Abstract
In the past several decades, a number of novel fluorescence image-guided surgery (FGS) contrast agents have been under development, with many in clinical translation and undergoing clinical trials. In this review, we have identified and summarized the contrast agents currently undergoing clinical translation. In total, 39 novel FGS contrast agents are being studied in 85 clinical trials. Four FGS contrast agents are currently being studied in phase III clinical trials and are poised to reach FDA approval within the next two to three years. Among all novel FGS contrast agents, a wide variety of probe types, targeting mechanisms, and fluorescence properties exists. Clinically available FGS imaging systems have been developed for FDA approved FGS contrast agents, and thus further clinical development is required to yield FGS imaging systems tuned for the variety of contrast agents in the clinical pipeline. Additionally, study of current FGS contrast agents for additional disease types and development of anatomy specific contrast agents is required to provide surgeons FGS tools for all surgical specialties and associated comorbidities. The work reviewed here represents a significant effort from many groups and further development of this promising technology will have an enormous impact on surgical outcomes across all specialties.
Keywords: fluorescence, image-guided surgery, fluorescence imaging system, clinical development, clinical trial, contrast agent, near-infrared fluorescence
1. Introduction
Fluorescence image-guided surgery (FGS) technologies have been under development for the past three decades. A movement that began with the development of clinically approved near-infrared (NIR) fluorescent agents indocyanine green (ICG) and methylene blue as vascular tracers has grown to become a multidisciplinary field studied by dozens of groups worldwide.1–4 Through the creation of targeted contrast agents and sensitive imaging systems, FGS has the potential to revolutionize surgery, improving surgical outcomes by enhancing visualization of tissues for resection, such as tumors, or preservation, such as nerves or vasculature. Utilizing compact and relatively low-cost imaging systems, FGS can be readily implemented into many procedures to bridge the gap between preoperative imaging, such as magnetic resonance imaging (MRI) and computed tomography (CT), and the current intraoperative reality.5–15 FGS systems have been successfully utilized in a wide variety of clinical applications to improve outcomes, including tumor resection, sentinel lymph node mapping, angiography, lymphography, and ureter and bile duct anatomic imaging.16–28 Additionally, a number of targeted contrast agents are under development to expand the applications of this promising technology. For example, 5-aminominolevulinic acid (5-ALA) and its fluorescent metabolite protoporphyrin IX (PpIX) has garnered clinical approval for glioma resection and has significantly enhanced complete resection rates and revolutionized neurosurgical treatment of brain tumors over the past decade.29 Many more targeted contrast agents are currently in the clinical pipeline, representing substantial effort by the FGS community to translate this promising technology to many surgical indications.
Considered new drugs by the FDA, novel FGS contrast agents face a lengthy and expensive approval process to reach clinical use. Completion of preclinical development to enable Investigational New Drug (IND) approval for first-in-human trials represents the first major hurdle for FGS contrast agent development. Subsequently, completion of Phase I, II, and III clinical trials requires exponentially more time, effort, and money to complete successfully prior to market approval. Finding the necessary investments and strategic partners to navigate this long and costly process is difficult, especially for FGS contrast agents as non-curative diagnostic agents for single use, which provide a low financial incentive for investment from commercial sources.30,31 Nonetheless, many startups, groups in academia, and even mid-scale biotechnology and medical device companies have worked towards clinical translation of a variety of novel FGS contrast agents. Herein, we review the current landscape of novel FGS contrast agents undergoing clinical translation and outline their integration with the current ecosystem of clinical, and pre-clinical, fluorescence imaging systems.
2. Clinically Approved Fluorescence Image-Guided Surgery Contrast Agents
A complete picture of the current clinical translation of FGS contrast agents cannot be achieved without including fluorescent agents with current clinical approval. Although few in number and most lacking specific targeting, these agents represent the majority of clinical work using FGS to date (Table 1). The majority of these contrast agents, Methylene Blue (MB), ICG, and Fluorescein, have been used as far back as 1891 for the treatment of diseases like malaria and as colorimetric reporters for tissue perfusion and angiography.32,33 Initial use of these agents for FGS dates back to 1947, when fluorescein was used to guide brain tumor resection.34 However, the agent used perhaps most frequently for FGS, ICG, has dominated the majority of clinical applications in recent years.
Table 1.
FDA-approved FGS contrast agents.
| Name | Description | Chemical Class | Excitation Wavelength | Emission Wavelength |
|---|---|---|---|---|
| Fluorescein | Visible fluorophore often used for angiography and glioma resection. No specific targeting mechanism | Xanthene | 494 nm | 521 nm |
| Methylene Blue (MB) | Near-infrared fluorophore used in many FGS applications. No specific targeting mechanism. | Thiazine | 668 nm | 688 nm |
| Indocyanine Green (ICG) | Near-infrared fluorophore with the broadest clinical adoption and use. No specific targeting mechanism | Cyanine | 780 nm | 820 nm |
| 5-aminominolevulinic acid (5-ALA) | Pro-drug that is metabolized to the visible fluorophore protoporphyrin IX (PplX) in cancer cells. Specifically targeted to cancer tissue. | Porphyrin | 380–440 nm | 620–640 nm |
ICG is a water soluble tricarbocyanine fluorophore that possesses bright NIR fluorescence properties with excitation at 780 nm and emission at 820 nm.35 The production and use of ICG dates back to 1955, when ICG was manufactured for use by Kodak Research Laboratories, and clinical approval dates back to 1956, when ICG was approved by the FDA for retinal angiography.33 ICG clears rapidly from the body and possesses an excellent safety profile, with LD50s between 50–80 mg/kg in animals. ICG is currently used for sentinel lymph node mapping, angiography, reconstructive surgery, cholangiography, and tumor imaging among other uses for FGS and other clinical practices.16,35–38
One FGS contrast agent was only recently approved, 5-ALA, and is unique among the clinically approved agents in that it provides targeted fluorescence imaging of cancer instead of providing contrast via passive mechanisms. 5-ALA is metabolized to the fluorescent porphyrin molecule PpIX following uptake in cancer cells, possessing visible fluorescence with excitation at 380–440 nm and emission at 620–640 nm.39,40 5-ALA was first studied for FGS in 1998 and reached FDA approval in 2017.41 Remarkably, 5-ALA enabled FGS has improved high grade glioma complete resection rates almost two-fold over white light imaging alone, resulting in increased overall progression free survival.23 These FGS contrast agents, while few in number and possessing suboptimal fluorescent and/or tissue uptake properties, have demonstrated the incredible utility of FGS to improve surgical outcomes. Looking forward, many new FGS contrast agents are under development and possess advanced targeting, physiochemical, and tissue uptake characteristics.
2. Novel Fluorescence Image-Guided Surgery Contrast Agents Under Clinical Development
At present, 39 novel FGS contrast agents are undergoing clinical trials in the United States. These agents account for 85 clinical trials registered in clinicaltrials.gov across a broad range of indications. These agents and their corresponding clinical trials are outlined in Table 2. These clinical trials represent a diverse field of researchers, clinicians, and industry partners undertaking the clinical translation of a variety of unique targeted FGS contrast agents. A number of probes utilize targeting moieties such as antibodies or peptides to obtain highly specific fluorescent signal.42 Others generate specific contrast via activatable fluorescence mechanisms using fluorophore quenching groups that are cleaved via enzymatic processes.43 Others still possess structure inherent targeting mechanisms where the fluorophore itself acts as the targeting moiety and fluorescent reporter.44–47 This diversity in probe design is evident in the molecular type of each contrast agent (Fig. 1), where the majority of contrast agents are small molecule based, followed closely by peptide and antibody based contrast agents.
Table 2.
