Abstract
Granulomatous polyangiitis (GPA) is a multiple systemic necrotizing vasculitis. Diagnosis of pulmonary nodules in GPA is still challenging in clinical practice, however, other extrapulmonary manifestations, serology, and histopathology may help the diagnosis of GPA. This case series was of limed GPA with one of the largest pulmonary nodules which had a poor treatment response in contrast with previous literature.
1. Introduction
Granulomatous polyangiitis (GPA), previously called Wegener's granulomatosis, is a multiple systemic necrotizing vasculitis first described by Friedrich Wegener in 1936 [1]. The median age at diagnosis of GPA is about 55 years old. It most commonly occurs in white patients (90%) without sexual difference [2]. Patients typically present with constitutional symptoms including fever, anorexia, malaise and weight loss [3]. Two interesting cases of GPA with large pulmonary nodules are described in this article.
2. Case 1
A 39-year old man was presented with painful sensation, redness and blurred vision of both eyes for 3 weeks. He also had multiple violaceous skin lesions on both arms and legs. His medical history was unremarkable. He was a non-smoker, but he was social alcohol drinker. He did not have a history of tuberculosis contact. He recalled no family history of cancer, tuberculosis, nor autoimmune diseases.
Physical examination revealed a temperature 38.1 °C, blood pressure 126/85 mmHg, pulse rate 90/minute, respiratory rate 16/minute, and O2 saturation 98% on ambient air. Ophthalmic examination revealed bilateral swollen lids, chemosis, ciliary injection, compatible with bilateral diffuse scleritis. He did not have an oral ulcer, saddle-nose deformity, sinus tenderness, or hair loss. Multiple pulpable painless violaceous papules were found along his legs and arms. The neurological, heart, lungs, abdomen, lymph nodes and musculoskeletal examinations were unremarkable.
The laboratory examination showed a hemoglobin of 10.3 g/dL, white blood cell count 11.9 × 103/μL (77% neutrophils, 11% lymphocytes), a platelet count 366 × 103/μL, CRP-QT 204.48, ESR 91 mm/hr, serum creatinine (Cr) 0.58 mg/dL, globulin 4.5 g/dL, albumin 3.6 g/dL, ALP 227 U/L (30–120), AST 39 U/L (0-32), ALT 25 U/L (0-25). Urinalysis revealed 2+ protein, dysmorphic RBC of 10–20 cells/HPF. A 24-h urine total protein was 1333 mg/volume. Hepatitis B, hepatitis C, and anti-HIV antibodies were negative. Serum VDRL, TPHA, RT-PCR for herpes virus, and QuantiFERON-TB gold tests were also negative. The c-ANCA (indirect immunofluorescent assay, IFA) and anti-PR3 antibodies (western blotting) were positive. Anti-RF antibody was positive 26.4 IU/L (0–20.0). Anti-MPO, ANA, anti-Ro and anti-La antibodies were negative.
Initially, infectious scleritis was suspected. An orbits CT showed left eye proptosis with bilateral scleritis, enlargement of bilateral lacrimal glands with mildly retrobulbar fat stranding and sinusitis at bilateral sphenoid and ethmoid sinuses. Nasal endoscopy revealed suppurative discharge in sphenoid and ethmoid recesses and nasal crust without mass. A pus culture was negative.
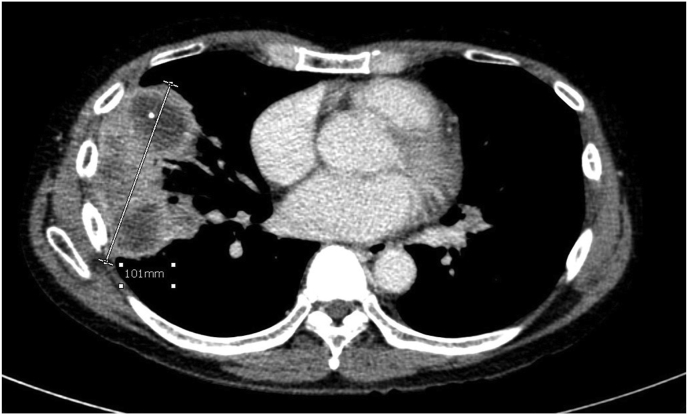
Chest radiography showed multiple pulmonary nodules in both lungs. A chest CT showed multiple lung masses with peripheral enhancements and perilesional ground glass opacities, the largest mass was 10.1 × 5.6 × 6.0 cm. Sub-centimeter mediastinal lymphadenopathy was noted (Fig. 1a). An abdominal CT showed 10.5-cm heterogenous enhancements of the spleen without space-occupying lesion. Liver and portal veins were unremarkable. Neither adenopathy nor ascites were seen.
