Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among reproductive age women. Although its cardinal manifestations include hyperandrogenism, oligo/anovulation, and/or polycystic ovarian morphology, PCOS women often display also notable metabolic comorbidities. An array of pathogenic mechanisms have been implicated in the etiology of this heterogeneous endocrine disorder; hyperandrogenism at various developmental periods is proposed as a major driver of the metabolic and reproductive perturbations associated with PCOS. However, the current understanding of the pathophysiology of PCOS-associated metabolic disease is incomplete, and therapeutic strategies used to manage this syndrome's metabolic complications remain limited.
Scope of review
This study is a systematic review of the potential etiopathogenic mechanisms of metabolic dysfunction frequently associated with PCOS, with special emphasis on the metabolic impact of androgen excess on different metabolic tissues and the brain. We also briefly summarize the therapeutic approaches currently available to manage metabolic perturbations linked to PCOS, highlighting current weaknesses and future directions.
Major conclusions
Androgen excess plays a prominent role in the development of metabolic disturbances associated with PCOS, with a discernible impact on key peripheral metabolic tissues, including the adipose, liver, pancreas, and muscle, and very prominently the brain, contributing to the constellation of metabolic complications of PCOS, from obesity to insulin resistance. However, the current understanding of the pathogenic roles of hyperandrogenism in metabolic dysfunction of PCOS and the underlying mechanisms remain largely incomplete. In addition, the development of more efficient, even personalized therapeutic strategies for the metabolic management of PCOS patients persists as an unmet need that will certainly benefit from a better comprehension of the molecular basis of this heterogeneous syndrome.
Keywords: PCOS, Androgen excess, Insulin resistance, Obesity, Poly-agonists, GLP-1
1. Introduction. PCOS: More than a reproductive condition
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder among premenopausal women. Globally, the incidence of this syndrome varies, ranging from 6% to 20% [1] depending on the diagnostic criteria applied, with higher prevalence in overweight or obese women and in specific ethnic groups [2,3]. PCOS is a complex and heterogeneous endocrinopathy characterized by a constellation of symptoms and clinical features, including hyperandrogenism (clinical or biochemical), ovarian dysfunction (menstrual irregularities), and polycystic ovarian morphology. Currently, there are several diagnostic criteria for PCOS that utilize different combinations of these clinical traits. According to the Rotterdam criteria, the most widely used for the clinical diagnosis of PCOS is defined by at least two of the three aforementioned clinical features [4]. PCOS is considered the leading cause of anovulatory infertility [5] and is therefore clinically associated with subfertility or infertility. However, the deleterious impact of this pathology is not confined to reproductive function, and metabolic function is also frequently compromised.
PCOS is closely linked to metabolic disorders such as obesity and insulin resistance (IR) [6]. A large proportion of women with PCOS are obese or overweight [7] and exhibit IR with associated compensatory hyperinsulinemia [8,9]. Of note, IR and hyperinsulinemia are metabolic traits that are also present in most lean women with PCOS. Hyperinsulinemia plays a prominent role in the development of some phenotypic features of PCOS and, together with β cell dysfunction, increases the risk of developing other metabolic abnormalities such as type 2 diabetes (T2D), hypertension, dyslipidemia, and cardiovascular diseases [10]. Importantly, the prevalence of these metabolic comorbidities is high in women with this disorder [11,12], and the concurrence of overweight or obesity and PCOS exacerbates not only metabolic complications [6,7], but also reproductive derangements associated with this endocrinopathy [13,14].
The pathophysiological mechanisms of PCOS are complex and not fully understood [10,15,16]. Several lines of evidence suggest that developmental, environmental, genetic, and epigenetic mechanisms are involved in the etiology of this endocrine disorder [17,18]. Although many aspects of its pathophysiology remain obscure, it is widely accepted that hyperandrogenism plays a fundamental role in the development of most of the reproductive and metabolic perturbations associated with PCOS. Androgen excess has a deleterious impact on metabolic homeostasis in women with PCOS, acting on different metabolic tissues such as the adipose tissue, liver, muscle, and pancreas as well as on the brain. However, a better understanding of the molecular mechanisms underlying the metabolic actions of androgens in PCOS is needed.
This study reviews the current literature to provide an overview of the main pathogenic mechanisms that may underlie metabolic dysregulation commonly linked to PCOS, with particular attention to the potential molecular mechanisms responsible for the metabolic impact of androgen excess in selective tissues. Special emphasis was placed on discussing findings from studies of various female rodent models of hyperandrogenism generated by exposures to different types of androgens at various doses and administration windows, which resulted in a multiplicity of PCOS-like symptoms resembling the heterogenous clinical presentation of the syndrome. In addition, data from other species (for example, sheep and non-human primates) have been also included when relevant. Finally, a brief recap is provided on the current therapeutic strategies for managing the metabolic complications of PCOS, which are urgently necessary for more effective treatment options, in particular subsets of PCOS patients.
2. Pathogenesis of PCOS: Putative mechanisms
Given its heterogeneous clinical presentation, different and eventually non-exclusive mechanisms are involved in the pathogenesis of PCOS and, particularly, in the generation of its common metabolic complications. These are depicted in Figure 1 and itemized below.
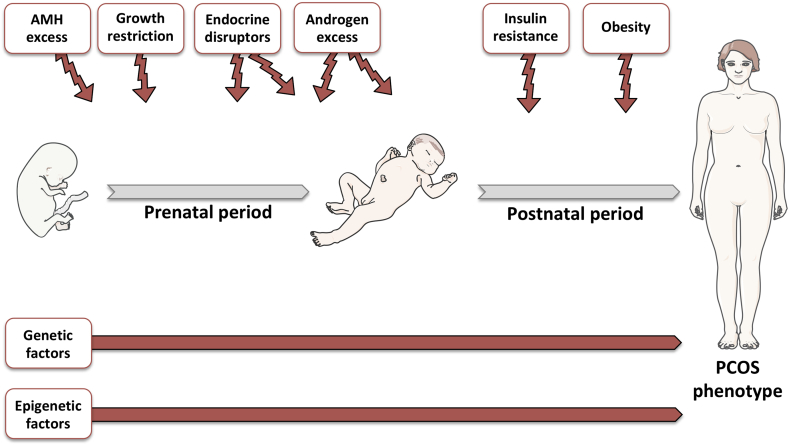
Figure 1.
Potential pathogenic factors of PCOS. During gestation, multiple factors including increased AMH levels, growth restriction, endocrine disruptors such as BPA, and androgen excess may predispose to the development of a PCOS-like phenotype in adulthood. During the postnatal period, exposure to endocrine disruptors and androgen excess and the development of obesity and insulin resistance are considered pathogenic factors that may also cause PCOS. Genetic and epigenetic factors may also increase the risk of developing PCOS. The figure was designed using tools provided by Servier Medical Art (https://smart.servier.com).
2.1. Androgen excess
Hyperandrogenemia is considered the main clinical hallmark of PCOS [14]. It is estimated that more than 80% of women who exhibit signs or symptoms of hyperandrogenism, including hirsutism, acne or alopecia, have PCOS [15]. Androgens are produced in the ovary and adrenal gland from a common precursor, cholesterol [19]. After a series of enzymatic reactions, cholesterol is converted into dehydroepiandrosterone (DHEA) and androstenedione. In the ovary, these reactions take place in the theca cells, whereas in the adrenal gland they occur in the adrenal cortex [20]. Cytochrome P450c17 is the rate-limiting enzyme for sex steroid synthesis in theca cells and the adrenal cortex. The activity of this enzyme is dose-dependently regulated by luteinizing hormone (LH) in the ovary and ACTH in the adrenal cortex. Cytochrome P450c17 possesses dual enzymatic activity, 17-hydroxylase and 17,20-lyase, essential for the conversion of pregnenolone into DHEA. The activity of P450c17 is allosterically modulated by the accessory protein cytochrome b5 (CytB5), which promotes 17,20-lyase activity. CytB5 is expressed in androgen-producing tissues such as the ovary, testis, and adrenal cortex. Of note, in the adrenal cortex, CytB5 is predominantly expressed in the zona reticularis, whereas P450c17 is expressed in both the zona reticularis and fasciculata [21]. This is why the adrenal conversion of pregnenolone into DHEA occurs in the zona reticularis. Subsequently, DHEA is converted into androstenedione by the action of the enzyme 3β-hydroxysteroid dehydrogenase type 2 (3β-HSD2) [20].
Androstenedione is the main precursor for the synthesis of testosterone and estrogen in both the ovaries and adrenal cortex. In the ovary, the conversion of androstenedione to testosterone occurs in the theca cells, and then testosterone is converted into estrogen in granulosa cells by cytochrome p450 aromatase [20]. This enzyme is predominantly expressed in granulosa cells and also catalyzes the direct conversion of androstenedione into estrone, which is metabolized into estradiol by 17 β-hydroxysteroid dehydrogenase type 1 (17βHSD1) [22]. Of note, transcription of the CYP19A1 gene, which encodes cytochrome p450 aromatase, is regulated by follicle-stimulating hormone (FSH) [23]. In the ovary, androgen production is regulated by LH in the theca cells, whereas estrogen synthesis from the androgens is regulated by FSH in granulosa cells [20]. The biosynthesis of both sex steroids is also modulated by intra- and extra-ovarian factors. Androgens and estradiol inhibit their own production via a paracrine negative feedback loop modulating the activity of cytochrome P450c17 in theca cells. Insulin and insulin-like growth factor-1 (IGF-1) are the major extra-ovarian factors that modulate sex steroid synthesis, acting as stimulating factors of androgen production by increasing the 17-hydroxylase and 17,20-lyase activity of P450c17 [22].
The most common biochemical perturbation in patients with PCOS is the elevation of circulating testosterone and androstenedione levels [24]. Compelling evidence suggests that the major source of androgens in women with PCOS is the ovary [25,26], although the adrenal gland might also contribute to androgen overproduction in a minority of patients [27,28]. Several studies have reported that an intrinsic abnormality in the steroidogenic machinery of the ovarian theca cells may be responsible for the increased androgen biosynthesis frequently associated with PCOS [19,29,30]. Furthermore, in vivo studies showed that the steroidogenic response to exogenous administration of LH is higher in women with PCOS either in basal conditions or after suppression of endogenous LH levels by a GnRH antagonist [29]. In line with these findings, in vitro studies have also demonstrated a steroidogenic hyperfunction in theca cells isolated from women with PCOS. These results demonstrated that theca cells from women with PCOS release more androgens than those of healthy women as a result of an upregulated expression of cytochrome P450c17, P450scc (a key rate-limiting enzyme in steroid production), 3β-HSD2, and 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5), also referred to as aldo-keto reductase type 1C3 (AKR1C3) [19,30].
In addition to this gonadal abnormality, it has been reported that neuroendocrine dysregulation may also contribute to enhanced androgen production in the ovary and participate in the pathogenesis of this disorder. PCOS patients normally exhibit elevated LH relative to FSH levels as a consequence of increased LH pulse frequency and amplitude [31]. This alteration in the LH pulse has been primarily attributed to a defect in the regulatory mechanisms of LH release by sex steroids. Thus, it has been reported that hyperandrogenism enhances hypothalamic gonadotropin releasing-hormone (GnRH) pulse frequency through the inhibition of sex steroid negative feedback on LH secretion [32], which ultimately leads to increased LH and androgen levels. In addition, other factors such as anti-Müllerian hormone (AMH) and insulin may contribute to neuroendocrine dysregulation. AMH levels are commonly elevated in women with PCOS as a result of the excessive accumulation of small antral follicles in their ovaries [33]. Interestingly, it has been demonstrated that GnRH neurons express AMH type II receptor, and this hormone stimulates GnRH neuron activity [34]. Hence, the rise in AMH levels in PCOS might promote GnRH release from the hypothalamus and contribute to hyperandrogenism. Insulin is another factor that may increase the frequency and amplitude of GnRH and LH pulse secretion by the upregulation of GnRH gene expression in hypothalamic GnRH neurons, an effect mediated via activation of the MAPK pathway [35]. Given the dominant role of hyperandrogenemia, its impact on the multiple metabolic derangements seen in PCOS patients is discussed in detail in Section 3.
