Abstract
Nerve damage plagues surgical outcomes and remains a major burden for patients, surgeons, and the healthcare system. Fluorescence image-guided surgery using nerve specific small molecule fluorophores offers a solution to diminish surgical nerve damage through improved intraoperative nerve identification and visualization. Oxazine 4 has shown superior nerve specificity in initial testing in vivo, while exhibiting a red shifted excitation and emission spectra compared to other nerve-specific fluorophores. However, Oxazine 4 does not exhibit near-infrared (NIR) excitation and emission, which would be ideal to improve penetration depth and nerve signal to background ratios for in vivo imaging. Successful development of a NIR nerve-specific fluorophore will require understanding of the molecular target of fluorophore nerve specificity. While previous small molecule nerve-specific fluorophores have demonstrated excellent ex vivo nerve specificity, Oxazine 4 ex vivo nerve specific fluorescence has been difficult to visualize. In the present study, we examined each step of the ex vivo fluorescence microscopy sample preparation procedure to discover how in vivo nerve-specific fluorescence is changed during ex vivo tissue sample preparation. Through step-by-step examination we found that Oxazine 4 fluorescence was significantly diminished by washing and mounting tissue sections for microscopy. A method to preserve Oxazine 4 nerve specific fluorescence ex vivo was determined, which can be utilized for visualization by fluorescence microscopy.
Keywords: nerve-specific fluorophore, fluorescence image-guided surgery, near-infrared (NIR), fluorophore development, ex vivo tissue analysis
1. INTRODUCTION
Nerve damage is a major morbidity associated with surgical outcomes, affecting up to 600,000 patients per year in the United States alone [1]. Surgery induced nerve damage affects quality of life and confers unnecessary costs to the healthcare system. Currently, there is no technology to aid in visualization of nerve tissue intraoperatively, with surgeons relying on visual inspection or palpation to identify important nerve structures intraoperatively. Fluorescence image-guided surgery offers a means of improving nerve identification in the surgical suite through the use of nerve specific small molecule fluorophores [2]. In preclinical studies, 6 classes of nerve specific small molecules have been shown to highlight nerve tissue following intravenous administration [3–14]. Of particular interest, an Oxazine dye, Oxazine 4, has demonstrated significant nerve specificity as well as red-shifted excitation and emission wavelengths [14]. Oxazine 4 is a promising candidate for further fluorophore development to synthesize a nerve-specific small molecule fluorophore with excitation and emission in the near-infrared (NIR) window (700–900 nm) due to the potential of the oxazine fluorophore scaffold to reach NIR wavelengths. NIR fluorescence is ideal for fluorescence image-guided surgery because endogenous tissue chromophore absorbance, scattering and fluorescence are all at local minima [15, 16]. Therefore fluorescent dyes in the NIR window enable visualization of their target tissues at greater depths due to increased light penetration as well as diminished autofluorescence.
Development of a NIR, nerve-specific fluorophore is particularly challenging because by definition nerve-specific fluorophores must be relatively small to cross the blood nerve barrier [17, 18], while NIR fluorophores must have a sufficient number of double bonds to reach these redder wavelengths, inherently increasing their molecular weight [6]. Therefore understanding the nerve-specific molecular target and mechanism of binding for current nerve-specific fluorophores such as Oxazine 4 is crucial to future development of NIR nerve-specific fluorophores. Initial testing of Oxazine 4 for nerve-specific fluorescence has demonstrated readily obtainable in vivo contrast following either systemic administration [14] or through the use of an optimized direct administration methodology (manuscript in review). However, interestingly, attempts to study Oxazine 4 nerve-specific fluorescence ex vivo have been fraught with difficulty, where the same ex vivo visualization has been successfully utilized with several other nerve specific small molecule fluorophores [5, 10, 14, 19] and with sections of resected tissue that had been stained in vivo. This lack of ex vivo fluorescent contrast makes studying the molecular target and nerve-specific mechanism of Oxazine 4 challenging. In the work presented herein, we report observations of Oxazine 4 nerve-specific fluorescence decrease ex vivo and quantify each step of the ex vivo fluorescence microscopy sample preparation procedure to determine the cause of the fluorescence loss in ex vivo tissue sections, with the overall goal of determining the best method for Oxazine 4 fluorescence preservation for molecular target and mechanism studies.