Fig. 1a.
Chest CT showed the largest pulmonary mass at lateral segment of right middle lobe.
Bilateral conjunctival debridement and administration of topical and systemic ceftazidime and vancomycin were done. The scleritis, however, did not improve, and no organisms were found, conjunctival recess resection was performed. Conjunctival tissues revealed focal multinucleated giant cells with necrotizing vasculitis. AFB stains were negative. The IgG4 to IgG ratio was 0.29:1. Tissues from violaceous skin lesions were compatible with leukocytoclastic vasculitis.
The CT-guided lung mass biopsies for cultures, PCR for Xpert MTB/RIF assay, PCR for 18s rRNA were negative. The lung tissues showed geographic necrosis with few multinucleated giant cells, special staining for AFB, GMS, PAS, and cultures were negative.
The overall findings were compatible with GPA by ACR/EULAR 2017 provisional classification criteria. Prednisolone 1 mg/kg/day and intravenous cyclophosphamide 800 mg were started. The scleritis and lung mass were improved. Bronchoscopy was done 6 days later, hypervascularization and inflammation were seen along the bronchus (Fig. 2a). A transbronchial biopsy was done at the lateral segment of right middle lobe, and showed organizing pneumonia foci, a few necrotic tissues, no chronic granulomatous inflammation, no neoplasm, no vasculitis lesions, special staining for AFB, GMS, PAS, and cultures were negative. The final diagnosis was GPA with diffuse scleral, sinopulmonary, splenic, and mild renal involvements.
Fig. 2.
a: Hypervascularization and inflammation of carina.
After the 3rd dose of cyclophosphamide, the bilateral scleritis deteriorated, the right lung mass was enlarged, and the patient also had a new onset of bilateral sensorineural hearing loss. ESR was raised from 29 to 58 mm/hr. Bronchoscopy revealed progression of bronchial inflammation, and a transbronchial biopsy showed necrotic tissue with neutrophil infiltration. The bronchoalveolar lavage for mycobacterial, fungal cultures, and PCR for herpes were negative. Intravenous rituximab 375 mg/m2 was given due to clinical deterioration. During treatment, he developed severe secondary infection, clinical deterioration, and then expired.
3. Case 2
A 58-year-old woman presented with progressive painless visual loss in both eyes for 1 month. She had low grade fever, non-productive cough, and weight loss for 6 months.
Her medical history was notable for right Bell's palsy and bilateral hearing loss 1 year ago and was improved after a short course of oral corticosteroid. She denied any history of tuberculosis contact. She acknowledged no family history of cancer, tuberculosis, nor autoimmune diseases.
Physical examination revealed body temperature 37.7 °C, blood pressure 133/80 mmHg, pulse rate 96/minute, and respiratory rate 18/minute. Ophthalmic examination revealed peripheral ulcerative keratitis, necrotizing scleritis, impending corneal perforation on both eyes, right visual acuity (VA) 3/60, left VA 6/24, normal intraocular pressure (IOP). The examination of ear-nose-throat (ENT), cardiovascular system, lung, abdomen, extremities, lymph nodes, skin and neurological system were unremarkable.
The laboratory examination showed hemoglobin 12.2 g/dL, white blood cell count 12,930 cell/mm3 (74.6% neutrophils, 18% lymphocytes, 5.7% monocytes, 0.9% eosinophils), platelet count 927,000/mm3, Cr 1.0 mg/dL, BUN 15.2 mg/dL, globulin 3.7 g/dL, albumin 3.8 g/dL, and no hepatitis. Urinalysis revealed trace protein, WBC 0-1 cell/HPF, and RBC 0-1 cell/HPF. Serum VDRL, TPHA, hepatitis B, hepatitis C, and anti-HIV antibodies were negative. Anti-RF antibody was 124.60 IU/ml (0–20 IU/ml) but Anti-CCP antibody was <7 U/mL. ANA was positive for nucleolar pattern with titer 1:320. The c-ANCA (IFA) and anti-PR3 antibody (western blotting) were positive.