2.2. Insulin resistance/hyperinsulinemia
IR is the most prevalent metabolic perturbation in women with PCOS, affecting 65–70% of all patients [9]. IR is defined as an impairment in the ability of insulin to mediate its metabolic actions. As a consequence of the perturbed insulin action, a higher amount of insulin is required to attain its metabolic effects, which results in increased production and release of insulin from pancreatic β cells. This is why IR is frequently associated with compensatory hyperinsulinemia [9,26,36].
IR and hyperinsulinemia are metabolic traits characteristic of lean and obese women with PCOS [8] and are considered important components in the pathogenesis of this endocrinopathy. Hyperinsulinemia contributes to androgen-dependent anovulation through different mechanisms. Insulin enhances the stimulating effects of LH on androgen production in ovarian theca cells [37,38]. In fact, insulin has been proposed to act as a co-gonadotropin and stimulate androgen biosynthesis in the ovary by activating P450c17 expression and activity in theca cells [22]. Of note, theca cells in women with PCOS are more sensitive to the hyperandrogenic effects of insulin than those in healthy women [39]. Despite peripheral IR, insulin sensitivity remains intact or may be even enhanced in ovaries in women with PCOS [37,38,40]. This observation suggests the presence of selective insulin resistance in PCOS that differentially affects the metabolic tissues and ovary.
Another mechanism by which insulin excess may contribute to increasing androgen levels is by inhibition of sex hormone-binding globulin (SHBG) release from the liver. SHBG is a circulating protein responsible for steroid transport in the blood and is the main binding protein for testosterone transport. However, unbound testosterone (free testosterone) is the biologically active androgen in circulation. In vivo and in vitro studies reported that insulin reduces SHBG release from the liver [41,42]. This attenuation of SHBG levels increases the bioavailability of free testosterone in the blood, thus promoting increased androgenic activity. Insulin has also been reported to stimulate GnRH-mediated gonadotropin release from the anterior pituitary [43] and, as discussed previously, to upregulate GnRH expression in hypothalamic GnRH neurons [35]. These pituitary and hypothalamic effects may also enhance ovarian androgen biosynthesis and compromise ovarian function.
Supporting the role of hyperinsulinemia as a pathogenic factor for the development of hyperandrogenism and PCOS, a variety of studies demonstrated that intervention with insulin-sensitizing drugs such as metformin or thiazolidinediones (TZDs) reduces circulating insulin and androgen levels, increases SHBG levels, and improves ovarian function in women with PCOS [36,[44], [45], [46], [47], [48], [49]].
2.3. Developmental factors
According to the Developmental Origin of Health and Disease (DOHaD) hypothesis proposed by Barker [50,51], environmental fluctuations during gestation and early postnatal life can permanently alter gene expression patterns and determine health status and susceptibility to disease in subsequent stages of development. This hypothesis postulates that during critical developmental stages of tissue and organ maturation, such as gestation, environmental changes result in permanent structural and physiological perturbations that may predispose to the occurrence of diseases later in life. This phenomenon, known as developmental programming, has been proposed as a potential pathogenic factor for PCOS.
Compelling evidence indicates that perinatal overexposure to androgens may play an important role in the pathogenesis of PCOS [[52], [53], [54], [55], [56], [57], [58], [59]]. Experimental studies of a variety of preclinical models such as monkeys, sheep, rats, and mice documented that prenatal exposure to androgens induces a PCOS-like phenotype in adult females characterized by altered gonadotropin release, hyperandrogenism, anovulation, insulin resistance, and other metabolic and reproductive disturbances [[53], [54], [55], [56], [57], [58], [59]]. Neonatal exposure to androgens has also been shown to cause cardiometabolic dysfunction in adult female mice similar to that observed in women with PCOS [59]. In humans, there is evidence that fetal overexposure to androgens due to certain pathologies such as congenital adrenal hyperplasia or androgen-secreting neoplasms causes permanent alterations in the pattern of gonadotropin secretion in girls [60,61]. Of note, female infants born from women with PCOS may also be at a greater risk of developing PCOS since the intrauterine milieu during pregnancy is likely to be hyperandrogenic in women with this syndrome [[62], [63], [64]]. In normal conditions, the fetus is protected against maternal androgens by the placental aromatase, which transforms androgens into estrogens, thus limiting androgen supply to the fetus. However, the steroidogenic pathway in placental tissue from women with PCOS seems to be dysregulated, showing increased activity of 3β-HDS1 and decreased activity of P450 aromatase [65]. Such alterations in the placental steroidogenic machinery may change the intrauterine environment and expose the fetus to abnormal androgen levels during gestation that might induce a PCOS-like phenotype in adulthood.
A recent report documented that pregnant women with PCOS exhibit increased circulating AMH levels compared with control women and demonstrated in an experimental model in mice that overexposure to this hormone during gestation may lead to fetal programming of the female offspring causing a PCOS-like reproductive phenotype in adulthood [66]. Thus, girls born from mothers with PCOS might be at a greater risk of developing this endocrine disorder not only due to the abnormal exposure to androgens during gestation, but also due to the intrauterine AMH excess.
Low birth weight as a consequence of intrauterine growth restriction has also been proposed as a contributing factor for the development of PCOS [67,68]. In this sense, it has been shown that the prevalence of symptoms of PCOS is higher in young adult women born small for gestational age compared with women with adequate body weight at birth, exhibiting altered insulin sensitivity, increased androgenic activity, and irregular menses [69,70]. These findings suggest that alterations in the intrauterine nutritional environment may also be considered a predisposing factor for the development of PCOS in humans.
2.4. Environmental factors
Environmental factors have been also implicated in the pathogenesis of PCOS. As previously discussed, insulin resistance and associated hyperinsulinemia are considered key pathological factors in the development of PCOS, and obesity is the most common cause of insulin resistance. Consequently, all environmental factors that can lead to overweight or obesity and alter insulin action may be involved in the etiology of this endocrine disorder. Diet and lifestyle are the main factors that may cause or exacerbate the metabolic and reproductive abnormalities of PCOS [71]. Sedentary lifestyle and inadequate dietary habits may promote obesity and insulin resistance and contribute to worsening the metabolic and reproductive features of PCOS. Supporting the role of these environmental elements in the pathogenesis of PCOS, it has been documented that an improvement in insulin sensitivity through lifestyle and diet modifications is sufficient to ameliorate some indexes of reproductive perturbations in obese women with PCOS such as anovulation and menstrual cycle irregularities [72,73].
In addition to these obesogenic factors, endocrine disruptors may also play a role in the pathogenesis of PCOS. Endocrine disruptors are natural or synthetic compounds that are ubiquitous in the environment and may interrupt the endocrine system. Exposure to these compounds, which can be found in plastic bottles, detergents, toys, cosmetics, pesticides, or metal cans, may result in adverse health effects. Bisphenol A (BPA) is the most characteristic endocrine disruptor whose effects on health have been extensively explored. This compound is a synthetic chemical with estrogenic activity. Preclinical studies of female rats, lambs, and monkeys showed that prenatal exposure to BPA may alter the hypothalamic-pituitary-ovarian axis and lead to a PCOS-like phenotype characterized by anovulation, polycystic ovaries, and infertility [[74], [75], [76]]. In humans, exposure to BPA during development has been associated with an increased risk of developing obesity, T2D, and reproductive disorders [77]. Interestingly, a recent meta-analysis reported a positive association between serum BPA levels and PCOS, suggesting that BPA may be involved in the development of insulin resistance and hyperandrogenism associated with PCOS [78].
2.5. Genetic factors
Many studies have suggested that genetic factors may be important in the etiology of this syndrome. Studies of probands with PCOS and their sisters revealed a high heritability of metabolic and endocrine features [79,80]. However, the most conclusive evidence for the influence of genetic factors on PCOS pathogenesis came from research involving monozygotic and dizygotic twins. These studies showed a strong contribution of familiar factors to the development of PCOS as reflected by a higher concordance of PCOS symptoms in monozygotic twins compared with dizygotic twins [81]. Additional studies have focused on the identification of susceptible genes that may underlie the intrinsic cause of PCOS. Recent genome-wide association studies (GWAS) conducted in different populations of women with PCOS identified an array of loci with a strong association with the development of PCOS [20]. Genes located in these susceptibility loci are related to the gonadotropic axis (LHCGR, LH receptor; FSHR, FSH receptor), ovarian androgen production (DENND1A), glucose metabolism (INSR, insulin receptor), vesicular trafficking and receptor recycling (RAB5B), and cell cycle regulation (THADA and HMGA2) [20]. While the results of the initial GWAS were not reproducible in all series, probably due to the heterogeneous nature of the syndrome and an insufficient number of patients recruited in some of these studies [20], recent analyses have demonstrated the consistent identification of altered gene loci linked to various metabolic and reproductive pathways; FSHR, THADA, and DENND1A are among the most robust candidates [82]. Nevertheless, these association studies explained only a modest fraction of the heritability of PCOS [20].
2.6. Epigenetic factors
As previously mentioned, unfavorable perinatal environment can permanently alter gene expression patterns and increase susceptibility to diseases later in life. In the context of PCOS, perinatal androgen exposure has been shown to induce a PCOS-like phenotype in adult females in a variety of species [[53], [54], [55], [56], [57], [58]]. Inappropriate epigenetic reprogramming has been identified as a potential underlying mechanism linking early overexposure to androgens and the development of PCOS in adulthood. Thus, prenatal androgenization in female rats, sheep, and rhesus monkeys has been associated with alterations in the pattern of methylation of relevant genes related to the steroidogenic pathway and reproductive and biological processes [[83], [84], [85], [86]]. These epigenetic modifications of specific promoters alter gene expression patterns and may increase the risk of developing PCOS in subsequent stages of development. Exposure to androgens during the early postnatal stage has also been shown to induce epigenetic changes in the promotion of genes involved in ovarian function such as luteinizing hormone receptor (LHr), peroxisome proliferator-activated receptor-γ (PPAR-γ), nuclear corepressor 1 (NCOR1), and histone deacetylase 3 (HDAC3) [87,88]. Collectively, these data suggest that alteration of the epigenome in the early stages of development may also be involved in the pathogenesis of PCOS.
3. Metabolic dysfunction in PCOS: Role of androgen excess
Androgen excess is regarded as the cardinal feature of PCOS. Hyperandrogenism plays a prominent role in the development of metabolic disturbances associated with PCOS, acting on peripheral tissues as well as at the central level (Figure 2). The major metabolic actions of androgen excess in different metabolic tissues and the brain is discussed in the forthcoming sections, with special focus on the putative underlying molecular mechanisms driving the metabolic complications of PCOS.
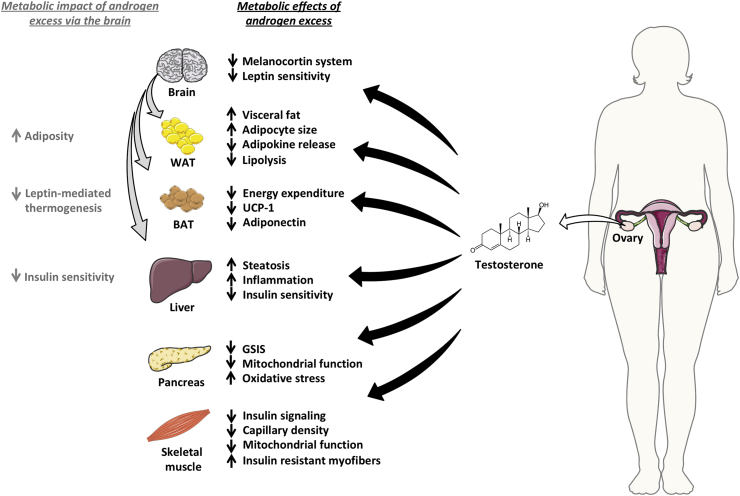
Figure 2.