2. MATERIALS & METHODS
2.1. Contrast Agents
Oxazine 4 perchlorate was obtained from Fisher Scientific Inc. (Pittsburgh, PA). BMB was synthesized using previously published methods [5]. Three formulations were tested to solubilize the lipophilic Oxazine 4 and BMB fluorophores, detailed as follows. (1) A serum/buffer solution containing 75% serum and 25% phosphate buffered saline (PBS) was used, (2) a previously reported formulation using 5% Kolliphor EL in D5W [14] was tested as well as (3) a previously reported co-solvent formulation containing 10% dimethyl sulfoxide (DMSO), 5% Kolliphor EL, 65% serum, and 20% phosphate buffered saline (PBS) was used [6]. Oxazine 4 has better solubility that BMB, with a peak absorbance in PBS, pH 7.4 at 616 nm and a peak emission at 635 nm [14]. BMB could not be solubilized in PBS, and spectral properties were instead measured in FBS, where peak absorbance at pH 7.4 was 390 nm and peak emission was at 501 nm [6].
2.2. Animals
Approval for the use of all animals in this study was obtained from the Institutional Animal Care and Use Committee (IACUC) at Oregon Health and Science University (OHSU). Male CD-1 mice weighing 22–24g were purchased from Charles River Laboratories (Wilmington, MA). Prior to surgery, mice were anaesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine (Patterson Veterinary, Devens, MA). The brachial plexus and sciatic nerves were surgically exposed by removal of overlaying adipose and muscle tissues for direct nerve staining and imaging. All surgeries were terminal, and exposed nerve and surrounding muscle and adipose tissue were resected either post staining or from unstained nerve sites following euthanasia for ex vivo staining studies.
2.3. Fluorescence Imaging Systems
A custom-built small animal imaging system capable of real-time color and fluorescence imaging was used to acquire murine in vivo images. The imaging system consisted of a QImaging EXi Blue monochrome camera (Surrey, British Columbia, CA) for fluorescence detection with a removable Bayer filter for collecting co-registered color and fluorescence images. A PhotoFluor II light source (89 North, Burlington, VT) was focused onto the surgical field through a liquid light guide and used unfiltered for white light illumination. For Oxazine 4 fluorescence excitation, the PhotoFluor II was filtered with a 620 ± 30 nm bandpass excitation filter. Resulting fluorescence was collected with a 700 ± 37.5 nm bandpass emission filter for image collection. Camera exposure times ranged from 2.5 – 50 ms for fluorescence image collection.
Ex vivo fluorescence microscopy images were acquired on an Axio Observer inverted fluorescence microscope (Zeiss, Thornwood, NY). A Photofluor II light source was used filtered with a 620 ± 30 nm or 405 ± 10 nm bandpass excitation filter for Oxazine 4 or BMB excitation, respectively. Fluorescence images were collected using an Axiocam 506 camera (Zeiss) where a 700 ± 37.5 nm or 550 ± 12.5 nm bandpass emission filter was used for image collection of Oxazine 4 or BMB fluorescence, respectively. All filters were obtained from Chroma Technology (Bellows Falls, VT). Images were captured with camera exposure times ranging from 1 – 10 sec and at magnifications of 10x or 40x. All images collected for comparison between treatment groups were acquired with the same exposure time and are displayed under equal normalized brightness and contrast levels where indicated.
2.4. Ex Vivo Nerve Specific Fluorescence Staining
Sciatic and brachial plexus nerves with surrounding muscle and adipose tissue were harvested from unstained nerve sites, fixed with 2% paraformaldehyde (PFA) for 12 h, snap frozen in optimal cutting temperature (OCT) compound with liquid nitrogen, and stored at −80 °C for ex vivo studies. Cryosections were cut at 10 μm onto Superfrost Plus slides (Fisherbrand, Fisher Scientific). Ex vivo nerve specific staining was completed as previously reported [5, 10, 14, 19]. Briefly, the tissue sections were washed once with PBS for 2 min, fixed with 2% PFA for 15 min, and then washed with PBS three times for 5 min, The tissue sections were then incubated with Oxazine 4 in the D5W formulation, co-solvent formulation, or serum/buffer solution at 100 μM fluorophore concentration for 20 min at room temperature or with BMB in the co-solvent formulation or serum/buffer solution at 100 μM fluorophore concentration for 20 min at room temperature. Control slides were incubated with blank versions of the formulations. Then, a blank version of the formulation not containing any fluorophore was used to wash the sections following fluorophore incubation twice for 5 min. Two additional washes were performed with PBS for 5 min. All stained slides were mounted with Fluoromount-G and imaged with the microscope as described above.
2.5. In Vivo Murine Nerve Staining
In vivo nerve staining with Oxazine 4 was completed as previously reported via systemic administration [14] or using an optimized direct administration methodology (manuscript in review). For systemic administration, 200 nmol (79.2 μg) of Oxazine 4 in the co-solvent formulation was administered intravenously via tail vein injection. Injections were performed 4 hours prior to imaging, providing the highest nerve to background tissue fluorescence [14]. For direct administration, 4.95 μg Oxazine 4 in the co-solvent formulation was incubated for 5 min on the nerve site, submerging all nerve and surrounding muscle and adipose tissue in the fluorophore solution. Following the removal of the fluorophore solution, 3 flush steps were performed with 3 flushes of PBS per step to remove non-specific fluorescence. Blank co-solvent formulation was incubated for 5 min on the nerve site. Following the blank co-solvent incubation, 3 additional flush steps were performed with 3 flushes of PBS per step. Color and fluorescence images were acquired upon completion of the direct administration staining procedure using the small animal imaging system.