The chest radiograph revealed multiple mass-like lesions. A chest CT showed two heterogenous enhancing masses, one 3.6 cm in size located at the lateral segment of the right middle lobe and the other was 4.4 cm in size located at the superior segment of the right lower lobe. A CT-guided lung mass biopsy was done at right lower lobe lesion. The pathological findings were one focus of necrotizing vasculitis. The AFB stain, gram stain, GMS stain, PAS stain, and cultures were all negative.
One-gram methylprednisolone was given for 3 days as initial therapy, followed by monthly intravenous 800-mg cyclophosphamide and prednisolone for 6 months. During treatment, the ophthalmic and pulmonary lesions were improved. After 6 months of therapy, she developed new mass-like lesions at right lower lungs without ophthalmic lesion. Bronchoscopy was done and revealed an ulcerative lesion along the carina through the right main bronchus. All microbiological testing was negative.
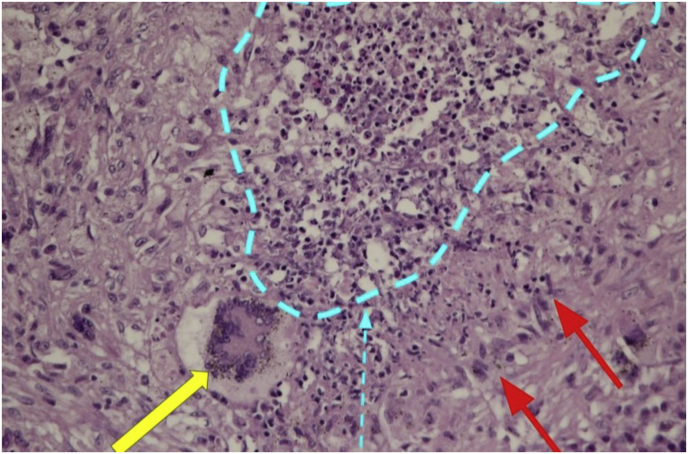
A right lower lobe wedge resection was performed due to isolated pulmonary lesions, and showed epithelioid histiocyte aggregation with central necrosis, eosinophils and multinucleated giant cells, lymphoplasmacytic infiltration in the vascular wall, compatible with granulomatous inflammation with destructive leukocytic angiitis. Special strains for AFB, GMS and PAS revealed no microorganisms (Fig. 1b). The final diagnosis was GPA with diffuse necrotizing scleritis, central airway, and lungs involvements by ACR/EULAR 2017 provisional classification criteria. Azathioprine and oral prednisolone were given for maintenance of remission. She was in remission state.
Fig. 1b.
Areas of multinucleated giant cells (yellow arrow), central necrosis (blue outlined area with arrow), and palisaded histiocytes (red arrow). (For interpretation of the reference to colour in this figure legend, the reader is referred to web version of this article.)
4. Discussion
Lung nodules are a common lower respiratory tract manifestation of GPA, they are usually multiple bilateral and generally tend to spare the apices. The size is typically 2–4 cm in diameter but can be as large as 10 cm4. Approximately, 25% of nodules larger than 2 cm have thick or thin wall cavitation, mimicking lung abscesses, metastases or septic infarcts [[4], [5],6]. A rapid incremental of gas-fluid levels is suggested superimposed on the infection. Hemorrhage secondary to small-vessel vasculitis may occur around the nodules and manifests as ground glass opacity on chest HRCT (halo sign) [7] which can mimic bronchioloalveolar carcinoma, hypervascular metastases or angioinvasive infection such as Asperillus fumigatus. Diffuse alveolar hemorrhage (DAH) due to capillaritis is a life-threatening manifestation seen with an estimated incidence of 7–45% [8]. A few necrotizing granulomas may be present, with angiocentric inflammatory changes. The inflammatory cells including neutrophils, lymphocytes, multinucleated giant cells and rarely eosinophils, are abundant in eosinophilic granulomatous polyangiitis (EGPA) [9]. Small areas of organizing pneumonia are often seen in association with small suppurative granulomas, foci of bland necrosis, multinucleated giant cells, and microabscesses [10]. The presence of vasculitis in an area of uninvolved lung tissue and necrosis of the vascular media are the findings that favors GPA over granulomatous infections [11].