Metabolic impact of androgen excess in PCOS. In women with PCOS, androgen excess has a detrimental impact on different metabolic tissues, including the adipose tissue (white and brown), liver, pancreas, and skeletal muscle. Androgen excess also impairs systemic metabolism via the brain, primarily increasing adiposity and reducing insulin sensitivity. The figure was created using tools provided by Servier Medical Art (https://smart.servier.com).
3.1. Effects of hyperandrogenism on the adipose tissue
3.1.1. White adipose tissue
3.1.1.1. Effects on fat distribution
A differential pattern of fat distribution exists between males and females. While females tend to store fat predominantly in the subcutaneous adipose depots (SAD) and especially in the gluteal and femoral fat pads, males accumulate fat in the visceral depots [89]. However, this pattern of fat accumulation is altered in hyperandrogenic women with PCOS. Clinical studies documented that women with PCOS exhibited increased global adiposity [90] and thickness of the intraperitoneal and mesenteric fat depots compared with control women [91]. The thickness of the intraperitoneal fat depots in these patients was positively correlated with circulating androgen levels, suggesting that androgen excess masculinizes the pattern of fat distribution, favoring visceral fat accumulation [91]. Additional findings have supported the role of androgens in body fat distribution in females, promoting increased visceral adiposity. In this sense, chronic administration of testosterone in female-to-male transsexuals has also been shown to increase visceral adiposity and decrease subcutaneous fat depots [92]. Furthermore, women with PCOS subjected to chronic intervention with flutamide, an anti-androgenic compound, exhibited reduced abdominal fat depots [93]. This alteration in regional fat distribution induced by androgens may have detrimental metabolic implications for PCOS patients, since increased visceral adiposity is considered a risk factor for the development of metabolic syndrome [94] and may contribute to aggravating metabolic abnormalities linked to this endocrinopathy. However, the molecular mechanisms involved in the increased abdominal adiposity induced by chronic exposure to androgens remain largely unknown. Preclinical studies of female mice suggested that androgen excess may perturb the ability of leptin to stimulate energy expenditure, which in turn may promote visceral fat accumulation [95]. However, further studies are needed to elucidate the underlying mechanisms associated with this effect of hyperandrogenism on patterns of fat accumulation.
3.1.1.2. Effects on adipocyte size and differentiation
Increasing evidence suggests that androgen excess enhances adipocyte size in SAD in PCOS women [96,97]. This adipocyte hypertrophy may lead to adipocyte dysfunction, since it has been suggested that enlarged adipocytes are more susceptible to inflammation, macrophage infiltration, and apoptotic processes [98,99], effects that may impair insulin sensitivity. These findings were confirmed in rodent studies in which overexposure to androgens during the early postnatal and peri-pubertal stage was associated with increased adipocyte size in subcutaneous and visceral fat depots and the development of insulin resistance in females [59,100,101]. However, prenatal exposure to testosterone has been associated with decreased adipocyte size in female sheep and monkeys [102,103], suggesting that the effects of hyperandrogenism on adipocyte cell size might depend not only on the species, but also on the nature of the androgen (for example, whether aromatizable or not) and the time window and/or duration of exposure.
Adipocyte differentiation is also influenced by hyperandrogenism. Androgens disrupt the differentiation of pre-adipocytes to mature adipocytes in female rats, sheep, and monkeys [[102], [103], [104]]. This androgen-driven inhibition of adipogenic differentiation has also been demonstrated in human pre-adipocytes, an effect that was partially reversed by the administration of anti-androgenic compounds [105,106]. The anti-adipogenic effects of androgens seem to be mediated by different mechanisms. The physiological functions of androgens are conveyed by the canonical androgen receptor (AR), a nuclear receptor expressed in multiple tissues, including the adipose tissue. Wnt signaling in fat cells plays an important role in cellular differentiation and growth. Activation of this intracellular pathway in adipocytes allows the translocation of β-catenin to the nucleus and the activation of a series of transcription factors that ultimately lead to the expression of Wnt-regulated target genes. It has been suggested that the Wnt signaling may function as an adipogenic switch that represses adipogenesis when activated and initiates adipogenic processes when inhibited [107,108]. AR activation may activate this pathway by inducing translocation of β-catenin to the nucleus, thus downregulating the expression of adipogenic transcription factors [109]. Another potential mechanism is related to the ability of ARA70, a ligand-enhanced coactivator, to interact with the AR and PPAR-γ. PPAR-γ is involved in adipocyte differentiation and function and its activation stimulates adipogenesis [110,111]. When the AR is activated, it competes with PPAR-γ to bind coactivator ARA70, thus limiting PPAR-γ effects on adipogenesis [112]. Studies of subcutaneous adipocytes isolated from nonobese women also demonstrated that androgens impair the differentiation of human adipocyte-derived stem cells to preadipocytes by altering bone morphogenic protein 4 (BMP4) activity, an effect that was reversed after flutamide administration [113].
3.1.1.3. Effects on lipolysis and adipokine secretion
Androgen excess also affects lipolytic regulation. Studies of differentiated pre-adipocytes demonstrated that androgens exert depot-specific lipolytic actions, causing a reduction of catecholamine-stimulated lipolysis particularly in subcutaneous adipocytes [114], an effect likely due to the downregulation of hormone-sensitive lipase (HSL) and β-2 adrenergic receptor expression [114]. In line with this, previous studies of isolated subcutaneous adipocytes reported that dihydrotestosterone (DHT) exposure reduced HSL expression [115]. In female rhesus monkeys, peri-pubertal exposure to testosterone has been shown to attenuate lipolysis and reduce HSL expression in visceral depots during the luteal phase of the menstrual cycle [116]. In addition, in the same experimental model, the combination of testosterone administration and consumption of a Western diet was reported to impair also β-adrenergic-stimulated lipolysis in white adipocytes and accelerate adipose tissue dysfunction [117]. Interestingly, this anti-lipolytic effect of androgens has also been documented in humans, wherein chronic intervention with testosterone reduced HSL expression in subcutaneous adipocytes from postmenopausal women [118]. This androgen-driven impairment of lipid metabolism in adipocytes impacts lipid storage capacity and may cause insulin resistance.
Of note, recent findings demonstrated that intra-adipose androgen production by the enzyme AKR1C3 plays a prominent role in adipose tissue dysfunction in PCOS [119]. This enzyme catalyzes the conversion of androstenedione to testosterone and is abundantly expressed in the adipose tissue. Studies of patients with PCOS showed increased local androgen production by AKR1C3 and lipid accumulation in the adipose tissue leading to lipotoxicity, insulin resistance, and compensatory hyperinsulinemia [119]. Interestingly, in vitro experiments showed that insulin upregulates AKR1C3 expression, which may exacerbate intra-adipose androgen production and generate a vicious cycle in the adipose tissue that may increase the metabolic risk in PCOS patients [119].
Androgens have also been shown to modulate adipokine production in the adipose tissue. Adiponectin is an adipocyte-derived adipokine with insulin-sensitizing features. An array of in vitro and in vivo studies have documented the ability of androgens to reduce circulating adiponectin levels [[120], [121], [122]], an effect that has been postulated as a key factor contributing to insulin resistance in PCOS women. In keeping with the beneficial effects of adiponectin on insulin sensitivity and metabolic health in PCOS, a recent report in mice showed that the overexpression of this adipokine in the adipose tissue prevents metabolic derangements linked to continuous exposure to DHT but had only minor effects on the reproductive profile in a mouse model of PCOS [123]. In addition to their effects on adiponectin levels, androgens also reduce circulating levels of other adipokines such as omentin-1. This adipokine also has insulin-sensitizing properties and its circulating levels have been negatively correlated with free testosterone levels in obese patients with PCOS [124]. Collectively, these findings demonstrated that hyperandrogenism attenuates adipokine levels with insulin-sensitizing properties that may have detrimental consequences on insulin sensitivity in women with PCOS.
3.1.2. Brown adipose tissue
Brown adipose tissue (BAT) is a metabolically active type of fat located in specific areas of the body whose main function is to protect important organs from hypothermia via the induction of adaptative thermogenesis [125]. BAT produces heat due to the presence of increased levels of uncoupling protein 1 (UCP-1) within the mitochondria, which promotes heat production by uncoupling aerobic respiration. BAT thermogenesis is regulated by several factors, including the environmental temperature, certain nutrients during the postprandial phase, physical activity, and the sympathetic nervous system, among others [126,127]. Of note, women with PCOS exhibit decreased BAT thermogenesis compared with controls, a reduction that has been negatively correlated with circulating androgen levels [128]. Evidence from in vitro and experimental studies revealed that androgens modulate BAT activity by regulating UCP-1 expression [129,130]. Testosterone reduces UCP-1 levels by downregulating the expression of PGC1-α, a master regulator of UCP-1 transcription [130], and inhibits leptin's ability to stimulate UCP-1 expression in BAT [95].
Recent findings proposed that dysfunctional BAT may be related to the development of PCOS. These studies demonstrated that peri-pubertal exposure to the androgen DHEA in rats induced some classical PCOS traits, including irregular menstrual cycles, polycystic ovarian morphology, and insulin resistance, and also reduced BAT activity. Surprisingly, BAT transplantation from control animals to PCOS rats was capable of reverting the PCOS-like phenotype, with not only metabolic, but also reproductive improvements [131]. BAT transplantation restored estrous cyclicity and improved metabolic homeostasis in DHEA-treated animals, effects that were mediated, at least partially, by the enhanced BAT activity and adiponectin release. Importantly, chronic exposure to adiponectin mimicked the metabolic and reproductive effects induced by BAT transplantation in DHEA-treated rats [131]. These findings are partially discordant with the modest effects of adiponectin on the reproductive profile of the PCOS-like mouse model previously mentioned [123]. This discrepancy might be due, at least partially, to the use of different PCOS models generated in different rodent species (rat vs mouse) by exposure to different androgens (DHEA vs DHT). Hence, the phenotypic presentation of PCOS-like symptoms might differ between these two PCOS models, and the reproductive actions of adiponectin may also vary. In addition, the source of adiponectin may also contribute to the observed differences; while in the DHEA study [131], recombinant adiponectin was daily administered to rats, in the DHT study [123], specific transgenic mice were engineered to overexpress adiponectin in the white adipose tissue. Hence, the differences in the dose and duration of exposure to adiponectin between the two models might also influence the reproductive effects of this adipokine. Nevertheless, these findings suggest that there may be a link among BAT activity, circulating adiponectin levels, and the development of PCOS, and highlight that BAT-activating compounds might be considered potential drug candidates for the treatment of this endocrinopathy. In line with this hypothesis, a recent report described that intervention with the natural compound rutin, a flavonoid that promotes BAT activation and ameliorates metabolic and reproductive phenotypes in DHEA-induced PCOS rats [132], thus emphasizing the therapeutic potential of BAT-activating compounds for the treatment of PCOS. However, it must be stressed that evidence linking BAT dysfunction and PCOS primarily comes from studies of rodent models in which BAT is likely to play a more prominent physiological role than in primates, including humans [133]. Nonetheless, the conclusive documentation of the presence of functional BAT in adult humans [[134], [135], [136]], where it plays a role in energy homeostasis [137], makes it plausible that rodent findings might be extrapolated to human pathophysiology, also in the case of PCOS. Additionally, the possibility that beige cells, which share numerous molecular features with canonical brown cells in humans [138], might be also pathophysiological contributors and/or therapeutic targets in PCOS warrants further investigation.
