Abstract
Background
The clinicopathological parameters associated with glucose transporter-1 (GLUT-1) expression in advanced gastric cancer are still controversial. This study aimed to determine the clinicopathological parameters and prognosis associated with GLUT-1 expression in advanced gastric cancer.
Material/Methods
The GLUT-1 expression level of 234 consecutive gastric cancer samples was detected by immunohistochemical staining and evaluated by semiquantitative analysis. The clinicopathological data and expression level of GLUT-1 of enrolled patients were retrospectively analyzed with univariate and multivariate analyses.
Results
Tumor size, depth of invasion, and Lauren classification were independent factors related to GLUT-1 expression (P<0.05). Within advanced gastric cancer, tumor size and Lauren type were independent factors associated with GLUT-1 (P=0.011, P<0.001, respectively). The mean survival time of GLUT-1-positive patients with stage M0 advanced gastric cancer who had undergone radical gastrectomy was shorter than that of GLUT-1-negative patients (61.26±6.12 versus 80.88±7.38, P=0.044). GLUT-1 was an independent prognosis factor in locally advanced gastric cancer patients who had undergone radical gastrectomy (hazard ratio [HR] 1.769, P=0.046). The mean survival time of adjuvant chemotherapy was significantly better than no adjuvant chemotherapy in the GLUT-1-positive group (71.10±6.88 versus 24.65±8.69, P<0.001) and in the GLUT-1 negative group (87.48±7.99 versus 49.39±11.71, P<0.001).
Conclusions
Tumor size and Lauren type independently affected GLUT-1 expression in advanced gastric cancer. GLUT-1 was not only related to poor prognosis but also predicted to be a metabolic biomarker for intestinal type in locally advanced gastric cancer. The relationship among GLUT-1, hepatic metastasis and chemotherapy regimens, and mechanism of chemotherapy responses related to GLUT-1 should be further investigated.
MeSH Keywords: Chemotherapy, Adjuvant; Glucose Transporter Type 1; Prognosis; Stomach Neoplasms
Background
Gastric cancer is the second most common cancer in Eastern Asia [1]. In Japan, mortality from gastric cancer may be reduced due to screening, although the incidence remains high [2,3]. A reduction in the incidence of gastric cancer has been reported in the Shanghai urban area [4]. However, mortality in this region has not decreased, resulting in a high rate of individuals with advanced gastric cancer [5]. The characteristics of advanced gastric cancer should be investigated to improve survival.
Glucose transporter-1 (GLUT-1) is a member of the major transporter superfamily [6] and regulates glucose distribution by controlling the direction of movement of glucose [7]. The transporter is expressed in numerous cell types, such as erythrocytes, brain cells, and muscle cells, at varying levels [8]. It is significant that for tissues depending on glucose for energy production that GLUT-1 is a highly expressed protein [9]. In addition, a variety of cancers, including lung cancer and colorectal carcinoma, overexpress GLUT-1 [10,11].
GLUT-1 positivity in gastric cancer is not high among malignant tumors and is only 19~29.5% in Japan [12,13], 16.9~43.0% in Korea [14–16], and 22.0~50.0% in Germany [17,18]. Depth of invasion, lymphatic permeation, venous invasion, lymph node metastasis, and hepatic metastasis were associated with s positive rate in Japan, where early gastric cancer is predominant among all gastric cancer cases [13]. The clinicopathological parameters associated with GLUT-1 expression in advanced gastric cancer in the Chinese population are still controversial. Therefore, this study aimed to explore the clinicopathological parameters and prognosis related to GLUT-1 in advanced gastric cancer.
Material and Methods
Patients
A total of 234 consecutive patients who did not undergo preoperative chemotherapy or radiotherapy between January 2008 and January 2014 at the Fifth People’s Hospital of Shanghai were enrolled in this study. All of them had pathologically confirmed disease after gastrectomy and lymph node dissection. All advanced stage cases underwent radical gastrectomy and D2 dissection. Adjuvant chemotherapy has been employed according to NCCN clinical practice guidelines. Advanced gastric cancer patients underwent chemotherapy protocol including mFOLFOX and SOX. All enrolled patients had an over 5-years follow-up period and examinations at regular intervals. The local Research Ethics Commission approved this study.
The staging system used in of this study was the American Joint Committee on Cancer, 8th edition. Histology was scored according to the World Health Organization (WHO) classification [19]. The characteristics of the patients are shown in Table 1.
Table 1.
