |
| Fowl pox | FPV | Poxviridae | Avipoxvirus | Modified live attenuated |
| Infectious bursal disease (Gumboro disease) | IBDV | Birnaviridae | Avibirnavirus | Inactivated/Attenuated/Recombinant herpesvirus-VP2 |
| Newcastle diseaseb | NDV | Paramyxoviridae | Avulavirus | Inactivated/Attenuated (lentogenic and mesogenic). Recombinant avian herpesvirus and avipoxvirus |
| Marek’s disease | MDV (GaHV-2) | Herpesviridae (α-herpesvirinae) | Gallid herpevirus-1 | Live attenuated |
| Turkey rhinotracheitis | aMPV | Paramyxoviridae | Metapneumovirus | Live attenuated/Inactivated |
| Lagomorph diseases | ||||
| Myxomatosis | MV | Poxviridae | Live attenuated | |
| Rabbit hemorrhagic disease | RHDV | Caliciviridae | Recombinant poxvirus | |
| Other infections | ||||
| Camelpox | Poxviridae | Inactivated/Attenuated | ||
| Bunyaviral infections (Akabane, Cache Valley, Schmallenberg, and Nairobi sheep disease) |
AKAVb CVVb SBVb NSDVb |
Bunyaviridae |
Orthobunyavirus Orthobunyavirus Orthobunyavirus Nairovirus |
Inactivated |
| Hendra and Nipah virus diseasesb |
HeV NiV |
Paramyxoviridae | Henipaviruses | Not available |
| Fish diseases | ||||
| Infection with HPR-deleted or HPR0 infectious salmon anemia virus | ISAV | Orhtomyxoviridae | Isavirus | Inactivated |
| Infection with salmonid alphavirus | SAV | Togaviridae | Alphavirus | Inactivated |
| Epizootic hematopoietic necrosis | EHNV | Iridoviridae | Ranavirus | Not available |
| Infectious hematopoietic disease | IHNV | Rhabdoviridae | Novirhabdovirus | Inactivated/DNA |
| Koi herpesvirus disease | KHV | Alloherpesviridae | Cyprinivirus | Live attenuated |
| Red sea bream iridoviral disease | RSIDV | Iridoviridae | Formalin inactivated | |
| Spring viraemia of carp | SVCV | Rhabdoviridae | Vesiculovirus | Not available |
| Viral hemorrhagic septicemia | VHSV | Rhabdoviridae | Novirhabdovirus | Not available |
| Mollusc diseases | ||||
|
Infection with Ostreid Herpesvirus 1 microvariants |
OsHV-1 | Herpesviridae | Not applicable | |
| Infection with abalone herpesvirus | AbHV | Herpesviridae (Malacoherpesviridae) | Not available | |
| Crustacean diseases | ||||
| Infectious hypodermal and hematopoietic necrosis | IHHNV | Parvoviridae | Brevidensovirus | Not developed |
| Infectious myonecrosis | IMNV | Totiviridae | Totivirus | Not developed |
| Taura syndrome | TSV | Dicistroviridae | Aparavirus in the Family | Not developed |
| White spot disease | WSSV | Nimaviridae | Whispovirus | Not developed |
| White tail disease (Infection by Macrobrachium rosenbergii nodavirus) | MrNV and XSV associate virus | Nodaviridae | Nodavirus | Not developed |
| Yellowhead disease | YHV | Roniviridae (O. Nidovirales) | Okavirus | Not developed |
| Amphibian diseases | ||||
| Infection with ranavirus | FV3 | Iridoviridae | Ranavirus | Not available |
aArthropod-borne virus (arbovirus)
bZoonotic disease
Fig. 1.
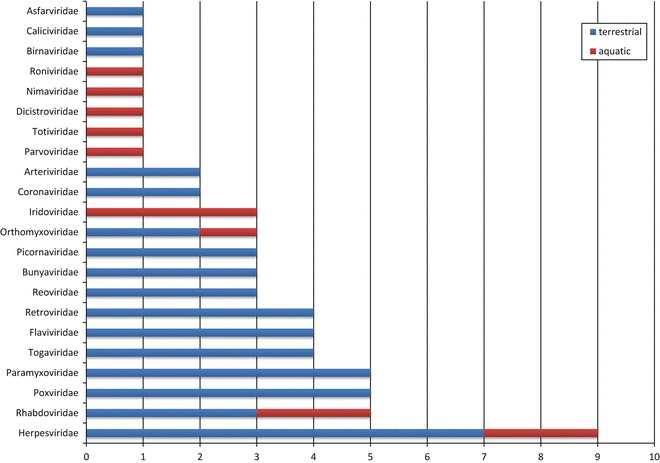
Virus families including members causing notifiable animal diseases. The figure depicts the number of pathogenic members from each virus family causing important diseases in terrestrial and aquatic animals
Immunology Matters
The objective of vaccination is to achieve a specific stimulation of the immune system enabling the host to mount an efficient (and desirably long-lived) memory immune response that can recognize the pathogen and eventually eliminate it once is present in the organism upon infection. This can be achieved providing the appropriate antigenic stimulus (the vaccine) to activate cellular mechanisms involved in recognition of the nonself. Thus, an efficient vaccine needs to be recognized as a nonself entity and, ideally, be able to stimulate innate immune responses that further “instruct” subsequent adaptive and memory responses. The first step is carried out either by infected or by specialized phagocytic cells (antigen presenting cells or APCs, including macrophages and dendritic cells) able to present antigenic determinants to näive (B and T) lymphocytes (Fig. 2).