3.2. Effects of hyperandrogenism on the liver
Cumulative evidence suggests a clear association between PCOS and the development of non-alcoholic fatty liver disease (NAFLD) [[139], [140], [141], [142], [143]]. NAFLD is considered the most common liver disease, encompassing a spectrum of hepatic alterations ranging from liver fat accumulation (steatosis) to non-alcoholic steatohepatitis (NASH) and cirrhosis, which, in certain cases, may progress to hepatocellular carcinoma [142,143]. The pathophysiology of NALFD in women with PCOS is related to the metabolic disturbances frequently associated with this syndrome such as insulin resistance, obesity, and dyslipidemia. Recent studies reported that hyperandrogenism per se might increase the risk of developing NAFLD in these patients. After statistical adjustment for body mass index (BMI) and IR, a case–control study documented that women with PCOS and hyperandrogenism were more prone to developing NAFLD compared with non-hyperandrogenic PCOS and healthy women [144]. Additional studies of hyperandrogenic women with PCOS also described a positive correlation between androgen excess and circulating alanine aminotransferase (ALT) levels, a marker of hepatocellular damage and NAFLD commonly used in clinical practice, independently of obesity [145,146]. An elevated free androgen index (FAI) has also been associated with increased prevalence of NAFLD in PCOS women, independent of age, BMI, and insulin resistance [140,147]. Thus, the available evidence strongly suggests that androgen excess might be considered an independent factor that may be involved in the development of NAFLD in PCOS women.
From a mechanistic perspective, few studies have addressed the putative mechanisms linking androgen excess and NAFLD development in women with PCOS. A recent study conducted on female rats demonstrated that hyperandrogenemia induced by the chronic administration of exogenous human chorionic gonadotropin (hCG) caused an imbalance between de novo lipogenesis and mitochondrial β-oxidation, affecting the PPAR-α/β-Srebp1/2-Acc1 axis, leading to liver fat accumulation [148]. Overexposure to androgens also exacerbated liver inflammation in these animals as reflected by the increased hepatic expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β and negatively affected insulin-mediated IRS-PI3K-Akt signaling [148]. Hyperandrogenism has also been shown to downregulate low-density lipoprotein receptor (LDL-R) expression in the adipose tissue of PCOS patients, an effect that may also promote liver fat accumulation [149]. According to a recent preclinical study of female rats, prenatal exposure to androgens may also cause NAFLD by altering liver lipid metabolism via modification of the PPAR system, thus increasing susceptibility to developing hepatic steatosis [150]. Despite these mechanistic findings linking hyperandrogenism and NAFLD, further preclinical studies are needed to assess the real contribution of androgens to the development of NAFLD in PCOS patients.
3.3. Effects of hyperandrogenism on the skeletal muscle
Hyperandrogenism may also have negative consequences on skeletal muscle in women with PCOS, primarily affecting insulin sensitivity. Various studies explored the association between androgen excess and insulin resistance in PCOS women, with a particular focus on the effects of androgens on whole body glucose uptake and insulin sensitivity assessed by euglycemic-hyperinsulinemic clamps. These studies revealed a reduction of insulin-stimulated glucose uptake in women exhibiting hyperandrogenism that was primarily attributable to skeletal muscle insulin resistance [151]. Initial mechanistic analyses conducted in cultured skeletal muscle cells from PCOS patients reported alterations in the insulin-signaling pathway characterized by decreased tyrosine phosphorylation and increased serine phosphorylation of the insulin receptor [152]. Subsequent reports confirmed that women with PCOS and hyperandrogenism showed abnormalities not only in the phosphorylation pattern of the insulin receptor, but also at different levels of the intracellular insulin pathway. Namely, skeletal muscle cells in women with PCOS exhibited reduced Akt/PKB and AS160 phosphorylation and activation, alterations that impair insulin-stimulated glucose uptake and consequently contribute to insulin resistance [153,154]. Interestingly, a recent study conducted on lean women with PCOS and hyperandrogenism described impaired insulin sensitivity in these patients compared with BMI-matched controls. However, activation of insulin signaling in muscle cells did not differ between women with PCOS and controls. The study concluded that reduced insulin sensitivity in lean PCOS patients might be associated with a decline in the circulating adiponectin levels that may modulate skeletal muscle insulin sensitivity via the AMPK system [155].
Experimental studies have also demonstrated the association between hyperandrogenism and insulin resistance in muscle cells in PCOS. In female rats, chronic treatment with testosterone reduced insulin-mediated glucose transport into the muscle, causing hyperinsulinemia and altering the relative number of myofibers in the skeletal muscle by decreasing the number of type 1 fibers and increasing the proportion of type 2 (fast twitch and insulin resistant) fibers [156]. Another study conducted on female rats reported that hyperandrogenism diminished capillary density in skeletal muscle, thereby hampering insulin access to muscle cells and delaying insulin action in this tissue [157]. Novel findings in a mouse model of PCOS induced by the chronic administration of DHEA indicated that androgen excess might deregulate the mammalian target of rapamycin complex 1 (mTORC1) autophagy pathway and impair mitochondrial function, which may contribute to reducing insulin sensitivity and glucose uptake in muscle cells [158]. Moreover, metabolomic analyses of skeletal muscle in the same PCOS model revealed that hyperandrogenism affected five metabolic pathways, some related to mitochondrial function and glucose metabolism [159]. These data demonstrate a potential role of mitochondrial dysfunction as pathogenic factor for androgen-mediated insulin resistance in the skeletal muscle in PCOS.
3.4. Effects of hyperandrogenism on the pancreas
As previously outlined, women with PCOS frequently exhibit insulin resistance with associated hyperinsulinemia and have a higher propensity of developing T2D. While the effect of androgen excess on systemic insulin sensitivity has been extensively explored, less attention has been paid to assessing the impact of hyperandrogenism on β cell function and insulin release. However, some evidence suggests that androgen excess may contribute to impairing glucose tolerance and β cell function in women with PCOS [160,161]. However, the mechanisms underpinning this androgenic effect on the pancreas remain poorly understood.
Experimental studies demonstrated the expression of androgen receptors in β cells [[162], [163], [164]], suggesting that androgens might have a direct impact on pancreatic function. Supporting this hypothesis, in vitro studies conducted on isolated islets from rats showed defective glucose-stimulated insulin secretion and mitochondrial dysfunction in islets treated with testosterone compared with controls, effects that were not detected in islets treated with testosterone but previously administered the anti-androgen flutamide [165]. Initial studies of female mice also reported that androgen excess may predispose to β cell dysfunction due to its ability to produce systemic oxidative stress [166], but did not clarify whether the detrimental effects of testosterone on β cell function were direct or indirect. Subsequent studies confirmed the direct effect of androgens on pancreatic function, both in males and females, by using mice lacking AR specifically in the β cells [164,167]. These studies revealed that wild-type female mice exposed to a Western diet and chronically treated with DHT in adulthood exhibited hyperinsulinemia and pancreatic β cell failure, effects that were not observed in female animals lacking AR in β cells and exposed to the same obesogenic factors [164]. The direct impact of androgens on β cells was further documented in cultured islets from female mice and women, with androgens producing insulin hypersecretion from β cells in response to glucose [164]. This study concluded that excessive AR activation in β cells may promote mitochondrial hyperfunction, oxidative damage, and insulin hypersecretion, effects that may predispose to β cell failure [164]. Additional studies of female sheep and rhesus monkeys also demonstrated that prenatal exposure to androgens may alter islet morphology and enhance β cell numbers and expansion in subsequent stages of development, which may promote insulin hypersecretion [168,169]. In women with PCOS, it was documented that hyperglycemia-induced oxidative stress in mononuclear cells (MNC) and glucose-stimulated nuclear factor-κB (NF-κB) activation in these immune cells were involved in β cell dysfunction in PCOS, suggesting that both oxidative stress and inflammatory processes are linked to β cell failure in these patients [170,171]. Of note, androgen excess enhances MNC sensitivity to glucose, thereby exacerbating glucose-stimulated oxidative stress and release of inflammatory factors from MNC [170,171]. These results indicate that androgen excess may promote β cell dysfunction in women with PCOS via direct and indirect mechanisms. However, additional experimental analyses are required to explore the associated underlying mechanisms.
3.5. Metabolic impact of hyperandrogenism via the central nervous system
In women, androgen excess also promotes metabolic dysfunction through its interaction with specific brain centers. Hyperandrogenism has a detrimental impact on leptin sensitivity at the central level and reduces leptin-mediated BAT thermogenesis by altering melanocortin signaling in the DMH and communication between the hypothalamic nucleus and BAT [95]. Such effects may compromise the weight-lowering properties of leptin and exacerbate the metabolic derangements associated with PCOS. It was also suggested that prenatal overexposure to androgens may increase the proportion of AgRP neurons in adulthood, which may affect energy intake and enhance adiposity [172]. Interestingly, it was demonstrated that AgRP and AR colocalizes in the hypothalamus, suggesting that androgens may alter metabolic homeostasis by acting on these neurons. In addition, prenatal exposure to androgens also reduced colocalization of AgRP and the insulin receptor [172], which may affect hepatic insulin sensitivity since insulin action in these neurons plays a prominent role in the regulation of hepatic glucose production [173]. Neonatal exposure to androgens was also shown to alter sexual differentiation of POMC neurons in female mice, thereby inducing reduced hypothalamic POMC expression and increased energy intake [174]. In this scenario, it is plausible that androgens act on the melanocortin system to impair systemic metabolism in women with PCOS.
Recent reports demonstrated the essential role of the neuroendocrine action of androgens in the development of the metabolic and reproductive traits associated with PCOS [164,175]. Thus, AR signaling in neurons appears to be crucial for the development of most metabolic and reproductive abnormalities associated with hyperandrogenism. This was illustrated by functional genomic approaches in which three mouse lines with site-specific loss of AR signaling were generated and compared: (i) a global AR knockout, (ii) a mouse model lacking AR specifically in neurons, and (iii) a mouse line lacking AR in granulosa cells. In these three lines, a standard protocol was applied to induce a PCOS-like phenotype in adulthood based on chronic exposure to DHT from weaning. Neither the mice lacking AR signaling in neurons nor the global knockout mice displayed key metabolic and reproductive traits associated with DHT treatment, whereas mice lacking AR signaling in granulosa cells developed obesity, dyslipidemia, and ovarian dysfunction [175]. Another study recently documented that chronic AR activation in the brain produced peripheral insulin resistance in female mice [164]. This study used a combination of loss-of-function models to explore the contribution of excessive AR activation in neurons and β cells to the development of T2D. The study's results showed that chronic AR activation in neurons was essential for the development of peripheral insulin resistance. While control females exposed to a Western diet and DHT exhibited hyperinsulinemia and insulin resistance, mice lacking AR in neurons and exposed to an obesogenic diet and androgen excess did not display such metabolic disturbances. These data suggest that androgen excess predisposes the development of T2D via the specific activation of AR in neurons, causing hepatic insulin resistance, and in β cells, leading to increased oxidative stress, insulin hypersecretion, and β cell failure [164]. Altogether, these findings provide evidence that the direct action of androgens on the brain is an important component for the development of the metabolic and reproductive derangements associated with PCOS.
4. Therapeutic strategies for the management of metabolic disturbances of PCOS
Currently, the therapeutic options for the treatment of the metabolic dysregulation associated with PCOS are limited. Although there are no specific therapies for the management of the metabolic perturbations in these patients, several recommendations and interventions similar to those employed for the management of obese and diabetic patients are frequently used for the treatment of obese PCOS women, including lifestyle changes, pharmacological treatment, and, in cases of morbid obesity and inefficacy of the previous interventions, bariatric surgery.