Novel FGS contrast agents under clinical development.
| Probe Name | Description | Fluorophore | Company/Group | Indications | Clinical Trial Phase | NCT# | Status |
|---|---|---|---|---|---|---|---|
| Antibody | |||||||
| SGM-10148−50 | Monoclonal antibody to carcinoembryonic antigen (CEA) labelled with 700nm BM104fluorophore. IV administration 4 days before surgery | BM104 | Surgimab | Colon Cancer | Phase 2 | NCT02973672 | completed |
| Rectum Cancer | Phase 2 | ||||||
| Pancreatic Cancer | Phase 2 | ||||||
| Metastatic Colorectal Cancer | Phase 2 | ||||||
| Recurrent Colorectal Carcinoma | Phase 2 | ||||||
| Colorectal Neoplasms | Phase 3 | NCT03659448 | recruiting | ||||
| Peritoneal Carcinomatosis | Phase 1 | NCT02784028 | recruiting | ||||
| Panitumumab-IRDye800CW51–54 | EGFR targeting antibody conjugated with IRDye800. IV administered over60min, 1–5 days prior to surgery | IRDye-800 | Rosenthal-Stanford | Pediatric Neoplasms | Phase 2 | NCT04085887 | not yet recruiting |
| Malignant Glioma | Phase 2 | NCT03510208 | recruiting | ||||
| Head and Neck Cancer | Phase 2 | NCT03405142 | recruiting | ||||
| Lung Cancer | Phase 2 | NCT03582124 | suspended (business decision) | ||||
| Pancreatic Cancer | Phase 2 | NCT03384238 | recruiting | ||||
| Cetuximab-IRDye800CW52,53,55–59 | EGFR targeting antibody conjugated with IRDye800. IV administered 4 days prior to surgery | IRDye-800 | Rosenthal-Stanford | Head and Neck Cancer | Phase 2 | NCT03134846 | recruiting |
| Pancreatic Cancer | Phase 2 | NCT02736578 | terminated (logistics) | ||||
| Esophageal Cancer | Phase 1 | NCT04161560 | recruiting | ||||
| Brain Cancer | Phase 2 | NCT02855086 | terminated (logistics) | ||||
| Bevacizumab-IRDye800CW52,60–62 | VEGF targeting antibody conjugated to IRDye800. IV administration 3 days prior to surgery at doses of 10, 25, or 50 mg | IRDye-800 | van Dam-Groningen | Adenomatous Polyposis | Phase 1 | NCT02113202 | completed |
| Rectal Cancer | Phase 1 | NCT01972373 | completed | ||||
| Breast Cancer | Phase 2 | NCT02583568 | completed | ||||
| Esophageal Cancer | Phase 2 | NCT03877601 | recruiting | ||||
| Hilar Cholangiocarcinoma | Phase 2 | NCT03620292 | recruiting | ||||
| Soft Tissue Sarcoma | Phase 2 | NCT03913806 | recruiting | ||||
| Pancreatic Cancer | Phase 2 | NCT02743975 | recruiting | ||||
| Endometriosis | Phase 1 | NCT02975219 | recruiting | ||||
| Inverted Papilloma | Phase 1 | NCT03925285 | recruiting | ||||
| Pituitary Adenoma | Phase 1 | NCT04212793 | not yet recruiting | ||||
| Carotid plaque instability | N/A | NCT03757507 | not yet recruiting | ||||
| lndium-111-DOTA-Labetuzumab-IRDye800CW63 | CEA targeting antibody conjugated with dual modality (SPECT/CT and fluorescence) tracers. Administered 6–7 days before surgery. | IRDye-800 | Boerman-Radbound University | Colorectal Cancer | Phase 2 | NCT03699332 | recruiting |
| lndium-111-DOTA-Girentuximab-IRDye800CW64 | Carbonic anhydrase IX (CAIX) targeting antibody conjugated with dual modality (SPECT/CT and fluorescence) tracers. Administered 7 days before surgery. | IRDye-800 | Boerman-Radbound University | Renal Carcinoma | Phase 1 | NCT02497599 | active, not recruiting |
| MDX1201-A488 | PSMA targeting antibody conjugated with Alexa Fluor 488. IV administration 4 days prior to surgery | AF488 | Zhumkhaw-ala - City of Hope Medical | Prostate Cancer | Phase 1 | NCT02048150 | active, not recruiting |
| ProstaFluor | PSMA targeting antibody huJ-591 conjugated to IR-800CW | IRDye-800 | Spectros | Prostate Cancer | Phase 1 | NCT01173146 | withdrawn - cost of antibody production increased, no supplemental funding obtained |
| Protein | |||||||
| Fluorescein conjugated wisteria floribunda | Fluorescein conjugated lectin. Sprayed onto colonic surface during surgery | Fluorescein | Yeung -Oxford | Colorectal Cancer | Phase 0 | NCT03070613 | enrolling by invitation |
| Affibody | |||||||
| ABY-02928,65–67 | EGFR targeting affibody labeled with IRDye800CW. Microdose injection 1–3 hours prior to surgery | IRDye-800 | Pogue -Dartmouth | Glioma | Phase 0 | NCT02901925 | suspended -awaiting FDA approval of eIND amendment |
| Primary soft tissue Sarcoma | Phase 0 | NCT03154411 | suspended -awaiting FDA approval of eIND amendment | ||||
| Head and Neck Cancer | Phase 0 | NCT03282461 | suspended -awaiting FDA approval of eIND amendment | ||||
| Peptide | |||||||
| BLZ-100 (tozuleristide)68−74 | Chlorotoxin (scorpion venom) with high affinity to matrix metalloprotease 2 (MMP-2) conjugated with NIR fluorophore. IV administration at least 1 hour before surgery | ICG | Blaze Bioscience | Soft Tissue Sarcoma | Phase 1 | NCT02464332 | withdrawn - not enough subjects enrolled |
| CNS Tumors | Phase 3 | NCT03579602 | recruiting | ||||
| Glioma | Phase 1 | NCT02234297 | completed | ||||
| Breast Cancer | Phase 1 | NCT02496065 | completed | ||||
| Skin Neoplasms | Phase 1 | NCT02097875 | completed | ||||
| AVB-62075,76 | Ratiometric MMP activatable peptide labeled with cy5 and cy7. Upon activation, cy5 is cleaved, and cy5 fluorescence increases. IV administration up to 24 hr before | Cy5& Cy7 (cy5 detected) | Avelas Biosciences | Breast Cancer | Phase 2 | NCT03113825 | recruiting |
| BBN-IRDye800CW76 | Peptide targeting gastrin-releasing peptide receptor (GRPR) labeled with IRDye 800 and radiotracer. IV administration 2 hr before surgery at 1 mg dose | IRDye-800 | Chen-NIH | Brain Cancer | Phase 0 | NCT02910804 | recruiting |
| EMI-13777 | Human hepatocyte growth factor receptor (c-MET) targeting peptide conjugated to cyanine based fluorophore (Cy5?). IV administration 1–3 hours before surgery | Cy5 | Edinburgh Molecular Imaging | Colon Cancer | Phase 2 | NCT03360461 | recruiting |
| Thyroid Cancer | Phase 1 | NCT03470259 | completed | ||||
| Barret Esophagus | Phase 1 | NCT03205501 | recruiting | ||||
| Lung Cancer | Phase 1 | NCT02676050 | not yet recruiting | ||||
| QRH-882260 Heptapeptide78 | Seven amino acid long peptide that binds to EGFR labeled with cy5. Orally administered and binds to tumor cells in Gl tract. | Cy5 | Wang-University of Michigan | Colon Cancer | Phase 1 | NCT03148119 | terminated (QRH to be used for other indications) |
| Cholangiocarcinoma | Phase 1 | NCT03438435 | recruiting | ||||
| Barret Esophagus | Phase 1 | NCT03589443 | completed | ||||
| Safety study | Phase 1 | NCT02574858 | completed | ||||