This present case series was of two cases of large pulmonary nodules in GPA, one of them had cavitation, and was approximately 10.1 cm. In diameter, which was one of the largest reported pulmonary nodules in GPA, and was initially suspected as lung metastases, or lung abscesses, which are more common in clinical practice. Both cases presented with ocular involvement which is seen in 15% at presentation. The scleritis is found in 40% of ocular involvements, it often presented with sudden onset of bilateral diffuse anterior scleral inflammation with moderate-to-severe pain, peripheral ulcerative keratitis and corneal ulcer are more commonly observed in GPA, other ocular involvements are orbital pseudotumor, nasolacrimal duct obstruction, conjunctivitis, keratitis, episcleritis, and uveitis [[12], [13], [14]]. All of cases had hearing impairment. Nasal crusting is the most common manifestation (>90%) [2], while chronic rhinosinusitis, nasal septal perforation, saddle-nose deformity, and serous otitis can also be found in GPA [15].
All of cases had central airway involvement, which usually occurs with ENT involvement. More than one-half of the GPA had bronchoscopic lesions, which includes bronchial stenosis (32%), inflammatory lesions without stenosis (24%), and isolated hemorrhage (24%), ulceration or pseudotumor (17%), and isolated purulent secretion (3%) [16]. The central airway lesions usually have good response to a combination of immunosuppressive therapy. The airway dilation (with or without stenting) is required only in case of airway stenosis [16]. More than one-half of the GPA had cutaneous manifestation. The leukocytoclastic angiitis, which is purpura involving the lower legs that may be accompanied by focal necrosis and ulceration, is the most common manifestation. Urticaria, livedo reticularis, erythema nodosum, pyoderma gangrenosum, and sweet syndrome may also be found [17]. The splenic infraction, resulted from occlusion of the distal parenchymal splenic arteries, is a rare manifestation [18], which may appear on CT scan as a hypodense lesion. The possible complications were bleeding, splenic rupture, and abscess [18].
Renal manifestation is common in GPA, which ranges from asymptomatic microscopic hematuria to rapidly progressive glomerulonephritis. Absence of renal manifestation within first two years of onset is present in 15–23% [19]. The classic triad of GPA, which is upper respiratory tract, lung and renal involvement, is present in only 13% [20].
Approximately 90% of GPA, 70% of microscopic polyangiitis (MPA) and 50% of EGPA have a positive ANCA [2]. The major of patients with GPA have autoantibodies against serine proteinase 3 (PR3), which have a diffuse cytoplasmic staining pattern (c-ANCA), while autoantibodies against myeloperoxidase (MPO), which are perinuclear staining pattern (p-ANCA), are found in MPA, EGPA, pauci-immune glomerulonephritis and the drug-induced ANCA-associated vasculitis. About 20% of GPA have the alternative ANCA, and at least 10% of GPA have a negative ANCA [21]. The PR3-ANCA is associated with nodular and central airway disease. The MPO-ANCA is associated with usual interstitial pneumonia (UIP), bronchiectasis, DAH, lymphadenopathy, pleural effusion and pulmonary venous congestion [22].
The EULAR/ERA-EDTA 2015 recommendations for the management of ANCA-associated vasculitis recommend a combination of glucocorticoids and either cyclophosphamide or rituximab for induction therapy of organ/life-threatening GPA. Plasma exchange should be considered in patients with a rapid progressive renal failure (Cr > 5.7 mg/dL), diffuse alveolar hemorrhage, or concomitantly positive anti-glomerular basement membrane (Anti-GBM) antibody [23]. The comparative trials (CYCLOP trials) have shown that oral daily (2 mg/kg/day) and intravenous cyclophosphamide (15 mg/kg every 2–3 weeks) for 3–6 months induces remission in 9 months at a similar rate (88.1 vs. 87.7%). Incidence of leukopenia was lower with a intravenous regimen [24]. Rituximab is an alternative to cyclophosphamide with a similar remission rate and adverse events [25,26]. However, the evidence of optimal dose, route, and duration of glucocorticoids remain uncertain. Intravenous methylprednisolone, typically in doses of 1000 mg daily for three days, is often used for severe end organ involvement, although the evidence base supporting this practice is weak [27].
