Abstract
The Ataxia-telangiectasia mutated (ATM) kinase responds to DNA double-strand breaks and other forms of cellular stress, including reactive oxygen species (ROS). Recent work in the field has uncovered links between mitochondrial ROS and ATM activation, suggesting that ATM acts as a sensor for mitochondrial derived ROS and regulates ROS accumulation in cells through this pathway. In addition, characterization of cells from Ataxia-telangiectasia patients as well as ATM-deficient mice and cell models suggest a role for ATM in modulating mitochondrial gene expression and function. Here we review ROS responses related to ATM function, recent evidence for ATM roles in mitochondrial maintenance and turnover, and the relationship between ATM and regulation of protein homeostasis.
Keywords: Ataxia, Mitochondria, Reactive oxygen species, DNA repair
1. Introduction
Ataxia-Telangiectasia Mutated (ATM) is a serine/threonine-specific protein kinase in a family of large, phosphatidylinositol 3-kinase-like kinases (PIKKs) [1]. Patients with the rare, autosomal recessive A-T disorder lack functional ATM kinase due to nonsense or missense mutations in the ATM gene [2] and exhibit progressive cerebellar neuronal degeneration, ataxia, immunodeficiency, increased risk of cancer, as well as metabolic deficiencies [3]. ATM is activated by DNA double strand breaks (DSBs) and phosphorylates many proteins involved in DNA repair, cell cycle checkpoints, and apoptosis [[3], [4], [5]]. Although DNA damage is a primary source of its activation, ATM can also be activated by reactive oxygen species (ROS) independently from the DNA DSBs [4,6]. In this review, we focus on the functions of ATM in cellular responses to ROS and in recent insights into the relationships between ATM activation and mitochondrial function, oxidative stress, and protein aggregation.
1.1. Cellular responses to oxidative stress
Oxidative stress comes from an imbalance between the generation of free radicals by cellular aerobic metabolism and the capacity for removal of these species. The predominant intracellular free radical ROS species include superoxide (O2•-), hydroxyl radicals (HO•), and nitric oxide (NO•), in addition to the more stable oxygen species hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) [[7], [8], [9]]. These ROS are produced predominantly during mitochondrial oxidative metabolism as well as during mixed-function oxidation by enzymes in the endoplasmic reticulum, and by enzymes catalyzing β-oxidation of fatty acids in the peroxisome. ROS can also be generated by exogenous sources such as xenobiotics, γ-irradiation, UV-irradiation, heavy metals, and pollutants [9,10].
The presence of these ROS molecules presents cellular challenges as their high reactivity can lead to the oxidative modification of many cellular components. ROS can generate DNA damage by reacting with the sugar phosphate backbone and pyrimidine bases, causing DNA breaks and base modifications [9,11]. ROS is also associated with cell death through a variety of mechanisms including caspase-dependent apoptosis pathways, as well as with necroptosis, which can be induced by disruption of membrane ion gradients followed by rupture of the plasma membrane after lipids in the membrane are attacked by free radicals [12,13].
To regulate ROS levels, eukaryotic cells have evolved two types of anti-oxidative pathways; 1) anti-oxidative enzymes, and 2) the production of reducing agents/anti-oxidants. The first pathway involves anti-oxidant catalytic enzymes, including superoxide dismutases (SODs), catalase, and glutathione peroxidase [[14], [15], [16], [17]]. One of the most important groups of anti-oxidative enzymes is the SOD family, including Cu/Zn-SOD, Mn-SOD, and EC-SOD enzymes, all of which convert superoxide radicals to H2O2 and O2 [14]. Hydrogen peroxide (H2O2) can then be reduced to H2O by glutathione peroxidases or by catalase [16]. In addition, thiol-containing enzymes such as thioredoxins (TRX), peroxiredoxins, and thioredoxin reductases (TrxR), are involved in maintaining intracellular anti-oxidant capacity [18]. Other enzymatic anti-oxidants include Glutathione-S-transferase enzymes [19], which catalyze conjugation of reduced glutathione (GSH) with exogenous compounds, and glutathione reductase, which recycles GSH from glutathione disulfide [20]. Reduced nicotinamide adenine dinucleotide phosphate (NADPH) plays an important role in the enzymatic anti-oxidant pathway as a protective cofactor for catalase, as a reducing agent for the TrxR/TRX system, and also as a co-substrate for glutathione reductase [[21], [22], [23]]. However, NADPH also can contribute to the production of superoxide and H2O2 through its role as a cofactor for the family of transmembrane NADPH oxidases that are prevalent in neutrophils and epithelial cells [24].
The second, broadly defined anti-oxidant pathway involves non-enzymatic neutralization of ROS via direct chemical reduction of oxidants. This includes low molecular weight compounds including GSH, alpha-tocopherol (Vitamin E), ascorbic acid (Vitamin C), and carotenoids (β-carotene) [21,[25], [26], [27]]. The primary cellular anti-oxidant is glutathione, present at 1 to 2 mM in most cell types [28], which becomes oxidized to glutathione disulfide (GSSG) in an oxidizing environment. GSH is a co-factor for anti-oxidant enzymes, including GST enzymes and glutathione peroxidase, which reduces H2O2 into H2O and O2 while producing oxidized GSSG [29,30]. Other important endogenous cellular antioxidants include Vitamin E, Vitamin C, β-carotene, uric acid, and ubiquinone [31]. These agents directly counteract free radicals by donating electrons to free radical species. For examples, vitamin C is capable of scavenging hydrogen peroxide, superoxide, and hydroxyl, peroxyl, and oxygen free radicals [26], vitamin E scavenges peroxyl radicals [27], and β-carotene reacts with superoxide, hydroxyl, and peroxyl radicals [21].
1.2. Mechanisms of ATM activation
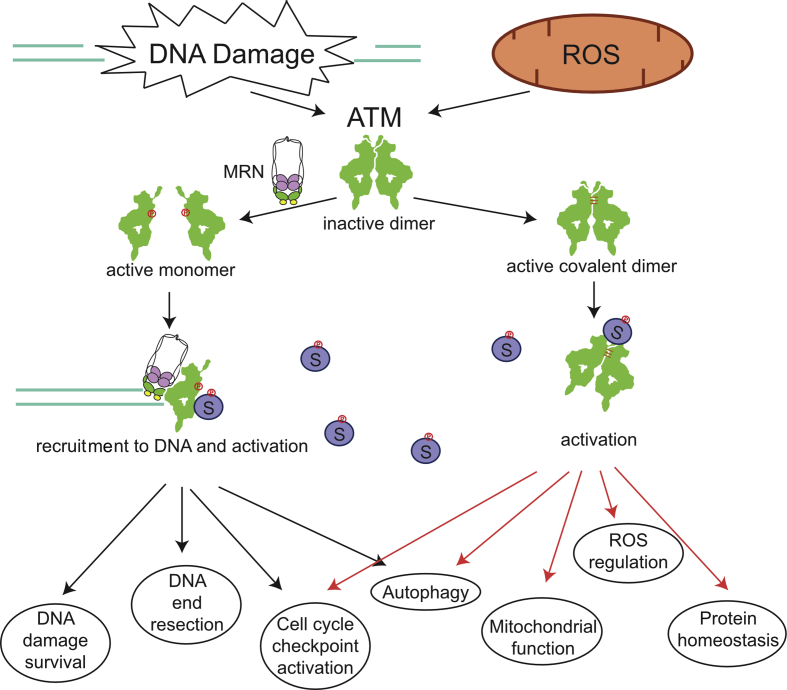
The ATM protein was shown to be activated by DNA damage many years ago and was subsequently shown to be recruited to and activated by DNA double-strand breaks through the Mre11-Rad50-Nbs1 (MRN) complex [3, 32]. ATM exists in a homodimeric catalytically-inactive form under normal conditions but is released into an active monomeric form when associated with the MRN complex and exposed to DSBs, an event that is coincident with auto-phosphorylation [[33], [34], [35]]. Other treatments have also been shown to evoke auto-phosphorylation and activity in the apparent absence of DNA breaks, namely chloroquine treatment, histone deacetylase inhibitors, or hypotonic conditions [33]. In addition, exposure to ROS trigger ATM activation in cells and in vitro through a pathway dependent on disulfide bonds between the two ATM monomers [6]. Oxidation of ATM produces a functional, active kinase that does not require the MRN complex, DNA ends, or autophosphorylation [6,36] (Fig. 1).