2.6. Ex Vivo Fluorescence Microscopy of Resected Nerve Tissue
Following completion of in vivo staining experiments, the sciatic nerve and brachial plexus nerves as well as the surrounding muscle and adipose tissues were harvested, fixed with 2% PFA for 12 h, snap frozen in OCT with liquid nitrogen, and cryosectioned into 10 μm sections onto Superfrost Plus slides. For initial microscopy testing, sections from tissue stained via direct administration were washed with PBS for 5 min to remove OCT and then mounted with Fluoromount – G prior to microscopy. For slide washing studies, sections from tissue stained via direct administration were imaged prior to, during, and following washing for 5 min with PBS (n = 2 sections). For slide mounting studies, sections from tissue stained via systemic administration were imaged prior to and following mounting with either Glycerol or Fluoromount – G without washing (n = 2 sections per group).
2.7. Quantification and Statistical Analysis
Region of interest analysis was performed on ex vivo fluorescence microscopy images to determine the fluorescence intensity from nerve, muscle, and adipose tissue. The nerve to muscle (N/M) and nerve to adipose (N/A) ratios were calculated from the intensity measurements for each tissue type. Mean nerve to background tissue ratios (N/M, N/A) were calculated for each group and significant differences between each group were evaluated using an unpaired two-tailed Student’s t test with equal variance. The α value was 0.05 for all analyses. Results were presented as mean ± standard deviation (S.D.). All statistical analysis was performed with GraphPad Prism (La Jolla, CA).
3. RESULTS & DISCUSSION
3.1. Ex vivo Oxazine 4 nerve tissue specificity
Mouse brachial plexus and sciatic nerve tissue sections were stained ex vivo with Oxazine 4 and BMB in several formulations. When compared to control tissue sections, the Oxazine 4 stained sections in the three formulations showed similar nerve to muscle fluorescence (Fig 1A and 1B). The only exception was an increase in overall