| KSP/QRH peptide dimer79 | EGFR and HER2 targeting peptide labelled with IRDye800. Orally administered or sprayed onto area of interest | IRDye-800 | Wang-University of Michigan | Barret Esophagus | Phase 1 | NCT03852576 | recruiting |
| Gl heptapeptide80 | Heptapeptide labeled with FITC. Orally administered or sprayed onto area of interest | FITC | Wang-University of Michigan | Barret Esophagus | Phase 1 | NCT01630798 | completed |
| KCC heptapeptide81 | Heptapeptide labeled with FITC. Orally administered or sprayed onto area of interest | FITC | Wang-University of Michigan | Colorectal Cancer | Phase 1 | NCT02156557 | completed |
| LS30182,83 | integrin receptor targeting octapeptide conjugated to NIR fluorophore cypate. IV administration 1 day prior to surgery. | Cypate | Achilefu -Washington University | Breast Cancer | Phase 2 | NCT02807597 | not yet recruiting |
| Pancreatic Cancer | Phase 2 | NCT04105062 | not yet recruiting | ||||
| Liver Cancer | Phase 2 | ||||||
| Gastric Cancer | Phase 2 | ||||||
| Gastrointestinal Stromal Cancer | Phase 2 | ||||||
| Metastatic Cancer | Phase 2 | ||||||
| RGD peptide -cy7 (ORL-1)84–88 | Alpha(v) beta(3) integrin targeting peptide labeled with cy7. Topically applied to skin surface. | Cy7 | Orlucent | Melanoma | NCT03535077 | recruiting | |
| Nanoparticle | |||||||
| cRGDY-PEG-Cy5.5-C*89 | Integrin-targeting, dual modality (PET & fluorescent) silica nanoparticle labeled with cy5.5. Injected at site of tumor before or during surgery | Cy5.5 | Patel - Sloan Kettering | Head and Neck Cancer | Phase 2 | NCT02106598 | recruiting |
| Breast Cancer | Phase 2 | ||||||
| Colorectal Cancer | Phase 2 | ||||||
| cRGD-ZW800–187,90 | Integrin-targeting silica nanoparticle labelled with ZW800. Injected 4–24 hours prior to surgery. | ZW-800 | Keereweer -Erasmus Medical Center | Head and Neck Cancer | Phase 2 | NCT04191460 | not yet recruiting |
| 64Cu-NOTA-PSMAi-PEG-Cy5.5-C*91 | PSMA targeting nanoparticle, multi-modality (PET, MRI, fluorescent) labelled with Cy5.5. | Cy5.5 | Memorial Sloan Kettering | Prostate Cancer | Phase 1 | NCT04167969 | recruiting |
| ONM-10092 | pH-sensitive micelles conjugated with ICG. IV administration on day of surgery. | ICG | OncoNano Medicine | Breast Cancer | Phase 2 | NCT03735680 | recruiting |
| Head and Neck Cancer | Phase 2 | ||||||
| Colorectal Cancer | Phase 2 | ||||||
| Bladder Cancer | Phase 2 | ||||||
| Prostate Cancer | Phase 2 | ||||||
| Ovarian Cancer | Phase 2 | ||||||
| Small Molecule | |||||||
| Demeclocycline93 | Antibiotic with UV abs/yellow fl. Administered orally for 2 day | Demeclocycline | Curry -Massachuset ts General | Brain Tumor | Phase 1 | NCT02740933 | not yet recruiting |
| IRDye-800BK94 | injected into urethra and imaged immediately | IRDye-800BK | Barnes -Oxford | Ureter Injury | Phase 2 | NCT03387410 | completed |
| LUM01595−97 | Cathepsin-activatable labeled with cy5 and fluorescent quencher linked by a pan-cathepsin protease cleavable peptide. IV injection 2–6 hrs prior to surgery at 1 mg/kg dose. | Cy5 | Lumicell | Breast Cancer | Phase 3 | NCT03686215 | recruiting |
| Colorectal Cancer | Phase 2 | NCT02584244 | recruiting | ||||
| Barret Esophagus | Phase 2 | ||||||
| Pancreatic Cancer | Phase 2 | ||||||
| Brain Cancer | Phase 1 | NCT03717142 | recruiting | ||||
| Prostate Cancer | Phase 1 | NCT03441464 | recruiting | ||||
| IS-00144 | Agent for use with daVinci robot | IS-001 | Intuitive Surgical | Hysterectomy -Ureter Injury | Phase 2 | NCT03937505 | recruiting |
| PARPi-FL98-100 | PARP1 inhibitor (olaparib) labeled with BODIPY-FL. Applied topically for basal cell carcinoma | BODIPY-FL | Reiner -Sloan Kettering | Oral Squamous Cell Carcinoma | Phase 2 | NCT03085147 | recruiting |
| HS-196101,102 | Heat shock protein 90 (HSP90) inhibitor labelled with NIRdye. IV administration | N/A | Lyerly - Duke | Solid Tumor | Phase 1 | NCT03333031 | recruiting |
| TMVP1-ICG | Used for SLN mapping, injected into the cervix | ICG | Ding Ma -Huazhong University | Cervical Cancer | Phase 1 | NCT03320772 | recruiting |
| EC17103−105 | Folate-FITC conjugate targeting folate receptor. IV administration 2–4 hours before surgery at 0.1 mg/kg dose | FITC | Singhal -University of Pennsylvania | Hyperparathyroidism | Phase 1 | NCT01996072 | completed |
| Breast Cancer | Phase 1 | NCT01994369 | completed | ||||
| Ovarian Cancer | Phase 1 | NCT02000778 | completed | ||||
| Renal Carcinoma | Phase 0 | NCT01778933 | completed | ||||
| Lung Cancer | Phase 1 | NCT01778920 | completed | ||||
| OTL38106–110 | Folate receptor alpha (Fra) targeting ligand (folic acid) conjugated to NIR fluorophore S0456. IV administration 2–3 hrs before surgery | S0456 | On Target Laboratories | Ovarian Cancer | Phase 3 | NCT03180307 | recruiting |
| Lung Cancer | Phase 2 | NCT02872701 | completed | ||||
| Lung Cancer | Phase 1 | NCT02769156 | recruiting | ||||
| Lung Cancer | Phase 1 | NCT02602119 | recruiting | ||||
| pituitary adenoma | Phase 1 | NCT02629549 | terminated (recruitment fulfilled) | ||||
| Malignancies in pituitary gland | Phase 1 | NCT02769533 | completed | ||||
| Bladder Cancer | Phase 1 | NCT02852252 | recruiting | ||||
| Rheumatoid Arthritis | Phase 1 | NCT03938701 | not yet recruiting | ||||
| Renal Carcinoma | Phase 1 | NCT02645409 | completed | ||||
| Li-COR Ureter Agent | IV administration during surgery at 0.06 mg/kg | IRDye-800BK | Li-COR | Ureter Injury | Phase 2 | NCT03106038 | completed |
| MB-10246,47 | Human plasma fluorescence tracer. | MB-102 | MediBeacon | Acute Kidney Injury | Phase 2 | NCT02772276 | recruiting |
| Aftobetin - HCI | Amyloid beta binding ligand. Administered via opthalmic ointment, eye lens imaged. | Aftobetin | Cognoptix | Alzheimer’s Disease | Phase 1 | NCT02928211 | recruiting |
| HS201 | HSP90 inhibitor connected by linker to verteporfin (imaging agent and photosensitizing agent). | Verteporfin | Lyerly - Duke | Solid Tumor | Phase 1 | NCT03906643 | not yet recruiting |
| LuminoMark | Fluorescence localization in patients with nonpalpable breast lesions - not clear if this is a functionalized version of ICG or tagged molecule | ICG | Hanlim Pharm. Co. Ltd. | Breast Cancer | Phase 2 | NCT03743259 | completed |
| Prosense/VM110111,112 | Near infrared fluorophore self-quenched and activated when cleaved by proteases, cathepsin B,L, and S and plasmin in cancer cells, fluorescent cleavage product detected. Combination product. | Cy5.5 | Weissleder -Harvard | Ovarian Cancer | Phase 1 | NCT03286062 | active, not recruiting |
| Pancreatic Cancer | Phase 1 | ||||||
Figure 1.