Fig. 1.
DNA damage and oxidation pathways that activate the ATM protein kinase. Double-strand breaks in DNA activate ATM through the MRN complex which monomerizes ATM into active subunits, recruits ATM to sites of double-strand breaks, and promotes recruitment of substrates (left). This form of activation leads to higher survival of DNA damage exposure, efficient processing of DNA ends for homologous recombination (resection), and DNA damage-dependent cell cycle checkpoint activation and macroautophagy. Activation of ATM by oxidation or other agents that promote disulfide bond formation between the ATM monomers promote activation of the kinase independently of MRN and DNA (right). The active form of the kinase is a covalent dimer, which regulates cellular ROS levels, mitophagy, protein homeostasis, and ROS-dependent autophagy.
Our recent studies characterized separation-of-function mutants of ATM for nuclear as well as cytoplasmic functions to determine which roles of ATM are dependent on each activation pathway. These experiments focused primarily on the ATM mutant C2991L (CL), deficient in oxidative activation [6], and the R2579A/R2580A (2RA) ATM mutant, deficient in MRN/DNA-dependent activation [36]. Expression and characterization of these ATM mutants in the human U2OS osteosarcoma cell line showed that cells expressing 2RA ATM are sensitive to ionizing radiation (IR) and the Top1 poison camptothecin (CPT) but not to sources of oxidative stress such as hydrogen peroxide or arsenite [36]. In contrast, cells expressing the CL ATM allele only show sensitivity to ROS-inducing agents. In addition, oxidative stress-induced checkpoint activation as well as macroautophagy were also compromised in cells expressing the CL allele, while cells expressing the 2RA allele were deficient in DNA damage-induced checkpoints and autophagy pathways. These results show that the two distinct pathways of ATM activation are responsible for signaling to diverse nuclear and cytosolic organelles and that each activation pathway functions separately. ATM also shows substrate specificity dependent on the activation pathway since oxidation-dependent ATM activation does not induce phosphorylation of H2AX, an established marker for DSBs, or phosphorylation of KAP1, a component of heterochromatin [6]. Thus, ATM activates specific downstream effectors as well as common substrates based on the type of cellular stress.
1.3. ATM as a sensor for the oxidative stress
ATM dysfunction is associated with accumulation of intracellular ROS, which has been suggested to contribute to cerebellar degeneration and other symptoms present in A-T patients [3,4,25]. Over the last three decades, numerous studies have demonstrated the functional consequences of ATM loss related to oxidative stress, as summarized below.
Early studies with cells from A-T patients showed that these lines are hypersensitive to ROS exposure [[37], [38], [39], [40], [41], [42]]. In addition, A-T patient cells and ATM-depleted cell lines in culture have been reported to exhibit higher levels of ROS and oxidation-related byproducts compared to normal cells [36,[43], [44], [45], [46]]. Several mouse models of ATM deficiency have also been created [[47], [48], [49], [50]], and although these generally show early T-cell lymphomas and no cerebellar degeneration, there are some indications of oxidation-related pathology. For instance, nitric oxide-dependent protein oxidation was observed in ATM-/- mouse brain as well as higher than normal levels of lipid peroxidation [51]. ATM (−/−) mouse hematopoietic stem cells (HSCs) showed significantly higher levels of intracellular ROS, especially H2O2, compared to normal HSCs [52]. Salicylate oxidation, caused by H2O2 and peroxynitrite, was elevated in cerebellum and basal ganglia but not in cortex from ATM (−/−) mice [53]. Superoxide levels, as measured by dihydroethidium (DHE) staining in vivo, were also increased in ATM-/- cerebellar Purkinje cells and neurons from hippocampal CA1 and substantia Nigra but not cortical neurons, suggesting that oxidative stress might be cell type-specific in the brain [53].
In ATM (−/−) mice, the levels of the thiol-containing compound GSH and its precursor, cysteine, were aberrant and suggested an age-dependent generation of oxidative stress in the absence of ATM [54]. The activity of thioredoxin, a small redox protein that responds to oxidative stress and catalyzes dithiol-disulfide exchange reactions, was also increased in the cerebella of ATM (−/−) mice. From these observations it was suggested that compensatory anti-oxidative mechanisms are upregulated in ATM-/- cells. In addition, a decrease in catalase activity was observed but Mn-SOD activity was increased in the cerebella of ATM (−/−) mice, suggesting higher H2O2 levels in these cells since catalase is a scavenger of H2O2 and SOD generates H2O2 from superoxide [54]. Similar phenomena were not only observed in the cerebella of ATM (−/−) mice [54], but also in human AT lymphoblast cells [55] as well as in A-T patient fibroblasts [56,57]. Another study also reported that ATM (−/−) astrocytes have elevated HSP70, Cu/Zn-SOD, and Mn-SOD, consistent with the interpretation that these cells experience increased oxidative stress, as well as high levels of extracellular-regulated kinase (ERK1/2) activation and defects in cell growth [58]. The total plasma antioxidant capacity and plasma levels of α-tocopherol, retinol, and ubiquinol in 10 A-T patients was slightly but significantly lower than healthy control patients [45]. This might affect A-T cells by making them more vulnerable to elevated levels of internal ROS.
Our studies with ATM-deficient cells and experiments with small molecule inhibitors of ATM have shown higher ROS levels, specifically H2O2, in cells with ATM deficiency [6,36]. Expression of ATM mutants that cannot be activated by oxidation (C2991L or R3047X) also results in high levels of ROS, but this is not observed with mutants that cannot be activated by DNA damage (R2579A/R2580A) [6,36]. This suggests that the oxidation pathway of ATM activation directly influences levels of ROS in human cells. From these in vivo and cell culture-based experiments, it is clear that ATM deficiency results in an elevation of cellular oxidative stress and in animal models it is clear that this is more severe in specific tissues. The R3047X mutation has been found in several A-T patients [[59], [60], [61]], an indication that loss of the oxidation pathway is sufficient for the neurodegeneration that is the primary characteristic of the disease. These specific patients have been noted as A-T "variants" due to intermediate radiosensitivity or reduced immunodeficiency, likely a result of the fact that they retain normal activation by the MRN complex through the canonical double-strand break pathway [6].