non-specific Oxazine 4 fluorescence in the D5W stained section (Fig 1B). Therefore, Oxazine 4 demonstrated little to no nerve specificity following ex vivo tissue section staining in any of the three tested formulations. In contrast, BMB, which has shown extensive nerve specificity using both ex vivo and in vivo staining methods in previous studies [5, 6, 10, 19], demonstrated positive nerve specificity in both tested formulations (Fig 1C). Importantly, Oxazine 4 in the D5W formulation has been used previously to stain nerve tissue sections ex vivo with reported positive nerve signal imaged by fluorescence microscopy [14]. Although this study replicates the experimental conditions of the previous study including tissue section thickness, tissue fixation method, incubated fluorophore concentration, and reported staining protocol, equivalent nerve to background tissue fluorescence was not obtained.
Figure 1.
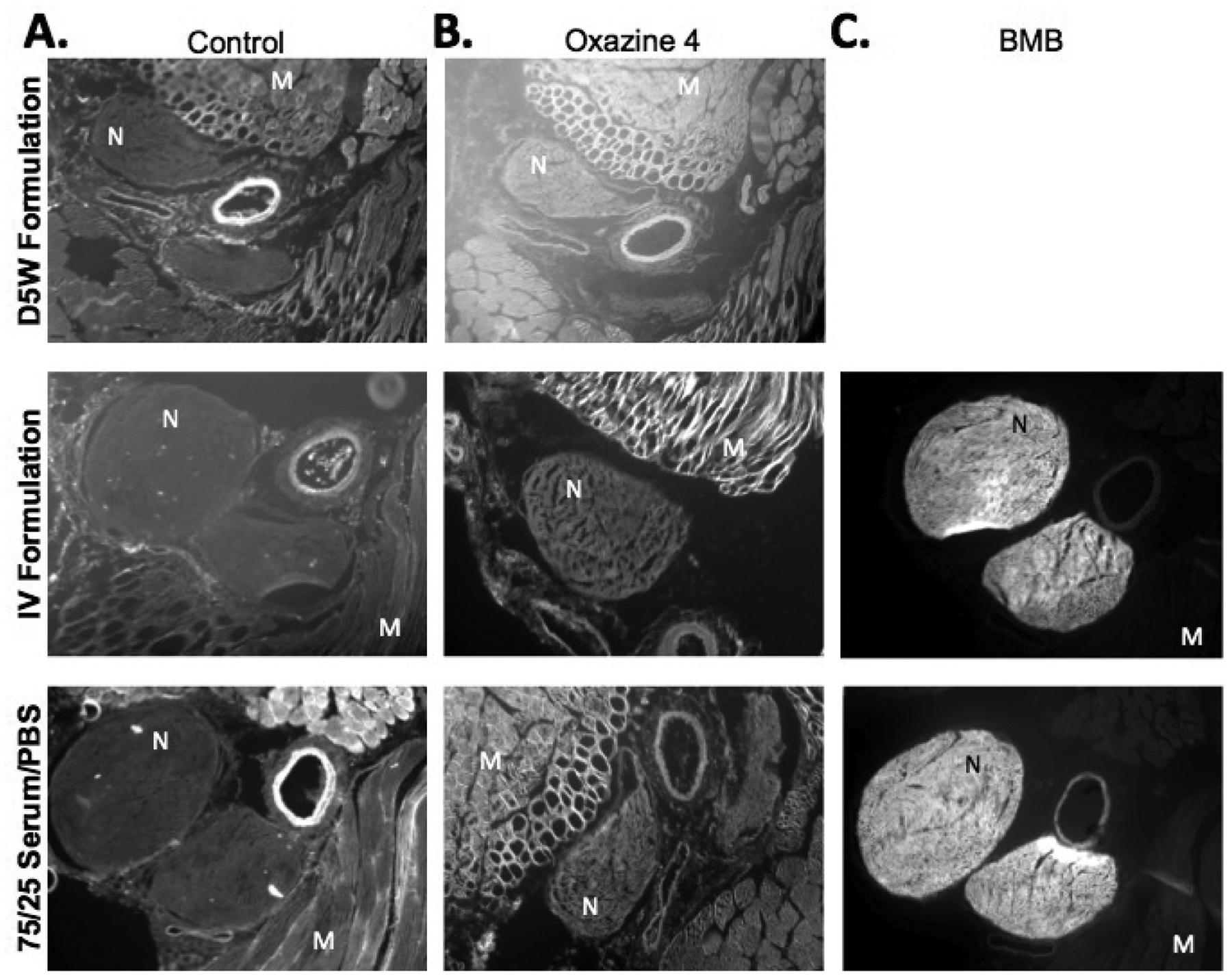
Microscopy images of tissue sections stained ex vivo with either (A) Blank formulations, (B) Oxazine 4 in D5W formulation, co-solvent IV formulation, and serum/buffer solution or (C) BMB in co-solvent IV formulation and serum/buffer solution. N = nerve tissue, M = muscle tissue.
3.2. Resected tissue nerve specific fluorescence preservation
In an attempt to visualize nerve-specific fluorescence ex vivo, nerve tissue stained in vivo with Oxazine 4 using the optimized direct administration methodology was resected, fixed, frozen, and sectioned for microscopy, where imaging data was collected at each step to follow the fluorescence signal (Fig 2). Nerve specific fluorescence was observed following direct administration of Oxazine 4 in vivo, which was retained upon resection, fixation, and freezing. However, preparation of the tissue for microscopy via sectioning and mounting onto glass slides resulted in loss of nerve specific fluorescence. This was confirmed by comparison of the Oxazine 4 stained tissue to control unstained tissue, which resulted in similar observed nerve to muscle fluorescence.
Figure 2.