Methotrexate and mycophenolate mofetil are an alternative to cyclophosphamide in non-organ-threatening GPA such as rhinosinusitis, or pulmonary nodules. The maintenance of remission therapy consists of azathioprine, rituximab, or methotrexate, which are given for 12–36 months for sustaining remission [[28], [29], [30]].
The predictors of mortality of GPA are dialysis dependent, older age, and a presence of organ involvement [31]. The predictors of severe late organ damage are heart involvement, Cr ≥ 1 mg/dL, and an increased ESR of 10 mm/hr31. The predictor of treatment resistance, which defined as progression of disease despite immunosuppressive therapy, is an increased age of 10 years [32], while female sex and Cr ≥ 1 mg/dL tended to be associated with treatment resistance [32]. The results of multivariable survival analysis for the risk of relapse, which defined as worsening of previous manifestations after a period of at least partial remission, in a patient with GPA based on clinical manifestations at the time of diagnosis with adjustment for the use of cyclophosphamide showed that cavity nodules trended to have an increased relapse rate but did not reach statistical significance, while a presence of anti-PR3 antibody, solid pulmonary nodules, ENT and renal involvement were not associated to the relapse rate [33].
The present case series were compatible with GPA by ACR/EULAR 2017 provisional classification criteria [34]. All of them were presented with large pulmonary nodules and multiorgan involvement. Both cases had initial response to the induction therapy. However, the first case had rapidly deteriorated, because he did not receive a combination of glucocorticoids and either cyclophosphamide or rituximab according to the guideline at the beginning because he was in the process of ruling out the infection, therefore he received only oral prednisolone (1 mg/kg/day) but after we confirm his diagnosis, we started intravenous cyclophosphamide as recommended, so we decided to give him rituximab for remission induction therapy after ruling out the infection. The second case had isolated pulmonary deterioration, so we decided to perform only wedge resection, and she was still in remission state.
The present case series were relapsed GPA in the patients who had large pulmonary nodules at presentation. Despite receiving an optimal treatment, the second case still had relapse. It was of interest to conduct the study of the size of pulmonary nodules in GPA and treatment responsiveness.
5. Conclusion
Diagnosis of pulmonary nodules in GPA is still challenging in clinical practice, however, other extrapulmonary manifestations, serology, and histopathology may help the diagnosis of GPA. This case series were of relapsed GPA with large pulmonary nodules. It was of interest to conduct the study of the size of pulmonary nodules and treatment responsiveness.
Declaration of competing interest
The authors of this case report have no conflict of interest.
Acknowledgements
The authors are grateful to the Faculty of Medicine, Khon Kaen University for the support and Professor James Arthur Will for assistance with the English-language presentation.
Footnotes
This case report was approved by the Human Research Ethics Committee of Khon Kaen University (HE631032).
References
- 1.Wegener F. Über eine eigenartige rhinogene Granulomatose mit besonderer Beteilgung des Arteriensytems und den Nieren. Beitr. Pathol. Anat. Allg. Pathol. 1939;102:36–68. [Google Scholar]
- 2.Seo P., Stone J.H. The antineutrophil cytoplasmic antibody-associated vasculitides. Am. J. Med. 2004;117(1):39. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Falk R.J., Hogan S., Carey T.S., Jennette J.C. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann. Intern. Med. 1990;113(9):656. doi: 10.7326/0003-4819-113-9-656. [DOI] [PubMed] [Google Scholar]