1.4. Pathogenesis generated by ATM deficiency and ROS
Although the pathogenesis of A-T is still not understood, it has been suggested that accumulated oxidative stress plays a critical role as in other neurodegenerative diseases [62]. ATM (−/−) astrocytes and neural stem cells exhibit growth defects and higher levels of senescence as well as earlier death in culture [63]. These phenotypes are likely caused by elevated ROS, since treatment with N-acetyl cysteine (NAC) suppressed these phenotypes [63]. These cell populations also showed enhanced activity of ERK1/2 and p38 mitogen-activated protein kinase (MAPK), as well as elevated levels of p16, p21, or p27, which were reversed by the addition of NAC or MAPK/ERK inhibitor [64]. ERKs and p38 MAPKs are subgroups within the larger MAPK family, and are involved in regulation of both cell growth and cell death as well as negative regulation of neuronal stem cell proliferation during early brain development [65,66]. ERK, JNK, and p38 MAPK enzymes have also known to be activated by ROS, including H2O2 [67]. Collectively, these results suggest that high levels of intracellular ROS in ATM-deficient cells coupled with hyperactivation of MAPK kinases could potentially contribute to reductions in neuronal stem cell proliferation and neuronal degeneration [63,64]. High levels of ROS generated in the absence of ATM function also result in defects in self-renewal of HSCs related to MAPK hyperactivity, based on observations that NAC or p38 MAPK inhibitor treatment extends the lifespan of HSCs and is accompanied by down-regulation of p16INK4a [[52], [68], [69]]. Taken together, these results suggest that ATM deficiency causes internal oxidative stress which results in pathology specific to stem cell and neuronal cell populations.
Another major source of oxidative stress in humans is the family of NADPH oxidase enzymes which can act as ROS producers in phagocytes and other cells by transferring an electron from NADPH to generate superoxide [24]. NOX4 levels are higher in A-T cells and ATM-depleted cells compared to wild-type cells, and depletion of NOX4 by siRNA was shown to suppress high ROS, oxidative DNA damage, and defects in replication and cell growth that occur during senescence in A-T cells [70]. This suggests that functional ATM is required to limit NOX4 levels and that elevation of internal ROS generated by NOX4 contributes to ROS-induced DNA damage and neurodegeneration [70].
ROS-dependent events are also key factors in inflammation [71]. Recent work using rats as a model system for ATM deficiency showed that loss or partial deficiency in ATM generate a high frequency of hind-limb paralysis (although no cerebellar generation) associated with neuroinflammation in the brain and spinal cord [72,73]. Cytosolic DNA was noted in cells from these animals, perhaps a source of pro-inflammatory signals in this model. A recent study of peripheral blood mononuclear cells from A-T patients also showed upregulation of many genes involved in inflammation and immune system regulation [74], consistent with the idea that chronic inflammation is associated with the disease [75] and observations that anti-inflammatory agents such as betamethasone can generate short-term improvement in A-T symptoms [76]. In general, it is not clear why the mouse, rat, and other mammalian A-T models [77] produce mostly subtle effects on cerebellar morphology and function but instead exhibit a range of other features.
1.5. Mitochondria and ROS
Mitochondrial oxidative metabolism is the major source of intracellular ROS [78,79]. Superoxide species are produced by transfer of high energy electrons to oxygen in the electron transfer chain (ETC) and other sites in mitochondria and H2O2 is generated by dismutation of superoxide [80]. This, in turn, can result in production of hydroxyl radicals. Therefore, mitochondrial defects have been closely connected to generation of free radicals, including hydroxyl radicals and superoxide, since they are generated as a by-product of oxidative metabolism [78].
Neurons have a high demand for energy and, accordingly, have high mitochondrial respiration and produce higher mitochondrial ROS basally compared to other cell types [81,82]. Oxidative DNA damage in the human brain increases with age and mtDNA damage is significantly higher than nuclear DNA damage in these cells [83,84]. Impaired mitochondrial function is particularly critical in neuronal cells since it causes a decrease in energy production and contributes to free radical production, which can result in neuronal death. The relationship between mitochondria, oxidative stress, and neuronal degeneration has been extensively studied in various neurodegenerative disorders, such as Parkinson’s disease, Alzheimer’s disease, Amyotrophic lateral sclerosis, and Huntington’s disease [9,82]. Cellular changes observed in these disorders have been shown to correlate with mitochondrial dysfunction and elevated levels of free radicals [[85], [86], [87], [88], [89]]. Since A-T shares some of the same molecular markers of chronic oxidative stress [25], mitochondrial function in ATM-deficient cells has been an area of active research.
1.6. Mitochondrial dysfunction in ATM-deficient cells
Several groups have shown that ATM deficiency results in mitochondrial dysfunction [36,[90], [91], [92], [93], [94]]. mRNA expression levels of mitochondrial DNA repair and ROS scavenging genes, TOP1mt, Prx3, SOD2, and PolG, are significantly upregulated in A-T cells compared to wild-type cells [90]. Another study showed that loss of ATM or its inhibition by small molecule inhibitors reduces expression levels of ribonucleotide reductase (RR) subunits and lowers mitochondrial DNA (mtDNA) levels, demonstrating that ATM is required for mtDNA homeostasis via RR maintenance [93]. These studies suggest that ATM is directly involved in the expression of genes required for mitochondrial function and mtDNA homeostasis (Fig. 2).
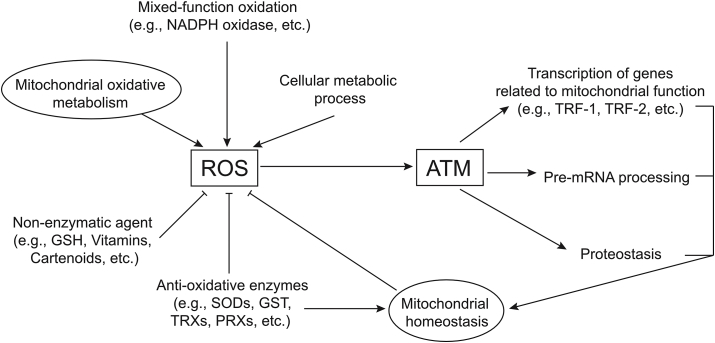
Fig. 2.
Summary of relationships between ROS and ATM functions. ROS is generated from many sources, most prominently mitochondrial oxidative metabolism but also NADPH oxidases and low levels from other cellular metabolic processes (see text for details). Cellular enzymes and small molecule anti-oxidants block accumulation of ROS, but excess levels activate ATM (see Fig. 1). Direct consequences of ATM oxidation include effects on protein homeostasis, pre-mRNA processing, and transcription of genes related to mitochondrial function.