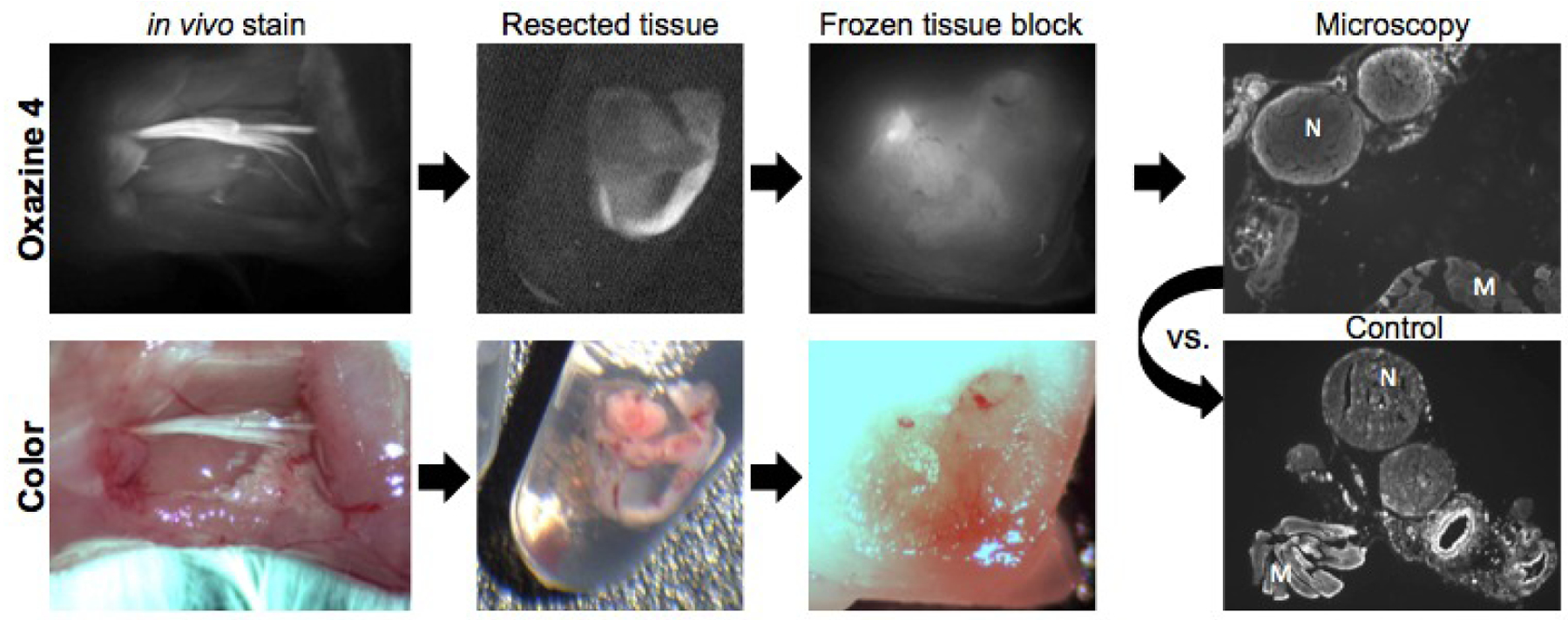
Schematic and images of resected tissue processed for fluorescence microscopy. In vivo images were collected upon completion of the optimized direct administration nerve staining method using Oxazine 4 on the mouse sciatic nerve. Resected tissue and frozen tissue block images were collected with the small animal imaging system. N = nerve, M = muscle.
3.3. Effect of tissue section washing and mounting on fluorescence in resected tissues
To determine the cause of the observed loss of fluorescence signal upon sample preparation for microscopy, tissue fluorescence was imaged before and after each step of the slide mounting process. Images and video were collected during the PBS wash step used to remove residual OCT (Fig 3). The mean nerve, muscle, and adipose tissue signal intensities were determined using region of interest (ROI) analysis and the corresponding nerve to background tissue ratios were calculated. Initially, nerve specific fluorescence was maintained in the unwashed and unmounted tissue sections (Fig 3A), however immediately upon applying the PBS wash a 20x decrease in nerve fluorescence was observed (Fig 3B). This fluorescence decrease was proportionally higher than the fluorescence decrease in muscle and adipose tissue fluorescence (Fig 3B). This caused a decrease in the N/M and N/A tissue ratios, yielding post wash images that were not representative of the original in vivo nerve specific fluorescence (Fig 3C). Post wash nerve fluorescence intensity and nerve to background tissue ratios were similar to control, unstained tissue section values, making it difficult to determine the nerve specificity of the fluorophore or the efficacy of the in vivo staining procedure.
Figure 3.