Fig. 2.
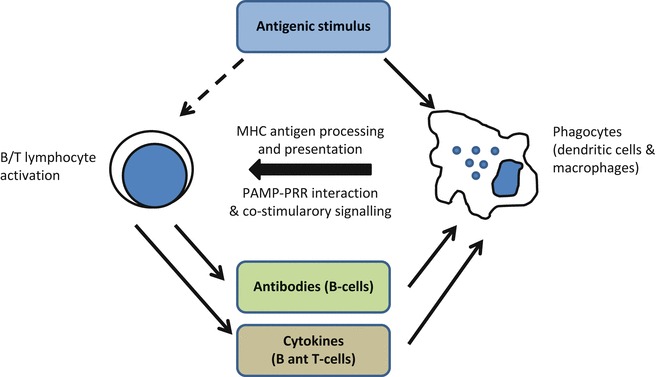
The cellular cooperation in the immune response. After vaccination, specialized phagocytes present the processed antigens to näive B or T-cells that may become activated only if proper co-stimulatory signals are produced (derived from the interaction of PAMPs with cellular PRRs). Activation drives lymphocytes to secrete soluble mediators and antibodies initiating inflammatory responses (adapted from [33])
Though the innate immune response is broadly reactive and unspecific it strongly conditions the magnitude and the composition of the specific (adaptive or acquired) immune responses. Cellular pathogen recognition receptors (cPRRs) either membrane bound (Toll-like-, C-type lectin- and scavenger receptors) or cytosolic (NOD-like and RIG-like receptors) of phagocytes eventually bind to pathogen associated molecular patterns (PAMPs) carried on infecting microbes [5]. In particular, encountering of pathogenic virus ligands (such as single or double stranded RNA) to intracellular PRRs activates the phagocytic cells from a normal quiescent state by inducing NFκB-mediated gene transcription of a number of co-stimulatory molecules, proinflammatory cytokines and chemokines as well as IRF-mediated transcription of type I-interferons (IFNs) and other cytokines such as IL-1β and TNF-α [6]. Other immune cell types such as the natural killer (NK) cells express functional TLRs specifically for detecting viral PAMPs and can be also activated by type-I interferons [7]. NK cells can eliminate cells in which expression of MHC molecules is reduced upon viral infection. NK cells secrete IFN-γ which in turn can enhance the phagocytic activity of macrophages and antigen presentation by mature dendritic cells (DCs), a key player in the bridging of innate and adaptive immunity. DCs signaling to naïve lymphocytes will determine whether these cells should be eventually involved in fighting against the viral infection. This fact is exploited by those vaccines based in attenuated viruses or in replicating live virus vectors where the initiation of innate immune responses greatly augments the quality and magnitude of the adaptive response in contrast to that elicited by vaccines based on inert antigens (inactivated virus or subunit vaccines). Recently, the central role of dendritic cells or APCs in regulating the immune response has made antigen targeting to these cells a major subject for specific immune stimulation aimed to improve vaccine efficacy as well as other forms of immunotherapy [8, 9].
Upon näive lymphocyte activation by interaction with DCs the specificity of the immune response is granted and clonal expansion of B and T-cells capable of recognizing the specific antigen will take place. A pool of specific lymphocytes containing memory and effector cells will be expanded as a primary response to the vaccine stimulus. Upon infection and virus antigen encounter the secondary response will be greatly increased, potentially leading to protection and long-lived immunity by specific effector and memory cells (Fig. 3). Therefore, the two main principles to be exploited by vaccines are necessarily specificity and memory. When designing vaccines the issue of specificity is crucial for the success of a vaccine and can be approached by an adequate selection of the antigen fraction, whole antigen or antigens of choice, being able to recall the memory lymphocyte pools produced in the primary responses after vaccination.
Fig. 3.