Despite a general consensus that ATM deficiency leads to abnormal mitochondria, reports have been quite variable with respect to specific parameters of mitochondrial health. Work in ATM-deficient human cell lines in culture (patient-derived fibroblasts as well as cells treated with ATM inhibitor) showed that the loss of ATM leads to reduced mitochondrial mass, although measurements were found to be dependent on the growth rate of the cells and the level of confluence in cell culture [93]. In contrast, another study with A-T patient lymphoblasts showed no change in total mitochondrial DNA content compared to wild-type cells, although mitochondrial respiratory function was reduced [90]. Lower mitochondrial mass was observed in human ATM-deficient cells in comparison to wild-type cells by Valentin-Vega et al, although increased numbers of mitochondria as well as increased mitochondrial mass were observed in newly established thymocytes from ATM-/- mice and a dependence on passage number and cell culture conditions was also noted [92]. In this study, impaired mitochondrial turnover (mitophagy) in ATM-deficient fibroblasts was also demonstrated after treatment with the mitochondrial uncoupler CCCP [92]. An in-depth study of the effect of ATM on mitochondrial respiration showed little difference in resting respiration rates but deficiencies in reserve capacity of mitochondria from ATM-deficient mice [94]. The issue of membrane potential in ATM-deficient cells is also reported variably in the literature, with some studies observing higher membrane potential while others report sub-populations with high membrane potential compared to wild-type cells, although the diversity of chemical probes, cells, and conditions used for these studies make it difficult to arrive at specific conclusions [90,92,94].
It is possible that deficiencies in mitophagy, reported by multiple groups [36,92,95], are largely responsible for differences in mitochondrial parameters but that this defect may have different consequences depending on the cell type and conditions of the assay used. In this regard, it is notable that the mitochondrial fraction of human A-T fibroblasts exhibit accumulation of Parkin in the absence of exogenous stress while normal fibroblasts do not [92]. Parkin is recruited to dysfunctional mitochondria and plays a critical role in the autophagic degradation of damaged mitochondria [96]. Consistent with these observations, we recently found that cells expressing an oxidation-deficient mutant ATM (C2991L) exhibit reduced mitochondrial membrane potential and mitophagy compared to cells expressing wild-type ATM [36]. These results align with the hypothesis that disruption of mitochondrial function in ATM-deficient cells is caused by loss of ROS-induced ATM functions. Chow et al also recently found that ROS stimulation of ATM, rather than DNA damage stimulation, leads to up-regulation of mitochondrial function [94], in agreement with this view.
Recent work from Shadel and colleagues also showed that menadione, which generates mitochondrial ROS, promotes formation of the oxidized, activated form of ATM and that this effect is relieved by the addition of MitoTEMPO, a mitochondrial antioxidant [97]. Mitochondria-derived hydrogen peroxide was shown to be responsible for the activation of ATM under these conditions, consistent with work from the Paull laboratory indicating that peroxide activates ATM in vitro as well as in human cells [36,43]. Zhang et al. also showed that deficiency in oxidative activation of ATM reduces antioxidant defenses in human cells by lowering RNA and protein levels of glucose-6-phosphate dehydrogenase (G6PD), an enzyme that plays an important antioxidant role by producing NADPH via the pentose phosphate pathway as well as the production of precursors for nucleotide synthesis [97]. Collectively, this work suggests that ATM acts as a sensor for mitochondrial derived ROS and that this function of the kinase may underlie some of its known effects on cellular metabolism.
Other recent studies have also suggested that specific mitochondrial regulatory factors are directly controlled by ATM. Herrup and colleagues showed that ATM phosphorylates Nuclear respiratory factor 1 (NRF1), a transcription factor required for expression of genes related to mitochondrial transcription, replication, and respiration [94]. ATM phosphorylation of NRF1 on T259 was shown to be required for its homodimerization, nuclear localization, and upregulation of genes promoting mitochondrial function in response to ATP-depletion-induced oxidative stress. Expression of phospho-mimic T259E NRF1 efficiently restored defects in mitochondrial bioenergetics in ATM-deficient neurons, including reduced expression of oxidative phosphorylation-related genes, mitochondrial DNA repair, replication, and transcription [94].
Nuclear erythroid 2-related factor (NRF2), a transcription factor that regulates antioxidant responses [98], was also recently shown to be dysregulated in cells lacking the histone variant H2AX, leading to increased levels of ROS and defects in antioxidant responses [99,100]. H2AX is one of the canonical targets of ATM during a DNA damage response [101]. Deletion of H2AX in the mouse results in impaired motor learning and balance, both of which can be reversed by treatment with the antioxidant NAC [100]. Loss of H2AX also leads to the loss of PGC-1α-dependent gene expression related to mitochondrial biogenesis, suggesting that H2AX indirectly controls mitochondrial homeostasis [102]. Although it is not yet clear if ATM phosphorylation of H2AX is involved in this pathway, these results suggest that histone H2AX and ROS-regulated transcription are important to maintain mitochondrial homeostasis.
Many studies have proven that ATM is predominantly present in the nucleus of most mammalian cells and functions there as a regulator of DNA damage responses [103,104]. However, several studies also showed that ATM is detected in the cytoplasm, including cytoplasmic vesicles and peroxisomes [57,105,106], and that as much as 50% of ATM is extranuclear in neuronal cells [57,[105], [106], [107]]. A recent study also showed that ATM is found in mitochondrial fractions and that treatment of mitochondrial dysfunction by CCCP, a mitochondrial oxidative phosphorylation uncoupler, causes ATM activation in the absence of DNA damage repair signaling [92]. This suggests that ATM might work directly on potential substrates in mitochondria, although localization of ATM within this organelle is still controversial and needs to be addressed in a definitive way in different cell types to resolve these issues. ATM has also been demonstrated to play a role in the peroxisome, an organelle that plays important roles in metabolism and generates (as well as eliminates) ROS species, particularly hydrogen peroxide [108]. ATM was found to be associated with peroxisomes many years ago [57] through a C-terminal region overlapping with the region implicated in ROS regulation and was later shown to regulate the turnover of peroxisomes (pexophagy) through phosphorylation of PEX5 [109].
Interestingly, there has also been a report of Tel1 (the budding yeast ortholog of ATM) functioning to modulate lifespan in response to mitochondrial ROS in yeast [110]. Although Δtel1 yeast strains do not exhibit sensitivity to oxidative stress [111] (T.P. unpublished observations), Schroeder et al found that Tel1 and Rad53 (ortholog of mammalian Chk2) mediate a hormetic response to mitochondrial ROS that extends yeast chronological lifespan by altering histone methylation patterns in heterochromatin [110]. Thus, the role of ATM in ROS sensing may be a more ancient function than previously assumed.