The relation between GLUT-1 expression and clinicopathological parameters in 234 gastric cancer cases.
| Clinicopathological parameters | GLUT-1 expression | P-value | |
|---|---|---|---|
| Negative (score 0) n=99 |
Positive (score 1~2) n=135 |
||
| Gender | 0.086 | ||
| Male | 63 (63.6%) | 100 (74.1%) | |
| Female | 36 (36.4%) | 35 (25.9%) | |
| Age (year) | 63±13 | 65±11 | 0.219a |
| Tumor diameter(cm) | 4.31±2.30 | 5.24±2.60 | 0.002a,* |
| Tumor localization | 0.013* | ||
| Upper | 10 (10.1%) | 34 (25.2%) | |
| Middle | 18 (18.2%) | 18 (13.3%) | |
| Lower | 71 (71.7%) | 83 (61.5%) | |
| pT | 0.088b | ||
| T1 (T1a, T1b) | 25 (25.3%) | 19 (14.1%) | |
| T2 | 14 (14.1%) | 14 (10.4%) | |
| T3 | 1 (1.0%) | 3 (2.2%) | |
| T4 (T4a, T4b) | 59 (59.6%) | 99 (73.3%) | |
| pN | 0.936 | ||
| N0 | 29 (29.3%) | 41 (30.4%) | |
| N1 | 15 (15.2%) | 24 (17.8%) | |
| N2 | 23 (23.2%) | 30 (22.2%) | |
| N3 (N3a, N3b) | 32 (32.3%) | 40 (29.6%) | |
| M | 0.009b,* | ||
| M0 | 97 (98.0%) | 120 (88.9%) | |
| M1 | 2 (2.0%) | 15 (11.1%) | |
| TNM stage | 0.133b | ||
| IA, IB | 25 (25.3%) | 25 (18.5%) | |
| IIA, IIB | 18 (18.2%) | 24 (17.8%) | |
| IIIA, IIIB, IIIC | 54 (54.5%) | 75 (55.6%) | |
| IV | 2 (2.0%) | 11 (8.1%) | |
| Clinical stage | 0.031* | ||
| Early | 25 (25.3%) | 19 (14.1%) | |
| Advanced | 74 (74.7%) | 116 (85.9%) | |
| Histology | <0.001b,* | ||
| Well differentiated | 18 (18.2%) | 32 (23.7%) | <0.001c,* |
| Moderately differentiated | 22 (22.2%) | 60 (44.4%) | |
| Poorly differentiated | 49 (49.5%) | 36 (26.7%) | |
| Signet-ring cell | 9 (9.1%) | 5 (3.7%) | |
| Mucinous | 1 (1.0%) | 2 (1.5%) | |
| Lauren type | <0.001* | ||
| Intestinal | 40 (40.4%) | 92 (68.1%) | |
| Diffuse | 59 (59.6%) | 43 (31.9%) | |
| Venous invasion | 0.061 | ||
| Positive | 62 (62.6%) | 100 (74.1%) | |
| Negative | 37 (37.4%) | 35 (25.9%) | |
| Lymphatic invasion | 0.919 | ||
| Positive | 71 (71.7%) | 96 (71.1%) | |
| Negative | 28 (28.3%) | 39 (28.9%) | |
| Perineural invasion | 0.047* | ||
| Positive | 51 (51.5%) | 87 (64.4%) | |
| Negative | 48 (48.5%) | 48 (35.6%) | |
Mann-Whitney U test;
Fisher’s exact test;
Comparison of different grade in adenocarcinoma.
P<0.05.
GLUT-1 – glucose transporter-1.
Experimental procedures
Paraffin-embedded samples of tumors were sliced into 4 μm-thick specimens. After deparaffinization and hydration, the slides were treated with 3% H2O2 for 10 minutes at room temperature. We used 0.01 M sodium citrate buffer (pH 6.0) at 100°C for 1 minute for antigen retrieval. The slides were incubated with primary monoclonal GLUT-1 antibody (ab40084, Abcam, UK, 1: 100). EnVision detection systems (Peroxidase/DAB, Rabbit/Mouse, DAKO) were used for staining. After treatment with the kits, the sections were counterstained with Mayer’s hematoxylin. Then, the specimens were dehydrated with gradient ethanol series and sealed with neutral balsam. For quality control, omission of the GLUT-1 antibody and use of isotype controls (ab18413, Abcam, UK) were performed.