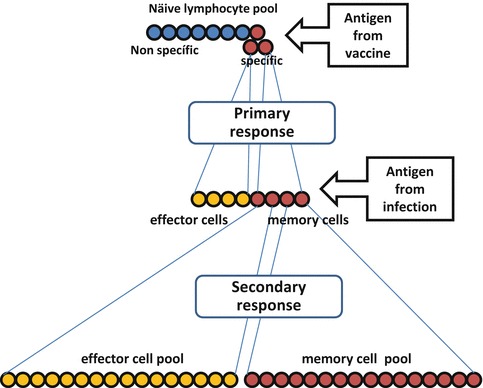
Vaccination exploits the induction of specificity and immunological memory. A primary clonal expansion of lymphocytes is produced upon activation of naïve T-cells by phagocytes primed with specific vaccine antigens/stimulus. Both effector and memory cell pools are generated that upon encounter with pathogen (infection) will undergo a massive secondary expansion of both cell pools (adapted from [33])
Previous identification of the correlates of protective immunity upon infection is one of the logical approaches for vaccine design, for example including relevant epitopes that induce neutralizing antibodies made upon infection and/or the key T-cell epitopes responsible for helper or cytotoxic functions [10]. Ideally, this knowledge should be derived from the pathogenesis of viral infection in the target species to which the vaccine is intended for but unfortunately these type of studies are often more difficult to perform than in laboratory animals (mainly due to genetic diversity of the outbred species, the lack of reagents and markers for cell phenotype characterization, and the limitation in the number of animals used for experimentation). Nonetheless, in some cases the pathogenesis of other animal models of disease (mainly rodents), correlates well enough with that of the target species and valuable information can be obtained about the immune mechanisms of protection. After the knowledge gathered in the past decades of virus research it becomes evident that, in a general sense, for virus with less complex pathogenesis a successful immunoprophylaxis could be obtained by the generation of an immune response against surface antigens displayed on virions and/or virus infected cells. For other virus (for example poxvirus, herpesvirus, asfivirus, respiratory viruses, and lentivirus) that have developed more complex pathogenesis (i.e., induction of persistence, replication in immune privileged tissues, using immune evasion strategies, induction of harmful host immune responses) the effective vaccine should elicit, in addition to neutralizing antibodies, specific T-cell responses [11].
Vaccine Technologies
A first classification of vaccines has been outlined above with respect to the level of immunogenicity elicited (inactivated/killed non-infectious versus live attenuated vaccines). Therefore two broad categories of antiviral vaccines can be considered with respect to the nature of the virus used (live or death) or the relevant antigen (whole or fractionated); in fact all licensed vaccines against viral diseases available to date (both for the medical and veterinary use) could fall in either one of these categories. This dichotomy helps to categorize vaccines into four general types (Table 2).
Table 2.
A proposed classification for the current vaccine technologies
| VIRUS | Whole antigen | Fraction/component |
|---|---|---|
| Death | Type I (inactivated, killed) | Type III (subunit, VLPs, genetic DNA or RNA, killed recombinant vectors) |
| Live | Type II (modified live attenuated, reverse genetics modified) | Type IV (recombinant viral vectors expressing antigens) |
In this categorization type I vaccines include those produced by means of inactivating methodologies while type II vaccines include all attenuated virus used as vaccines, including those generated by reverse genetics. Type III and IV vaccines include those in which only components or a fraction of the pathogen is used as the vaccine antigen. Thus, type III vaccines would include subunit vaccines, including carrier micro/nanoparticle and virus-like particles (VLPs) vaccines produced by recombinant technologies in both prokaryotic and eukaryotic cells and inactivated recombinant vectors expressing heterologous vaccine antigens. In this category, both nucleic acid vaccines as well as peptide based vaccines could be also included. Finally, the type IV vaccines are those delivered by a live viral vector that codes and expresses particular (selected) heterologous vaccine antigens. Obviously, from all these categories further classifications can be made, depending on the formulation of the vaccine (for example, inactivated vaccines can be subdivided into those composed of whole inactivated infected cultures or purified virus fractions), and the type of adjuvant used to augment the immune responses. Attenuated vaccines could include those natural virus isolates with reduced virulence, or attenuated virus generated by serial passages, or virus rescued by means of a reverse genetics approach. Nucleic acid vaccines can be based on DNA plasmids or self-replicating RNA molecules launched by a DNA plasmid encoding a viral replicon. For each technology several methodologies for production or antigen expression can be used and further modifications and formulations applied, therefore the potential combinations that can be tested experimentally are many. The choice of one or another may depend on the experimental (preclinical) data obtained in models of infection if available. Further classification of vaccine technologies could be done on the basis of the main type of immunity provided (mucosal, systemic, humoral or cellular), preferred delivery method (oral, parenteral) or prime-boost combination (see Table 3).
Table 3.