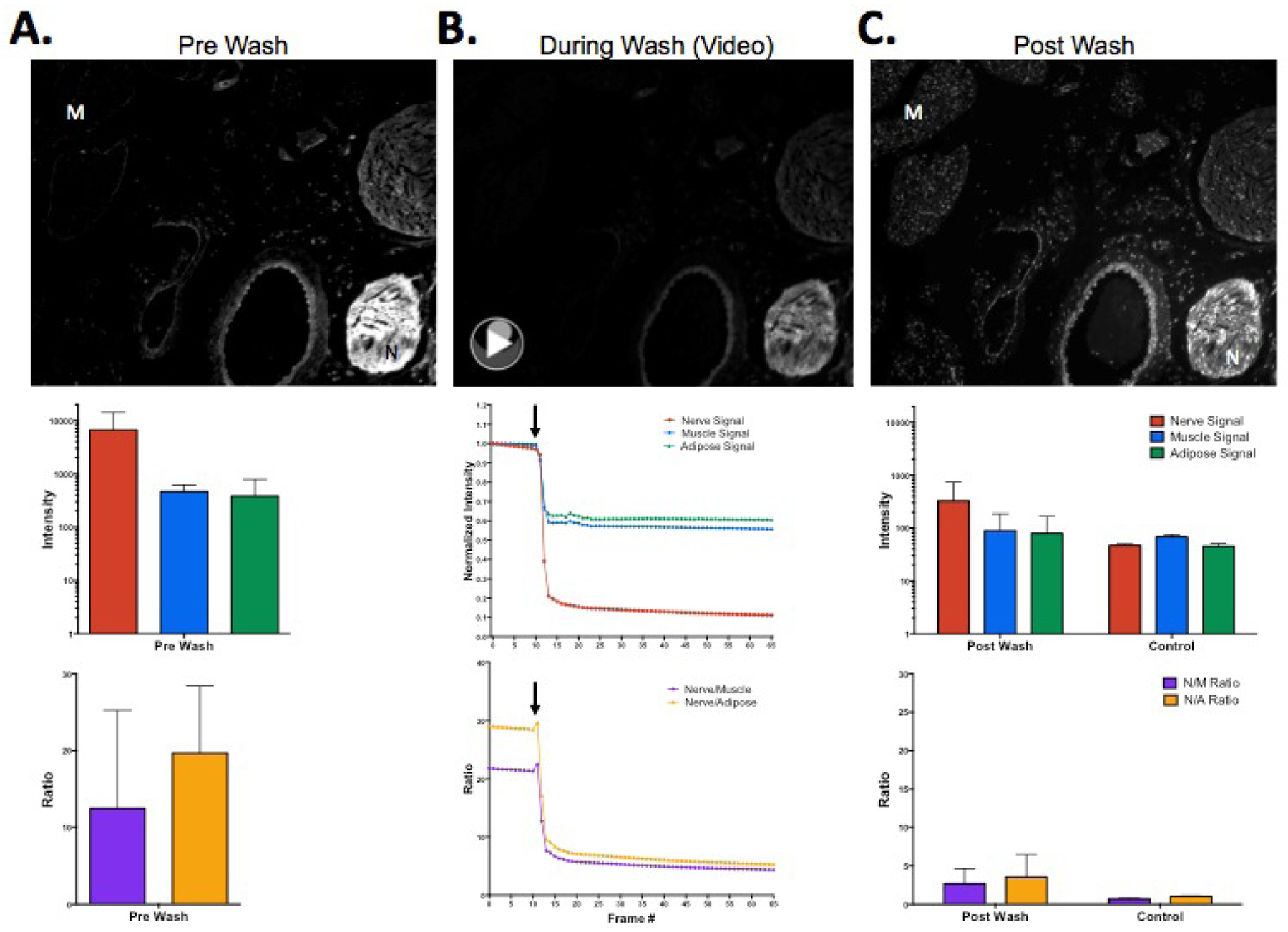
Representative fluorescence microscopy images and video collected (A) prior to washing, (B) during washing, and (C) post washing of tissue stained with Oxazine 4 via direct administration. The corresponding fluorescence intensity values and nerve to background tissue ratios were determined using ROI analysis. Black arrows indicate the time point at which the wash solution was applied. N = nerve, M = muscle, N/M = nerve to muscle ratio, N/A = nerve to adipose ratio.http://dx.doi.org/10.1117/12.2214204.1
The effect of mounting unwashed tissue sections collected from tissue that had been stained in vivo via systemic administration with Glycerol or Fluoromount-G was also tested. Mounting slides with glycerol maintained the fluorescence as it was observed prior to mounting (Fig 4A) where the N/M ratios remained relatively unchanged (Fig 4B). By comparison, mounting slides with Fluoromount-G caused a loss in overall fluorescence intensity as well as significantly diminished nerve specific fluorescence (Fig 4A), returning the nerve to muscle ratios to around 1, similar to control unstained tissue (Fig 4B). We hypothesized that the aqueous nature of Fluoromount-G washed Oxazine 4 from the tissue in a similar way that PBS removed Oxazine 4 fluorescence, diluting the fluorophore into the mounting media. This hypothesis was supported by the significant non-specific fluorescence observed in the non-normalized Fluoromount-G image (Fig 4A). The post mount N/M ratios were significantly higher for slides mounted with Glycerol compared to the Fluoromount-G (p = 0.0004) (Fig 4B) and the nerve tissue staining pattern of Oxazine 4 remained visible in higher magnification images of the tissue (40x) (Fig 4A).
Figure 4.