4.1. Lifestyle interventions and pharmacological approaches
As previously discussed, most women with PCOS are obese or overweight, exhibit insulin resistance associated with compensatory hyperinsulinemia, and have an increased risk of developing T2D. The most recommended first therapeutic option for the management of the metabolic perturbations associated with PCOS is diet and exercise, with the aim of reducing body weight, since it has been shown that reduction in body weight improves metabolic homeostasis and hyperandrogenism in women with PCOS [176]. Nevertheless, most obese patients with PCOS fail to achieve substantial weight loss through lifestyle modifications. For those patients, the use of insulin-sensitizing drugs, such as metformin, is the preferred therapeutic approach. The aim of this therapy is to improve insulin sensitivity to reduce circulating insulin levels and attenuate insulin-mediated stimulation of androgen production in the ovary. In this context, it was shown that intervention with metformin improves insulin sensitivity and regulates menstrual cycles, ameliorates ovulation rates, and may also improve hyperandrogenemia in women with PCOS [44,49]. However, metformin therapy has modest effects on body weight and adiposity [177], and controversial results on its efficiency has been reported in PCOS patients [[178], [179], [180], [181]], a phenomenon that might be related, at least partially, to heterogeneity in responses among different subgroups of PCOS women.
TZDs are alternative pharmacological therapies for the treatment of the metabolic and reproductive abnormalities associated with PCOS. TZDs are insulin-sensitizing drugs that activate PPAR-γ, a nuclear receptor whose activation improves insulin sensitivity primarily in the adipose tissue and skeletal muscle [48,182]. Treatment with these pharmacotherapies has been shown to ameliorate insulin sensitivity, reduce insulin levels, and improve several reproductive parameters in prenatally androgenized female sheep and monkeys as well as in women with PCOS [48,183,184]. However, similar to metformin, TZDs have a negligible effect on body weight or may even promote weight gain [185], which may limit the use of this therapy to lean or overweight PCOS patients who exhibit hyperandrogenism and hyperinsulinemia.
Glucagon-like peptide-1 (GLP-1) analogs have recently emerged as novel anti-diabetic drugs with excellent therapeutic profiles, improving glycemic control and insulin resistance together with weight loss [186,187]. Given the importance of reducing body weight and improving insulin sensitivity in obese and overweight women with PCOS, several studies assessed the metabolic and reproductive effects of this gut hormone in this patient population. These fragmentary studies revealed that treatment with GLP1 reduces body weight, increases menstrual frequency, and may improve hyperandrogenemia in obese women with PCOS [[188], [189], [190]]. Interestingly, a recent meta-analysis provided evidence that GLP-1 therapy may be more effective than metformin for improving insulin sensitivity and other metabolic parameters [191], suggesting that GLP-1 might be considered a more suitable pharmacological option for the clinical management of obese patients with PCOS. Of note, the combined action of both pharmacotherapies, GLP-1 and metformin, may be more effective than the individual action of each drug for the treatment of the metabolic and reproductive disturbances linked to PCOS [192] and may even improve metabolic outcomes in women who previously exhibited deficient responses to metformin [193]. Nevertheless, despite these promising results, additional analyses are required to evaluate the reproductive and metabolic efficacy and safety of this drug combination in obese women with PCOS.
4.2. Bariatric surgery
Bariatric surgery is considered an effective intervention for the treatment of morbid obesity. This surgical procedure improves body weight, IR, and other metabolic parameters [194,195]. However, bariatric surgery is associated with short- and long-term risks. Although the risks of this intervention have decreased over time due to the development of novel approaches (for example, laparoscopy), bariatric surgery is primarily recommended for extremely obese patients who exhibit metabolic comorbidities and do not achieve therapeutic goals after lifestyle and pharmacological intervention.
In the context of PCOS, several studies reported the beneficial effects of bariatric surgery on metabolic and reproductive function. A recent meta-analysis focusing on nine different studies and encompassing 234 obese patients with PCOS documented that bariatric surgery improved BMI, circulating glucose levels, IR, and hypertension in PCOS patients [194]. The surgery also improved hyperandrogenism and associated clinical symptoms and ameliorated menstrual cycles and ovulation rates [194]. Experimental studies also documented the metabolic and reproductive effects of bariatric surgery in a rat model of PCOS induced by chronic exposure to DHT. After the intervention, the animals showed reduced body weight and adiposity together with improved glucose and triglycerides levels. Bariatric surgery also restored estrous cyclicity in this PCOS model, but only when DHT levels returned to normal [196].
The specific mechanisms by which bariatric surgery improves the metabolic and reproductive profile in obese PCOS patients remain unclear. A potential mechanism may be related to the marked reduction in body weight that patients display after intervention, which is associated with an improvement in IR, a reduction in circulating insulin levels, and, consequently, with a decline in circulating androgen levels and an increase in SHBG levels [194]. This improvement in hyperandrogenism may be related to the restoration of menstrual cycles and ovulation. Another mechanism that may explain the metabolic and reproductive effects of bariatric surgery may be related to the alterations in the secretion patterns of gastrointestinal hormones frequently observed after surgery. Namely, several days after surgery there is an increase in circulating GLP-1 and peptide YY levels, which may have beneficial metabolic and eventually reproductive consequences due to the ability of these gut peptides to reduce food intake [194].
5. Conclusions and future directions
In conclusion, epidemiological studies of humans and observations of preclinical models indicate that multiple factors are involved in the pathogenesis of PCOS, including developmental, environmental, genetic, and epigenetic elements. Although it is widely accepted that hyperandrogenism is the cardinal endocrine perturbation driving the development and manifestation of PCOS traits, further mechanistic analyses must be undertaken to dissect the specific metabolic and reproductive effects of androgen excess on metabolic and reproductive tissues as well as the brain. The development of novel PCOS experimental models may be of significant interest to explore the pathogenic mechanisms of this endocrine disorder and characterize the metabolic impact of endogenous hyperandrogenism. In particular, the generation of preclinical models of PCOS exhibiting endogenous hyperandrogenemia is of special relevance, considering that the vast majority of the currently available PCOS models are induced by exogenous administration of androgens in different stages of development. The development of these experimental models may help elucidate different aspects of the disease and may serve as preclinical tools to assess the putative anti-androgenic and anti-obesogenic effects of novel therapeutic candidates.
To date, several pharmacological therapies have been used for the management of the metabolic abnormalities observed in PCOS. Pharmacotherapies such as metformin or TZDs aim to reduce androgenic activity and improve metabolic homeostasis and have been shown to be effective in specific patient populations. However, there is a considerable demand for the development of novel, safer, and more effective therapeutic approaches against the metabolic dysregulation frequently linked to PCOS. In this scenario, GLP-1 receptor agonists, alone or in combination with other metabolic hormones, are emerging as potential therapies for obese women with PCOS due to their anti-diabetic and weight-lowering properties, which may offer an attractive therapeutic option for these patients. In this context, we forecast that in the near future, experimental and clinical studies will address the potential therapeutic utility of novel unimolecular GLP-1-based poly-agonists for the treatment of the metabolic and reproductive abnormalities associated with PCOS. The metabolic efficacy and safety of these unimolecular multi-agonists, as well as their superior metabolic efficiency compared with GLP-1 receptor agonists, was recently demonstrated in different preclinical models of obesity [197], and some of these drugs are currently in clinical evaluation for the treatment of T2D. Hence, the potential use of this novel class of multi-agonist therapies holds promising opportunities for the metabolic management of obese patients with PCOS.
Acknowledgments
Research activities at the authors' laboratory related to the contents of this work were funded by grants BFU2017-83934-P (to MT-S; Ministry of Economy and Competitiveness, Spain, co-funded with EU funds from the FEDER Program), project PIE14-00005 (to MT-S; Flexi-Met, Carlos III Health Institute, Ministry of Health, Spain), and project PI-0358-2018-FIB (to MAS-G; FIBICO). CIBER is an initiative of the Carlos III Health Institute (Ministry of Health, Spain).
Conflict of interest
None declared.
References
- 1.Yildiz B.O., Bozdag G., Yapici Z., Esinler I., Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Human Reproduction. 2012;27(10):3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 2.Lim S.S., Davies M.J., Norman R.J., Moran L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human Reproduction Update. 2012;18(6):618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 3.Ding T., Hardiman P.J., Petersen I., Wang F.F., Qu F., Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. OncoTarget. 2017;8(56):96351–96358. doi: 10.18632/oncotarget.19180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotterdam, E.A.-S.P.C.W.G. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Human Reproduction. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 5.Franks S. Assessment and management of anovulatory infertility in polycystic ovary syndrome. Endocrinology and Metabolism Clinics of North America. 2003;32(3):639–651. doi: 10.1016/s0889-8529(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert E.W., Tay C.T., Hiam D.S., Teede H., Moran L.J. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clinical Endocrinology (Oxf) 2018 doi: 10.1111/cen.13828. [DOI] [PubMed] [Google Scholar]
- 7.Moran C., Arriaga M., Rodriguez G., Moran S. Obesity differentially affects phenotypes of polycystic ovary syndrome. The Internet Journal of Endocrinology. 2012:317241. doi: 10.1155/2012/317241. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legro R.S., Castracane V.D., Kauffman R.P. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstetrical and Gynecological Survey. 2004;59(2):141–154. doi: 10.1097/01.OGX.0000109523.25076.E2. [DOI] [PubMed] [Google Scholar]
- 9.Marshall J.C., Dunaif A. Should all women with PCOS be treated for insulin resistance? Fertility and Sterility. 2012;97(1):18–22. doi: 10.1016/j.fertnstert.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bednarska S., Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what's new? Advances in Clinical and Experimental Medicine. 2017;26(2):359–367. doi: 10.17219/acem/59380. [DOI] [PubMed] [Google Scholar]
- 11.Moran L.J., Misso M.L., Wild R.A., Norman R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Human Reproduction Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 12.Yang R., Yang S., Li R., Liu P., Qiao J., Zhang Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reproductive Biology and Endocrinology. 2016;14(1):67. doi: 10.1186/s12958-016-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquali R., Gambineri A., Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113(10):1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Rojas J., Chavez M., Olivar L., Rojas M., Morillo J., Mejias J. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. International Journal of Reproductive Medicine. 2014;2014:719050. doi: 10.1155/2014/719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirmans S.M., Pate K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical Epidemiology. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badawy A., Elnashar A. Treatment options for polycystic ovary syndrome. International Journal of Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenichel P., Rougier C., Hieronimus S., Chevalier N. Which origin for polycystic ovaries syndrome: genetic, environmental or both? Annales d'Endocrinologie. 2017;78(3):176–185. doi: 10.1016/j.ando.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Morreale H.F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 19.Nelson V.L., Legro R.S., Strauss J.F., 3rd, McAllister J.M. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Molecular Endocrinology. 1999;13(6):946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 20.Crespo R.P., Bachega T., Mendonca B.B., Gomes L.G. An update of genetic basis of PCOS pathogenesis. Archives Endocrinology Metabol. 2018;62(3):352–361. doi: 10.20945/2359-3997000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dharia S., Slane A., Jian M., Conner M., Conley A.J., Parker C.R., Jr. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biology of Reproduction. 2004;71(1):83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfield R.L., Ehrmann D.A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine Reviews. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parakh T.N., Hernandez J.A., Grammer J.C., Weck J., Hunzicker-Dunn M., Zeleznik A.J. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proceedings of the National Academy of Sciences of the U S A. 2006;103(33):12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franks S. The ubiquitous polycystic ovary. Journal of Endocrinology. 1991;129(3):317–319. doi: 10.1677/joe.0.1290317. [DOI] [PubMed] [Google Scholar]