General features of laboratory (experimental) vaccine technologies
| Type | Type of modification | Production platform | Delivery method | Adjuvants | Dosage | Immunity provided | Safety |
|---|---|---|---|---|---|---|---|
| I. Inactivated | Physical, Chemical | Eukaryotic cell culture | Parenteral | Chemical | Repeated | Humoral and Th responses | +++ |
| II. Live Attenuated |
Physical Chemical mutagens, Reverse genetics, Tissue propagation (in vitro in vivo) |
Cell culture | Parenteral | none |
Single Repeated |
Humoral, and cellular including CTL responses | + |
| IIIa. Subunit & carrier technologies, glycoconjugate and peptide vaccines, microparticle and nanoparticle formulations, virus-like particles | Prokaryotic cell culture, Eukaryotic cell culture, Plant based. Chemical synthesis | Parenteral/mucosal | Chemical/Molecular | Repeated | Humoral and Th | ++++ | |
| IIIb. Nucleic acid | VpG, delivery, liposome | Prokaryotic cell culture | Parenteral | Molecular | Repeated | Humoral and cellular | +++ |
| IV. Viral Vector based | Mammalian, Insect, Plant cell culture | Parenteral | None/molecular |
Single Repeated |
Humoral and | +++ |
Type I Vaccine Technologies
Inactivated (killed) antiviral vaccines have being used for long and are based on the disruption of the ability of a virus to replicate by generally chemical or physical methods. Among chemical methods used, formaldehyde and organic compounds such as cyclic esters (β-propiolactone) or binary ethylenimine (BEI) have been most widely used. Other cross-linking agents such as glutaraldehyde can be an option for the inactivation but its use has not been as wide as formaldehyde. Two main caveats of the use of cross-linking agents for vaccine preparation can be cited; the first one is the potential for aggregation leading to disruption or modification of antigenic epitopes possibly accounting for the reduced immunogenicity of these vaccines, usually requiring two or three booster doses to maintain adequate and lasting levels of protective immunity. Another problem is the risk for incomplete inactivation leading to exacerbation of disease if the partially (or suboptimal) induced immunity cooperates with infectivity by mechanisms such as antibody dependent enhancement (ADE). In this case, monocytes or macrophages (Fc-receptors bearing cells) can be infected by virus complexed to non-neutralizing antibodies, a process described in dengue virus infections [12]. Finally, another issue with inactivated vaccines is overcoming the differentiation of infected and vaccinated animals not to interfere with the surveillance diagnostics. While formaldehyde reacts primarily with proteins, β-propiolactone (BPL) and binary ethylenimine (BEI) modify mainly DNA or RNA so BPL is expected to maintain a high immunogenicity during the inactivation of viruses. However it has been reported that BPL may also and react to some amino acids including cysteine, methionine, and histidine so certain modification of proteins may also affect the immunogenicity of BPL vaccines. Similarly BEI has been also shown to react with proteins [13]. This compound is used widely for the inactivation of foot and mouth disease virus (FMDV) in the preparation of vaccines. Nonetheless, inactivated vaccines remain as a leading methodology for vaccine production (both for human and veterinary use) in part due to the effectiveness of adjuvants (mainly aluminum salts) in the vaccine formulations overcoming the main issue of limited immunity. In fact, this technology may benefit from other inactivation approaches such as the use of hydrogen peroxide or protonating compounds, such as diethyl-pyrocarbonate (DEPC). Hydrogen peroxide could inactivate both DNA and RNA viruses (vaccinia virus, LCMV, WNV and YFV) with little damage to the antigenic structure, thus minimizing the effect on immunogenicity. More interestingly, this inactivation approach rendered vaccines able to induce both humoral (neutralizing antibodies) and cellular immune responses including WNV and LCMV specific CD8+ cytotoxic T-cells [14, 15]. Using a histidine-protonating agent such as DEPC it was reported the abolishment of vesicular stomatitis virus (VSV) infectivity and pathogenicity in mice. These animals survived a further lethal challenge and this protection was associated to the induction of neutralizing antibodies [16] although no further reports have arose since the first description. In spite of the advances made in different technologies for stimulating the immune responses the classic inactivation methodology is still broadly used to manufacture many vaccines for veterinary use, in part since manufacturers need to balance carefully the investment needed to adapt their traditional manufacturing processes to the new technologies and the expected profitability. Other classical inactivation techniques by physical methods have been exposure to several types of radiation: thermal, electromagnetic or ionizing. UV radiation has been one of the most used in human vaccine manufacturing.