1.7. ATM deficiency and suppression by antioxidants
There have been several attempts to test connections between accumulation of ROS and the clinical phenotype of A-T patients by treatment with antioxidants. Treatment of ATM (−/−) mice with a catalytic antioxidant EUK-189, a salen (N,N′-bis(salicylidene)ethylenediamine) -manganese complex with superoxide dismutase/catalase activities), corrects rotarod performance, decreases brain fatty acids, and increases lifespan [112]. In vitro, Purkinje neurons from both ATM (−/−) mice and mice expressing an ATM mutant allele (7666del9/7666del9, a nine-nucleotide in-frame deletion that is also a common human ATM mutation in A-T patients) show significantly reduced survival and reduced dendritic differentiation compared with cells from ATM (+/+) mice [113]. These phenotypes were corrected by treatment with the antioxidant isoindoline nitroxide (CTMIO), suggesting that oxidative stress plays a key role in defective cerebellar phenotypes in ATM-deficient animals. Treatment of ATM −/− mice with tempol, a nitroxide antioxidant and superoxide dismutase (SOD) mimetic, also increased lifespan, reduced ROS, restored mitochondrial membrane potential, and resulted in an increased latency of thymic lymphomas [91]. These results suggest that correction of internal oxidative stress in ATM deficiency results in at least partial suppression of some aspects of the A-T phenotype, most notably neuronal and stem cell-specific deficiencies.
Experiments using mouse models showed that ATM is also required for the self-renewal of hematopoietic stem cells and the proliferation of T cells, populations which exhibit much lower survival in the absence of ATM function yet can be rescued by treatment with the antioxidant N-acetyl cysteine (NAC) [52,114]. A follow-up study also showed that NAC treatment of ATM-deficient mouse embryonic fibroblasts prevents premature senescence, aberrant T cell receptor rearrangement, impaired class switch recombination, and results in increased survival [68]. Taken together, these diverse reports suggest that aberrant levels of ROS are responsible for at least some of the pathology observed in ATM-deficient or ATM mutant cells.
1.8. Protein aggregation and oxidation
Several neurodegenerative diseases that are more prevalent in the human population compared to A-T are characterized by pronounced neuronal protein aggregation, including Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease [115]. Although the aggregation-prone factors are distinct and the brain region affected in each disease is clearly different, issues related to protein homeostasis and protein oxidation have been broadly implicated in disease pathogenesis for all of these disorders [116].
Considering these attributes, observations that loss of functional ATM leads to oxidative damage to protein and lipids is potentially very important [25,51,52]. For instance, ATM-deficient mice, despite lacking an overt neurological phenotype, show elevated levels of nitrotyrosine modifications in the brain as well as higher levels of lipid peroxidation compared to wild-type mice [51]. Human cells depleted of ATM also exhibit progressive increases in oxidized (carbonylated) proteins as well as increased nuclear proteasome activity upon ATM removal that are consistent with proteostatic stress [117].
Widespread protein aggregation was also recently shown to occur in A-T patient cells, human cancer cell lines depleted of ATM, and cells expressing the C2991L mutant ATM protein that is incapable of activation by oxidative stress [36]. This aggregation was monitored using biochemical fractionation and mass spectrometry-based identification of detergent-resistant protein aggregates. Appearance of the aggregates was amplified by the addition of low-level oxidative stress (arsenite), and was relieved by NAC treatment, demonstrating that defects in protein homeostasis are at least partially dependent on increased ROS in cells lacking ATM or expressing non-functional ATM variants [36]. Approximately 500 proteins were found to be present at statistically significant levels compared to wild-type cells, particularly when ROS levels were elevated. In addition, several of the polypeptides identified among the aggregated fraction are known as familial sources of neuronal toxicity in other neurodegenerative disorders, including C9ORF72, FUS, and TDP43 [[118], [119], [120]]. These observations show that ATM proteostasis dysfunction is related to oxidative stress and suggest the possibility that A-T neuropathology may be related to disruption of normal protein homeostasis.
Lastly, another mechanism potentially underlying the downstream responses of ATM to oxidative stress is through RNA splicing since ATM has been implicated in pre-mRNA processing [121]. The recent study showed that UV-irradiated cells changed spicing widely and up to 40% of this change was partly dependent on ATM activity [121]. If proteins containing disordered regions and low-complexity domains are affected by these splicing deficiencies, this could also contribute to overall protein aggregation since intrinsically disordered proteins are particularly sensitive to oxidation conditions [122].
1.9. Concluding remarks
Although the connections between ATM dysfunction and accumulation of internal oxidative stress have been widely reported, the mechanisms underlying an involvement of ATM in ROS homeostasis are still unclear. ATM has several hundred targets and affects potentially several thousands of associated proteins [36,123,124], so one obvious explanation is that it may phosphorylate and thus directly regulate many enzymes involved in the regulation of ROS levels, some of which have been mentioned here. In addition, mitochondrial ROS is likely regulated by ATM indirectly through transcription of genes related to mitochondrial functions and metabolism (e.g. PPP) as discussed above. Further work is clearly needed to define the exact mechanism of neuronal toxicity observed in A-T patients and to determine the relationship between oxidative stress, DNA damage, defects in protein homeostasis, and mitochondrial abnormalities in order to work toward a cure for this disorder.
Author disclosure statement
No competing financial interests exist.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Gerald Shadel and the members of the Paull laboratory for helpful comments. The work of the authors was funded in part by the Howard Hughes Medical Institute.
Abbreviations
- ATM
Ataxia-telangiectasia mutated
- ROS
reactive oxygen species
- PIKKs
phosphatidylinositol 3-kinase-like kinases
- DSB
double strand break
- TRX
thioredoxin
- TrxR
thioredoxin reductase
- GSH
reduced glutathione
- NADPH
Reduced nicotinamide adenine dinucleotide phosphate
- GSSG
glutathione disulfide
- MRN
Mre11-Rad50-Nbs1
- CL
C2991L allele of ATM, deficient in oxidative activation (6).
- 2RA
R2579A/R2580A allele of ATM, deficient in MRN-mediated activation (36).