The analyses were conducted by experienced pathologists blinded to the patients’ clinical information. Positive tumor cells were identified by staining of the cell membrane (Figure 1). The GLUT-1 expression level was evaluated by semiquantitative assessment [20,21]. The scoring system criteria were as follows: 0 as <1% positive tumor cells; 1 as 1~30% positive tumor cells; and 2 as >30% positive tumor cells.
Figure 1.

Glucose transporter-1 (GLUT-1) positive staining in gastric adenocarcinoma cell membrane (200×).
Statistical analysis
Continuous variables are expressed as mean values with standard deviation or range. Chi-squared test or Fisher’s exact test was performed to determine the relationship between GLUT-1 expression and clinicopathological parameters such as gender and tumor localization. Age and tumor diameter of the GLUT-1-positive and GLUT-1-negative groups were compared with the Mann-Whitney U test. Multivariate analyses of GLUT-1 expression and potentially significant factors (P<0.10) were performed with logistic regression analysis, which was verified by stepwise regression of all clinicopathological parameters. The 132 patients with stage M0 advanced gastric cancer who had undergone radical gastrectomy (dissected lymph nodes ≥20, R0 resection) were included in the survival analysis with the Kaplan-Meier method and log-rank test. Cox regression multivariate analysis included the clinicopathological parameters for survival analysis. P<0.05 was considered to be statistically significant. SPSS software, version 16.0, was used for all statistical analyses.
Results
In these gastric cancer patients, the overall positive rate of GLUT-1 expression was 57.69% (135 out of 234 patients). The relationship between GLUT-1 and clinicopathological parameters is shown in Table 1. Tumor diameter, localization, M stage, and histology/Lauren type were associated with GLUT-1 expression in advanced gastric cancer (Table 2). Tumor diameter, M stage, and Lauren type were also independent factors (Table 3).
Table 2.
The relation between GLUT-1 expression and clinicopathological parameters in 190 advanced gastric cancer cases.
| Clinicopathological parameters | GLUT-1 expression | P-value | |
|---|---|---|---|
| Negative (score 0) n=74 |
Positive (score 1~2) n=116 |
||
| Gender | 0.191 | ||
| Male | 49 (66.2%) | 87 (75.0%) | |
| Female | 25 (33.8%) | 29 (25.0%) | |
| Age (year) | 63±14 | 66±11 | 0.099a |
| Tumor diameter (cm) | 4.78±2.35 | 5.68±2.48 | 0.007a,* |
| Tumor localization | 0.039* | ||
| Upper | 9 (12.2%) | 32 (27.6%) | |
| Middle | 14 (18.9%) | 16 (13.8%) | |
| Lower | 51 (68.9%) | 68 (58.6%) | |
| pT | 0.382b | ||
| T2 | 14 (18.9%) | 14 (12.1%) | |
| T3 | 1 (1.4%) | 3 (2.6%) | |
| T4 (T4a, T4b) | 59 (79.7%) | 99 (85.3%) | |
| pN | 0.328 | ||
| N0 | 9 (12.2%) | 26 (22.4%) | |
| N1 | 13 (17.5%) | 21 (18.1%) | |
| N2 | 21 (28.4%) | 29 (25.0%) | |
| N3 (N3a, N3b) | 31 (41.9%) | 40 (34.5%) | |
| M | 0.012b,* | ||
| M0 | 72 (97.3%) | 101 (87.1%) | |
| M1 | 2 (2.7%) | 15 (12.9%) | |
| TNM stage | 0.233b | ||
| IB | 3 (4.0%) | 7 (6.0%) | |
| IIA, IIB | 15 (20.3%) | 23 (19.8%) | |
| IIIA, IIIB, IIIC | 54 (73.0%) | 75 (64.7%) | |
| IV | 2 (2.7%) | 11 (9.5%) | |
| Histology | <0.001b,* | ||
| Well differentiated | 8 (10.8%) | 20 (17.3%) | <0.001b,c,* |
| Moderately differentiated | 16 (21.6%) | 57 (49.1%) | |
| Poorly differentiated | 43 (58.1%) | 34 (29.3%) | |
| Signet-ring cell | 6 (8.1%) | 3 (2.6%) | |
| Mucinous | 1 (1.4%) | 2 (1.7%) | |
| Lauren type | <0.001* | ||
| Intestinal | 24 (32.4%) | 77 (66.4%) | |
| Diffuse | 50 (67.6%) | 39 (33.6%) | |
| Venous invasion | 0.085 | ||
| Positive | 55 (74.3%) | 98 (84.5%) | |
| Negative | 19 (25.7%) | 18 (15.5%) | |
| Lymphatic invasion | 0.100 | ||
| Positive | 65 (87.8%) | 91 (78.4%) | |
| Negative | 9 (12.2%) | 25 (21.6%) | |
| Perineural invasion | 0.265 | ||
| Positive | 50 (67.6%) | 87 (75.0%) | |
| Negative | 24 (32.4%) | 29 (25.0%) | |
Mann-Whitney U test;
Fisher’s exact test;
Comparison of different grade in adenocarcinoma.