Type II Vaccine Technologies
Live attenuated virus vaccines are among the most successful forms of vaccines particularly with regards to immunogenic character. The ability to replicate makes these vaccines stronger inducers of innate responses, a feature that critically may influence the outcome of the acquired immune responses as discussed above. Several veterinary and companion animal vaccines are based on attenuated viruses and these types of vaccines are used in the human side as well. The common feature shared by attenuated virus vaccines is the lost of virulence factors while the immunogenicity is maintained. Traditional methods for development of attenuated vaccines were the serial passage or propagation of the virus in heterologous cell cultures or in brain tissue from rodents, suckling mice, rabbits or goats, in particular for veterinary use. Propagation in different tissue usually ends up with a change of tropism. For example, hepatotropic viruses passaged in brain tissues were unable to replicate in liver though acquired neurovirulence. A different approach is to induce mutations with mutagenic compounds such us nucleoside analogous. Temperature sensitive mutants grown at lower temperatures were then unable to replicate at normal temperatures in the hosts. The main advantages of attenuated vaccines over inactivated or killed vaccines or subunit vaccines can be related with a wider presentation of epitopes since, obviously, more proteins will be expressed as a consequence of virus replication into the infected host cell (in the infected cell protein fragments will be presented through MHC-I), and also with the possibility of administration by similar or natural routes of infection (i.e., nasal/mucosal route for influenza vaccines). The immune responses elicited are also similar to that of infections, including triggering of innate immune responses, as well as humoral and/or cellular responses. Importantly, the costs of generation and manufacturing these types of vaccines are usually affordable for the veterinary vaccine markets. On the other side, possible disadvantages of attenuated virus vaccines are the genetic instability, allowing reversion to virulence or lost of replicating phenotype, problems related with immunosuppressed individuals, or deleterious effects of some attenuated vaccines when used in gestating animals. This usually accounts for those vaccines obtained by methods in which the inactivation process is not fully controllable or understood (i.e., serial propagation in tissue culture). Table 4 summarizes advantages and disadvantages between killed and attenuated vaccines. For diseases affecting several species a vaccine that is safe for a specific ruminant host might not be safe for swine. In general terms it is generally accepted that inactivated vaccines offer less safety problems than attenuated vaccines. Advances in the knowledge of pathogen biology, immunology and molecular biology allowed to carry out more rational vaccine designs so novel alternatives to the attenuated type of vaccines have been developed. Particularly for RNA viruses, the generation of reverse genetic systems (i.e., the ability to rescue fully infectious virus from cloned viral genomes and transcripts) [17] has allowed to develop novel attenuated vaccines with enhanced safety features. For DNA viruses defining virulence and/or immunomodulatory genes allowed its deletion by homologous recombination techniques [18]
Table 4.
Most recognizable pros and cons of inactivated and attenuated vaccines
| Inactivated vaccines | Attenuated vaccines | ||
|---|---|---|---|
| PROS | CONS | PROS | CONS |
| No risk of infection | May potentiate disease (paramyxovirus, lentivirus, coronavirus vaccines) | Systemic and local immune activation. Humoral and cellular immune responses | Presence of adventitious agents |
| No residual adventitious agents | Parenteral administration (No mucosal immunity) | Durable immunity | May cause illness |
| Low rate of CTL responses | Effective immunity | May loose attenuation | |
| Low immunity | Low cost of production | Spread to contacts | |
| Need boosting doses | Easy administration | May loose infectivity | |
| Expensive manufacturing | Herd immunity (most if vaccine spreads) |
Storage limited Risk for pregnancy |
|
| Single dose administration | Interference with live virus (preexisting immunity). Presence of defective interfering particles | ||
| Discrimination of vaccinates and infected animals more difficult | |||
| Immunosuppression | |||
In most of the cases the modification of these genomes allowed the introduction to these vaccines of an important characteristic for veterinary vaccines: the possibility of differentiate infected from vaccinated animals [19]. This is particularly important when surveillance diagnostic is implemented for example to maintain the condition of a disease-free country.
Type III Vaccine Technologies
Once identified, protective antigen fractions or components from whole pathogens can be isolated and/or produced by cloning and expression in heterologous systems (bacterial, yeast, plant, eukaryotic cell). We include in this category both subunit particulate and nucleic acid vaccines. With this approach the specificity of the immune response generated is maximized but the magnitude of the immune response tends to be lower than that of attenuated vaccines. Thus, immune adjuvants, targeting strategies or prime boost regimens might be considered to enhance the immune responses.
Subunit vaccines have several advantages over conventional attenuated vaccines in particular regarding safety and production. Most used systems to produces subunit vaccines are based on bacteria, yeast, insect or mammalian cells. More recently other systems based on non-fermentative approaches such as live organisms have been developed, particularly plants or insects. In plants two main alternatives have been developed, either genetically modified or expressing transiently antigens encoded by plant virus or bacterial vectors. In live insects (Lepidoptera) recombinant baculoviruses can be used to infect insect larvae and transgenic silkworms can be also generated (reviewed in [20]). A particular feature of subunit vaccines is the possibility of generation of virus like particles (VLPs) by co-expression of capsid proteins constituent of virions, but devoid of ribonucleoproteins. Like the viral capsids, the VLPs are composed of a geometrically arranged array of proteins, forming repetitive structures against which soluble antibodies and/or B-cell receptors can interact with high avidity. These structures are thus good inductors of T-cell independent responses. In addition the VLPs can be also internalized and processed by APCs to induce both Th and CTL responses, therefore having the potential to stimulating broader immune responses than monomeric forms of protein subunits. Another advantage of VLPs is that they can be produced in a variety of expression systems (baculovirus, poxvirus, alphavirus replicons, plants, Salmonella, E. coli, yeasts, and so on) and can be engineered in order to even express foreign epitopes or immune-stimulatory molecules in the form of chimeric-VLPs, or by covalent linking of immunomodulators (either linear or cyclic peptides, haptens, glycans). VLPs can be obtained from enveloped viruses by budding from cells expressing the VLP components (such in the case of influenza virus). A more specialized technique is the reconstitution of viral envelopes in unilamelar liposomes, termed virosomes. These synthetic structures can be also complemented with immune-stimulatory conjugates or even heterologous molecules such as DNA, siRNA, antibody fragments (reviewed in [20]). Perhaps from the veterinary vaccine perspective, the generation of VLP subunit vaccines and derivatives is being hampered by the higher costs for production precluding a more generalized use as a vaccine production technology.