- CPT
camptothecin
- HSC
hematopoietic stem cell
- DHE
dihydroethidium
- NAC
N-acetyl cysteine
- MAPK
mitogen-activated protein kinase
- ETC
electron transfer chain
- G6PD
glucose-6-phosphate dehydrogenase
- RR
ribonucleotide reductase
- mtDNA
mitochondrial DNA
- NRF1
Nuclear respiratory factor 1
- NRF2
Nuclear erythroid 2-related factor
References
- 1.Paull T.T. Mechanisms of ATM activation. Annu. Rev. Biochem. 2015;84:711–738. doi: 10.1146/annurev-biochem-060614-034335. [DOI] [PubMed] [Google Scholar]
- 2.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Canc. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 3.Shiloh Y., Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 4.Ditch S., Paull T.T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem. Sci. 2011;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.H., Paull T.T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 6.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 7.Ray P.D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:2407. doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. IJCB. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jena N.R. DNA damage by reactive species: mechanisms, mutation and repair. J. Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 12.Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 13.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A.-F., Teixeira M., Valentine J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkman H.N., Gaetani G.F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. [Google Scholar]
- 17.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta BBA - Gen. Subj. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Hanschmann E.-M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 20.Couto N., Wood J., Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkman H.N., Rolfo M., Ferraris A.M., Gaetani G.F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 1999;274:13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 23.Mustacich D., Powis G. Thioredoxin reductase. Biochem. J. 2000;346(Pt 1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Meitzler J.L., Antony S., Wu Y., Juhasz A., Liu H., Jiang G., Lu J., Roy K., Doroshow J.H. NADPH oxidases: a perspective on reactive oxygen species production in tumor biology. Antioxidants Redox Signal. 2014;20:2873–2889. doi: 10.1089/ars.2013.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barzilai A., Rotman G., Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair Amst. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 26.Sies H., Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 27.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspect. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- 30.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta BBA - Gen. Subj. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Bouayed J., Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stracker T.H., Petrini J.H. The MRE11 complex: starting from the ends. Nat. Rev. Mol. Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 34.Lee J.H., Paull T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 35.Baretić D., Pollard H.K., Fisher D.I., Johnson C.M., Santhanam B., Truman C.M., Kouba T., Fersht A.R., Phillips C., Williams R.L. Structures of closed and open conformations of dimeric human ATM. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J.-H., Mand M.R., Kao C.-H., Zhou Y., Ryu S.W., Richards A.L., Coon J.J., Paull T.T. ATM directs DNA damage responses and proteostasis via genetically separable pathways. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aan5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi M., Rosin M.P., Anderson C.K. Response of fibroblast cultures from ataxia-telanglectasia patients to oxidative stress. Canc. Lett. 1990;54:43–50. doi: 10.1016/0304-3835(90)90089-g. [DOI] [PubMed] [Google Scholar]
- 38.Ward A.J., Olive P.L., Burr A.H., Rosin M.P. Response of fibroblast cultures from ataxia-telangiectasia patients to reactive oxygen species generated during inflammatory reactions. Environ. Mol. Mutagen. 1994;24:103–111. doi: 10.1002/em.2850240205. [DOI] [PubMed] [Google Scholar]
- 39.Green M.H., Marcovitch A.J., Harcourt S.A., Lowe J.E., Green I.C., Arlett C.F. Hypersensitivity of ataxia-telangiectasia fibroblasts to a nitric oxide donor. Free Radic. Biol. Med. 1997;22:343–347. doi: 10.1016/s0891-5849(96)00336-x. [DOI] [PubMed] [Google Scholar]
- 40.Shiloh Y., Tabor E., Becker Y. Abnormal response of ataxia-telangiectasia cells to agents that break the deoxyribose moiety of DNA via a targeted free radical mechanism. Carcinogenesis. 1983;4:1317–1322. doi: 10.1093/carcin/4.10.1317. [DOI] [PubMed] [Google Scholar]
- 41.Yeo A.J., Fantino E., Czovek D., Wainwright C.E., Sly P.D., Lavin M.F. Loss of ATM in airway epithelial cells is associated with susceptibility to oxidative stress. Am. J. Respir. Crit. Care Med. 2017;196:391–393. doi: 10.1164/rccm.201611-2210LE. [DOI] [PubMed] [Google Scholar]
- 42.Yeo A.J., Henningham A., Fantino E., Galbraith S., Krause L., Wainwright C.E., Sly P.D., Lavin M.F. Increased susceptibility of airway epithelial cells from ataxia-telangiectasia to S. pneumoniae infection due to oxidative damage and impaired innate immunity. Sci. Rep. 2019;9:2627. doi: 10.1038/s41598-019-38901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 44.Takao N., Li Y., Yamamoto K. Protective roles for ATM in cellular response to oxidative stress. FEBS Lett. 2000;472:133–136. doi: 10.1016/s0014-5793(00)01422-8. [DOI] [PubMed] [Google Scholar]
- 45.Reichenbach J., Schubert R., Schwan C., Muller K., Bohles H.J., Zielen S. Anti-oxidative capacity in patients with ataxia telangiectasia. Clin. Exp. Immunol. 1999;117:535–539. doi: 10.1046/j.1365-2249.1999.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichenbach J., Schubert R., Schindler D., Muller K., Bohles H., Zielen S. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxidants Redox Signal. 2002;4:465–469. doi: 10.1089/15230860260196254. [DOI] [PubMed] [Google Scholar]
- 47.Barlow C., Hirotsune S., Paylor R., Liyanage M., Eckhaus M., Collins F., Shiloh Y., Crawley J.N., Ried T., Tagle D., Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 48.Kuljis R.O., Xu Y., Aguila M.C., Baltimore D. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci U A. 1997;94:12688–12693. doi: 10.1073/pnas.94.23.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elson A., Wang Y., Daugherty C.J., Morton C.C., Zhou F., Campos-Torres J., Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci U A. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borghesani P.R. From the Cover: abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97:3336–3341. doi: 10.1073/pnas.050584897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barlow C., Dennery P.A., Shigenaga M.K., Smith M.A., Morrow J.D., Roberts L.J., Wynshaw-Boris A., Levine R.L. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci U A. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N., Ikeda Y., Mak T.W., Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 53.Quick K.L., Dugan L.L. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann. Neurol. 2001;49:627–635. [PubMed] [Google Scholar]
- 54.Kamsler A., Daily D., Hochman A., Stern N., Shiloh Y., Rotman G., Barzilai A. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Canc. Res. 2001;61:1849–1854. [PubMed] [Google Scholar]
- 55.Lee K.H., Abe S., Yanabe Y., Matsuda I., Yoshida M.C. Superoxide dismutase activity and chromosome damage in cultured chromosome instability syndrome cells. Mutat. Res. 1990;244:251–256. doi: 10.1016/0165-7992(90)90137-9. [DOI] [PubMed] [Google Scholar]