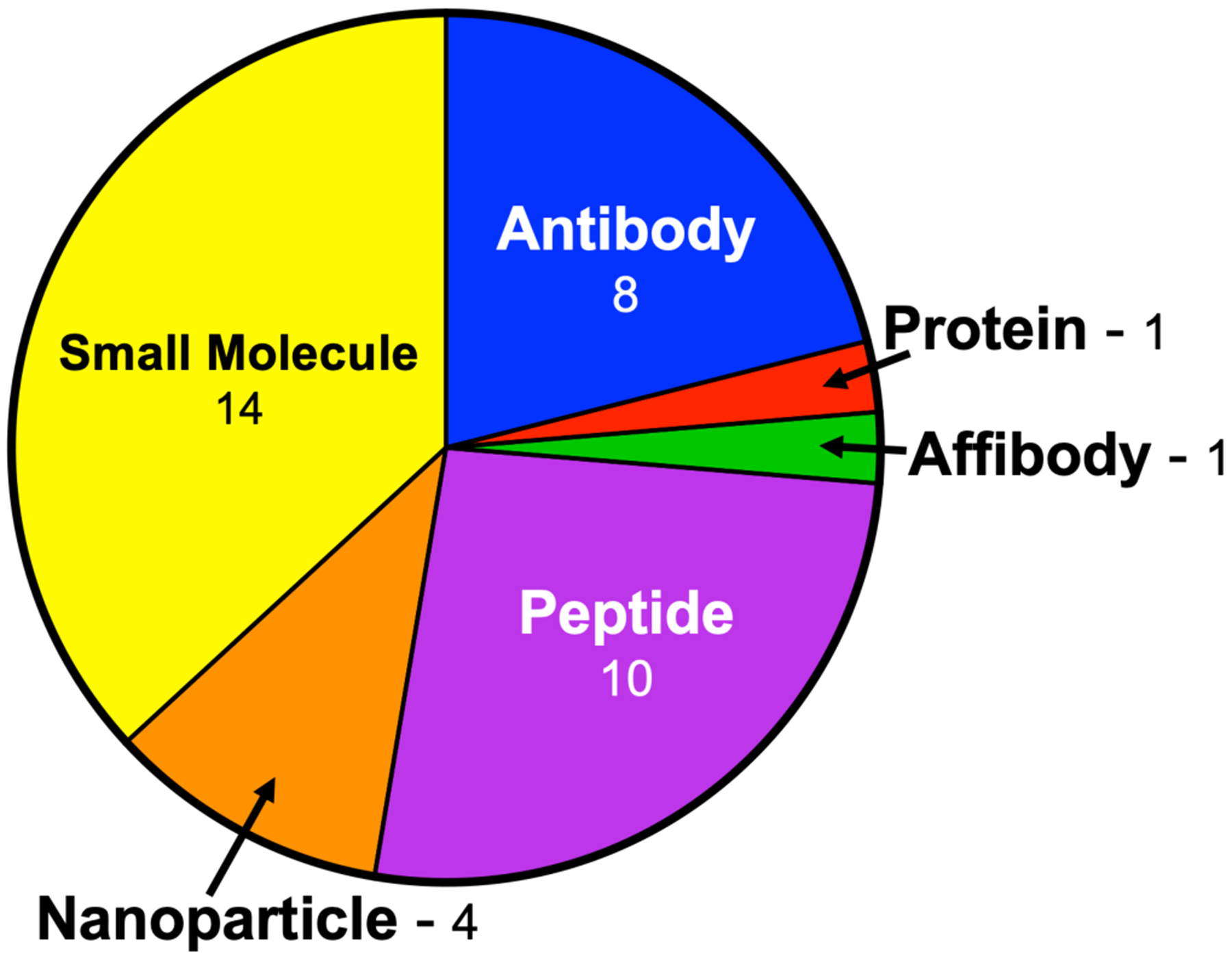
A pie chart of novel FGS contrast agent currently undergoing clinical translation split by probe type. The number of each agent type is listed next to the type.
From this diverse group of contrast agents for FGS, a handful have completed significant development through phase II clinical trials on the pathway to FDA approval. The clinical trial phase of each probe is outlined in Fig. 2. Currently, SGM-101, BLZ-100, LUM015, and OTL38 have reached phase III clinical trials (NCT03659448, NCT03579602, NCT03686215, and NCT03180307, respectively). SGM-101 is a CEA targeting antibody labeled with a NIR fluorophore under phase III testing for colorectal neoplasms. SGM-101 has demonstrated a high degree of specificity in primary tumor tissue and metastases, with mean tumor to background ratios of 1.6 and 1.7, respectively.50 BLZ-100 is composed of a chlorotoxin peptide covalently bound to ICG that demonstrates high affinity for matrix metalloprotease 2 (MMP-2) under phase III testing for pediatric central nervous system (CNS) tumors. In phase I testing, BLZ-100 demonstrated low toxicity, with no observed adverse events, and positive fluorescence signal in tumor tissue that was positively correlated with the dose of the imaging agent and grade of the cancer.74 OTL38 is a small molecule probe consisting of folic acid, a ligand for folate receptor alpha, conjugated to the NIR fluorophore S0456 that is in phase III clinical studies for ovarian cancer. In phase II studies OTL38 identified cancer lesions with a sensitivity of 97.97%, where 48.3% of patients had at least one additional lesion identified using FGS over white light alone.110 These probes represent every major type of targeted FGS contrast agent and are leading the way for clinical translation of many others.
Figure 2.

A pie chart highlighting the clinical trial phase of the novel FGS contrast agents currently undergoing clinical translation. The number of agents in each phase is listed.
For all other FGS contrast agents under clinical translation, probes in phase I clinical trials make up the majority of clinical studies, followed closely by phase II studies (Fig. 2). One option for easing the regulatory burden for first-in-human studies of new agents is to utilize the food and drug administration’s (FDA’s) exploratory investigational new drug (eIND) pathway. First in human (FIH) studies conducted under an eIND require significantly less preclinical toxicology testing by allowing researchers to administer “microdoses” denoted as less than 100 μg or 30 nmol per administration for small molecule or protein products, respectively.113 Due to the lower administered dose, substantially fewer preclinical toxicology studies are required prior to FIH clinical trials as compared to traditional translation under an IND, allowing proof of concept phase 0 studies to be performed with relative ease and significantly decreased financial burden. This alternative route to clinical use has been utilized with success in the recent phase 0 clinical trials of ABY-029, a promising tumor targeted FGS affibody probe, BBN-IRDye800CW, a dual modality PET/FGS contrast agent for brain cancer resection, fluorescein conjugated wisteria floribunda (WFA), a topically applied lectin for colon cancer resection, and EC17, a small molecule folate-FITC conjugate targeting folate receptor for renal carcinoma resection.66,67 The eIND pathway offers a promising alternative for ease of clinical translation for new FGS imaging probes, decreasing the financial burden to obtaining FIH results.
3. Fluorescence Spectral Properties and Compatibility with Clinical Imaging Systems
Additional diversity among the novel FGS contrast agents undergoing clinical translation is found in analysis of the fluorescence reporters’ spectral properties (Fig. 3). A number of fluorophores have been utilized to provide contrast for each agent and those with reported excitation and emission are graphed in Fig. 3A along with the number of probes using each fluorophore. With fluorescence centered around 800 nm, IRDye800CW is the most often utilized fluorophore, providing contrast for more than double the number of probes than any other fluorophore. Examining the distribution of all 39 probes among the respective fluorescence NIR imaging channels (700 and 800 nm) and visible channel, just over half (20 probes) utilize fluorophores with excitation and emission wavelengths centered around 800 nm, with 9 probes using fluorophores with excitation and emission wavelengths centered around 700 nm and 10 probes using fluorophores with excitation and/or emission wavelengths in the visible range (Fig. 3B).
Figure 3. A.