P<0.05.
GLUT-1 – glucose transporter-1.
Table 3.
The logistic regression analysis of GLUT-1 expression with clinicopathological parameters in 190 advanced gastric cancer cases.
| Parameters | Regression coefficient (95% CI) | OR (95% CI) | P-value |
|---|---|---|---|
| Gender | −0.310 (−0.709~0.089) | 0.734 (0.336~1.602) | 0.437 |
| Age | 0.012 (−0.003~0.027) | 1.012 (0.982~1.043) | 0.453 |
| Tumor diameter | 0.198 (0.120~0.276) | 1.220 (1.047~1.420) | 0.011* |
| Tumor localization | −0.272 (−0.494~−0.050) | 0.762 (0.493~1.177) | 0.221 |
| pT | 0.306 (0.051~0.561) | 1.358 (0.825~2.236) | 0.229 |
| pN | −0.369 (−0.621~−0.117) | 0.691 (0.422~1.133) | 0.143 |
| M | 2.168 (1.280~3.056) | 8.744 (1.535~49.819) | 0.015* |
| Lauren type (intestinal =. diffuse) | 1.338 (0.980~1.696) | 3.810 (1.888~7.687) | <0.001* |
| Venous invasion | −0.682 (−1.230~−0.134) | 0.506 (0.173~1.481) | 0.214 |
| Lymphatic invasion | 0.356 (−0.358~1.070) | 1.427 (0.352~5.790) | 0.618 |
| Perineural invasion | −0.079 (−0.555~0.397) | 0.924 (0.364~2.349) | 0.869 |
P<0.05.
OR – odds ratio; CI – confidence interval; GLUT-1 – glucose transporter-1.
The median follow-up period was 48.45 months. The mean survival time of GLUT-1-positive patients with stage M0 advanced gastric cancer who had undergone radical gastrectomy was shorter than that of GLUT-1-negative patients (61.26±6.12 versus 80.88±7.38, P=0.044) (Figure 2). The hepatic metastasis hazard curve of GLUT-1-positive patients was higher than that of negative patients (Figure 3). Age, tumor diameter, pT, pN, GLUT-1, and adjuvant chemotherapy were related to survival time in our univariate analysis (Table 4). GLUT-1 was an independent prognosis factor in locally advanced gastric cancer undergone radical gastrectomy (hazard ratio [HR] 1.769, P=0.046) (Table 5).
Figure 2.

The survival curves of the stage M0 advanced gastric cancer patients who had undergone radical gastrectomy (dissecting lymph nodes ≥20, R0 resection), glucose transporter-1 (GLUT-1) positive and negative.
Figure 3.

The hepatic metastasis hazard curves of the stage M0 advanced gastric cancer patients who had undergone radical gastrectomy (dissecting lymph nodes ≥20, R0 resection), glucose transporter-1 (GLUT-1) positive and negative.
Table 4.
Kaplan-Meier survival analysis in stage M0 advanced gastric cancer after radical gastrectomy (dissected lymph nodes ≥20, R0 resection).