- 25.Wajchenberg B.L., Achando S.S., Okada H., Czeresnia C.E., Peixoto S., Lima S.S. Determination of the source(s) of androgen overproduction in hirsutism associated with polycystic ovary syndrome by simultaneous adrenal and ovarian venous catheterization. Comparison with the dexamethasone suppression test. Journal of Clinical Endocrinology & Metabolism. 1986;63(5):1204–1210. doi: 10.1210/jcem-63-5-1204. [DOI] [PubMed] [Google Scholar]
- 26.Franks S., Gilling-Smith C., Watson H., Willis D. Insulin action in the normal and polycystic ovary. Endocrinology and Metabolism Clinics of North America. 1999;28(2):361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 27.Moran C., Reyna R., Boots L.S., Azziz R. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertility and Sterility. 2004;81(1):126–131. doi: 10.1016/j.fertnstert.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A., Woods K.S., Bartolucci A.A., Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS) Clinical Endocrinology (Oxf) 2005;62(6):644–649. doi: 10.1111/j.1365-2265.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 29.Gilling-Smith C., Story H., Rogers V., Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clinical Endocrinology (Oxf) 1997;47(1):93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 30.Wickenheisser J.K., Nelson-DeGrave V.L., McAllister J.M. Human ovarian theca cells in culture. Trends in Endocrinology and Metabolism. 2006;17(2):65–71. doi: 10.1016/j.tem.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nature Reviews Endocrinology. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 32.Blank S.K., McCartney C.R., Marshall J.C. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Human Reproduction Update. 2006;12(4):351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 33.Walters K.A., Gilchrist R.B., Ledger W.L., Teede H.J., Handelsman D.J., Campbell R.E. New perspectives on the pathogenesis of PCOS: neuroendocrine origins. Trends in Endocrinology and Metabolism. 2018 doi: 10.1016/j.tem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Cimino I., Casoni F., Liu X., Messina A., Parkash J., Jamin S.P. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nature Communications. 2016;7:10055. doi: 10.1038/ncomms10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H.H., DiVall S.A., Deneau R.M., Wolfe A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Molecular and Cellular Endocrinology. 2005;242(1–2):42–49. doi: 10.1016/j.mce.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Nestler J.E. Metformin for the treatment of the polycystic ovary syndrome. New England Journal of Medicine. 2008;358(1):47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 37.Baillargeon J.P., Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertility and Sterility. 2007;88(4):886–893. doi: 10.1016/j.fertnstert.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baillargeon J.P., Nestler J.E. Commentary: polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? Journal of Clinical Endocrinology & Metabolism. 2006;91(1):22–24. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestler J.E., Jakubowicz D.J., de Vargas A.F., Brik C., Quintero N., Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. Journal of Clinical Endocrinology & Metabolism. 1998;83(6):2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 40.Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocrine Reviews. 1991;12(1):3–13. doi: 10.1210/edrv-12-1-3. [DOI] [PubMed] [Google Scholar]
- 41.Nestler J.E., Powers L.P., Matt D.W., Steingold K.A., Plymate S.R., Rittmaster R.S. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1991;72(1):83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 42.Plymate S.R., Matej L.A., Jones R.E., Friedl K.E. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. Journal of Clinical Endocrinology & Metabolism. 1988;67(3):460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 43.Adashi E.Y., Hsueh A.J., Yen S.S. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108(4):1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- 44.Velazquez E.M., Mendoza S., Hamer T., Sosa F., Glueck C.J. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43(5):647–654. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 45.Dunaif A., Scott D., Finegood D., Quintana B., Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1996;81(9):3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 46.Katsiki N., Hatzitolios A.I. Insulin-sensitizing agents in the treatment of polycystic ovary syndrome: an update. Current Opinion in Obstetrics and Gynecology. 2010;22(6):466–476. doi: 10.1097/GCO.0b013e32833e1264. [DOI] [PubMed] [Google Scholar]
- 47.Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome--a reappraisal. Nature Clinical Practice Endocrinology & Metabolism. 2008;4(5):272–283. doi: 10.1038/ncpendmet0787. [DOI] [PubMed] [Google Scholar]
- 48.Froment P., Touraine P. Thiazolidinediones and fertility in polycystic ovary syndrome (PCOS) PPAR Research. 2006;2006:73986. doi: 10.1155/PPAR/2006/73986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathan N., Sullivan S.D. The utility of metformin therapy in reproductive-aged women with polycystic ovary syndrome (PCOS) Current Pharmaceutical Biotechnology. 2014;15(1):70–83. doi: 10.2174/1389201015666140330195142. [DOI] [PubMed] [Google Scholar]
- 50.Barker D.J., Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 51.Barker D.J., Gluckman P.D., Godfrey K.M., Harding J.E., Owens J.A., Robinson J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 52.Filippou P., Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Human Reproduction Update. 2017;23(4):421–432. doi: 10.1093/humupd/dmx013. [DOI] [PubMed] [Google Scholar]
- 53.Abbott D.H., Tarantal A.F., Dumesic D.A. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. American Journal of Primatology. 2009;71(9):776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbott D.H., Barnett D.K., Bruns C.M., Dumesic D.A. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Human Reproduction Update. 2005;11(4):357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 55.Demissie M., Lazic M., Foecking E.M., Aird F., Dunaif A., Levine J.E. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. American Journal of Physiology. Endocrinology and Metabolism. 2008;295(2):E262–E268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foecking E.M., Szabo M., Schwartz N.B., Levine J.E. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biology of Reproduction. 2005;72(6):1475–1483. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- 57.Cernea M., Padmanabhan V., Goodman R.L., Coolen L.M., Lehman M.N. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277–3291. doi: 10.1210/en.2014-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbott D.H., Zhou R., Bird I.M., Dumesic D.A., Conley A.J. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocrine Development. 2008;13:145–158. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nohara K., Waraich R.S., Liu S., Ferron M., Waget A., Meyers M.S. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. American Journal of Physiology. Endocrinology and Metabolism. 2013;304(12):E1321–E1330. doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehrmann D.A., Barnes R.B., Rosenfield R.L. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocrine Reviews. 1995;16(3):322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 61.Barnes R.B., Rosenfield R.L., Ehrmann D.A., Cara J.F., Cuttler L., Levitsky L.L. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. Journal of Clinical Endocrinology & Metabolism. 1994;79(5):1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 62.Palomba S., Marotta R., Di Cello A., Russo T., Falbo A., Orio F. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clinical Endocrinology. 2012;77(6):898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- 63.Sir-Petermann T., Maliqueo M., Angel B., Lara H.E., Perez-Bravo F., Recabarren S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction. 2002;17(10):2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 64.Puttabyatappa M., Cardoso R.C., Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring's health. Molecular and Cellular Endocrinology. 2016;435:29–39. doi: 10.1016/j.mce.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maliqueo M., Lara H.E., Sanchez F., Echiburu B., Crisosto N., Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2013;166(2):151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Tata B., Mimouni N.E.H., Barbotin A.L., Malone S.A., Loyens A., Pigny P. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nature Medicine. 2018;24(6):834–846. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cresswell J.L., Barker D.J., Osmond C., Egger P., Phillips D.I., Fraser R.B. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997;350(9085):1131–1135. doi: 10.1016/s0140-6736(97)06062-5. [DOI] [PubMed] [Google Scholar]
- 68.Ibanez L., de Zegher F., Potau N. Premature pubarche, ovarian hyperandrogenism, hyperinsulinism and the polycystic ovary syndrome: from a complex constellation to a simple sequence of prenatal onset. Journal of Endocrinological Investigation. 1998;21(9):558–566. doi: 10.1007/BF03350781. [DOI] [PubMed] [Google Scholar]
- 69.Pandolfi C., Zugaro A., Lattanzio F., Necozione S., Barbonetti A., Colangeli M.S. Low birth weight and later development of insulin resistance and biochemical/clinical features of polycystic ovary syndrome. Metabolism. 2008;57(7):999–1004. doi: 10.1016/j.metabol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 70.Melo A.S., Vieira C.S., Barbieri M.A., Rosa E.S.A.C., Silva A.A., Cardoso V.C. High prevalence of polycystic ovary syndrome in women born small for gestational age. Human Reproduction. 2010;25(8):2124–2131. doi: 10.1093/humrep/deq162. [DOI] [PubMed] [Google Scholar]
- 71.Diamanti-Kandarakis E., Kandarakis H., Legro R.S. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30(1):19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- 72.Huber-Buchholz M.M., Carey D.G., Norman R.J. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. Journal of Clinical Endocrinology & Metabolism. 1999;84(4):1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 73.Kiddy D.S., Hamilton-Fairley D., Bush A., Short F., Anyaoku V., Reed M.J. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clinical Endocrinology (Oxf) 1992;36(1):105–111. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez M., Bourguignon N., Lux-Lantos V., Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environmental Health Perspectives. 2010;118(9):1217–1222. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collet S.H., Picard-Hagen N., Viguie C., Lacroix M.Z., Toutain P.L., Gayrard V. Estrogenicity of bisphenol a: a concentration-effect relationship on luteinizing hormone secretion in a sensitive model of prepubertal lamb. Toxicological Sciences. 2010;117(1):54–62. doi: 10.1093/toxsci/kfq186. [DOI] [PubMed] [Google Scholar]
- 76.Kurian J.R., Keen K.L., Kenealy B.P., Garcia J.P., Hedman C.J., Terasawa E. Acute influences of bisphenol A exposure on hypothalamic release of gonadotropin-releasing hormone and Kisspeptin in female rhesus monkeys. Endocrinology. 2015;156(7):2563–2570. doi: 10.1210/en.2014-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rezg R., El-Fazaa S., Gharbi N., Mornagui B. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environment International. 2014;64:83–90. doi: 10.1016/j.envint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Hu Y., Wen S., Yuan D., Peng L., Zeng R., Yang Z. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecological Endocrinology. 2018;34(5):370–377. doi: 10.1080/09513590.2017.1405931. [DOI] [PubMed] [Google Scholar]
- 79.Franks S., Webber L.J., Goh M., Valentine A., White D.M., Conway G.S. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. Journal of Clinical Endocrinology & Metabolism. 2008;93(9):3396–3402. doi: 10.1210/jc.2008-0369. [DOI] [PubMed] [Google Scholar]