Instead of using whole proteins as antigens, immunogenic epitopes previously identified allows to design synthetic peptides to direct more specifically the immune response. Known B and T-cell peptides and combinations can be included in a peptide vaccine design [21]. One of the advantages of peptides over subunit protein vaccines is the simple production, storage and distribution, as well as the flexibility to introduce modifications or mutations (for those highly changing viruses). In spite of these advantages, peptide vaccination is not yet a generalized approach since it needs a deeper knowledge of the protective immune responses in the host species and the intrinsic lower immunogenicity of peptides over whole proteins. However immunogenicity can be enhanced by multimerization strategies [22] or by the use of micro/nano particulate delivery of covalently attached peptides including or not targeting signals to facilitate interaction with immune cell receptors.
Genetic vaccines were discovered upon gene therapy experiments by Wolff and Felgner when intended to deliver DNA into muscle cells by using cationic lipids containing DNA [23]. In fact DNA uptake was produced even in the absence of lipids and expressed the encoded protein. Thus transcriptional units encoding HA antigens were placed under control of a viral promoter (CMV) so a DNA vaccine against influenza was first described in 1993 [24]. Usually DNA vaccines are delivered by intramuscular or intradermal injections. In the first case muscle cells can be directly transfected and express the protein. Dendritic cells present in the interstitial spaces could uptake the soluble antigen, or take up cells killed by the vaccine, or even being transfected directly. On the other hand, the cytosolic expression of the protein enables its MHC-I processing in either muscle or dendritic cells. MHC upregulation is one of the consequences of innate immunity stimulation by unmethylated CpG motifs upon TLR-9 receptor engagement. The main advantages of DNA vaccines are the ease to design and produce, allows differentiation of vaccinated and infected animals (DIVA), antigen is processed naturally, mimicking the immune response induced by virus replication thus stimulating the development of both cellular and humoral immune response. Finally, as with other vaccine strategies, DNA allows combining several antigens, targeting signals, or immunostimulatory molecules (cytokines and chemokines) to improve the immune response elicited. DNA vaccination has been so far successful in mice models of disease. The only DNA vaccines licensed to date have been against WNV in horses and VHS in salmonids [20]. However, experimental DNA vaccination in large animals against livestock viral diseases still needs further optimization in order to achieve stronger immune responses (the amount of plasmid needed for immunization may represent a serious disadvantage). This handicap could be addressed by the use of stronger promoters, replicon based plasmids (Alphavirus), increasing plasmid uptake efficiency or by co-delivery of immune-stimulatory molecules. Nonetheless, it remains a very attractive way for a rationale design of vaccines, combining the simplicity of production and the potential use in combined vaccine approaches such as prime boost.
Type IV Vaccine Technologies
Recombinant viral vectors constitute a very important platform for vaccine design and experimental vaccination approaches. Virtually any infectious, non-pathogenic, virus can be used to express foreign genes, provided a system for recombinant incorporation and expression has been developed. This has been achieved for different RNA viruses that were previously attenuated by using reverse genetics systems or in DNA virus by means of homologous recombination techniques. Among the DNA viruses used to deliver vaccine antigens Poxvirus (from both orthopoxvirus and parapoxvirus genus), Herpesvirus, Adenovirus and Baculovirus have been the most widely used in experimental vaccine trials. The main advantage of DNA viruses over RNA viruses is related with the higher stability of DNA genomes, greater insertional sites and availability of BAC-DNA clones available making engineering and rescuing of recombinant virus a conventional laboratory task. Additional features include the cytoplasmic replication (with the exception of herpesviruses) and the induction of long lived humoral and cellular immune responses, with emphasis on the strong CD8-T-cell activation that is mediated by attenuated poxvirus and adenovirus infections. On the RNA virus side, several viruses from different families have been used as foreign gene carriers: Alphavirus, Bunyavirus, Coronavirus, Flavivirus, Paramyxovirus, Retroviruses, Rhabdovirus [25]. This has been possible by the establishment of reverse genetics technologies allowing the rescue of infectious virus from a copy of its genome. Paramyxoviruses are very potent inducers of humoral and cellular immune responses conferring complete long-life protection when used as attenuated vaccines. They allow interchange of nucleoproteins or envelope glycoproteins between related family members giving rise to chimeric viruses for use as bivalent marker attenuated vaccines. In addition they can accommodate additional genetic information for expression of foreign antigens maintaining stability during propagation in cell culture, therefore they can be used also to immunize against pathogenic paramyxovirus and other infectious agents [25]. Attenuated rhabdoviruses (generated by manipulation of the viral glycoprotein and phosphoprotein and/or genome order rearrangement) offer similar characteristics for use as a vector for delivery of foreign genes, including the induction of innate and adaptive immune responses. One additional advantage of this type of vectors is the absence of seropositivity in both human and animal populations [25]. Replication deficient alphavirus have been also modified to express foreign antigens for use as vaccines, and in cancer and gene therapy studies. An interesting characteristic of alphaviruses is the induction of mucosal protective immune responses [26, 27]. For some bunyavirus the identification of virulence genes nonessential for growth in vitro allowed replacement for reporter genes or other viral antigens [28, 29]. As attenuated viruses they are capable of sustain limited replication in the host’s enabling the initiation of innate immune responses against the transgene. All this examples outline the number of strategies than can be selected when designing attenuated vector vaccines as well as the possibility to design marker vaccines to elicit protection against several virus pathogens simultaneously (multivalent vaccines).