| Parameters | Mean survival time, months (95%CI) | P-value |
|---|---|---|
| Gender | 0.793 | |
| Male | 68.39 (57.516~79.265) | |
| Female | 67.96 (50.524~85.393) | |
| Age (year) | <0.001* | |
| <65 | 91.08 (78.064~104.100) | |
| ≥65 | 51.37 (39.539~63.202) | |
| Tumor diameter (cm) | 0.003* | |
| <5 | 81.28 (68.810~93.754) | |
| ≥5 | 56.26 (43.277~69.233) | |
| Tumor localization | 0.073 | |
| Upper | 36.67 (21.558~51.772) | |
| Middle | 74.96 (52.284~97.628) | |
| Lower | 72.83 (61.413~84.251) | |
| pT | 0.006* | |
| T2 | 101.73 (84.760~118.691) | |
| T3~4 (T4a, T4b) | 62.65 (52.27~73.02) | |
| pN | 0.002* | |
| N0 | 77.86 (59.137~96.587) | |
| N1 | 99.64 (80.68~118.61) | |
| N2 | 63.72 (47.12~80.33) | |
| N3 (N3a, N3b) | 43.59 (30.21~59.96) | |
| Lauren type | 0.763 | |
| Intestinal | 69.39 (56.784~81.993) | |
| Diffuse | 68.74 (54.751~82.735) | |
| Venous invasion | 0.059 | |
| Positive | 64.72 (54.178~75.261) | |
| Negative | 69.86 (55.660~84.068) | |
| Lymphatic invasion | 0.363 | |
| Positive | 74.77 (56.08~93.45) | |
| Negative | 67.23 (56.46~78.00) | |
| Perineural invasion | 0.052 | |
| Positive | 63.77 (52.674~74.857) | |
| Negative | 69.07 (56.085~82.064) | |
| GLUT-1 | 0.044* | |
| Positive | 61.26 (49.256~73.256) | |
| Negative | 80.88 (66.418~95.348) | |
| Adjuvant chemotherapy | <0.001* | |
| Yes | 78.16 (67.763~88.563) | |
| No | 35.74 (19.779~51.692) |
P<0.05.
CI – confidence interval; GLUT-1 – glucose transporter-1.
Table 5.
Cox regression multivariate analysis in stage M0 advanced gastric cancer after radical gastrectomy (dissected lymph nodes ≥20, R0 resection).
| Parameters | HR (95% CI) | P-value |
|---|---|---|
| Gender | 1.065 (0.605~1.876) | 0.826 |
| Age | 1.043 (1.016~1.071) | 0.001* |
| Tumor diameter | 1.094 (0.994~1.205) | 0.067 |
| Tumor localization | 0.641 (0.452~0.910) | 0.013* |
| pT | 1.408 (0.901~2.201) | 0.133 |
| pN | 2.840 (1.755~4.597) | <0.001* |
| Lauren type | 0.778 (0.467~1.296) | 0.335 |
| Venous invasion | 0.644 (0.226~1.839) | 0.411 |
| Lymphatic invasion | 0.171 (0.051~0.576) | 0.004* |
| Perineural invasion | 1.404 (0.646~3.052) | 0.392 |
| GLUT-1 | 1.769 (1.010~3.096) | 0.046* |
| Adjuvant chemotherapy | 0.468 (0.253~0.866) | 0.016* |
P<0.05.
GLUT-1 – glucose transporter-1.
The mean survival time of the adjuvant chemotherapy group was significantly better than the no adjuvant chemotherapy group in the GLUT-1-positive and negative groups (Figure 4). The mean survival time for patients in the adjuvant chemotherapy and no adjuvant chemotherapy groups in the GLUT-1-positive group was 71.10±6.88 and 24.65±8.69 months, respectively; whereas in the GLUT-1-negative the mean survival time was 87.48±7.99 and 49.39±11.71 months, respectively.
Figure 4.
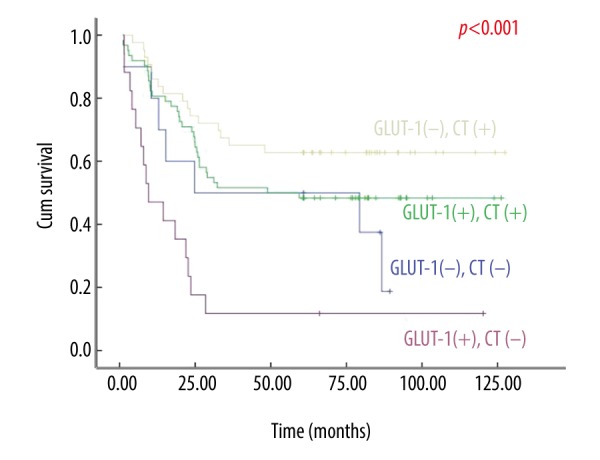
The survival curves of the stage M0 advanced gastric cancer patients who had undergone radical gastrectomy (dissecting lymph nodes ≥20, R0 resection), glucose transporter-1 (GLUT-1) positive and negative, adjuvant chemotherapy (CT(+)) and no adjuvant chemotherapy (CT(−)).
Discussion
Reprogramming energy metabolism is one of the canonical hallmarks of cancer [22]. Glucose metabolism is not only essential for human survival but also associated with carcinogenesis [23]. GLUT-1 is a typical transporter involved in metabolism and has been further elucidated with xyIE reporter analyses [24]. Gastric cancer and other malignant tumors have been found to be related to GLUT-1 [11,13,25–28]. The Warburg phenotype, which is GLUT-1 positive, is the most common phenotype in triple-negative breast cancer and corresponds to a poor prognosis [29]. The GLUT-1 positive rate of other digestive tract neoplasms, such as colorectal and esophageal carcinoma, is higher (90~100%) [11,30]. However, there have been discrepancies among several studies on GLUT-1 expression in gastric cancer. Therefore, we analyzed its expression in various situation in our study.