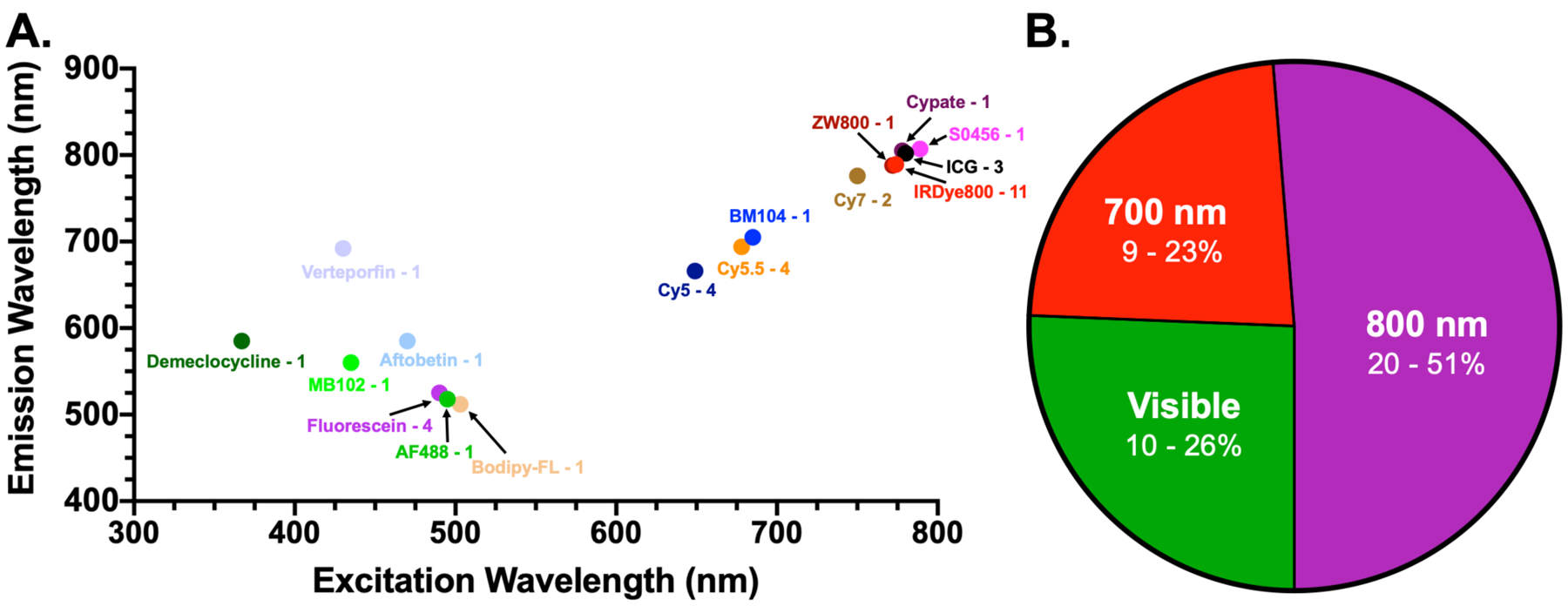
Fluorescence excitation and emission wavelengths and B. imaging channel distribution of all fluorophores utilized by all novel FGS contrast agents undergoing clinical translation. The number of probes using each fluorophore are listed next to their names and the number of fluorophores in each channel are listed as well as the percentage of the total that number represents.
Assessing the compatibility of these fluorophores with clinical imaging systems requires a review of the currently clinically approved fluorescence imaging systems. Table 3 outlines the FGS imaging systems that are FDA approved or under development/clinical translation for human use. Notably, all FDA approved imaging systems are developed with fluorescence imaging capabilities compatible with the current FDA approved FGS contrast agents. Thus, the majority of systems are tuned for ICG fluorescence imaging, with excitation and emission wavelengths centered around 800 nm. Few FDA approved imaging systems possess capabilities for imaging fluorescein and PpIX fluorescence, and only two approved systems, the Fluobeam and Quest Spectrum, possess capabilities for imaging MB in the 700 nm channel. Thus, there exists a gap between novel FGS contrast agents’ fluorescence properties and the imaging capabilities of clinically approved FGS imaging systems, where those contrast agents outside the 800 nm channel are lacking adequate options for spectrally tuned imaging systems. This gap exists likely due to the regulatory process for FGS imaging systems, where the majority of systems have achieved 510(k) clearance via predicate devices by showing substantial equivalence with already approved systems, most often the Stryker SPY imaging system that first obtained approval in 2005. However, a number of imaging systems are under development preclinically that could provide imaging capabilities for a wide range of fluorophores and incentive for approval of these systems will grow as novel FGS contrast agents with fluorescence outside of the 800 nm channel reach FDA approval (Table 3). For example, of the four novel FGS contrast agents in phase III clinical trials, two possess fluorescence properties outside the 800 nm channel including SGM-101, which uses BM104 as its fluorescent reporter with excitation and emission in the 700 nm channel, and LUM015, which utilizes Cy5 as its fluorescent reporter with excitation and emission in visible/NIR 700 nm channel.
Table 3.
Current FGS imaging systems.
| System Name | Description | Company/Group | Excitation (nm) | Emission (nm) | Approved? |
|---|---|---|---|---|---|
| Fluobeam | Hand held NIR imaging system (800nm channel approved, 700nm channel (680nm ex - 700 nm em developed) | Fluoptics Imaging | 750 | 800 longpass | Yes |
| PDE/Photodynamic EyeNeo(ll) | Hand held NIR imaging, uses LEDs | Hammamatsu/Mitaka | 760 | 820 longpass | Yes |
| Firefly | Endoscopic fluorescence imaging system for robotic surgery | Intuitive Surgical | 805 | 805 blocking | Yes |
| Image 1S Camera System | Endoscopic fluorescence imaging system | Karl Storz | N/A | N/A | Yes |
| Vitom II | Karl Storz Image 1s camera system, but used in open surgery as a microscope | Karl Storz | N/A | N/A | Yes |
| GLOW800 | Leica Surgical Microscope accessory (M530) | Leica | 790 | 835 | Yes |
| FL560 | Leica Surgical Microscope accessory (M530) | Leica | 460–500 | 510 longpass | Yes |
| FL800 | Leica Surgical Microscope accessory (M530) | Leica | 700–800 | 820–860 | Yes |
| FL400 | Leica Surgical Microscope accessory (M530) | Leica | 380–430 | 444 longpass | Yes |
| Pinpoint | Endoscopic fluorescence imaging system (“deep red” [for MB] system used in clinical trials) | Novadaq/Stryker | 805 | 825–850 | Yes |
| SPY PHI | Hand held fluorescence imaging system | Novadaq/Stryker | 805 | 825–850 | Yes |
| SPY Elite | On cart for open surgery fluorescence imaging system | Novadaq/Stryker | 805 | 825–850 | Yes |
| AIM (Advanced Imaging Modality) | Stryker’s AIM camera combined with SPY, SPY Elite, Pinpoint | Stryker | 805 | 825–850 | Yes |
| Visera Elite II | NIR and narrow band imaging in endoscope (Approved in Europe but not US?) | Olympus | N/A | N/A | Yes |
| Artemis | Hand held NIR fluorescence imaging system | Quest Medical Imaging | N/A | N/A | Yes |
| Spectrum | Replaced Artemis, hand held with 700 and 800 nm imaging channel, uses LEDs | Quest Medical Imaging | N/A | 700–830, 830–1000 | Yes |
| VS3 IR/lridium System | 3D endoscope or open surgical system with NIR fluorescence channel | VisionSense/Medtronic | 805 | 825–850 | Yes |
| Yellow 560 | Zeiss Surgical Microscope accessory (Kinevo 900) | Zeiss | 460–500 | 550–700 | Yes |
| Infrared 800 | Zeiss Surgical Microscope accessory (Kinevo 900) | Zeiss | 700–780 | 820–900 | Yes |
| Blue 400 | Zeiss Surgical Microscope accessory (Kinevo 900) | Zeiss | 400–410 | 620–710 | Yes |
| Fluorescence Goggle System | Augmented reality goggle system for FGS | Achilefu (Wash U) | 780 | N/A | No |
| OPAL | Light projection system for visualization on tissue surface in open surgery | Akers (Wash U) | 780 | 785 longpass | No |
| GXMI Navigator | Cart based system | Chinese Academy of Sciences | 760 | 810–870 | No |
| FLARE | Cart based system for open surgery, 700 & 800 nm channel (mini-FLARE latest iteration) | Curadel | 656–678, 745–779 | 689–725, 803–853 | No |
| IC-flow | Handheld imaging system | Diagnostic Green | 780 | N/A | No |
| HyperEye Medical System | Hand held fluorescence imaging system | Mizuho Medical Company | 760–780 | 800–850 | No |
| Solaris | Filtered LED light source, on cart | PerkinElmer | 488, 667, 743, 757 | 516–523, 692–742, 770–809, 784 LP | No |
| Visual Navigator | Hand held system | SH System | 740 | 820 | No |
| Explorer Air | Multispectral imaging platform (currently in clinical trials in EU, formerly SurgOptix T-3) | Surgvision | 520, 800 | N/A | No |
4. Clinical Indications of Novel Fluorescence-Guided Surgery Contrast Agents
Analysis of the clinical indication studied in all clinical trials using novel FGS contrast agent reveals an overwhelming majority of agents targeted to cancer for enhanced detection and/or resection. 75, or 88%, of all novel FGS contrast agent clinical trials are underway for cancer related indications, while 6, or 7%, are underway for other disease related indications and only 4, or 5%, are underway for enhanced anatomical preservation. Thus, while great progress has been made in the field of FGS contrast agent development for improved cancer detection and resection, there remains a need for surgical treatment of non-cancer diseases and preservation of normal tissue function that can be enhanced using FGS. Interestingly, some cancer targeting agents have been employed in the treatment of other diseases, such as hyperparathyroidism (EC17, NCT01996072), carotid plaque instability (Bevacizumab-IRDye800CW, NCT03757507), or rheumatoid arthritis (OTL38, NCT03938701). Continued expansion of the many novel cancer targeted FGS contrast agents for other diseases could provide an excellent foundation for increasing the impact of FGS outside surgical oncology. Developing probes targeted to important anatomical structures, such as ureters or nerves, however, requires separate development efforts to identify targeting moieties for these non-diseased and intact tissues. Although, such development efforts possess a strong value and are worth undertaking, as injury to non-diseased tissues is responsible for a plethora of surgical comorbidities that plague patient outcomes and present an enormous cost to the healthcare system. For instance, intraoperative nerve damage affects up to 63 million patients worldwide annually, causing pain or loss of function and significantly affecting quality of life.114,115 These rates remain high despite efforts to improve nerve sparing through complex surgical techniques and nerve detection technologies in procedures that have a high incidence of injury.115–123 Due to this need, several classes of nerve specific fluorophores have been studied for FGS preclinical.10,124–134 Further clinical translation of these and other anatomy targeted FGS contrast agents will improve surgical outcomes overall and could be used in synergy with cancer or disease specific agents to comprehensively benefit surgical goals.
5. Conclusion
Remarkable progress has been made in the past several decades not only in the field of optical imaging and biophotonics, but in the fields of fluorophore chemistry and FGS contrast agent development. An array of novel FGS contrast agents are under clinical translation, and many more are in preclinical development, providing evidence for a rapidly expanding field that is poised to significantly affect the surgical practice. There is an incredible diversity of FGS contrast agent molecular type, targeting mechanism, and fluorescence properties, owing to the efforts of a diverse field of physicists, chemists, clinicians, biologists, and engineers. Continued development towards clinical approval of these novel FGS contrast agents and FIH clinical studies of non-cancerous disease specific and anatomy specific FGS contrast agents will enable significant advancement in the field of surgery as a whole and ultimately improve patient outcomes across many surgical specialties.