New Approaches for Vaccine Design
The conventional approaches for vaccine design are often not sufficient to provide immunity against highly variable pathogens or when T-cell immunity is crucial for protection. Tools from molecular biology integrating systems biology (genomics, proteomics, structural biology) approaches allow researchers to identify ways to improve the quality of vaccines or identify repertories of potentially protective antigens. For example, high throughput sequencing can identify the presence of adventitious viral pathogens in commercial vaccines, or defective genomes in cell culture lines used for vaccine production. Structural modeling of the interaction of neutralizing antibodies and/or antibody fragments with antigen can uncover the molecular signatures defining protective epitopes (cryptic (hidden) epitopes or involving quaternary structures) being another approach for vaccine antigen (or antiviral compounds) design. Additionally, novel flow and mass cytometry technologies [30] may help to gain deeper knowledge of specific cell types involved in protective immune responses for each viral disease. Finally, integrating data of vaccine trials, including vaccine antigens, adjuvant usage or in silico epitope prediction algorithms, may allow development of platforms for experimental vaccine antigen candidates [31]. Though these approaches are far from being generalized they hold promise on the future of rationale vaccine design for some relevant viral diseases [32].
Concluding Remarks
Transition of successful experimental vaccines to industrial production and manufacturing may become a bottle neck in vaccinology since veterinary vaccines need to fulfill several important requisites, among them environmental and safety issues, manufacturing costs and marketability prospects. Considering that most novel vaccine technologies (other than killed or attenuated vaccines) need to adapt the current production processes, many vaccines will never develop further enough to reach market. Nonetheless, animal vaccine research is a very attractive research field with many advantages and complexity over human vaccine field. Firstly, due the larger number of target animal species or segments (ruminant livestock, poultry, porcine, equine, companion animals, aquaculture, and other animal vaccines), secondly, the lack of deep knowledge in the immune mechanisms and lack of reagents adds more difficulties if immune response characterization is needed. The possibility to test the efficacy of the vaccine prototypes in the target species and study the immune response evoked is one important difference that can speed the process of vaccine development over that of human vaccines. Another important advantage is the possibility of testing more innovative approaches that can be further tested for human vaccine development.
The following chapters illustrate a number of different techniques to provide antigen delivery in order to develop vaccines against viral diseases. Though the number of techniques is not exhaustive, the ones showed can be considered most currently used by laboratory researchers in the field of animal health. The reader will find useful examples for application to a particular viral disease since most of the techniques can be virtually applied to any virus pathogen. Among them, representative protocols for each of the broad categories for vaccine technologies discussed above. More discursive chapters are also included related to different techniques and protocols for analyses of the immune responses, the use of adjuvants as an essential part of vaccines based on non-live organisms, and experiences on the use of DNA vaccination in large animals.
Contributor Information
Alejandro Brun, Phone: +3434+34 91 620 23 00-179, FAX: +3434+34 91 620 22 47, Email: brun@inia.es.
Alejandro Brun, Email: brun@inia.es.