In our study, the positive rate of GLUT-1 expression in gastric cancer samples was similar to that reported in previous studies [12–16,31–38]. Moreover, the positive rate in the advanced stage group was significantly higher than that in the early stage group. The difference in the ratio of advanced to early stage cancer patients among studies may result in a higher positive rate found in Chinese studies, including ours, than found in studies from other East Asian countries [12–16,31–38]. The ratio in our study was significantly higher than that of other large case studies [13]. The differences in the epidemiological characteristics are likely caused by the varying strategies for diagnosing and treating gastric cancer. For example, an advanced screening system for gastric cancer in Japan may lead to a lower proportion of individuals with advanced gastric cancer than found in other East Asian countries [2,3].
Tumor diameter and pT stage were independent factors associated with GLUT-1 expression in all gastric cancer cases, and they were positively correlated with each other. The growth of gastric lesions in perpendicular directions determined the independence of these variables [39]. Tumor diameter was also an independent factor in advanced stage gastric cancer, which suggested that GLUT-1 expression was associated with further tumor enlargement.
Korean studies have suggested that the intestinal type of gastric cancer is related to GLUT-1 [14,16,40]. Our study also obtained a similar result: multivariate analysis of overall or advanced stage gastric cancer revealed that the positive rate of GLUT-1 in intestinal gastric cancer was higher than that in the diffuse type. Lauren proposed his classification of gastric carcinoma in 1965 [41]. This histological classification is simplified, accessible, and reproducible to the benefit of research [42]. Both the Lauren classification and the WHO classification have been commonly used in gastric cancer studies, which can be correlated to each other [42]. The mucinous and signet-ring cell types in the WHO classification are mostly categorized as diffuse type in the Lauren classification. The positive rate of GLUT-1 expression in the 2 types was lower than that in the differentiated type [13,31]. Therefore, the lower rate in the diffuse type was probably the result of the characteristics of mucinous and signet-ring cell gastric cancer. GLUT-1 might be a potential metabolic biomarker for the intestinal type in advanced gastric cancer.
There have been different results from previous Chinese studies on the relationship between GLUT-1 expression and differentiated type in gastric cancer [31–35]. Wei et al. found that the GLUT-1 positive rate in well-differentiated types of gastric cancers was higher than that in moderately and poorly differentiated types [31]. Nevertheless, other researchers presented distinct results that showed the rate of poorly differentiated and undifferentiated type was higher [33–35]. Our study demonstrated that GLUT-1 expression was positively correlated with differentiation grade. The positive rate of moderately differentiated type was the highest of all the types, which was in line with the results reported by Yu et al. [32]. Such contradictory results suggest that GLUT-1 is probably a molecular factor that is not dependent on differentiation.
Kawamura et al. found that GLUT-1 expression was associated with poor survival in gastric cancer patients using multivariate analyses [13]. Jung et al. in Korea also affirmed that GLUT-1-positive gastric cancer patients had a shorter mean survival time [16]. Nevertheless, studies in China have not clearly shown that GLUT-1 expression is related to poor prognosis [31,35]. This inconsistency may be associated with differences in the epidemiological characteristics of gastric cancer between patients in China and those in other East Asian countries. Patients with advanced stage gastric cancer are predominant in China due to the country’s strategy on diagnosing and treating cancer. Moreover, the development of multi-disciplinary treatments with new drugs, devices, and techniques has improved the survival of advanced gastric cancer [43]. This study only showed that GLUT-1 positivity was associated with poor survival in stage M0 advanced gastric cancer patients who had undergone radical gastrectomy. Therefore, the epidemiological characteristics limited the application of our work in overall stages of gastric cancer. The hepatic metastasis hazard curve of GLUT-1-positive stage M0 advanced gastric cancer after radical gastrectomy was higher than that of the GLUT-1-negative group, and GLUT-1 expression was associated with the intestinal type of advanced gastric cancer.