Figure 4.

A pie chart highlighting the broad clinical indication of the novel FGS contrast agents currently undergoing clinical translation. The number of agents targeted for each indication is listed next to it.
Acknowledgements
This work was funded by the National Institute of Biomedical Imaging and Bioengineering (R01EB021362).
References
- 1.Sheridan RL, et al. Burn depth estimation by use of indocyanine green fluorescence: initial human trial. J Burn Care Rehabil 16, 602–604 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Hongo K, Kobayashi S, Okudera H, Hokama M & Nakagawa F Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurol Res 17, 89–93 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Scheider A [Indocyanine green angiography with an infrared scanning laser ophthalmoscope. Initial clinical experiences]. Ophthalmologe 89, 27–33 (1992). [PubMed] [Google Scholar]
- 4.Arens C, Malzahn K, Dias O, Andrea M & Glanz H [Endoscopic imaging techniques in the diagnosis of laryngeal carcinoma and its precursor lesions]. Laryngorhinootologie 78, 685–691 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Lee BT, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plastic and reconstructive surgery 126, 1472–1481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tummers QR, et al. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 40, 850–858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troyan SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Annals of surgical oncology 16, 2943–2952 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashitate Y, Stockdale A, Choi HS, Laurence RG & Frangioni JV Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy. The Journal of surgical research 176, 7–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbeek FP, et al. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: a first in human experience. The Journal of urology 190, 574–579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs-Strauss SL, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Molecular imaging 10, 91–101 (2011). [PMC free article] [PubMed] [Google Scholar]
- 11.Hirche C, et al. An experimental study to evaluate the Fluobeam 800 imaging system for fluorescence-guided lymphatic imaging and sentinel node biopsy. Surgical innovation 20, 516–523 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Gotoh K, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. Journal of surgical oncology 100, 75–79 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa H, et al. Visualization of the Stomach’s Arterial Networks During Esophageal Surgery Using the HyperEye Medical System. Anticancer research 35, 6201–6205 (2015). [PubMed] [Google Scholar]
- 14.Weissleder R & Pittet MJ Imaging in the era of molecular oncology. Nature 452, 580–589 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangioni JV New technologies for human cancer imaging. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 26, 4012–4021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitai T, Inomoto T, Miwa M & Shikayama T Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12, 211–215 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Peek MC, Charalampoudis P, Anninga B, Baker R & Douek M Blue dye for identification of sentinel nodes in breast cancer and malignant melanoma: a systematic review and meta-analysis. Future Oncol 13, 455–467 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Jeschke S, et al. Visualisation of the lymph node pathway in real time by laparoscopic radioisotope- and fluorescence-guided sentinel lymph node dissection in prostate cancer staging. Urology 80, 1080–1086 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Chang DW, Suami H & Skoracki R A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plastic and reconstructive surgery 132, 1305–1314 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, et al. Indocyanine green angiography for intra-operative assessment in vascular surgery. Eur J Vasc Endovasc Surg 43, 426–432 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Boni L, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29, 2046–2055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stummer W, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42, 518–525; discussion 525–516 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7, 392–401 (2006). [DOI] [PubMed] [Google Scholar]
- 24.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med 17, 1315–1319 (2011). [DOI] [PubMed] [Google Scholar]
- 25.van der Vorst JR, et al. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J Gastrointest Surg 4, 180–184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Taher M, van den Bos J, Schols RM, Bouvy ND & Stassen LP Fluorescence Ureteral Visualization in Human Laparoscopic Colorectal Surgery Using Methylene Blue. J Laparoendosc Adv Surg Tech A 26, 870–875 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Ankersmit M, et al. Fluorescent Imaging With Indocyanine Green During Laparoscopic Cholecystectomy in Patients at Increased Risk of Bile Duct Injury. Surgical innovation 24, 245–252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samkoe KS, et al. Application of Fluorescence-Guided Surgery to Subsurface Cancers Requiring Wide Local Excision: Literature Review and Novel Developments Toward Indirect Visualization. Cancer Control 25, 1073274817752332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadjipanayis CG, Widhalm G & Stummer W What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 77, 663–673 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal EL, et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J Nucl Med 57, 144–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogue BW, et al. Advancing Molecular-Guided Surgery through probe development and testing in a moderate cost evaluation pipeline. Proc SPIE Int Soc Opt Eng 9311(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schirmer RH, Adler H, Pickhardt M & Mandelkow E “Lest we forget you — methylene blue …”. Neurobiology of aging 32, 2325.e2327–2325.e2316 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Nagaya T, Nakamura YA, Choyke PL & Kobayashi H Fluorescence-Guided Surgery. Frontiers in Oncology 7(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore GE Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 106, 130–131 (1947). [DOI] [PubMed] [Google Scholar]
- 35.Alander JT, et al. A Review of Indocyanine Green Fluorescent Imaging in Surgery. International Journal of Biomedical Imaging 2012, 1–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamby P, et al. Evaluation of the vascular integrity of free flaps based on microcirculation imaging techniques. Clinical Hemorheology and Microcirculation 39, 253–263 (2008). [PubMed] [Google Scholar]
- 37.Newman M & Samson M The Application of Laser-Assisted Indocyanine Green Fluorescent Dye Angiography in Microsurgical Breast Reconstruction. Journal of Reconstructive Microsurgery 25, 021–026 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Cho SS, Salinas R & Lee JYK Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Frontiers in Surgery 6(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widhalm G, et al. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. Journal of Neurosurgery, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stepp H & Stummer W 5-ALA in the management of malignant glioma. Lasers in Surgery and Medicine 50, 399–419 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Walter S, et al. Intraoperative Detection of Malignant Gliomas by 5-Aminolevulinic Acid-induced Porphyrin Fluorescence. Neurosurgery 42, 518–526 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Staderini M, Megia-Fernandez A, Dhaliwal K & Bradley M Peptides for optical medical imaging and steps towards therapy. Bioorganic & medicinal chemistry 26, 2816–2826 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Mochida A, Ogata F, Nagaya T, Choyke PL & Kobayashi H Activatable fluorescent probes in fluorescence-guided surgery: Practical considerations. Bioorganic & medicinal chemistry 26, 925–930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farnam RW, Arms RG, Klaassen AH & Sorger JM Intraoperative ureter visualization using a near-infrared imaging agent. Journal of biomedical optics 24(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munhenzva IR, et al. Assessment of human pancreas cancer tissue and precursor lesions via a fluorophore with inherent PDAC selectivity. Methods 168, 35–39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh J-J, Rochelle Riley I & Dorshow RB Clinical analysis and quantitation of MB-102, a novel fluorescence tracer agent, in human plasma. Analytical Methods 10, 2376–2383 (2018). [Google Scholar]