- 4.Allen S.D., Harvey C.J. Imaging of Wegener's granulomatosis. Br. J. Radiol. 2007;80:757–765. doi: 10.1259/bjr/34705892. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius E.S., Stone J.H., Hellman D.B., Fishman E.K. Wegener's granulomatosis: CT evolution of pulmonary parenchymal findings in treated disease. Crit. Rev. Comput. Tomogr. 2004;45:67–85. [PubMed] [Google Scholar]
- 6.Lohrmann C., Uhl M., Kotter E., Burger D., Ghanem N., Langer M. Pulmonary manifestations of Wegener granulomatosis: CT findings in 57 patients and a review of the literature. Eur. J. Radiol. 2005;53:471–477. doi: 10.1016/j.ejrad.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y., Lee K.S., Jung K.J., Han J., Kim J.S., Suh J.S. Halo sign on high-resolution CT: findings in spectrum of pulmonary diseases with pathologic correlation. J. Comput. Assist. Tomogr. 1999;23:622–626. doi: 10.1097/00004728-199907000-00025. [DOI] [PubMed] [Google Scholar]
- 8.See C.Q., Jaffe H.A., Schraufnagel D.E. Dyspnea and hemoptysis develop in a young man with prostitis. Chest. 2005;128:3625–3628. doi: 10.1378/chest.128.5.3625. [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein A.L., Locke W.K. Solitary lung lesions in Wegener's granulomatosis. Pathologic findings and clinical significance in 25 cases. Am. J. Surg. Pathol. 1995;19(5):545. [PubMed] [Google Scholar]
- 10.Travis W.D., Hoffman G.S., Leavitt R.Y., Pass H.I., Fauci A.S. Surgical pathology of the lung in Wegener's granulomatosis. Review of 87 open lung biopsies from 67 patients. Am. J. Surg. Pathol. 1991;15(4):315. doi: 10.1097/00000478-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Yousem S.A. Wegener's granulomatosis. In: Churg A.M., Myers J.L., Tazelaar H.D., Wright J.L., editors. Thurlbeck's Pathology of the Lung. 3rd. Thieme; New York: 2005. p. 371. [Google Scholar]
- 12.Hoffman G.S., Kerr G.S., Leavitt R.Y. Wegener's granulomatosis: an analysis of 158 patients. Ann. Intern. Med. 1992;116(6):488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 13.Pakrou N., Selva D., Leibovitch I. Wegener's granulomatosis: ophthalmic manifestations and management. Semin. Arthritis Rheum. 2006;35(5):284–292. doi: 10.1016/j.semarthrit.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Cocho L., Gonzalez-Gonzalez L.A., Molina-Prat N., Doctor P., Sainz-de-la-Maza M., Foster C.S. Scleritis in patients with granulomatosis with polyangiitis (Wegener) Br. J. Ophthalmol. 2016 Aug;100(8):1062–1065. doi: 10.1136/bjophthalmol-2015-307460. [DOI] [PubMed] [Google Scholar]
- 15.Polychronopoulos V.S., Prakash U.B., Golbin J.M., Edell E.S., Specks U. Airway involvement in Wegener's granulomatosis. Rheum. Dis. Clin. N. Am. 2007;33(4):755. doi: 10.1016/j.rdc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Cordier J.-F., Valeyre D., Guillevin L., Loire R., Crechot J.-M. Pulmonary Wegener's granulomatosis: a clinical and imaging study of 77 cases. Chest. 1990;97:906–912. doi: 10.1378/chest.97.4.906. [DOI] [PubMed] [Google Scholar]
- 17.Daoud M.S., Gibson L.E., DeRemee R.A., Specks U., el-Azhary R.A., Su W.P. Cutaneous Wegener's granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J. Am. Acad. Dermatol. 1994;31(4):605. doi: 10.1016/s0190-9622(94)70224-1. [DOI] [PubMed] [Google Scholar]
- 18.Martusewicz-Boros M., Baranska I., Wiatr E., Bestry I., Roszkowski-Sliz K. Asymptomatic appearance of splenic infarction in Wegener's granulomatosis. Pol. J. Radiol. 2011;76(2):43–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Seo P., Stone J.H. The antineutrophil cytoplasmic antibody-associated vasculitides. Am. J. Med. 2004;117(1):39. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Li C., Li J. Thoracic manifestation of Wegener's granulomatosis: computed tomography findings and analysis of misdiagnosis. Exp. Ther. Med. 2018;16(1):413–419. doi: 10.3892/etm.2018.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagen E.C., Daha M.R., Hermans J., Andrassy K., Csernok E., Gaskin G., Lesavre P., Lüdemann J., Rasmussen N., Sinico R.A., Wiik A., van der Woude F.J. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998;53(3):743. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 22.Mohammad A.J., Mortensen K.H., Babar J., Smith R., Jones R.B., Nakagomi D., Sivasothy P., Jayne D.R.W. Pulmonary involvement in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: the influence of ANCA subtype. J. Rheumatol. 2017;44(10):1458. doi: 10.3899/jrheum.161224. [DOI] [PubMed] [Google Scholar]