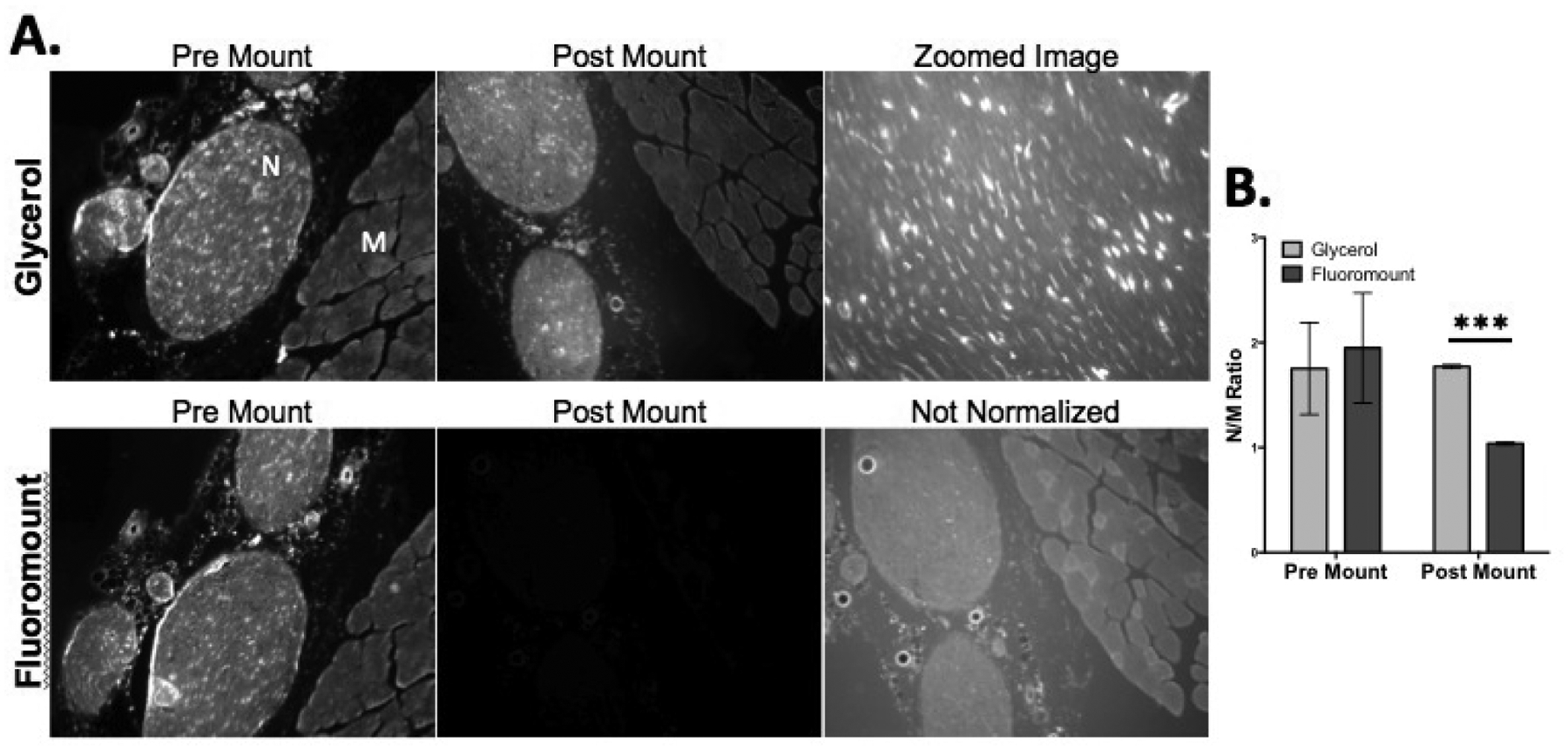
(A) Representative fluorescence microscopy images of resected tissue stained in vivo via systemic administration collected prior to mounting with either glycerol or Fluoromount-G. (B) The corresponding N/M ratios were calculated from intensity values determined using ROI analysis. Zoomed image indicated 40x magnification. Pre mount and post mount images were displayed with equal normalized brightness and contrast between the first two image columns. N = nerve, M = muscle. *** = P value < 0.001.
Overall we determined that to maintain the nerve specific fluorescence obtained from in vivo nerve staining the resected tissues can be sectioned for microscopy, but must remain unwashed and can be mounted with glycerol for imaging. Performing any washing on the tissue caused Oxazine 4 to be removed from the nerve tissue and mounting with Fluoromount-G caused specifically bound Oxazine 4 to be leeched from the tissue and diluted in the mounting media. This fluorescence loss occurred even after fixing the stained tissue in 2% PFA, which we hypothesized would fix Oxazine 4 specifically associated with proteins in place via crosslinking the fluorophore with the protein at the free amine present in the Oxazine 4 chemical structure [14]. Of note the fluorescence was only washed out if the tissue had been sectioned into thin sections for microscopy. These results explain the inability of Oxazine 4 to stain nerve tissue sections ex vivo and provide evidence that Oxazine 4 preferentially accumulates in nerve tissue via a different mechanism than other nerve specific fluorophores that remained bound to nerve tissue sections ex vivo [5, 10, 14]. Specifically, the results suggest that Oxazine 4 does not specifically accumulated in nerve tissue in vivo upon systemic or direct administration by means of a specific molecular interaction or protein binding reaction, but rather by a mechanism that requires the entire tissue structure to remain intact for specificity.