- 56.Vuillaume M., Calvayrac R., Best-Belpomme M., Tarroux P., Hubert M., Decroix Y., Sarasin A. Deficiency in the catalase activity of xeroderma pigmentosum cell and simian virus 40-transformed human cell extracts. Canc. Res. 1986;46:538–544. [PubMed] [Google Scholar]
- 57.Watters D., Kedar P., Spring K., Bjorkman J., Chen P., Gatei M., Birrell G., Garrone B., Srinivasa P., Crane D.I., Lavin M.F. Localization of a portion of extranuclear ATM to peroxisomes. J. Biol. Chem. 1999;274:34277–34282. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 58.Liu N., Stoica G., Yan M., Scofield V.L., Qiang W., Lynn W.S., Wong P.K.Y. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Investig. J. Tech. Methods Pathol. 2005;85:1471–1480. doi: 10.1038/labinvest.3700354. [DOI] [PubMed] [Google Scholar]
- 59.Gilad S., Chessa L., Khosravi R., Russell P., Galanty Y., Piane M., Gatti R.A., Jorgensen T.J., Shiloh Y., Bar-Shira A. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am. J. Hum. Genet. 1998;62:551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chessa L., Petrinelli P., Antonelli A., Fiorilli M., Elli R., Marcucci L., Federico A., Gandini E. Heterogeneity in ataxia-telangiectasia: classical phenotype associated with intermediate cellular radiosensitivity. Am. J. Med. Genet. 1992;42:741–746. doi: 10.1002/ajmg.1320420524. [DOI] [PubMed] [Google Scholar]
- 61.Toyoshima M., Hara T., Zhang H., Yamamoto T., Akaboshi S., Nanba E., Ohno K., Hori N., Sato K., Takeshita K. Ataxia-telangiectasia without immunodeficiency: novel point mutations within and adjacent to the phosphatidylinositol 3-kinase-like domain. Am. J. Med. Genet. 1998;75:141–144. [PubMed] [Google Scholar]
- 62.Gatti R.A., Becker-Catania S., Chun H.H., Sun X., Mitui M., Lai C.H., Khanlou N., Babaei M., Cheng R., Clark C., Huo Y., Udar N.C., Iyer R.K. The pathogenesis of ataxia-telangiectasia. Learning from a Rosetta Stone. Clin. Rev. Allergy Immunol. 2001;20:87–108. doi: 10.1385/CRIAI:20:1:87. [DOI] [PubMed] [Google Scholar]
- 63.Kim J., Wong P.K. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J. Biol. Chem. 2009;284:14396–14404. doi: 10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J., Wong P.K.Y. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells Dayt. Ohio. 2009;27:1987–1998. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- 65.Sato K., Hamanoue M., Takamatsu K. Inhibitors of p38 mitogen-activated protein kinase enhance proliferation of mouse neural stem cells. J. Neurosci. Res. 2008;86:2179–2189. doi: 10.1002/jnr.21668. [DOI] [PubMed] [Google Scholar]
- 66.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Son Y., Kim S., Chung H.-T., Pae H.-O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 68.Ito K., Takubo K., Arai F., Satoh H., Matsuoka S., Ohmura M., Naka K., Azuma M., Miyamoto K., Hosokawa K., Ikeda Y., Mak T.W., Suda T., Hirao A. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J. Immunol. 2007;178:103–110. doi: 10.4049/jimmunol.178.1.103. [DOI] [PubMed] [Google Scholar]
- 69.Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y., Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 70.Weyemi U., Redon C.E., Aziz T., Choudhuri R., Maeda D., Parekh P.R., Bonner M.Y., Arbiser J.L., Bonner W.M. NADPH oxidase 4 is a critical mediator in Ataxia telangiectasia disease. Proc. Natl. Acad. Sci. U.S.A. 2015;112:2121–2126. doi: 10.1073/pnas.1418139112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quek H., Luff J., Cheung K., Kozlov S., Gatei M., Lee C.S., Bellingham M.C., Noakes P.G., Lim Y.C., Barnett N.L., Dingwall S., Wolvetang E., Mashimo T., Roberts T.L., Lavin M.F. Rats with a missense mutation in Atm display neuroinflammation and neurodegeneration subsequent to accumulation of cytosolic DNA following unrepaired DNA damage. J. Leukoc. Biol. 2017;101:927–947. doi: 10.1189/jlb.4VMA0716-316R. [DOI] [PubMed] [Google Scholar]
- 73.Quek H., Luff J., Cheung K., Kozlov S., Gatei M., Lee C.S., Bellingham M.C., Noakes P.G., Lim Y.C., Barnett N.L., Dingwall S., Wolvetang E., Mashimo T., Roberts T.L., Lavin M.F. A rat model of ataxia-telangiectasia: evidence for a neurodegenerative phenotype. Hum. Mol. Genet. 2017;26:109–123. doi: 10.1093/hmg/ddw371. [DOI] [PubMed] [Google Scholar]
- 74.McGrath-Morrow S.A., Ndeh R., Collaco J.M., Rothblum-Oviatt C., Wright J., O’Reilly M.A., Singer B.D., Lederman H.M. Inflammation and transcriptional responses of peripheral blood mononuclear cells in classic ataxia telangiectasia. PloS One. 2018;13 doi: 10.1371/journal.pone.0209496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaki-Dizaji M., Akrami S.M., Azizi G., Abolhassani H., Aghamohammadi A. Inflammation, a significant player of Ataxia-Telangiectasia pathogenesis? Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al. 2018;67:559–570. doi: 10.1007/s00011-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 76.Hasegawa S., Kumada S., Tanuma N., Tsuji-Hosokawa A., Kashimada A., Mizuno T., Moriyama K., Sugawara Y., Shirai I., Miyata Y., Nishida H., Mashimo H., Hasegawa T., Hosokawa T., Hisakawa H., Uematsu M., Fujine A., Miyata R., Sakuma H., Kashimada K., Imai K., Morio T., Hayashi M., Mizutani S., Takagi M. Long-term evaluation of low-dose betamethasone for ataxia telangiectasia. Pediatr. Neurol. 2019;100:60–66. doi: 10.1016/j.pediatrneurol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Beraldi R., Chan C.-H., Rogers C.S., Kovács A.D., Meyerholz D.K., Trantzas C., Lambertz A.M., Darbro B.W., Weber K.L., White K.A.M., Rheeden R.V., Kruer M.C., Dacken B.A., Wang X.-J., Davis B.T., Rohret J.A., Struzynski J.T., Rohret F.A., Weimer J.M., Pearce D.A. A novel porcine model of ataxia telangiectasia reproduces neurological features and motor deficits of human disease. Hum. Mol. Genet. 2015;24:6473–6484. doi: 10.1093/hmg/ddv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nissanka N., Moraes C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592:728–742. doi: 10.1002/1873-3468.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolisetty S., Jaimes E. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int. J. Mol. Sci. 2013;14:6306–6344. doi: 10.3390/ijms14036306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watts M.E., Pocock R., Claudianos C. Brain energy and oxygen metabolism: emerging role in normal function and disease. Front. Mol. Neurosci. 2018;11 doi: 10.3389/fnmol.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592:692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 83.Canugovi C., Misiak M., Ferrarelli L.K., Croteau D.L., Bohr V.A. The role of DNA repair in brain related disease pathology. DNA Repair. 2013;12:578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeppesen D.K., Bohr V.A., Stevnsner T. DNA repair deficiency in neurodegeneration. Prog. Neurobiol. 2011;94:166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith E.F., Shaw P.J., De Vos K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 86.Winklhofer K.F., Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 87.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Carmo C., Naia L., Lopes C., Rego A.C. Mitochondrial dysfunction in Huntington’s disease. Adv. Exp. Med. Biol. 2018;1049:59–83. doi: 10.1007/978-3-319-71779-1_3. [DOI] [PubMed] [Google Scholar]
- 89.Burté F., Carelli V., Chinnery P.F., Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 90.Ambrose M., Goldstine J.V., Gatti R.A. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum. Mol. Genet. 2007;16:2154–2164. doi: 10.1093/hmg/ddm166. [DOI] [PubMed] [Google Scholar]