- 23.Yates M., Watts R.A., Bajema I.M. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 24.de Groot K., Harper L., Jayne D.R., Flores Suarez L.F., Gregorini G., Gross W.L., Luqmani R., Pusey C.D., Rasmussen N., Sinico R.A., Tesar V., Vanhille P., Westman K., Savage C.O., EUVAS (European Vasculitis Study Group) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann. Intern. Med. 2009;150(10):670. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stone J.H., Merkel P.A., Spiera R., Seo P., Langford C.A., Hoffman G.S., Kallenberg C.G., St Clair E.W., Turkiewicz A., Tchao N.K., Webber L., Ding L., Sejismundo L.P., Mieras K., Weitzenkamp D., Ikle D., Seyfert-Margolis V., Mueller M., Brunetta P., Allen N.B., Fervenza F.C., Geetha D., Keogh K.A., Kissin E.Y., Monach P.A., Peikert T., Stegeman C., Ytterberg S.R., Specks U., RAVE-ITN Research Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010;363(3):221. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones R.B., Tervaert J.W., Hauser T., Luqmani R., Morgan M.D., Peh C.A., Savage C.O., Segelmark M., Tesar V., van Paassen P., Walsh D., Walsh M., Westman K., Jayne D.R. European Vasculitis Study Group. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 2010;363(3):211. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 27.Wallace Zachary S., Miloslavsky Eli M. Management of ANCA associated vasculitis. BMJ. 2020;368:m421. doi: 10.1136/bmj.m421. [DOI] [PubMed] [Google Scholar]
- 28.Jayne D., Rasmussen N., Andrassy K., Bacon P., Tervaert J.W., DadonienéJ, Ekstrand A., Gaskin G., Gregorini G., de Groot K., Gross W., Hagen E.C., Mirapeix E., Pettersson E., Siegert C., Sinico A., Tesar V., Westman K., Pusey C., European Vasculitis Study Group A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N. Engl. J. Med. 2003;349(1):36. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 29.Pagnoux C., Mahr A., Hamidou M.A., Boffa J.J., Ruivard M., Ducroix J.P., Kyndt X., Lifermann F., Papo T., Lambert M., Le Noach J., Khellaf M., Merrien D., Puéchal X., Vinzio S., Cohen P., Mouthon L., Cordier J.F., Guillevin L., French Vasculitis Study Group Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N. Engl. J. Med. 2008;359(26):2790. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 30.Guillevin L., Pagnoux C., Karras A., Khouatra C., Aumaître O., Cohen P., Maurier F., Decaux O., Ninet J., Gobert P., Quémeneur T., Blanchard-Delaunay C., Godmer P., Puéchal X., Carron P.L., Hatron P.Y., Limal N., Hamidou M., Ducret M., Daugas E., Papo T., Bonnotte B., Mahr A., Ravaud P., Mouthon L., French Vasculitis Study Group Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 2014;371(19):1771. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 31.Koldingsnes W., Nossent H. Predictors of survival and organ damage in Wegener's granulomatosis. Rheumatology. 2002;41:572–581. doi: 10.1093/rheumatology/41.5.572. [DOI] [PubMed] [Google Scholar]
- 32.Pagnoux C., Hogan S.L., Chin H., Jennette J.C., Falk R.J., Guillevin L., Nachman P.H. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008 Sep;58(9):2908–2918. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell B., Mohan S., Chahal R., Carette S., Pagnoux C. Prognostic significance of cavitary lung nodules in granulomatosis with polyangiitis (Wegener's): a Clinical Imaging Study of 225 Patients. Arthritis Care Res. 2018 Jul;70(7):1082–1089. doi: 10.1002/acr.23443. [DOI] [PubMed] [Google Scholar]
- 34.Yoo J., Kim H.J., Ahn S.S., Jung S.M., Song J.J., Park Y.B. The utility of the ACR/EULAR 2017 provisional classification criteria for granulomatosis with polyangiitis in Korean patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin. Exp. Rheumatol. 2018;36(Suppl 111):85–87. [PubMed] [Google Scholar]