4. CONCLUSION
The findings of this study outline a method for visualizing nerve specific fluorescence provided by Oxazine 4 via in vivo administration using ex vivo fluorescence microscopy. In addition, the findings provide an explanation for the inability to stain nerve tissue sections ex vivo using Oxazine 4 as well as provide useful mechanistic information toward understanding the nerve-specificity of Oxazine 4. Through further analysis of the interaction between Oxazine 4 and nerve tissue, an improved understanding of the nerve-specific mechanism will be obtained and used to direct NIR nerve-specific fluorophore development.
ACKNOWLEDGEMENTS
We would like to thank Meaghan McCoy for experimental assistance. This study was supported by grants from the Oregon Clinical and Translational Research Institute and the National Institute of Biomedical Imaging and Bioengineering (#K01-EB-010201, SLG).
REFERENCES
- [1].Burke S, and George D Shorten, “When pain after surgery doesn’t go away…,” Biochemical Society Transactions, 37(1), 318–322 (2009). [DOI] [PubMed] [Google Scholar]
- [2].Gibbs SL, “Near infrared fluorescence for image-guided surgery,” Quant Imaging Med Surg, 2(3), 177–87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu C, Wei J, Tian D et al. , “Molecular probes for imaging myelinated white matter in CNS,” J Med Chem, 51(21), 6682–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang C, Popescu DC, Wu C et al. , “In situ fluorescence imaging of myelination,” J Histochem Cytochem, 58(7), 611–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gibbs SL, Xie Y, Goodwill HL et al. , “Structure-activity relationship of nerve-highlighting fluorophores,” PLoS One, 8(9), e73493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gibbs-Strauss SL, Nasr KA, Fish KM et al. , “Nerve-highlighting fluorescent contrast agents for image-guided surgery,” Mol Imaging, 10(2), 91–101 (2011). [PMC free article] [PubMed] [Google Scholar]
- [7].Stankoff B, Wang Y, Bottlaender M et al. , “Imaging of CNS myelin by positron-emission tomography,” Proc Natl Acad Sci U S A, 103(24), 9304–9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cotero VE, Siclovan T, Zhang R et al. , “Intraoperative fluorescence imaging of peripheral and central nerves through a myelin-selective contrast agent,” Mol Imaging Biol, 14(6), 708–17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cotero VE, Kimm SY, Siclovan TM et al. , “Improved Intraoperative Visualization of Nerves through a Myelin-Binding Fluorophore and Dual-Mode Laparoscopic Imaging,” PLoS One, 10(6), e0130276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bajaj A, LaPlante NE, Cotero VE et al. , “Identification of the protein target of myelin-binding ligands by immunohistochemistry and biochemical analyses,” J Histochem Cytochem, 61(1), 19–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gibbs-Strauss SL, Vooght C, Fish KM et al. , “Molecular imaging agents specific for the annulus fibrosus of the intervertebral disk,” Mol Imaging, 9(3), 128–40 (2010). [PubMed] [Google Scholar]
- [12].Meyers JR, MacDonald RB, Duggan A et al. , “Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels,” J Neurosci, 23(10), 4054–65 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang C, Wu C, Popescu DC et al. , “Longitudinal near-infrared imaging of myelination,” J Neurosci, 31(7), 2382–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park MH, Hyun H, Ashitate Y et al. , “Prototype nerve-specific near-infrared fluorophores,” Theranostics, 4(8), 823–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chance B, “Near-infrared images using continuous, phase-modulated, and pulsed light with quantitation of blood and blood oxygenation,” Ann N Y Acad Sci, 838, 29–45 (1998). [DOI] [PubMed] [Google Scholar]
- [16].Vahrmeijer AL, Hutteman M, van der Vorst JR et al. , “Image-guided cancer surgery using near-infrared fluorescence,” Nat Rev Clin Oncol, 10(9), 507–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fagerholm U, “The highly permeable blood-brain barrier: an evaluation of current opinions about brain uptake capacity,” Drug Discov Today, 12(23–24), 1076–82 (2007). [DOI] [PubMed] [Google Scholar]
- [18].Waterhouse RN, “Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents,” Mol Imaging Biol, 5(6), 376–89 (2003). [DOI] [PubMed] [Google Scholar]
- [19].Hackman KM, Doddapaneni BS, Barth CW et al. , “Polymeric Micelles as Carriers for Nerve-Highlighting Fluorescent Probe Delivery,” Mol Pharm, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