References
- 1.Gutierrez AH, Spero DM, Gay C, Zimic M, De Groot AS. New vaccines needed for pathogens infecting animals and humans: one Health. Hum Vaccin Immunother. 2012;8(7):971–978. doi: 10.4161/hv.20202. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP. Vaccines against diseases transmitted from animals to humans: a one health paradigm. Vaccine. 2013;31(46):5321–5338. doi: 10.1016/j.vaccine.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njeumi F, Taylor W, Diallo A, Miyagishima K, Pastoret PP, Vallat B, Traore M. The long journey: a brief review of the eradication of rinderpest. Rev Sci Tech. 2012;31(3):729–746. doi: 10.20506/rst.31.3.2157. [DOI] [PubMed] [Google Scholar]
- 4.Heymann DL. Ebola: learn from the past. Nature. 2014;514:299–300. doi: 10.1038/514299a. [DOI] [PubMed] [Google Scholar]
- 5.Olive C. Pattern recognition receptors: sentinels in innate immunity and targets of new vaccine adjuvants. Expert Rev Vaccines. 2012;11(2):237–256. doi: 10.1586/erv.11.189. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3(6):920–940. doi: 10.3390/v3060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92(3):256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez B, Poderoso T, Alonso F, Ezquerra A, Dominguez J, Revilla C. Antigen targeting to APC: from mice to veterinary species. Dev Comp Immunol. 2013;41(2):153–163. doi: 10.1016/j.dci.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv. 2013;2013:869718. doi: 10.1155/2013/869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham BS, Crowe JE Jr, Ledgerwood JE (2013) Immunization against viral diseases. In: Knipe DM, Howley PM (eds) Fields Virology, 6th edn, vol 1
- 12.Slifka MK. Vaccine-mediated immunity against dengue and the potential for long-term protection against disease. Front Immunol. 2014;5:195. doi: 10.3389/fimmu.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uittenbogaard JP, Zomer B, Hoogerhout P, Metz B. Reactions of beta-propiolactone with nucleobase analogues, nucleosides, and peptides: implications for the inactivation of viruses. J Biol Chem. 2011;286(42):36198–36214. doi: 10.1074/jbc.M111.279232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18(6):974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto AK, Richner JM, Poore EA, Patil PP, Amanna IJ, Slifka MK, Diamond MS. A hydrogen peroxide-inactivated virus vaccine elicits humoral and cellular immunity and protects against lethal West Nile virus infection in aged mice. J Virol. 2013;87(4):1926–1936. doi: 10.1128/JVI.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stauffer F, De Miranda J, Schechter MC, Carneiro FA, Salgado LT, Machado GF, Da Poian AT. Inactivation of vesicular stomatitis virus through inhibition of membrane fusion by chemical modification of the viral glycoprotein. Antiviral Res. 2007;73(1):31–39. doi: 10.1016/j.antiviral.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Neumann G, Whitt MA, Kawaoka Y. A decade after the generation of a negative-sense RNA virus from cloned cDNA: what have we learned? J Gen Virol. 2002;83(Pt 11):2635–2662. doi: 10.1099/0022-1317-83-11-2635. [DOI] [PubMed] [Google Scholar]
- 18.Kit S. Genetically engineered vaccines for control of Aujeszky’s disease (pseudorabies) Vaccine. 1990;8(5):420–424. doi: 10.1016/0264-410X(90)90240-M. [DOI] [PubMed] [Google Scholar]
- 19.van Oirschot JT. Diva vaccines that reduce virus transmission. J Biotechnol. 1999;73(2–3):195–205. doi: 10.1016/S0168-1656(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 20.Brun A, Barcena J, Blanco E, Borrego B, Dory D, Escribano JM, Le Gall-Recule G, Ortego J, Dixon LK. Current strategies for subunit and genetic viral veterinary vaccine development. Virus Res. 2011;157(1):1–12. doi: 10.1016/j.virusres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Cubillos C, de la Torre BG, Jakab A, Clementi G, Borras E, Barcena J, Andreu D, Sobrino F, Blanco E. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J Virol. 2008;82(14):7223–7230. doi: 10.1128/JVI.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23(12):1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 23.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 24.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 25.Brun A, Albina E, Barret T, Chapman DA, Czub M, Dixon LK, Keil GM, Klonjkowski B, Le Potier MF, Libeau G, Ortego J, Richardson J, Takamatsu HH. Antigen delivery systems for veterinary vaccine development. Viral-vector based delivery systems. Vaccine. 2008;26(51):6508–6528. doi: 10.1016/j.vaccine.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vajdy M, Gardner J, Neidleman J, Cuadra L, Greer C, Perri S, O’Hagan D, Polo JM. Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles. J Infect Dis. 2001;184(12):1613–1616. doi: 10.1086/324581. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Hu KF, Rozell B, Orvell C, Morein B, Liljestrom P. Vaccination with recombinant alphavirus or immune-stimulating complex antigen against respiratory syncytial virus. J Immunol. 2002;169(6):3208–3216. doi: 10.4049/jimmunol.169.6.3208. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami T, Won S, Peters CJ, Makino S. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J Virol. 2006;80(6):2933–2940. doi: 10.1128/JVI.80.6.2933-2940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oreshkova N, Cornelissen LA, de Haan CA, Moormann RJ, Kortekaas J. Evaluation of nonspreading Rift Valley fever virus as a vaccine vector using influenza virus hemagglutinin as a model antigen. Vaccine. 2014;32(41):5323–5329. doi: 10.1016/j.vaccine.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S. Highly multiparametric analysis by mass cytometry. J Immunol Methods. 2010;361(1–2):1–20. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Xiang Z. Databases and in silico tools for vaccine design. Methods Mol Biol. 2013;993:115–127. doi: 10.1007/978-1-62703-342-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakaya HI, Pulendran B. Systems vaccinology: its promise and challenge for HIV vaccine development. Curr Opin HIV AIDS. 2012;7(1):24–31. doi: 10.1097/COH.0b013e32834dc37b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivan M, Jonathan B; David K. Roitt M (1989) Immunology, 2nd edn. Harper & Row