The chemotherapy regime 5-fluorouracil (5-FU) has been shown to affect GLUT-1 on gastric cancer cell in vitro, which indicates that GLUT-1 may be partly associated with responses to 5-FU chemotherapy in gastric cancer [44]. Lu et al. suggested that GLUT-1 may be a marker for gastric cancer sensitivity to ascorbate in chemotherapy with in vitro and in vivo experiments [45]. Our work indicated that GLUT-1-positive patients might be subject to benefit from chemotherapy based on clinical data.
Although our work suggested that GLUT-1 might be associated with hepatic metastasis from gastric cancer and chemotherapy responses, there were a few limitations to our study, including the study was a small single center retrospective study and advanced gastric cancer was the predominantly patient diagnosis (especially pT4 staging). The relationship between GLUT-1, hepatic metastasis and chemotherapy, and mechanism of chemotherapy responses related to GLUT-1 should be further investigated.
Conclusions
GLUT-1 expression in advanced stage gastric cancer was significantly higher than that in early stage gastric cancer. Tumor size and Lauren type independently affected GLUT-1 expression in advanced gastric cancer. GLUT-1 was not only related to poor prognosis but also predicted to be a metabolic biomarker for the Lauren classification in locally advanced gastric cancer.
Footnotes
Conflict of interest
None.
Source of support: Nature Science Foundation of Minhang District, 2014MHZ032 and 2013MHZ017
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lee KJ, Inoue M, Otani T, et al. Gastric cancer screening and subsequent risk of gastric cancer: A large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118(9):2315–21. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 3.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38(4):259–67. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 4.Fei SJ, Xiao SD. Diet and gastric cancer: A case-control study in Shanghai urban districts. Chin J Dig Dis. 2006;7(2):83–8. doi: 10.1111/j.1443-9573.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Wu C, Zheng Y, et al. [Time trends and characteristics of gastric cancer incidence in urban Shanghai]. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28(9):875–80. [in Chinese] [PubMed] [Google Scholar]
- 6.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62(1):1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina RA, Owen GI. Glucose transporters: Expression, regulation and cancer. Biol Res. 2002;35(1):9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- 8.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br J Nutr. 2003;89(1):3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 9.Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24(6):650–54. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes M, Brown RW, Stephenson M, et al. Overexpression of GLUT1 and GLUT3 in stage I non-small cell lung carcinoma is associated with poor survival. Cancer. 1997;80(6):1046–51. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: A marker for poor prognosis. Cancer. 1998;83(1):34–40. doi: 10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi Y, Marat D, Saito A, et al. Expression of facilitative glucose transporters in gastric tumors. Hepatogastroenterology. 1999;46(28):2683–89. [PubMed] [Google Scholar]
- 13.Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: Association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92(3):634–41. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Kim WS, Kim YY, Jang SJ, et al. Glucose transporter 1 (GLUT1) expression is associated with intestinal type of gastric carcinoma. J Korean Med Sci. 2000;15(4):420–24. doi: 10.3346/jkms.2000.15.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur H, Xuan Y, Kim YB, et al. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol. 2013;42(1):44–54. doi: 10.3892/ijo.2012.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung JH, Im S, Jung ES, et al. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci. 2013;10(9):1217–23. doi: 10.7150/ijms.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlosser HA, Drebber U, Urbanski A, et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer. 2017;20(1):83–91. doi: 10.1007/s10120-015-0577-x. [DOI] [PubMed] [Google Scholar]
- 18.Berlth F, Monig S, Pinther B, et al. Both GLUT-1 and GLUT-14 are independent prognostic factors in gastric adenocarcinoma. Ann Surg Oncol. 2015;(Suppl 3):S822–31. doi: 10.1245/s10434-015-4730-x. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton S, Aaltonen L. Pathology and genetics of tumours of the digestive system. IARCP; 2000. [Google Scholar]
- 20.Yamada A, Oguchi K, Fukushima M, et al. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: Relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20(9):597–604. doi: 10.1007/BF02984657. [DOI] [PubMed] [Google Scholar]