- 80.Legro R.S., Driscoll D., Strauss J.F., 3rd, Fox J., Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the U S A. 1998;95(25):14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vink J.M., Sadrzadeh S., Lambalk C.B., Boomsma D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. Journal of Clinical Endocrinology & Metabolism. 2006;91(6):2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 82.Hiam D., Moreno-Asso A., Teede H.J., Laven J.S.E., Stepto N.K., Moran L.J. The genetics of polycystic ovary syndrome: an overview of candidate gene systematic reviews and genome-wide association studies. Journal of Clinical Medicine. 2019;8(10) doi: 10.3390/jcm8101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salehi Jahromi M., Hill J.W., Ramezani Tehrani F., Zadeh-Vakili A. Hypomethylation of specific CpG sites in the promoter region of steroidogeneic genes (GATA6 and StAR) in prenatally androgenized rats. Life Sciences. 2018;207:105–109. doi: 10.1016/j.lfs.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 84.Zhang D., Cong J., Shen H., Wu Q., Wu X. Genome-wide identification of aberrantly methylated promoters in ovarian tissue of prenatally androgenized rats. Fertility and Sterility. 2014;102(5):1458–1467. doi: 10.1016/j.fertnstert.2014.07.1203. [DOI] [PubMed] [Google Scholar]
- 85.Guo X., Puttabyatappa M., Thompson R.C., Padmanabhan V. Developmental programming: contribution of epigenetic enzymes to antral follicular defects in the sheep model of PCOS. Endocrinology. 2019;160(10):2471–2484. doi: 10.1210/en.2019-00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu N., Kwon S., Abbott D.H., Geller D.H., Dumesic D.A., Azziz R. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PloS One. 2011;6(11) doi: 10.1371/journal.pone.0027286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu J.Q., Zhu L., Liang X.W., Xing F.Q., Schatten H., Sun Q.Y. Demethylation of LHR in dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Molecular Human Reproduction. 2010;16(4):260–266. doi: 10.1093/molehr/gap089. [DOI] [PubMed] [Google Scholar]
- 88.Qu F., Wang F.F., Yin R., Ding G.L., El-Prince M., Gao Q. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. Journal of Molecular Medicine (Berlin) 2012;90(8):911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 89.Bouchard C., Despres J.P., Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocrine Reviews. 1993;14(1):72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 90.Evans D.J., Barth J.H., Burke C.W. Body fat topography in women with androgen excess. International Journal of Obesity. 1988;12(2):157–162. [PubMed] [Google Scholar]
- 91.Borruel S., Fernandez-Duran E., Alpanes M., Marti D., Alvarez-Blasco F., Luque-Ramirez M. Global adiposity and thickness of intraperitoneal and mesenteric adipose tissue depots are increased in women with polycystic ovary syndrome (PCOS) Journal of Clinical Endocrinology & Metabolism. 2013;98(3):1254–1263. doi: 10.1210/jc.2012-3698. [DOI] [PubMed] [Google Scholar]
- 92.Elbers J.M., Asscheman H., Seidell J.C., Megens J.A., Gooren L.J. Long-term testosterone administration increases visceral fat in female to male transsexuals. Journal of Clinical Endocrinology & Metabolism. 1997;82(7):2044–2047. doi: 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- 93.Gambineri A., Patton L., Vaccina A., Cacciari M., Morselli-Labate A.M., Cavazza C. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. Journal of Clinical Endocrinology & Metabolism. 2006;91(10):3970–3980. doi: 10.1210/jc.2005-2250. [DOI] [PubMed] [Google Scholar]
- 94.Kwon H., Kim D., Kim J.S. Body fat distribution and the risk of incident metabolic syndrome: a longitudinal Cohort study. Scientific Reports. 2017;7(1):10955. doi: 10.1038/s41598-017-09723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nohara K., Laque A., Allard C., Munzberg H., Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring) 2014;22(6):1477–1484. doi: 10.1002/oby.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dimitriadis G.K., Kyrou I., Randeva H.S. Polycystic ovary syndrome as a proinflammatory state: the role of adipokines. Current Pharmaceutical Design. 2016;22(36):5535–5546. doi: 10.2174/1381612822666160726103133. [DOI] [PubMed] [Google Scholar]
- 97.Echiburu B., Perez-Bravo F., Galgani J.E., Sandoval D., Saldias C., Crisosto N. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids. 2018;130:15–21. doi: 10.1016/j.steroids.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Escobar-Morreale H.F., Luque-Ramirez M., Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertility and Sterility. 2011;95(3):1048–1058. doi: 10.1016/j.fertnstert.2010.11.036. e1041-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carmina E., Chu M.C., Moran C., Tortoriello D., Vardhana P., Tena G. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertility and Sterility. 2008;89(3):642–648. doi: 10.1016/j.fertnstert.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 100.Manneras L., Cajander S., Holmang A., Seleskovic Z., Lystig T., Lonn M. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 101.Perello M., Castrogiovanni D., Giovambattista A., Gaillard R.C., Spinedi E. Impairment in insulin sensitivity after early androgenization in the post-pubertal female rat. Life Sciences. 2007;80(19):1792–1798. doi: 10.1016/j.lfs.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Puttabyatappa M., Lu C., Martin J.D., Chazenbalk G., Dumesic D., Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on steroidal machinery and cell differentiation markers in visceral adipocytes of female sheep. Reproductive Sciences. 2018;25(7):1010–1023. doi: 10.1177/1933719117746767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keller E., Chazenbalk G.D., Aguilera P., Madrigal V., Grogan T., Elashoff D. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155(7):2696–2703. doi: 10.1210/en.2014-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 105.Gupta V., Bhasin S., Guo W., Singh R., Miki R., Chauhan P. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Molecular and Cellular Endocrinology. 2008;296(1–2):32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blouin K., Nadeau M., Perreault M., Veilleux A., Drolet R., Marceau P. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clinical Endocrinology. 2010;72(2):176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 107.Bennett C.N., Ross S.E., Longo K.A., Bajnok L., Hemati N., Johnson K.W. Regulation of Wnt signaling during adipogenesis. Journal of Biological Chemistry. 2002;277(34):30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 108.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 109.Singh R., Artaza J.N., Taylor W.E., Braga M., Yuan X., Gonzalez-Cadavid N.F. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147(1):141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kliewer S.A., Lenhard J.M., Willson T.M., Patel I., Morris D.C., Lehmann J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 111.Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B.M., Evans R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 112.Heinlein C.A., Ting H.J., Yeh S., Chang C. Identification of ARA70 as a ligand-enhanced coactivator for the peroxisome proliferator-activated receptor gamma. Journal of Biological Chemistry. 1999;274(23):16147–16152. doi: 10.1074/jbc.274.23.16147. [DOI] [PubMed] [Google Scholar]
- 113.Chazenbalk G., Singh P., Irge D., Shah A., Abbott D.H., Dumesic D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78(9):920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dicker A., Ryden M., Naslund E., Muehlen I.E., Wiren M., Lafontan M. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47(3):420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 115.Anderson L.A., McTernan P.G., Harte A.L., Barnett A.H., Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diabetes, Obesity and Metabolism. 2002;4(3):209–213. doi: 10.1046/j.1463-1326.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 116.Varlamov O., Chu M.P., McGee W.K., Cameron J.L., O'Rourke R.W., Meyer K.A. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154(11):4126–4135. doi: 10.1210/en.2013-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varlamov O., Bishop C.V., Handu M., Takahashi D., Srinivasan S., White A. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Human Reproduction. 2017;32(9):1892–1902. doi: 10.1093/humrep/dex244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zang H., Ryden M., Wahlen K., Dahlman-Wright K., Arner P., Linden Hirschberg A. Effects of testosterone and estrogen treatment on lipolysis signaling pathways in subcutaneous adipose tissue of postmenopausal women. Fertility and Sterility. 2007;88(1):100–106. doi: 10.1016/j.fertnstert.2006.11.088. [DOI] [PubMed] [Google Scholar]
- 119.O'Reilly M.W., Kempegowda P., Walsh M., Taylor A.E., Manolopoulos K.N., Allwood J.W. AKR1C3-Mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3327–3339. doi: 10.1210/jc.2017-00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishizawa H., Shimomura I., Kishida K., Maeda N., Kuriyama H., Nagaretani H. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 121.Manneras-Holm L., Leonhardt H., Kullberg J., Jennische E., Oden A., Holm G. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2011;96(2):E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 122.van Houten E.L., Kramer P., McLuskey A., Karels B., Themmen A.P., Visser J.A. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861–2869. doi: 10.1210/en.2011-1754. [DOI] [PubMed] [Google Scholar]
- 123.Benrick A., Chanclon B., Micallef P., Wu Y., Hadi L., Shelton J.M. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proceedings of the National Academy of Sciences of the U S A. 2017;114(34):E7187–E7196. doi: 10.1073/pnas.1708854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ozgen I.T., Oruclu S., Selek S., Kutlu E., Guzel G., Cesur Y. Omentin-1 level in adolescents with polycystic ovarian syndrome. Pediatrics International. 2019;61(2):147–151. doi: 10.1111/ped.13761. [DOI] [PubMed] [Google Scholar]
- 125.Sacks H., Symonds M.E. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62(6):1783–1790. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.M. U.D., Saari T., Raiko J., Kudomi N., Maurer S.F., Lahesmaa M. Postprandial oxidative metabolism of human Brown fat indicates thermogenesis. Cell Metabolism. 2018;28(2):207–216 e203. doi: 10.1016/j.cmet.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 127.Marzetti E., D'Angelo E., Savera G., Leeuwenburgh C., Calvani R. Integrated control of brown adipose tissue. Heart Metabolism. 2016;69:9–14. [PMC free article] [PubMed] [Google Scholar]
- 128.Shorakae S., Jona E., de Courten B., Lambert G.W., Lambert E.A., Phillips S.E. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clinical Endocrinology (Oxf) 2019;90(3):425–432. doi: 10.1111/cen.13913. [DOI] [PubMed] [Google Scholar]
- 129.Monjo M., Rodriguez A.M., Palou A., Roca P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144(11):4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 130.Rodriguez-Cuenca S., Monjo M., Gianotti M., Proenza A.M., Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(1):E340–E346. doi: 10.1152/ajpendo.00175.2006. [DOI] [PubMed] [Google Scholar]
- 131.Yuan X., Hu T., Zhao H., Huang Y., Ye R., Lin J. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the U S A. 2016;113(10):2708–2713. doi: 10.1073/pnas.1523236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu T., Yuan X., Ye R., Zhou H., Lin J., Zhang C. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. The Journal of Nutritional Biochemistry. 2017;47:21–28. doi: 10.1016/j.jnutbio.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 133.Liu X., Cervantes C., Liu F. Common and distinct regulation of human and mouse brown and beige adipose tissues: a promising therapeutic target for obesity. Protein Cell. 2017;8(6):446–454. doi: 10.1007/s13238-017-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 135.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 137.Ravussin E., Galgani J.E. The implication of brown adipose tissue for humans. Annual Review of Nutrition. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Herz C.T., Kiefer F.W. Adipose tissue browning in mice and humans. Journal of Endocrinology. 2019;241(3):R97–R109. [PubMed] [Google Scholar]
- 139.Rocha A.L.L., Faria L.C., Guimaraes T.C.M., Moreira G.V., Candido A.L., Couto C.A. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta-analysis. Journal of Endocrinological Investigation. 2017;40(12):1279–1288. doi: 10.1007/s40618-017-0708-9. [DOI] [PubMed] [Google Scholar]
- 140.Kim J.J., Kim D., Yim J.Y., Kang J.H., Han K.H., Kim S.M. Polycystic ovary syndrome with hyperandrogenism as a risk factor for non-obese non-alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics. 2017;45(11):1403–1412. doi: 10.1111/apt.14058. [DOI] [PubMed] [Google Scholar]