- 47.Achilefu S, et al. Modeling of transdermal fluorescence measurements from first-in-human clinical trials for renal function determination using fluorescent tracer agent MB-102. in Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications IX (2017). [Google Scholar]
- 48.Gutowski M, et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surgical Oncology 26, 153–162 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Boogerd LSF, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. The Lancet Gastroenterology & Hepatology 3, 181–191 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Hoogstins CES, et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Annals of surgical oncology 25, 3350–3357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath CH, Deep NL, Sweeny L, Zinn KR & Rosenthal EL Use of Panitumumab-IRDye800 to Image Microscopic Head and Neck Cancer in an Orthotopic Surgical Model. Annals of surgical oncology 19, 3879–3887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korb ML, et al. Use of monoclonal antibody–IRDye800CW bioconjugates in the resection of breast cancer. Journal of Surgical Research 188, 119–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao RW, et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics 8, 2488–2495 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marston JC, et al. Panitumumab-IRDye800CW for Fluorescence-Guided Surgical Resection of Colorectal Cancer. Journal of Surgical Research 239, 44–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day KE, Sweeny L, Kulbersh B, Zinn KR & Rosenthal EL Preclinical Comparison of Near-Infrared-Labeled Cetuximab and Panitumumab for Optical Imaging of Head and Neck Squamous Cell Carcinoma. Molecular Imaging and Biology 15, 722–729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warram JM, et al. Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. British Journal of Neurosurgery 29, 850–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenthal EL, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clinical Cancer Research 21, 3658–3666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenthal EL, et al. Sensitivity and Specificity of Cetuximab-IRDye800CW to Identify Regional Metastatic Disease in Head and Neck Cancer. Clinical Cancer Research 23, 4744–4752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tummers WS, et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Annals of surgical oncology 25, 1880–1888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terwisscha van Scheltinga AGT, et al. Intraoperative Near-Infrared Fluorescence Tumor Imaging with Vascular Endothelial Growth Factor and Human Epidermal Growth Factor Receptor 2 Targeting Antibodies. Journal of Nuclear Medicine 52, 1778–1785 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Harlaar NJ, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. The Lancet Gastroenterology & Hepatology 1, 283–290 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Lamberts LE, et al. Tumor-Specific Uptake of Fluorescent Bevacizumab–IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clinical Cancer Research 23, 2730–2741 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Hekman MCH, et al. Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model. J Nucl Med 58, 706–710 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Hekman MC, et al. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 8, 2161–2170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samkoe KS, et al. Measuring microdose ABY-029 fluorescence signal in a primary human soft-tissue sarcoma resection. Proc SPIE Int Soc Opt Eng 10862(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samkoe KS, et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: a Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use. Mol Imaging Biol 19, 512–521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott JT, et al. Microdose fluorescence imaging of ABY-029 on an operating microscope adapted by custom illumination and imaging modules. Biomed Opt Express 7, 3280–3288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veiseh M, et al. Tumor Paint: A Chlorotoxin:Cy5.5 Bioconjugate for Intraoperative Visualization of Cancer Foci. Cancer Research 67, 6882–6888 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Butte PV, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurgical Focus 36(2014). [DOI] [PubMed] [Google Scholar]
- 70.Kittle DS, et al. Fluorescence-guided Tumor Visualization Using the Tumor Paint BLZ-100. Cureus (2014). [Google Scholar]
- 71.Fidel J, et al. Preclinical Validation of the Utility of BLZ-100 in Providing Fluorescence Contrast for Imaging Spontaneous Solid Tumors. Cancer Research 75, 4283–4291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baik FM, et al. Fluorescence Identification of Head and Neck Squamous Cell Carcinoma and High-Risk Oral Dysplasia With BLZ-100, a Chlorotoxin-Indocyanine Green Conjugate. JAMA Otolaryngology–Head & Neck Surgery 142(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dintzis SM, et al. Real-time Visualization of Breast Carcinoma in Pathology Specimens From Patients Receiving Fluorescent Tumor-Marking Agent Tozuleristide. Archives of Pathology & Laboratory Medicine 143, 1076–1083 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Patil CG, et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 85, E641–E649 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Miampamba M, et al. Sensitive in vivo Visualization of Breast Cancer Using Ratiometric Protease-activatable Fluorescent Imaging Agent, AVB-620. Theranostics 7, 3369–3386 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unkart JT, et al. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Annals of surgical oncology 24, 3167–3173 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Burggraaf J, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nature Medicine 21, 955–961 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Li Z, et al. In vivo fluorescence imaging of hepatocellular carcinoma xenograft using near-infrared labeled epidermal growth factor receptor (EGFR) peptide. Biomedical Optics Express 7(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Gao Z, Li G & Wang TD Dual-modal in vivo fluorescence and photoacoustic imaging using a heterodimeric peptide. Chemical Communications 54, 13196–13199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joshi BP, et al. Design and Synthesis of Near-Infrared Peptide for in Vivo Molecular Imaging of HER2. Bioconjugate Chemistry 27, 481–494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joshi BP, et al. Detection of Sessile Serrated Adenomas in the Proximal Colon Using Wide-Field Fluorescence Endoscopy. Gastroenterology 152, 1002–1013.e1009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones JE, Busi SB, Mitchem JB, Amos-Landgraf JM & Lewis MR Evaluation of a Tumor-Targeting, Near-Infrared Fluorescent Peptide for Early Detection and Endoscopic Resection of Polyps in a Rat Model of Colorectal Cancer. Molecular imaging 17(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Achilefu S, et al. Synthesis, In Vitro Receptor Binding, and In Vivo Evaluation of Fluorescein and Carbocyanine Peptide-Based Optical Contrast Agents. Journal of medicinal chemistry 45, 2003–2015 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Huang R, et al. Integrin v 3-Targeted IRDye 800CW Near-Infrared Imaging of Glioblastoma. Clinical Cancer Research 18, 5731–5740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verbeek FPR, et al. Near-Infrared Fluorescence Imaging of Both Colorectal Cancer and Ureters Using a Low-Dose Integrin Targeted Probe. Annals of surgical oncology 21, 528–537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng H, et al. Precise integrin-targeting near-infrared imaging-guided surgical method increases surgical qualification of peritoneal carcinomatosis from gastric cancer in mice. Oncotarget 8(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Handgraaf HJM, et al. Real-time near-infrared fluorescence imaging using cRGD-ZW800–1 for intraoperative visualization of multiple cancer types. Oncotarget 8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, et al. Near-Infrared Fluorescent Peptides with High Tumor Selectivity: Novel Probes for Image-Guided Surgical Resection of Orthotopic Glioma. Molecular pharmaceutics 16, 108–117 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Sun Y, et al. Novel dual-function near-infrared II fluorescence and PET probe for tumor delineation and image-guided surgery. Chem Sci 9, 2092–2097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi HS, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nature biotechnology 31, 148–153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng H, Wang H, Wang M, Li Z & Wu Z Synthesis and Evaluation of 64Cu-DOTA-NT-Cy5.5 as a Dual-Modality PET/Fluorescence Probe to Image Neurotensin Receptor-Positive Tumor. Molecular pharmaceutics 12, 3054–3061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Witjes M, et al. Fluorescence Guided Surgery Using the pH-Activated Micellar Tracer ONM-100: First-In-Human Proof of Principle in Head and Neck Squamous Cell Carcinoma. Journal of Oral and Maxillofacial Surgery 77(2019). [Google Scholar]