- 91.Schubert R., Erker L., Barlow C., Yakushiji H., Larson D., Russo A., Mitchell J.B., Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004;13:1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 92.Valentin-Vega Y.A., Maclean K.H., Tait-Mulder J., Milasta S., Steeves M., Dorsey F.C., Cleveland J.L., Green D.R., Kastan M.B. Mitochondrial dysfunction in ataxia telangiectasia. Blood. 2012;119(6):1490–1500. doi: 10.1182/blood-2011-08-373639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eaton J.S., Lin Z.P., Sartorelli A.C., Bonawitz N.D., Shadel G.S. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J. Clin. Invest. 2007;117:2723–2734. doi: 10.1172/JCI31604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chow H.-M., Cheng A., Song X., Swerdel M.R., Hart R.P., Herrup K. ATM is activated by ATP depletion and modulates mitochondrial function through NRF1. J. Cell Biol. 2019;218:909–928. doi: 10.1083/jcb.201806197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang E.F., Kassahun H., Croteau D.L., Scheibye-Knudsen M., Marosi K., Lu H., Shamanna R.A., Kalyanasundaram S., Bollineni R.C., Wilson M.A., Iser W.B., Wollman B.N., Morevati M., Li J., Kerr J.S., Lu Q., Waltz T.B., Tian J., Sinclair D.A., Mattson M.P., Nilsen H., Bohr V.A. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metabol. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McWilliams T.G., Muqit M.M. PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 2017;45:83–91. doi: 10.1016/j.ceb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y., Lee J.-H., Paull T.T., Gehrke S., D’Alessandro A., Dou Q., Gladyshev V.N., Schroeder E.A., Steyl S.K., Christian B.E., Shadel G.S. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aaq0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S., Pommier Y. GammaH2AX and cancer. Nat. Rev. Canc. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weyemi U., Paul B.D., Snowman A.M., Jailwala P., Nussenzweig A., Bonner W.M., Snyder S.H. Histone H2AX deficiency causes neurobehavioral deficits and impaired redox homeostasis. Nat. Commun. 2018;9:1526. doi: 10.1038/s41467-018-03948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burma S., Chen B.P., Murphy M., Kurimasa A., Chen D.J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 102.Weyemi U., Paul B.D., Bhattacharya D., Malla A.P., Boufraqech M., Harraz M.M., Bonner W.M., Snyder S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2019;116:7471–7476. doi: 10.1073/pnas.1820245116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown K.D., Ziv Y., Sadanandan S.N., Chessa L., Collins F.S., Shiloh Y., Tagle D.A. The ataxia-telangiectasia gene product, a constitutively expressed nuclear protein that is not up-regulated following genome damage. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1840–1845. doi: 10.1073/pnas.94.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lakin N.D., Weber P., Stankovic T., Rottinghaus S.T., Taylor A.M., Jackson S.P. Analysis of the ATM protein in wild-type and ataxia telangiectasia cells. Oncogene. 1996;13:2707–2716. [PubMed] [Google Scholar]
- 105.Barlow C., Ribaut-Barassin C., Zwingman T.A., Pope A.J., Brown K.D., Owens J.W., Larson D., Harrington E.A., Haeberle A.M., Mariani J., Eckhaus M., Herrup K., Bailly Y., Wynshaw-Boris A. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci U A. 2000;97:871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim D.S., Kirsch D.G., Canman C.E., Ahn J.H., Ziv Y., Newman L.S., Darnell R.B., Shiloh Y., Kastan M.B. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci U A. 1998;95:10146–10151. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J., Han Y.R., Plummer M.R., Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr. Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker C.L., Pomatto L.C.D., Tripathi D.N., Davies K.J.A. Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol. Rev. 2018;98:89–115. doi: 10.1152/physrev.00033.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J., Tripathi D.N., Jing J., Alexander A., Kim J., Powell R.T., Dere R., Tait-Mulder J., Lee J.H., Paull T.T., Pandita R.K., Charaka V.K., Pandita T.K., Kastan M.B., Walker C.L. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat. Cell Biol. 2015;17:1259–1269. doi: 10.1038/ncb3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schroeder E.A., Raimundo N., Shadel G.S. Epigenetic silencing mediates mitochondria stress-induced longevity. Cell Metabol. 2013;17:954–964. doi: 10.1016/j.cmet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Higgins V.J., Alic N., Thorpe G.W., Breitenbach M., Larsson V., Dawes I.W. Phenotypic analysis of gene deletant strains for sensitivity to oxidative stress. Yeast Chichester Engl. 2002;19:203–214. doi: 10.1002/yea.811. [DOI] [PubMed] [Google Scholar]
- 112.Browne S.E., Roberts L.J., Dennery P.A., Doctrow S.R., Beal M.F., Barlow C., Levine R.L. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Radic. Biol. Med. 2004;36:938–942. doi: 10.1016/j.freeradbiomed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Chen P., Peng C., Luff J., Spring K., Watters D., Bottle S., Furuya S., Lavin M.F. Oxidative stress is responsible for deficient survival and dendritogenesis in purkinje neurons from ataxia-telangiectasia mutated mutant mice. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:11453–11460. doi: 10.1523/JNEUROSCI.23-36-11453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bagley J., Singh G., Iacomini J. Regulation of oxidative stress responses by ataxia-telangiectasia mutated is required for T cell proliferation. J. Immunol. Baltim. 1950;178:4757–4763. doi: 10.4049/jimmunol.178.8.4757. 2007. [DOI] [PubMed] [Google Scholar]
- 115.Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 116.Gandhi J., Antonelli A.C., Afridi A., Vatsia S., Joshi G., Romanov V., Murray I.V.J., Khan S.A. Protein misfolding and aggregation in neurodegenerative diseases: a review of pathogeneses, novel detection strategies, and potential therapeutics. Rev. Neurosci. 2019;30:339–358. doi: 10.1515/revneuro-2016-0035. [DOI] [PubMed] [Google Scholar]
- 117.Poletto M., Yang D., Fletcher S.C., Vendrell I., Fischer R., Legrand A.J., Dianov G.L. Modulation of proteostasis counteracts oxidative stress and affects DNA base excision repair capacity in ATM-deficient cells. Nucleic Acids Res. 2017;45:10042–10055. doi: 10.1093/nar/gkx635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prasad A., Bharathi V., Sivalingam V., Girdhar A., Patel B.K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019;12 doi: 10.3389/fnmol.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balendra R., Isaacs A.M. C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 2018;14:544–558. doi: 10.1038/s41582-018-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng H., Gao K., Jankovic J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 2014;10:337–348. doi: 10.1038/nrneurol.2014.78. [DOI] [PubMed] [Google Scholar]
- 121.Tresini M., Warmerdam D.O., Kolovos P., Snijder L., Vrouwe M.G., Demmers J.A., van Ij W.F., Grosveld F.G., Medema R.H., Hoeijmakers J.H., Mullenders L.H., Vermeulen W., Marteijn J.A. The core spliceosome as target and effector of non-canonical ATM signalling. Nature. 2015;523:53–58. doi: 10.1038/nature14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Darling A.L., Uversky V.N. Intrinsic disorder and posttranslational modifications: the darker side of the biological dark matter. Front. Genet. 2018;9 doi: 10.3389/fgene.2018.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S.P., Elledge S.J. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 124.Bensimon A., Schmidt A., Ziv Y., Elkon R., Wang S.Y., Chen D.J., Aebersold R., Shiloh Y. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci. Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]