- 141.Wu J., Yao X.Y., Shi R.X., Liu S.F., Wang X.Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reproductive Health. 2018;15(1):77. doi: 10.1186/s12978-018-0519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cerda C., Perez-Ayuso R.M., Riquelme A., Soza A., Villaseca P., Sir-Petermann T. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Journal of Hepatology. 2007;47(3):412–417. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 143.Gambarin-Gelwan M., Kinkhabwala S.V., Schiano T.D., Bodian C., Yeh H.C., Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clinical Gastroenterology and Hepatology. 2007;5(4):496–501. doi: 10.1016/j.cgh.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 144.Jones H., Sprung V.S., Pugh C.J., Daousi C., Irwin A., Aziz N. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2012;97(10):3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 145.Chen M.J., Chiu H.M., Chen C.L., Yang W.S., Yang Y.S., Ho H.N. Hyperandrogenemia is independently associated with elevated alanine aminotransferase activity in young women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2010;95(7):3332–3341. doi: 10.1210/jc.2009-2698. [DOI] [PubMed] [Google Scholar]
- 146.Vassilatou E., Lafoyianni S., Vryonidou A., Ioannidis D., Kosma L., Katsoulis K. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Human Reproduction. 2010;25(1):212–220. doi: 10.1093/humrep/dep380. [DOI] [PubMed] [Google Scholar]
- 147.Cai J., Wu C.H., Zhang Y., Wang Y.Y., Xu W.D., Lin T.C. High-free androgen index is associated with increased risk of non-alcoholic fatty liver disease in women with polycystic ovary syndrome, independent of obesity and insulin resistance. International Journal of Obesity (Lond) 2017;41(9):1341–1347. doi: 10.1038/ijo.2017.116. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y., Meng F., Sun X., Sun X., Hu M., Cui P. Hyperandrogenism and insulin resistance contribute to hepatic steatosis and inflammation in female rat liver. OncoTarget. 2018;9(26):18180–18197. doi: 10.18632/oncotarget.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baranova A., Tran T.P., Afendy A., Wang L., Shamsaddini A., Mehta R. Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS) Journal of Translational Medicine. 2013;11:133. doi: 10.1186/1479-5876-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abruzzese G.A., Heber M.F., Ferreira S.R., Velez L.M., Reynoso R., Pignataro O.P. Prenatal hyperandrogenism induces alterations that affect liver lipid metabolism. Journal of Endocrinology. 2016;230(1):67–79. doi: 10.1530/JOE-15-0471. [DOI] [PubMed] [Google Scholar]
- 151.Navarro G., Allard C., Xu W., Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23(4):713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dunaif A., Xia J., Book C.B., Schenker E., Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. Journal of Clinical Investigation. 1995;96(2):801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hojlund K., Glintborg D., Andersen N.R., Birk J.B., Treebak J.T., Frosig C. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57(2):357–366. doi: 10.2337/db07-0706. [DOI] [PubMed] [Google Scholar]
- 154.Stepto N.K., Moreno-Asso A., McIlvenna L.C., Walters K.A., Rodgers R.J. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Unraveling the conundrum in skeletal muscle? Journal of Clinical Endocrinology & Metabolism. 2019 doi: 10.1210/jc.2019-00167. [DOI] [PubMed] [Google Scholar]
- 155.Hansen S.L., Svendsen P.F., Jeppesen J.F., Hoeg L.D., Andersen N.R., Kristensen J.M. Molecular mechanisms in skeletal muscle underlying insulin resistance in women who are lean with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2019;104(5):1841–1854. doi: 10.1210/jc.2018-01771. [DOI] [PubMed] [Google Scholar]
- 156.Holmang A., Svedberg J., Jennische E., Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. American Journal of Physiology. 1990;259(4 Pt 1):E555–E560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- 157.Holmang A., Niklasson M., Rippe B., Lonnroth P. Insulin insensitivity and delayed transcapillary delivery of insulin in oophorectomized rats treated with testosterone. Acta Physiologica Scandinavica. 2001;171(4):427–438. doi: 10.1046/j.1365-201X.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 158.Song X., Shen Q., Fan L., Yu Q., Jia X., Sun Y. Dehydroepiandrosterone-induced activation of mTORC1 and inhibition of autophagy contribute to skeletal muscle insulin resistance in a mouse model of polycystic ovary syndrome. OncoTarget. 2018;9(15):11905–11921. doi: 10.18632/oncotarget.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen Q., Bi H., Yu F., Fan L., Zhu M., Jia X. Nontargeted metabolomic analysis of skeletal muscle in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Molecular Reproduction and Development. 2019;86(4):370–378. doi: 10.1002/mrd.23111. [DOI] [PubMed] [Google Scholar]
- 160.Zhang B., Wang J., Shen S., Liu J., Sun J., Gu T. Association of androgen excess with glucose intolerance in women with polycystic ovary syndrome. BioMed Research International. 2018;2018:6869705. doi: 10.1155/2018/6869705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu W., Morford J., Mauvais-Jarvis F. Emerging role of testosterone in pancreatic beta cell function and insulin secretion. Journal of Endocrinology. 2019 doi: 10.1530/JOE-18-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rae M., Grace C., Hogg K., Wilson L.M., McHaffie S.L., Ramaswamy S. The pancreas is altered by in utero androgen exposure: implications for clinical conditions such as polycystic ovary syndrome (PCOS) PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harada N., Yotsumoto Y., Katsuki T., Yoda Y., Masuda T., Nomura M. Fetal androgen signaling defects affect beta cell mass and function, leading to glucose intolerance in high-fat diet-fed male rats. American Journal of Physiology. Endocrinology and Metabolism. 2019 doi: 10.1152/ajpendo.00173.2019. [DOI] [PubMed] [Google Scholar]
- 164.Navarro G., Allard C., Morford J.J., Xu W., Liu S., Molinas A.J. Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight. 2018;3(12) doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang H., Wang X., Zhu Y., Chen F., Sun Y., Han X. Increased androgen levels in rats impair glucose-stimulated insulin secretion through disruption of pancreatic beta cell mitochondrial function. The Journal of Steroid Biochemistry and Molecular Biology. 2015;154:254–266. doi: 10.1016/j.jsbmb.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 166.Liu S., Navarro G., Mauvais-Jarvis F. Androgen excess produces systemic oxidative stress and predisposes to beta cell failure in female mice. PloS One. 2010;5(6) doi: 10.1371/journal.pone.0011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Navarro G., Xu W., Jacobson D.A., Wicksteed B., Allard C., Zhang G. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metabolism. 2016;23(5):837–851. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramaswamy S., Grace C., Mattei A.A., Siemienowicz K., Brownlee W., MacCallum J. Developmental programming of polycystic ovary syndrome (PCOS): prenatal androgens establish pancreatic islet alpha/beta cell ratio and subsequent insulin secretion. Scientific Reports. 2016;6:27408. doi: 10.1038/srep27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nicol L.E., O'Brien T.D., Dumesic D.A., Grogan T., Tarantal A.F., Abbott D.H. Abnormal infant islet morphology precedes insulin resistance in PCOS-like monkeys. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Malin S.K., Kirwan J.P., Sia C.L., Gonzalez F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic beta cell dysfunction in polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2014;99(1):322–329. doi: 10.1210/jc.2013-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Malin S.K., Kirwan J.P., Sia C.L., Gonzalez F. Pancreatic beta cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-kappaB activation and systemic inflammation. American Journal of Physiology. Endocrinology and Metabolism. 2015;308(9):E770–E777. doi: 10.1152/ajpendo.00510.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sheppard K.M., Padmanabhan V., Coolen L.M., Lehman M.N. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the Ewe. Journal of Neuroendocrinology. 2011;23(5):401–411. doi: 10.1111/j.1365-2826.2011.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Konner A.C., Janoschek R., Plum L., Jordan S.D., Rother E., Ma X. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metabolism. 2007;5(6):438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 174.Nohara K., Zhang Y., Waraich R.S., Laque A., Tiano J.P., Tong J. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152(4):1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Caldwell A.S.L., Edwards M.C., Desai R., Jimenez M., Gilchrist R.B., Handelsman D.J. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the U S A. 2017;114(16):E3334–E3343. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lim S.S., Hutchison S.K., Van Ryswyk E., Norman R.J., Teede H.J., Moran L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews. 2019;3:CD007506. doi: 10.1002/14651858.CD007506.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–128. doi: 10.1177/2042018810380215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aubuchon M., Lieman H., Stein D., Cohen H.W., Isaac B., Adel G. Metformin does not improve the reproductive or metabolic profile in women with polycystic ovary syndrome (PCOS) Reproductive Sciences. 2009;16(10):938–946. doi: 10.1177/1933719109340925. [DOI] [PubMed] [Google Scholar]
- 179.Sturrock N.D., Lannon B., Fay T.N. Metformin does not enhance ovulation induction in clomiphene resistant polycystic ovary syndrome in clinical practice. British Journal of Clinical Pharmacology. 2002;53(5):469–473. doi: 10.1046/j.1365-2125.2002.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Legro R.S., Barnhart H.X., Schlaff W.D., Carr B.R., Diamond M.P., Carson S.A. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. New England Journal of Medicine. 2007;356(6):551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 181.Palomba S., Orio F., Jr., Falbo A., Manguso F., Russo T., Cascella T. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2005;90(7):4068–4074. doi: 10.1210/jc.2005-0110. [DOI] [PubMed] [Google Scholar]
- 182.Girard J. [Mechanisms of action of thiazolidinediones] Diabetes & Metabolism. 2001;27(2 Pt 2):271–278. [PubMed] [Google Scholar]
- 183.Veiga-Lopez A., Lee J.S., Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151(8):4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou R., Bruns C.M., Bird I.M., Kemnitz J.W., Goodfriend T.L., Dumesic D.A. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reproductive Toxicology. 2007;23(3):438–448. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Du Q., Yang S., Wang Y.J., Wu B., Zhao Y.Y., Fan B. Effects of thiazolidinediones on polycystic ovary syndrome: a meta-analysis of randomized placebo-controlled trials. Advances in Therapy. 2012;29(9):763–774. doi: 10.1007/s12325-012-0044-6. [DOI] [PubMed] [Google Scholar]
- 186.Tuduri E., Lopez M., Dieguez C., Nadal A., Nogueiras R. Glucagon-like peptide 1 analogs and their effects on pancreatic islets. Trends in Endocrinology and Metabolism. 2016;27(5):304–318. doi: 10.1016/j.tem.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 187.Muller T.D. The potential of glucagon-like peptide 1 to reverse high-fat, high-sugar diet-related metabolic damage. Expert Review of Endocrinology and Metabolism. 2014;9(4):293–295. doi: 10.1586/17446651.2014.914850. [DOI] [PubMed] [Google Scholar]
- 188.Lamos E.M., Malek R., Davis S.N. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Review of Clinical Pharmacology. 2017;10(4):401–408. doi: 10.1080/17512433.2017.1292125. [DOI] [PubMed] [Google Scholar]
- 189.Rasmussen C.B., Lindenberg S. The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Frontiers in Endocrinology (Lausanne) 2014;5:140. doi: 10.3389/fendo.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jensterle M., Kravos N.A., Pfeifer M., Kocjan T., Janez A. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones (Athens) 2015;14(1):81–90. doi: 10.1007/BF03401383. [DOI] [PubMed] [Google Scholar]
- 191.Han Y., Li Y., He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reproductive BioMedicine Online. 2019;39(2):332–342. doi: 10.1016/j.rbmo.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 192.Elkind-Hirsch K., Marrioneaux O., Bhushan M., Vernor D., Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2670–2678. doi: 10.1210/jc.2008-0115. [DOI] [PubMed] [Google Scholar]
- 193.Jensterle Sever M., Kocjan T., Pfeifer M., Kravos N.A., Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. European Journal of Endocrinology. 2014;170(3):451–459. doi: 10.1530/EJE-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li Y.J., Han Y., He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surgery for Obesity and Related Diseases. 2019;15(6):942–950. doi: 10.1016/j.soard.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 195.Menguer R.K., Weston A.C., Schmid H. Evaluation of metabolic syndrome in morbidly obese patients submitted to laparoscopic bariatric surgery: comparison of the results between roux-En-Y gastric bypass and sleeve gastrectomy. Obesity Surgery. 2017;27(7):1719–1723. doi: 10.1007/s11695-017-2547-3. [DOI] [PubMed] [Google Scholar]
- 196.Ressler I.B., Grayson B.E., Seeley R.J. Metabolic, behavioral, and reproductive effects of vertical sleeve gastrectomy in an obese rat model of polycystic ovary syndrome. Obesity Surgery. 2014;24(6):866–876. doi: 10.1007/s11695-013-1153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brandt S.J., Gotz A., Tschop M.H., Muller T.D. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides. 2018;100:190–201. doi: 10.1016/j.peptides.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]