- 93.Wirth D, Smith TW, Moser R & Yaroslavsky AN Demeclocycline as a contrast agent for detecting brain neoplasms using confocal microscopy. Physics in Medicine and Biology 60, 3003–3011 (2015). [DOI] [PubMed] [Google Scholar]
- 94.van den Bos J, Al-Taher M, Bouvy ND & Stassen LPS Near-infrared fluorescence laparoscopy of the ureter with three preclinical dyes in a pig model. Surgical Endoscopy 33, 986–991 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitley MJ, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Science Translational Medicine 8, 320ra324–320ra324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith BL, et al. Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system. Breast Cancer Research and Treatment 171, 413–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuneo KC, et al. Imaging Primary Mouse Sarcomas After Radiation Therapy Using Cathepsin-Activatable Fluorescent Imaging Agents. International Journal of Radiation Oncology*Biology*Physics 86, 136–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Irwin CP, et al. PARPi-FL - a Fluorescent PARP1 Inhibitor for Glioblastoma Imaging. Neoplasia 16, 432–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kossatz S, et al. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Scientific Reports 6(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sobol RW, Kossatz S, Weber WA & Reiner T Optical Imaging of PARP1 in Response to Radiation in Oral Squamous Cell Carcinoma. PloS one 11(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barrott Jared J., et al. Optical and Radioiodinated Tethered Hsp90 Inhibitors Reveal Selective Internalization of Ectopic Hsp90 in Malignant Breast Tumor Cells. Chemistry & Biology 20, 1187–1197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crouch BT, et al. Exploiting heat shock protein expression to develop a non-invasive diagnostic tool for breast cancer. Scientific Reports 9(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nature Medicine 17, 1315–1319 (2011). [DOI] [PubMed] [Google Scholar]
- 104.Guzzo TJ, et al. Intraoperative Molecular Diagnostic Imaging Can Identify Renal Cell Carcinoma. Journal of Urology 195, 748–755 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Tummers QRJG, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget 7(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoogstins CES, et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clinical Cancer Research 22, 2929–2938 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Keating JJ, et al. Intraoperative near-infrared fluorescence imaging targeting folate receptors identifies lung cancer in a large-animal model. Cancer 123, 1051–1060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahalingam SM, et al. Evaluation of Novel Tumor-Targeted Near-Infrared Probe for Fluorescence-Guided Surgery of Cancer. Journal of medicinal chemistry 61, 9637–9646 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Predina JD, et al. An open label trial of folate receptor-targeted intraoperative molecular imaging to localize pulmonary squamous cell carcinomas. Oncotarget 9(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Randall LM, Wenham RM, Low PS, Dowdy SC & Tanyi JL A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer. Gynecologic Oncology 155, 63–68 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Thukkani AK & Jaffer FA Intravascular near-infrared fluorescence molecular imaging of atherosclerosis. Am J Nucl Med Mol Imaging 3, 217–231 (2013). [PMC free article] [PubMed] [Google Scholar]
- 112.Jaffer FA, et al. Two-Dimensional Intravascular Near-Infrared Fluorescence Molecular Imaging of Inflammation in Atherosclerosis and Stent-Induced Vascular Injury. Journal of the American College of Cardiology 57, 2516–2526 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamashita S & Sugiyama Y New strategy for drug development with exploratory IND studies: scientific basis and future directions. Advanced drug delivery reviews 63, 493 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Bouyer-Ferullo S Preventing perioperative peripheral nerve injuries. AORN J 97, 110–124 e119 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Antoniadis G, et al. Iatrogenic nerve injuries: prevalence, diagnosis and treatment. Dtsch Arztebl Int 111, 273–279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ficarra V, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 62, 405–417 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Ghavamian R, Knoll A, Boczko J & Melman A Comparison of operative and functional outcomes of laparoscopic radical prostatectomy and radical retropubic prostatectomy: single surgeon experience. Urology 67, 1241–1246 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Lopez A, et al. Intraoperative Optical Biopsy during Robotic Assisted Radical Prostatectomy Using Confocal Endomicroscopy. The Journal of urology 195, 1110–1117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boyette LB, et al. Fiberoptic imaging of cavernous nerves in vivo. The Journal of urology 178, 2694–2700 (2007). [DOI] [PubMed] [Google Scholar]
- 120.Rais-Bahrami S, et al. Optical coherence tomography of cavernous nerves: a step toward real-time intraoperative imaging during nerve-sparing radical prostatectomy. Urology 72, 198–204 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Aron M, et al. Second prize: preliminary experience with the Niris optical coherence tomography system during laparoscopic and robotic prostatectomy. J Endourol 21, 814–818 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Ukimura O, Magi-Galluzzi C & Gill IS Real-time transrectal ultrasound guidance during laparoscopic radical prostatectomy: impact on surgical margins. The Journal of urology 175, 1304–1310 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Ponnusamy K, Sorger JM & Mohr C Nerve mapping for prostatectomies: novel technologies under development. J Endourol 26, 769–777 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Wu C, et al. Molecular probes for imaging myelinated white matter in CNS. Journal of medicinal chemistry 51, 6682–6688 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang C, et al. In situ fluorescence imaging of myelination. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 58, 611–621 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gibbs SL, et al. Structure-activity relationship of nerve-highlighting fluorophores. PloS one 8, e73493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stankoff B, et al. Imaging of CNS myelin by positron-emission tomography. Proceedings of the National Academy of Sciences of the United States of America 103, 9304–9309 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cotero VE, et al. Intraoperative fluorescence imaging of peripheral and central nerves through a myelin-selective contrast agent. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging 14, 708–717 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cotero VE, et al. Improved Intraoperative Visualization of Nerves through a Myelin-Binding Fluorophore and Dual-Mode Laparoscopic Imaging. PloS one 10, e0130276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bajaj A, et al. Identification of the protein target of myelin-binding ligands by immunohistochemistry and biochemical analyses. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 61, 19–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibbs-Strauss SL, et al. Molecular imaging agents specific for the annulus fibrosus of the intervertebral disk. Molecular imaging 9, 128–140 (2010). [PubMed] [Google Scholar]
- 132.Meyers JR, et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. The Journal of neuroscience: the official journal of the Society for Neuroscience 23, 4054–4065 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, et al. Longitudinal near-infrared imaging of myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 2382–2390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park MH, et al. Prototype nerve-specific near-infrared fluorophores. Theranostics 4, 823–833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