- 21.Alakus H, Batur M, Schmidt M, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31(6):532–38. doi: 10.1097/MNM.0b013e32833823ac. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18(6):598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Zeng X, Yan C, et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature. 2012;490(7420):361–66. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Kaida H, Kawahara A, et al. The relationship between GLUT-1 and vascular endothelial growth factor expression and 18F-FDG uptake in esophageal squamous cell cancer patients. Clin Nucl Med. 2012;37(5):447–52. doi: 10.1097/RLU.0b013e31823924bb. [DOI] [PubMed] [Google Scholar]
- 26.Shim BY, Jung JH, Lee KM, et al. Glucose transporter 1 (GLUT1) of anaerobic glycolysis as predictive and prognostic values in neoadjuvant chemoradiotherapy and laparoscopic surgery for locally advanced rectal cancer. Int J Colorectal Dis. 2013;28(3):375–83. doi: 10.1007/s00384-012-1542-3. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman RL, Fogt F, Burke M, et al. Assessment of GLUT-1 expression in cholangiocarcinoma, benign biliary lesions and hepatocellular carcinoma. Oncol Rep. 2002;9(4):689–92. [PubMed] [Google Scholar]
- 28.Hussein YR, Bandyopadhyay S, Semaan A, et al. GLUT-1 expression correlates with basal-like breast cancer. Transl Oncol. 2011;4(6):321–27. doi: 10.1593/tlo.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Kim DH, Jung WH, et al. Metabolic phenotypes in triple-negative breast cancer. Tumour Biol. 2013;34(3):1699–1712. doi: 10.1007/s13277-013-0707-1. [DOI] [PubMed] [Google Scholar]
- 30.Tohma T, Okazumi S, Makino H, et al. Overexpression of glucose transporter 1 in esophageal squamous cell carcinomas: A marker for poor prognosis. Dis Esophagus. 2005;18(3):185–89. doi: 10.1111/j.1442-2050.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- 31.Wei B, Chen L, Li J. [Expression of glucose transporter 1 in gastric carcinoma and metastatic lymph nodes and its association with prognosis]. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12(3):277–80. [in Chinese] [PubMed] [Google Scholar]
- 32.Yu Y, Hu J, Fang Y. Clinical pathological significance of GLUT-1 protein expression in gastric carcinoma. China Medicine. 2009;4(11):845–46. [Google Scholar]
- 33.Chen Y, Liu Q, Huang J, et al. GLUT1 expression and clinicopathological significance in gastric cancer. Clinical Focus. 2012;27(8):694–95. [Google Scholar]
- 34.Li W, Song X, Sun S, et al. Expressions of HIF-1α, GLUT1 and PCNA in gastric cancer and clinical significance. Chinese Journal of Gastroenterology and Hepatology. 2008;17(3):223–25. [Google Scholar]
- 35.Qian Y, Shi H. HIF-1α and GLUT1 expression and significance in gastric cancer. Zhejiang Clinical Medical Journal. 2013;(10):1444–47. [Google Scholar]
- 36.Zhang P, Wu J, Zhang H, et al. HIF-1α, VEGF and GLUT1 protein expression in gastric carcinoma. Acta Universitatis Medicinalis Anhui. 2010;45(1):86–89. [Google Scholar]
- 37.Sun J. Expression and clinical significance of Survivin and Glut 1 protein in gastric carcinoma. Clinical Medicine of China. 2013;29(2):176–78. [Google Scholar]
- 38.Xu Q. The interaction between glucose transporter-1(GLUT-1) and expression of Hrd-1 and Derlin-1 in gastric cancer [Master Degree] Southeast University. 2013 [Google Scholar]
- 39.Yin C, Nan Y, Lu T, et al. Clinicopathological parameters influence assessment of FDG SPECT in gastric cancer. Hepatogastroenterology. 2015;62(139):762–65. [PubMed] [Google Scholar]
- 40.Choi BH, Song HS, An YS, et al. Relation between fluorodeoxyglucose uptake and glucose transporter-1 expression in gastric signet ring cell carcinoma. Nucl Med Mol Imaging. 2011;45(1):30–35. doi: 10.1007/s13139-010-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y. [Evaluation of traditional pathological classification at molecular classification era for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17(1):18–20. [in Chinese] [PubMed] [Google Scholar]
- 43.Ji J, Li Z, Shen L, et al. Attaching importance to the effect of multidisciplinary team in the standardized treatment of gastric cancer. Chinese Journal of Practical Surgery. 2014;(7):592–94. [Google Scholar]
- 44.Won HJ, Ha TK, Kwon SJ, et al. Differential effects of 5-fluorouracil on glucose transport and expressions of glucose transporter proteins in gastric cancer cells. Anticancer Drugs. 2010;21(3):270–76. doi: 10.1097/CAD.0b013e328334562c. [DOI] [PubMed] [Google Scholar]
- 45.Lu YX, Wu QN, Chen DL, et al. Pharmacological ascorbate suppresses growth of gastric cancer cells with GLUT1 overexpression and enhances the efficacy of oxaliplatin through redox modulation. Theranostics. 2018;8(5):1312–26. doi: 10.7150/thno.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]


